Abstract
Slit diaphragms are essential components of the glomerular filtration apparatus, as changes in these junctions are the hallmark of proteinuric diseases. Slit diaphragms, considered specialized adherens junctions, contain both unique membrane proteins (e.g., nephrin, podocin, and Neph1) and typical adherens junction proteins (e.g., P-cadherin, FAT, and catenins). Whether slit diaphragms also contain tight junction proteins is unknown. Here, immunofluorescence, immunogold labeling, and cell fractionation demonstrated that rat slit diaphragms contain the tight junction proteins JAM-A (junctional adhesion molecule A), occludin, and cingulin. We found these proteins in the same protein complexes as nephrin, podocin, CD2AP, ZO-1, and Neph1 by cosedimentation, coimmunoprecipitation, and pull-down assays. PAN nephrosis increased the protein levels of JAM-A, occludin, cingulin, and ZO-1 several-fold in glomeruli and loosened their attachment to the actin cytoskeleton. These data extend current information about the molecular composition of slit diaphragms by demonstrating the presence of tight junction proteins, although slit diaphragms lack the characteristic morphologic features of tight junctions. The contribution of these proteins to the assembly of slit diaphragms and potential signaling cascades requires further investigation.
Slit diaphragms are specialized cell-cell junctions located between mature podocytes that have fascinated cell biologists and nephrologists for more than 40 yr.1 In contrast to podocytes, most other epithelial cells have junctional complexes composed of tight junctions and adherens junctions. Slit diaphragms originate from typical apical junctional complexes between primordial epithelia of the early S-shaped body. These junctional complexes migrate in a zipper-like fashion to the base of the cell where tight junctions persist as interdigitation of the foot processes begins.2,3 Slit diaphragms appear during the capillary loop stage and gradually replace tight junctions. In many diseases associated with proteinuria and foot process loss or effacement, there is a rerun in reverse of this developmental sequence, and tight junctions reappear between adjoining foot processes.4–6
Major progress has been made recently in establishing the molecular make-up of the slit diaphragms. Several integral membrane proteins, including nephrin,7 podocin,8 and Neph1,9 not found in other junctions, have been identified as slit diaphragm components. Slit diaphragms are currently looked upon as signaling platforms in which nephrin and Neph1 transduce major signals that serve to maintain the filtration slits and to regulate podocyte shape through interaction of slit diaphragm proteins with the actin cytoskeleton.10 Mutations in nephrin,7 Neph1,9 and podocin8 have been linked to diseases associated with foot process effacement and proteinuria. In addition to these specialized slit diaphragm proteins, a number of other proteins that are associated with junctions in other locations are also concentrated at the slit diaphragms, including the adherens junction proteins P-cadherin,11 FAT,12 β-catenin,11 and p120 catenin;13 scaffold proteins such as ZO-1,14,15 CD2AP,16 MAGI-2,17 and CASK;13 and actin binding proteins, including IQGAP17 and α-actinin 4.17,18 Because slit diaphragms share some morphologic features with adherens junctions and contain P-cadherin and catenins, slit diaphragms are assumed to represent modified adherens junctions.11 However, several scaffold proteins that are often associated with tight junctions (i.e., ZO-1,14,15 MAGI-1,19 MAGI-2,17 and CASK13) are present at slit diaphragms and have been shown to associate with nephrin. Based on their derivation from typical tight junctions2 and the fact that they are replaced by tight junctions in nephrosis,4,6 we reasoned that slit diaphragms might also contain membrane proteins normally associated with tight junctions.
In this paper, we used morphological, biochemical, and bioinformatics techniques to investigate the expression of representative tight junction proteins in glomeruli in situ and in slit diaphragm-enriched fractions. Here, we document the presence of several tight junction proteins in slit diaphragms and demonstrate their interactions with slit diaphragm proteins in both normal and PAN nephrotic rats. The presence of tight junction proteins in slit diaphragms adds a new dimension to understanding the organization and functions of these junctions.
RESULTS
JAM-A is Present in the Foot Processes of Podocytes
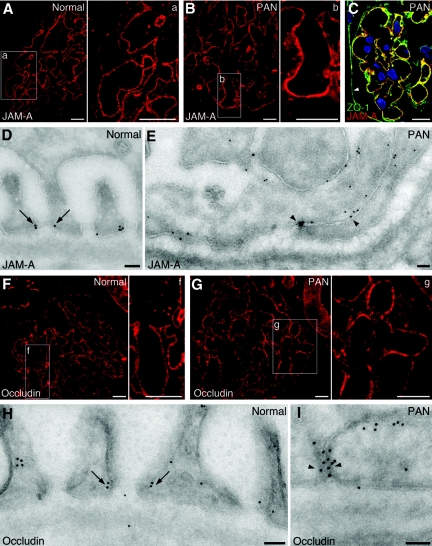
Previously, we established that the scaffolding proteins ZO-114 and CASK13 are present in foot processes of podocytes. In other epithelial cells, JAM-A, an integral membrane protein of tight junctions,20 directly interacts with both ZO-121 and CASK.22 This led us to investigate whether JAM-A is also present in the slit diaphragms of podocytes. Immunofluorescence labeling of semithin cryosections with a JAM-A-specific antibody (Figure S1) revealed that JAM-A is found in glomeruli from both normal and PAN-treated rats (Figure 1, A and B). By double labeling, we observed that JAM-A was distributed in a punctate pattern along the foot process layer of podocytes where it corresponds in distribution to that of ZO-1 (Figure 1C). Immunoelectron microscopy established that JAM-A is indeed concentrated along the slit diaphragms in normal glomeruli (Figure 1D) and is associated with the tight junctions that appear between the podocytes in PAN nephrotic glomeruli (Figure 1E).4
Figure 1.
AM-A and occludin are present between foot processes of podocytes of normal and PAN nephrotic rats. By immunofluorescence, both JAM-A (A, a, and B, b) and occludin (F, f and G, g) are distributed in a punctate pattern along normal rat glomerular capillaries and in glomeruli from 7 d PAN-nephrotic rats (PAN). The boxed areas in A, B, F, and G are enlarged in a, b, f, and g. (C) By double labeling, JAM-A (red) and ZO-1 (green) colocalize (yellow) along glomerular capillaries at the base of the podocytes. Note that parietal cells (arrowhead) stain for ZO-1 but not for JAM-A. Nuclei were stained with DAPI (blue). (D) JAM-A and occludin (H) (10 nm gold) are seen to be concentrated at the slit diaphragms (arrows) in normal glomeruli. In PAN glomeruli, JAM-A (E) and occludin (I) are concentrated along the extensive tight or occluding junctions (arrowheads) (Bar = 10 μm in A-C and F-G; Bar = 50 nm in D, E, H, and I)
Occludin is Also Present at Slit Diaphragms
Next, we investigated whether occludin, another tight junction protein, is also present on foot processes of podocytes and obtained similar findings to those obtained for JAM-A. By immunofluorescence, occludin was distributed in a punctate pattern along glomerular capillaries in both normal (Figure 1F) and PAN-nephrotic (Figure 1G) rats. By immunoelectron microscopy, occludin was concentrated at slit diaphragms between foot processes in normal glomeruli (Figure 1H) and at tight or occluding junctions in PAN glomeruli (Figure 1I).
Tight Junction Proteins Cofractionate with Slit Diaphragm Markers in Slit Diaphragm-Enriched Fractions Prepared from Normal Glomeruli
Using a procedure worked out for isolation of apical junctional complexes from MDCK cells,23 we prepared slit-diaphragm-enriched fractions from normal rat glomeruli on self-forming linear 10 to 20 to 30% iodixanol density gradients. We cross-linked proteins before homogenization to avoid partial loss of peripheral membrane proteins and disassembly of protein complexes during preparation of junctional fractions.
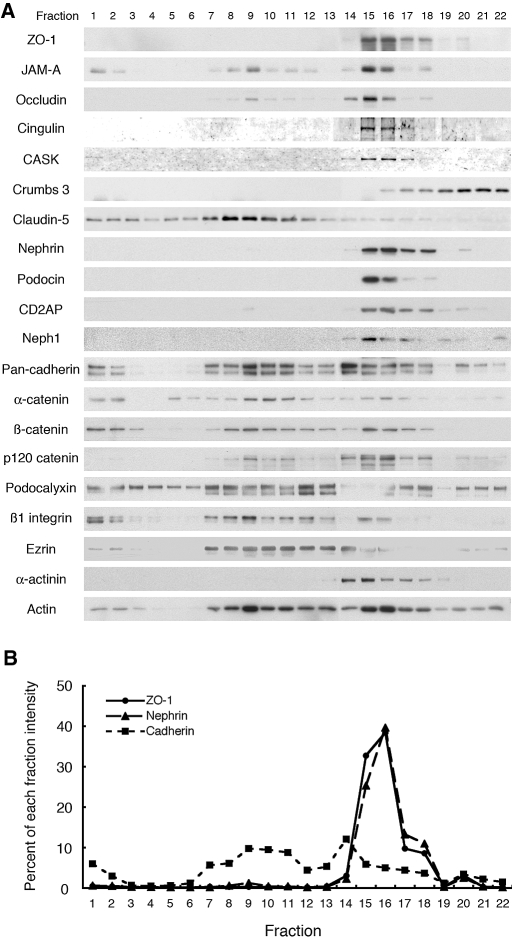
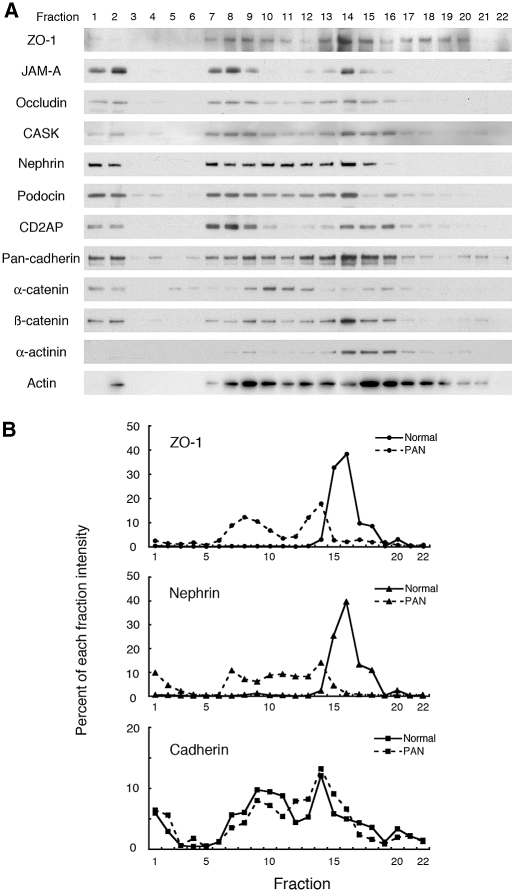
We examined the distribution of 20 proteins in these gradients that are expressed in different domains of the foot processes of podocytes (Figure 2A). Proteins in the same membrane domains cofractionate in the gradient, whereas proteins in different domains sediment at different densities.23,24 In normal glomeruli, the tight junction proteins JAM-A, occludin, cingulin, ZO-1, and CASK cofractionated in fractions 15 to 18 with slit diaphragm markers, nephrin, podocin, CD2AP, and Neph1 (Figure 2A). In contrast, adherens junction proteins (cadherins and catenins) were broadly distributed (fractions 7 to 18) in the gradients and partially overlapped with slit diaphragm-enriched fractions (Figure 2B). Claudin-5, a specific marker for endothelial cell tight junctions25 sedimented in much lighter fractions (fractions 1 to 12), and Crumbs 3, also a tight junction protein, sedimented in heavier fractions than slit diaphragms. Podocalyxin, an apical membrane protein on foot processes,14 and β1 integrin, a basal protein,26 were also diffusely distributed in the gradients. Podocalyxin cofractionated with its binding partner, ezrin,27,28 and α-actinin partially cofractionated with its binding partners, α-catenin and β1 integrin. The finding that tight junction proteins are concentrated in slit diaphragm-enriched fractions suggests that they are associated with slit diaphragms.
Figure 2.
Distribution of junctional fractions from normal rat glomeruli in iodixanol density gradient centrifugation. (A) A Postnuclear supernatant (PNS) prepared from glomeruli isolated from normal rats was mixed with an equal amount of iodixanol [final concentration 30% (wt/vol)] and overlaid with equal volumes of 20% and 10% iodixanol. After centrifugation at 350,000 × g for 3 h at 4°C, fractions were collected from the top, separated by SDS-PAGE, and immunoblotted for the indicated 20 proteins expressed in foot processes of podocytes. In normal rat glomeruli, the tight junction proteins ZO-1, JAM-A, occludin, cingulin, and CASK cofractionate with the slit diaphragm enriched fractions marked by nephrin, podocin, CD2AP, and Neph1 in fractions 15 to 18. By contrast, the adherens junction proteins, cadherin and α-, β-, and p120 catenins, are broadly distributed in the iodixanol gradients (fractions 7 to 18) and only partially cofractionate with slit diaphragm proteins. Crumbs 3, another tight junction protein, is concentrated in heavier fractions (18–22) than other tight junction and slit diaphragm markers. Claudin-5, a specific marker for endothelial tight junctions, is concentrated in lighter fractions (1–12). Podocalyxin, an apical domain protein in foot processes, and β1 integrin, a basal protein, are broadly distributed in the gradient but are concentrated mostly in lighter fractions (1–13). Podocalyxin cofractionates with its binding partner, ezrin (8–14). (B) Densitometric analysis showing the % of the total nephrin (triangles with dashed line), pan-cadherin (squares with dotted line), and ZO-1 (circles with solid line) present in the fractions shown in “A.” The distribution of ZO-1 (used as a tight junction marker) in each of the fractions is similar to that of nephrin (slit diaphragm marker) but not to that of pan-cadherin (adherens junction marker).
ZO-1, JAM-A, Occludin, and Cingulin Cosediment in Sucrose Gradients with Slit Diaphragm Proteins, but not Adherens Junction Proteins
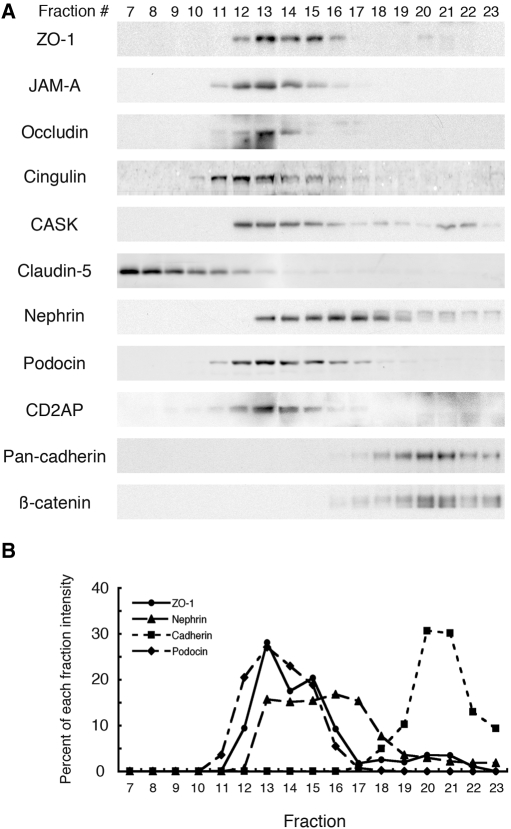
Cosedimentation analysis of detergent-solubilized proteins is commonly used to gain information on whether proteins associate with one another in the same multiprotein complexes.17,29 When we subjected glomerular lysates to velocity gradient centrifugation on sucrose gradients, we found that the tight junction proteins ZO-1, JAM-A, occludin, cingulin, and CASK cosedimented in fractions 12 to 16 with nephrin, podocin, and CD2AP (Figure 3). In contrast, cadherin and β-catenin cosedimented in heavier fractions (fractions 19 to 23), and claudin-5, a specific marker for tight junctions of endothelial cells, sedimented in lighter fractions (fractions 7 to 10). These results suggest that tight junction proteins are present in the same protein complexes as slit diaphragm proteins.
Figure 3.
Tight junction proteins cosediment in the same protein complexes as slit diaphragm proteins, but not adherens junction proteins. Glomeruli isolated from normal rats were lysed in 0.5% Nonidet P-40 and 0.25% Triton X-100, and the lysate was loaded on top of a 5 to 25% discontinuous sucrose gradient and centrifuged as described in Concise Methods. Fractions were collected from the top of the gradient and separated by SDS-PAGE followed by immunoblotting with the indicated antibodies. (A) ZO-1, JAM-A, occludin, and cingulin are concentrated in fractions 12 to 16 and cosediment with nephrin, podocin, CD2AP, and CASK. In contrast, cadherin and β-catenin distributed in heavier fractions (19–23) of the gradient. Claudin-5, a marker for endothelial tight junctions, is concentrated in lighter fractions (7–10). (B) Densitometric analysis of ZO-1 (circles with solid line), nephrin (triangles with dashed line), pan-cadherin (squares with dotted line), and podocin (diamonds with hatched line). ZO-1 partially cosediments with nephrin and podocin but not with pan-cadherin.
Tight Junctions Proteins are Present in Nephrin Multiprotein Complexes
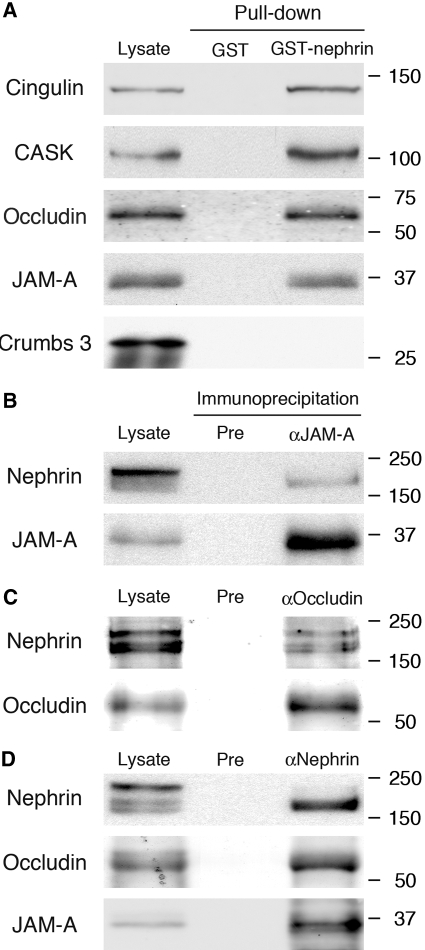
To establish whether tight junction and slit diaphragm proteins are found in the same protein complexes, we carried out pulldown and coimmunoprecipitation assays. GST-nephrin pulled down JAM-A, occludin, cingulin, and CASK, but not crumbs 3 from glomerular lysates (Figure 4A). Moreover, we detected nephrin in immunoprecipitates obtained with either JAM-A (Figure 4B) or occludin (Figure 4C) antibodies. Similarly, we detected JAM-A and occludin in immunoprecipitates obtained with anti-nephrin IgG (Figure 4D). These results demonstrate that JAM-A, occludin, and cingulin, but not crumbs 3, are components of slit diaphragm protein complexes.
Figure 4.
Tight junction proteins form a protein complex with nephrin but not crumbs 3. (A) JAM-A, occludin, cingulin, and CASK are pulled down with GST-nephrin tail from normal glomerular lysates. In contrast, crumbs 3 was not pulled down. Isolated glomeruli from normal rats were lysed in 1% Nonidet P-40 with 100 μmol/L potassium iodide. Twenty micrograms nephrin cytoplasmic domain fused to GST (GST-nephrin) or GST alone (GST) were incubated with 400 μg glomerular lysate at 4°C for 16 h and immunoblotted with JAM-A, occludin, cingulin, and CASK antibodies. (B to D) Nephrin can be coimmunoprecipitated with anti-JAM-A (B) and anti-occludin (C) IgG. Similarly, JAM-A and occludin can be coimmunopreciptated with anti-nephrin IgG (D). Isolated glomeruli from normal rats were lysed in 1% Triton X-100 and 0.5% Nonidet P-40. Immunoprecipitation was performed on 500 μg glomerular lysate from normal rats with JAM-A (αJAM-A), occludin (αOccludin), nephrin (αNephrin), or preimmune (Pre) IgG. Glomerular lysates (25 μg) were included as controls.
Expression of Tight Junction Proteins is Increased in PAN Nephrosis
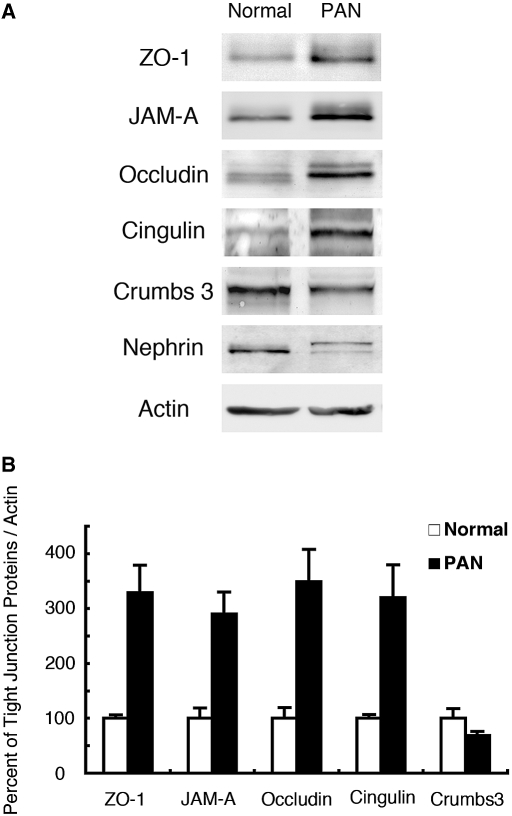
Slit diaphragms are known to decrease in number and be replaced by tight junctions in nephrosis.6,4 To find out whether the expression of tight junction proteins is changed in nephrosis, we performed immunoblotting on glomerular lysates from normal versus PAN-treated rats (Figure 5). ZO-1, JAM-A, occludin, and cingulin were dramatically increased (approximately 330%, 290%, 350%, and 320%, respectively) in PAN-treated rats. By contrast, both crumbs 3 and nephrin were decreased (approximately 70%) (Figure 5, A and B).
Figure 5.
Comparative immunoblot analysis of expression of tight junction proteins in glomeruli from normal versus PAN nephrotic rats. (A) The protein levels of ZO-1, JAM-A, occludin, and cingulin are dramatically increased in glomerular lysates from PAN-treated rats (PAN) compared with normal, whereas expression of crumbs 3 and nephrin are decreased. β-actin was used as a control for protein load. Glomeruli isolated from two rats were lysed in RIPA buffer; 20 μg glomerular lysate was immunoblotted with the indicated antibodies. We performed experiments three times with similar results. (B) Densitometric analyses of data in “A” showing expression of tight junction proteins relative to β-actin in normal (open bars) and PAN (closed bars) glomeruli. Data are mean ± SEM values.
Redistribution in Iodixanol Gradients of Tight Junction and Slit Diaphragm Proteins Prepared from Nephrotic Glomeruli
Next, we compared the behavior of junctional membranes prepared from PAN nephrotic versus normal rats after separation on iodixanol density gradients. In glomeruli from 7 d PAN-treated rats, ZO-1, JAM-A, and occludin as well as nephrin, podocin, and CD2AP were less dense as they floated up and were broadly distributed across fractions 7 to 15 (Figure 6, A and B), whereas in normal glomeruli, they were restricted to fractions 15 to 18 (see Figure 2). The distribution of cadherins and catenins was unchanged as they were broadly distributed (Figure 6B). These results indicate that membrane domains containing the slit diaphragms and tight junctions obtained from nephrotic glomeruli are less dense than those from normal glomeruli.
Figure 6.
Iodixanol density gradient centrifugation of postnuclear supernatant prepared from glomeruli of PAN nephrotic rats. (A) In glomeruli from PAN-treated rats, fractions containing ZO-1, JAM-A, occludin, and CASK and the slit diaphragm markers, nephrin, podocin, and CD2AP, float up to regions of lower density and are broadly distributed in fractions 7 to 16. In normal glomeruli, they are restricted to fraction 15 to 18 (compare with Figure 2). In contrast, the distribution of adherens junction proteins (cadherins, α- and β-catenins) is similar in normal and PAN rats. (B) Densitometric analysis demonstrating that the distribution of nephrin and ZO-1 is shifted to lighter fractions in PAN (dashed lines) compared with normal (solid lines) glomeruli (data from Figure 2A). There is little change in the distribution of cadherins.
Tight Junction and Slit Diaphragm Proteins Dissociate from the Actin Cytoskeleton in Nephrotic Glomeruli
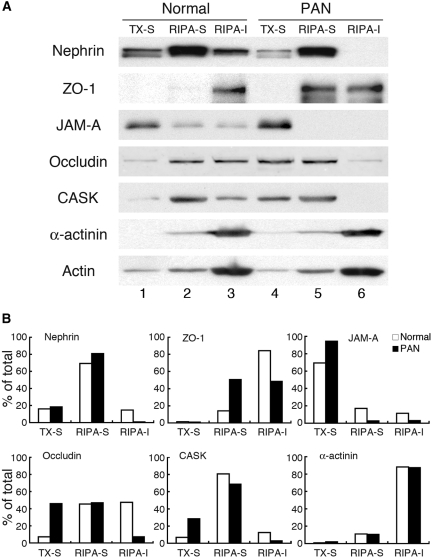
Next, we used sequential detergent extraction to assess the interactions between tight junction and slit diaphragm proteins and the actin cytoskeleton in normal and nephrotic glomeruli.30 Glomeruli isolated from normal and PAN-treated rats were sequentially solubilized in 0.5% Triton X-100 and RIPA buffer, and the distribution of junctional proteins in Triton X-100-soluble, RIPA-soluble, and RIPA-insoluble fractions was determined. In normal glomerular lysates we found nephrin, JAM-A, occludin, and CASK in all three fractions and ZO-1 and α-actinin mainly distributed in the RIPA-insoluble fraction (Figure 7). With PAN treatment, we saw a significant increase in the detergent extractability of nephrin, ZO-1, JAM-A, occludin, and CASK, as greater amounts of these proteins were detected in the Triton-soluble or RIPA-soluble fractions, and only ZO-1 was detected in the RIPA-insoluble fraction. By contrast, the distribution of α-actinin and actin did not change noticeably after PAN treatment. These results indicate that nephrin, ZO-1, JAM-A, occludin, and CASK (but not α-actinin) partially dissociate from the actin cytoskeleton in glomeruli from PAN rats. Subsequent results obtained after extraction with potassium iodide (KI), an actin depolymerizing agent,27 supported this conclusion and showed that the ratios of actin-associated proteins were decreased in PAN glomeruli (Figure S3).
Figure 7.
Sequential detergent extraction of proteins from glomeruli of normal and PAN-treated rats. (A) Immunoblot of normal and PAN-treated glomeruli after sequential detergent extraction. In normal glomeruli, nephrin, JAM-A, occludin, and CASK distributed in all three fractions (lanes 1 to 3), whereas ZO-1 and α-actinin mainly distributed in the RIPA-insoluble (RIPA-I) (lane 3) fraction. PAN treatment resulted in a significant increase in the detergent solubility of nephrin, ZO-1, JAM-A, occludin, and CASK in that increased amounts of these proteins were found in the Triton-soluble (compare lanes 1 and 4) and RIPA-soluble fraction (compare lanes 2 and 5), and only ZO-1 was detected in the RIPA-insoluble fraction (lane 6). The distribution of α-actinin and actin in each fraction is similar in normal and PAN rats. Glomeruli isolated from normal and PAN-treated rats were sequentially solubilized in 0.5% Triton X-100 and RIPA lysis buffer and separated into Triton X-100-soluble (TX-S), RIPA-soluble (RIPA-S), and RIPA-insoluble fractions, followed by immunoblotting. (B) Densitometric analysis showing the % of total protein in the Triton X-100-soluble, RIPA-soluble, and RIPA-insoluble fractions in normal (open bars) and PAN rats (closed bars) in “A.” Each bar indicates the percent of the total band intensity in each fraction.
Analysis of Protein-Protein Interaction Map
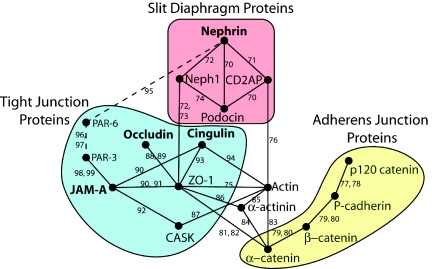
To better understand the molecular links between slit diaphragm, tight junction, and adherens junction proteins, we took advantage of the interologous interaction database,31,32 which is an amalgam of several databases containing protein-protein interactions validated from the literature and predicted interactions based on high throughput studies across multiple species. Our resulting protein-protein interaction map (Figure 8) contains only experimentally validated interactions. It shows that the closest link between nephrin and JAM-A, occludin, and cingulin is through Neph1 and ZO-1. A second possible connection is through PAR-6 and PAR-3. The entire group of tight junction proteins studied is on average only two protein-protein interactions away from a slit diaphragm protein. This is in keeping with our finding that tight junction proteins are enriched in the same fractions as slit diaphragm proteins. In contrast, adherens junction proteins have no direct link to slit diaphragm proteins. They are linked only indirectly through actin or ZO-1. On average, 3.5 protein-protein interactions are required to reach the closest slit diaphragm protein from an adherens junction protein, which is in keeping with our finding of a relative lack of enrichment of adherens junction proteins in slit diaphragm fractions (Figure 2). In addition to the direct interactions, a number of indirect interactions occur among all three groups of proteins, especially via kinases (not shown). Such signaling interactions imply coordination among the slit diaphragm, tight junction, and adherens junction groups of proteins.
Figure 8.
Proposed protein-protein interaction map for the podoctye slit diaphragm. Interactions between proteins analyzed in this study are shown based on interactions listed in the I2D database32,69 and validated in the literature (references shown). The slit diaphragm (red) and tight junction (blue) groups of proteins are linked by a direct interaction between Neph1 and ZO-1. This is the shortest link between nephrin and occludin, cingulin, and JAM-A (shown in bold). An alternative interaction may occur between nephrin and JAM-A via PAR-3 and PAR-6 (shown with dashed line and smaller font), which were not examined experimentally in the current study. In contrast to tight junction proteins, the adherens junction proteins studied lack direct interactions with slit diaphragm proteins, but indirect interactions are present (e.g., α-catenin is linked indirectly to CD2AP through actin and indirectly to Neph1 through ZO-1).
DISCUSSION
In this study, we report data obtained from cell fractionation, immunofluorescence, and immunoelectron microscopy establishing the presence of the tight junction proteins JAM-A, occludin, and cingulin in slit diaphragms of normal glomeruli. We have also shown that these tight junction proteins are found in the same multiprotein complexes as nephrin because they cosediment with nephrin in sucrose gradients and interact with nephrin in immunoprecipitation and pulldown assays.
The presence of tight junction proteins in glomerular junctions has wide functional implications. Until now, the glomerular slit diaphragms have been regarded to be highly specialized adherens junctions whose unique permeability properties are created by the presence of nephrin, podocin, and Neph1–3 that are specific components of slit diaphragms.11 We have shown that three proteins that usually make up tight junctions, occludin, JAM-A, and cingulin, are also expressed in slit diaphragms, and yet morphologically recognizable tight junctions are absent. We have further shown that in PAN nephrosis protein levels of JAM-A, occludin, and cingulin (Figure 5) as well as ZO-115 are increased at a time when nephrin and podocin expression goes down,33,34 expression of claudin-6 increases,35 and tight junctions form. From these findings, it is tempting to speculate that the expression of slit diaphragm proteins and their incorporation into tight junction protein complexes late in glomerular development may facilitate the formation of mature slit diaphragms. Conversely, decreased expression of nephrin33 together with increased expression of occludin, JAM-A, cingulin, and claudin-6 may facilitate the assembly of typical tight junctions in PAN nephrosis.
Based on the above, the discovery of tight junction proteins at slit diaphragms has led to the following working hypothesis: In developing glomeruli, nephrin, podocin, and Neph1 are synthesized late in the capillary loop stage, form complexes with tight junction proteins, facilitate maturation of tight junctions into slit diaphragms, and prevent assembly of tight junctions in normal, mature glomeruli. In PAN nephrotic glomeruli, slit diaphragm proteins are reduced, and tight junction proteins are increased, allowing tight junctions to assemble.
Some time ago, Caulfield et al4 found that junctions formed in PAN nephrosis have the morphologic features characteristic of tight junction proteins by routine EM and freeze fracture. Our findings that the protein levels of JAM-A, occludin, and cingulin as well as ZO-115 are increased in glomeruli from PAN-nephrotic rats is in keeping with the presence of tight junctions. Furthermore, our results based on sequential detergent extraction and KI treatment indicate that nephrin, JAM-A, occludin, CASK, and ZO-1 partially dissociate from the actin cytoskeleton in PAN nephrosis. The association between slit diaphragm proteins and membrane proteins of the foot processes and the actin cytoskeleton is highly dynamic, and tight junction proteins can be added to the growing list of membrane proteins whose attachment to actin is required for maintenance of the normal organization of the foot processes and filtration slits. Previously, nephrin,31 podocalyxin28 (apical domain protein), and α3β1 integrin36 (basal domain protein) were found to dissociate from the actin cytoskeleton under pathologic conditions associated with foot process effacement, and it was suggested or implied that this is the cause of the structural changes (effacement) of the foot processes.
Until now, the regulation of the functions of slit diaphragms has been centered on nephrin signaling. However, the presence of tight junctions has important functional implications for signaling from slit diaphragms, as tight junctions serve as signaling centers that play important roles in orchestrating cell polarity, cell proliferation, and differentiation as well as regulating paracellular permeability in other epithelia.37–39 Tight junctions are composed of transmembrane proteins (occludin, JAMs, claudins) and scaffolding proteins (ZO-1, ZO-2, ZO-3, cingulin, MAGI-1, 2, 3) that link the integral membrane proteins to the actin cytoskeleton.37,40 They are also closely associated with signaling molecules (e.g., aPKC, Rab3B, Rab13, Gαs, Gα12, GEFH1) and polarity complexes (e.g., PAR-3, PAR-6, Crumbs, PALS-1).37–39 Occludin and claudins are believed to be responsible for the classical sealing function of tight junctions.41 Interestingly, occludin interacts with c-yes, a Src-family kinase, in MDCK cells,42 and c-yes can phosphorylate nephrin at slit diaphragms.43
JAM-A20 is believed to participate in both the establishment of cell polarity and in tight junction formation.20,44,45 JAM-A can bind several PDZ domain proteins, including ZO-1 and CASK. Recently, several JAM family members, JAM-4 and the Coxackievirus/adenovirus receptor (CAR), were reported to be present in foot processes of podocytes,46,47 but whether JAM-4 is associated with slit diaphragms is controversial.19,48 Cingulin interacts with several junctional and cytoskeletal proteins including ZO-1, ZO-2, ZO-3, AF-6, myosin,49 and actin.50 The function of cingulin has not fully been established, but it has been suggested to regulate cell proliferation.51 Although claudins are expressed in developing and PAN nephrotic glomeruli35 as well as the junctions between parietal epithelial cells,52 they have not been convincingly localized at slit diaphragms in normal glomeruli.21,22,53–55
Recently, it was reported that in nephrosis the polarity of foot processes is lost,56 which correlates with our finding of decreased levels of crumbs 3. Intriguingly, we could not detect an association between crumbs 3 (a polarity protein associated with the tight junction)57 and nephrin in pulldown and cofractionation assays, and in contrast to the tight junction proteins studied, the expression of crumbs 3 is decreased in PAN nephrosis.
Our protein interaction map suggests that ZO-1 may be the key protein that links tight junction proteins and slit diaphragm proteins and in turn links both to the cytoskeleton. We previously reported that ZO-114,15 and CASK13,17 are present at slit diaphragms and foot processes of podocytes. Our results indicate that CASK forms a complex with nephrin and behaves the same as tight junction proteins in cosedimentation assays (see Figure 4). In other epithelia, these MAGUK scaffold proteins serve to cluster occludin,58 claudins,59 and JAMs21 at tight junctions and to anchor them to the actin cytoskeleton.60–62
A key unanswered question that has puzzled us6 and others for many years is why increased permeability of glomeruli and proteinuria are associated with conversion of slit diaphragms to tight junctions. Among the possible factors to be considered are that 1) the tight junctions in nephrotic glomeruli are discontinuous with gaps that allow protein leakage; 2) damage to the GBM results in increased permeability of the GBM;6,63 3) damage to the GBM results in areas of focal detachment of podocytes from the GBM; and 4) detachment of junctional proteins from the actin cytoskeleton. Our current results as well as those of others emphasize that detachment of tight junction and slit diaphragm proteins from the actin cytoskeleton is a common denominator of the podocyte's response to injury.
In conclusion, the present study documents the presence of tight junction proteins in the slit diaphragms and nephrin multiprotein complexes, and thus, significantly extends current information on the molecular composition of slit diaphragms by demonstrating that they are a highly specialized variant of the tight junction. The tight junction proteins JAM-A, occludin, ZO-1, cingulin, and CASK are known to be components of unique and extensive signaling networks. Thus, their presence in slit diaphragms as well as in the modified occluding junctions seen in nephrosis provides mechanistic insights into the assembly of these junctions and greatly extends their potential protein interactions and signaling repertoire.
CONCISE METHODS
Materials
Chemical reagents were from Sigma (St. Louis, MO) or Fisher Biotech (Tustin, CA), and detergents were from Sigma or Calbiochem (San Diego, CA). Kodak Biomax MR film was obtained from Fisher Biotech.
Antibodies
Mouse anti-nephrin mAb (043; for immunoblotting)13 and rabbit anti-nephrin 029 (for immunoprecipitaton)64 were raised against the extracellular domain of rat nephrin (amino acids 749 to 1030) and the cytoplasmic tail of nephrin, respectively. Rabbit anti-podocin (P0372) and mouse anti-β-actin mAb (AC-15) were from Sigma. Rabbit anti-CD2AP polyclonal IgG (R209) was raised against amino acids 331 to 637 of mouse CD2AP.65 Mouse anti-JAM-A mAb (for immunofluorescence) and rabbit anti-JAM-A (for immunoblotting) were obtained from Dr. Charles Parkos (Emory University) and Invitrogen (Carlsbad, CA), respectively. Rabbit anti-Neph1 and anti-β-catenin antibodies were obtained from Drs. Lawrence Holzman (University of Michigan) and James Nelson (Stanford University), respectively. Affinity-purified rabbit anti-pan-cadherin (recognizes P-, N-, E-, and R-cadherins), anti-α-catenin, anti-ZO-1, anti-occludin, anti-JAM-C, anti-CASK (calcium/calmodulin-dependent serine protein kinase), and mouse anti-claudin-5 IgG were from Invitrogen. Rabbit anti-cingulin and anti-crumbs 3 antibodies were obtained from Dr. Sandra Citi (University of Geneva, Switzerland) and Dr. André Le Bivic (Faculté des Sciences de Luminy, France), respectively. Rabbit anti-JAM-B antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti-p120 catenin was from BD Transduction Laboratories (BD Biosciences, San Jose, CA), and mouse anti-podocalyxin mAb 5A was described previously.66 Rabbit anti-ezrin (3C12) was purchased from NeoMarkers (Fremont, CA), mouse α-actinin mAb (clone AT6.172) was purchased from Chemicon (Temecula, CA), and horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse IgG were from Promega (Madison, WI).
Preparation of Glomerular Lysates
Glomerular fractions (containing > 95% glomeruli; see Supplementary Figure S1) were isolated from kidney cortices of male Sprague-Dawley rats (150 g; Charles River Laboratories, Boston, MA) by graded sieving as described previously.27 Glomerular lysates were prepared by incubation of isolated glomeruli in either 1% Nonidet P-40, 20 mmol/L HEPES, pH 7.5, 150 mmol/L NaCl, 100 mmol/L potassium iodide (KI)27 (for GST pulldown), in 1% TX-100, 0.5% Nonidet P-40, 150 mmol/L NaCl, 10 mmol/L Tris-HCl, pH 7.6, 1 mmol/L ethylenediamine-tetraacetic acid (EDTA), 1 mmol/L ethylene glycol-bis(oxyethylenenitrilo)tetraacetic acid (EGTA)13 (for immunoprecipitation), or in 0.1% SDS, 0.5% deoxycholate, 1% TX-100, 20 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM EDTA (for immunoblotting of tight junction proteins) supplemented with 1x Complete, EDTA-free proteinase inhibitor cocktail (Roche, Mannheim, Germany), 50 mmol/L sodium fluoride, and 1 mmol/L sodium vanadate at 4°C for 60 min. Detergent-insoluble material was removed by centrifugation (10,000 × g for 10 min).
Induction of PAN Nephrosis
Male rats (150 g) were injected once intraperitoneally with PAN (15 mg/100 g body weight) as described previously. Animals were sacrificed on day 7 after injection. All animal experiments were done according to the NIH Guidelines for the Care and Use of Laboratory Animals.
SDS-PAGE and Immunoblotting
Protein concentration was measured by Quick Start™ Bradford Dye Reagent (Bio-Rad Laboratories, Inc., Hercules, CA). Proteins were separated on 8 or 10% SDS-PAGE under reducing conditions and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore Corp., Bedford, MA) using a Minigel-Transfer-Unit (Bio-Rad Laboratories) as described previously.30 β-actin was used as an internal control. Protein bands were detected by enhanced chemiluminescence (Supersignal; Pierce Biotechnology Inc., Rockford, IL) and quantified using Image J analysis software (NIH, Bethesda, MD).
Immunofluorescence Microscopy
Kidneys were immersion fixed with 2% paraformaldehyde (PFA) for 30 min. For preparation of semithin sections, PFA-fixed kidneys were cryoprotected and frozen in liquid nitrogen.67 Semithin cryosections (0.5 μm) were cut with a Leica Ultracut UCT microtome equipped with an FCS cryoattachment at −100°C and incubated with primary antibodies overnight at 4°C followed by detection with Alexa 594 goat anti-rabbit and anti-mouse IgG in PBS containing 5% fetal calf serum for 2 h at room temperature. Samples were examined with a Zeiss Axiophot microscope (Carl Zeiss Inc., Thornwood, NY). Images were collected with the ORCA-ER camera (Hamamatsu, Bridgewater, NJ) using Scion Image Version 1.59 (Scion Corp., Frederick, MA) and processed using Adobe Photoshop CS3 (Adobe Systems, San Jose, CA).
Immunoelectron Microscopy
Rat kidneys were fixed in 4% PFA for 45 min followed by 15 min in 8% PFA, cryoprotected in sucrose and frozen in liquid nitrogen as above. Ultrathin cryosections (80 nm) were cut and processed as described previously.27 Sections were incubated with rabbit anti-occludin, JAM-A, or cingulin IgG for 16 h at 4°C followed by incubation with anti-rabbit gold conjugates (10 nm) for 2 h at room temperature. Sections were then adsorption-stained and examined with a JOEL EX-II electron microscope.30
GST-Pulldown Assays
GST and GST-nephrin tail fusion proteins were expressed in Escherichia coli BL21 (DE3) (Stratagene, LA Jolla, CA) and purified on glutathione-Sepharose beads (Amersham Biosciences) as described previously.13 Glomerular lysates were precleared for 1 h at 4°C, followed by incubation at 4°C for 16 h with GST-nephrin tail or GST alone (20 μg each) immobilized on beads. Beads were washed five times with lysis buffer and boiled in 2x sample buffer for immunoblotting.
Coimmunoprecipitation
Glomerular lysates were precleared with protein G plus/protein A-agarose beads (Calbiochem, San Diego, CA) at 4°C for 1 h and incubated with anti-nephrin, anti-JAM-A, anti-occludin, or preimmune IgG (2 μg) for 16 h at 4°C. Immune complexes were bound to protein G plus/protein A-agarose beads at 4°C for 1 h, washed three times with lysis buffer, and boiled in 2x sample buffer for immunoblotting.
Preparation of Junctional Fractions
Enriched junctional fractions were prepared on self-forming linear 10 to 20 to 30% iodixanol density gradients24 as reported previously for isolation of junctions from MDCK cells.23 Isolated glomeruli were chemically cross-linked with dithiobis (succinimidylpropionate) (DSP; Pierce Chemical) before mechanical breakage of glomeruli.68 Freshly prepared 200 μg/ml DSP in PBS was added to the isolated glomeruli for 20 min at room temperature with gentle rocking followed by quenching in quenching buffer (120 mM NaCl, 10 mM Tris, pH 7.4, +50 mM NaH4Cl). After cross-linking, glomeruli were homogenized in 0.25 M sucrose in 20 mM HEPES-KOH, pH 7.2, 90 mM KOAc, 2 mM Mg(OAc)2 buffer using a loose Dounce homogenizer (20 strokes). Glomerular homogenates were transferred to microcentrifuge tubes and centrifuged at 1000 × g for 10 min to obtain the postnuclear supernatant (PNS). The PNS was mixed with equal amounts of iodixanol [final concentration 30% (wt/vol); Nycomed, Oslo, Norway] and overlaid with equal volumes of 20% and 10% iodixanol. After centrifugation at 58,000 rpm (350,000 × g) for 3 h at 4°C in a Beckman Coulter SW 60 Ti rotor, fractions were collected from the top of the gradient. Gradients were highly reproducible and fraction densities comparable between gradients.
Cosedimentation Assays
Velocity gradient centrifugation was performed as described previously.29 Isolated glomeruli were lysed in 0.5% Nonidet P-40, 0.25% Triton X-100, 10 mmol/L Tris-HCl, pH 7.6, 150 mmol/L NaCl, 1 mmol/L EDTA with 1x Complete, 50 mmol/L sodium fluoride, and 1 mmol/L sodium vanadate, homogenized with very loose Dounce homogenizer (3 strokes), and incubated at 4°C for 90 min. Detergent-insoluble material was removed by centrifugation (15,000 × g for 15 min at 4°C). Glomerular lysates were applied on top of a 5 to 10 to 15 to 20 to 25% discontinuous sucrose gradient. Sucrose solutions were prepared in lysis buffer containing 0.05% Nonidet P-40 and 0.025% Triton X-100. After centrifugation at 44,000 rpm (200,000 × g) for 15 h at 4°C in Beckman Coulter SW 60 Ti rotor, fractions were collected from the top of the gradient and their sedimentation coefficients were assessed using protein molecular weight standards (Sigma) with known S values.
Differential Detergent Extraction of Glomeruli
Sequential extraction of proteins was carried out as described previously.30 In brief, glomeruli were lysed in 500 μl Triton X-100 lysis buffer (0.5% TX-100, 20 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM EDTA with 1x Complete, 50 mmol/L sodium fluoride, and 1 mmol/L sodium vanadate) at 4°C for 30 min and centrifuged at 15,000 × g at 4°C for 30 min. The insoluble pellet was resuspended in the same volume of RIPA buffer (0.1% SDS, 0.5% deoxycholate, 1% TX-100, 20 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM EDTA with 1x Complete, 50 mmol/L sodium fluoride, and 1 mmol/L sodium vanadate) at 4°C for 30 min and centrifuged as above. The resultant pellet was solubilized by sonication in the same volume of 1x sample buffer as the supernatant, and equal volumes of the fractions were analyzed by immunoblotting.
Actin Depolymerization
Isolated glomeruli were extracted on ice for 30 min with lysis buffer (1% Triton X-100, 20 mM Tris-HCl, pH 6.2, 10 mM NaCl, and 1.5 mM MgCl2).30 Insoluble material was separated by centrifugation at 15,000 × g at 4°C for 30 min and then incubated with 0.6 M potassium iodide (KI) in the same buffer to depolymerize F-actin.27,68 The supernatants containing actin-associated proteins released by KI were separated by centrifugation as above. The resultant pellet was solubilized by sonication in the same volume of sample buffer, and equal volumes of each fraction were analyzed by immunoblotting.
Creation of the Protein-Protein Interaction Map
The protein-protein interaction map was based on protein-protein interactions listed in the I2D database32,69 and validated in the literature. For simplicity it was restricted mainly to direct protein-protein interactions between proteins studied in this manuscript. The layout of the map was designed using NAViGaTOR software (http://ophid.utoronto.ca/navigator/).
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant DK17724 (to M.G.F).
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “How to Build a Tight but Permeable Glomerular Junction,” on pages 1420–1421.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Pavenstadt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Reeves W, Caulfield JP, Farquhar MG: Differentiation of epithelial foot processes and filtration slits: Sequential appearance of occluding junctions, epithelial polyanion, and slit membranes in developing glomeruli. Lab Invest 39: 90–100, 1978 [PubMed] [Google Scholar]
- 3.Reeves WH, Kanwar YS, Farquhar MG: Assembly of the glomerular filtration surface. Differentiation of anionic sites in glomerular capillaries of newborn rat kidney. J Cell Biol 85: 735–753, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caulfield JP, Reid JJ, Farquhar MG: Alterations of the glomerular epithelium in acute aminonucleoside nephrosis. Evidence for formation of occluding junctions and epithelial cell detachment. Lab Invest 34: 43–59, 1976 [PubMed] [Google Scholar]
- 5.Kurihara H, Anderson JM, Farquhar MG: Increased Tyr phosphorylation of ZO-1 during modification of tight junctions between glomerular foot processes. Am J Physiol 268: F514–524, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Farquhar MG, Palade GE: Glomerular permeability. II. Ferritin transfer across the glomerular capillary wall in nephrotic rats. J Exp Med 114: 699–716, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein—nephrin is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, Mathur BN, Turner CA, Geske R, Montgomery CA, Starbuck M, Brandt M, Gupta A, Ramirez-Solis R, Zambrowicz BP, Powell DR: Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol 21: 4829–4836, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benzing T: Signaling at the slit diaphragm. J Am Soc Nephrol 15: 1382–1391, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Reiser J, Kriz W, Kretzler M, Mundel P: The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol 11: 1–8, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Inoue T, Yaoita E, Kurihara H, Shimizu F, Sakai T, Kobayashi T, Ohshiro K, Kawachi H, Okada H, Suzuki H, Kihara I, Yamamoto T: FAT is a component of glomerular slit diaphragms. Kidney Int 59: 1003–1012, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Lehtonen S, Lehtonen E, Kudlicka K, Holthofer H, Farquhar MG: Nephrin forms a complex with adherens junction proteins and CASK in podocytes and in Madin-Darby canine kidney cells expressing nephrin. Am J Pathol 165: 923–936, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnabel E, Anderson JM, Farquhar MG: The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol 111: 1255–1263, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurihara H, Anderson JM, Kerjaschki D, Farquhar MG: The altered glomerular filtration slits seen in puromycin aminonucleoside nephrosis and protamine sulfate-treated rats contain the tight junction protein ZO-1. Am J Pathol 141: 805–816, 1992 [PMC free article] [PubMed] [Google Scholar]
- 16.Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS: Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286: 312–315, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Lehtonen S, Ryan JJ, Kudlicka K, Iino N, Zhou H, Farquhar MG: Cell junction-associated proteins IQGAP1, MAGI-2, CASK, spectrins, and alpha-actinin are components of the nephrin multiprotein complex. Proc Natl Acad Sci U S A 102: 9814–9819, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kos CH, Le TC, Sinha S, Henderson JM, Kim SH, Sugimoto H, Kalluri R, Gerszten RE, Pollak MR: Mice deficient in alpha-actinin-4 have severe glomerular disease. J Clin Invest 111: 1683–1690, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirabayashi S, Mori H, Kansaku A, Kurihara H, Sakai T, Shimizu F, Kawachi H, Hata Y: MAGI-1 is a component of the glomerular slit diaphragm that is tightly associated with nephrin. Lab Invest 85: 1528–1543, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E: Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol 142: 117–127, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bazzoni G, Martinez-Estrada OM, Orsenigo F, Cordenonsi M, Citi S, Dejana E: Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J Biol Chem 275: 20520–20526, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Estrada OM, Villa A, Breviario F, Orsenigo F, Dejana E, Bazzoni G: Association of junctional adhesion molecule with calcium/calmodulin-dependent serine protein kinase (CASK/LIN-2) in human epithelial caco-2 cells. J Biol Chem 276: 9291–9296, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Vogelmann R, Nelson WJ: Fractionation of the epithelial apical junctional complex: reassessment of protein distributions in different substructures. Mol Biol Cell 16: 701–716, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeaman C: Ultracentrifugation-based approaches to study regulation of Sec6/8 (exocyst) complex function during development of epithelial cell polarity. Methods 30: 198–206, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Morita K, Sasaki H, Furuse M, Tsukita S: Endothelial claudin: Claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol 147: 185–194, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerjaschki D, Ojha PP, Susani M, Horvat R, Binder S, Hovorka A, Hillemanns P, Pytela R: A beta 1-integrin receptor for fibronectin in human kidney glomeruli. Am J Pathol 134: 481–489, 1989 [PMC free article] [PubMed] [Google Scholar]
- 27.Orlando RA, Takeda T, Zak B, Schmieder S, Benoit VM, McQuistan T, Furthmayr H, Farquhar MG: The glomerular epithelial cell anti-adhesin podocalyxin associates with the actin cytoskeleton through interactions with ezrin. J Am Soc Nephrol 12: 1589–1598, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Schmieder S, Nagai M, Orlando RA, Takeda T, Farquhar MG: Podocalyxin activates RhoA and induces actin reorganization through NHERF1 and Ezrin in MDCK cells. J Am Soc Nephrol 15: 2289–2298, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Biemesderfer D, Dekan G, Aronson PS, Farquhar MG: Biosynthesis of the gp330/44-kDa Heymann nephritis antigenic complex: Assembly takes place in the ER. Am J Physiol 264: F1011–1020, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Takeda T, McQuistan T, Orlando RA, Farquhar MG: Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest 108: 289–301, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan H, Takeuchi E, Taylor GA, McLaughlin M, Brown D, Salant DJ: Nephrin dissociates from actin, and its expression is reduced in early experimental membranous nephropathy. J Am Soc Nephrol 13: 946–956, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Brown KR, Jurisica I: Online predicted human interaction database. Bioinformatics 21: 2076–2082, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Kawachi H, Koike H, Kurihara H, Yaoita E, Orikasa M, Shia MA, Sakai T, Yamamoto T, Salant DJ, Shimizu F: Cloning of rat nephrin: Expression in developing glomeruli and in proteinuric states. Kidney Int 57: 1949–1961, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Kawachi H, Koike H, Kurihara H, Sakai T, Shimizu F: Cloning of rat homologue of podocin: Expression in proteinuric states and in developing glomeruli. J Am Soc Nephrol 14: 46–56, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Zhao L, Yaoita E, Nameta M, Zhang Y, Cuellar LM, Fujinaka H, Xu B, Yoshida Y, Hatakeyama K, Yamamoto T: Claudin-6 localized in tight junctions of rat podocytes. Am J Physiol Regul Integr Comp Physiol 294: R1856–1862, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R: Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development 122: 3537–3547, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Aijaz S, Balda MS, Matter K: Tight junctions: Molecular architecture and function. Int Rev Cytol 248: 261–298, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Zahraoui A, Louvard D, Galli T: Tight junction, a platform for trafficking and signaling protein complexes. J Cell Biol 151: F31–36, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matter K, Balda MS: Signalling to and from tight junctions. Nat Rev Mol Cell Biol 4: 225–236, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Shin K, Fogg VC, Margolis B: Tight junctions and cell polarity. Annu Rev Cell Dev Biol 22: 207–235, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Mitic LL, Van Itallie CM, Anderson JM: Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: Lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol 279: G250–254, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Chen YH, Lu Q, Goodenough DA, Jeansonne B: Nonreceptor tyrosine kinase c-Yes interacts with occludin during tight junction formation in canine kidney epithelial cells. Mol Biol Cell 13: 1227–1237, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verma R, Wharram B, Kovari I, Kunkel R, Nihalani D, Wary KK, Wiggins RC, Killen P, Holzman LB: Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J Biol Chem 278: 20716–20723, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Rehder D, Iden S, Nasdala I, Wegener J, Brickwedde MK, Vestweber D, Ebnet K: Junctional adhesion molecule-a participates in the formation of apico-basal polarity through different domains. Exp Cell Res 312: 3389–3403, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Mandell KJ, McCall IC, Parkos CA: Involvement of the junctional adhesion molecule-1 (JAM1) homodimer interface in regulation of epithelial barrier function. J Biol Chem 279: 16254–16262, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Hirabayashi S, Tajima M, Yao I, Nishimura W, Mori H, Hata Y: JAM4, a junctional cell adhesion molecule interacting with a tight junction protein, MAGI-1. Mol Cell Biol 23: 4267–4282, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagai M, Yaoita E, Yoshida Y, Kuwano R, Nameta M, Ohshiro K, Isome M, Fujinaka H, Suzuki S, Suzuki J, Suzuki H, Yamamoto T: Coxsackievirus and adenovirus receptor, a tight junction membrane protein, is expressed in glomerular podocytes in the kidney. Lab Invest 83: 901–911, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Harita Y, Miyauchi N, Karasawa T, Suzuki K, Han GD, Koike H, Igarashi T, Shimizu F, Kawachi H: Altered expression of junctional adhesion molecule 4 in injured podocytes. Am J Physiol Renal Physiol 290: F335–344, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Cordenonsi M, D'Atri F, Hammar E, Parry DA, Kendrick-Jones J, Shore D, Citi S: Cingulin contains globular and coiled-coil domains and interacts with ZO-1, ZO-2, ZO-3, and myosin. J Cell Biol 147: 1569–1582, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D'Atri F, Citi S: Cingulin interacts with F-actin in vitro. FEBS Lett 507: 21–24, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Guillemot L, Citi S: Cingulin regulates claudin-2 expression and cell proliferation through the small GTPase RhoA. Mol Biol Cell 17: 3569–3577, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S: Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol 13: 875–886, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Ebnet K, Schulz CU, Meyer Zu Brickwedde MK, Pendl GG, Vestweber D: Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J Biol Chem 275: 27979–27988, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Ebnet K, Suzuki A, Horikoshi Y, Hirose T, Meyer Zu Brickwedde MK, Ohno S, Vestweber D: The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM). EMBO J 20: 3738–3748, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohno S: Intercellular junctions and cellular polarity: The PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr Opin Cell Biol 13: 641–648, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Hartleben B, Schweizer H, Lubben P, Bartram MP, Moller CC, Herr R, Wei C, Neumann-Haefelin E, Schermer B, Zentgraf H, Kerjaschki D, Reiser J, Walz G, Benzing T, Huber TB: Neph-Nephrin proteins bind the Par3-Par6-atypical Protein kinase C (aPKC) complex to regulate podocyte cell polarity. J Biol Chem 283: 23033–23038, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hong Y, Stronach B, Perrimon N, Jan LY, Jan YN: Drosophila Stardust interacts with Crumbs to control polarity of epithelia but not neuroblasts. Nature 414: 634–638, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM: The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 273: 29745–29753, 1998 [DOI] [PubMed] [Google Scholar]
- 59.Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S: Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol 147: 1351–1363, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fanning AS, Ma TY, Anderson JM: Isolation and functional characterization of the actin binding region in the tight junction protein ZO-1. FASEB J 16: 1835–1837, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Cohen AR, Woods DF, Marfatia SM, Walther Z, Chishti AH, Anderson JM: Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells. J Cell Biol 142: 129–138, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boettner B, Govek EE, Cross J, Van Aelst L: The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin. Proc Natl Acad Sci U S A 97: 9064–9069, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caulfield JP, Farquhar MG: The permeability of glomerular capillaries of aminonuceoside nephrotic rats to graded dextrans. J Exp Med 142: 61–83, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahola H, Heikkila E, Astrom E, Inagaki M, Izawa I, Pavenstadt H, Kerjaschki D, Holthofer H: A novel protein, densin, expressed by glomerular podocytes. J Am Soc Nephrol 14: 1731–1737, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Lehtonen S, Ora A, Olkkonen VM, Geng L, Zerial M, Somlo S, Lehtonen E: In vivo interaction of the adapter protein CD2-associated protein with the type 2 polycystic kidney disease protein, polycystin-2. J Biol Chem 275: 32888–32893, 2000 [DOI] [PubMed] [Google Scholar]
- 66.Miettinen A, Dekan G, Farquhar MG: Monoclonal antibodies against membrane proteins of the rat glomerulus. Immunochemical specificity and immunofluorescence distribution of the antigens. Am J Pathol 137: 929–944, 1990 [PMC free article] [PubMed] [Google Scholar]
- 67.McCaffery JM, Farquhar MG: Localization of GTPases by indirect immunofluorescence and immunoelectron microscopy. Methods Enzymol 257: 259–279, 1995 [DOI] [PubMed] [Google Scholar]
- 68.Yeaman C, Grindstaff KK, Nelson WJ: Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells. J Cell Sci 117: 559–570, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brown KR, Jurisica I: Unequal evolutionary conservation of human protein interactions in interologous networks. Genome Biol 8: R95, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P: Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest 108: 1621–1629, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shih NY, Li J, Cotran R, Mundel P, Miner JH, Shaw AS: CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. Am J Pathol 159: 2303–2308, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu G, Kaw B, Kurfis J, Rahmanuddin S, Kanwar YS, Chugh SS: Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J Clin Invest 112: 209–221, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huber TB, Schmidts M, Gerke P, Schermer B, Zahn A, Hartleben B, Sellin L, Walz G, Benzing T: The carboxyl terminus of Neph family members binds to the PDZ domain protein zonula occludens-1. J Biol Chem 278: 13417–13421, 2003 [DOI] [PubMed] [Google Scholar]
- 74.Sellin L, Huber TB, Gerke P, Quack I, PAvenstadt H, Walz G: NEPH1 defines a novel family of podocin interacting proteins. FASEB J 17: 115–117, 2003 [DOI] [PubMed] [Google Scholar]
- 75.Fanning AS, Ma TY, Anderson JM: Isolation and functional characterization of the actin- binding region in the tight junction protein ZO-1. FASEB J 16: 1835–1837, 2002 [DOI] [PubMed] [Google Scholar]
- 76.Welsch T, Endlich N, Gokce G, Doroshenko E, Simpson JC, Kriz W, Shaw AS, Endlich K: Association of CD2AP with dynamic actin on vesicles in podocytes. Am J Physiol Renal Physiol 289: F1134–1143, 2005 [DOI] [PubMed] [Google Scholar]
- 77.Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J, Zhang Z: Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol 14: 8333–8342, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Miyazawa K, Kitamura N, Johnson KR, Wheelock MJ, Matsuyoshi N, Takeichi M, Ito F: Association of p120, a tyrosine kinase substrate, with E-cadherin/catenin complexes. J Cell Biol 128: 949–957, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H: Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci 107(Pt 12): 3655–3663, 1994 [DOI] [PubMed] [Google Scholar]
- 80.Jou TS, Stewart DB, Stappert J, Nelson WJ, Marrs JA: Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex. Proc Natl Acad Sci U S A 92: 5067–5071, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Itoh M, Yonemura S, Nagafuchi A, Tsukita S: A 220-kD undercoat-constitutive protein: its specific localization at cadherin-based cell-cell adhesion sites. J Cell Biol 115: 1449–1462, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Itoh M, Nagafuchi A, Moroi S, Tsukita S: Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol 138: 181–192, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wheelock MJ, Knudsen KA: Cadherins and associated proteins. In Vivo 5: 505–513, 1991 [PubMed] [Google Scholar]
- 84.Knudsen KA, Soler AP, Johnson KR, Wheelock MJ: Interaction of α-actinin with the cadherin/catenin cell-cell adhesion complex via α-catenin. J Cell Biol 130: 67–77, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maruyama K, Ebashi S: Alpha-actinin, a new structural protein from striated muscle. II. Action on actin. J Biochem 58: 13–19, 1965 [DOI] [PubMed] [Google Scholar]
- 86.Chen VC, Li X, Perreault H, Nagy JI: Interaction of zonula occludens-1 (ZO-1) with α-actinin-4: application of functional proteomics for identification of PDZ domain-associated proteins. J Proteome Res 5: 2123–2134, 2006 [DOI] [PubMed] [Google Scholar]
- 87.Hata Y, Butz S, Sudhof TC: CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci 16: 2488–2494, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM: The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 273: 29745–29753, 1998 [DOI] [PubMed] [Google Scholar]
- 89.Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S: Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol 127: 1617–1626, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bazzoni G, Martinez-Estrada OM, Orsenigo F, Cordenonsi M, Citi S, Dejana E: Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J Biol Chem 275: 20520–20526, 2000 [DOI] [PubMed] [Google Scholar]
- 91.Ebnet K, Schulz CU, Meyer Zu Brickwedde MK, Pendl GG, Vestweber D: Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J Biol Chem 275: 27979–27988, 2000 [DOI] [PubMed] [Google Scholar]
- 92.Martinez-Estrada OM, Villa A, Breviario F, Orsenigo F, Dejana E, Bazzoni G: Association of junctional adhesion molecule with calcium/calmodulin-dependent serine protein kinase (CASK/LIN-2) in human epithelial caco-2 cells. J Biol Chem 276: 9291–9296, 2001 [DOI] [PubMed] [Google Scholar]
- 93.Cordenonsi M, D'Atri F, Hammar E, Parry DA, Kendrick-Jones J, Shore D, Citi S: Cingulin contains globular and coiled-coil domains and interacts with ZO-1, ZO-2, ZO-3, and myosin. J Cell Biol 147: 1569–1582, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.D'Atri F, Citi S: Cingulin interacts with F-actin in vitro. FEBS Lett 507: 21–24, 2001 [DOI] [PubMed] [Google Scholar]
- 95.Hartleben B, Schweizer H, Lubben P, Bartram MP, Moller CC, Herr R, Wei C, Neumann-Haefelin E, Schermer B, Zentgraf H, Kerjaschki D, Reiser J, Walz G, Benzing T, Huber TB: Neph-Nephrin proteins bind the Par3-Par6-atypical protein kinase C (aPKC) complex to regulate podocyte cell polarity. J Biol Chem 283: 23033–23038, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Izumi Y, Hirose T, Tamai Y, Hirai S, Nagashima Y, Fujimoto T, Tabuse Y, Kemphues KJ, OHno S: An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J Cell Biol 143: 95–106, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Joberty G, Petersen C, Gao L, Macara IG: The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol 2: 531–539, 2000 [DOI] [PubMed] [Google Scholar]
- 98.Ebnet K, Suzuki A, Horikoshi Y, Hirose T, Meyer Zu Brickwedde MK, OHno S, Vestweber D: The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM). EMBO J 20: 3738–3748, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Itoh M, Sasaki H, Furuse M, Ozaki H, Kita T, Tsukita S: Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J Cell Biol 154: 491–497, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.