Abstract
Recent epidemiological studies suggest a positive association between exposure to the environmental pollutant cadmium (Cd) and the incidence and severity of diabetes. In this review, we examine the literature suggesting a relationship between Cd exposure, elevated blood glucose levels, and the development of diabetes. In addition we review human and animal studies indicating that Cd potentiates or exacerbates diabetic nephropathy. We also review the various possible cellular mechanisms by which Cd may alter blood glucose levels. In addition, we present some novel findings from our own laboratories showing that Cd elevates fasting blood glucose levels in an animal model of subchronic Cd exposure before overt signs of renal dysfunction are evident. These studies also show that Cd reduces insulin levels and has direct cytotoxic effects on the pancreas. Together, these findings indicate that Cd may be a factor in the development of some types of diabetes and they raise the possibility that Cd and diabetes-related hyperglycemia may act synergistically to damage the kidney.
Keywords: cadmium, diabetes, fasting blood glucose, insulin, A1C
Introduction
Diabetes and diabetes-related kidney disease are serious health problems that are the cause of growing concern in many parts of the world. It is estimated the total number of people with diabetes worldwide will rise from 171 million in 2000 to an estimated 366 million by the year 2030 (Wild et al., 2004). The projected increase is mainly due to an epidemic of type II diabetes that has been attributed to environmental factors such as greater urbanization and industrialization as well as longer life expectancies, and this increase is likely to occur even if the prevalence of obesity remains constant (Wild et al., 2004).
One of the most serious complications of diabetes is chronic kidney disease, also known as diabetic nephropathy. Diabetic nephropathy is associated with albuminuria, decreased creatinine clearance, altered glomerular morphology and tubular degeneration (Schrijvers et al., 2004). Approximately 30-40% of type II diabetic patients will develop diabetic nephropathy, and it is now the most common cause of end stage renal failure in the Western world (Schrijvers et al., 2004). While diabetic nephropathy is most commonly associated with the more severe and advanced stages of type II diabetes, there is increasing concern that even early stages of the disease, which are sometimes referred to as prediabetes, may be associated with increased risk of kidney disease.
Prediabetes is an intermediate stage of diabetes that bridges normal blood glucose levels and the blood glucose levels associated with type II diabetes. The American Diabetes Association defines prediabetes as a fasting blood glucose level between 100 and 125 mg/dl (Aroda and Ratner, 2008). Several studies have shown that sustained prediabetic elevations of blood glucose may lead to significant renal dysfunction as evidenced by altered creatinine clearance, albuminuria and renal cell injury (Pugliese et al., 1989; Pontes Andersen et al., 2008).
Type II diabetes and diabetic nephropathy are clearly chronic progressive diseases that are associated with a combination of genetic, lifestyle and environmental factors. While many risk factors have been identified, such as obesity, diet and other lifestyle factors, it is highly likely that there are as yet unidentified environmental factors that influence whether or not an individual will become diabetic, or whether mild or incipient diabetes progresses to a more advanced disease state. In this context, the growing volume of evidence suggesting that Cd may play a role in the development and progression of diabetes and diabetes-related kidney disease could be especially significant.
Cd is an important nephrotoxic pollutant, that poses increasing risks to populations in many parts of the world (Diawara et al., 2006; Kim et al., 2004; Cackovic et al., 2009; Yang et al., 2007 and reviewed by Jarup in this issue). With chronic exposure, Cd accumulates in the epithelial cells of the proximal tubule of the kidney. When a threshold concentration of 150-200 μg/g tissue is reached, Cd causes a generalized dysfunction of the proximal tubule characterized by polyuria, low molecular weight proteinuria and glucosuria (Jarup, 2002; Lauwerys et al., 1984; Satarug and Moore, 2004). As a result of the extensive use of Cd in industry and its extensive dissemination in the environment, numerous studies have focused on the identification of the early stages of Cd-induced kidney injury in exposed human populations (Prozialeck et al., 2007; Bernard, 2004; Prozialeck et al. 2009 in this issue). In some cases, the results of these epidemiological studies have suggested a possible link between Cd exposure and diabetes.
Epidemiological Studies
Results of a study of 31 to 60 year olds from the emerging industrial nation of Pakistan showed that diabetic males (N = 196) had significantly higher blood and urinary levels of Cd than non-diabetic males (N = 238) (Afridi et al., 2008). These differences were evident in both smokers (N = 209) and non-smokers (N = 225) (Afridi et al., 2008). The Third National Health and Nutrition Examination Survey (NHANES III), which examined 8,722 U.S. citizens over age 40, revealed a significant association between elevations in urinary Cd levels and increases in fasting blood glucose levels (110 - 126 mg/dl, N = 610) as well as the numbers of individuals diagnosed with type II diabetes (N = 1207) (Schwartz et al., 2003).
Results of other epidemiological studies suggest that Cd exposure may potentiate the effects diabetes on the kidney. In a study of 122 men and women aged 18 - 85 in the Torres Strait Islands off the coast of Australia Haswell-Elkins et al., (2008) found a statistically significant correlation between urinary Cd levels and albuminuria in individuals with type II diabetes, but found no such correlation in non-diabetic individuals. This population has a notoriously high rate of diabetes and they are exposed to Cd by consuming contaminated seafood (Haswell-Elkins et al., 2007). In another study of 229 type II diabetic patients (92 men and 137 women) in China, Chen et al. (2006), found a 3 - 4 fold increase in the prevalence of urinary indicators of proximal tubule dysfunction in individuals with elevated urinary Cd levels. In another study from Belgium, Buchet et al. (1990) found a significant association between the urinary biomarkers of renal injury (NAG and β2-microglobulin), Cd exposure and diabetes as determined by a multivariate correlation analysis of data from a sampling of 1699 men and women aged 20 - 80. In a similar study of 820 Swedish women between the ages of 53 and 64, multiple linear regression analysis showed statistically significant associations between urinary α1-microglobulin, urinary Cd and diabetes (Akesson et al., 2005).
These epidemiological studies indicate Cd may exacerbate the harmful renal effects of diabetes and vice versa. However, due to numerous other confounding environmental factors inherent in these epidemiological studies, it is difficult to firmly establish any cause and effect relationships. For example, other environmental toxins such as arsenic (Navas-Acien et al., 2008), bisphenol A (Lang et al., 2008) or a generalized inflammation and/or oxidative stress response (Lamb and Goldstein, 2008) may further contribute and increase the probability that an individual will become diabetic. Additional epidemiological studies are needed to rule out the effects of such confounding variables on the possible link between Cd and diabetes. However, results of experimental studies in animals have provided more direct evidence for such a link.
Animal Studies
Experimental studies using animal models of Cd exposure have shown that Cd has diabetogenic effects in both acute and subchronic exposure models. For example, Bell et al., (1990) found that 30 min after acute exposure to a single dose of Cd (0.84 mg/kg, i.p.) plasma glucose levels became significantly elevated in non-fasted rats. In a study involving subchronic exposure, rats given daily doses of Cd (1.0 mg/kg) by oral gavage for 45 days exhibited significantly elevated fasting blood glucose levels (Merali and Singhal, 1980). In other subchronic studies, rats subcutaneously injected with between 1.0 and 2.0 mg/kg Cd daily for 7 to 14 days developed significant increases in blood glucose levels (Lei et al., 2007; Chapatwala et al., 1982). Other studies using similar dosing protocols revealed significant abnormalities in glucose tolerance tests in Cd-treated rats (Han et al., 2003; Merali and Singhal, 1975).
In a study utilizing alloxan-induced diabetic animals, Chandra et al., (1985) showed that Cd exacerbated diabetic hyperglycemia. In this study, fasting blood glucose levels were 4 fold higher in alloxan-induced diabetic animals that were also treated with Cd (2mg/kg/day for 21 days), while there was only a 2 fold increase in blood glucose in the alloxan-alone treated animals as compared to controls (Chandra et al., 1985). In a similar study utilizing streptozotocin-induced diabetic animals, Bernard et al., (1991) found that fasting blood glucose levels were 5 fold higher in the diabetic animals. However, they found no added effect on blood glucose levels in diabetic animals that were also treated with Cd (100 ppm) given in drinking water for 75 days. By contrast in a study involving longer term Cd exposure, streptozotocin-induced diabetic animals given Cd (100 ppm) in drinking water for 90 days had a doubling of urinary NAG levels as compared to the non-diabetic Cd exposed group (Jin et al., 1999). In addition, the accumulation of Cd in kidney tissue in the streptozotocin-induced diabetic animals was approximately 2 fold higher as compared to non-diabetic and Cd treated animals (Jin et al., 1999). While there are some minor inconsistencies, the preponderance of evidence from these animal studies suggests an apparent association and synergistic relationship between the renal dysfunction resulting from Cd exposure and experimentally-induced models of diabetes.
Experimental Studies from Our Laboratory
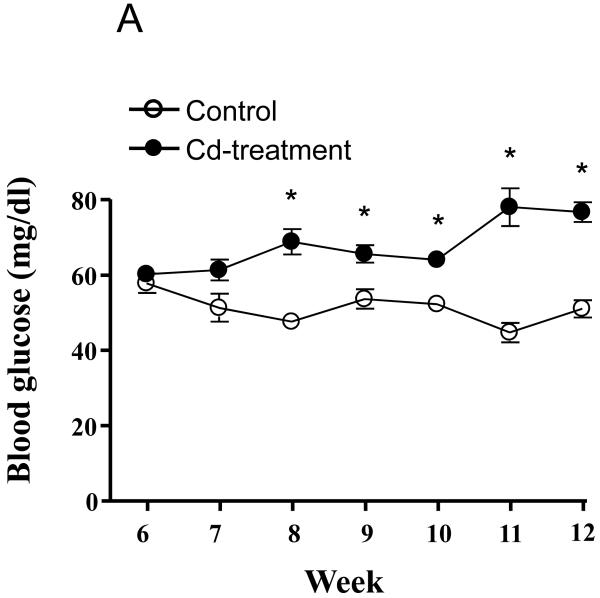
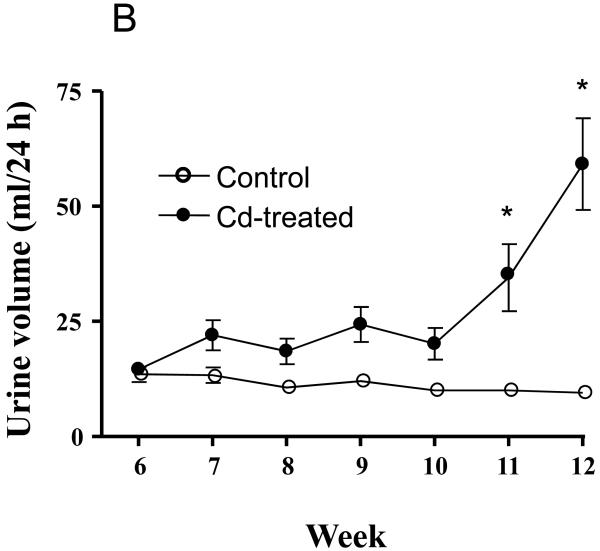
The results of the forgoing epidemiological and animal studies strongly suggest a possible link between chronic Cd exposure and the development of diabetes. In conjunction with our own ongoing studies on the mechanisms of Cd-induced nephrotoxicity (see Prozialeck et al., this issue), we have begun to examine the possible association between Cd-induced alterations in glucose metabolism and kidney injury. These preliminary studies utilized a well-established model of subchronic Cd exposure. Adult Sprague-Dawley rats were given daily subcutaneous injections of CdCl2 at a Cd dose of 0.6 mg/Kg 5 days each week, while control animals received injections of 0.25-0.40 ml isotonic saline, over a total period of 12 weeks. Animals were monitored for weekly changes in fasting blood glucose levels and urine volume. Figure 1 shows the time course for changes in fasting blood glucose levels (1A) and urine volume (1B) in control and Cd-treated animals. For Cd treated animals, significant increases in fasting blood glucose levels were first detected after 8 weeks of Cd treatment. This was 2 weeks prior to the onset of changes in urine volume. Table 1 summarizes the effects of 12 weeks of Cd exposure on blood levels of glycosylated hemoglobin A (%A1C), fasting serum insulin levels and pancreatic Cd concentrations. Cd exposure resulted in increased %A1C levels and about a 50% reduction in levels of fasting serum insulin. Our finding that Cd can decrease insulin levels is in agreement with Lei et al., (2007) who found dose-dependent decreases in insulin gene mRNA levels in pancreata of Cd-treated rats however, the same study did not find significant changes in serum insulin. However, it is important to note that the Lei et al., study involved much shorter exposure to Cd (10 days) than the present study (12 weeks). After 12 weeks, the pancreatic concentration of Cd was 61.5 μg/g of tissue, whereas Cd was not detectable in pancreatic tissue from control animals. Although Cd accumulates in the pancreas, it is at levels much below that of liver or kidney tissue (Lei et al., 2007).
Figure 1. Effects of Cd on the time course for changes in fasting blood glucose (A) and urine volume (B).
Animals were treated with Cd (0.6 mg/kg/day, 5 days a week for 12 weeks). Weekly, 24 h fasting blood glucose values were measured using an Equaline brand blood glucose meter with disposable test strips. Values are mean ± SE. An asterisk (*) denotes significant differences from week matched control mean values (two-way ANOVA and post-hoc Tukey’s test, p ≤ 0.05, n = 3-6 for each data point).
Table 1. Blood glucose related parameters and accumulation of pancreatic Cd from 12 week Cd treated (0.6mg/kg/day of Cd in the form of CdCl2) and control animals.
| Parameter | Control | Cd-treated |
|---|---|---|
| %A1C | 4.6 ± 0.09 | 5.0 ± 0.05 * |
| Serum Insulin | 0.56 ± 0.08 | 0.23 ± 0.06 * |
| Pancreatic Cd concentration | Not detected | 61.5 ± 10.5 |
Percent glycosylated hemoglobin A (%A1C) was determined by A1CNow+ monitor kit (Diabetic Express, Eastlake, OH, USA, catalog # 01-38) using the manufacturer’s recommended protocol (Bayer Corporation, Pittsburg, PA, USA). Serum insulin levels (ng/ml) from fasted animals were quantified using a commercially available ELISA kit according to the manufacturer’s recommended protocol (Crystal Chem Inc., Downers Grove, IL, USA catalog # 90060). Pancreatic Cd levels (μg/g of tissue) were determined by Chemical Solutions Incorporated (Mechanicsburg, PA, USA) using a Perkin Elmer DRCII inductively coupled plasma mass spectrometer to analyze extracts of ashed pancreatic tissue samples.
The accumulation of Cd in the pancreas and reduction of serum insulin levels suggest a possible direct toxic effect of Cd on the pancreas. Figure 2 shows representative images from H&E stained sections of the pancreas taken from control and 12 week Cd-treated animals. Under low power magnification (10X objective) the α- and β-cells located in the islets of Langerhans (white arrows) from Cd-treated animals (B) appeared to have separated from each other as evidenced by the presence of gaps between the cells. Such gaps are absent in sections from saline-control animals (A). Under high power magnification, the cells within the islets of Langerhans from 12 week Cd-treated animals have an irregular appearance with an apparent decrease in cell body volume and what appeared to be vacuoles present in the cells (D) as compared to tissue from control animals (C). Furthermore, there appear to be red blood cell infiltration within the islets of Langerhans (black arrows) in tissue from Cd-treated animals (D), such infiltrates are absent in the sections from saline-control animals (C). Under high power magnification, the acinar cells (black circles) of the exocrine pancreas from Cd treated animals (D) also had an irregular appearance that may also further indicate possible loss of cell-cell adhesion as compared to saline control animals (C). These results show that 12 weeks of Cd exposure caused massive disruption of pancreatic cell morphology and possibly altered cell-cell adhesion.
Figure 2. Histopathological analysis of the effects of Cd on pancreatic tissue.
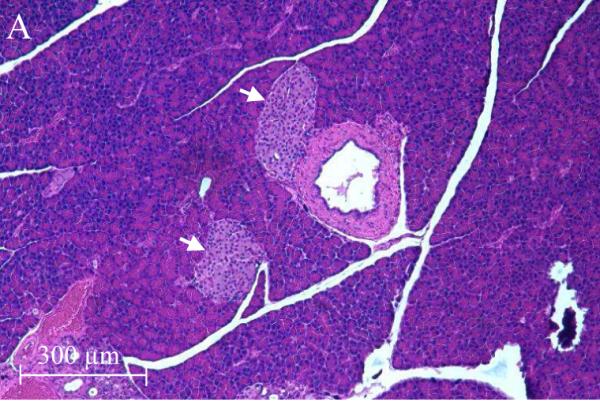
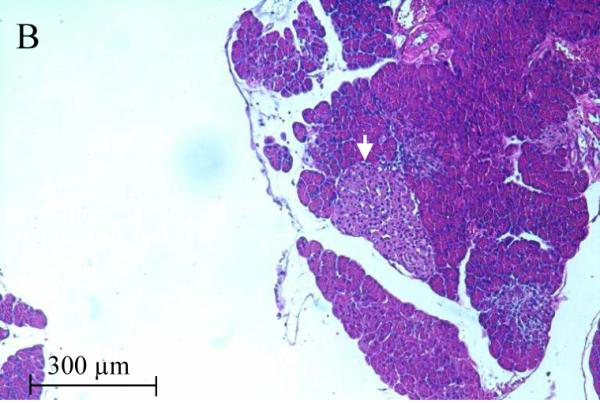
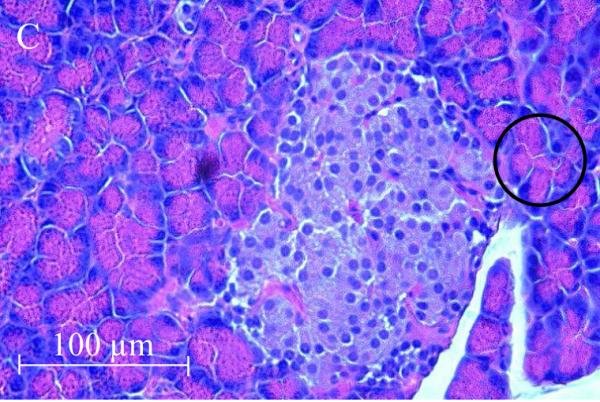
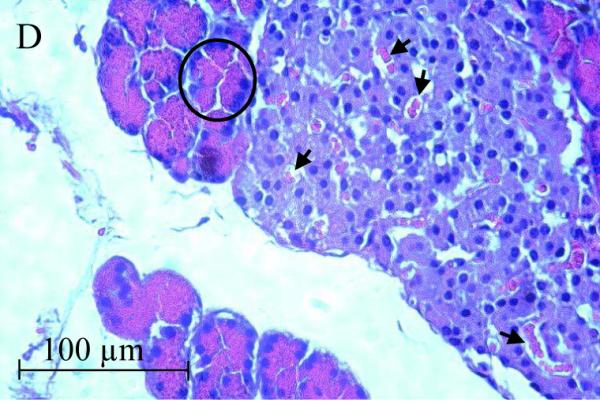
Animals were treated with Cd (0.6 mg/kg/day, 5 days a week). After 12 weeks of Cd treatment approximately 100mg of fresh pancreas was removed from each animal and placed in 6 ml of 10 % formalin (Fisher Sci. cat. # SF98-4) for fixation. Weeks later, the tissue was sent to a contract lab (AML Laboratories, Baltimore, MD) where it was, embedded in paraffin, then sectioned to a thickness of 5μm, and stained with hematoxylin and eosin. H&E stained sections were viewed under standard bright field illumination. Digital images of representative sections were captured with a Spot digital camera (Diagnostic Instruments, Sterling Heights, MI, USA) using the Image-Pro Plus software package (Media Cybernetics, Silver Spring, MD, USA) as described by Edwards et al., (2007). Low magnification images (10X objective) showed individual islets of Langerhans within the pancreatic tissue (white arrows) from saline control animals (A) and Cd-treated animals (B). Higher magnification images (40X objective) showed cells within the islets of Langerhans from saline-control (C) and Cd-treated animals (D). Red blood cells (black arrows) may be seen in the islets of Langerhans from 12 week Cd-treated animals (D). Acinar cells are located adjacent to the islets of Langerhans are denoted by black circles.
Cellular mechanisms of Cd-induced diabetes
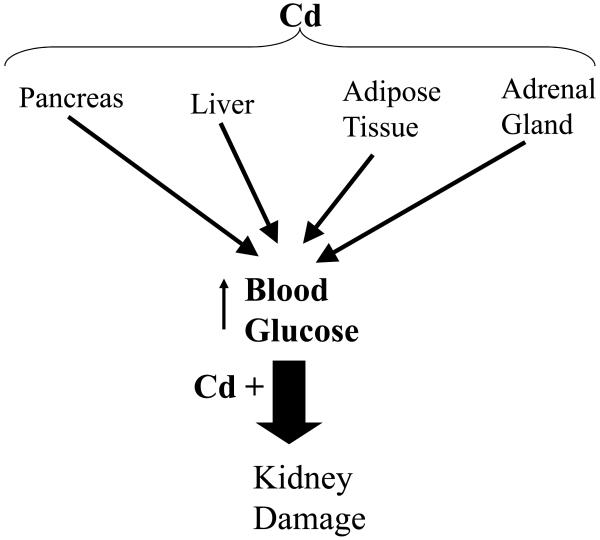
There are a multitude of cellular and physiological mechanisms by which Cd could alter blood glucose levels. Some of these potential mechanisms are summarized in Figure 3. Theoretically, Cd could affect glucose metabolism by acting on a variety of different organs including: pancreas, liver, adipose tissue and the adrenal gland. Our results suggest a direct effect of Cd on the pancreas and there is evidence that Cd can alter insulin release from pancreatic β-cells. In pancreatic islets isolated from obese-hyperglycemic mice, Cd was rapidly taken up in pancreatic tissue (Nilsson et al., 1986). In that study, low levels of (5μM) Cd exposure resulted in an enhanced rate of glucose-stimulated insulin release, whereas high Cd levels (20μM) resulted in a significantly diminished rate of insulin release. Within the liver, sub-chronic Cd exposure has been shown to increase the activity all four of the enzymes responsible for gluconeogenesis (Merali and Singhal, 1975; Chapatwala et al., 1982). Cd also induced glucose metabolism and lipogenesis, and mimicked the effects of insulin in isolated rat adipocytes (Yamamoto et al., 1986). Furthermore, cultured adipocytes that were isolated from rats previously exposed to Cd had decreased expression of the glucose transporter (GLUT4) and reduced glucose transport activity (Han et al., 2003). In the adrenal gland, Cd has been shown to enhance the release of catecholamines, and this may indirectly result in increased blood glucose levels (Shanbaky et al., 1978). Cd exerts direct effects on glucose metabolism in the kidney as well. In primary cultures of mouse renal cortical cells, Cd decreased both glucose uptake and expression of SGLT1, a Na+-dependent glucose symporter, at concentrations of Cd that did not cause cell death (Blumenthal et al., 1998). Furthermore, rats given Cd at a dose of 0.75 mg/kg/day Cd for 2 weeks resulted in elevated activities of enzymes responsible for gluconeogenesis in kidney tissue (Chapatwala et al., 1982). Further studies are needed to sort out the roles of these various organs and their interrelationships in mediating the actions of Cd on glucose metabolism.
Figure 3. Schematic diagram summarizing potential mechanisms of Cd-induced elevated in blood glucose levels and subsequent renal damage.
Following Cd exposure, adipose, pancreatic, and liver tissues along with the adrenal gland, become injured leading to altered glucose metabolism and/or glucose uptake that ultimately results in increased blood glucose. Elevated blood glucose levels coupled with the direct effects of Cd on renal tissue eventually leads to kidney dysfunction and damage.
Summary
Results of both human and animal studies suggest an association between Cd exposure, elevated blood glucose levels and the development of diabetes and diabetes-related kidney disease. In addition, many epidemiological and experimental studies suggest that Cd potentiates or exacerbates diabetic nephropathy. While the link between Cd exposure and elevated blood glucose levels has been established, the mechanisms responsible for Cd-induced changes in blood glucose levels have yet to be elucidated. Of the many diverse mechanisms by which Cd may increase blood glucose levels outlined above, there is considerable evidence that Cd can injure cells within the islets of Langerhans and subsequently influence insulin levels. Preliminary data from the present study show that significant elevations of blood glucose levels occur prior to changes in overt renal dysfunction. This would suggest that Cd-induced elevation in fasting blood glucose levels may in fact contribute to, the etiology of Cd-induced renal dysfunction. Further study is needed to determine the etiology of Cd-induced changes in blood glucose levels, pancreatic β-cell damage and subsequent changes in insulin release.
Acknowledgements
This work was supported by NIH grants ES 006478 to W.C.P.. The authors thank Peter Lamar for his excellent technical assistance and Victoria Sears for her help in preparing the manuscript.
Footnotes
Conflict of Interest Statement
None of the other authors had any conflicts of interest pertaining to the work described in the manuscript.
References
- Afridi HI, Kazi TG, Kazi N, Jamali MK, Arain MB, Jalbani N, Baig JA, Sarfraz RA. Evaluation of status of toxic metals in biological samples of diabetes mellitus patients. Diabetes Res.Clin.Pract. 2008;80:280–288. doi: 10.1016/j.diabres.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Akesson A, Lundh T, Vahter M, Bjellerup P, Lidfeldt J, Nerbrand C, Samsioe G, Stromberg U, Skerfving S. Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ.Health Perspect. 2005;113:1627–1631. doi: 10.1289/ehp.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroda VR, Ratner R. Approach to the patient with prediabetes. J.Clin.Endocrinol.Metab. 2008;93:3259–3265. doi: 10.1210/jc.2008-1091. [DOI] [PubMed] [Google Scholar]
- Bell RR, Early JL, Nonavinakere VK, Mallory Z. Effect of cadmium on blood glucose level in the rat. Toxicol.Lett. 1990;54:199–205. doi: 10.1016/0378-4274(90)90184-n. [DOI] [PubMed] [Google Scholar]
- Bernard A. Renal dysfunction induced by cadmium: biomarkers of critical effects. Biometals. 2004;17:519–523. doi: 10.1023/b:biom.0000045731.75602.b9. [DOI] [PubMed] [Google Scholar]
- Bernard A, Schadeck C, Cardenas A, Buchet JP, Lauwerys R. Potentiation of diabetic glomerulopathy in uninephrectomized rats subchronically exposed to cadmium. Toxicol.Lett. 1991;58:51–57. doi: 10.1016/0378-4274(91)90190-h. [DOI] [PubMed] [Google Scholar]
- Blumenthal SS, Ren L, Lewand DL, Krezoski SK, Petering DH. Cadmium decreases SGLT1 messenger RNA in mouse kidney cells. Toxicol.Appl.Pharmacol. 1998;149:49–54. doi: 10.1006/taap.1997.8353. [DOI] [PubMed] [Google Scholar]
- Buchet JP, Lauwerys R, Roels H, Bernard A, Bruaux P, Claeys F, Ducoffre G, de Plaen P., Staessen J, Amery A. Renal effects of cadmium body burden of the general population. Lancet. 1990 Sep 22;336:699–702. doi: 10.1016/0140-6736(90)92201-r. [DOI] [PubMed] [Google Scholar]
- Cackovic M, Kalinic N, Vadjic V, Pehnec G. Heavy metals and acidic components in total deposited matter in sibenik and national park kornati, croatia. Arch.Environ.Contam Toxicol. 2009;56:12–20. doi: 10.1007/s00244-008-9169-7. [DOI] [PubMed] [Google Scholar]
- Chandra SV, Kalia K, Hussain T. Biogenic amines and some metals in brain of cadmium-exposed diabetic rats. J.Appl.Toxicol. 1985;5:378–381. doi: 10.1002/jat.2550050608. [DOI] [PubMed] [Google Scholar]
- Chapatwala KD, Boykin M, Butts A, Rajanna B. Effect of intraperitoneally injected cadmium on renal and hepatic gluconeogenic enzymes in rats. Drug Chem.Toxicol. 1982;5:305–317. doi: 10.3109/01480548209041060. [DOI] [PubMed] [Google Scholar]
- Chen L, Lei L, Jin T, Nordberg M, Nordberg GF. Plasma metallothionein antibody, urinary cadmium, and renal dysfunction in a Chinese type 2 diabetic population. Diabetes Care. 2006;29:2682–2687. doi: 10.2337/dc06-1003. [DOI] [PubMed] [Google Scholar]
- Diawara MM, Litt JS, Unis D, Alfonso N, Martinez L, Crock JG, Smith DB, Carsella J. Arsenic, cadmium, lead, and mercury in surface soils, Pueblo, Colorado: implications for population health risk. Environ.Geochem.Health. 2006;28:297–315. doi: 10.1007/s10653-005-9000-6. [DOI] [PubMed] [Google Scholar]
- Han JC, Park SY, Hah BG, Choi GH, Kim YK, Kwon TH, Kim EK, Lachaal M, Jung CY, Lee W. Cadmium induces impaired glucose tolerance in rat by down-regulating GLUT4 expression in adipocytes. Arch.Biochem.Biophys. 2003 May 15;413:213–220. doi: 10.1016/s0003-9861(03)00120-6. [DOI] [PubMed] [Google Scholar]
- Haswell-Elkins M, Imray P, Satarug S, Moore MR, O’dea K. Urinary excretion of cadmium among Torres Strait Islanders (Australia) at risk of elevated dietary exposure through traditional foods. J.Expo.Sci.Environ.Epidemiol. 2007;17:372–377. doi: 10.1038/sj.jes.7500520. [DOI] [PubMed] [Google Scholar]
- Haswell-Elkins M, Satarug S, O’Rourke P, Moore M, Ng J, McGrath V, Walmby M. Striking association between urinary cadmium level and albuminuria among Torres Strait Islander people with diabetes. Environ.Res. 2008;106:379–383. doi: 10.1016/j.envres.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Jarup L. Cadmium overload and toxicity. Nephrol.Dial.Transplant. 2002;17(Suppl 2):35–39. doi: 10.1093/ndt/17.suppl_2.35. [DOI] [PubMed] [Google Scholar]
- Jin T, Nordberg G, Sehlin J, Wallin H, Sandberg S. The susceptibility to nephrotoxicity of streptozotocin-induced diabetic rats subchronically exposed to cadmium chloride in drinking water. Toxicology. 1999 Dec 20;142:69–75. doi: 10.1016/s0300-483x(99)00135-3. [DOI] [PubMed] [Google Scholar]
- Kim KH, Choi BJ, Yun ST, Hwang SJ. Studies of spatial and temporal distribution characteristics of TSP-bound trace metals in Seoul, Korea. Environ.Pollut. 2004;127:323–333. doi: 10.1016/j.envpol.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Lamb RE, Goldstein BJ. Modulating an oxidative-inflammatory cascade: potential new treatment strategy for improving glucose metabolism, insulin resistance, and vascular function. Int.J.Clin.Pract. 2008;62:1087–1095. doi: 10.1111/j.1742-1241.2008.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008 Sep 17;300:1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- Lauwerys RR, Bernard A, Roels HA, Buchet JP, Viau C. Characterization of cadmium proteinuria in man and rat. Environ.Health Perspect. 1984;54:147–152. doi: 10.1289/ehp.8454147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei LJ, Jin TY, Zhou YF. Insulin expression in rats exposed to cadmium. Biomed.Environ.Sci. 2007;20:295–301. [PubMed] [Google Scholar]
- Merali Z, Singhal RL. Protective effect of selenium on certain hepatotoxic and pancreotoxic manifestations of subacute cadmium administration. J.Pharmacol.Exp.Ther. 1975;195:58–66. [PubMed] [Google Scholar]
- Merali Z, Singhal RL. Diabetogenic effects of chronic oral cadmium adminstration to neonatal rats. Br.J.Pharmacol. 1980;69:151–157. doi: 10.1111/j.1476-5381.1980.tb10895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA. 2008 Aug 20;300:814–822. doi: 10.1001/jama.300.7.814. [DOI] [PubMed] [Google Scholar]
- Nilsson T, Rorsman F, Berggren PO, Hellman B. Accumulation of cadmium in pancreatic beta cells is similar to that of calcium in being stimulated by both glucose and high potassium. Biochim.Biophys.Acta. 1986 Oct 10;888:270–277. doi: 10.1016/0167-4889(86)90225-9. [DOI] [PubMed] [Google Scholar]
- Pontes Andersen CC, Holmstrup P, Buschard K, Flyvbjerg A. Renal alterations in prediabetic rats with periodontitis. J.Periodontol. 2008;79:684–690. doi: 10.1902/jop.2008.070433. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Vaidya VS, Liu J, Waalkes MP, Edwards JR, Lamar PC, Bernard AM, Dumont X, Bonventre JV. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int. 2007;72:985–993. doi: 10.1038/sj.ki.5002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese G, Tilton RG, Speedy A, Chang K, Santarelli E, Province MA, Eades D, Sherman WR, Williamson JR. Effects of very mild versus overt diabetes on vascular haemodynamics and barrier function in rats. Diabetologia. 1989;32:845–857. doi: 10.1007/BF00297449. [DOI] [PubMed] [Google Scholar]
- Satarug S, Moore MR. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ.Health Perspect. 2004;112:1099–1103. doi: 10.1289/ehp.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijvers BF, De Vriese AS, Flyvbjerg A. From hyperglycemia to diabetic kidney disease: the role of metabolic, hemodynamic, intracellular factors and growth factors/cytokines. Endocr.Rev. 2004;25:971–1010. doi: 10.1210/er.2003-0018. [DOI] [PubMed] [Google Scholar]
- Schwartz GG, Il’yasova D, Ivanova A. Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care. 2003;26:468–470. doi: 10.2337/diacare.26.2.468. [DOI] [PubMed] [Google Scholar]
- Shanbaky IO, Borowitz JL, Kessler WV. Mechanisms of cadmium- and barium-induced adrenal catecholamine release. Toxicol.Appl.Pharmacol. 1978;44:99–105. doi: 10.1016/0041-008x(78)90288-0. [DOI] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Wada O, Ono T, Ono H. Cadmium stimulates glucose metabolism in rat adipocytes. J.Inorg.Biochem. 1986;27:221–226. doi: 10.1016/0162-0134(86)80063-0. [DOI] [PubMed] [Google Scholar]
- Yang QW, Li H, Long FY. Heavy metals of vegetables and soils of vegetable bases in Chongqing, Southwest China. Environ.Monit.Assess. 2007;130:271–279. doi: 10.1007/s10661-006-9395-2. [DOI] [PubMed] [Google Scholar]