Abstract
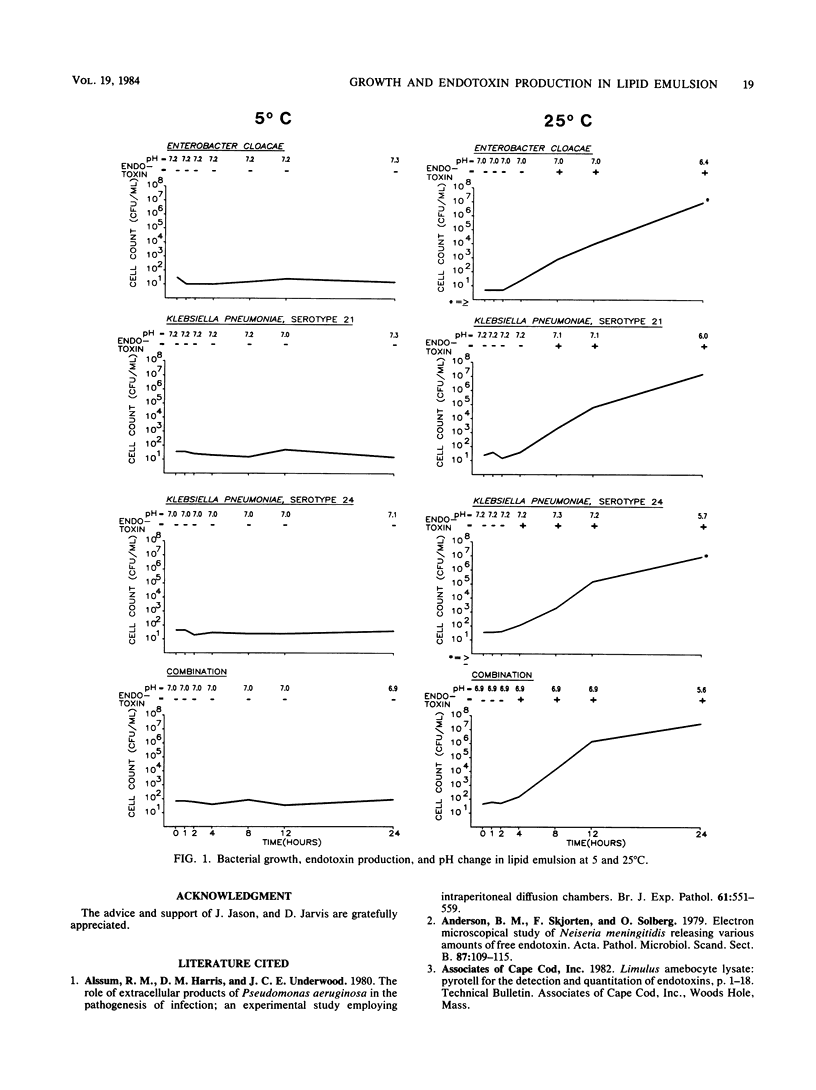
Klebsiella pneumoniae serotypes 21 and 24 and Enterobacter cloacae were responsible for an outbreak of polymicrobial bacteremia associated with the receipt of lipid emulsion. Since it is recommended that lipid emulsion be kept refrigerated between uses, we undertook a study to determine the growth characteristics of these organisms in lipid emulsion at 5 and 25 degrees C and to examine the use of alternative measurements (pH and endotoxin) to determine contamination by viable and nonviable microorganisms. The bacteria survived but did not proliferate at 5 degrees C; no endotoxin was detected, and the pH remained unchanged. In contrast, after a 2-h lag phase, all three organisms proliferated rapidly when incubated at 25 degrees C and reached concentrations of greater than or equal to 10(7) CFU/ml at 24 h. A decrease in pH was detected after proliferation to 10(7) CFU/ml. Endotoxin was detected after proliferation reached 10(2) CFU/ml. The amount of endotoxin elaborated by the three organisms differed and ranged from 0.013 ng per 8 X 10(2) CFU/ml to 1.3 ng per 2 X 10(3) CFU/ml at 8 h. Our findings show that these microorganisms do not proliferate at refrigerator temperature in lipid emulsion, but can reach significant levels at room temperature. It is, therefore, important to keep lipid emulsion refrigerated between uses. Furthermore, when lipid emulsion contamination is suspected, endotoxin and pH determinations should be considered as possible adjunctive tests while results of bacterial cultures are pending. The results of the present study are applicable to only selected gram-negative bacteria and may not apply to gram-positive bacteria and fungi. However, these data demonstrate that measurement of pH and detection of endotoxin is quite useful when lipid emulsion contamination occurs with selected gram-negative bacteria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Ssum R. M., Harris D. M., Underwood J. C. The role of extracellular products of Pseudomonas aeruginosa in the pathogenesis of infection; an experimental study employing intraperitoneal diffusion chambers. Br J Exp Pathol. 1980 Dec;61(6):551–559. [PMC free article] [PubMed] [Google Scholar]
- Andersen B. M., Skjørten F., Solberg O. Electron microscopical study of Neisseria meningitidis releasing various amounts of free endotoxin. Acta Pathol Microbiol Scand B. 1979 Apr;87B(2):109–115. doi: 10.1111/j.1699-0463.1979.tb02412.x. [DOI] [PubMed] [Google Scholar]
- Goldmann D. A., Martin W. T., Worthington J. W. Growth of bacteria and fungi in total parenteral nutrition solutions. Am J Surg. 1973 Sep;126(3):314–318. doi: 10.1016/s0002-9610(73)80115-1. [DOI] [PubMed] [Google Scholar]
- Guzman R. J., Kuo H. S. Pyrogen testing of an intravenous fat emulsion. Prog Clin Biol Res. 1979;29:333–337. [PubMed] [Google Scholar]
- Jarvis W. R., Highsmith A. K., Allen J. R., Haley R. W. Polymicrobial bacteremia associated with lipid emulsion in a neonatal intensive care unit. Pediatr Infect Dis. 1983 May-Jun;2(3):203–208. doi: 10.1097/00006454-198305000-00006. [DOI] [PubMed] [Google Scholar]
- McKee K. T., Jr, Melly M. A., Greene H. L., Schaffner W. Gram-negative bacillary sepsis associated with use of lipid emulsion in parenteral nutrition. Am J Dis Child. 1979 Jun;133(6):649–650. doi: 10.1001/archpedi.1979.02130060089023. [DOI] [PubMed] [Google Scholar]
- Melly M. A., Meng H. C., Schaffner W. Microbiol growth in lipid emulsions used in parenteral nutrition. Arch Surg. 1975 Dec;110(12):1479–1481. doi: 10.1001/archsurg.1975.01360180049010. [DOI] [PubMed] [Google Scholar]



