Abstract
Within the glomerulus, the scaffolding protein nephrin bridges the actin-rich foot processes that extend from adjacent podocytes to form the slit diaphragm. Mutations affecting a number of slit diaphragm proteins, including nephrin, cause glomerular disease through rearrangement of the actin cytoskeleton and disruption of the filtration barrier. We recently established that the Nck family of Src homology 2 (SH2)/SH3 cytoskeletal adaptor proteins can mediate nephrin-dependent actin reorganization. Formation of foot processes requires expression of Nck in developing podocytes, but it is unknown whether Nck maintains podocyte structure and function throughout life. Here, we used an inducible transgenic strategy to delete Nck expression in adult mouse podocytes and found that loss of Nck expression rapidly led to proteinuria, glomerulosclerosis, and altered morphology of foot processes. We also found that podocyte injury reduced phosphorylation of nephrin in adult kidneys. These data suggest that Nck is required to maintain adult podocytes and that phosphotyrosine-based interactions with nephrin may occur in foot processes of resting, mature podocytes.
Podocytes are unique epithelial cells within the kidney glomerulus that comprise the outermost layer of the blood filtration barrier.1 Upon differentiation, podocytes extend numerous actin-based foot processes from their cell bodies that interdigitate and surround the glomerular capillary wall. At the interface of adjacent foot processes, a specialized intercellular junction known as the slit diaphragm is formed. The slit diaphragm apparently contributes to the morphology of foot processes through physical connection to the underlying actin cytoskeleton, as mutations affecting numerous slit diaphragm-associated proteins lead to actin rearrangement and foot process effacement.2 In addition to genetic alterations, injury to podocytes as a consequence of diseases such as diabetes and hypertension as well as inflammation may also result in impaired slit diaphragm filtration leading to loss of protein in the urine (proteinuria) and subsequent renal failure.3
Nephrin, encoded by NPHS1, is a transmembrane protein of the Ig superfamily that forms a key structural component of the slit diaphragm. Nephrin also functions as an intracellular signaling scaffold to recruit proteins such as podocin and CD2AP to the podocyte membrane.4 Upon clustering of adjacent nephrin molecules, the intracellular domain of nephrin becomes tyrosine phosphorylated by Src family kinases.5,6 We and others have recently demonstrated that multiple phosphorylated tyrosine residues on nephrin can directly bind the Nck adaptor proteins.7–10 Nck is a subfamily of two highly related proteins, Nck1 (also Nckα) and Nck2 (also Nckβ or Grb4), which are composed of three Src homology 3 (SH3) domains followed by a carboxy-terminal SH2 domain.11,12 The Nck SH2 domain binds optimally to phosphorylated YDxV motifs on nephrin, whereas the SH3 domains interact with a wide array of effector proteins such as N-WASp that regulate cytoskeletal dynamics.13 Accordingly, recruitment of Nck to phosphorylated nephrin induces localized actin polymerization.7–10
Through conditional deletion of Nck2 in podocytes of Nck1-null embryonic mice, we have previously shown that expression of Nck in developing mouse podocytes is required for the formation of foot processes.7 These mice present with congenital nephrotic syndrome, similar to that observed in mice deficient in nephrin,14,15 suggesting that Nck proteins might provide a link between nephrin and the actin cytoskeleton during the development of podocytes. While it is clear that Nck signaling is important in developing podocytes, whether it has a similar role in maintaining podocyte structure and function throughout life has remained less apparent. To address this, we have now investigated the role of Nck proteins in the maintenance of podocyte foot processes in the adult kidney. Using a strategy that allows deletion of Nck2 expression in a conditional and inducible fashion, we found that Nck proteins are also required in the adult glomerulus to maintain proper podocyte morphology. We have also developed a series of antibodies that specifically recognize individual phosphorylated nephrin YDxV sites, which we have used to confirm nephrin tyrosine phosphorylation in adult kidneys. Together these results support an emerging hypothesis that nephrin phosphorylation is retained in differentiated podocytes and that connection to the actin cytoskeleton via Nck may be an ongoing requirement to maintain the functional integrity of the slit diaphragm.
RESULTS
Strategy for a Conditional and Inducible Podocyte-Specific Nck Knockout
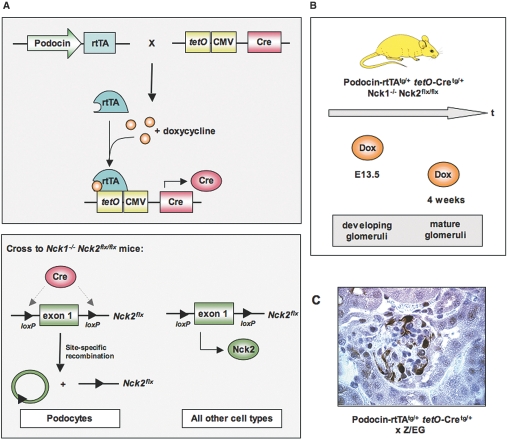
To restrict deletion of Nck to adult podocytes, we have utilized a conditional inducible expression system (Tet-On) in which Nck can be deleted in a time- and cell-specific manner. In this system, the reverse tetracycline-controlled transcriptional activator (rtTA) is placed under the control of the podocyte-specific podocin promoter, such that rtTA is only produced in kidney podocytes (Figure 1A).16 A second transgene uses the tetO promoter elements upstream of a minimal CMV promoter to drive expression of Cre recombinase. These transgenes were bred into a background that is homozygous for both the Nck1-null allele and the Nck2 floxed allele.7 In the absence of doyxcycline, rtTA cannot bind to tetO; as a consequence, no Cre protein is produced, and the mice retain expression of Nck2 in podocytes. However, upon administration of the tetracycline analog doxycycline, rtTA can now bind tetO to initiate transcription of Cre recombinase, resulting in deletion of Nck2 selectively in podocytes. Doxycycline can be administered at any timepoint to remove Nck2 protein from either developing or established Nck1-null podocytes (Figure 1B). Since Nck2 is excised from the mouse genome, removal of doxycycline does not restore Nck2 expression. To generate the appropriate experimental animals, we intercrossed mice carrying both the podocin-rtTA transgene (tg/+) and the tetO-Cre transgene (tg/+) with mice homozygous for the Nck1-null (-) allele and the Nck2 floxed (flx) allele (Figure 1A). We then backcrossed animals positive for all four modified alleles to Nck1-/-, Nck2flx/flx mice to generate podocin-rtTAtg/+, tetO-Cretg/+, Nck1-/-, Nck2flx/flx experimental animals (hereafter designated conditional Nck mutant mice).
Figure 1.
Generation of a conditional knockout of Nck in adult podocytes, using the Tet-On system. (A) Binary transgenic system in which one transgene places the rtTA under the control of the podocyte-specific podocin promoter, while a second transgene uses tetO promoter elements upstream of a minimal CMV promoter to drive expression of Cre recombinase. rtTA requires the tetracycline analog doxycycline to bind tetO and produce Cre protein. These transgenes are introduced into mice homozygous for the Nck1-null (-) allele and the Nck2 floxed (flx) allele. Upon doxycycline treatment, Cre recombinase induces recombination between the loxP sites on the Nck2 allele, resulting in deletion of Nck2 selectively in podocytes of Nck1 null mice. Since Cre is not produced in any other cell types, they will continue to express Nck2 protein. Loss of Nck2 in podocytes is irreversible. (B) Mutant mice were exposed to doxycycline at E13.5 to delete Nck2 in developing podocytes or at 4 wk of age to delete Nck2 in established podocytes. (C) Binary transgenic mice were intercrossed with the Z/EG reporter strain to demonstrate podocyte-restricted expression of Cre recombinase. Cre-mediated excision results in expression of GFP, which is visualized by staining kidney sections with anti-GFP antibodies.
We assessed the efficiency of doxycycline-regulated Cre expression by crossing the double transgenic podocin-rtTAtg/+, tetO-Cretg/+ mice to the Z/EG transgenic reporter strain, which carries a loxP-flanked lacZ cassette.17 Upon Cre-mediated excision of lacZ, a second reporter protein, eGFP, is expressed. Immunostaining of kidney sections using anti-GFP antibody indicates that over 80% of podocytes were positive for the excision event (Figure 1C).
Doxycycline-Mediated Nck Deletion in Podocytes at E13.5 Results in Congenital Nephrosis
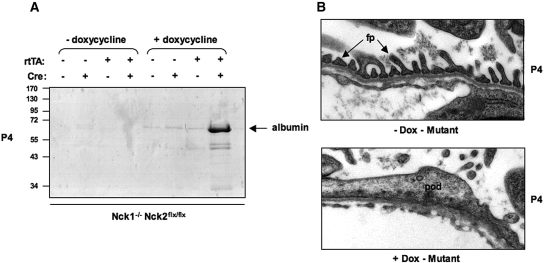
We have previously demonstrated that loss of Nck2 expression in podocytes of Nck1-null mice using podocin-Cre results in congenital nephrosis.7 In these animals, Cre recombinase (under the control of the podocin promoter) becomes active at the capillary loop stage of nephrogenesis, which begins as early as embryonic day 13.5 (E13.5) in mice. Thus, to ensure that the conditional inducible strategy could recapitulate the phenotype observed previously with the traditional Cre breeding approach, we administered doxycycline to pregnant female animals at E13.5 to initiate Cre-mediated excision of Nck2 in podocytes. We crossed female Nck1-/-, Nck2flx/flx mice with male conditional Nck mutant mice (described above) and provided doxycycline in the drinking water beginning at E13.5, continuing throughout gestation and nursing. All offspring from these crosses are thus Nck1-/-, Nck2flx/flx, with one or no copies of the rtTA and/or tetO-Cre transgenes. SDS-PAGE urinalysis at postnatal day 4 (P4) showed large amounts of albumin in the urine of rtTA and tetO-Cre double positive pups that had been treated with doxycycline during gestation (Figure 2A). In contrast, we observed no albuminuria in single positive pups treated with doxycycline or in double positive pups that had not been exposed to doxycycline (Figure 2A). Electron micrographic examination of kidneys from rtTA and tetO-Cre double positive pups at P4 showed extensive foot process fusion in pups exposed to doxycycline, whereas we found well-separated foot processes with clear slit diaphragms in nontreated double-positive pups (Figure 2B). Together these findings indicate that administration of doxycycline to conditional Nck mutant mice at E13.5 results in congenital nephrosis, consistent with our previous report using podocin-Cre. Moreover, the lack of any kidney defects in control littermate animals suggests that the expression of Cre is tightly regulated. This strategy is thus a valid approach to examine the function of Nck proteins in adult mouse podocytes.
Figure 2.
Doxycycline-mediated Cre induction and loss of Nck expression in developing podocytes results in nephrotic syndrome. (A) SDS-PAGE analysis of urine from littermates at postnatal day 4 (P4). All mice are Nck1-/- Nck2flx/flx, and carrying neither, one or both Podocin-rtTA and tetO-Cre transgenes, as indicated. Double transgenic Nck1-/- Nck2flx/flx mice (referred to as “mutant”) develop albuminuria when exposed to doxycycline in utero at E13.5. Proteinuria is not seen in untreated mutants, or single transgenic control littermates exposed to doxycycline. (B) Transmission electron micrographs of the glomerular filtration barrier in mutant mice exposed to doxycycline in utero or left untreated. Well-separated foot processes (fp) with intervening slit diaphragms are observed in untreated mutant mice (upper panel), whereas mice exposed to doxycycline to induce Cre-mediated Nck2 excision show flattened podocytes (pod) with an absence of slit diaphragms (lower panel).
Loss of Nck Expression in Adult Podocytes Leads to Rapid Proteinuria, Glomerulosclerosis and Foot Process Effacement
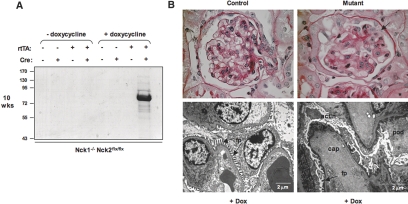
To investigate a potential role for Nck expression in adult mouse podocytes, we exposed conditional Nck mutant mice and appropriate control littermates to doxycycline at 4 wk of age. At this time point, nephrogenesis is complete—podocytes possess completely differentiated foot processes and glomeruli are fully functional. Mice were treated with doxycycline for a period of 2 wk and analyzed 4 wk later. We observed significant proteinuria in conditional Nck mutant mice that had been exposed to doxycycline (Figure 3A). Urinalysis reagent strips also showed the presence of blood in the urine of some mutant mice, indicating damage to the glomerular filtration barrier. Similar to our observations in embryonic mice shown in Figure 2, we detected no protein in the urine of single positive adult mice treated with doxycycline or in double positive mice that had not been exposed to doxycycline (Figure 3A).
Figure 3.
Genetic deletion of Nck expression in established podocytes demonstrates an ongoing requisite role for Nck signaling at the slit diaphragm. (A) SDS-PAGE shows proteinuria in mutant mice exposed to doxycycline at 4 wk of age to induce Nck2 deletion, but not in untreated mutants, or single transgenic control littermates. All mice are Nck1-/- Nck2flx/flx, and carrying neither, one or both Podocin-rtTA and tetO-Cre transgenes, as indicated. Mice were analyzed at 10 wk of age. (B) PAS staining of kidney sections reveals prominent glomerulosclerosis in mutant mice, with tuft adhesions to Bowman's capsule (arrow) and protein deposits in the tubules (asterisk). Electron micrographs show widespread podocyte (pod) effacement with electron dense areas along the basement membrane consistent with collapsed actin (actin), although distinct foot processes (fp) can still be observed around some capillary (cap) loops. Normal glomerular morphology and foot process architecture in a doxycycline-treated littermate control is shown for comparison.
Kidneys from conditional Nck mutant mice exposed to doxycycline were pale and sclerotic, and histologic analysis revealed a number of defects consistent with end-stage renal disease. The glomeruli in mutant mice exhibited widespread sclerosis as well as adhesions (synechiae) with Bowman's capsule in regions with podocyte loss, and protein casts in the surrounding tubules could also be observed (Figure 3B). None of these changes were observed in control littermates (Figure 3B). Ultrastructural examination of the glomeruli in these same mutant mice revealed extensive foot process fusion and flattening (Figure 3B), and electron dense areas in the podocyte along the basement membrane are consistent with collapsed actin filaments (Figure 3B). We did not observe any changes to the endothelium in mutant glomeruli. All control mice exhibited regular foot processes surrounding the basement membrane as well as endothelial fenestrations (Figure 3B). We exposed a total of 13 conditional Nck mutant mice to doxycycline at 4 wk of age, and all but one displayed proteinuria upon urinalysis. No untreated animals developed proteinuria during the course of these studies. Taken together, these results indicate that Nck proteins are required for the maintenance of foot processes in adult kidney podocytes.
Foot Process Alterations in Adult Mice Lacking Nck Expression in Podocytes
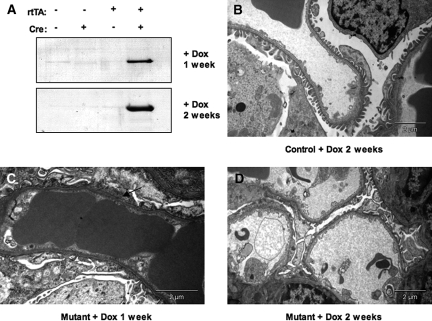
To determine the onset of this phenotype, we analyzed mutant mice and control littermates at weekly intervals following exposure to doxycycline, beginning at 4 wk of age as described above. One week after induction with doxycycline, conditional Nck mutant mice exhibited albuminuria (Figure 4A) as well as focal areas of foot process fusion and broadening (Figure 4C). The degree of proteinuria and structural damage to podocytes were greater after 2 wk of doxycycline induction with areas of diffuse foot process fusion (Figures 4D). These abnormalities in foot process morphology were accompanied by a concomitant increase in proteinuria (Figure 4A). The architecture of the glomerular filtration barrier remained normal in treated littermate controls at all time points (Figure 4B). These studies confirm that expression of Nck in podocytes is critical to retain the structure and function of the adult glomerular filtration barrier.
Figure 4.
Early alterations in glomerular morphology and function upon loss of Nck expression in adult podocytes. Adult mice (4 wk of age) were exposed to doxycycline and analyzed after 1 or 2 wk. (A) An increase in proteinuria is seen at these timepoints in mutant mice, but not in doxycycline-treated littermate controls. (B) Foot process architecture is maintained in control mice exposed to doxycycline. (C and D) By contrast, conditional Nck mutants exhibit focal areas of foot process fusion and broadening (arrow) at 1 wk after doxycycline exposure, and these ultrastructural changes are widespread after 2 wk of induction with areas of diffuse foot process fusion. Fenestrated endothelial cells are relatively spared in mutant mice.
Development of Nephrin Phospho-Specific Antibodies
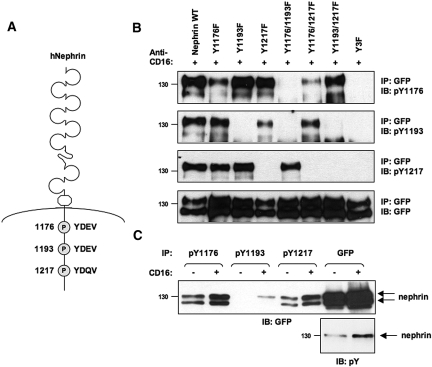
The results from our genetic experiments indicate that Nck expression is critical in adult podocytes to retain foot process morphology. We thus hypothesized that Nck may interact with nephrin in both developing and established podocyte foot processes, and that this interaction is required to maintain connections with the underlying actin cytoskeleton. Increasing evidence suggests that nephrin is tyrosine phosphorylated in normal glomeruli of adult rodents.6,7,9,18 To investigate the tyrosine phosphorylation status of nephrin in the adult kidney and its potential ability to associate with Nck, we generated a series of antibodies that specifically recognize each of the three Nck binding sites found on mouse and human nephrin (Figure 5A). We used peptides encompassing each tyrosine phosphorylated YDxV site on nephrin to immunize rabbits and generate rabbit monoclonal antibodies (RAbMAbs). We assessed the specificity of each affinity-purified antibody using a series of human nephrin proteins harboring tyrosine (Y) to phenylalanine (F) substitutions at amino acid positions 1176, 1193, and 1217. In each of these proteins, the intracellular domain of nephrin (which includes a GFP tag) has been fused to the extracellular domain of the Ig receptor CD16 and the transmembrane domain of CD7 to facilitate activation of nephrin signaling upon addition of CD16 antibody. Chimeric proteins were expressed in HEK 293T cells, and lysates from CD16-stimulated cells were immunoprecipitated with anti-GFP antibody and subjected to immunoblotting with the phospho-nephrin antibodies. We have previously used this approach to demonstrate that nephrin is tyrosine phosphorylated on multiple YDxV sites following CD16 stimulation.7 Figure 5B shows that all three Nck binding sites on nephrin are indeed tyrosine phosphorylated upon CD16-induced clustering, as each phospho-specific antibody can detect the wild-type (WT) protein. The nephrin pY1193 antibody appears to be specific to this Tyr residue, as it does not recognize the Y1193F mutant, nor does it detect the Y1176/1193F or Y1193/1217F double mutants (Figure 5B). Similarly, the pY1217 antibody also appears to be specific, as it does not recognize the Y1217F mutant, or the Y1176/1217F or Y1193/1217F double mutants (Figure 5B). In contrast, the pY1176 antibody appears to display weak cross-reactivity with the other YDEV motif at Tyr 1193, as it can detect low levels of the Y1176F mutant but not the Y1176/1193F double mutant (which still contains the YDQV motif at Tyr 1217) (Figure 5B). However, since there was no reduction in intensity with the Y1193F mutant when compared with the dramatic reduction with the Y1176F mutant, it suggests that this antibody has high affinity for Tyr 1176. None of the antibodies could detect the Y3F triple mutant, indicating their specificity for phosphorylated YDxV sites on nephrin. We also examined the ability of these antibodies to specifically immunoprecipitate phosphorylated chimeric nephrin from lysates of transfected HEK 293T cells. Indeed, each of these antibodies showed an ability to immunoprecipitate chimeric nephrin, and in each case, this was markedly increased following anti-CD16 cross-linking, suggesting that nephrin stimulation increases phosphorylation at each YDxV site (Figure 5C). We observed similar patterns of phosphorylation with chimeric nephrin proteins bearing a Myc-epitope tag in place of GFP (data not shown). These results confirm that nephrin phosphorylation induced by CD16 clustering in HEK 293T cells occurs on all three Nck binding sites, and that these reagents are specific to the multiple YDxV sites on nephrin.
Figure 5.
Conserved tyrosine residues on nephrin are recognized by phospho-specific antibodies. (A) The intracellular region of human nephrin contains a series of conserved tyrosine residues within YDxV motifs that can bind the Nck SH2 domain. (B) Peptides encompassing each tyrosine phosphorylated YDxV motif on nephrin were used to immunize rabbits and generate rabbit monoclonal antibodies (RAbMAbs). HEK 293T cells were transfected with a series of GFP-tagged chimeric human nephrin proteins with tyrosine (Y)-to-phenylalanine (F) substitutions at amino acid positions 1176, 1193, and 1217. Nephrin phosphorylation was induced by CD16 clustering, and lysates were immunoprecipitated (IP) with GFP antibodies to isolate nephrin. Separated proteins were immunoblotted (IB) with affinity-purified phospho-nephrin antibodies or GFP. Both the nephrin pY1193 and pY1217 antibodies appear to be specific to each respective Tyr residue, while the pY1176 antibody appears to recognize YDEV (1176 and also 1193 to a limited extent) but not YDQV (1217) sites. None of the antibodies could detect the Y3F triple mutant, indicating their specificity for phosphorylated YDxV sites on nephrin. Exposure time for the pY1176 and pY1193 blots is 30 min, and exposure time for the pY1217 blot is 10 min. (C) HEK 293T cells expressing wildtype (WT) chimeric nephrin were stimulated with anti-CD16 or left unstimulated, and lysates were immunoprecipitated (IP) with each phospho-nephrin antibody or GFP. Separated proteins were immunoblotted (IB) with GFP or phosphotyrosine (pY) antibodies. Nephrin clustering results in increased tyrosine phosphorylation on each YDxV site.
Nephrin Phosphorylation is Maintained in the Adult Glomerulus
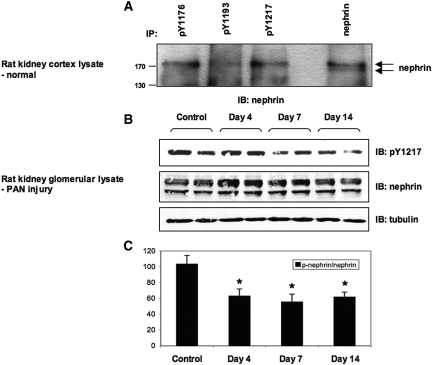
We next wanted to utilize these reagents to pursue the possibility that endogenous nephrin might be phosphorylated in adult rodent kidneys under normal physiologic conditions. Rat nephrin contains a single YDEV site (corresponding to human Tyr 1193) as well as the YDQV site (corresponding to human Tyr 1217), while mouse nephrin contains all three YDxV sites. To bypass any potential artifacts that could arise in the sieving procedure wherein glomeruli are isolated from intact kidneys, we chose to first prepare lysates directly from kidney cortex. Cortical lysates from normal adult rat kidneys were immunoprecipitated with various phospho- or pan-nephrin antibodies followed by anti-nephrin immunoblotting. Endogenous nephrin appears as a doublet of 180 and 170 kD, and the upper band is tyrosine phosphorylated (Figure 6A).7 As shown in Figure 6A, each of the phospho-nephrin antibodies specifically immunoprecipitated the 180-kD phosphorylated species. Although a site corresponding to human Tyr 1176 is not present in rat nephrin, the ability of the pY1176 antibody to cross-react with the corresponding rat Tyr 1193 site likely allows this antibody to immunoprecipitate phosphorylated nephrin from rat kidney lysates. These data clearly demonstrate that a basal level of nephrin tyrosine phosphorylation exists in normal adult rat kidneys. Similar results were obtained with mouse kidney lysates, where each antibody again detected the 180-kD phosphorylated nephrin species (data not shown), and we found no reactivity with IgG alone. Taken together, these results indicate that the Nck binding sites on nephrin are phosphorylated in normal adult kidney.
Figure 6.
Nephrin is phosphorylated on YDxV sites in normal adult kidneys and a decrease is associated with podocyte injury. (A) Adult rat kidney cortex lysates were immunoprecipitated (IP) with each phospho-nephrin antibody or pan-nephrin, and separated proteins were immunoblotted (IB) with nephrin antibodies. Endogenous nephrin appears as a doublet of 180 and 170 kD, and each of the phospho-nephrin antibodies detected the 180-kD tyrosine-phosphorylated upper species. (B) Glomerular lysates from rats with PAN nephrosis at various time-points or untreated control rats were immunoblotted with phospho-nephrin or nephrin antibodies. We utilized the pY1217 antibody as it displays superior sensitivity. Equivalent amounts of lysate are indicated by tubulin. Data shown are representative of four independent experiments. (C) Densitometric quantitation of phosphorylated nephrin normalized to total nephrin (p-nephrin/nephrin) indicates a decrease in nephrin phosphorylation on the YDQV site upon podocyte injury. *P < 0.05 versus control, n = 4.
Altered dynamics of nephrin tyrosine phosphorylation have been observed during foot process effacement in vivo.8,9,18 To investigate whether these changes in phosphorylation occur on the YDxV sites, we examined nephrin phosphorylation in the puromycin aminonucleoside (PAN) nephrosis model of podocyte injury. We prepared glomerular extracts from PAN-treated rats at multiple time-points corresponding to the onset (day 4) and peak period (day 7 and 14) of proteinuria. Phosphorylation on the YDQV site (human Tyr 1217, corresponding to rat Tyr 1228) was clearly observed in untreated control rats, and it decreased significantly over the course of PAN nephrosis (Figure 6B). Expression of nephrin itself was not changed (Figure 6B). Upon normalizing for the amount of total nephrin in each lysate, the reduction in phosphorylation on this site was nearly 50% during the peak of proteinuria (Figure 6C). These findings, therefore, indicate a decrease in nephrin tyrosine phosphorylation on Nck binding sites upon podocyte injury.
DISCUSSION
The glomerular filtration barrier depends on the presence of actin-based foot processes and an intact slit diaphragm for proper function. We have previously demonstrated that the Nck cytoskeletal adaptor proteins are necessary in developing podocytes for the initial formation of podocyte foot processes,7 and that these proteins link phosphorylated nephrin to the actin cytoskeleton.7–10 We now extend these studies to demonstrate that Nck proteins are also required in mature podocytes, as deletion of Nck2 expression in podocytes of adult Nck1-null mice leads to rapid onset of proteinuria with accompanying foot process effacement. Within 1 wk after doxycycline-induced Nck elimination in podocytes, mutant mice developed proteinuria and after 6 wk showed characteristic features of end-stage renal disease. Our findings, therefore, suggest a continuing role for Nck signaling in the mature glomerulus to maintain proper podocyte morphology.
The physiologic importance of Nck signaling in mature podocytes is consistent with our earlier finding that Nck is associated with tyrosine phosphorylated nephrin in adult glomeruli.7 Our results do not directly demonstrate that development of renal disease in mice lacking Nck expression in adult podocytes is a consequence of impaired nephrin signaling, and it is quite plausible that Nck might be involved in actin signaling from multiple podocyte cell surface proteins. Given the profound defects in motility and formation of actin-rich protrusions such as membrane ruffles and lamellipodia in mouse embryonic fibroblasts lacking Nck expression, it is likely that Nck is an essential component of the cellular actin organization machinery.19–21 Nonetheless, the presence of phosphorylated nephrin in the normal mature kidney strongly suggests that alterations in the interaction between nephrin and Nck could contribute to disruption of the glomerular filtration barrier. To further investigate the nature of this persistent nephrin phosphorylation, we have developed and characterized a series of phospho-specific antibodies that recognize each of the conserved Nck SH2 domain binding motifs on nephrin. Using these three antibodies, we demonstrated that all of the YDxV motifs on nephrin appear to be phosphorylated in adult rat and mouse kidneys. As each of these sites has a similar affinity for the Nck SH2 domain,7,10 these observations correlate well with the notion that multiple Nck SH2 domain bindings motifs on nephrin act cooperatively to initiate and sustain robust levels of actin polymerization.10
Two other laboratories recently reported in vivo phosphorylation of the Nck binding sites on nephrin. Uchida et al.22 developed two antibodies that recognize the conserved Nck binding sites on rat nephrin (corresponding to human Tyr 1193 and Tyr 1217), while Verma et al.8 developed a single antibody that recognizes both YDEV sites on mouse nephrin (corresponding to human Tyr 1176 and Tyr 1193). Interestingly, although Verma et al.8 could detect nephrin phosphorylation in developing glomeruli at the capillary loop stage, they were unable to detect nephrin phosphorylation on the Nck binding sites in adult glomeruli. It was, therefore, speculated that since nephrin was not phosphorylated in mature mouse podocytes, it may connect to actin via phosphorylation-independent mechanisms in established podocytes. These findings contrast with those reported here and in Uchida et al.,22 and may reflect differences in the specificity and sensitivity of these various antibodies. Accordingly, it is important to note that this latter antibody does not appear to recognize the third Nck binding site on mouse and human nephrin,8 which is contained within a YDQV motif and is conserved on rat nephrin. Taken together, our data support those of Uchida et al.22 and are consistent with a model in which nephrin is phosphorylated on the Nck binding sites in both developing and mature podocytes, thereby providing a continuous and dynamic connection to the actin machinery that controls foot process morphology.
Phosphorylation of Tyr 1193 appears to be particularly sensitive to nephrin clustering, as very little phosphorylation of this site could be seen in unstimulated cells when compared with Tyr 1176 and Tyr 1217 (Figure 5C). While we must consider that this reduction could be due to lower antibody affinity, it is interesting to note that the phosphorylation status of Tyr 1193 may be an important regulator of nephrin signaling, through controlling the expression of nephrin on the cell surface. Phosphorylation of this site has been shown by Quack and colleagues23 to function as a switch to determine whether podocin or β-arrestin2 is recruited to nephrin. Phosphorylation of Tyr 1193 by Src kinases Fyn and Yes induces podocin binding and AP-1 transactivation.18,23 In contrast, unphosphorylated nephrin or a Y1193F mutant can interact with β-arrestin2, and this interaction is disrupted in the presence of Yes kinase. Association of nephrin with β-arrestin2 promotes nephrin endocytosis and reduces nephrin signaling. This “switch” at Tyr 1193 is, therefore, hypothesized to function as a mechanism by which podocytes can sense the structural integrity of the slit diaphragm. Unpaired nephrin molecules, such as those that are mislocalized or have lost extracellular contacts, will not be phosphorylated on this residue, thus promoting interaction with β-arrestin2 and nephrin internalization. Indeed, in our experiments where nephrin is not clustered via CD16 antibodies, phosphorylation of Tyr 1193 was virtually undetectable, consistent with the potential for β-arrestin2 binding and nephrin endocytosis. Phosphorylation of Tyr 1193 is also reduced in rats with foot process effacement.22
Alterations in nephrin phosphorylation may be an important indicator of slit diaphragm integrity during glomerular disease. Our results showing a reduction in phosphorylation on the YDQV site in PAN-induced nephrosis confirm those of Uchida et al., where foot process effacement and proteinuria coincide with a decrease in phosphorylation on both Nck binding sites on rat nephrin.22 Moreover, the interaction between nephrin and Nck is reduced in glomeruli isolated from rats with PAN-induced nephrosis,9 and a decrease in the amount of F-actin is also observed.22 A reduction in phosphorylation of the YDQV site on nephrin is also associated with human minimal change nephrosis.22 These findings raise the intriguing possibility that loss of connection between nephrin and Nck may contribute to foot process effacement in such patients. In contrast, other models of podocyte damage, including protamine sulfate perfusion, passive Heymann nephritis and 27A antibody injection, result in an increase in nephrin phosphorylation,8,18,24 and it is plausible that such hyperphosphorylation of nephrin may also contribute to nephropathy under certain circumstances. Alternatively, hyperphosphorylation of certain tyrosine residues may be required at certain time points in the course of recovery as a compensatory mechanism. The phospho-specific nephrin antibodies described here will be a useful tool for deciphering how nephrin phosphorylation and Nck binding are regulated under both physiologic and pathologic conditions in the glomerulus.
Regulation of actin cytoskeleton dynamics is of critical importance for podocyte structure and function,25 and it is becoming clear that multiple tyrosine phosphorylation-dependent pathways may converge at the slit diaphragm to accomplish this. The SH2 domain-containing p85 regulatory subunit of phosphatidylinositol 3′kinase was recently shown to be recruited to a phosphorylated tyrosine residue on nephrin that is distinct from the Nck binding sites.26 Activation of nephrin-p85 signaling results in a decrease in stress fiber formation and an increase in lamellipodia formation in cultured podocytes, which may be analogous to foot process formation in vivo.26 This role for p85 downstream of nephrin (rather than a survival function) is consistent with the observation that podocyte survival is not affected during glomerular development in nephrin-null mice.27 In addition to nephrin, tyrosine phosphorylation of the related protein Neph1 has also been demonstrated.28,29 Phosphorylated Neph1 binds the SH2 adaptor protein Grb2, and this interaction promotes actin polymerization.28 In contrast to nephrin, Neph1 phosphorylation is increased in PAN-induced nephrosis.29 Further studies will undoubtedly demonstrate how these various signaling pathways cooperate with Nck to stabilize the podocyte cytoskeleton.
In summary, although the role of phosphotyrosine-based nephrin signaling at the slit diaphragm is incompletely understood, our data indicate that the phosphotyrosine-binding adaptor protein Nck is essential for podocyte function in both the embryo and the adult. Our results also support the emerging hypothesis that nephrin phosphorylation must be maintained at the slit diaphragm of adult podocytes. These phosphosites likely allow proper localization of signaling proteins such as Nck to the actin cytoskeleton of podocyte foot processes. Such a direct link between nephrin and the actin cytoskeleton would allow highly dynamic control over the structural integrity of the slit diaphragm and foot process morphology, in both developing and “quiescent” podocytes as well as in response to glomerular injury. Ultimately, the ability to specifically monitor nephrin phosphorylation may allow for better classification of renal biopsy changes and the assignment of more focused therapies to patients with glomerular disease.
CONCISE METHODS
Generation of Nck Conditional Inducible Mutant Mice
Nck1-/-, Nck2flx/flx mice have been described previously.7,30 Double homozygous animals were crossed to transgenic mice expressing both the reverse tetracycline transactivator (rtTA) under the control of the podocyte-specific Podocin (NPHS2) promoter (Podocin-rtTA) and the Cre recombinase driven by a minimal CMV promoter, fused downstream of tet operator sequences (each with tg/+ genotype).16 We induced Cre expression by administration of doxycycline (4 mg/ml; Sigma, St. Louis, MO) in the drinking water, supplemented with 5% (w/v) sucrose, for 1 to 2 wk as indicated. We exchanged the water twice per week and covered the bottles with aluminum foil. We housed experimental and littermate control mice together and collectively treated them with doxycycline, as indicated. Animal husbandry was carried out in accordance with Canadian Council of Animal Care protocols.
Induction of PAN Nephrosis
We obtained male Sprague Dawley rats (250 to 300 g) from Charles River (St. Constant, QU). We used a single injection of puromycin aminonucleoside (50 mg/kg body wt) to induce podocyte injury, as described previously.26
Urine Analysis
We passively collected urine from indicated mice and analyzed 2 μl by SDS-PAGE followed by Coomassie Blue staining to detect urinary proteins. We also used urinalysis reagent strips (Bayer HealthCare, Toronto, ON, Canada) to identify mice with proteinuria (> 1.0 g/L).
Histologic and Electron Microscopic Analysis
Kidneys were isolated and fixed in 10% formalin/PBS followed by paraffin embedding. Four-micrometer sections were cut, stained with periodic acid Schiff (PAS), examined, and photographed with a DC200 Leica camera and Leica DMLB microscope (Leica Microsystems Inc., Richmond Hill, ON, Canada). Immunofluorescence staining was performed on paraffin sections using rabbit polyclonal anti-GFP antibody (Molecular Probes, Eugene, OR) at 1:2000 followed by counterstaining with hematoxylin.16 Tissue for electron microscopy was fixed in 1.5% glutaraldehyde, postfixed with 1% osmium tetroxide, dehydrated in graded ethanols, and embedded in Spurr epoxy resin (Canemco Inc.). Ultrathin sections were stained with uranyl acetate and lead citrate and examined with a Philips CM 100 electron microscope (Philips Electron Optics, Hillsboro, OR).
Generation of Phospho-Specific Nephrin Antibodies
We generated rabbit monoclonal antibodies (RAbMAbs) in collaboration with Epitomics® (Burlingame, CA). Briefly, peptides specific to phosphorylated mouse nephrin were synthesized and used to immunize rabbits. Upon screening for sera reactive with phosphorylated nephrin expressed from HEK293T cells, B cells were isolated from spleens and used to generate rabbit hybridoma cells. Pooled hybridoma cells were next screened for reactivity with phosphorylated nephrin, followed by screening of clonal lines from positive pools. A single production clone for each antibody was selected for final immunoanalysis of nephrin phosphorylation, with 0.5 μg of phosphoantibody used for immunoprecipitation and 0.07 μg/ml used for immunoblotting. We used the following peptides: AFPGHLpYDEVERC (mouse Y1191 = human Y1176); GVWGPLpYDEVQMC (mouse Y1208 = human Y1193); and DPRGIpYDQVAADC (mouse Y1232 = human Y1217). Antibodies are labeled with human numbering throughout the text for consistency.
Cell Culture
We grew HEK 293T cells in high glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT). Transient transfections were performed using polyethyleneimine.
Plasmids
Construction of CD16/7-nephrin(WT)IC and CD16/7-nephrin(Y3F)IC have been described previously.7 Here, we amplified the intracellular domains of human nephrin containing tyrosine to phenylalanine substitutions at one or two YDxV sites by PCR to generate in-frame fusions with the extracellular domain of CD16 and the intracellular domain of CD7 (CD16/7) followed by a carboxy-terminal EGFP epitope tag. We confirmed all constructs by DNA sequencing.
Immunoprecipitations and Immunoblotting
Immunoprecipitation and immunoblotting procedures have been described previously.7 Briefly, for phosphorylation analyses and co-immunoprecipitation experiments, cells were starved overnight and stimulated for 10 min at 37°C using a 1 μg/ml dilution of anti-CD16 antibody. We prepared lysates from transfected cells, rodent kidney cortex biopsies, or rat glomeruli using PLC lysis buffer supplemented with fresh protease inhibitors [50 mM HEPES (pH 7.5), 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA, 10 mM NaPPi, 100 mM NaF, supplemented with 1 mM sodium orthovanadate, 1 mM PMSF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin]. We obtained the following antibodies from commercial sources: polyclonal anti-GFP 290 (Abcam, Cambridge, MA) and monoclonal anti-phosphotyrosine clone 4G10 (Upstate-Millipore, Bedford, MA). Polyclonal antibodies to rat nephrin have been described previously.18 We quantified protein content in immunoblots using scanning densitometry (Image J software, NIH, Bethesda, MD) and used the t-test to determine significant differences between two groups.
DISCLOSURES
None.
Acknowledgments
We are grateful to J. Kopp for providing Podocin-rtTA transgenic mice. We also thank D. Holmyard for electron microscopy and P. DeFields and G. Mbamalu for technical contributions. This work was supported by grants from the Kidney Foundation of Canada (to N.J. and T.T.) and the Canadian Institutes for Health Research (CIHR, to T.P., S.E.Q., and T.T.). N.J. is the recipient of a Kidney Research Scientist Core Education and National Training (KRESCENT) Program New Investigator Award and a Natural Sciences and Engineering Research Council of Canada (NSERC) University Faculty Award. L.A.N. and M.A.F. received support from NSERC. T.T. holds a scholarship from the Fonds de la recherché en santé du Québec. S.E.Q. holds a Tier 2 Canada Research Chair in Vascular and Metabolic Biology. T.P. is a distinguished scientist of the CIHR.
This work was previously presented in part at the annual meeting of the American Society of Nephrology, November 2 through 5, 2007 in San Francisco, CA, and published in abstract form.
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “The Promise of Well-Being: Stay in Shape with N(i)ck,” on pages 1425–1427.
References
- 1.Pavenstadt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Michaud JL, Kennedy CR: The podocyte in health and disease: Insights from the mouse. Clin Sci (Lond) 112: 325–335, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Patrakka J, Tryggvason K: Nephrin—A unique structural and signalling protein of the kidney filter. Trends Mol Med 13: 396–403, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Lahdenpera J, Kilpelainen P, Liu XL, Pikkarainen T, Reponen P, Ruotsalainen V, Tryggvason K: Clustering-induced tyrosine phosphorylation of nephrin by Src family kinases. Kidney Int 64: 404–413, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Verma R, Wharram B, Kovari I, Kunkel R, Nihalani D, Wary KK, Wiggins RC, Killen P, Holzman LB: Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J Biol Chem 278: 20716–20723, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, Huang H, Larose L, Li SS, Takano T, Quaggin SE, Pawson T: Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440: 818–823, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB: Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest 116: 1346–1359, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Zhu J, Aoudjit L, Latreille M, Kawachi H, Larose L, Takano T: Rat nephrin modulates cell morphology via the adaptor protein Nck. Biochem Biophys Res Commun 349: 310–316, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Blasutig IM, New LA, Thanabalasuriar A, Dayarathna TK, Goudreault M, Quaggin SE, Li SS, Gruenheid S, Jones N, Pawson T: Phosphorylated YDXV motifs and Nck SH2/SH3 adaptors act cooperatively to induce actin reorganization. Mol Cell Biol 28: 2035–2046, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Fan J, Woodley DT: Nck/Dock: An adapter between cell surface receptors and the actin cytoskeleton. Oncogene 20: 6403–6417, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Buday L, Wunderlich L, Tamas P: The Nck family of adapter proteins: regulators of actin cytoskeleton. Cell Signal 14: 723–731, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Higgs HN, Pollard TD: Regulation of actin polymerization by Arp2/3 complex and WASp/Scar proteins. J Biol Chem 274: 32531–32534, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Putaala H, Soininen R, Kilpelainen P, Wartiovaara J, Tryggvason K: The murine nephrin gene is specifically expressed in kidney, brain and pancreas: Inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet 10: 1–8, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Rantanen M, Palmen T, Patari A, Ahola H, Lehtonen S, Astrom E, Floss T, Vauti F, Wurst W, Ruiz P, Kerjaschki D, Holthofer H: Nephrin TRAP mice lack slit diaphragms and show fibrotic glomeruli and cystic tubular lesions. J Am Soc Nephrol 13: 1586–1594, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE: VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novak A, Guo C, Yang W, Nagy A, Lobe CG: Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis 28: 147–155, 2000 [PubMed] [Google Scholar]
- 18.Li H, Lemay S, Aoudjit L, Kawachi H, Takano T: SRC-family kinase Fyn phosphorylates the cytoplasmic domain of nephrin and modulates its interaction with podocin. J Am Soc Nephrol 15: 3006–3015, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Bladt F, Aippersbach E, Gelkop S, Strasser GA, Nash P, Tafuri A, Gertler FB, Pawson T: The murine Nck SH2/SH3 adaptors are important for the development of mesoderm-derived embryonic structures and for regulating the cellular actin network. Mol Cell Biol, 23: 4586–4597, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivera GM, Antoku S, Gelkop S, Shin NY, Hanks SK, Pawson T, Mayer BJ: Requirement of Nck adaptors for actin dynamics and cell migration stimulated by platelet-derived growth factor B. Proc Natl Acad Sci U S A 103: 9536–9541, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruusala A, Pawson T, Heldin CH, Aspenström P: Nck adapters are involved in the formation of dorsal ruffles, cell migration, and Rho signaling downstream of the platelet-derived growth factor beta receptor. J Biol Chem, 283: 30034–30044, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uchida K, Suzuki K, Iwamoto M, Kawachi H, Ohno M, Horita S, Nitta K: Decreased tyrosine phosphorylation of nephrin in rat and human nephrosis. Kidney Int 73: 926–932, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Quack I, Rump LC, Gerke P, Walther I, Vinke T, Vonend O, Grunwald T, Sellin L: beta-Arrestin2 mediates nephrin endocytosis and impairs slit diaphragm integrity. Proc Natl Acad Sci U S A 103: 14110–14115, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simons M, Schwarz K, Kriz W, Miettinen A, Reiser J, Mundel P, Holthofer H: Involvement of lipid rafts in nephrin phosphorylation and organization of the glomerular slit diaphragm. Am J Pathol 159: 1069–1077, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P: Actin up: Regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 17: 428–437, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Zhu J, Sun N, Aoudjit L, Li H, Kawachi H, Lemay S, Takano T: Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int 73: 556–566, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Done SC, Takemoto M, He L, Sun Y, Hultenby K, Betsholtz C, Tryggvason K: Nephrin is involved in podocyte maturation but not survival during glomerular development. Kidney Int 73: 697–704, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Garg P, Verma R, Nihalani D, Johnstone DB, Holzman LB: Neph1 cooperates with nephrin to transduce a signal that induces actin polymerization. Mol Cell Biol 27: 8698–8712, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harita Y, Kurihara H, Kosako H, Tezuka T, Sekine T, Igarashi T, Hattori S: Neph1, a component of the kidney slit diaphragm, is tyrosine-phosphorylated by the Src family tyrosine kinase and modulates intracellular signalling by binding to Grb2. J Biol Chem 283: 9177–9186, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fawcett JP, Georgiou J, Ruston J, Bladt F, Sherman A, Warner N, Saab BJ, Scott R, Roder JC, Pawson T: Nck adaptor proteins control the organization of neuronal circuits important for walking. Proc Natl Acad Sci U S A 104: 20973–20978, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]