Abstract
Although previous studies have shown that Braille reading and other tactile-discrimination tasks activate the visual cortex of blind and sighted people [1–5], it is not known whether this kind of cross-modal reorganization is influenced by retinotopic organization. We have addressed this question by studying S, a visually impaired adult with the rare ability to read print visually and Braille by touch. S had normal visual development until age six years, and thereafter severe acuity reduction due to corneal opacification, but no evidence of visual-field loss. Functional magnetic resonance imaging (fMRI) revealed that, in S’s early visual areas, tactile information processing activated what would be the foveal representation for normally-sighted individuals, and visual information processing activated what would be the peripheral representation. Control experiments showed that this activation pattern was not due to visual imagery. S’s high-level visual areas which correspond to shape- and object-selective areas in normally-sighted individuals were activated by both visual and tactile stimuli. The retinotopically specific reorganization in early visual areas suggests an efficient redistribution of neural resources in the visual cortex.
Results
Perceptual experience changes the physiological and functional architecture of a developing brain [6]. Brain imaging studies have shown that the visual cortex in blind people is active in Braille reading and other tactile tasks, suggesting cross-modal plasticity [1–5]. Disruption of the visual cortex using transcranial magnetic stimulation (TMS) worsens blind people’s performance in both Braille reading and tactile discrimination tasks [3, 7]. However, the precise role of the visual cortex in tactile processing remains controversial. At least two explanations have been suggested for the involvement of the visual cortex in tactile processing. One explanation is that spatial [8–10] or visual [2, 11] imagery plays an important role in the involvement of the visual cortex in tactile tasks in early- and later- blind people. On the other hand, since tactile tasks activate the visual cortex not only in blind people, but also in sighted people, the visual cortex has been hypothesized to be a multimodal spatial processor [12, 13]. Short-term visual deprivation by blindfolding sighted people facilitates Braille learning [14] and results in the recruitment of the visual cortex for tactile processing [12, 15, 16]. A potential explanation of this fast cross-modal plasticity is that latent connections between the primary somatosensory cortex and the visual cortex are unmasked when the dominating retinogeniculate visual inputs are blocked.
However, these two explanations do not take into account the functional and spatial organization of the visual cortex. Early visual cortices are known to have retinotopic organization [17, 18]. Neurons representing different retinal eccentricities in the early visual cortices have different spatial frequency tuning [19, 20]. Foveal neurons have a smaller average receptive field size [21, 22] and are more tuned to high spatial frequencies. They are capable of processing visual information at very high spatial frequencies. Cortical neurons representing peripheral vision have larger receptive fields [21, 22] and are more sensitive to the lower range of spatial frequencies.
Visual impairment due to diseases in the early visual pathways often causes acuity reduction and results in selective deprivation of higher spatial-frequency inputs to the visual cortex. It is possible that the more severe input deprivation in the foveal cortical regions, compared with the peripheral cortical regions, might influence the recruitment pattern of visual cortex for tactile processing. If so, visually-impaired people might exhibit a retinotopically specific reorganization of visual cortex in which some regions are retained for visual processing while other regions are re-assigned to touch or other sensory modalities.
We report findings on S, a visually-impaired person who has the rare ability of reading both print visually and Braille by touch. Examination of S’s visual cortex using fMRI provides a unique opportunity for testing the proposed explanations for tactile processing in visual cortex. If S’s impaired vision and skilled Braille-reading result in multimodal sharing of the visual cortex, it is important to determine whether the same neurons participate in both vision and touch or whether S’s visual cortex exhibits a retinotopically specific segregation of function for vision and touch. Findings on this special case will provide important information about the extent of specificity in cross-modal cortical plasticity.
Case description
S had normal visual development and acuity until age six years, presumably resulting in normal retinotopic organization in his early visual areas [23, 24]. He then acquired severe bilateral corneal opacification, secondary to Stevens-Johnson Syndrome. The vision in his better (right) eye has remained fairly stable since. Clinical examinations show no evidence for nystagmus in S, and he is capable of stable fixation (Supplemental Figure S1 and S2). Photopic electroretinograms showed no retinal defects in S and a tangent field test confirmed that there is no central-visual-field loss (Supplementary Figure S3)
At the time of testing at age 56 years, he had Snellen acuity 20/1000, Pelli-Robson contrast sensitivity 1.00 log unit, and a full visual field. S is a university professor who reads highly magnified print on a daily basis at tested speeds from 44 to 100 words per minute. He started to read Braille at the age of seven and is a competent Braille reader, reading daily at a tested speed of 110 words per minute.
Lack of activation in S’s foveal confluence by visual stimuli
Blood-oxygen-level-dependent (BOLD) response to different stimuli was measured by fMRI. We attempted retinotopic mapping in S using variants of a standard technique. We presented four 3° thick annuli at eccentricities of, 1.5°, 4.5°, 7.5° and 10.5°, and four 45 deg wedges with radius of 12° at the horizontal and vertical meridians. However, the resulting fMRI activation maps failed to reveal retinotopic maps in S’s occipital cortex. Instead, these stimuli produced activation maps similar to a 26° disk stimulus (Supplementary Figure S4). It is likely that S’s cornea produced sufficient light scatter across S’s retina to render the retinotopic mapping stimuli ineffective. Therefore, the visual areas of S were identified mainly by their anatomical locations [18, 25].
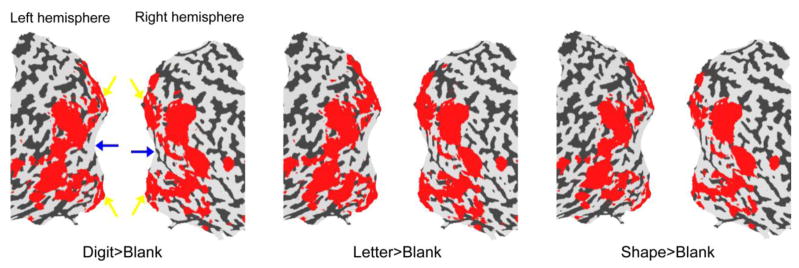
Figure 1 shows the regions that were activated by visually presented digits, letters and simple shapes when S performed a categorization task. These three kinds of stimuli induced similar patterns of cortical activations in both hemispheres: the visually activated regions formed a band that surrounded the occipital pole, but the occipital pole was not activated. A similar pattern was also found when S passively viewed a flickering red/green disk, a flickering black/white disk or moving dots (Supplementary Figure S4).
Figure 1.
fMRI activation maps by visual stimuli in S. Statistical significance maps (thresholded at corrected p < 0.01) are shown on the flattened surface reconstruction of the posterior part of S’s brain. Red regions were activated by visually presented digits (left), letters (middle) and shapes (right) contrasting with blank intervals. S’s foveal and peripheral representations in V1 are indicated by blue and yellow arrows respectively.
The visually inactive and active regions in S’s visual cortex closely match the foveal and peripheral representations in the early visual areas respectively [18] (note that the active regions also include some high-level visual areas extending into temporal and parietal lobes). Since S had no central-visual-field loss, the lack of activity in the foveal confluence is surprising.
S’s retina receives a severely blurred and low-contrast image due to the degraded optics of his eyes. Due to the different spatial frequency tuning properties of foveal and peripheral neurons [17–22], the loss of high spatial frequency content might result in a preferential activation of the peripheral cortical regions representing peripheral vision during visual stimulation. We tested that idea by measuring cortical response to various visual stimuli in normally-sighted control participants who wore diffuser goggles that simulated S’s retinal image quality. The foveal confluence of these normally-sighted controls was activated during visual stimulation (see Supplementary Figure S4). Therefore, we conclude that the foveal confluence in S has been disengaged from normal processing of visual information.
Double dissociation in S’s early visual areas
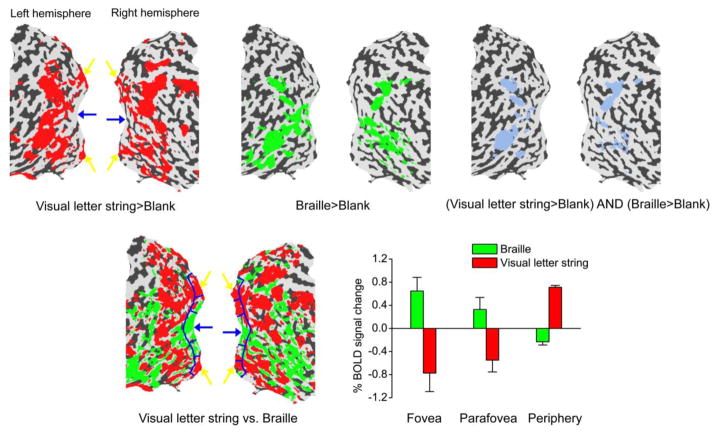
We then studied S’s cortical activity during both visual and Braille lexical decision tasks. S read Braille letter strings of three characters and indicated whether each of the strings was a word. S also performed the same lexical decision task with highly magnified visually displayed letter strings. The Braille task and the visual task were performed within the same fMRI scans, in different blocks separated by blank rest periods. Figure 2 shows the fMRI activation maps indicating the BOLD contrast between the stimulus conditions and the blank condition (upper left and upper middle panels), and the contrast between the Braille and the visual lexical decision conditions (lower left panel). Red and green regions indicate stronger BOLD response to the visual task and the Braille task respectively. The foveal confluence is preferentially activated during the Braille task. The peripheral cortical representation is preferentially activated during the visual task.
Figure 2.
fMRI activation maps by visual letter string and Braille in S. Statistical significance maps (thresholded at corrected p < 0.01) are shown on the flattened surface reconstruction of the posterior part of S’s brain. Upper left: Red regions were activated by visual letter strings contrasting with blank intervals. Upper middle: Green regions were activated by Braille contrasting with blank intervals. Upper right: Blue regions were activated by both visual letter strings and Braille contrasting with blank intervals. Lower left: Visual letter string and Braille conditions were contrasted with each other. Red regions showed higher response to visual letter strings than to Braille, and green regions showed the opposite. S’s foveal and peripheral representations in V1 are indicated by blue and yellow arrows respectively. Lower right: BOLD signals evoked by Braille and visual letter strings relative to blank intervals in three V1 subregions (Talairach coordinates shown in parentheses) – fovea (left: −21, −95,−4; right: 17, −95, −8), parafovea (left: −6, −94, −9; right: 4, −92, −7) and periphery (left: −5, −75, −6; right: 3, −75, −1). The three subregions are delineated by blue curves in the lower left panel. Error bars denote 1 standard error of mean across scans.
Three contiguous regions of interest (ROIs) with roughly the same size were defined along the calcarine sulcus (see the lower left panel of Figure 2), including its lower and upper banks, where early visual areas V1/V2 are usually found. These three ROIs would presumably have corresponded to S’s fovea (0°–1.5°), parafovea (1.5°–5°) and periphery (5°–12°) if there had been no cortical reorganization. The Talairach coordinates [25] of the fovea ROI are close to a previous measurement in normally-sighted individuals [18]. In line with the cortical activation maps, the BOLD signals evoked by the Braille task and the visual task relative to the blank intervals showed opposite trends from fovea to periphery (lower right panel of Figure 2). The BOLD signals were elevated during the Braille task at the foveal confluence and the visual task at the peripheral representation, but the BOLD signals were suppressed during the Braille task at the peripheral representation and the visual task at the foveal confluence. The foveal activation by the Braille task seemed to show a tendency for left lateralization, which is consistent with a report by Burton and colleagues [4].
In addition to the double dissociation in early visual areas, we also found a sharing of cortical resources between Braille reading and visual tasks in other visual areas. The blue regions in the upper right panel of Figure 2 were activated during both the Braille and the visual lexical decision tasks. The shared regions are located in dorsal and lateral occipital areas, ventral occipito-temporal areas and intraparietal sulcus, which closely match the reported locations of visual object- and shape-selective areas [26, 27]. Some visual object- and shape-selective areas (e.g. lateral occipital complex LOC) have also been found to be activated by tactile object perception tasks in normally sighted people [28]. It appears that S’s Braille processing finds its way not only into the early visual areas, but also into the high-level areas that are normally involved in visual shape perception.
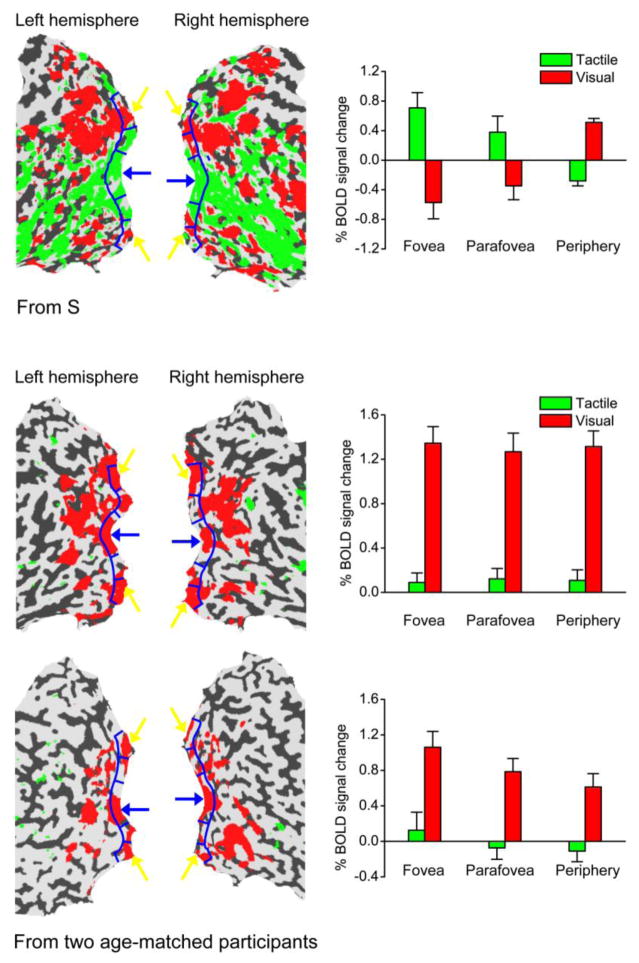
We next asked whether the activation of S’s foveal confluence is dependent on the linguistic content of the Braille task. To address this, we measured BOLD response in S while he performed a non-Braille tactile task and its visual counterpart. S was asked to make a symmetry/asymmetry judgment for simple geometrical shapes presented either visually or tactually. This tactile task induced a similar fMRI activation map to the map in the Braille task (see Figure 3), which suggests that S’s foveal activation to tactile inputs is due to tactile perceptual processing rather than a top-down influence from linguistic processing specific to Braille reading.
Figure 3.
fMRI activation maps by visual and tactile shapes in S and two age-matched control participants. Statistical significance maps (thresholded at corrected p < 0.01) are shown on the flattened surface reconstruction of the posterior part of participants’ brains. Left: Visual shape and tactile shape conditions were contrasted with each other. Red regions showed higher response to visual shape than to tactile shape, and green regions showed the opposite. Participants’ foveal and peripheral representations in V1 are indicated by blue and yellow arrows respectively. Right: BOLD signals evoked by tactile and visual shapes relative to blank intervals in three V1 subregions (fovea, parafovea and periphery), which are delineated by blue curves on the flattened surfaces. Error bars denote 1 standard error of mean across scans.
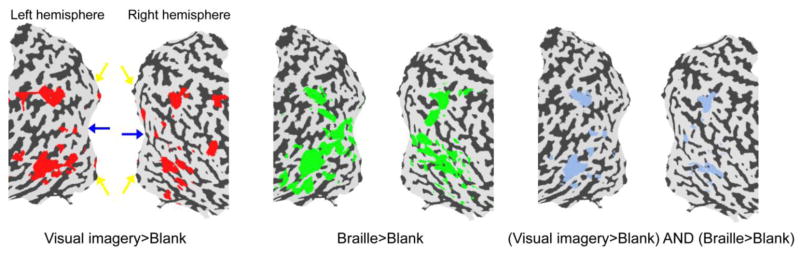
In order to evaluate the possible role of visual imagery in this double dissociation phenomenon in S’s early visual cortex, we measured S’s BOLD response to a visual-imagery task. S heard a spoken word and imagined the word in a pre-specified color (red/green) or case (upper/lower) (We equated auditory stimulation between stimulus blocks and blank blocks. See Methods.) We found sporadic activations in S’s early visual cortex in this task, but they were outside of S’s foveal confluence (left panel of Figure 4). In addition, the imagery task produced substantial activations in S’s high-level visual cortex, including the dorsal and lateral occipital areas, ventral occipito-temporal areas, and intraparietal sulcus, which significantly overlapped with those areas activated by the Braille task (right panel of Figure 4). It should be noted that our visual imagery task might not be the best one to activate V1, but it was designed to best match the Braille task.
Figure 4.
fMRI activation maps by visual imagery and Braille in S. Statistical significance maps (thresholded at corrected p < 0.01) are shown on the flattened surface reconstruction of the posterior part of S’s brain. Left: Red regions were activated by visual imagery contrasting with blank intervals. Middle: Green regions were activated by Braille contrasting with blank intervals. Right: Blue regions were activated by both visual imagery and Braille contrasting with blank intervals. S’s foveal and peripheral representations in V1 are indicated by blue and yellow arrows respectively.
Control experiments with normally-sighted participants
All normally-sighted control participants wore diffuser goggles to simulate S’s retinal image quality during fMRI testing. The diffuser goggles reduced their Snellen acuity to 20/1000, approximately matching S’s visual acuity. Two young controls participated in the visual and Braille lexical decision scans. Since the young controls could not read Braille, they were asked to feel the Braille symbols and to count the dots, instead of performing the Braille lexical decision task. Two age- and gender-matched controls (males aged 56 and 59 years) participated in the same visual and tactile shape categorization scans as S. In all control participants, we found no evidence for tactile activation of the foveal confluence (Figure 3 and Supplementary Figure S5).
Discussion
Our findings about S show that experience-dependent cortical reorganization can be remarkably specific. S’s retinogeniculate pathway ceased to deliver fine-grained visual information to his visual cortex after age six years. Eventually, the neurons normally adept at resolving visual details were recruited for fine discrimination of tactile details. The rest of the visual neurons continued to process coarse visual information. Thus, we interpret our findings as evidence for a visual and tactile experience specific cortical reorganization that is guided by both the availability of input information as well as the inherent functional specialization of the neurons involved. S’s early exposure to the high tactile spatial resolution demanded by Braille reading and other tactile tasks might have been the trigger for cross-modal “takeover” of the foveal representation. His visual cortex has responded to the development of skills in tactile pattern analysis, especially Braille reading, and the concomitant experience of partial visual deprivation by reorganizing cross-modally at the foveal confluence.
Previous studies [4, 29] found that Braille reading in blind people preferentially recruited their peripheral representations, different from the foveal activation in S. This might be due to S’s special visual and tactile experience, and perhaps different study designs as well. Accompanying the foveal activation by tactile stimuli and the peripheral activation by visual stimuli in S, there were corresponding peripheral and foveal suppressions of BOLD signal. Negative BOLD signals are pervasive in functional brain imaging studies, but the origin remains controversial [30, 31]. Whether the negative BOLD signals in S are an epiphenomenon or have a functional role remains unresolved.
Although visual imagery involves V1 [32] and is retinotopically specific [33], we did not find evidence supporting foveal activation by visual imagery in S. And non-visual mental imagery cannot explain the double dissociation in S’s visual cortex too. Nor is this double dissociation explained by the hypothesis that the visual cortex is a multimodal spatial processor, which predicts that the visual and tactile modalities share the neural resources and activate overlapping regions of the visual cortex. Unlike the cortical reorganization studies of blind people [1–5] or the visual deprivation study of blindfolded sighted people [12, 15], our results indicate that the unmasking of connections between the somatosensory cortex and visual cortex can be very specific and functionally adaptive. The recruitment of the visual cortex for touch seems optimal: only those visual neurons that are not critical for S’s remaining low-resolution vision are recruited for tactile processing.
We considered two possible artifactual explanations based on stimulus size for the activation and suppression pattern in S’s visual cortex. First, because most of our visual stimuli covered a large portion of the field, it might be argued that stimulation of both foveal and peripheral regions of the visual field could lead to competitive interaction between these regions in the cortex and result in foveal suppression by peripheral cortical responses. A related argument is that S attended only to the global outline of large stimuli, accounting for peripheral activation and foveal suppression. But S’s data from the retinotopic mapping experiment counter these explanations. In this experiment, a small annulus and a large annulus activated the same peripheral area in S’s cortex, and attending to a small stimulus did not selectively activate S’s foveal projection. Also, S’s ability to read printed text implies that he pays attention to internal features as well as bounding contours of patterns. Second, it might be argued that Braille symbols are physically small, and stimulation with larger embossed patterns might result in tactile activation of more peripheral portions of S’s visual cortex. We believe this is not the case. In our tactile experiments, we used Braille letters and embossed geometrical shapes, which we believe are typical patterns for tactile processing on the fingertip. Recognizing Braille letters requires the ability to process very fine tactile information. On the other hand, since the geometrical shape stimuli were at least six times larger in area than Braille letters, much coarser tactile information processing is adequate for making symmetry/asymmetry judgments for the geometrical shapes. Both large (geometrical shapes) and small (Braille) stimuli evoked similar foveal activation and peripheral suppression in the cortex. This finding suggests that the tactile activation of foveal cortex in S was not limited to fine tactile information processing.
The findings about S may have implications for sight-restoration procedures. What would be the prognosis for S’s visual function if a surgical procedure could provide him with good optical image quality? The reorganization of S’s visual cortex makes it likely that cortical resources would not be available for high-resolution visual analysis, even if the retinogeniculate pathway remained capable of encoding high-resolution features. The disappointing visual outcomes after “sight-restoration” surgery, reported in the case studies by Gregory and Wallace [34], Sacks [35] and Fine et al. [36] of long-term severe visual impairment, are consistent with this likely outcome (but see also [37]). On the other hand, it remains possible that sight restoration late in life might be accompanied by vision reclaiming some of the cortical areas that it has lost. Data from the rare case studies available to date, while suggestive, are inadequate for a definitive conclusion about the capabilities of the visual system for reorganization following sight restoration in adulthood.
In summary, our study of S has demonstrated a multimodal “visual” cortex with dissociable functions. In the midst of an increasing amount of evidence for a plastic brain, our findings show a remarkably specific cortical adaptation to sensory experience. Despite the retinogeniculate inputs to the early visual areas, it appears that tactile afferent inputs are able to make use of unused portions of visual cortex in a functionally appropriate fashion. We suggest that the division of early visual areas in S reflects an optimal distribution of cortical resources. As Braille reading is a tactile task that requires high spatial resolution, the remapping of the foveal confluence for Braille reading is beneficial. At the same time, the preserved peripheral cortical representation in the early visual areas is adequate for processing the severely blurred retinal inputs.
Supplementary Material
Acknowledgments
S is the fourth author. We thank Thomas A. Carlson and Serena Thompson for their assistance in the data collection, Deyue Yu for conducting the tangent field measurements on S, Allen M.Y. Cheong for conducting the fixation-stability measurements on S, Scott O. Murray and Bosco S. Tjan for their comments on earlier drafts of this manuscript. This study was supported by a University of Minnesota Doctoral Dissertation Fellowship to S.-H.C. and a U. S. National Institutes of Health (NIH) grant EY002934 to G.E.L.. The 3T magnetic resonance scanner at the Center for Magnetic Resonance Research of the University of Minnesota was supported by NIH National Center for Research Resources (NCRR) P41 RR008079 and the Mental Illness and Neuroscience Discovery (MIND) Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sadato N, Pascual-Leone A, Grafman J, Ibanez V, Deiber MP, Dold G, Hallett M. Activation of the primary visual cortex by Braille reading in blind subjects. Nature. 1996;380:526–528. doi: 10.1038/380526a0. [DOI] [PubMed] [Google Scholar]
- 2.Buchel C, Price C, Frackowiak RS, Friston K. Different activation patterns in the visual cortex of late and congenitally blind subjects. Brain. 1998;121:409–419. doi: 10.1093/brain/121.3.409. [DOI] [PubMed] [Google Scholar]
- 3.Cohen LG, Weeks RA, Sadato N, Celnik P, Ishii K, Hallett M. Period of susceptibility for cross-modal plasticity in the blind. Ann Neurol. 1998;45:451–460. doi: 10.1002/1531-8249(199904)45:4<451::aid-ana6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 4.Burton H, Snyder AZ, Conturo TE, Akbudak E, Ollinger JM, Raichle ME. Adaptive changes in early and late blind: a fMRI study of Braille reading. J Neurophysiol. 2002;87:589–607. doi: 10.1152/jn.00285.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadato N, Okada T, Honda M, Yonekura Y. Critical period for cross-modal plasticity in blind humans: a functional MRI study. NeuroImage. 2002;16:389–400. doi: 10.1006/nimg.2002.1111. [DOI] [PubMed] [Google Scholar]
- 6.Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen LG, Celnik P, Pascual-Leone A, Corwell B, Falz L, Dambrosia J, Honda M, Sadato N, Gerloff C, Catalá MD, et al. Functional relevance of cross-modal plasticity in blind humans. Nature. 1997;389:180–183. doi: 10.1038/38278. [DOI] [PubMed] [Google Scholar]
- 8.De Volder AG, Toyama H, Kimura Y, Kiyosawa M, Nakano H, Vanlierde A, Wanet-Defalque MC, Mishina M, Oda K, Ishiwata K, et al. Auditory triggered mental imagery of shape involves visual association areas in early blind humans. NeuroImage. 2001;14:129–139. doi: 10.1006/nimg.2001.0782. [DOI] [PubMed] [Google Scholar]
- 9.Vanlierde A, De Volder AG, Wanet-Defalque MC, Veraart C. Occipital-parietal cortex activation during visuo-spatial imagery in early blind humans. NeuroImage. 2003;19:698–709. doi: 10.1016/s1053-8119(03)00153-8. [DOI] [PubMed] [Google Scholar]
- 10.Lambert S, Sampaio E, Mauss Y, Scheiber C. Blindness and brain plasticity: contribution of mental imagery? An fMRI study Cogn Brain Res. 2004;20:1–11. doi: 10.1016/j.cogbrainres.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Zhang M, Weisser VD, Stilla R, Prather SC, Sathian K. Multisensory cortical processing of object shape and its relations to mental imagery. Cogn Affect Behav Neurosci. 2004;4:251–259. doi: 10.3758/cabn.4.2.251. [DOI] [PubMed] [Google Scholar]
- 12.Pascual-Leone A, Hamilton R. The metamodal organization of the brain. Prog Brain Res. 2001;134:427–445. doi: 10.1016/s0079-6123(01)34028-1. [DOI] [PubMed] [Google Scholar]
- 13.Merabet L, Thut G, Murray B, Andrews J, Hsiao S, Pascual-Leone A. Feeling by sight or seeing by touch? Neuron. 2004;42:173–179. doi: 10.1016/s0896-6273(04)00147-3. [DOI] [PubMed] [Google Scholar]
- 14.Kauffman T, Theoret H, Pascual-Leone A. Braille character discrimination in blindfolded human subjects. Neuroreport. 2002;13:571–574. doi: 10.1097/00001756-200204160-00007. [DOI] [PubMed] [Google Scholar]
- 15.Merabet LB, Swisher JD, McMains SA, Halko MA, Amedi A, Pascual-Leone A, Somers DC. Combined activation and deactivation of visual cortex during tactile sensory processing. J Neurophysiol. 2007;97:1633–1641. doi: 10.1152/jn.00806.2006. [DOI] [PubMed] [Google Scholar]
- 16.Merabet LB, Rizzo JF, Amedi A, Somers DC, Pascual-Leone A. What blindness can tell us about seeing again: merging neuroplasticity and neuroprostheses. Nat Rev Neurosci. 2005;6:71–77. doi: 10.1038/nrn1586. [DOI] [PubMed] [Google Scholar]
- 17.Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb Cortex. 1997;7:181–192. doi: 10.1093/cercor/7.2.181. [DOI] [PubMed] [Google Scholar]
- 18.Dougherty RF, Koch VM, Brewer AA, Fischer B, Modersitzki J, Wandell BA. Visual field representation and locations of visual areas V1/2/3 in human visual cortex. J Vis. 2003;3:586–598. doi: 10.1167/3.10.1. [DOI] [PubMed] [Google Scholar]
- 19.De Valois RL, Albrecht DG, Thorell LG. Spatial frequency selectivity of cells in macaque visual cortex. Vision Res. 1982;22:545–559. doi: 10.1016/0042-6989(82)90113-4. [DOI] [PubMed] [Google Scholar]
- 20.Tootell RBH, Silverman MS, Hamilton SL, Switkes E, De Valois RL. Functional anatomy of macaque striate cortex. V Spatial frequency. J Neurosci. 1988;8:1610–1624. doi: 10.1523/JNEUROSCI.08-05-01610.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith AT, Singh KD, Williams AL, Greenlee MW. Estimating receptive field size from fMRI data in human striate and extrastriate visual cortex. Cereb Cortex. 2001;11:1182–1190. doi: 10.1093/cercor/11.12.1182. [DOI] [PubMed] [Google Scholar]
- 22.Dumoulin SO, Wandell BA. Population receptive field estimates in human visual cortex. NeuroImage. 2008;39:647–660. doi: 10.1016/j.neuroimage.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin E, Joeri P, Loenneker T, Ekatodramis D, Vitacco D, Hennig J, Marcar VL. Visual processing in infants and children studied using functional MRI. Pediatr Res. 1999;46:135–140. doi: 10.1203/00006450-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Conner IP, Sharma S, Lemieux SK, Mendola JD. Retinotopic organization in children measured with fMRI. J Vis. 2004;4:509–523. doi: 10.1167/4.6.10. [DOI] [PubMed] [Google Scholar]
- 25.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- 26.Kourtzi Z, Kanwisher N. Representation of perceived object shape by the human lateral occipital complex. Science. 2001;293:1506–1509. doi: 10.1126/science.1061133. [DOI] [PubMed] [Google Scholar]
- 27.Fang F, He S. Cortical response to invisible objects in the human dorsal and ventral pathways. Nat Neurosci. 2005;8:1380–1385. doi: 10.1038/nn1537. [DOI] [PubMed] [Google Scholar]
- 28.Amedi A, Jacobson G, Hendler T, Malach R, Zohary E. Convergence of visual and tactile shape processing in the human lateral occipital complex. Cereb Cortex. 2002;12:1202–1212. doi: 10.1093/cercor/12.11.1202. [DOI] [PubMed] [Google Scholar]
- 29.Burton H. Visual cortex activity in early and late blind people. J Neurosci. 2003;23:4005–4011. doi: 10.1523/JNEUROSCI.23-10-04005.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devor A, Ulbert I, Dunn AK, Narayanan SN, Jones SR, Andermann ML, Boas DA, Dale AM. Coupling of the cortical hemodynamic response to cortical and thalamic neuronal activity. Proc Natl Acad Sci USA. 2005;102:3822–3827. doi: 10.1073/pnas.0407789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9:569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- 32.Kosslyn SM, Pascual-Leone A, Felician O, Camposano S, Keenan JP, Thompson WL, Ganis G, Sukel KE, Alpert NM. The role of area 17 in visual imagery: convergent evidence from PET and rTMS. Science. 1999;284:167–170. doi: 10.1126/science.284.5411.167. [DOI] [PubMed] [Google Scholar]
- 33.Slotnick SD, Thompson WL, Kosslyn SM. Visual mental imagery induces retinotopically organized activation of early visual areas. Cereb Cortex. 2005;15:1570–1583. doi: 10.1093/cercor/bhi035. [DOI] [PubMed] [Google Scholar]
- 34.Gregory RL, Wallace JG. Exp Psychological Soc. Cambridge: Heffer and Sons; 1963. Recovery from early blindness: a case study. Monograph 2. [Google Scholar]
- 35.Sacks O. An Anthropologist on Mars. New York: Vintage Books, Random House; 1995. To see and not see. [Google Scholar]
- 36.Fine I, Wade AR, Brewer AA, May MG, Goodman DF, Boynton GM, Wandell BA, MacLeod DI. Long-term deprivation affects visual perception and cortex. Nat Neurosci. 2003;6:915–916. doi: 10.1038/nn1102. [DOI] [PubMed] [Google Scholar]
- 37.Ostrovsky Y, Andalman A, Sinha P. Vision following extended congenital blindness. Psychol Sci. 2006;17:1009–1014. doi: 10.1111/j.1467-9280.2006.01827.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.