Non-alcoholic fatty liver disease (NAFLD) is associated with obesity and insulin resistance. The condition disproportionately affects Hispanic Americans. The aims of this study were to examine the risk factors for and heritability of NAFLD in 795 Hispanic American and 347 African American adults participating in the IRAS Family Study. Computed tomography scans of the abdomen were evaluated centrally for measures of liver-spleen (LS) density ratio and abdominal fat distribution. Other measures included insulin sensitivity (SI) calculated from a frequently sampled intravenous glucose tolerance test and various laboratory measures. Statistical models which adjust for familial relationships were estimated separately for the two ethnic groups. Heritability was calculated using a variance components approach. The mean age of the cohort was 49 years (range 22−84); 66% were female. NAFLD (LS ratio < 1) was more common in Hispanic Americans (24%) than African Americans (10%). NAFLD was independently associated with SI and visceral adipose tissue area in both ethnic groups although the proportion of explained variance was considerably higher in the Hispanic models. Adiponectin contributed significantly in the African American models while triglycerides and PAI-1 contributed only in the Hispanic models. Liver density was modestly heritable in both ethnic groups (h2 ∼ 0.35). In summary, the prevalence of NAFLD was twofold greater in Hispanic than African Americans. Certain correlates of NAFLD were similar between the ethnic groups, while others were distinct. NAFLD was modestly heritable. These findings suggest that NAFLD may have a differing environmental and/or genetic basis in these ethnic groups.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is closely associated with obesity, type 2 diabetes (T2DM), and the metabolic syndrome (1-3), conditions which are increasing in prevalence in the United States (4-6). The condition is more common in Hispanic Americans than either African Americans or Caucasians (7, 8), possibly due to the high prevalence of the associated metabolic abnormalities in the former population.
NAFLD is often benign, characterized by excess triglyceride accumulation in hepatocytes among persons who do not abuse alcohol; however, it can lead to inflammation, fibrosis, and eventually, cirrhosis (9, 10). Even within its most common form, NAFLD is associated with increased mortality (10), and an increased risk of CVD among persons with T2DM, independent of classical risk factors (11).
Despite the increasing prevalence and the potential for serious clinical outcomes, the epidemiology and genetics of NAFLD are not well defined. Previous studies have often relied on surrogate measures of NAFLD (e.g., liver function tests), have had limited assessments of metabolic factors and adipose tissue distribution, and have not studied family members.
The present study was undertaken to fill these gaps in the etiology of NAFLD. The IRAS Family Study is a large family study in which 1142 adult men and women of Hispanic or African American descent have had the presence or absence of NAFLD uniformly defined using computed tomography (CT) scanning of the abdomen. The cohort members were studied extensively for a large panel of metabolic and physical measures including CT-measured abdominal adiposity and directly quantified insulin sensitivity. Furthermore, the family design allows an assessment of the heritability of NAFLD. Thus, the objectives of this report are to describe the correlates and heritability of NAFLD in Hispanic and African Americans.
Methods and Procedures
The IRAS Family Study was designed to explore genetic and epidemiologic contributions to abdominal adiposity and glucose homeostasis traits among Hispanic and African Americans using a family-based design (12). Large families were recruited between 2000−2002 at study centers in San Antonio, TX (Hispanics); San Luis Valley, CO (Hispanics); and Los Angeles, CA (African Americans); with probands identified from both the parent study (IRAS [13]) as well as the general population. Families were recruited based upon family size, not disease status. A follow-up examination was conducted approximately five years after the baseline examination, at which time computed tomography (CT) scanning of the liver and spleen was obtained. The Institutional Review Boards approved the protocol and informed consent was provided by each subject.
Imaging of the liver and spleen were obtained under a standardized protocol and scans were read centrally (University of Colorado Health Sciences Center). In brief, a single axial CT image was obtained at or near the T11/T12 level (14). The reading center quantified the density of the liver and spleen as visualized in the slice. The ratio of liver density to spleen density (LS ratio) was calculated; LS ratio < 1.0 has become an accepted cutpoint for a discrete outcome of NAFLD (1). All CT images were coded for pathology and image quality; poor quality studies were excluded from analysis.
Fat mass in the abdominal region was obtained by CT at the L4/L5 vertebral regions under a standardized protocol. Scans were read centrally at the reading center for subcutaneous (SAT) and visceral (VAT) adipose tissue. Percent total body fat was obtained by DEXA scan.
Insulin sensitivity was determined using a frequently sampled intravenous glucose tolerance test (FSIGTT), with modification from the published protocol (15). An injection of regular insulin, rather than tolbutamide, was used to ensure adequate plasma insulin levels for the accurate computation of insulin sensitivity across a broad range of glucose tolerance (16). In addition, a reduced sampling protocol was employed for efficiency (17). Insulin sensitivity, expressed as the insulin sensitivity index (SI), acute insulin response (AIR), and disposition index (DI= SI × AIR) were calculated using minimal model analysis software (MINMOD; 18). Approximately 15% of the participants did not complete the FSIGTT due to scheduling difficulties.
Adiponectin concentration was measured using a radioimmunoassay (Linco Research, St. Charles, MO) (19). C-reactive protein (CRP) was measured using an ultra-sensitive competitive immunoassay (antibodies and antigens from Calbiochem, La Jolla, CA) (20). Plasma glucose was measured using the glucose oxidase technique on an autoanalyzer. Plasma insulin was measured using the dextran-charcoal radioimmunoassay (21). Triglyceride (TG) concentrations were measured from plasma using enzymatic methods. High density lipoprotein (HDL) cholesterol was measured using the direct method (22). Alanine transaminase (ALT), aspartate transaminase (AST), and gamma-glutamyl transpeptidase (GGT) were determined by enzymatic colorimetric assays using a Chemistry Analyzer Model ATAC 8000 (Elan Diagnostic Co.). Fibrinogen was measured in citrated plasma with a modified clot-rate assay (23). PAI-1 was measured in citrated plasma using a two-site immunoassay (24, 25).
Height and weight were measured to the nearest 0.5 cm and 0.1 kg, respectively. Body mass index (BMI) was calculated at weight (kg)/height (m)2. Waist circumference was measured at the natural indentation or at a level midway between the iliac crest and the lower edge of the rib cage. The metabolic syndrome was defined using standard criteria (26). Usual consumption of beer, wine, and liquor in the past year was assessed by self-report. Exclusions were made for usual alcohol consumption which exceeded two drinks/day in men and one drink/day in women. History of liver disease was not collected. Diabetes was defined as a single fasting glucose measure exceeding 126 mg/dl or use of hypoglycemic medications.
Statistical Methods
NAFLD is presented as both a dichotomous variable (LS ratio < 1 and ≥ 1) and as a continuous variable (LS ratio). We also present results for liver density unadjusted for spleen density (27). All analyses were conducted separately in the two race/ethnic groups for two reasons: a major difference in the prevalence of NAFLD, and observed differences in risk factor relationships. All risk factors and outcomes presented here were obtained at the follow-up examination with the exception of the FSIGT parameters, adiponectin, and CRP, which were only available from the baseline examination. We consider these factors to be sufficiently novel to include, even though they were collected five years prior to the liver parameters.
Tests of significance for descriptive statistics and Spearman correlation coefficients were all corrected for familial correlations. To determine whether the observed ethnic differences in the prevalence of NAFLD was present across the range of values for a risk factor, we performed subgroup analyses by dichotomizing VAT and SI at the median using the entire cohort.
Multivariable generalized estimating equations (GEE1) were used to determine the set of independent variables that were associated with LS ratio. Two sets of models are presented. A Basic Model was designed to include a minimal set of covariates that incorporate the primary risk phenotypes: obesity and insulin resistance. A Full Model evaluated a broader set of covariates including those in the Basic Model. Variables were selected for the Full Model if they reached statistical significance (p<0.05) in one or both ethnic specific models. Age, sex, study center (Hispanic model only), and BMI were retained in the models irrespective of their contribution to explained variance. GEE1 is a standard approach to the analysis of correlated data such as family data and can be intuitively thought of as similar to linear regression models except that they account for the correlation among pedigrees. Participants with diabetes were excluded from correlation analyses (Table 2) and multivariable GEE1 models (Table 3).
Table 2.
Unadjusted Spearman correlation coefficients between risk factors and liver-spleen ratio, and p-value for ethnic interaction, IRAS Family Study, excluding persons with diabetes
| Hispanic | African American | ||||
|---|---|---|---|---|---|
| r | P-value | r | P-value | p-value for interaction | |
| Age at follow-up (yrs) | 0.09 | 0.03 | −0.09 | 0.13 | 0.07 |
| Liver Density (HU) | 0.84 | <0.0001 | 0.76 | <.0001 | 0.29 |
| BMI (kg/m2) | −0.44 | <0.0001 | −0.13 | 0.03 | <.0001 |
| VAT (cm3) | −0.43 | <0.0001 | −0.24 | <.0001 | 0.002 |
| SAT (cm3) | −0.27 | <0.0001 | −0.07 | 0.27 | <.0001 |
| Percent Total Body Fat | −0.07 | 0.09 | 0.06 | 0.30 | 0.02 |
| Fasting insulin (mg/dl) | −0.43 | <0.0001 | −0.23 | 0.0001 | <.0001 |
| Fasting glucose (mg/dl) | −0.27 | <0.0001 | −0.05 | 0.39 | 0.0001 |
| HDL (mg/dl) | 0.38 | <0.0001 | 0.14 | 0.02 | 0.02 |
| TG (mg/dl) | −0.30 | <0.0001 | −0.19 | 0.001 | <.0001 |
| PAI-1 (ng/ml) | −0.40 | <0.0001 | −0.09 | 0.13 | <.0001 |
| ALT (IU/L) | −0.46 | <0.0001 | −0.24 | 0.0001 | 0.0001 |
| AST (IU/L) | −0.32 | <0.0001 | −0.17 | 0.003 | 0.02 |
| GGT (IU/L) | −0.30 | <0.0001 | −0.22 | 0.0002 | 0.36 |
| Insulin Sensitivity Index * | 0.43 | <0.0001 | 0.25 | <.0001 | 0.0007 |
| Acute Insulin Response * | −0.22 | <0.0001 | −0.08 | 0.22 | 0.01 |
| Disposition Index * | 0.22 | <0.0001 | 0.15 | 0.01 | 0.002 |
| Adiponectin (mg/ml) * | 0.31 | <0.0001 | 0.29 | <.0001 | 0.91 |
| CRP (mg/ml) * | −0.27 | <0.0001 | −0.08 | 0.17 | 0.01 |
Data obtained five years prior to NAFLD assessment.
Table 3.
Correlates of NAFLD as measured by liver-spleen density ratio in multivariate models by ethnic group, IRAS Family Study, excluding persons with diabetes
| Basic Model | Full Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Hispanic** N=620 R2=0.27 |
African American N=266 R2=0.11 |
Hispanic** N=576 R2=0.33 |
African American N=259 R2=0.14 |
|||||
| B | P-value | B | p-value | B | P-value | B | p-value | |
| Age | 0.004 | <0.0001 | 0.0012 | 0.08 | 0.004 | <0.0001 | 0.0008 | 0.30 |
| Sex (female, ref) | −0.03 | 0.10 | −0.02 | 0.24 | −0.02 | 0.26 | 0.0003 | 0.99 |
| Insulin Sensitivity Index* | 0.11 | <0.0001 | 0.07 | 0.005 | 0.08 | <0.0001 | 0.046 | 0.09 |
| VAT (cm2) | −0.0015 | <.0001 | −0.0007 | 0.02 | −0.0009 | 0.004 | −0.0006 | 0.06 |
| BMI (kg/m2) | −0.004 | 0.10 | 0.0 | 0.59 | −0.003 | 0.26 | 0.001 | 0.65 |
| Fasting glucose (mg/dl) | −0.003 | 0.05 | 0.001 | 0.55 | ||||
| Adiponectin (mg/ml) | 0.0002 | 0.91 | 0.005 | 0.04 | ||||
| TG (mg/dl)* | −0.10 | <.0001 | −0.03 | 0.13 | ||||
| PAI-1 (ng/ml)* | −0.04 | 0.01 | −0.005 | 0.74 | ||||
log transformed
Hispanic models were additionally adjusted for study center; non-significant in both models.
Heritability was estimated using a multipoint variance component procedure implemented in the SOLAR software package (28). Models adjusted for age, sex, and SI. We included SI in this minimal set of covariates because SI was strongly correlated to LS ratio in both ethnic groups.
Results
Ninety-five percent (95%) of IRAS Family Study participants completed an abdominal CT study at the follow-up examination (1364/1433). Forty-four scans were excluded from this analysis due to pathology or poor image quality. One-hundred seven participants were excluded from the analysis for excessive consumption of alcohol and 51 participants were excluded because they were missing data on the key measures. Thus, this analysis includes 1142 participants (795 Hispanic and 347 African American) from 129 families (87 Hispanic and 42 African American).
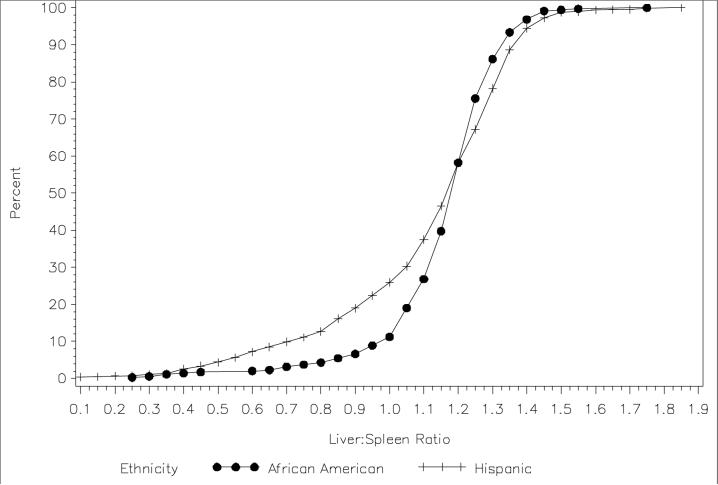
The average age of the cohort was 49 years; 63% were female (Table 1). African Americans had higher BMI and SAT, but lower VAT than Hispanics. African Americans were more insulin resistant than Hispanics. The prevalence of the metabolic syndrome was higher in Hispanics than African Americans (33% vs 19%), whereas the prevalence of diabetes (17%) was similar in both groups. NAFLD was observed in 24% of Hispanics (190/795) and 10% of African Americans (34/347). This trend of a lower prevalence of NAFLD among African Americans persisted across subgroups of VAT and SI (data not shown). Similarly, mean LS ratio was lower in Hispanics than in African Americans (Figure 1).
Table 1.
Baseline characteristics of the IRAS Family Study, means and standard deviations (or percentages)
| Entire Cohort (n=1142) | Hispanic American (n=795) | African American (n=347) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean(Std) | Hispanic | African American | p-value | L/S Ratio ≥ 1 (Normal) | L/S Ratio < 1 (NAFLD) | p-value | L/S Ratio ≥ 1 (Normal) | L/S Ratio < 1 (NAFLD) | p-value |
| N | 795 | 347 | - | 605 | 190 | 313 | 34 | - | |
| Gender (% female) | 63.6 | 60.5 | 0.39 | 66.8 | 53.7 | 0.0007 | 61 | 55.9 | 0.54 |
| Age at follow-up (yrs) | 48.0 (14.1) | 50.3 (13.9) | 0.07 | 48.1 (14.7) | 47.4 (11.9) | 0.62 | 50.2 (14.1) | 52 (12.1) | 0.44 |
| Liver:Spleen (LS) Ratio | 1.1 (0.3) | 1.2 (0.2) | 0.0007 | 1.2 (0.1) | 0.7 (0.2) | <.0001 | 1.2 (0.1) | 0.8 (0.2) | <.0001 |
| Liver Density (HU) | 51.9 (12.5) | 55.5 (8.6) | <.0001 | 57.6 (6.2) | 33.8 (10.3) | <.0001 | 57.5 (5.1) | 37 (11.4) | <.0001 |
| BMI (kg/m2) | 29.6 (6.0) | 31.4 (6.9) | 0.0008 | 28.5 (5.8) | 32.9 (5.5) | <.0001 | 31.2 (6.9) | 33.8 (7.2) | 0.04 |
| VAT (cm3) | 116.7 (62.4) | 103.3 (58.7) | 0.002 | 104.5 (58.2) | 155.5 (59.8) | <.0001 | 100.2 (57.9) | 131.6 (58.9) | 0.0001 |
| SAT (cm3) | 368.4 (160.3) | 401.7 (194.5) | 0.02 | 348.5 (154.7) | 432 (161.5) | <.0001 | 395.4 (191.9) | 459.1 (211.7) | 0.09 |
| Percent Total Body Fat | 34.1 (8.7) | 32.5 (9.7) | 0.03 | 33.7 (8.9) | 35.3 (7.9) | 0.07 | 32.3 (9.8) | 33.7 (9.1) | 0.38 |
| Fasting insulin (mg/dl) | 22.0 (16.9) | 20.2 (14.1) | 0.19 | 19.2 (14.4) | 31 (20.9) | <.0001 | 19.4 (13.8) | 28.1 (14.6) | 0.002 |
| Fasting glucose (mg/dl) | 103.8 (38.2) | 97.2 (32.8) | 0.0662 | 99.3 (32.6) | 118.1 (49.6) | <.0001 | 95.7 (31.3) | 112.2 (43.2) | 0.02 |
| HDL (mg/dl) | 47.0 (13.1) | 53.1 (13.6) | <.0001 | 48.7 (12.7) | 41.7 (13.3) | <.0001 | 53.8 (13.4) | 45.7 (12.7) | 0.001 |
| TG (mg/dl) | 159.6 (93.8) | 106.8 (74.3) | <.0001 | 146.1 (80.9) | 202.7 (116.8) | <.0001 | 100.6 (54.4) | 170.7 (168) | 0.0003 |
| PAI-1 (ng/ml) | 39.2 (34.1) | 27.8 (25.1) | <.0001 | 33.2 (29.3) | 58.5 (40.5) | <.0001 | 26.7 (24.1) | 39 (32.3) | 0.003 |
| ALT (IU/L) | 26.1 (15.3) | 22.6 (11.2) | 0.0002 | 22.3 (10.5) | 38.2 (21.1) | <.0001 | 21.6 (9.5) | 33.6 (18.8) | <.0001 |
| AST (IU/L) | 24.3 (9.8) | 24.3 (8.2) | 0.73 | 22.6 (6.9) | 29.8 (14.5) | <.0001 | 23.5 (6) | 31.5 (18.6) | 0.0006 |
| GGT (IU/L) | 41.3 (52.9) | 39.0 (49.4) | 0.65 | 35.8 (29.5) | 58.8 (92.6) | <.0001 | 37.4 (49.3) | 55.8 (47.7) | <.0001 |
| Metabolic Syndrome (%) | 33.3 | 19.3 | <.0001 | 24.8 | 60.5 | <.0001 | 16.9 | 41.2 | 0.0003 |
| Diabetes (%) | 17.4 | 17.6 | 0.97 | 14.7 | 25.9 | 0.01 | 16.1 | 33.3 | 0.003 |
| Insulin Sensitivity Index* [× 10−4 (min−1 · UU−1 · ml−1)] | 2.0 (1.8) | 1.4 (1.2) | 0.001 | 2.3 (1.9) | 1 (1) | <.0001 | 1.5 (1.2) | 0.8 (0.6) | <.0001 |
| Acute Insulin Response* (pmol · ml−1 · min−1) | 705.3 (647.7) | 877.6 (807.0) | 0.005 | 678.4 (594.5) | 789.2 (786.8) | 0.42 | 868.7 (812.4) | 958 (763.5) | 0.59 |
| Disposition Index (SI × AIR) * | 1177.5 (1180.1) | 1165.6 (1188.4) | 0.90 | 1333.2 (1230.1) | 692.6 (843.3) | <.0001 | 1203.4 (1203.9) | 824.9 (990) | 0.004 |
| Adiponectin (mg/ml)* | 13.5 (7.0) | 8.6 (4.7) | <.0001 | 14.2 (6.8) | 11.3 (7.4) | <.0001 | 8.9 (4.8) | 6.3 (3.1) | <.0001 |
| CRP (mg/ml)* | 3.5 (4.4) | 4.1 (5.0) | 0.27 | 3.3 (4.4) | 4.3 (4.5) | <.0001 | 4 (5) | 5.5 (4.8) | 0.01 |
Data obtained five years prior to NAFLD assessment.
Figure 1.
Cumulative distribution of liver-spleen density ratio in Hispanic and African Americans, IRAS Family Study, 2005−2007
Univariate associations between NAFLD and risk factors were similar in Hispanics and African Americans and included increased total and abdominal body fat, glucose dysregulation and insulin resistance, inflammatory markers, HDL and triglycerides, liver enzyme levels, metabolic syndrome, and diabetes (Table 1). Risk factors not associated with NAFLD included age, percent total body fat by DXA, and acute insulin response.
Correlation coefficients between the continuous measure of NAFLD (LS ratio) and the metabolic and physical measures were consistently stronger in the Hispanic sample than in the African American sample (Table 2). Significant ethnic interactions were observed for nearly all risk factors (interaction p-values ranging from 0.02 −<0.0001).
In Hispanics, the minimal set of covariates explained 27% of the variance in LS ratio; age, SI, and VAT were significant covariates in this model (Table 3). In African Americans, the minimal set of covariates explained 11% of the variance; SI and VAT were significant covariates in the model. BMI was not a significant independent correlate of LS ratio in either ethnic group, despite statistically significant unadjusted correlation coefficients (Table 2). Several additional measures were found to be significant covariates of LS ratio in the full models. In Hispanics, the Full Model explained 33% of the variance in LS ratio and included age and SI as positive independent correlates, and VAT, TG, and PAI-1 as negative independent correlates. In African Americans, the Full Model explained 14% of the variance in LS ratio and included only adiponectin as a positive independent correlate. SI and VAT had borderline significant associations with LS ratio in this smaller ethnic sample (p<0.10). Fasting insulin, AIR, HDL, and CRP were not significant multivariate correlates in either ethnic group and were thus not included in the Full Models.
The residual heritability of LS ratio was statistically significant (p < 0.02) in both ethnic groups: 0.21 (p<0.0001) in Hispanics and 0.19 (p=0.02) in African Americans (Table 4). A small proportion of the variance in LS ratio was explained by covariates (10% to 15%). The residual heritability of liver density was considerably higher, estimated as 0.35 in Hispanics and 0.32 in African Americans (both p<0.001), as was the proportion of variance explained by covariates (16 % to 19%).
Table 4.
Heritability estimates for liver-spleen density ratio and liver density, IRAS Family Study
| Cohort | Heritability | SE | P-value | Proportion of Variance Explained by Covariates* | |
|---|---|---|---|---|---|
| Liver-spleen ratio | Hispanic | 0.21 | 0.07 | <0.0001 | 0.15 |
| African American | 0.19 | 0.12 | 0.02 | 0.10 | |
| Liver density | Hispanic | 0.35 | 0.07 | <0.0001 | 0.19 |
| African American | 0.32 | 0.13 | 0.001 | 0.16 |
Model covariates include age, sex, and insulin sensitivity.
Discussion
In this study of 1142 participants of the IRAS Family Study, we observed a higher prevalence of NAFLD in Hispanic Americans (24%) than in African Americans (10%). The independent correlates of NAFLD included only two that were similar across the two ethnic groups (insulin sensitivity and VAT) and several that were different. Age, TG and PAI-1 were additional correlates of NAFLD in Hispanics, while adiponectin was independently associated only in African Americans. These factors explained a large portion of the variance of NAFLD in the Hispanic cohort (33%), but far less of the variance in the African American cohort (14%). NAFLD was modestly heritable in both ethnic groups (approximately 20% when assessed by LS ratio and 35% when assessed by liver density).
The high prevalence of NAFLD in Hispanics relative to African Americans and Caucasians has been reported in several multi-ethnic studies (7, 29). Considering a condition for which risk has been attributed to central obesity, insulin resistance, and diabetes, we expected to observe a high prevalence of NAFLD in the IRAS Family Study Hispanic cohort. Indeed, one of four Hispanic participants was determined to have NAFLD. Paradoxically, central obesity, insulin resistance, and diabetes were similar or more frequent in African Americans than Hispanics in this study and others (30), yet African Americans had a dramatically lower prevalence of NAFLD. Browning et al (7), observing a similar paradox, assessed whether the ethnic difference in the prevalence of hepatic steatosis was due to differences in the frequency of risk factors. In subgroup analyses (low BMI or insulin-sensitive by HOMA), African Americans persisted in a lower prevalence of hepatic steatosis compared to Hispanics. We have extended this finding by showing, using our detailed phenotypes, that even among subgroups with low VAT or insulin sensitivity, African Americans have a lower prevalence of hepatic steatosis than Hispanics. This discrepancy between the environment and outcome suggests that NAFLD may have important, yet unidentified, genetic or environmental predictors. Characteristics which could be explored to understand the mechanisms responsible for ethnic differences in NAFLD include dietary intake and environmental toxins.
Our large sample size and extensive phenotyping allowed us to examine whether there were different predictors of NAFLD across ethnic groups. Indeed, only two of the risk factors, insulin resistance and visceral adipose tissue area, were significant independent predictors of NAFLD in both groups. Point estimates for risk factors contained in the multivariate models were consistently lower among African Americans compared to Hispanics, and, in addition to the smaller sample size, resulted in few significant associations. Furthermore, very little of the variance in LS ratio was explained in African Americans with our extensive panel of highly detailed phenotypes (r2=0.14) compared to Hispanics (r2=0.33). This is the first report directly comparing risk factor profiles for NAFLD across ethnic groups. These findings suggest that there may be fundamental differences in the etiology of NAFLD in African Americans and Hispanics.
NAFLD has been shown to be strongly associated with general and abdominal obesity as measured by BMI and waist (7). However, in studies that have directly measured the area of the abdominal fat depots, a stronger relationship is observed between NAFLD and visceral adiposity than with overall obesity (1, 31). Our large study extends these findings by showing that in Hispanics the variance in fatty liver is better explained by insulin resistance and visceral adiposity than by measures of overall obesity. (BMI and percent total body fat were not significant correlates of LS ratio in either ethnic group). These findings further emphasize the fundamental association of NAFLD with abdominal adiposity and the insulin resistance syndrome.
Insulin resistance was the strongest and most consistent correlate of NAFLD in this bi-ethnic population. Univariate correlations between SI and LS ratio were 0.43 and 0.25, (Hispanics and African Americans, respectively), associations which persisted in the multivariate models, albeit reduced to non-significance in the African American cohort (p=0.09). We also examined another parameter from the FSIGT: acute insulin response. AIR yielded a statistically significant univariate association with LS ratio in Hispanics; however, this association did not persist in the multivariate model. Disposition index was highly correlated with LS ratio in both groups (p≤0.01) in univariate analysis; we did not include it in the multivariate model due to its collinearity with SI
An association between NAFLD and low adiponectin levels was observed in both the African American and Hispanic samples, persisting as an independent effect only in the African American cohort. This association of fatty liver with markedly lower plasma adiponectin has been previously reported (32, 33) and emphasizes the importance of adipose tissue to the development of NAFLD. The lack of an independent effect in Hispanics is puzzling; it may reflect the considerably higher values of adiponectin in this ethnic group relative to African Americans (19).
A positive association between age and LS ratio was unexpected. There are no consistent observations between age and NAFLD in the literature, perhaps due to the limited number of large population-based cohorts in which this question has been explored. Regarding associations with inflammatory markers, we observed strong associations with PAI-1 in Hispanics. CRP, however, was not independently associated with NAFLD in either ethnic cohort. While this lack of association for CRP may be a result of the time difference between its measurement and the liver parameters, its effect might also be mediated through VAT. PAI-1 is of particular interest in that it is secreted by adipose tissue and is an inhibitor of fibrinolysis. These findings are in agreement with others that NAFLD is an inflammatory process (34).
This is the first study to estimate the contribution of genetic factors to variation in NAFLD as assessed using CT. We found modest heritabilities of approximately 20% for LS ratio. Heritabilities were considerably higher for liver density unadjusted for spleen density (32% and 35%), suggesting that the LS ratio incorporates more variability associated with the environment, and liver density is more strongly genetic. The only other reports of heritability come from studies in which biochemical liver function enzymes were measured (35, 36). Our findings of modest heritability support future efforts to map genes which contribute to NAFLD.
Our study has several important strengths. We have comprehensively phenotyped a large cohort using direct measure of insulin resistance and insulin secretion, inflammatory markers, and adipose tissue distribution. Several previous studies of NAFLD have relied on surrogate measures of insulin resistance and BMI. Our multi-ethnic cohort allowed us to examine differences in risk factor profiles among groups for whom the prevalence of NAFLD differs substantially. Finally, the family study design allowed us to assess the familial context of NAFLD. An important limitation is reduced power in the African American sample compared to the Hispanic sample. Caution was taken to minimize over-interpretation of non-significant findings. Another limitation is the five-year time difference between the collection of several measures and the assessment of NAFLD. We chose to incorporate these novel parameters in our analysis but recognize that they were not collected coincident with the NAFLD assessment. Despite this time lag, one of these measures, SI, has the strongest association with LS ratio, supporting its strength as a predictor variable.
In conclusion, NAFLD appears to be part of the broader metabolic syndrome characterized by insulin resistance and abdominal adiposity in Hispanic Americans and African Americans. Consistent with other reports, NAFLD was less prevalent in African Americans, a finding that could not be explained by different frequencies of risk factors. Notably, risk factors had a much weaker impact on the variability of fatty liver among African Americans than in Hispanics. Moderate heritability estimates were observed in both ethnic groups and suggest that these families may be valuable to efforts for mapping genes which contribute to NAFLD. Continued exploration of these ethnic differences may yield valuable clues to the etiology of this prevalent condition.
Acknowledgements
This work was supported by National Institutes of Health grants numbered: R01HL060944, R01HL061019, R01HL060919, R01HL060894, R01HL061210; and the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332). The investigators thank Dr. David E. Kelley for his comments on the computed tomography scanning and reading protocol.
Abbreviations
- ALT
alanine transaminase
- AST
aspartate transaminase
- BMI
body mass index
- CRP
c-reactive protein
- CT
computed tomography
- GEE
generalized estimating equation
- GGT
gamma-glutamyl transpeptidase h2, heritability
- HDL
high density lipoprotein
- IRAS
Insulin Resistance Atherosclerosis Study
- LS ratio
liver-spleen ratio
- MINMOD
minimal model
- NAFLD
non-alcoholic fatty liver disease
- NCEP
National Cholesterol Education Panel
- PAI-1
plasminogen activator inhibitor 1
- SAT
subcutaneous adipose tissue
- SOLAR
Sequential Oligogenic Linkage Analysis Routines
- SI
insulin sensitivity index
- TG
triglycerides
- VAT
visceral adipose tissue
Footnotes
Disclosure statement: We have no conflicts of interest to declare.
References
- 1.Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab. 2003;285:E906–E916. doi: 10.1152/ajpendo.00117.2003. [DOI] [PubMed] [Google Scholar]
- 2.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40:S5–S10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 3.Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, Nakajima T, Sarui H, Shimazaki M, Kato T, Okuda J, Ida K. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among U.S. adults. Diabetes Care. 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 5.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999−2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 6.Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, Saydah SH, Williams DE, Geiss LS, Gregg EW. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999−2002. Diabetes Care. 2006;29:1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 7.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 8.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 9.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 10.Adams LA, Lymp JF, St. Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: A Population-Based Cohort Study. Gastroenterology. 2005;129:113–p121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Targher G, Bertolini L, Rodella S, Tessari R, Zenari L, Lippi G, Arcaro G. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diab Care. 2007;30:2119–2121. doi: 10.2337/dc07-0349. [DOI] [PubMed] [Google Scholar]
- 12.Henkin L, Bergman RN, Bowden DW, Ellsworth DL, Haffner SM, Langefeld CD, Mitchell BD, Norris JM, Rewers M, Saad MF, Stamm E, Wagenknecht LE, Rich SS. Genetic epidemiology of insulin resistance and visceral adiposity: the IRAS Family Study Design and Methods. Ann Epidemiol. 2003;13:211–217. doi: 10.1016/s1047-2797(02)00412-x. [DOI] [PubMed] [Google Scholar]
- 13.Wagenknecht LE, Mayer E, Rewers M, Haffner S, Selby J, Borok GM, Henkin L, Howard G, Savage PJ, Saad M, Bergman RN, Hamman R. The Insulin Resistance Atherosclerosis Study (IRAS): Objectives, design, and recruitment results. Ann Epidemiol. 1995;5:464–472. doi: 10.1016/1047-2797(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 14.Davidson LE, Kuk JL, Church TS, Ross R. Protocol for measurement of liver fat by computed tomography. J Appl Physiol. 2006;100:864–868. doi: 10.1152/japplphysiol.00986.2005. [DOI] [PubMed] [Google Scholar]
- 15.Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocrine Rev. 1985;6:45–86. doi: 10.1210/edrv-6-1-45. [DOI] [PubMed] [Google Scholar]
- 16.Welch S, Gebhart SSP, Bergman RN, Phillips LS. Minimal model analysis of intravenous glucose tolerance test derived insulin sensitivity in diabetic subjects. J Clin Endocrinol Metab. 1990;71:1508–1518. doi: 10.1210/jcem-71-6-1508. [DOI] [PubMed] [Google Scholar]
- 17.Steil GM, Volund A, Kahn SE, Bergman RN. Reduced sample number for calculation of insulin sensitivity and glucose effectiveness from the minimal model: suitability for use in population studies. Diabetes. 1993;42:250–256. doi: 10.2337/diab.42.2.250. [DOI] [PubMed] [Google Scholar]
- 18.Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsitivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed. 1986;23:113–122. doi: 10.1016/0169-2607(86)90106-9. [DOI] [PubMed] [Google Scholar]
- 19.Hanley AJG, Bowden D, Wagenknecht L, Balasubramanyam A, Langefeld C, Saad MF, Rotter JI, Guo X, Chen YI, Bryer-Ash M, Norris JM, Haffner SM. Associations of adiponectin with body fat distribution and insulin sensitivity in non-diabetic Hispanic and African Americans. J Clin Endocrinol Metab. 2007;92:2665–71. doi: 10.1210/jc.2006-2614. [DOI] [PubMed] [Google Scholar]
- 20.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–58. [PubMed] [Google Scholar]
- 21.Herbert V, Lau K, Gottlieb C, Bleicher S. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965;25:1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- 22.Sugiuchi H, Uji Y, Okabe H, Irie T, Uekama K, Kayahara N, Miyauchi K. Direct measurement of high-density lipoprotein cholesterol in serum with polyethylene glycol-modified enzymes and sulfated alpha-cyclodextrin. Clin Chem. 1995;41:717–723. [PubMed] [Google Scholar]
- 23.Clauss A. Rapid physiological coagulation method for the determination of fibrinogen. Acta Haematol. 1957;17:237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 24.Macy EM, Meilahn EN, Declerck PJ, Tracy RP. Sample preparation for plasma measurement of plasminogen activator inhibitor-1 antigen in large population studies. Arch Pathol Lab Med. 1993;117:67–70. [PubMed] [Google Scholar]
- 25.Declerck PJ, Collen D. Measurement of plasminogen activator inhibitor 1 (PAI-1) in plasma with various monoclonal antibody-based enzyme-linked immunosorbent assays. Thromb Res Suppl. 1990;10:3–9. doi: 10.1016/0049-3848(90)90373-k. [DOI] [PubMed] [Google Scholar]
- 26.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 27.Kodama Y, Ng CS, Wu TT, Ayers GD, Curley SA, Abdalla EK, Vauthey JN, Charnsangavej C. Comparison of CT methods for determining the fat content of the liver. Am Roentg Soc. 2007;188:1307–1312. doi: 10.2214/AJR.06.0992. [DOI] [PubMed] [Google Scholar]
- 28.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, Manos MM, Terrault NA. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41:372–379. doi: 10.1002/hep.20554. [DOI] [PubMed] [Google Scholar]
- 30.Haffner SM, D'Agostino RB, Jr., Saad MF, Rewers M, Mykkanen L, Selby J, Howard G, Savage PJ, Hamman R, Wagenknecht LE, Bergman RN. Increased insulin resistance and insulin secretion in non-diabetic African Americans and Hispanics compared with non-Hispanic whites: the Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45:742–748. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 31.Church TS, Kuk JL, Ross R, Priest EL, Biltoff E, Blair SN. Association of cardio-respiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterol. 2006;130:2023–2030. doi: 10.1053/j.gastro.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Kim SG, Kim HY, Seo JA, Lee KW, Oh JH, Kim NH, Choi KM, Baik SH, Choi DS. Relationship between serum adiponectin concentration, pulse wave velocity and nonalcoholic fatty liver disease. Eur J Endocrinol. 2005;152:225–31. doi: 10.1530/eje.1.01842. [DOI] [PubMed] [Google Scholar]
- 33.Targher G, Bertolini L, Zenari L. Hypoadiponectinemia is closely associated with nonalcoholic hepatic steatosis in obese subjects. Diab Care. 2004;27:2085–2086. doi: 10.2337/diacare.27.8.2085. [DOI] [PubMed] [Google Scholar]
- 34.Targher G, Bertolini L, Scala L, Zoppini G, Zenari L, Falezza G. Non-alcoholic hepatic steatosis and its relation to increased plasma biomarkers of inflammation and endothelial dysfunction in non-diabetic men. Role of visceral adipose tissue. Diabet Med. 2005;22:1354–1358. doi: 10.1111/j.1464-5491.2005.01646.x. [DOI] [PubMed] [Google Scholar]
- 35.Brouwers MC, Cantor RM, Kono N, Yoon JL, van der Kallen CJ, Bilderbeek-Beckers MA, van Greevenbroek MM, Lusis AJ, de Bruin TW. Heritability and genetic loci of fatty liver in familial combined hyperlipidemia. J Lipid Res. 2006;47:2799–2807. doi: 10.1194/jlr.M600312-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Bathum L, Petersen HC, Rosholm JU, Hyltoft Petersen P, Vaupel J, Christensen K. Evidence for a substantial genetic influence on biochemical liver function tests: results from a population-based Danish twin study. Clin Chem. 2001;47:81–87. [PubMed] [Google Scholar]