Abstract
As a result of the widespread use of Cd in industry and its extensive dissemination in the environment, there has been considerable interest in the identification of early biomarkers of Cd-induced kidney injury. Kim-1 is a transmembrane glycoprotein that is not detectable in normal kidney, but is up-regulated and shed into the urine following ischemic or nephrotoxic injury. Recent studies utilizing a sub-chronic model of Cd exposure in the rat have shown that Kim-1 is an early urinary marker of Cd-induced kidney injury. Kim-1 was detected in the urine 4–5 weeks before the onset of proteinuria and 1–3 weeks before the appearance of urinary metallothionein and Clara cell protein 16, which are standard markers of Cd nephrotoxicity. In the present study, we have compared the time course for the appearance of Kim-1 in the urine with the time course for the appearance of alpha glutathione-S-transferase (α-GST), N-acetyl-β-D-glucoseamidase (NAG) and Cd, each of which have been used or proposed as urinary markers of Cd nephrotoxicity. Adult male Sprague-Dawley rats were given daily subcutaneous injections of 0.6 mg (5.36 μmoles)/kg Cd, 5 days per week for up to 12 weeks. One day each week, 24 hour urine samples were collected and analyzed for protein, creatinine and the various markers. The results showed that significant levels of Kim-1 appeared in the urine as early as 6 weeks into the treatment protocol and then continued to rise for the remainder of the 12 week treatment period. By contrast, significant levels of α-GST and NAG did not appear in the urine until 8 and 12 weeks, respectively, while proteinuria was not evident until 10 weeks. The urinary excretion of Cd was below the level of detection until week 4 and then showed a slow, linear increase over the next 6 weeks before increasing markedly between weeks 10 and 12. These results provide additional evidence that Kim-1 is a sensitive biomarker of the early stages of Cd-induced proximal tubule injury.
Keywords: cadmium, kidney, Kim-1, alpha glutathione-S-transferase, N-acetyl glucose amidase
Introduction
Cd is an important industrial and environmental pollutant that can exert adverse effects on multiple organ systems (Bernard and Lauwerys, 1984; Jarup et al., 1998; Morselt, 1991). However, with the chronic, low-level patterns of exposure that are common in humans, the kidney is the primary target of toxicity, where Cd causes a generalized dysfunction of the proximal tubule characterized by polyuria and low molecular weight proteinuria (Jarup, 2002; Kjellstrom, 1986; Lauwerys et al., 1984; Piscator, 1986). Several recent studies have highlighted the fact that adverse renal effects of Cd may result from even low levels of exposure and that children and individuals with confounding health conditions, such as diabetes, may be especially susceptible (Akesson et al., 2005; Friedman et al., 2006; Hellstrom et al., 2001; Jarup, 2002; Satarug et al., 2003; Satarug and Moore, 2004).
As a result of the widespread use of Cd in industry and its extensive dissemination in the environment, much attention has been focused on the identification of urinary biomarkers of the early stages of Cd-nephrotoxicity (Abe et al., 2001; Bernard, 2004; Mueller et al., 1998; Nakajima et al., 2005; Roels et al., 1999; Shaikh and Smith, 1986). Some of the urinary biomarkers that have been used for this purpose include the Cd-binding protein metallothionein (Shaikh et al., 1990), low molecular weight proteins such as β-2 microglobulin (Lauwerys et al., 1984) and Clara cell protein-16 (CC-16) (Bernard et al., 1994), and even Cd itself (Bernard, 2004; Mueller et al., 1998).
While these markers have been used to monitor Cd toxicity in humans and experimental animals, several problems remain. For example, the urinary excretion of metallothionein and Cd are markers of Cd exposure as well as proximal tubular injury, and identifying the critical levels of urinary metallothionein or Cd to indicate the onset of tubular injury has been problematic (Chen et al., 2006; Nakajima et al., 2005; Shaikh and Tohyama, 1984; Shaikh et al., 1990; Shaikh and Smith, 1986; Sugihira et al., 1986; Suwazono et al., 2006). Moreover, the urinary excretion of proteins such as β2-microglobulin and CC-16 can be influenced by actions of toxicants on organs other than the kidney (Halatek et al., 2005; Hantson et al., 2008; Shaikh and Smith, 1986). Most significantly, these current markers only identify relatively late stages of Cd-induced kidney injury. By the time these markers appear in the urine, the injury to the kidney is generally considered to be irreversible and untreatable (Kobayashi et al., 2006; Wu et al., 2004). Thus, there is a need for better early biomarkers of Cd-induced kidney injury.
Kim-1 is a type I transmembrane protein that is not detectable in normal kidney but is expressed at high levels in proximal tubule epithelial cells after ischemic or toxic injury (Ferguson et al., 2008; Han and Bonventre, 2004; Ichimura et al., 2004; Vaidya et al., 2008). The ectodomain of Kim-1 is shed into the urine and has been shown to be a sensitive marker of renal injury induced by a variety of agents including cisplatin, S-(1,1,2,2,-tetrafluorethyl)-L-cysteine, folic acid (Ichimura et al., 2004; Vaidya et al., 2006), Hg (Zhou et al., 2008), chromium (Zhou et al., 2008) and cyclosporine (Perez-Rojas et al., 2006). The evidence for the utility of Kim-1 as an early marker of kidney injury is so compelling that the United States Food and Drug Administration and European Medicines Agency have recently adopted Kim-1 as a standard biomarker for the preclinical safety evaluation of novel drug candidates (FDA, 2008).
In a recent study utilizing a sub-chronic model of Cd exposure in rats, we showed that Kim-1 is a very early urinary marker of Cd-induced kidney injury (Prozialeck et al., 2007). In that study, urinary levels of Kim-1 were found to be elevated 1–3 weeks before the appearance of metallothionein and Clara cell protein-16, which are traditional markers of Cd toxicity, and 4 weeks before the onset of overt proteinuria.
While our original studies with Kim-1 were in progress and being prepared for publication, several studies were published highlighting the utility of other urinary biomarkers of Cd nephrotoxicity. Two of the urinary markers that were highlighted in this context were the proximal-tubule derived enzymes NAG (Moriguchi et al., 2003; Suwazono et al., 2006; Teeyakasem et al., 2007) and α-GST (Garcon et al., 2004). Results of those studies, which involved Cd exposed human populations, indicated that NAG and α-GST each outperformed traditional markers of Cd toxicity such as β2-microglubulin. In light of these observations, the present studies were undertaken in order to directly evaluate the utility of Kim-1, α-GST and NAG as early biomarkers of Cd-induced renal injury. These studies involved the use of a well-established model of Cd nephrotoxicity in rats and the direct comparison of the patterns of excretion of the various markers in relation to urinary levels of Cd.
Methods
The Cd-treatment protocol was identical to that described previously (Prozialeck et al., 2007). Male Sprague-Dawley rats weighing 250–300 g were given daily subcutaneous injections of 0.6 mg (5.36 μmoles)/kg Cd, 5 days per week, for up to 12 weeks as described previously (Prozialeck et al., 2007). One day each week, 24-h urine samples were collected and alliquoted. Some of the urine samples were stabilized in a special buffer (Argutus Medical, Dublin, Ireland) for the analysis of α-GST. All samples were then frozen and stored at −80ºC until they were analyzed for the various markers. All of the analyses were conducted within 6 months after the collection and storage of the urine samples. Each of the markers that were examined have been shown to be stable under these storage conditions (Waikar et al., 2007 and product information from Argutus Medical, Dublin Ireland). The entire treatment protocol was repeated a total of 4 times. Whenever possible, data from the various treatment protocols were pooled so that the total n for the various parameters ranged from a minimum of 5 to a maximum of 22. The pooled data includes the data from our previous study (Prozialeck et al., 2007) plus data from another treatment protocol that was completed since the publication of the previous paper. All animal treatment protocols were reviewed and approved by Midwestern University’s Animal Care and Use Committee.
Determination of Kim-1, NAG, α-GST and Cd
Urine samples were coded so that individuals performing the analyses were blinded as to the identity of the samples. Kim-1 protein was measured in the Vaidya/Bonventre laboratory using Microsphere-based Luminex xMAP™ technology as described previously (Prozialeck et al., 2007; Vaidya et al., 2005). Urinary levels of NAG were determined by using commercially available colorimetric assay kits (#875406, Roche Applied Science, Indianapolis, IN) according to the manufacturer’s recommendedprotocol.
Levels of α-GST were determined using commercially available kits (BIO 64RAT Argutus Medical, Dublin, Ireland) according to the manufacturer’s protocol. Urinary levels of Cd were determined by Chemical Solutions Incorporated (Mechanicsburg, PA) using a Perkin Elmer DRCII inductively coupled plasma mass spectrometer to analyze nitric acid extracts of heat-digested urine samples. The level of Cd detection was approximately 0.034 ppb and all assays were performed in triplicate.
Statistical analysis
Statistical analyses were performed using the Sigma Stat computer program (Version V2.03, Systat Software Inc., Point Richmond, CA). Data for the various urinary parameters were analyzed by the non-parametric Kruskal-Wallis test and Dunn’s posthoc test for multiple comparisons. The rationale for the use of these specific tests have been described previously (Prozialeck et al., 2007).
Results
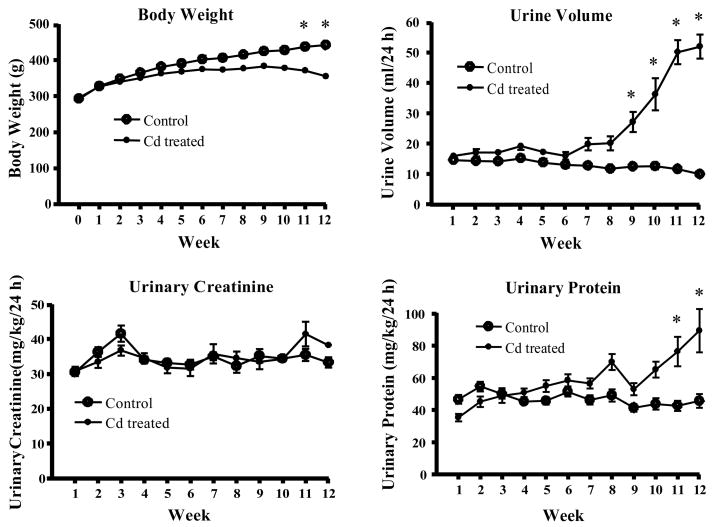
Figure 1 summarizes the effects of Cd on body weight, urine volume, urinary protein and urinary creatinine. Over the course of treatment, the Cd-treated animals gained significantly less weight than the control animals. Accordingly, data for the various urinary parameters were adjusted for body weight. After 9–10 weeks of exposure, the Cd-treated animals developed significant polyuria and proteinuria, with no change in urinary creatinine excretion, effects that are characteristic of Cd-induced proximal tubule injury. The magnitude and time course for these effects are similar to those described in previous studies (Goyer et al., 1989; Liu et al., 1998; Prozialeck et al., 2007; Shaikh et al., 1999; Suzuki, 1980).
Figure 1. Effects of Cd on body weight, urine volume, urinary creatinine and urinary protein.
Male Sprague-Dawley rats received daily subcutaneous injections of Cd (0.6 mg/kg) for up to 12 weeks. One day each week, animals were weighed, 24h urine samples were collected and analyzed for creatinine and protein as described in the Methods section. Values represent the mean ± SE. An * denotes significant differences from week matched control values (p < 0.05) as determined by the non-parametric Kruskal-Wallis test and Dunn’s posthoc test for multiple comparisons. n = 36 for weeks 1–6 and 24 for weeks 7–12.
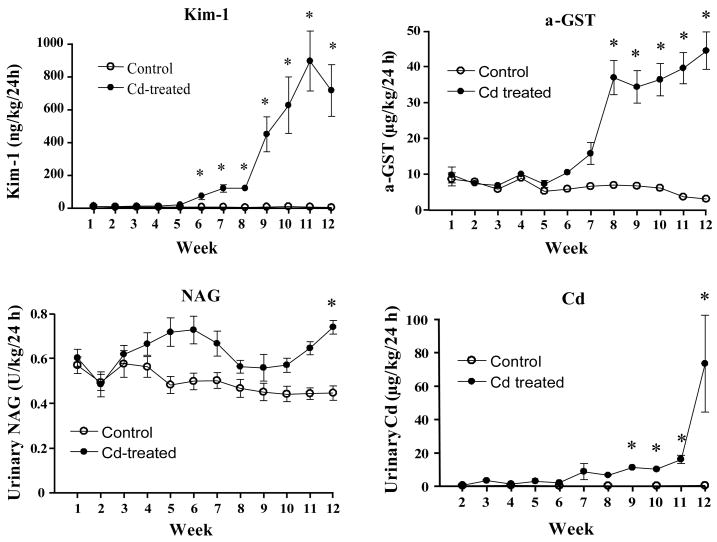
Figure 2 shows the changes in the urinary levels of Kim-1, α-GST, NAG and Cd over the 12 week Cd treatment period. Significantly elevated levels of Kim-1 (about a 15 fold increase over control levels) were present in the urine after 6 weeks of Cd exposure and then continued to rise slightly over the next two weeks. Between weeks 8 and 9, levels of Kim-1 increased markedly and remained elevated throughout the rest of the treatment period. By contrast, increases in the excretion of α-GST were not statistically significant until week 8, about 2 weeks later than the increase in Kim-1 excretion. The levels of α-GST then increased only slightly more over the remainder of the treatment period. Urinary excretion of NAG did not change significantly, until week 12, when it was increased by about 30%. The urinary levels of Cd were below the level of detection in all of the control samples. In the samples from the Cd-treated animals, the urinary excretion of Cd was also below the level of detection until week 4, at which point very low, but detectible, levels of Cd were present. Over the next 5 weeks urinary Cd levels continued to rise, in a slow, linear manner. Between 11 and 12, the urinary excretion of Cd increased markedly. Of all the markers, Cd and Kim-1, which not detectable or are present only at very low levels in control samples, exhibited the largest magnitude of increases in response to Cd exposure. However, the major increase in Cd excretion occurred 3–4 weeks after the increase in Kim-1 excretion.
Figure 2. Effects of Cd treatment on the urinary excretion of Kim-1, α-GST, NAG and Cd.
Animals were treated with Cd (0.6 mg/kg, 5 days per week) for up to 12 weeks and weekly urine samples were analyzed for levels of Kim-1, α-GST, NAG and Cd as described in the Methods section. Values represent the mean ± SE. An * denotes significant differences from week matched control values (p < 0.05) as determined by the non-parametric Kruskal-Wallis test and Dunn’s posthoc test for multiple comparisons For the Kim-1 data, n = 22 for weeks 1–6 and 16 for weeks 7–12; for α-GST, n=9–12; for NAG, n=11–12 and for Cd n=5–6 for each data point.
Discussion
Results of our previous studies showed that Kim-1 is a more sensitive and earlier urinary biomarker of Cd-induced proximal tubule injury than either urinary metallothionein or CC-16, which have long been used as traditional markers of Cd nephrotoxicity (Prozialeck et al., 2007). The results of the present studies indicate that Kim-1 is also a more sensitive marker of Cd-nephrotoxicity than urinary Cd, NAG and α-GST.
In addition to being a very early marker of Cd-induced kidney injury, Kim-1 offers several other advantages as a biomarker. First, it is not expressed, to any significant degree, in non-injured kidney, making it relatively easy to detect any Cd-induced increase in expression. Secondly, it is very stable in urine, even when frozen at −80ºC for months, and unlike α-GST, it does not require the use of special preservatives. Third, it is conserved across species (Vaidya et al., 2008), which could facilitate the extrapolation of the results of studies in animals to the results of studies on human populations and vice versa. With regard to the latter point, it is interesting to note that while NAG has been a useful early marker of Cd toxicity in humans (Jin et al., 1999; Moriguchi et al., 2003; Suwazono et al., 2006; Teeyakasem et al., 2007), our results as well as studies from other laboratories (Groten et al., 1994; Viau et al., 1986) indicate that it does not perform as well in rat models of Cd-induced kidney injury.
In considering the utility of these various markers, it is also important to note that their presence in the urine is indicative of different events in the pathophysiology of nephrotoxic injury [for reviews see (Ferguson et al., 2008; Vaidya et al., 2008)]. α-GST is a cytosolic enzyme that is expressed primarily in the epithelial cells of the proximal tubule (Sundberg et al., 1994). The appearance of α-GST in urine is thought to result from the leakage of cytosolic contents when proximal tubule epithelial cells die and/or slough off into the urine (Ferguson et al., 2008; Sundberg et al., 1994; Vaidya et al., 2008). However, results of recent studies from the our laboratory indicate that at the time the Cd-induced increase in the urinary excretion of α-GST occurs, there is no evidence of necrosis in the proximal tubule. Those studies, which are described in the accompanying article by Prozialeck et al. in this journal suggest that the Cd- increased urinary excretion of α-GST may be due to the shedding of viable or apoptotic cells into the urine.
The urinary excretion of Cd is both a marker of Cd exposure and proximal tubule injury (Suzuki, 1980; Bernard, 2004; Mueller et al., 1998). Under normal conditions, circulating Cd which is bound to low molecular weight materials such as metallothionein cysteine or glutathione, is filtered at the glomerulus and efficiently taken up by the epithelial cells of the proximal tubule (Bridges and Zalups, 2005) and only small amounts are excreted in the urine (Shaikh et al., 1990; Suzuki, 1980). During this stage of exposure the presence of Cd in the urine results from the normal turnover and shedding of epithelial cells and is mainly a reflection of the level of Cd exposure (Suzuki, 1980). However, over time, the concentration of Cd in the epithelial cells increases to the point that more cells begin to die and slough off into the urine. It is at this point that the urinary excretion of Cd increases markedly (Suzuki, 1980). Our results show that Cd begins to appear in the urine after 2–4 weeks of exposure. The urinary levels of Cd then slowly rise until weeks 9–10, at which point there is a marked increase in Cd excretion. This surge in the urinary excretion of Cd coincides with the onset of polyuria and proteinuria. This pattern is similar to that reported by other investigators (Dudley et al., 1985; Goyer et al., 1989; Suzuki, 1980) and is also very similar to the pattern of metallothionein excretion that we observed previously (Prozialeck et al., 2007). These findings are consistent with the hypothesis that the early, linear phases of Cd and metallothionein excretion are a reflection of Cd exposure, whereas the later rises in excretion are a reflection of Cd-induced tubular injury.
By contrast, Kim-1 is expressed by dedifferentiated proximal tubule epithelial cells after ischemic or toxic injury [for reviews see (Ferguson et al., 2008; Vaidya et al., 2008)]. Kim-1 functions as a regulator of cell-cell adhesion and phagocytosis at a time when the dedifferentiated regenerating cells of the injured proximal tubule relocate to denuded patches of the basement membrane to phagocytize cellular debri and reform a continuous epithelial layer (Bailly et al., 2002; Ichimura et al., 2008). This process is associated with the proteolytic cleavage of the ectodomain of Kim-1 into the urine (Bailly et al., 2002). The finding that Kim-1 appears in the urine of the Cd treated animals before markers of necrosis such as α-GST and Cd is consistent with recent observations that the early stages of Cd nephrotoxicity may involve changes in proximal tubule function that occur before overt necrosis of epithelial cells (Prozialeck et al., 2003; 2007). This topic is considered in greater detail in the accompanying publication by Prozialeck et al. in this journal. The fact that Kim-1 can be detected in urine before the onset of lethal injury to most proximal tubule epithelial cells could have important implications regarding the reversibility and potential treatment of Cd-induced kidney disease.
Acknowledgments
This work was supported by NIH grants ES 006478 to W.C.P.; DK 039773, DK 072831 and DK 074099 to J.V.B., K99/R00 ES016723 grant by NIEHS to V.S.V. The authors thank Peter Lamar for his excellent technical assistance and Victoria Sears for her help in preparing the manuscript.
Footnotes
Conflict of Interest Statement
Dr. Bonventre is co-inventor on Kim-1 patents. None of the authors had any conflicts of interest pertaining to the work described in the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe T, Kobayashi E, Okubo Y, Suwazono Y, Kido T, Shaikh ZA, Nogawa K. Application of path analysis to urinary findings of cadmium-induced renal dysfunction. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2001;36:75–87. doi: 10.1081/ese-100000473. [DOI] [PubMed] [Google Scholar]
- Akesson A, Lundh T, Vahter M, Bjellerup P, Lidfeldt J, Nerbrand C, Samsioe G, Stromberg U, Skerfving S. Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ Health Perspect. 2005;113:1627–1631. doi: 10.1289/ehp.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly V, Zhang Z, Meier W, Cate R, Sanicola M, Bonventre JV. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J Biol Chem. 10182002;277:39739–39748. doi: 10.1074/jbc.M200562200. [DOI] [PubMed] [Google Scholar]
- Bernard A. Renal dysfunction induced by cadmium: biomarkers of critical effects. Biometals. 2004;17:519–523. doi: 10.1023/b:biom.0000045731.75602.b9. [DOI] [PubMed] [Google Scholar]
- Bernard A, Lauwerys R. Cadmium in human population. Experientia. 2151984;40:143–152. doi: 10.1007/BF01963577. [DOI] [PubMed] [Google Scholar]
- Bernard AM, Thielemans NO, Lauwerys RR. Urinary protein 1 or Clara cell protein: a new sensitive marker of proximal tubular dysfunction. Kidney Int Suppl. 1994;47:S34–S37. [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. Molecular and ionic mimicry and the transport of toxic metals. Toxicol Appl Pharmacol. 512005;204:274–308. doi: 10.1016/j.taap.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Jin T, Huang B, Nordberg G, Nordberg M. Critical exposure level of cadmium for elevated urinary metallothionein--an occupational population study in China. Toxicol Appl Pharmacol. 8152006;215:93–99. doi: 10.1016/j.taap.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Dudley RE, Gammal LM, Klaassen CD. Cadmium-induced hepatic and renal injury in chronically exposed rats: likely role of hepatic cadmium-metallothionein in nephrotoxicity. Toxicol Appl Pharmacol. 3151985;77:414–426. doi: 10.1016/0041-008x(85)90181-4. [DOI] [PubMed] [Google Scholar]
- FDA. European Medicines Agency to Consider Additional Test Results when Assessing New Drug Safety. 2008 http://www.fda.gov/bbs/topics/NEWS/2008/NEW01850.html.
- Ferguson MA, Vaidya VS, Bonventre JV. Biomarkers of nephrotoxic acute kidney injury. Toxicology. 3202008;245:182–193. doi: 10.1016/j.tox.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman LS, Lukyanova EM, Kundiev YI, Shkiryak-Nizhnyk ZA, Chislovska NV, Mucha A, Zvinchuk AV, Oliynyk I, Hryhorczuk D. Anthropometric, environmental, and dietary predictors of elevated blood cadmium levels in Ukrainian children: Ukraine ELSPAC group. Environ Res. 2006;102:83–89. doi: 10.1016/j.envres.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Garcon G, Leleu B, Zerimech F, Marez T, Haguenoer JM, Furon D, Shirali P. Biologic markers of oxidative stress and nephrotoxicity as studied in biomonitoring of adverse effects of occupational exposure to lead and cadmium. J Occup Environ Med. 2004;46:1180–1186. doi: 10.1097/01.jom.0000141665.22881.69. [DOI] [PubMed] [Google Scholar]
- Goyer RA, Miller CR, Zhu SY, Victery W. Non-metallothionein-bound cadmium in the pathogenesis of cadmium nephrotoxicity in the rat. Toxicol Appl Pharmacol. 1989;101:232–244. doi: 10.1016/0041-008x(89)90272-x. [DOI] [PubMed] [Google Scholar]
- Groten JP, Koeman JH, van Nesselrooij JH, Luten JB, van Fentener V, Stenhuis WS, van Bladeren PJ. Comparison of renal toxicity after long-term oral administration of cadmium chloride and cadmium-metallothionein in rats. Fundam Appl Toxicol. 1994;23:544–552. doi: 10.1006/faat.1994.1139. [DOI] [PubMed] [Google Scholar]
- Halatek T, Gromadzinska J, Wasowicz W, Rydzynski K. Serum clara-cell protein and beta2-microglobulin as early markers of occupational exposure to nitric oxides. Inhal Toxicol. 2005;17:87–97. doi: 10.1080/08958370590899460. [DOI] [PubMed] [Google Scholar]
- Han WK, Bonventre JV. Biologic markers for the early detection of acute kidney injury. Curr Opin Crit Care. 2004;10:476–482. doi: 10.1097/01.ccx.0000145095.90327.f2. [DOI] [PubMed] [Google Scholar]
- Hantson P, Bernard A, Hermans C. Kinetics and determinants of the changes of CC16, a lung secretory protein in a rat model of toxic lung injury. Clin Toxicol(Phila) 2008;46:230–238. doi: 10.1080/15563650701449448. [DOI] [PubMed] [Google Scholar]
- Hellstrom L, Elinder CG, Dahlberg B, Lundberg M, Jarup L, Persson B, Axelson O. Cadmium exposure and end-stage renal disease. Am J Kidney Dis. 2001;38:1001–1008. doi: 10.1053/ajkd.2001.28589. [DOI] [PubMed] [Google Scholar]
- Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286:F552–F563. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- Jarup L. Cadmium overload and toxicity. Nephrol Dial Transplant. 2002;17(Suppl 2):35–39. doi: 10.1093/ndt/17.suppl_2.35. [DOI] [PubMed] [Google Scholar]
- Jarup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure--a review of the literature and a risk estimate. Scandinavian Journal of Work and Environmental Health. 1998;24(Suppl 1):1–51. [PubMed] [Google Scholar]
- Jin T, Nordberg G, Wu X, Ye T, Kong Q, Wang Z, Zhuang F, Cai S. Urinary N-acetyl-beta-D-glucosaminidase isoenzymes as biomarker of renal dysfunction caused by cadmium in a general population. Environ Res. 1999;81:167–173. doi: 10.1006/enrs.1999.3959. [DOI] [PubMed] [Google Scholar]
- Kjellstrom T. Renal Effects. In: Friberg L, Elinder C-G, Kjellstrom T, Nordberg GF, editors. Cadmium and Health: A Toxicological and Epidemiological Appraisal. CRC Press; Boca Raton, FL: 1986. pp. 21–109. [Google Scholar]
- Kobayashi E, Suwazono Y, Uetani M, Inaba T, Oishi M, Kido T, Nishijo M, Nakagawa H, Nogawa K. Estimation of benchmark dose as the threshold levels of urinary cadmium, based on excretion of total protein, beta2-microglobulin, and N-acetyl-beta-D-glucosaminidase in cadmium nonpolluted regions in Japan. Environ Res. 2006;101:401–406. doi: 10.1016/j.envres.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Lauwerys RR, Bernard A, Roels HA, Buchet JP, Viau C. Characterization of cadmium proteinuria in man and rat. Environ Health Perspect. 1984;54:147–152. doi: 10.1289/ehp.8454147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu Y, Habeebu SS, Klaassen CD. Susceptibility of MT-null mice to chronic CdCl2-induced nephrotoxicity indicates that renal injury is not mediated by the CdMT complex. Toxicol Sci. 1998;46:197–203. doi: 10.1006/toxs.1998.2541. [DOI] [PubMed] [Google Scholar]
- Moriguchi J, Ezaki T, Tsukahara T, Furuki K, Fukui Y, Okamoto S, Ukai H, Sakurai H, Shimbo S, Ikeda M. Comparative evaluation of four urinary tubular dysfunction markers, with special references to the effects of aging and correction for creatinine concentration. Toxicol Lett. 8282003;143:279–290. doi: 10.1016/s0378-4274(03)00181-4. [DOI] [PubMed] [Google Scholar]
- Morselt AF. Environmental pollutants and diseases. A cell biological approach using chronic cadmium exposure in the animal model as a paradigm case. Toxicology. 1991;70:1–132. doi: 10.1016/0300-483x(91)90102-7. [DOI] [PubMed] [Google Scholar]
- Mueller PW, Price RG, Finn WF. New approaches for detecting thresholds of human nephrotoxicity using cadmium as an example. Environ Health Perspect. 1998;106:227–230. doi: 10.1289/ehp.98106227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Kobayashi E, Suwazono Y, Uetani M, Oishi M, Inaba T, Kido T, Shaikh ZA, Nogawa K. Excretion of urinary cadmium, copper, and zinc in cadmium-exposed and nonexposed subjects, with special reference to urinary excretion of beta2-microglobulin and metallothionein. Biol Trace Elem Res. 2005;108:17–31. doi: 10.1385/bter:108:1-3:017. [DOI] [PubMed] [Google Scholar]
- Perez-Rojas J, Blanco JA, Cruz C, Trujillo J, Vaidya VS, Uribe N, Bonventre JV, Gamba G, Bobadilla NA. Mineralocorticoid receptor blockade confers renoprotection in preexisting chronic cyclosporine nephrotoxicity. Am J Physiol Renal Physiol. 7112006 doi: 10.1152/ajprenal.00147.2006. [DOI] [PubMed] [Google Scholar]
- Piscator M. The nephropathy of chronic cadmium poisoning. In: Foulkes EC, editor. Cadmium, Handb Exp Pharmacol. Springer-Verlag; New York: 1986. pp. 197–194. [Google Scholar]
- Prozialeck WC, Lamar PC, Lynch SM. Cadmium alters the localization of N-cadherin, E-cadherin, and beta-catenin in the proximal tubule epithelium. Toxicol Appl Pharmacol. 2003;189:180–195. doi: 10.1016/s0041-008x(03)00130-3. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Vaidya VS, Liu J, Waalkes MP, Edwards JR, Lamar PC, Bernard AM, Dumont X, Bonventre JV. Kidney injury molecule-1 (Kim-1) as an early biomarker of cadmium nephrotoxicity. Kidney International. 2007;72:985–993. doi: 10.1038/sj.ki.5002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels HA, Hoet P, Lison D. Usefulness of biomarkers of exposure to inorganic mercury, lead, or cadmium in controlling occupational and environmental risks of nephrotoxicity. Ren Fail. 1999;21:251–262. doi: 10.3109/08860229909085087. [DOI] [PubMed] [Google Scholar]
- Satarug S, Baker JR, Urbenjapol S, Haswell-Elkins M, Reilly PE, Williams DJ, Moore MR. A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett. 1312003;137:65–83. doi: 10.1016/s0378-4274(02)00381-8. [DOI] [PubMed] [Google Scholar]
- Satarug S, Moore MR. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environmental Health Perspectives. 2004;112:1099–1103. doi: 10.1289/ehp.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh ZA, Ellis KJ, Subramanian KS, Greenberg A. Biological monitoring for occupational cadmium exposure: the urinary metallothionein. Toxicology. 1990;63:53–62. doi: 10.1016/0300-483x(90)90068-r. [DOI] [PubMed] [Google Scholar]
- Shaikh ZA, Smith LM. Biological indicators of cadmium exposure and toxicity. Experientia Suppl. 1986;50:124–130. doi: 10.1007/978-3-0348-7238-6_16. [DOI] [PubMed] [Google Scholar]
- Shaikh ZA, Tohyama C. Urinary metallothionein as an indicator of cadmium body burden and of cadmium-induced nephrotoxicity. Environ Health Perspect. 1984;54:171–174. doi: 10.1289/ehp.8454171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh ZA, Vu TT, Zaman K. Oxidative stress as a mechanism of chronic cadmium-induced hepatotoxicity and renal toxicity and protection by antioxidants. Toxicol Appl Pharmacol. 211999;154:256–263. doi: 10.1006/taap.1998.8586. [DOI] [PubMed] [Google Scholar]
- Sugihira N, Tohyama C, Murakami M, Saito H. Significance of increase in urinary metallothionein of rats repeatedly exposed to cadmium. Toxicology. 1986;41:1–9. doi: 10.1016/0300-483x(86)90099-5. [DOI] [PubMed] [Google Scholar]
- Sundberg A, Appelkvist EL, Dallner G, Nilsson R. Glutathione transferases in the urine: sensitive methods for detection of kidney damage induced by nephrotoxic agents in humans. Environ Health Perspect. 1994;102(Suppl 3):293–296. doi: 10.1289/ehp.94102s3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwazono Y, Sand S, Vahter M, Filipsson AF, Skerfving S, Lidfeldt J, Akesson A. Benchmark dose for cadmium-induced renal effects in humans. Environ Health Perspect. 2006;114:1072–1076. doi: 10.1289/ehp.9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y. Cadmium metabolism and toxicity in rats after long-term subcutaneous administration. J Toxicol Environ Health. 1980;6:469–482. doi: 10.1080/15287398009529866. [DOI] [PubMed] [Google Scholar]
- Teeyakasem W, Nishijo M, Honda R, Satarug S, Swaddiwudhipong W, Ruangyuttikarn W. Monitoring of cadmium toxicity in a Thai population with high-level environmental exposure. Toxicol Lett. 3302007;169:185–195. doi: 10.1016/j.toxlet.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463–493. doi: 10.1146/annurev.pharmtox.48.113006.094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya VS, Ramirez V, Bobadilla NA, Bonventre JV. A microfluidics based assay to measure kidney injury molecule-1 (Kim-1) in the urine as a biomarker for early diagnosis of acute kidney injury. J Am Soc Nephrol. 2005;16:192A. [Google Scholar]
- Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290:F517–F529. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- Viau C, Bernard A, Ouled A, Lauwerys R. Determination of rat beta 2-microglobulin in urine and in serum. II Application of its urinary measurement to selected nephrotoxicity models. J Appl Toxicol. 1986;6:191–195. doi: 10.1002/jat.2550060310. [DOI] [PubMed] [Google Scholar]
- Waikar SS, Vaidya VS, Ferguson MA. Stability and collection requirements of urinary biomarkers of acute kidney injury. J Am Soc Nephrol. 2007;18:576A. [Google Scholar]
- Wu X, Su S, Zhai R, Chen K, Jin T, Huang B, Zhou Y, Ge X, Wei G, Liao R. Lack of reversal effect of EDTA treatment on cadmium induced renal dysfunction: a fourteen-year follow-up. Biometals. 2004;17:435–441. doi: 10.1023/b:biom.0000029440.23884.d6. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Vaidya VS, Brown RP, Zhang J, Rosenzweig BA, Thompson KL, Miller TJ, Bonventre JV, Goering PL. Comparison of kidney injury molecule-1 and other nephrotoxicity biomarkers in urine and kidney following acute exposure to gentamicin, mercury, and chromium. Toxicol Sci. 2008;101:159–170. doi: 10.1093/toxsci/kfm260. [DOI] [PMC free article] [PubMed] [Google Scholar]