Abstract
The goal of the present functional magnetic resonance imaging (fMRI) study was to investigate whether and to what extent brain regions involved in high-confidence recognition (HCR) versus low-confidence recognition (LCR) overlap or separate from each other. To this end, we performed conjunction analyses involving activations elicited during high-confidence hit, low-confidence hit, and high-confidence correct-rejection responses. The analyses yielded 3 main findings. First, sensory/perceptual and associated posterior regions were common to HCR and LCR, indicating contribution of these regions to both HCR and LCR activity. This finding may help explain why these regions are among the most common in functional neuroimaging studies of episodic retrieval. Second, medial temporal lobe (MTL) and associated midline regions were associated with HCR, possibly reflecting recollection-related processes, whereas specific prefrontal cortex (PFC) regions were associated with LCR, possibly reflecting executive control processes. This finding is consistent with the notion that the MTL and PFC networks play complementary roles during episodic retrieval. Finally, within posterior parietal cortex, a dorsal region was associated with LCR, possibly reflecting top-down attentional processes, whereas a ventral region was associated with HCR, possibly reflecting bottom-up attentional processes. This finding may help explain why functional neuroimaging studies have found diverse parietal effects during episodic retrieval. Taken together, our findings provide strong evidence that HCR versus LCR, and by implication, recollection versus familiarity processes, are represented in common as well as specific brain regions.
Keywords: fMRI, memory, high confidence recognition, low confidence recognition, recollection, familiarity
1. Introduction
1.1 Brain regions associated with memory confidence
The ability to distinguish an old event from a new event is fundamental to recognition memory. In an attempt to explain this process, functional neuroimaging studies have investigated brain regions showing greater activity for the old items that were correctly classified as “old” (hits) than for the new items that were correctly classified as “new” (correct rejections). These studies have related successful recognition activity to several brain regions, including prefrontal, parietal, sensory/perceptual, and, occasionally, medial temporal regions (for reviews: Cabeza and Nyberg, 2002; Rugg and Henson, 2002; Henson, 2005). However, recognition memory is not an ‘all-or-none’ process, but varies in the degree of subjective confidence that an event has been previously experienced. For example, subjective confidence in recognition memory would be high with vivid remembering of specific contextual details, but would be low with a weak feeling of oldness in the absence of contextual details. The goal of the present functional magnetic resonance imaging study was to investigate whether and to what extent brain regions involved in high-confidence recognition (HCR) versus low-confidence recognition (LCR) overlap or separate from each other.
A few prior fMRI studies have investigated the issue of brain regions involved in high- versus low-confidence recognition memory. However, their implication for the present study is ambiguous because the pertinent analyses were often collapsed across old and new items (Henson et al., 2000), across correct and incorrect responses (Chua et al., 2006), or across all four (hit, miss, false alarm, correct rejection) response types (Moritz et al., 2006). Findings of such analyses could reflect neural correlates of confidence per se rather than neural correlates of memory confidence. In addition, one of these studies (Chua et al.; 2006) has addressed metamemory process of confidence assessment rather than memory confidence per se. Thus, surprisingly little research has been performed to directly compare brain regions involved in high- versus low-confidence correct recognition. Despite these limitations, results of the prior studies have provided some evidence that brain regions involved in HCR versus LCR are at least partially separate from each other. Greater activation for high-confidence than low-confidence responses has been shown in medial temporal lobe (MTL) and associated midline regions (Chua et al., 2006; Moriz et al., 2006), whereas the reverse has been found in right dorsal prefrontal cortex (PFC) and superior posterior parietal cortex (PPC) regions (Henson et al., 2000; Moriz et al., 2006). High- and low-confidence recognition memories are likely to share at least some brain regions given that both reflect explicit memory rather than fundamentally different types of memory (e.g., explicit versus implicit memory). Yet, prior studies have not addressed regions common to HCR and LCR, focusing only on regions specific to HCR versus LCR. Thus, virtually no specific information is available regarding what, if any, brain regions are common to HCR and LCR processes.
A larger number of fMRI studies (e.g., Henson et al., 1999; Eldridge et al., 2000; Wheeler and Buckner, 2004; Yonelinas et al., 2005; Daselaar et al., 2006) have investigated the related issue of brain regions involved in recollection (i.e., vivid remembering of specific contextual details) versus familiarity (i.e., a feeling of oldness in the absence of contextual details). These studies are relevant for the present study because, although there is no one-to-one direct correspondence, on average, recollection versus familiarity aligns more with high- versus low-confidence responses, respectively (e.g., Dunn, 2004; Wixted and Stretch, 2004). Yet, a caution is necessary because familiarity-based responses are associated with a wide range of confidence responses (e.g., Yonelinas, 2001, 2002). The prior relevant studies have provided evidence that brain regions involved in recollection versus familiarity are at least partially separate from each other. A recent review of these studies by Skinner and Fernandes (2007) has suggested that the two functions are characterized by different patterns of activity in frontal, parietal, sensory, and medial temporal regions. Apart from ongoing debate about whether recollection and familiarity depend on qualitatively similar or different psychological processes (e.g., Yonelinas, 2001, 2002; Dunn, 2004; Wixted and Stretch, 2004), the two functions are likely to share at least some brain regions given that both reflect explicit memory. Yet, most relevant prior studies have primarily focused on the distinct brain regions associated with recollection versus familiarity, with no explicit consideration of common regions shared by the two functions. For example, direct, mutual contrasts between recollection- versus familiarity-related activities, which are among the most frequently used contrasts in these studies (e.g., Henson et al., 1999; Eldridge et al., 2000; Wheeler and Buckner, 2004), do not address common regions. Thus, very little specific information is available regarding what, if any, brain regions are common to recollection and familiarity processes.
1.2 Measuring brain regions associated with HCR and LCR
In the present study, participants studied a series of‘mini’ word lists during the encoding phase, and during the test phase, they performed an old/new recognition test that included studied words (old) as well as unrelated, nonstudied words (new). At test, participants provided not only their memory for the word, but also their degree of confidence in their decision. They indicated their decision by pressing one of four keys according to whether they judged the word to be ‘definitely old’, ‘probably old’, ‘probably new’, or ‘definitely new’. In an effort to identify brain regions involved in HCR versus LCR, we focused on the following 3 trial types: high-confidence hit (HH), low-confidence hit (LH), and high-confidence correct rejection (N). We used high-confidence correct rejections as a ‘baseline’ condition rather than using a mixture of high- and low-confidence correct rejections, to maximize sensitivity of the critical contrasts (see Section 4.4). We addressed brain regions (i) common to HCR and LCR by conjunction (), (ii) specific to HCR by conjunction (), and (iii) specific to LCR by conjunction ().
1.3 Predicted model of brain regions associated with HCR and LCR
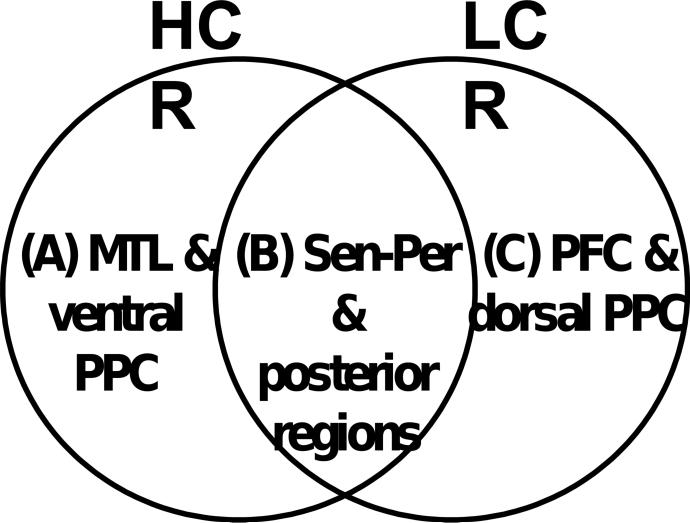
We made the following predictions regarding common and specific brain regions associated with HCR and LCR. Fig. 1 illustrates these predictions. First, there is strong evidence that MTL and associated midline regions (e.g., retrosplenial cortex) play important roles in high-strength memories. For example, lesions in hippocampus could abolish recollection memory in both humans (e.g., Yonelinas et al., 2002) and animals (e.g., Fortin et al., 2004), and functional neuroimaging studies often find HCR-related activity (Chua et al., 2006; Moriz et al., 2006) as well as recollection-related activity within MTL (for a review: Diana et al., 2007). Thus, we predicted that regions specific to HCR would include mainly MTL and associated midline regions (see Fig. 1A). Second, there is evidence that regions earlier in the processing stream, such as sensory/perceptual regions, play important roles in low-strength memories. Familiarity, which, in general, leads to lower confidence responses than does recollection, is sensitive to perceptual manipulations (e.g., changing the presentation modality; Toth, 1996). Event-related potential (ERP) studies (e.g., Rugg et al., 2002) have shown that the familiarity ERP correlate (300−500 ms) peaks earlier than the recollection ERP correlate (500−800 ms). Functional neuroimaging studies have implicated visual processing and associated posterior regions to familiarity, along with other regions (Skinner and Fernandes, 2007). Supposing that high-strength memories involve the activation of additional brain regions compared to low-strength memories (i.e., redundancy model; Joordens and Merikle, 1993; Knowlton, 1998), regions supporting LCR should also be active during HCR. Thus, we predicted that regions common to HCR and LCR would include mainly sensory/perceptual and associated posterior regions that are rich in sensory/perceptual input (e.g., PPC; see Fig. 1B). Finally, executive control processes should be strongly engaged when memories are weak, whereas there should be little need for control processing when memory strength is maximal. Consistent with this idea, functional neuroimaging evidence indicates a complementary relationship between PFC and MTL activity during episodic memory retrieval (Moscovitch, 1992; Buckner and Wheeler, 2001; Rudy et al., 2005). Thus, we predicted that regions specific to LCR would include mainly specific PFC sites (see Fig. 1C).
Figure 1.
Venn diagram depicting the predictions regarding common and specific brain regions in high-confidence recognition (HCR) and low-confidence recognition (LCR). The predictions are: (A) the medial temporal lobe (MTL) and ventral posterior parietal cortex (PPC) regions are specific to HCR activity; (B) sensory-perceptual and associated posterior regions are common to HCR and LCR activity; and (C) prefrontal cortex (PFC) and dorsal PPC regions are specific to LCR activity.
We also made specific predictions regarding PPC regions based on the newly proposed Attention to Memory (AtoM) model (Cabeza, 2008; Cabeza et al., 2008; Ciaramelli et al., 2008). Although PPC is frequently activated during episodic memory retrieval, damage to this region does not markedly impair episodic memory (Simons et al., 2008). Moreover, parietal activations overlap in episodic memory retrieval and other cognitive tasks (Cabeza et al., 2003), and there is indication that parietal retrieval effects are differential between dorsal versus ventral regions (Wagner et al., 2005). To address these and other findings, the AtoM model links the contribution of ventral and dorsal PPC to episodic memory retrieval to their presumed roles in attention. Specifically, the hypothesis proposes that dorsal PPC mediates top-down attentional processes guided by retrieval goals, whereas ventral PPC mediates bottom-up attentional processes captured by salient retrieval output. In the present study, HCR may be associated with stronger capture of bottom-up attention resources because the strength of memory is maximal, whereas LCR may require greater top-down attentional resources because the demand for executive control processes is maximal. Thus, we predicted that ventral PPC would be specific to HCR (see Fig. 1A), whereas dorsal PPC would be specific to LCR (see Fig. 1C).
Finally, given verbal nature of our memory task, we anticipated that our predicted activations would be more commonly found in the left brain than in the right brain.
2. Results
2.1 Behavioral results
The mean proportions and reaction times of old and new items attracting each response type are given in Table 1, excluding response omissions. Collapsed across high- and low-confidence responses, corrected recognition scores (hits – false alarms) were .53 (.72 − .19), indicating good discrimination between old and new items. Corrected recognition scores were .44 for high-confidence responses and .09 for low-confidence responses. The corrected recognition scores were significantly above chance levels of performance in both high-confidence (t11 = 16.5, P < .001) and low-confidence responses (t11 = 2.3, P < .05), confirming that even low-confidence responses were more than guesses. Thus, we anticipated that fMRI signals associated with LCR ‘hit’ responses would activate a distinct memory network rather than a network associated with guessing or chance responses. An ANOVA on reaction times for the 3 critical trial types indicated a significant difference (F2, 22 = 33.8, P < .001). Reaction times were significantly shorter in HH versus LH (t11 = 9.2, P < .001) and HH versus N responses (t11 = 6.6, P < .001). There was no significant difference in reaction times between LH and N responses (t11 < 1).
Table 1.
Behavioral results: Mean proportion of trials and reaction time (SD)
| Response |
|||||
|---|---|---|---|---|---|
| Item | Sure old | Unsure old | Unsure new | Sure new | |
| Proportion of trials | |||||
| Old | .49 (.11) | .23 (.09) | .18 (.09) | .08 (.07) | |
| New | .05 (.04) | .14 (.11) | .50 (.21) | .28 (.20) | |
| Reaction time (ms) | |||||
| Old | 1240 (140) | 1490 (164) | 1542 (189) | 1527 (264) | |
| New | 1502 (388) | 1534 (257) | 1500 (179) | 1467 (209) | |
2.2 fMRI results
To investigate common and distinct brain regions in HCR versus LCR, conjunction analyses involving HH, LH, and N conditions were performed (see Section 4.4). The results of these analyses are shown in Table 2. There were 3 main sets of findings.
Table 2.
Brain regions common and specific to high-confidence recognition (HCR) and low-confidence recognition (LCR) activations
| Contrast | Talairach |
||||||
|---|---|---|---|---|---|---|---|
| Region | H | BA | x | y | z | Voxels | t |
| Both HCR and LCR activity | |||||||
| Parieto-occipital cortex | B | 31/7/19 | 27 | −72 | 46 | 391 | 5.84 |
| Parieto-occipital cortex | L | 7/19 | −34 | −64 | 46 | 55 | 3.81 |
| Ventral occipital cortex | L | 18 | −15 | −74 | −3 | 71 | 5.84 |
| Post. parahippocampal cortex | L | 27 | −15 | −33 | 2 | 15 | 3.50 |
| HCR activity | |||||||
| Hippocampus | L | − | −30 | −37 | −7 | 23 | 4.39 |
| Hippocampus | R | − | 30 | −19 | −8 | 16 | 4.00 |
| Anterior cingulate | R | 32 | 11 | 40 | −11 | 21 | 3.04 |
| Retrosplenial cortex | L | 30 | −11 | −50 | 34 | 29 | 3.90 |
| Ventral PPC | L | 39 | −49 | −69 | 25 | 75 | 4.87 |
| LCR activity | |||||||
| Anterior PFC | L | 10 | −30 | 48 | 15 | 15 | 3.34 |
| Posterior PFC | L | 44/6 | −42 | 19 | 20 | 15 | 4.24 |
| Dorsolateral PFC | R | 6 | 30 | 7 | 56 | 27 | 3.83 |
| Dorsal PPC/ precuneus | B | 7 | 11 | −61 | 52 | 135 | 7.10 |
H, hemisphere; L, left; R, right; B, bilateral; BA, Brodmann area; Post., posterior; PPC, posterior parietal cortex; PFC, prefrontal cortex.
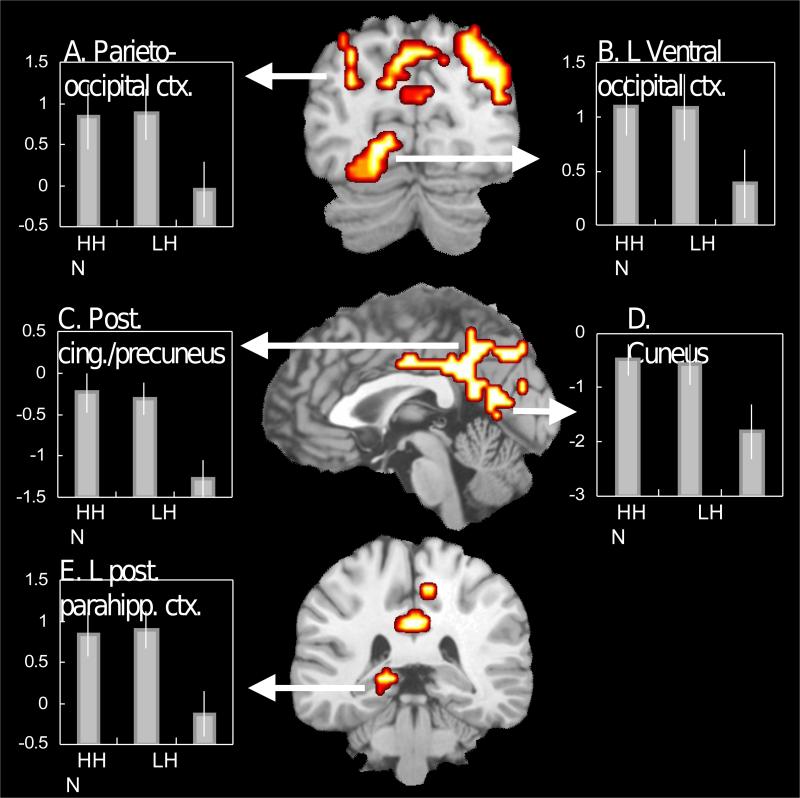
First, as predicted, regions common to HCR and LCR were found mainly in sensory/perceptual regions and posterior regions that are rich in sensory/perceptual input. As illustrated by Fig. 2, bilateral parieto-occipital (BAs 7, 19; Fig. 2A), left ventral occipital (BA 18: Fig. 2B), posterior cingulate/precuneus (BAs 31, 7; Fig. 2C), cuneus (BA 18, Fig. 2D), and left posterior parahippocampal regions (BA 27: Fig. 2E) contributed to both HCR and LCR. Thus, all regions common to HCR and LCR were in posterior cortex.
Figure 2.
Brain regions associated with both high-confidence and low-confidence recognition activity. The bar graphs display mean parameter estimates across all significant voxels. The error bars indicate ±1 SE. HH = high-confidence hit; LH = low-confidence hit; N = high-confidence correct rejection responses.
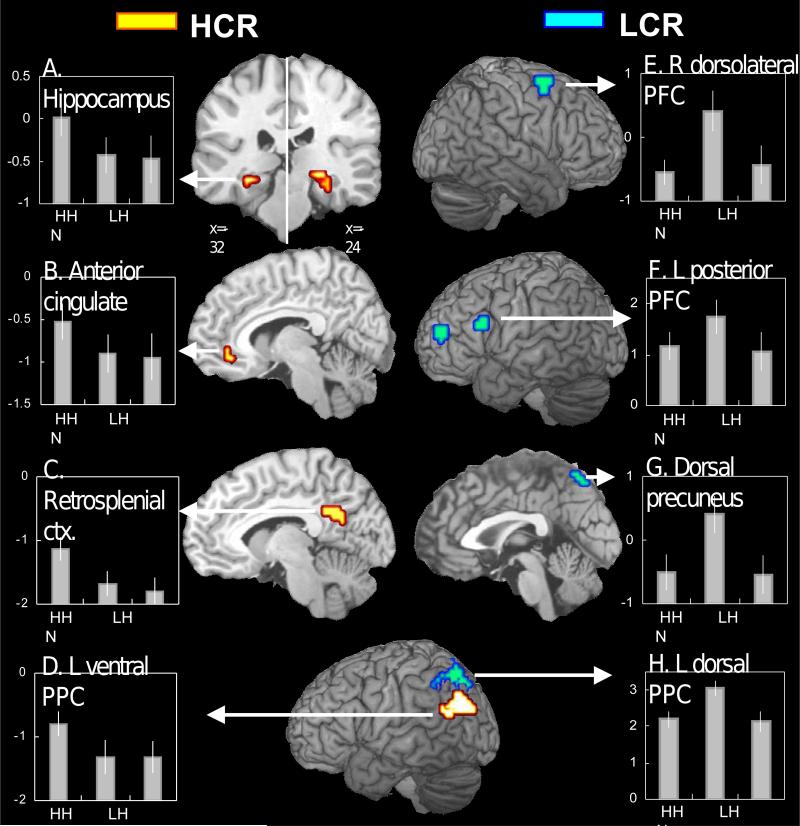
Second, also as predicted, regions specific to HCR were found mainly in MTL and associated midline regions. As illustrated by Fig. 3, bilateral hippocampus (Fig. 3A), anterior cingulate (BA 32: Fig. 3B), and retrosplenial cortex regions (BA 30: Fig. 3C) were specific to HCR. In addition, consistent with our prediction based on the AtoM model, a left ventral PPC region (BA 39: Fig. 3D) was specific to HCR. A follow-up ROI analysis (see Section 4.4) was performed to test whether there was a functional dissociation between the hippocampal region specific to HCR (Fig. 3A) and the posterior parahippocampal region common to HCR and LCR (Fig. 2E). The dissociation was confirmed by a significant region × response type (HH, LH, N) interaction in mean activity (F2, 22 = 4.2, P < .03).
Figure 3.
Brain regions associated with high-confidence recognition (HCR) activity (yellow) and low-confidence recognition (LCR) activity (blue). See Fig. 2 legend for explanation of the bar graphs.
Third, also as predicted, regions specific to LCR were found mainly in PFC. As illustrated by Fig. 3, right dorsolateral PFC (BA 6: Fig. 3E), left anterior PFC (BA 10), and left posterior PFC regions (BAs 44, 6: Fig. 3F) were specific to LCR. In addition, confirming our prediction based on the AtoM model, bilateral dorsal PPC (BA 7: Fig. 3H) and dorsal precuneus regions (BA 7; Fig. 3G) were specific to LCR. As noted in the Behavioral results, reaction times were significantly longer for LH versus HH. However, ‘time on task’ is unlikely to adequately account for the present pattern of findings, because (i) reaction times were significantly longer for N versus HH responses, but the two conditions showed similar levels of activation in all LCR-specific regions, and (ii) there was no significant across-subjects correlation between reaction time (LH minus HH) and signal change (LH minus HH) in all but one LCR-specific region (left posterior PFC, r = .71, P < .05)
As noted above, consistent with our prediction based on the AtoM model, a left ventral PPC region was associated with HCR, whereas bilateral dorsal PPC and precuneus regions were associated with LCR. In addition, bilateral parieto-occipital regions were associated with both HCR and LCR. The dorsal PPC regions were superior to the intraparietal sulcus, the ventral PPC region was centered on the angular gyrus, and the parieto-occipital regions were in-between and represented more widely in posterior regions. Follow-up ROI analyses (see Section 4.4) were performed to test functional dissociations among the three regions. The triple dissociation was confirmed by a significant region × response type interaction within left PPC in dorsal versus ventral PPC regions (F2, 22 = 15.7, P < .001), dorsal PPC versus parieto-occipital regions (F2, 22 = 9.3, P < .01), and ventral PPC versus parieto-occipital regions (F2, 22 = 12.3, P < .001).
3. Discussion
The goal of the present study was to investigate whether and to what extent brain regions involved in HCR versus LCR overlap, or are separate from each other. To this end, we performed conjunction analyses involving activations elicited during high-confidence hit, low-confidence hit, and high-confidence correct rejection responses. The results revealed common regions for HCR and LCR as well as subregions differentially involved in each function. Specifically, there were 3 main findings. First, regions common to HCR and LCR were found mainly in sensory/perceptual and associated posterior regions. Second, regions specific to HCR were found mainly in MTL and associated midline regions, whereas regions specific to LCR were observed mainly in PFC regions. Third, a ventral PPC region was specific to HCR, whereas a dorsal PPC region was specific to LCR. These 3 sets of findings are discussed in separate sections below.
3.1 Common regions for HCR and LCR
Consistent with our first prediction, common regions for HCR and LCR activity were found almost exclusively in sensory/perceptual regions and associated posterior regions that are rich in sensory/perceptual input. These regions included cuneus, left ventral occipital, bilateral parieto-occipital, posterior cingulate, precuneus, and left posterior parahippocampal regions. Neuroanatomical studies in monkeys have established that the posterior cingulate has strong connections with visuo-spatial areas (Kobayashi and Amaral, 2003), the precuneus with the parieto-occipitial visual area (Leichnetz, 2001), and the posterior parahippocampal cortex with several uni- and polymodal regions (Suzuki and Amaral, 1994). Because prior functional neuroimaging studies have rarely addressed common regions for HCR and LCR, no evidence from prior studies is directly relevant to the present finding of common regions. Yet, it is important that activations in similar sensory/perceptual and associated PPC regions are among the most commonly found in functional neuroimaging studies of episodic retrieval (for a review: Cabeza and Nyberg, 2002). Robust activations of these regions during episodic retrieval fit well with the current evidence that the regions contribute to both HCR and LCR activity.
As discussed below, HCR was supported by additional activation of MTL and associated midline regions, indicating that activations in the common regions, by themselves, do not support HCR, but only LCR. Thus, the association of common regions mainly with sensory/perceptual regions is consistent with the hypothesis that sensory/perceptual processes play important roles in low-strength memories, and by implication, familiarity. Although a conceptual process may also contribute to low-strength memories, there is evidence from behavioral as well as ERP studies linking familiarity to sensory-perceptual processing (e.g., Toth, 1996; Rugg et al., 2002; Yonelinas, 2002). Although some prior findings (Wheeler et al., 2000; Woodruff et al., 2005) have shown activation of sensory-perceptual cortex associated with vivid remembering, our findings emphasize that activations of these regions are necessary, but not sufficient for high-strength memories. Most likely, each common region mediates differential subcomponents of episodic retrieval activity. For example, activations of ventral occipital and parieto-occipital regions may reflect ‘reinstatement’ of encoding-related brain activity (Nyberg et al., 2000). Consistent with this idea, additional ‘inclusive masking’ analyses involving both encoding and retrieval phase data (joint probability = 0.033 × 0.033 = .001) revealed overlapping activity in ventral occipital (BAs 18, 19; 126 voxels) and occipito-parietal cortex (BAs 19, 7; 46 voxels), and no other regions. Activation of more anterior regions in the processing stream may reflect recovery of more abstract information.
3.2 Regions specific to HCR and LCR
Consistent with our second prediction, regions specific to HCR were found mainly in MTL and associated midline regions, including hippocampus, retrosplenial cortex, and anterior cingulate regions. These regions overlap those found in prior investigations of recollection-related activity (e.g., Eldridge et al., 2000; Wheeler and Buckner, 2004; Yonelinas et al., 2005), indicating that activation within theses regions may reflect recollection memory processes, which often accompany high-confidence recognition. The idea that the hippocampus is involved in recollection is also supported by the finding that this region shows greater activity for true than false memories (Kim and Cabeza, 2007b). Though some midline regions were specific to HCR, others were associated with both HCR and LCR. First, the hippocampus was specific to HCR, whereas a posterior parahippocampal region was common to both HCR and LCR. This dissociation is generally in agreement with clinical and fMRI evidence linking recollection to the hippocampus and familiarity to the surrounding cortex (e.g., Brown and Aggleton, 2001; Daselaar et al., 2006). Second, a retrosplenial region was specific to HCR, whereas a posterior cingulate region was common to both HCR and LCR. This dissociation is consistent with (i) evidence that the retrosplenial cortex has strong connections with posterior MTL, whereas the posterior cingulate has dense connections with visuo-spatial areas (Kobayashi and Amaral, 2003), (ii) evidence that lesions of the retrosplenial cortex could cause amnesia (Valenstein et al., 1987), (iii) fMRI evidence indicating different roles during episodic retrieval between the two regions (Yonelinas et al., 2005; Daselaar et al., 2006).
Consistent with our third prediction, regions specifically associated with LCR were found mainly within PFC, including left anterior, left posterior, and right dorsolateral regions. We interpret the roles of these PFC regions as reflecting executive control processes that are contingent upon LCR rather than in support of LCR. This interpretation fits well with Herron et al.'s (2004) finding that ‘old/new’ effects in all PFC regions are sensitive to the ratio of old to new items (e.g., 75:25 versus 25:75) within a recognition test. Also consistent with this interpretation, PFC activity during episodic retrieval is increased when MTL yields weak memory signals (Moscovitch, 1992; Buckner and Wheeler, 2001; Rudy et al., 2005).
The PFC executive processing may involve not only top-down control processes, but also bottom-up evaluation of memory signals eminating from sensory-perceptual and MTL regions. Both ideas are consistent with our previous finding that several PFC regions showed greater activity for false than for true memories (Kim and Cabeza, 2007b). More generally, different frontal regions are most likely to be involved in different aspects of executive control processing. For example, anterior PFC may reflect keeping in mind a main goal during iterative retrieval searches (Koechlin et al., 1999), whereas left posterior PFC may more specifically reflect controlled search processes when remembering is difficult (e.g., Velanova et al., 2003; Wheeler and Buckner, 2003). The right dorsolateral PFC region may mediate sustained attentional demands associated with a difficult task (Cabeza et al., 2003), or monitoring demands associated with low-confidence retrieval (Henson et al., 2000; Fleck et al., 2006).
Separation of MTL and PFC regions into HCR- and LCR-specific regions is consistent with the notion that MTL and the PFC network play complementary roles during episodic memory retrieval (Moscovitch, 1992; Buckner and Wheeler, 2001; Rudy et al., 2005). When MTL yields abundant raw memory materials (HCR), there would be minimal need for controlled retrieval processes mediated by PFC regions. On the other hand, when MTL yields weak memory signals (LCR), there would be greater demand for controlled retrieval attempts mediated by PFC regions. Thus, MTL and the PFC network may play complementary roles during episodic memory retrieval. Consistent with this view, negative coupling between MTL and PFC activity during episodic memory retrieval has been reported in both human (Bunge et al., 2004) and animal studies (Bontempi et al., 1999). We also found a complementary MTL and PFC relationship when comparing activity for true and false memories (Kim and Cabeza, 2007b).
3.3 Dissociation within the posterior parietal cortex
The AtoM model (Cabeza, 2008; Cabeza et al., 2008; Ciaramelli et al., 2008) predicts that dorsal PPC activity should increase when memories are weak (greater demands of top-down attention), whereas ventral PPC activity should increase when memories are strong (stronger capture of bottom-up attention). In line with this prediction, we found that a ventral PPC region was specific to HCR, whereas a dorsal PPC region was specific to LCR. Also consistent with the AtoM model, the dorsal PPC region co-activated with PFC regions, whereas the ventral PPC region co-activated with MTL. As noted before, bilateral parieto-occipital regions were associated with both HCR and LCR activities. The finding of a triple dissociation among ventral, dorsal, and caudal PPC regions could potentially clarify the diverse parietal retrieval effects that have been reported in functional neuroimaging literature (Wagner et al., 2005).
The value of the AtoM model in accounting for parietal effects during episodic retrieval has been confirmed in a recent meta-analysis of fMRI studies (Ciaramelli et al., 2008). Consistent with the AtoM model, this meta-analysis found that dorsal PPC activity tended to be greater when the need for retrieval attempt was supposedly maximal (e.g., familiarity, source memory), whereas ventral PPC activity tended to be greater when the attentional capture by memory contents was supposedly maximal (e.g., strong memories, recollection). Another piece of evidence in line with the AtoM model is Berryhill et al.'s (2007) report that patients with bilateral ventral PPC lesions have largely intact memories, but experience deficits in spontaneous attention to the contents of retrieved memories. This ‘memory neglect’ syndrome provides direct support to the AtoM model. Other accounts of ventral PPC activity include the ‘output buffer’ hypothesis (Vilberg and Rugg, 2008), which attributes ventral PPC activity to the maintenance of retrieved content. However, this hypothesis cannot easily explain high-confidence ventral PPC activity common to ‘old’ and ‘new’ responses reported in some prior fMRI studies (Daselaar et al., 2006; Yonelinas et al., 2005) and memory neglect syndrome described above.
3.4 Theoretical implications and conclusions
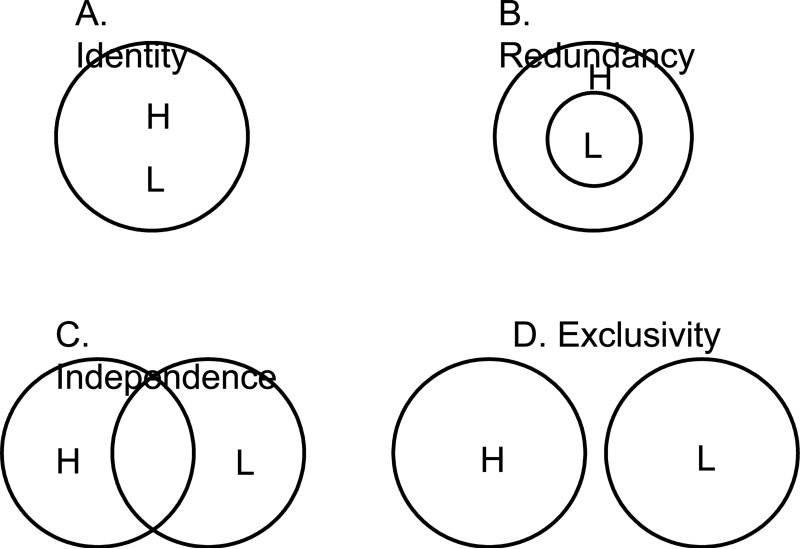
There is theoretical debate about whether HCR and LCR (and perhaps more frequently, recollection and familiarity) depend on the same or different psychological processes (e.g., Yonelinas, 2001, 2002; Dunn, 2004; Wixted and Stretch, 2004), and by implication, on the same or different neural processes (e.g., Montaldi et al., 2006; Skinner and Fernandes, 2007). Fig. 4 depicts four models of the relationship between HCR and LCR processes. The current findings rule out an identity model in which the two functions share completely redundant neural processes (Fig. 4A), as well as an exclusivity model in which the two functions share no common processes (Fig. 4D). Less certain is whether the current findings support a redundancy model in which all LCR processes are also active during HCR (Fig. 4B), or an independence model in which the two functions involve common as well as specific processes (Fig. 4C). Given that a redundancy model predicts a single dissociation involving HCR, whereas an independence model predicts double dissociations involving both HCR and LCR, the present findings may appear to be consistent with an independence model. However, if specific-LCR activations reflect processes that are contingent upon LCR rather than in support of LCR as we suggested above, the present findings are more in line with a redundancy model. Because the present study has provided no direct evidence regarding nature of specific-LCR activations, more studies are needed to definitely distinguish between redundancy and independence models. Finally, to the extent that recollection and familiarity reflect, on average, different levels of memory strength, the current theoretical implication couched in terms of high- and low-strength memories is also relevant to models of the relationship between recollection and familiarity processes.
Figure 4.
Venn diagrams depicting four models of the relationship between high-confidence recognition (H) and low-confidence recognition (L) processes.
In conclusion, the present study directly compared brain regions involved in HCR and LCR. The results showed common regions for these two functions as well as subregions specific to each function. The study yielded 3 main sets of findings. First, sensory/perceptual and associated regions were common to HCR and LCR, indicating involvement of these regions in both HCR and LCR activity. This finding may help explain why these regions are among the most frequently reported in functional neuroimaging studies of episodic memory retrieval. Second, MTL and associated regions were associated with HCR, possibly reflecting recollection-related processes, whereas PFC regions were associated with LCR, possibly reflecting executive control processes. Separation of MTL and PFC regions into HCR- and LCR-specific regions is consistent with the notion that MTL and the PFC network play complementary roles during episodic memory retrieval. Finally, a ventral PPC region was associated with HCR, whereas a dorsal PPC region was associated with LCR, consistent with the AtoM model (Cabeza, 2008). This finding may help explain why functional neuroimaging studies have found diverse parietal effects during episodic memory retrieval. Taken together, our findings provide strong evidence that HCR and LCR, and by implication, recollection and familiarity processes, are represented in common as well as specific brain regions.
4. Experimental Procedures
4.1 Participants
Sixteen young adults participated in the experiment. They were healthy, right-handed, native English speakers, with no history of neurological or psychiatric episodes. All subjects gave informed consent to a protocol approved by the Duke University Institutional Review Board. In an effort to identify neural correlates of HCR and LCR, the analyses focused on the following 3 trial types: high-confidence hit, low-confidence hit, and high-confidence correct rejection. Four subjects were excluded from the analyses due to sparse number (<10) of high-confidence correct rejection responses. Thus, the reported results are based on the data from the remaining 12 subjects (6 female; age range 18−31).
4.2 Behavioral Methods
The stimulus materials were 72 categorical 6-word lists selected from category norms (Battig and Montague, 1969; Yoon et al., 2004). Each list consisted of the 6 most typical instances (e.g., cow, pig, horse, chicken, sheep, goat) of a natural/artificial category (e.g., farm animal), with minor exceptions. In each list, the last four typical instances were used as encoding stimuli (Old words), whereas the first and the second typical instances were used as ‘critical lures’ (Lure words) in the test phase. Additionally, semantically unrelated words, matched in letter number, frequency, and concreteness to the category words, were used as distractors (New words) in the test phase. The categories were carefully chosen so that their instances did not overlap. Thus, both ‘farm animal’ and ‘wild animal’ categories were included in the stimulus set, but ‘four-legged animal’ was not included.
During the study phase, participants viewed 82 ‘mini’ word lists one by one, each consisting of a category name and 4 of the most typical members of the category. The words were displayed in colors to promote the encoding of sensory/perceptual information (Cabeza et al., 2001). Each list was presented for 4 sec. The subjects’ task was to decide whether all 4 or only 3 instances belonged to the category by pressing one of the two keys in a response box using their right hand. In 72 ‘critical’ trials, all 4 words were members of the category, whereas in 10 ‘catch’ trials, only 3 of the 4 words belonged to the category. The test phase, which started approximately 10 min after completion of the study phase, consisted of 6 scans. There were a total of 288 Old-word, 144 New-word, and 144 Lure-word trials across all scans. Lure-word trials were not considered in the analyses of the present study due to an extremely low rate of high-confidence correct rejection responding (7 subjects had less than 10 trials of this type). Trials were presented in a predetermined, pseudo-random order. In each trial, a word was shown for 2 sec, followed by a fixation cross for 1 sec. A fixation period, ranging from 1.5 sec to 4.5 sec, was interspersed across both study and test trials to ‘jitter’ the onset times of trials and allow event-related fMRI analyses. All words in the test phase were displayed in white color against black background. Subjects responded by pressing one of four keys according to whether the word was judged to be ‘definitely old’, ‘probably old’, ‘probably new’, or ‘definitely new’. Thus, in one-step response, subjects provided their memory for the word (old/new) as well as their confidence level in their decision (definitely/probably).
4.3 fMRI Procedures
MRI scanning was conducted using a 4-T GE magnet. Scanner noise was reduced with earplugs, and head motion was reduced with foam pads and headbands. Stimuli were presented with liquid-crystal display goggles. Anatomical scanning started with a T2-weighted sagittal localizer series. The anterior (AC) and posterior commissures (PC) were identified in the midsagittal slice, and 34 contiguous oblique slices were prescribed parallel to the AC-PC plane. High-resolution T1-weighted structural images were collected with a 500-msec repetition time (TR), a 14-msec echo time (TE), a 24-cm field of view (FOV), a 2562 matrix, 68 slices, and a slice thickness of 1.9 mm. Functional images were acquired using an inverse spiral sequence with a 1500-msec TR, a 6-msec TE, a 24-cm FOV, a 642 matrix, and a 60° flip angle. Thirty-four contiguous slices were acquired with the same slice prescription as the anatomical images. Slice thickness was 3.75 mm, resulting in cubic 3.75 mm3 isotropic voxels.
Although scanning took place during encoding and retrieval, the present study focuses on fMRI data collected during retrieval. The fMRI data collected during encoding is reported elsewhere (Kim and Cabeza, 2007a). Image processing and analyses were performed using SPM2 software (www.fil.ion.ucl.ac.uk/spm/). After discarding the first 6 volumes, the functional images were slice-timing corrected and motion-corrected, and then spatially normalized to the Montreal Neurological Institute (MNI) templates implemented in SPM2. The coordinates were later converted to Talairach and Tournoux's (1988) space. Subsequently, the functional images were spatially smoothed using an 8 mm isotropic Gaussian kernel, and resliced to a resolution of 3.75 mm3 isotropic voxels.
4.4 fMRI Analyses
Trial-related fMRI activity was first modeled by convolving a vector of the onset times of the stimuli with a canonical hemodynamic response function (HRF). The general linear model (GLM), as implemented in SPM2, was used to model the effects of interest and other confounding effects (e.g., head movement and magnetic field drift). Trials were coded based on item status (Old words, New words) and subjects’ responses (definitely old, probably old, probably new, definitely new). In an effort to identify neural correlates of HCR and LCR, 3 critical trial types were selected a priori for further analyses: (1) high-confidence hit (HH), (2) low-confidence hit (LH), and (3) high-confidence correct rejection (N). We used high-confidence correct rejections as a ‘baseline’ instead of low-confidence correct rejection or a mixture of high- and low-confidence correct rejections. A critical advantage of using high-confidence correct rejections as a baseline is that they are supposed to involve the lowest level of information recovery to maximize detection of HCR and LCR activity. For each subject, Statistical Parametric Maps (SPM) pertaining to the effects of interest were identified and subsequently integrated across subjects using a random-effects model.
As stated in the Introduction section, the aim of the present study was to compare brain regions involved in HCR versus LCR. To this end, 3 group analyses were conducted using random effects models. First, to identify regions commonly involved in HCR and LCR, we performed 3 steps: (i) created a T-map identifying greater activity for HH versus N and a T-map identifying greater activity for LH versus N; (ii) inclusively masked the 2 T-maps, each with a statistical threshold of P < .033, uncorrected. Since the two probabilities are not independent, this results in joint probability greater than .001 (= .033 × .033). We applied a relatively lenient threshold because our analyses were mainly hypothesis driven focusing on regions depicted in Fig 1., and because we applied an extent threshold considerably greater than conventionally used, i.e., 5 voxels (see below); (iii) eliminated from the conjunction map those voxels showing differential activity between HH and LH (P < .20). Note that in the last step, a more lenient threshold yields a more stringent elimination. Second, to identify regions involved in only HCR, we performed 3 steps: (i) created a T-map identifying greater activity for HH versus N and a T-map identifying greater activity for HH versus LH ; (ii) inclusively masked the 2 T-maps, each with a statistical threshold of P < .033, uncorrected; (iii) eliminated from the conjunction map those voxels showing greater activity for LH versus N (P < .20). Finally, to identify regions involved in only LCR, similar 3 steps were performed. In all 3 group analyses, the extent threshold was set at at least 15 contiguous voxels. A height threshold of .033 with an extent threshold of 15 voxels yields a false positive probability of about .002 per voxel, according to Monte Carlo simulations of spatially correlated data (Forman et al. 1995).
In addition to SPM-based group contrasts, follow-up ROI analyses were performed for certain significant clusters in the group contrast analyses. From each subject and ROI, the mean parameter estimate across all significant voxels was extracted for the 3 critical trial types, respectively. These parameter estimates were subject to repeated measures analysis-of-variance (ANOVAs). For all follow-up ROI analyses, the significance threshold was set at P < .05.
As noted above, in addition to old and new words, the recognition test included ‘lure words’ that were semantically related to studied lists and yielded too few correct rejections to be included in the current analyses. These lure word trials generated very high false alarm rates, and were the critical items in a previous study focused on false memory processes (Kim and Cabeza, 2007b). Although the two studies had different goals and investigated different fMRI contrasts, some findings are related and are considered in the Discussion section. A caveat for the current study is whether the presence of false items in the recognition test affected the processing of old and new items. We believe this is not a problem because the inclusion of false items in a recognition test may affect the placement of the decision criterion (e.g., make decisions more conservative) but it is unlikely to affect the nature of the retrieval processes per se.
Acknowledgements
This research was supported by a Daegu University research grant in 2007 to H.K., and National Institutes of Health Grant AG19731 and AG23770 to R.C. We thank Amber Baptiste for participant recruitment, Rakesh Arya for technical assistance, and Matt Lowder for proofreading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Battig WF, Montague WE. Category norms for verbal items in 56 categories: a replication and extension of the Connecticut norms. J. Exp. Psychol. 1969;80:1–46. [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal lobe and episodic memory: bilateral damage causes impaired free recall of autobiographical memory. J. Neurosci. 2007;27:14415–14423. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi B, Laurent-Demir C, Destrade C, Jaffard R. Time dependent reorganization of brain circuitry underlying long-term memory storage. Nature. 1999;400:671–674. doi: 10.1038/23270. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Wheeler ME. The cognitive neuroscience of remembering. Nat. Rev. Neurosci. 2001;2:624–634. doi: 10.1038/35090048. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Burrows B, Wagner AD. Prefrontal and hippocampal contributions to visual associative recognition: interactions between cognitive control and episodic retrieval. Brain Cogn. 2004;56:141–152. doi: 10.1016/j.bandc.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Role of posterior parietal regions in episodic memory retrieval: the dual attentional processes hypothesis. Neuropsychologia. 2008;46:1813–1827. doi: 10.1016/j.neuropsychologia.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat. Rev. Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Prince S, Rice H, Weissman D, Nyberg L. Attention-related activity during episodic memory retrieval: a cross-function fMRI study. Neuropsychologia. 2003;41:390–399. doi: 10.1016/s0028-3932(02)00170-7. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Seeing the forest through the trees: the cross-function approach to imaging cognition. In: Zani A, Proverbio A, editors. The cognitive electrophysiology of mind and brain. Academic Press; San Diego: 2002. pp. 41–68. [Google Scholar]
- Cabeza R, Rao SM, Wagner AD, Mayer AR, Schacter DL. Can medial temporal lobe regions distinguish true from false? an event-related functional MRI study of veridical and illusory recognition memory. Proc. Natl. Acad. Sci. U.S.A. 2001;98:4805–4810. doi: 10.1073/pnas.081082698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua EF, Schacter DL, Rand-Giovannetti E, Sperling RA. Understanding metamemory: neural correlates of the cognitive process and subjective level of confidence in recognition memory. Neuroimage. 2006;29:1150–1160. doi: 10.1016/j.neuroimage.2005.09.058. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Grady CL, Moscovitch M. Cueing and detecting memory: a hypothesis on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46:1828–1851. doi: 10.1016/j.neuropsychologia.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. Triple dissociations in the medial temporal lobes: recollection, familiarity, and novelty. J. Neurophysiol. 2006;96:1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn. Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Dunn JC. Remember-know: a matter of confidence. Psychol. Rev. 2004;111:524–542. doi: 10.1037/0033-295X.111.2.524. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nat. Neurosci. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Fleck MS, Daselaar SM, Dobbins IG, Cabeza R. Role of prefrontal and anterior cingulate regions in decision-making processes shared by memory and nonmemory tasks. Cereb. Cortex. 2006;16:1623–1630. doi: 10.1093/cercor/bhj097. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431:188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA. A mini-review of fMRI studies of human medial temporal lobe activity associated with recognition memory. Q. J. Exp. Psychol. 2005;58B:340–360. doi: 10.1080/02724990444000113. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Dolan RJ. Confidence in recognition memory for words: dissociating right prefrontal roles in episodic retrieval. J. Cogn. Neurosci. 2000;12:913–923. doi: 10.1162/08989290051137468. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J. Neurosci. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron JE, Henson RNA, Rugg MD. Probability effects on the neural correlates of retrieval success: an fMRI study. Neuroimage. 2004;21:302–310. doi: 10.1016/j.neuroimage.2003.09.039. [DOI] [PubMed] [Google Scholar]
- Joordens S, Merikle PM. Independence or redundancy? two models of conscious and unconscious influences. J. Exp. Psychol. Gen. 1993;122:462–467. [Google Scholar]
- Kim H, Cabeza R. Differential contributions of prefrontal, medial temporal, and sensory-perceptual regions to true and false memory formation. Cereb. Cortex. 2007a;17:2143–2150. doi: 10.1093/cercor/bhl122. [DOI] [PubMed] [Google Scholar]
- Kim H, Cabeza R. Trusting our memories: dissociating the neural correlates of confidence in veridical versus illusory memories. J. Neurosci. 2007b;27:12190–12197. doi: 10.1523/JNEUROSCI.3408-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton BJ. The relationship between remembering and knowing: a cognitive neuroscience perspective. Acta Psychol. 1998;98:253–265. doi: 10.1016/s0001-6918(97)00045-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex. II. Cortical afferents. J. Comp. Neurol. 2003;466:48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR. Connections of the medial posterior parietal cortex (area 7m) in the monkey. Anat. Rec. 2001;263:215–236. doi: 10.1002/ar.1082. [DOI] [PubMed] [Google Scholar]
- Montaldi D, Spencer TJ, Roberts N, Mayes AR. The neural system that mediates familiarity memory. Hippocampus. 2006;16:504–520. doi: 10.1002/hipo.20178. [DOI] [PubMed] [Google Scholar]
- Moritz S, Gläscher J, Sommer T, Büchel C, Braus DF. Neural correlates of memory confidence. Neuroimage. 2006;33:1188–1193. doi: 10.1016/j.neuroimage.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working-with-memory: a component process model based on modules and central systems. J. Cogn. Neurosci. 1992;4:257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Habib R, McIntosh AR, Tulving E. Reactivation of encoding-related brain activity during memory retrieval. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11120–11124. doi: 10.1073/pnas.97.20.11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Biedenkapp JC, O'Reilly RC. Prefrontal cortex and the organization of recent and remote memories: an alternative view. Learn. Mem. 2005;12:445–446. doi: 10.1101/lm.97905. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Henson RNA. Episodic memory retrieval: an (event-related) functional neuroimaging perspective. In: Parker AE, Wilding EL, Bussey T, editors. The cognitive neuroscience of memory encoding and retrieval. Psychology Press; New York: 2002. pp. 3–38. [Google Scholar]
- Rugg MD, Herron JE, Morcom AM. Electrophysiological studies of retrieval processing. In: Squire LR, Schacter DL, editors. Neuropsychology of memory. 3rd ed. Guilford Press; New York: 2002. pp. 154–165. [Google Scholar]
- Simons JS, Peers PV, Hwang DY, Ally BA, Fletcher PC, Budson AE. Is the parietal lobe necessary for recollection in humans? Neuropsychologia. 2008;46:1185–1191. doi: 10.1016/j.neuropsychologia.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Skinner EI, Fernandes MA. Neural correlates of recollection and familiarity: a review of neuroimaging and patient data. Neuropsychologia. 2007;45:2163–2179. doi: 10.1016/j.neuropsychologia.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the Macaque Monkey: cortical afferents. J. Comp. Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- Toth JP. Conceptual automaticity in recognition memory: levels-of-processing effects on familiarity. Can. J. Exp. Psychol. 1996;50:123–138. doi: 10.1037/1196-1961.50.1.123. [DOI] [PubMed] [Google Scholar]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain. 1987;110:1631–1646. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- Velanova K, Jacoby LL, Wheeler ME, McAvoy MP, Petersen SE, Buckner RL. Functional-anatomic correlates of sustained and transient processing components engaged during controlled retrieval. J. Neurosci. 2003;23:8460–8470. doi: 10.1523/JNEUROSCI.23-24-08460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: a review of evidence from event-related fMRI. Neuropsychologia. 2008;46:1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner R. Parietal lobe contributions to episodic memory retrieval. Trends Cogn. Sci. 2005;9:445–452. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional dissociation among components of remembering: control, perceived oldness, and content. J. Neurosci. 2003;23:3869–3880. doi: 10.1523/JNEUROSCI.23-09-03869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Buckner RL. Memory's echo: vivid remembering reactivates sensory-specific cortex. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11125–11129. doi: 10.1073/pnas.97.20.11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixted JT, Stretch V. In defense of the signal detection interpretation of remember/know judgments. Psychon. Bull. Rev. 2004;11:616–641. doi: 10.3758/bf03196616. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Johnson JD, Uncapher MR, Rugg MD. Content-specificity of the neural correlates of recollection. Neuropsychologia. 2005;43:1022–1032. doi: 10.1016/j.neuropsychologia.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Components of episodic memory: the contribution of recollection and familiarity. Phil. Trans. R. Soc. Land. B. 2001;356:1363–1374. doi: 10.1098/rstb.2001.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. J. Mem. Lang. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauve MJ, Widaman KF, Knight RT. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat. Neurosci. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J. Neurosci. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C, Feinberg F, Hu P, Gutchess AH, Hedden T, Chen H, Jing Q, Cui Y, Park DC. Category norms as a function of culture and age: comparisons of item responses to 105 categories by American and Chinese adults. Psychol. Aging. 2004;19:379–393. doi: 10.1037/0882-7974.19.3.379. [DOI] [PubMed] [Google Scholar]