Abstract
Introduction
Postmortem studies have repeatedly found decreased density and number of glia in cortical regions, including the prefrontal and cingulate areas from depressed patients. However, it is unclear whether this glial loss plays a direct role in the expression of depressive symptoms.
Methods
To address this question, we characterized the effects of pharmacologic glial ablation in the prefrontal cortex (PFC) of adult rat on behavioral tests known to be effected by stress or antidepressant treatments: sucrose preference test (SPT), novelty suppressed feeding test (NSFT), forced swim test (FST) and two-way active avoidance test (AAT). We established the dose and time course for the actions of an astrocyte specific toxin, L-alpha-aminoadipic acid (L-AAA), and compared the behavioral effects of this gliotoxin with the effects of an exitotoxic (ibotenate) lesion and to the effects of chronic stress.
Results
The results demonstrate that L-AAA infusions induced anhedonia in SPT, anxiety in NSF and helplessness in FST and AAT. These effects of L-AAA were similar to chronic unpredictable stress (CUS) -induced depressive-like behaviors in these tests. However, ibotenate-induced neurotoxic lesion of the PFC had no effect in these behavioral tests.
Conclusion
The results demonstrate that glial ablation in the PFC is sufficient to induce depressive-like behaviors similar to chronic stress and support the hypothesis that loss of glia contributes to the core symptoms of depression.
Keywords: stress, glia, prefrontal cortex, gliotoxin, anhedonia, helplessness
INTRODUCTION
Brain imaging studies have reported that both the hippocampus and prefrontal cortex (PFC) undergo selective volume reductions in several stress-related neuropsychiatric illnesses, particularly in major depressive disorder (MDD) (1–3). Growing evidence suggests that glial loss and neuronal atrophy may contribute to these volume reductions, and may underlie, in part, the cognitive dysfunction that is a core symptom of depression (4; 5). Indeed, one of the most consistent findings in postmortem studies of depressed patients is a decrease in the density and number of glia, as well as a reduction in the size of neuronal cell bodies, in cortical regions, including the prefrontal and cingulate areas (6–10). The decreases in glial density are accompanied by a reduction of astrocytic markers, such as GFAP (11) and glutamine synthetase (12). These observations support the hypothesis that cellular alterations in the PFC contribute to the symptoms of depression (5; 13–16), but it is unclear whether glial changes are a consequence or cause of illness.
To address this question, we first characterized the effects of chronic unpredictable stress (CUS), a well documented animal model of depression (17; 18), on the density of astrocytes in the rat prelimbic cortex (PLC), and on standard behavioral tests of depression and anxiety. We then investigated the effects of pharmacologic glial ablation in the PFC in the same behavioral tests. For these studies, we use L-Alpha-Aminoadipic Acid (L-AAA), a gliotoxin specific for astrocytes (19; 20). L-AAA enters cells via a Na+-dependent transporter and blocks essential cellular functions involving glutamate, including protein synthesis and energetic metabolism thereby inducing glial death (20). L-AAA infusions in vivo induce a transitory ablation of astrocytes (2–4h and lasts for ~3 days) (19; 21–23). We also compared the effects of L-AAA with a neurotoxic lesion in the PFC. The results demonstrate that CUS reduces glial density in the PFC and that the pharmacologic depletion of cortical astrocytes, but not neurons, produces anhedonia and helplessness, similar to the behavioral consequences of CUS.
MATERIALS and METHODS
Animals
Male Sprague-Dawley rats (Charles River, MA) were housed under a 12-hour light/12-hour dark cycle at constant temperature (25°C) with free access to food and water except when animals are subjected to light disturbance or deprivation stressors during the CUS procedure. Animals weighed between 250–300 g at the beginning of the experiment. Animal use procedures were in accordance with the Yale University Care and Use of laboratory animals (YACUC) guidelines.
CUS procedure
CUS is an experimental procedure in which animals are exposed to a variable sequence of 10 mild and unpredictable stressors, 2 per days for 35 days. The stressors were: cage rotation, light on, light off, cold stress, isolation, food and water deprivation, stroboscope, cage tilt, odor. This stress sequence was adapted from our recent study (24).
Surgery and toxin infusions
Cannula guides were implanted into the PLC (coordinates: AP + 3.2, DL −0.5, Depth −4 from Bregma) of adult rats anaesthetized with xylazine/ketamine (80/6 mg/kg, i.m.) using a stereotaxic frame. After 1 week of recovery, drugs and saline were infused bilaterally into the PFC using a 1mm projection cannula injector once daily for 2 days at a rate of 0.15μl/min. The volume of infusion was 0.5μl for each side. The number of animals per group is indicated after exclusion all animals showing incorrect cannula placement determined by histology.
Two doses of 50 and 100μg/μl of L-AAA (Sigma, St. Louis, MO) were used for this study and are within the range used in the literature (19; 21; 23). Ibotenic acid (Sigma) was infused twice at a dose of 5μg/μl to obtain a lesion similar to previous reports (25) and to keep the number of infusions consistent with that used for L-AAA.
Time course and experimental design
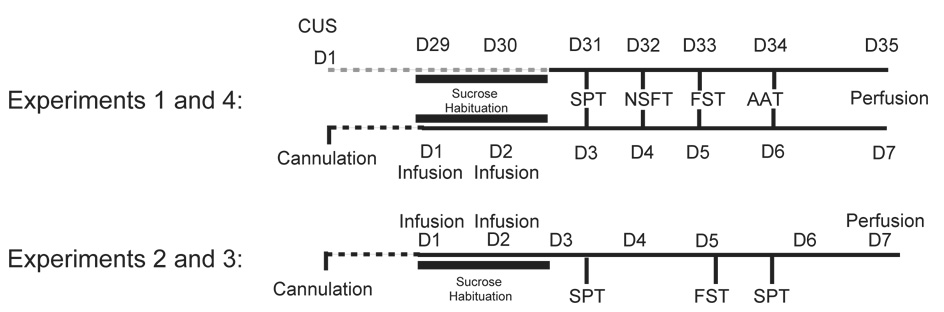
Experiment 1 (Figure 1) examines the influence of CUS compared to home cage controls (HCC, handled daily) on the sucrose preference test (SPT), novelty suppressed feeding test (NSFT), forced swim test (FST), and two-way active avoidance test (AAT) on 4 consecutive days (n=8/group). All behavioral tests were performed during the day to avoid bias of dark-cycle locomotor activity changes (26). The order of the tests was chosen in the minimize test interactions by introducing the least stressful tasks first (fluid and food deprivation) and the more stressful/invasive tasks last (swimming and foot shock). Animals were perfused on day 35, 24h after the AAT and density of astrocytes in the PLC was quantified.
Figure 1.
Experimental design. Experiment 1, animals were subjected to the chronic unpredictable stress (CUS) procedure and tested in sucrose preference test (SPT), novelty suppressed feeding test (NSFT), forced swim test (FST) and active avoidance test (AAT). Experiment 4, animals were infused with saline, L-AAA or ibotenate and then subjected to the same 4 behavioral tests. Experiments 2 and 3 animals were infused with 50 and 100μg/μl of L-AAA into the PFC (Experiment 2) or with 100μg/μl of L-AAA or 5 μg/μl ibotenate (7 days after cannula implantation), and were then subjected to the SPT and FST immediately followed by a second SPT.
Experiment 2 examines the dose and time course of L-AAA effects on SPT and FST (Figure 1). Animals were infused with 50 or 100μg/μl L-AAA on day 1 and day 2. On day 3 SPT was measured and on day 5, animals were tested in FST followed by a second SPT to determine if the gliotoxin lesion is still effective.
Experiment 3 utilizes the same protocol to compare the effects of gliotoxic to neurotoxic lesions in the PFC. Animals were infused with L-AAA (100μg/μl) or ibotenate (5μg/μl) and tested in SPT and FST, followed by a second SPT.
Experiment 4 utilized the same toxin infusion conditions in another cohort of animals and examined SPT, NSFT, FST and AAT (same tests as CUS in Experiment 1). In all studies, locomotor activity was measured on day 3 and right before the SPT. Animals were perfused on day 7.
Behavioral Tests
Locomotor activity was measured for 30 min in a cage equipped with automated activity meters (Digiscan animal activity monitor; Omnitech Electronics, Columbus, OH) as previously described (27).
SPT was conducted as previously described [25, 28, 29]. Briefly, animals were habituated for 48 hours to 1% sucrose (Sigma), and following a 4 hr deprivation period then preference for sucrose (1%) 6 or water (identical bottles) was determined for 1-hour. The second SPT did not include sucrose habituation.
NSFT was performed after 24 h of food-deprivation in an open field, and latency to feed was determined as previously described (27). Home cage food intake was also measured as a control.
FST is a standard test used as a screen for antidepressant-like compounds (28; 29) and immobility was determined in the present study as previously described (27; 30).
AAT was performed in shuttle boxes (Med Associates, VT) as previously described (30). Briefly, animals received 30 randomized escapable foot shocks (0.65mA), with the first 5 trials requiring one crossing to terminate the foot shock (FR-1) and the remaining 25 trials requiring 2 crossings (FR-2) (31–33).
Immunohistochemistry
Processing and immunohistochemistry was conducted as previously described (24; 25). Briefly, postfixed brains were sectioned (40 μm sections, coordinates 4.7 to 1.7 mm from bregma), and every 6th section was mounted on polylysine slides for NeuN or GFAP immunochemistry using mouse anti-neuronspecific nuclear protein (NeuN; 1:1000, Chemicon, UK) or rabbit anti-glial fibrillary acidic protein antibody (GFAP; 1:500, Dako, Denmark)(25; 34).
Quantification
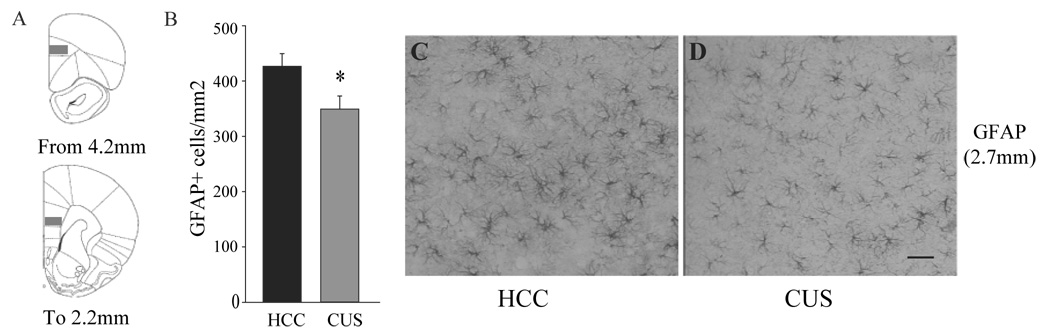
Density of GFAP-positive cells was measured on 6–7 sections per animal (at the coordinates indicated for each experiment) using Using Stereo Investigator software (MicroBrightField, Williston, VT) and a 1X0.5 mm square contour placed over the PLC (Figure 3A). All GFAP-positive cells within the contour were counted under high magnification and results are expressed as mean ± s.e.m. number of GFAP-positive cells per mm2. Results are expressed as mean ± s.e.m. number of GFAP-positive cells per mm2.
Figure 3.
Effects of chronic unpredictable stress (CUS) on density of GFAP-positive cells in the prelimbic cortex (PL). Density of GFAP-positive cells was quantified in the PL of animals subjected to CUS and compared to home cage controls (HCC). Numbers of GFAP-positive cells were quantified throughout the PLC as shown in A (gray squares). (B) Results are expressed as mean ± s.e.m. of GFAP-positive cells/mm2. * P<0.05 compared to HCC. Representative GFAP stained sections from HCC (C) and CUS treated (D) animals are illustrated at approximately 2.7 mm from bregma. Scale bar = 200 μm
Statistical analysis
Statistical differences were determined by analysis of variance (ANOVA, Statview 5) followed by Scheffe post hoc analysis. The F values and experimental degrees of freedom are included in Results. For experiments with two groups, a Student t-test was used. The level of statistical significance was set at P<0.05. ANOVA repeated measure was used when animals are subjected to the second SPT.
RESULTS
Effects of CUS on behavior and GFAP-positive cells in the PFC
CUS has been successfully used in our laboratory to assess changes in gliogenesis in the PFC (24). In the present study, CUS exposed animals were consecutively tested in 4 depressive-like behaviors within a 4-day period (Experiment 1, Figure 1). The results confirm that the ratio of sucrose/water consumed was significantly decreased in CUS animals (−80%) compared to HCC animals (P<0.001, Figure 2A) whereas the total fluid consumption during the 1h SPT was not different. After the SPT, food was removed and 24h later, and the effect of CUS on NSFT was determined. We found a significant 20% increase in latency to feed in the CUS animals (P<0.05, Figure 2B). There was no difference in home cage food consumption (not shown). In the FST (conducted on day 33), CUS animals spent significantly more time immobile than HCC (+58%, P<0.01, Figure 2C). In the AAT, CUS exposure increased escape failures by 2.5-fold compared to HCC (P<0.05, Figure 2D). There was no difference in locomotor activity between HCC and CUS animals (HCC: 2139±239, CUS: 2347±241, mean±s.e.m. number of beambreaks). Together, the results demonstrate that CUS induces deficits in these 4 behavioral tests that are known to be effected by stress and antidepressant treatment.
Figure 2.
Effects of chronic unpredictable stress (CUS) on anhedonia, anxiety and helplessness. CUS and home cage control (HCC) animals were consecutively tested in sucrose preference (A, day 31), novelty suppressed feeding (B, day 32), forced swim (C, day 33) and active avoidance (D, day 34) tests. Results are expressed as mean ± s.e.m. ratio sucrose vs. water consumed (A), latency to feed in seconds (B), time spent immobile in seconds (C) and number of escape failures (D). * P<0.05, ** P<0.01, compared to CTR.
We also analyzed the density of GFAP-positive cells in the PLC of HCC and CUS animals. The results demonstrate a significant decrease (−19%) in the number of GFAP-positive cells/mm2 in the CUS exposed animals compared to HCC (Figure 3B–D). This decrease was homogeneous and was observed throughout the anterior-posterior extent of the PLC (Figure 3A).
Effects of Glial Ablation in the PFC on Behavior
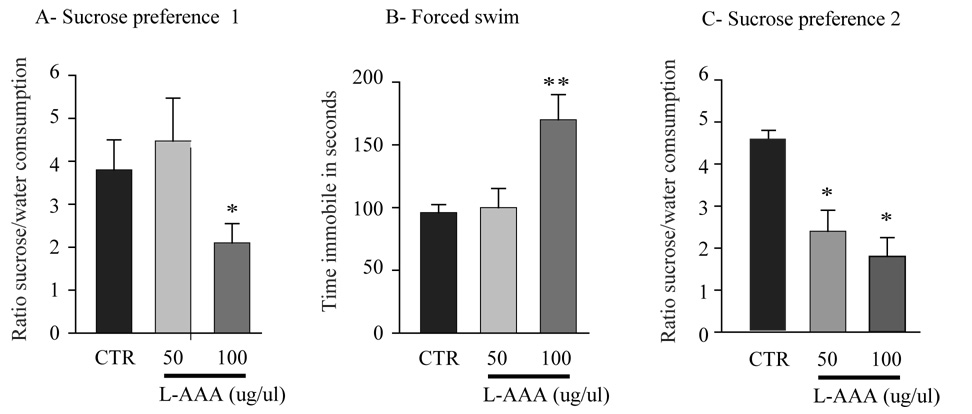
To determine whether glial loss in the PFC contributes to the expression of depressive-like behaviors, we first analyzed the behavioral effects of 50 or 100μg/μl L-AAA infusions on SPT. The lower dose was previously found to induce a transitory glial ablation in 7 to 24h with total recovery at 48h, determined by an increase of the number of astrocytes (gliosis) within the lesion site (22; 23), but to have little or no effect on neurons (19; 20). Because this short time course of L-AAA toxicity, we infused 50μg/μl L-AAA twice in a 24h interval, as well as 2x100μg/μl dose in a 24h interval (Figure 1). Sucrose intake was monitored during the 2 days of sucrose habituation and a trend for a decrease was observed on day 2 (data not shown). Figure 4A illustrates the effects of L-AAA on SPT on day 3. Animals treated with 50μg/μl L-AAA show no change on the ratio sucrose/water consumed compared to saline infused animals. However, a 45% decrease of the ratio of sucrose/water consumed was found at the 100μg/μl L-AAA dose (F2,17 =4.2, P<0.05; n =6 or 7/group). Total fluid consumption did not differ during the 1h test, nor did water consumption monitored for the two following days. Locomotor activity was not significantly different (Saline: 2534±205; L-AAA-50: 2803±251; L-AAA-100: 2635±262, mean±s.e.m. number of beambreaks)
Figure 4.
Effects of L-AAA infusions in the PFC on sucrose preference and forced swim tests. Two doses of L-AAA were tested (50 and 100μg/μl) and compared to saline infused animals (CTR). Rats were subjected to the sucrose preference test after 4h fluid deprivation (A, day 3), to forced swim test (B, day 5), and a second sucrose preference test (C, day 5). Results are expressed as mean ± s.e.m. ratio sucrose to water consumed (A and C) and time spent immobile in seconds (B). * P<0.05, ** P<0.01, compared to CTR.
In the FST (conducted on day 5) 100μg/μl L-AAA treated animals showed a 55% increase in the time spent immobile (F2,17 =4.8, P<0.05, Figure 4B) whereas there was no effect at the 50μg/μl dose. Considering the transitory action of L-AAA, we went on to verify the effects of L-AAA on sucrose preference after the FST on day 5. We observed a 61% decrease in the ratio of sucrose/water consumed during the test in the 100μg/μl L-AAA group and a 48% decrease of sucrose preference in the 50μg/μl L-AAA group (ANOVA repeated measures, treatment effect: F2,17 =8.26, P<0.05, Figure 4C). These changes in the SPT and FST on D5 demonstrate that the effects of L-AAA last for at least 5 days.
Effects of gliotoxin vs. neurotoxin in the PFC
To examine the possibility that the effects observed after L-AAA infusion are due to neuronal cell death, we analyzed the behavioral consequences of a neuronal lesion (Experiment 3, Figure 1). In this experiment, we first compared infusions of 100μg/μl L-AAA to 5μg/μl of ibotenate, which induces neuronal cell death by excitotoxity. The results and magnitude of effects of L-AAA infusions in rat PFC were very similar to those observed in experiment 2 (n=7–8 /group). Indeed, the L-AAA animal group showed a 43% decrease in SPT (F2,19=3.9; P<0.05), a ~60% increase in immobility (F2,19=3.4; P<0.01) and a 69% decrease in the second SPT (F2,19=7.6; P<0.01). Animals receiving ibotenate infusions in the PFC did not differ from the saline infused animals in the three tests (data not shown).
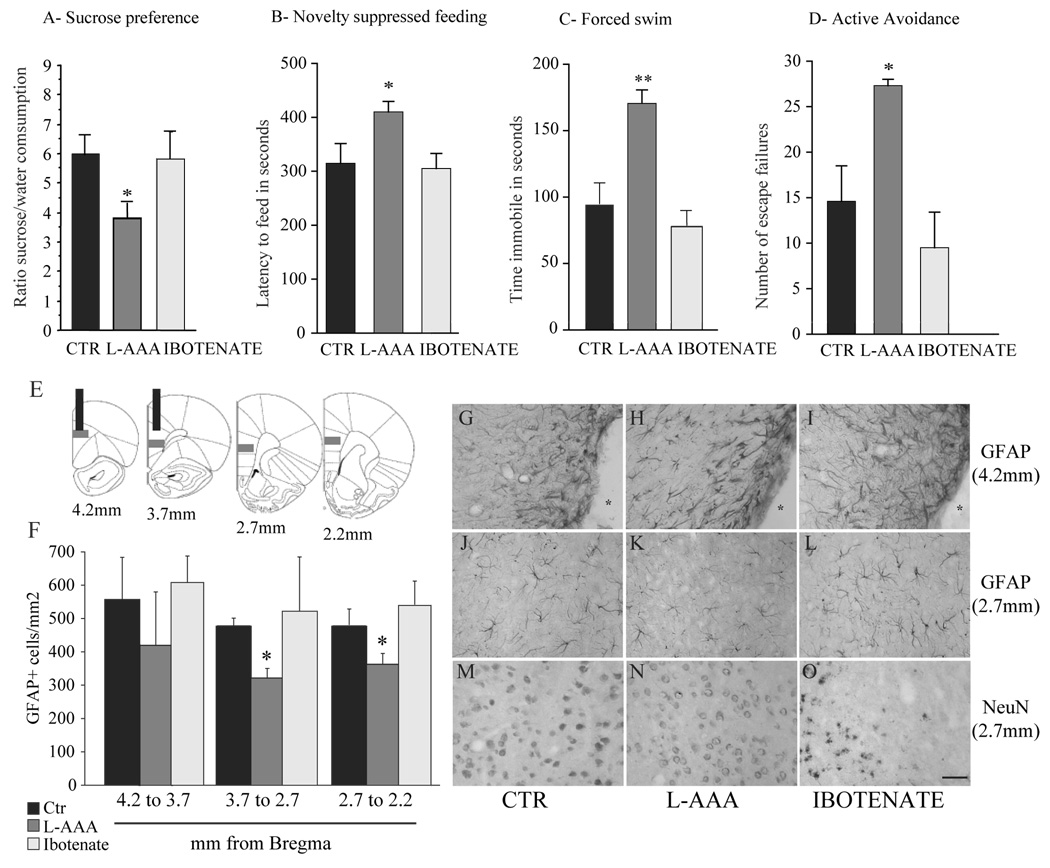
We then analyzed, in another cohort of animals, the effects of L-AAA or ibotenate infusions in the PFC on all 4 behaviors in which CUS animals showed deficits, using the same order and protocols (Experiment 4, Figure 1). We found that while ibotenate lesion had no effect on any of these behavioral tests, L-AAA infusions in the PFC decreased preference in the SPT by 33% (F1,18=4.5, P<0.05), decreased latency to feed in the NSFT by 28% (F1,18=4.48, P<0.05), increased in immobility in the FST by 72% (F1,18= 3.96, P<0.01), and increased escape failures in the AAT by ~2-fold (F1,18=4.48, P<0.05) (Figure 5A–D). There were no differences in home cage food intake, weight or total locomotor activity (Saline: 3029±205, Ibotenate: 3038±418, L-AAA: 2801±361, mean±s.e.m. number of beambreaks, n=7–8 /group).
Figure 5.
Influence of L-AAA or ibotenate infusions in the PFC on behavior, astroglia and neurons. Animals received infusions of saline (CTR), L-AAA or ibotenate as indicated and were consecutively tested in sucrose preference (A, day 3), novelty suppressed feeding (B, day 4), forced swim (C, day 5) and active avoidance (D, day 6) tests. Results are expressed as mean ± s.e.m. ratio sucrose vs. water consumed (A), latency to feed in seconds (B), time spent immobile in seconds (C) and number of escape failures (D). * P<0.05, ** P<0.01, compared to CTR. Density of GFAP-positive cells was quantified in the PLC of animals subjected to L-AAA and ibotenate animals and compared to CTR. Number of GFAP-positive cells was quantified throughout the PLC as described in E (gray squares), and results are expressed as mean ± s.e.m. number of GFAP-positive cells/mm2 (F) * P<0.05, compared to CTR. Representative micrographs of GFAP (G–K) or NeuN immunostained sections (MO) showing the effects of L-AAA (H, K, N)) or ibotenate (I, L, O) are shown. The cannula site (4.2 mm from bregma) is marked by a star (*, G-I). Consequences of L-AAA or ibotenate on GFAP or NeuN immunostaining in fields away from the cannula site (level 2.7 mm from bregma) compared to saline infusions are shown (J–O). Scale bar = 100 μm.
Effects of gliotoxin on the density of GFAP-positive cells in the PFC
We also quantified the effects of infusions of L-AAA or ibotenate on the density of GFAP-positive cells in the PLC on animals from Experiment 4. An overall 23% decrease of the number of GFAP-positive cells/mm2 was found in the PLC of animals infused with 100μg/μl L-AAA. Analysis of the different levels of PFC demonstrates that the effect was greatest away from the infusion site, as a result of reactive gliosis to cannula placement. L-AAA infused animals showed significant 35% and 20% decreases of GFAP-positive cell density at anterior-posterior levels away from the cannula track corresponding to levels 3.7 to 2.7 mm and 2.7 to 2.2 mm from bregma, respectively (Figures 5E, 5F, 5J–L). No significant differences were observed at levels where the cannula tracks were visible, at levels from 4.0 to 3.7 mm from bregma (Figures 5E, 5F, G–I). No significant change in number of GFAP-positive cells was observed in the PLC of ibotenate infused animals (Figures 5E, 5F, 5I, 5L).
L-AAA did not induce a noticeable neuronal loss in the PFC, determined by analysis of NeuN-positive cells, although there were signs of cell stress, including the presence of vacuoles and increased neuronal cell body size. This suggests that L-AAA may either have some direct effects on neurons or that ablation of astrocytes indirectly influences neuronal integrity. Ibotenate infusions produced a discernable decrease in the number of NeuN-positive cells in every animal (Figure 5M–O; qualitative assessment of immunostained sections).
DISCUSSION
This study provides experimental evidences that CUS induces reduction in glial cell density in the PLC of adult rat. The results also demonstrate that selective pharmacological ablation of cortical glia in the PFC produces behavioral deficits similar to those induced by CUS. More precisely, we demonstrate that L-AAA-induced glial ablation in the PFC is sufficient to induce depressive-like behaviors in four different paradigms (SPT, NSFT, FST, and AAT) effected by stress and antidepressant treatment. Moreover, these behavioral effects are not mimicked by excitotoxic neuronal loss produced by infusions of ibotenate into the PFC. Our data support the hypothesis that loss of glia in the PFC may contribute to the expression of depressive behaviors.
The CUS procedure used in this study was similar to that utilized previously (24; 35). The CUS paradigm is an animal model of depression with high predictive, face and construct validity (17; 18) and is thought to simulate stressful life events that promote the development of human depression and behavioral outcomes similar to that observed in depressed patients. We first confirmed that this stress sequence reduces sucrose preference (24; 27; 35), assessing the hedonic state of stressed animals (36). Our CUS procedure also increases immobility in FST, consistent with a previous report utilizing a similar FST paradigm (30). In addition, we extend the behavioral characterization and demonstrate that CUS animals exhibit increased anxiety- and helplessness-like behaviors in the NSFT and AAT. Although we can not exclude a possible interaction between the tests performed in this study, similar magnitude of CUS effects were found in recent studies conducted in our laboratory when animals are only tested in FST or AAT (unpublished data).
Growing evidence in clinical and preclinical studies has implicated glial anomalies in the pathophysiology of mood disorders. Postmortem studies of depressed patients demonstrate reductions in number and/or density of glial cells in the several cortical areas including cingulate, orbital, and dorsolateral PFC (8; 9; 37). These early studies were based on counting of Nissl stained sections and did not identify glial subtypes. However, reductions of GFAP immunoreactivity/protein or mRNA levels were found in brain regions of patients with mood disorders (38; 39), as well as reductions in the number GFAP-positive cells in the frontal cortex of MDD patients (11; 40). In the current study, we now demonstrate that CUS, a valid rodent model of depression, causes a reduction in the number of astrocytes in the PLC. This result extends to the PFC the glial atrophy and loss observed in hippocampus after 28 days of psychosocial stress (41) and demonstrates that repeated stress exposure has deleterious effects on glial morphology and survival.
Current theories propose that reductions of cortical or hippocampal glia contribute to the neural adaptations induced by stress exposure, and thereby contribute to neuronal dysfunction responsible for the expression of depressive-like behavioral deficits (4; 42). This hypothesis is directly supported by the results of the present study. We demonstrate that L-AAA infusions decrease the density of astrocytes in the PLC and provoke a reduction of length and complexity of the processes of GFAP-positive cells, suggesting astrocytic atrophy. The L-AAA-induced glial loss is associated with reduced sucrose preference, a phenotype that usually takes weeks to appear when animals are subjected to stress. This depressive-like phenotype was confirmed in two other behavioral models, the FST and AAT. In addition, the results demonstrate that L-AAA infusions in PFC increase the latency to feed in NSFT, a test of the anxiety to feed that is responsive to chronic, but not acute antidepressant treatments (43). Collectively, our results demonstrate that glial loss in the rat PFC produces changes in neuronal function that contribute to the expression of depressive-like deficits in tests that assess anhedonia, helplessness and anxiety.
Considering the role of astrocytes in nurturing neurons and maintaining synaptic transmission, it is not surprising that postmortem studies have reported neuronal alterations in the PFC of MDD patients. Indeed, reductions in the size of neuronal cell bodies were found in the several layers and cortical areas (6; 8; 10; 37). In the current study, L-AAA-infusions altered the cellular morphology of neurons, suggesting that glial loss and atrophy influences the heath and function of neurons. This hypothesis is supported by the reduced glial density found in this study and by reports of neuronal atrophy resulting from stress (44; 45). A similar hypothesis proposes that stress and stress-hormones alter glial function, consequently inducing neuronal atrophy and impairment in the PFC and the symptoms of depression (4; 10).
In line with this hypothesis, glial dysfunction could be a factor that also exacerbates the effects of stress. This possibility is supported by the observation that although the low dose of L-AAA had no effect on the first SPT, an anhedonic-like state appeared in the second SPT after the animals were subjected to the FST. This suggests that exposure to the acute stress of the FST reveals a depressive-like behavior that is usually seen only after chronic stress. Although the low L-AAA dose did not result in marked glial changes in the PFC (not shown), a previous report found that it was sufficient to inhibit glutamate transport and glutamine synthetase activity (22). Together, these results and the fact that the magnitude of the anhedonic effect of the high dose also increases after FST suggest that glial dysfunction is a susceptibility factor for stress. Indeed, L-AAA-induced alteration of glutamate transport or glutamine synthesis may exacerbate stress-induced glutamate neurotransmission and excitability, phenomenon associated with stress-induced neuronal atrophy (46; 47).
A recent study found that that neuronal loss occurs in brains of patients with MDD, notably the calbindin-immunoreactive GABAergic neurons of the dorsolateral PFC (48). However, we found that ibotenate-induced neuronal cell death in the PFC does not induce a depressive-like phenotype in SPT and FST. In addition, the neuronal loss induced by ibotenate infusions did not increase the susceptibility for stress, as no effect was observed in the second SPT after the FST. We also confirmed that PFC excitotoxic lesions do not increase anxiety or performance in the AAT, as previously reported after PFC lesions (49; 50). These findings are surprising as we expected that the ibotenate lesions would result in effects opposite to that observed with the gliotoxin. Indeed, astrocytes play a critical role in the uptake of glutamate (51), and L-AAA infusion is reported to decrease glutamate transport and glutamine synthethase (22). Thus, L-AAA and ibotenate would be expected to have opposite effects on brain regions receiving afferents from the PFC. Astrocytic dysfunction should provoke an over-activation of the PFC neurons and an increase in glutamate in the PFC projections, while neuronal cell death would result in loss of these projections. The absence of effects of the neurotoxic lesion may be due to the lack of specificity regarding the neuronal subtypes affected (i.e., principle neurons as well as interneurons) or to the size of the lesion. In any case, the fact that ibotenate lesions do not cause behavioral effects provides further evidence that glial dysfunction may precede neuronal adaptive changes (different from neuronal loss) and that these secondary alterations of neuronal function in the PFC and the projections of the PFC are probability similar to the ones induced by CUS, accounting for the similar behavioral phenotypes with these two conditions.
This study is a first step toward understanding the role of cortical glial loss in the pathophysiology of depression. However, it also raises methodological concerns that need to be mentioned. First, because of the physical proximity of glial processes and neurons and the dynamic interactions between these two cell types, any change in glial number or morphology will also influence neuronal function. One major limitation of pharmacologic glial ablation is that it will result in neuronal dysfunction. This approach was chosen because selective glial ablation by L-AAA models the loss of glia and some cellular alterations observed in depression and stress-related disorders (4; 12; 52; 53). Second, there is a concern about the effects of intracerebral infusions on astroglia. We opted for cannula implantation followed by one week of recovery before toxin infusions, a time after which most of the initial reactive gliosis has occurred, although additional gliosis in response to injector insertion and drug diffusion may still take place. To address these issues we are currently developing cell ablation models using genetic approaches that do not require in situ infusions of toxins. These less intrusive and more discrete approaches will ultimately lead to a more complete understanding of the role of glia in the actions of stress and depression.
Acknowledgments
This work was supported by United States Public Health Service Grants MH45481 and 2 PO1 MH25642, Veterans Administration National Center Grant for Post-Traumatic Stress Disorder, and the Connecticut Mental Health Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors reported no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- 2.Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- 3.Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- 4.Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets. 2007;6:219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banasr M, Duman RS. Regulation of neurogenesis and gliogenesis by stress and antidepressant treatment. CNS Neurol Disord Drug Targets. 2007;6:311–320. doi: 10.2174/187152707783220929. [DOI] [PubMed] [Google Scholar]
- 6.Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- 7.Cotter DR, Pariante CM, Everall IP. Glial cell abnormalities in major psychiatric disorders: the evidence and implications. Brain Res Bull. 2001;55:585–595. doi: 10.1016/s0361-9230(01)00527-5. [DOI] [PubMed] [Google Scholar]
- 8.Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- 9.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- 11.Miguel-Hidalgo JJ, Baucom C, Dilley G, Overholser JC, Meltzer HY, Stockmeier CA, et al. Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol Psychiatry. 2000;48:861–873. doi: 10.1016/s0006-3223(00)00999-9. [DOI] [PubMed] [Google Scholar]
- 12.Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A. 2005;102:15653–15658. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duman RS. Structural alterations in depression: cellular mechanisms underlying pathology and treatment of mood disorders. CNS Spectr. 2002;7:140–142. 144–147. doi: 10.1017/s1092852900017454. [DOI] [PubMed] [Google Scholar]
- 14.Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- 15.Manji HK, Duman RS. Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics. Psychopharmacol Bull. 2001;35:5–49. [PubMed] [Google Scholar]
- 16.Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, et al. Preclinical models: status of basic research in depression. Biol Psychiatry. 2002;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- 17.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 18.Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- 19.Takada M, Hattori T. Fine structural changes in the rat brain after local injections of gliotoxin, alpha-aminoadipic acid. Histol Histopathol. 1986;1:271–275. [PubMed] [Google Scholar]
- 20.Brown DR, Kretzschmar HA. The glio-toxic mechanism of alpha-aminoadipic acid on cultured astrocytes. J Neurocytol. 1998;27:109–118. doi: 10.1023/a:1006947322342. [DOI] [PubMed] [Google Scholar]
- 21.McBean GJ. Intrastriatal injection of DL-alpha-aminoadipate reduces kainate toxicity in vitro. Neuroscience. 1990;34:225–234. doi: 10.1016/0306-4522(90)90316-v. [DOI] [PubMed] [Google Scholar]
- 22.McBean GJ. Inhibition of the glutamate transporter and glial enzymes in rat striatum by the gliotoxin, alpha aminoadipate. Br J Pharmacol. 1994;113:536–540. doi: 10.1111/j.1476-5381.1994.tb17022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khurgel M, Koo AC, Ivy GO. Selective ablation of astrocytes by intracerebral injections of alpha-aminoadipate. Glia. 1996;16:351–358. doi: 10.1002/(SICI)1098-1136(199604)16:4<351::AID-GLIA7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, Duman RS. Chronic Unpredictable Stress Decreases Cell Proliferation in the Cerebral Cortex of the Adult Rat. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Risterucci C, Coccurello R, Banasr M, Stutzmann JM, Amalric M, Nieoullon A. The metabotropic glutamate receptor subtype 5 antagonist MPEP and the Na+ channel blocker riluzole show different neuroprotective profiles in reversing behavioral deficits induced by excitotoxic prefrontal cortex lesions. Neuroscience. 2006;137:211–220. doi: 10.1016/j.neuroscience.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 26.Gorka Z, Moryl E, Papp M. Effect of chronic mild stress on circadian rhythms in the locomotor activity in rats. Pharmacol Biochem Behav. 1996;54:229–234. doi: 10.1016/0091-3057(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 27.Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci U S A. 2007;104:4647–4652. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porsolt RD, Anton N, Blavet N, Jalfre M. Behavioral dispair test: a new model sensitive to antidepressant treatment. Eur J Pharmacol. 1979;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 29.Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 30.Haidkind R, Eller M, Harro M, Kask A, Rinken A, Oreland L, et al. Effects of partial locus coeruleus denervation and chronic mild stress on behaviour and monoamine neurochemistry in the rat. Eur Neuropsychopharmacol. 2003;13:19–28. doi: 10.1016/s0924-977x(02)00076-7. [DOI] [PubMed] [Google Scholar]
- 31.Rasmusson AM, Shi L, Duman R. Downregulation of BDNF mRNA in the hippocampal dentate gyrus after re-exposure to cues previously associated with footshock. Neuropsychopharmacology. 2002;27:133–142. doi: 10.1016/S0893-133X(02)00286-5. [DOI] [PubMed] [Google Scholar]
- 32.Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- 33.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banasr M, Hery M, Brezun JM, Daszuta A. Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. Eur J Neurosci. 2001;14:1417–1424. doi: 10.1046/j.0953-816x.2001.01763.x. [DOI] [PubMed] [Google Scholar]
- 35.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willner P, Muscat R, Papp M. An animal model of anhedonia. Clin Neuropharmacol. 1992;15(Pt A) Suppl 1:550A–551A. doi: 10.1097/00002826-199201001-00286. [DOI] [PubMed] [Google Scholar]
- 37.Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- 38.Webster MJ, Knable MB, Johnston-Wilson N, Nagata K, Inagaki M, Yolken RH. Immunohistochemical localization of phosphorylated glial fibrillary acidic protein in the prefrontal cortex and hippocampus from patients with schizophrenia, bipolar disorder, and depression. Brain Behav Immun. 2001;15:388–400. doi: 10.1006/brbi.2001.0646. [DOI] [PubMed] [Google Scholar]
- 39.Johnston-Wilson NL, Sims CD, Hofmann JP, Anderson L, Shore AD, Torrey EF, et al. Disease-specific alterations in frontal cortex brain proteins in schizophrenia, bipolar disorder, and major depressive disorder. The Stanley Neuropathology Consortium. Mol Psychiatry. 2000;5:142–149. doi: 10.1038/sj.mp.4000696. [DOI] [PubMed] [Google Scholar]
- 40.Si X, Miguel-Hidalgo JJ, O'Dwyer G, Stockmeier CA, Rajkowska G. Age-dependent reductions in the level of glial fibrillary acidic protein in the prefrontal cortex in major depression. Neuropsychopharmacology. 2004;29:2088–2096. doi: 10.1038/sj.npp.1300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Czeh B, Simon M, Schmelting B, Hiemke C, Fuchs E. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2006;31:1616–1626. doi: 10.1038/sj.npp.1300982. [DOI] [PubMed] [Google Scholar]
- 42.Czeh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? : Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci. 2007 doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 44.Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 45.Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol Psychiatry. 2000;48:755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- 47.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 48.Rajkowska G, O'Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology. 2007;32:471–482. doi: 10.1038/sj.npp.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lacroix L, Broersen LM, Weiner I, Feldon J. The effects of excitotoxic lesion of the medial prefrontal cortex on latent inhibition, prepulse inhibition, food hoarding, elevated plus maze, active avoidance and locomotor activity in the rat. Neuroscience. 1998;84:431–442. doi: 10.1016/s0306-4522(97)00521-6. [DOI] [PubMed] [Google Scholar]
- 50.Maaswinkel H, Gispen WH, Spruijt BM. Effects of an electrolytic lesion of the prelimbic area on anxiety-related and cognitive tasks in the rat. Behav Brain Res. 1996;79:51–59. doi: 10.1016/0166-4328(95)00261-8. [DOI] [PubMed] [Google Scholar]
- 51.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 52.Sattler R, Rothstein JD. Targeting an old mechanism in a new disease-protection of glutamatergic dysfunction in depression. Biol Psychiatry. 2007;61:137–138. doi: 10.1016/j.biopsych.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 53.Sanacora G, Rothman DL, Mason G, Krystal JH. Clinical studies implementing glutamate neurotransmission in mood disorders. Ann N Y Acad Sci. 2003;1003:292–308. doi: 10.1196/annals.1300.018. [DOI] [PubMed] [Google Scholar]