Abstract
Rats were tested on a hippocampus dependent win-shift working memory task in familiar or novel environments after receiving bilateral ventral tegmental area infusions of baclofen. Baclofen infusion disrupted working memory performance in both familiar and novel environments. In addition, baclofen infusion selectively disrupted short-term working memory in the novel environment. This experiment confirms selective ventral tegmental area support of accurate performance during a context dependent spatial navigation task.
Keywords: Hippocampus, Ventral tegmental area, Working memory, Context
Hippocampus (HPC) is necessary to perform a variety of spatial memory tasks. Intrahippocampal infusions of dopamine (DA) agonists improve performance on spatial tasks [27], while 6-OHDA lesions of HPC impair performance on spatial tasks [10]. D1 receptor knock-out mice show spatial learning impairments [2] and D2 antagonism in ventral HPC disrupts spatial working memory performance [34]. Thus, DA appears necessary to perform spatial tasks efficiently. The mechanisms by which DA regulates spatial performance are likely related to plasticity dependent processes that are facilitated by DA. For example, DA enhances long-term potentiation (LTP) in the CA1 region of hippocampus [20]. D1/D5 receptor agonists also enhance the stability of place fields in HPC [17].
HPC also plays a role in novelty detection [22] and context discrimination [25]. Similarly, DA is known for its association with novelty detection. Novelty facilitates DA release [14,23] and exposure to novel environments facilitates LTP in HPC via D1 receptor activation [20]. Furthermore, D1 antagonism influences HPC place fields in a context dependent way by causing them to become less reliable and specific and to reorganize to a greater extent during changes in spatial context [12]. Therefore, DA neuronal activity (from ventral tegmental area (VTA) and substantia nigra pars compacta (SNc)) may provide HPC with signals that stabilize responses after exposure to novel situations [33] or changes in context. It is proposed that HPC and VTA form a functional loop whereby novelty signals from HPC are relayed to VTA to influence novelty responses of DA neurons. Indeed, HPC stimulation by NMDA receptor agonists increases the number of active DA neurons in VTA [5]. The novelty signal may be conveyed back to HPC from VTA to facilitate LTP and learning [21]. Overall, DA may enhance performance on HPC dependent tasks by providing an attention or teaching signal that facilitates changes in cellular processes and behavior that are necessary for new learning.
Much of the evidence that supports this theory of interactions between the VTA and HPC comes from studies that pharmacologically manipulate the HPC DA system. The present study tests whether HPC DA alterations within the physiological range result in impaired context-dependent spatial learning. Specifically, we hypothesize that VTA inactivation will impair performance on a spatial working memory task. Given the greater effect of D1 antagonism after context manipulations on place field stability [12], we also hypothesize that the impairment in performance will be exacerbated following changes in context.
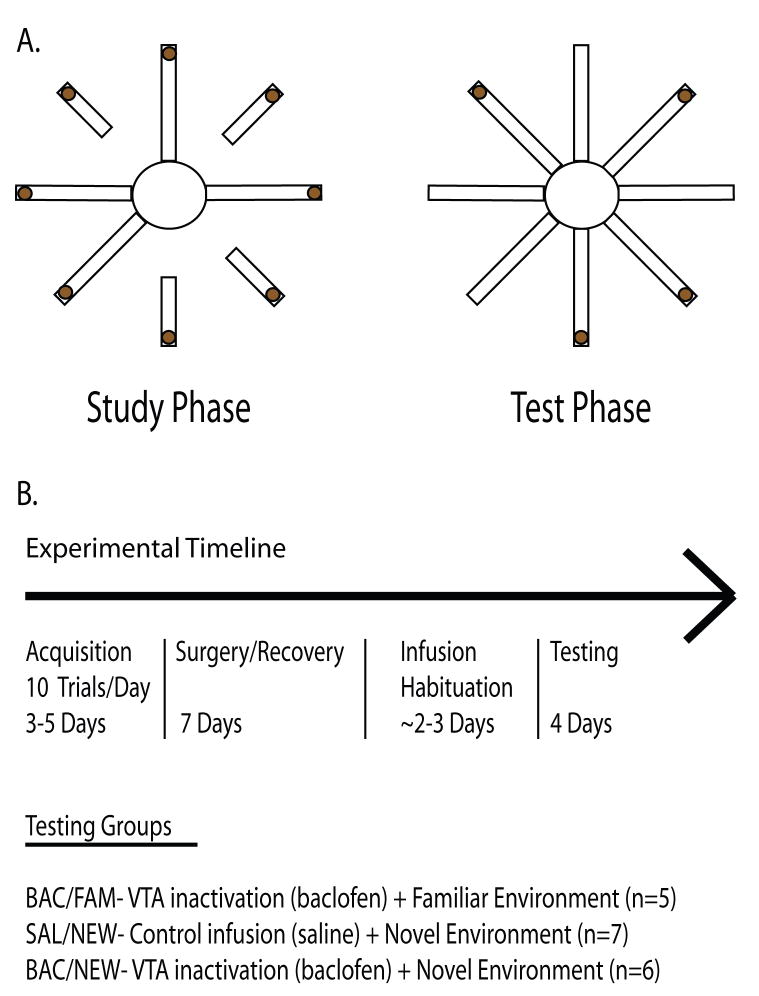
Long-Evans rats (N=18) were housed according to University of Washington’s Institutional Animal Care and Use guidelines. Rats were first habituated to the testing environment and on the eight arm radial maze (for detailed methods see ref. 12). Following habituation, rats were trained on a non-delayed win shift task that consisted of two phases per trial, a study phase and a test phase (Fig. 1A). Unlike a continuous foraging task, the study phase consists of forced arm entries; this makes the test phase of each trial more cognitively demanding than the study phase. Each testing session consisted of ten consecutive trials separated by a 1.5 min inter-trial interval. Re-entries to previously visited arms were classified as one of two types of working memory errors. Across-phase errors (APE) were defined as re-entries to arms that were visited in the study phase. Within-phase errors (WPE) were defined as re-entries to arms that were visited in the test phase. APE and WPE were used to distinguish between short and long term working memory errors. Less time passes between arm entries when WPE are made, hence we consider these to reflect short term working memory errors. In contrast, APE reflect errors regarding choices made earlier in the trial. Latencies to complete the trials were recorded with a stopwatch.
Figure 1.
A. Schematic of one trial of the win-shift spatial working memory task. During each trial of the study phase four randomly chosen arms are presented individually to the rat. After the fourth arm is visited, eight arms are presented and the rat forages for the remaining chocolate milk reward. B. Experimental timeline. Before surgery, each group of rats is trained to asymptotic performance. After habituating to the infusion procedure, rats are re-trained to asymptotic levels. Testing takes place over four days; rats receive either baclofen or saline infusions into VTA prior to testing in a novel environment, or baclofen infusions prior to testing in a familiar environment.
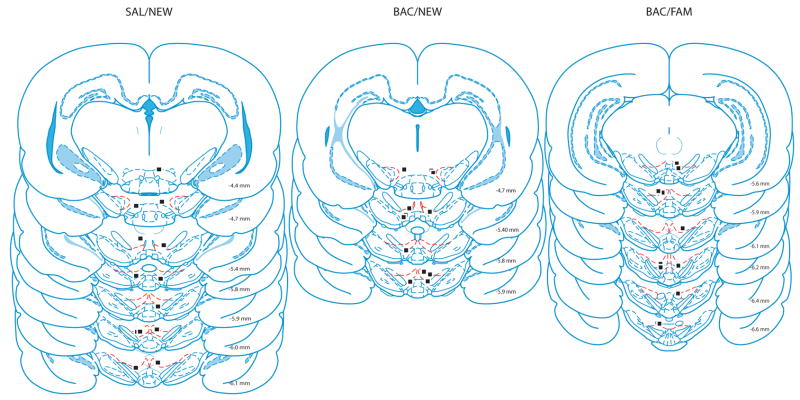
Rats were trained to asymptotic performance of less than one error per trial across 10 trials regardless of type of error (Fig 1B). Then, rats were implanted bilaterally with 25 GA guide cannula targeting VTA (AP −5.3mm and ML +/− .08mm from Bregma, and 6.5mm dorsal to the brain surface). Rats were allowed to recover for seven days before being habituated to the infusion procedure. During this habituation rats received infusions of .5 μl of saline over the course of one min through a 33 GA infusion cannula that extended 1 mm beyond the guide cannula tip prior to being tested for ten consecutive trials in the familiar testing environment. After reaching post-surgical asymptotic performance, rats were trained in one of three different conditions. The first group (n=5) received daily infusions of baclofen (a GABAB agonist) and was tested for four consecutive days for ten trials per day in the same environment that they were trained in (BAC/FAM). The second and third groups received daily saline (n=7) or baclofen (n=6) infusions and were tested in a different maze room with new extra-maze cues for four consecutive days (SAL/NEW and BAC/NEW, respectively). The last day of performance during the infusion habituation procedure served as the SAL/FAM group. Experimenters were blind to the experimental condition for rats being tested in the novel environment. After the fourth day of testing all rats were transcardially perfused with 9% saline followed by a 10% formalin/saline solution. Brains were sliced at 50 μm on a cryostat and stained with cresyl violet to verify cannula placement (Fig 2).
Figure 2.
Locations of infusion cannula tips [28].
Repeated measures analysis of variance (ANOVA) was used to identify significant differences in the average number of errors made per trial across testing sessions as well as between groups. Greenhouse-Geisser corrections were used if sphericity assumptions were violated. Three separate repeated measures ANOVAs were used to determine whether there were significant differences in the average number of errors made per trial within each condition across testing sessions. There was a significant effect of day of testing in the BAC/NEW group (F(4,20)=2.51, p=.01), and the BAC/FAM group (F(4,16), p<.01). For the SAL/NEW group, the effect of testing day was not significant (F(4,24)=2.88, p=.12). Post-hoc planned pairwise comparisons between test day 1 and the last day of infusion habituation (SAL-FAM control condition), and test day 1 and the last day of testing, showed that BAC/FAM rats made significantly more errors on test day 1 than SAL/FAM and the last day of testing. BAC/NEW rats also made more errors on test day 1 than SAL/FAM and the last day of testing. These results suggest VTA inactivation impaired working memory performance in either familiar or new environments. An overall repeated measures ANOVA revealed a significant effect of condition (Fig. 3; F(2,15)= 4.37, p=.03) and day of testing (F(4,60)=13.27, p<.01). Collapsing across days, BAC/NEW rats (1.08±11) made significantly more errors than SAL/NEW rats (.66±10), suggesting that VTA inactivation impaired new learning. However, there were no significant differences between groups across individual days of testing. Overall, VTA inactivation caused rats to make significantly more errors on the first testing day regardless of whether rats experienced a change in context. Performance was not affected by a change in context alone.
Figure 3.
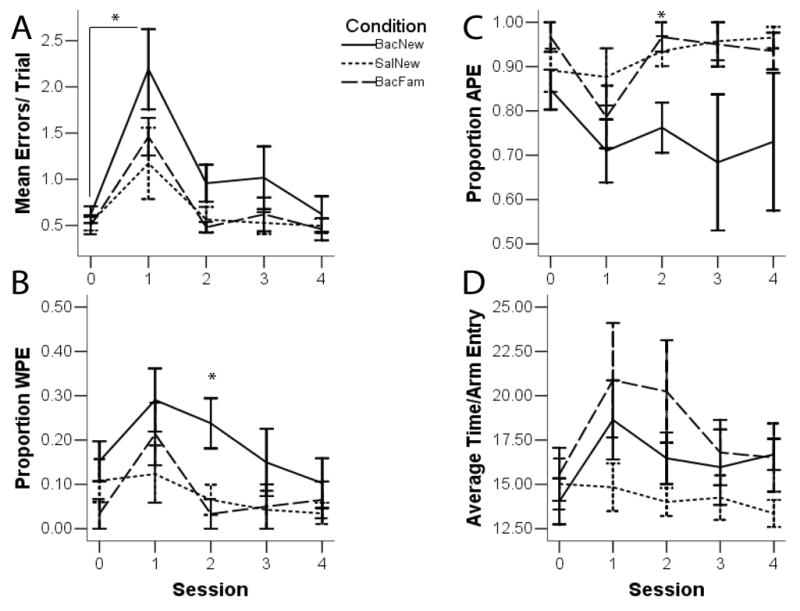
Working memory performance from the last day of infusion habituation (Session 0, or SAL/FAM) through the last day of testing (Session 4). A. Average errors- BAC/NEW rats made more errors than SAL/NEW rats. All rats receiving baclofen infusions made significantly more errors on the first day of testing. B. WPE- BAC/NEW rats made more WPEs than BAC/FAM and SAL/NEW rats. This effect was significant on the second day of testing. C. APE- BAC/NEW rats made fewer APEs than BAC/FAM and SAL/NEW rats. This effect was significant on the second day of testing. D. Movement- There were no significant differences between groups.
Although the number of errors was similar between BAC/NEW and BAC/FAM groups, it is possible that differences existed in the type of errors committed. Therefore, repeated measures ANOVA were conducted to compare between groups the types of errors made across days of testing. There was a significant effect of testing session, F(4,60), p=.01, and condition, F(2,15), p<.01, in terms of the proportion of errors that were WPE in nature (Fig 2). Bonferroni corrected pairwise comparisons revealed that regardless of condition rats made more WPE on the first day of testing (.21±.04) than the last day of testing (.07±.03), indicating that the short-term memory component of the working memory task was most affected initially, and that the number of WPE declined after additional test days. Collapsing across days, the BAC/NEW group in particular (.19±.02) made significantly more WPE than both the BAC/FAM (.08±.02) and SAL/NEW (.06±.02) groups, suggesting that VTA inactivation paired with a change in context had a greater effect on short-term working memory. Bonferroni corrected post-hoc analysis indicated that the BAC/NEW group continued to make significantly more WPE than the SAL/NEW group and the BAC/FAM group on the second day of testing (F(2,15), p=.01), suggesting that the change in context paired with VTA inactivation induced a prolonged short-term working memory deficit.
There was no significant effect of testing session per se (F(4,60), p=.25) on the proportion of errors that were APE in nature, and yet there was a significant effect of condition (F(2,15), p<.01). Collapsing across days, Bonferroni corrected pairwise comparisons revealed that rats in the BAC/NEW group (.75±.04) made fewer APE than both the BAC/FAM (.92±.04) and SAL/NEW (.94±.03) groups. The BAC/NEW group made significantly fewer APE than the SAL/NEW group and the BAC/FAM group on the second day of testing (F(2,15), p=.01). In summary, rats in the BAC/NEW group made more WPE (and fewer APE) than rats in either the BAC/FAM or SAL/NEW conditions. Conversely, rats in the BAC/FAM or SAL/NEW conditions made more APE than WPE compared to the BAC/NEW group. The BAC/NEW animals were therefore making more short-term working memory errors throughout the first two days of testing.
Given the involvement of VTA in movement and motivation, repeated measures ANOVA was conducted on the average time per arm entry. There were no significant differences between groups (F(2,15), p=.08) or across days of testing (F(4,60), p=.06).
The finding that inactivating VTA impairs performance on the spatial win-shift working memory task is consistent with previous work showing that DA in HPC is involved in spatial learning and working memory functions. The overall performance deficit was gone by the second day of testing and may be explained by compensatory mechanisms. SNc provides an alternative source of DA and it is possible for this structure to compensate for the loss of VTA activity by providing sufficient DA to support HPC dependent spatial working memory performance. Moreover, VTA inactivation, when combined with a change in the testing environment, selectively disrupted short-term working memory performance. Although the deficits were on the order of fractions of errors, rats performing this task at asymptote in familiar environments rarely commit WPE and even a small increase in this type of error is unusual (unpublished observations). The impairment in performance was gone after the second day of testing, suggesting that VTA may be more selective for engaging processes that are necessary for accurate processing of very recent information, the type that might be held in a short-term working memory buffer. The involvement of VTA seems time limited since after the second exposure to the novel environment VTA inactivation no longer had an effect.
VTA inactivation resulted in an overall deficit in spatial working memory performance on the first test day regardless of the context conditions. Changes in VTA projections to regions other than HPC, including prefrontal cortex (PFC) and nucleus accumbens (NAcc), may account for this result. DA levels in PFC are critically involved in working memory processes [30,31]. It has been shown that activity of single neurons in PFC is correlated with accurate performance on working memory tasks [9,29] and this activity is modulated by DA receptors [35]. In addition, DA input to PFC is necessary for PFC-HPC LTP [13]. Furthermore, D1 receptor mediated PFC-HPC interactions are crucial for accurate performance on delayed spatial working memory tasks [31]. However, since this circuit appears to be selective to delayed spatial working memory tasks, it is unlikely that VTA inactivation in this paradigm disrupted working memory performance via direct effects on PFC.
It is well known that VTA also sends many direct projections to NAcc which is involved in both delayed and non-delayed spatial working memory tasks [32]. Lesions to NAcc impair performance on spatial tasks and place cells have been recorded in NAcc [19], suggesting a role for this structure in spatial processing. DA antagonists infused into NAcc also disrupt spatial working memory [7]. However, DA agonists and antagonists infused into NAcc have also been found to have no effect on spatial working memory [18]. At any rate, the deficits seen in response to VTA inactivation in a familiar environment may not be due to direct effects on NAcc function per se. A more likely explanation could be that compromised function of the HPC and NAcc due to loss of VTA activity led to the behavioral impairments observed. Indeed HPC and NAcc interactions modulate performance on non-delayed spatial working memory tasks [6] and DA modulates HPC induced spiking activity in NAcc [3] but more direct future experiments are necessary to test the involvement of DA modulation of HPC and NAcc interactions during spatially guided behaviors.
Our data support the hypothesis that VTA plays an important role in context-dependent spatial learning. They also show that physiologically relevant alterations of VTA (presumably DA) input to HPC is sufficient to impair HPC dependent function. An issue to be resolved in the future is how DA comes to play a selective role in processing learning related information after a context change. It has been hypothesized that HPC determines the extent and/or nature of a context shift [25]. Such an output should be crucial for determining future behavioral choices. There are several HPC efferent routes that may be involved, an important one of which is likely the output to NAcc [21]. Contralateral disconnections of HPC and NAcc disrupt context conditioning in a spatial task [15]. Contextual information from HPC is transmitted via NAcc to the ventral pallidum (VP) [21], which has direct connections with VTA and the pedunculopontine tegmental area (PPTg). Both VP and PPTg have been shown to regulate DA neuron burst firing in VTA [8,24,26]. Lesions of PPTg and VP also impair performance on spatial working memory tasks [4,16]. HPC dependent excitation of VTA is also blocked by application of glutamate receptor antagonists in NAcc but not by TTX application into PFC [5]. However, PFC has been shown to regulate HPC activation of NAcc neurons [1]. Furthermore, PFC neurons have been shown to respond to changes in context while rats are performing a spatial working memory task [29]. It is unknown, then, whether the context dependent impairment in performance revealed in this experiment is due to communication deficits from HPC to PFC, HPC to NAcc or both, and if DA modulates this impairment. Furthermore, more studies are needed to determine the possible roles for VP and PPTg in spatial context processing, Nevertheless, this experiment supports the hypothesis that, VTA activity is important for accurate performance on a win-shift spatial working memory task. VTA support of accurate performance during a goal directed spatial navigation task appears stronger and more selective for short-term components of working memory, a process that is especially important when there is a change in context.
Acknowledgments
This work was supported by NIMH grant MH58755 and thoughtful discussions with Min Jung Kim and Emily Clark.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Belujon P, Grace AA. Critical role of the prefrontal cortex in the regulation of hippocampus-accumbens information flow. J Neurosci. 2008;28:9797–805. doi: 10.1523/JNEUROSCI.2200-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Ghundi M, Fletcher PJ, Drago J, Sigley DR, O’Dowd BF, George SR. Spatial learning deficits in dopamine D1 receptor knockout mice. Eur J Pharmacol. 1999;27:95–96. doi: 10.1016/s0014-2999(99)00573-7. [DOI] [PubMed] [Google Scholar]
- 3.Floresco SB, Blaha CD, Yang CR, Phillips AG. Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. J Neurosci. 2001;21:2851–60. doi: 10.1523/JNEUROSCI.21-08-02851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Floresco SB, Braaksma DN, Phillips AG. Involvement of the ventral pallidum in working memory tasks with or without a delay. Ann N Y Acad Sci. 1999;877:711–6. doi: 10.1111/j.1749-6632.1999.tb09308.x. [DOI] [PubMed] [Google Scholar]
- 5.Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Floresco SB, Seamans JK, Phillips AG. A selective role for dopamine in the nucleus accumbens of the rat in random foraging but not delayed spatial win-shift-based foraging. Behav Brain Res. 1996;80:161–8. doi: 10.1016/0166-4328(96)00031-9. [DOI] [PubMed] [Google Scholar]
- 8.Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–73. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 9.Funahashi S, Chafee MV, Goldman-Rakic PS. Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature. 1993;365:753–756. doi: 10.1038/365753a0. [DOI] [PubMed] [Google Scholar]
- 10.Gasbarri A, Sulli A, Innocenzi R, Pacitti C, Brioni JD. Spatial memory impairment induced by lesion of the mesohippocampal dopaminergic system in the rat. Neuroscience. 1996;74:1037–1044. doi: 10.1016/0306-4522(96)00202-3. [DOI] [PubMed] [Google Scholar]
- 11.Gasbarri A, Verney C, Innocenzi R, Campana E, Pacitti C. Mesolimbic dopaminergic neurons innervating the hippocampal formation in the rat: a combined retrograde tracing and immunohistochemical study. Brain Res. 1994;668:71–79. doi: 10.1016/0006-8993(94)90512-6. [DOI] [PubMed] [Google Scholar]
- 12.Gill KM, Mizumori SJY. Context-dependent modulation by D1 receptors: differential effects in hippocampus and striatum. Behav Neurosci. 2006;120:377–392. doi: 10.1037/0735-7044.120.2.377. [DOI] [PubMed] [Google Scholar]
- 13.Gurden H, Tassin JP, Jay TM. Integrity of the mesocortical DA system is necessary for complete expression of in vivo hippocampal-prefrontal cortex long-term potentiation. Neuroscience. 1999;94:1019–1027. doi: 10.1016/s0306-4522(99)00395-4. [DOI] [PubMed] [Google Scholar]
- 14.Horvitz JC, Stewart T, Jacobs BL. Burst activity of ventral tegmental dopamine neurons is elicited by sensory stimuli in the awake cat. Brain Res. 1997;759:251–258. doi: 10.1016/s0006-8993(97)00265-5. [DOI] [PubMed] [Google Scholar]
- 15.Ito R, Robbins TW, Pennartz CM, Everitt BJ. Functional interaction between the hippocampus and nucleus accumbens shell is necessary for the acquisition of appetitive spatial context conditioning. J Neurosci. 2008;28:6950–6959. doi: 10.1523/JNEUROSCI.1615-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keating GL, Winn P. Examination of the role of the pedunculopontine tegmental nucleus in radial maze tasks with or without a delay. Neuroscience. 2002;112:687–96. doi: 10.1016/s0306-4522(02)00108-2. [DOI] [PubMed] [Google Scholar]
- 17.Kentros CG, Agnihotri NT, Streater S, Hawkins RD, Kandel ER. Increased attention to spatial context increases both place field stability and spatial memory. Neuron. 2004;42:283–295. doi: 10.1016/s0896-6273(04)00192-8. [DOI] [PubMed] [Google Scholar]
- 18.Kim JS, Levin ED. Nicotinic, muscarinic and dopaminergic actions in the ventral hippocampus and the nucleus accumbens: effects on spatial working memory in rats. Brain Res. 1996;725:231–40. doi: 10.1016/0006-8993(96)00213-2. [DOI] [PubMed] [Google Scholar]
- 19.Lavoie AM, Mizumori SJ. Spatial, movement- and reward-sensitive discharge by medial ventral striatum neurons of rats. Brain Res. 1994;638:157–68. doi: 10.1016/0006-8993(94)90645-9. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- 21.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11:551–568. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- 23.Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol. 1992;67:145–163. doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- 24.Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31:1356–61. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- 25.Mizumori SJ, Ragozzino KE, Cooper BG, Leutgeb S. Hippocampal representational organization and spatial context. Hippocampus. 1999;9:444–51. doi: 10.1002/(SICI)1098-1063(1999)9:4<444::AID-HIPO10>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 26.Pan WX, Hyland BI. Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. J Neurosci. 2005;25:4725–32. doi: 10.1523/JNEUROSCI.0277-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Packard MG, White NM. Dissociation of hippocampus and caudate nucleus memory systems by posttraining intracerebral injection of dopamine agonists. Behav Neurosci. 1991;105:295–306. doi: 10.1037//0735-7044.105.2.295. [DOI] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 2009. [Google Scholar]
- 29.Pratt WE, Mizumori SJ. Neurons in rat medial prefrontal cortex show anticipatory rate changes to predictable differential rewards in a spatial memory task. Behav Brain Res. 2001;123:65–183. doi: 10.1016/s0166-4328(01)00204-2. [DOI] [PubMed] [Google Scholar]
- 30.Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–50. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- 31.Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seamans JK, Phillips AG. Selective memory impairments produced by transient lidocaine-induced lesions of the nucleus accumbens in rats. Behav Neurosci. 1994;108:456–468. doi: 10.1037//0735-7044.108.3.456. [DOI] [PubMed] [Google Scholar]
- 33.Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- 34.Wilkerson A, Levin ED. Ventral hippocampal dopamine D1 and D2 systems and spatial working memory in rats. Neuroscience. 1999;89:734–749. doi: 10.1016/s0306-4522(98)00346-7. [DOI] [PubMed] [Google Scholar]
- 35.Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–5. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]