Abstract
The main goal of the paper was to assess the pattern of risk factors having an impact on the onset of early wheezing phenotypes in the birth cohort of 468 two-year olds and to investigate the severity of respiratory illness in the two-year olds in relation to both wheezing phenotypes, environmental tobacco smoke (ETS) and personal PM2.5 exposure over pregnancy period (fine particulate matter). The secondary goal of the paper was to assess possible association of early persistent wheezing with the length of the baby at birth. Pregnant women were recruited from ambulatory prenatal clinics in the first and second trimester of pregnancy. Only women 18–35 years of age, who claimed to be non-smokers, with singleton pregnancies, without illicit drug use and HIV infection, free from chronic diseases were eligible for the study. In the statistical analysis of respiratory health of children multinomial logistic regression and zero-inflated Poisson regression models were used. Approximately one third of the children in the study sample experienced wheezing in the first two years of life and in about two third of cases (67%) the symptom developed already in the first year of life. The early wheezing was easily reversible and in about 70% of infants with wheezing the symptom receded in the second year of life. The adjusted relative risk ratio (RRR) of persistent wheezing increased with maternal atopy (RRR = 3.05; 95%CI: 1.30 – 7.15), older siblings (RRR = 3.05; 95%CI: 1.67 – 5.58) and prenatal ETS exposure (RRR= 1.13; 95%CI: 1.04 – 1.23), but was inversely associated with the length of baby at birth (RRR = 0.88; 95%CI: 0.76 – 1.01). The adjusted incidence risk ratios (IRR) of coughing, difficult breathing, runny/stuffy nose and pharyngitis/tonsillitis in wheezers were much higher than that observed among non-wheezers and significantly depended on prenatal PM2.5 exposure, older siblings and maternal atopy. The study shows a clear inverse association between maternal age or maternal education and respiratory illnesses and calls for more research efforts aiming at explanation of factors hidden behind proxy measures of quality of maternal care of babies. The data support the hypothesis that burden of respiratory symptoms in early childhood and possibly in later life may be programmed already in prenatal period when the respiratory system is completing its growth and maturation.
Keywords: wheezing phenotypes, respiratory symptoms, prenatal and postnatal environmental air quality, birth cohort study
Introduction
There is a good body of epidemiologic evidence that asthma and other respiratory diseases are a major health issue in childhood. They account for leading causes of visits to physicians and hospitalization with asthma being one of the main and increasing causes of hospitalization in young children and adolescents in many countries The prevalence of asthma and wheezing symptoms in infants and children varies widely between populations and there is much controversy concerning the nature and meaning of early wheezing for respiratory health in the course of adult life (1 – 4). Wheeze originates in airways which may be narrowed by compression or by intrabronchial or intraluminal obstruction (inflammatory mucosal edema, secretions or spasm), which cause an increase in velocity of gas through them with resultant oscillation. It is also suggested that wheezing lower respiratory illness (LRI) in the first year of life is a consequence of anatomically small airway unrelated to the later development of atopic asthma (5).
Up to now, most epidemiologic research on respiratory health in early childhood was focused on postnatal ETS and other environmental insults (6 – 18). However, the lung is unusual in that its development is incomplete at birth and respiratory function must undergo a very rapid and dramatic change at birth (19). It is becoming clear that not only perinatal and early childhood periods constitute a particularly vulnerable time during which air pollutants may exert harmful effects respiratory tract of infants (20 – 26). In the intrauterine period, during which the lung is developing and maturing, even very subtle influences on airway fetal development can have a lasting impact on the risks of respiratory disease later in life (27, 28).
The primary aim of the study was to establish the overall pattern of prenatal environmental risk factors (ETS and fine particulate matter) related to the onset of wheezing phenotypes and severity of respiratory illness in early childhood. The impact of prenatal air quality on the severity of respiratory illness measured by the duration of various respiratory symptoms in the first two years of life was adjusted to potential confounding factors such as gender of child, maternal age, education and maternal atopy, parity, moldy/damp home or socioeconomic status of the family. The secondary goal of the paper was to assess possible association of early persistent wheezing with the size of the baby at birth.
Material and Methods
This study uses data from an earlier established birth cohort of children in Krakow being the part of the collaborative study with Columbia University in New York. The design of the study and the detailed selection of the population have been described previously (29). Pregnant women were recruited from ambulatory prenatal clinics in the first and second trimester of pregnancy. Only women 18–35 years of age, who claimed to be non-smokers, with singleton pregnancies, without illicit drug use and HIV infection, free from chronic diseases such as diabetes or hypertension, and residents of Krakow for at least one year prior to pregnancy were eligible for the study. Prior to participation, women read and signed an informed consent. The Ethical Committee of the Jagiellonian University approved the research.
Upon enrolment, a detailed questionnaire was administered to each woman at the entry to the study to solicit information on demographic data, house characteristics, medical and reproductive history, occupational hazards, and smoking practices of others present in the home. A total of 505 pregnant women enrolled to the study born their children between January 2001 and February 2004, but the analysis has been based on 468 subjects with complete data.
Prenatal environmental tobacco smoke (ETS) was measured by a average number of cigarettes smoked daily in the presence of mother over pregnancy period and postnatal ETS by a number of cigarettes smoked daily at home in the presence of child over two years in postnatal period. The definition of moldy/damp household was based on questions regarding noticeable moisture stains and visible mould growth on the walls within the household at the interview taken at the end of the two-year follow up. Maternal atopy was assumed in the case that the mother reported allergic skin disorders or allergic related respiratory diseases. Maternal education (years of schooling) was treated as a proxy for the socio-economic status.
After delivery, newborns were followed-up every three months over two years and trained interviewers have carried out detailed standardized interviews on infants’ health at each three-month visit. All interviews have been performed with the mothers of infants. Respiratory outcomes variables included the following individual symptoms: 1. wheezing or whistling in the chest irrespective of respiratory infection, 2. cough with or without cold,.3. difficult breathing, 4. symptoms of angina/pharyngitis, 5. runny/stuffy nose. For each of the symptom its duration (days) in each 3-month period was recorded in the questionnaire. The severity of respiratory symptoms was expressed by the overall number of days with a given symptom experienced by the child in the first two years of life.
Data on wheezing with whistling on children chest during each of 8 time points was used to identify four mutually exclusive patterns of wheeze between birth and 2 years: 0. never wheezers (no wheezing at any of the 8 time points); 1. transient early wheeze (wheezed at any time at 0–12 months but not thereafter); 2. late wheeze (onset of wheeze between 13 and 24 months). 3. persistent wheezing, which developed in the first year of life and continued to be present in the second year of the follow-up.
Dosimetry of prenatal personal exposure to fine particles
During the second trimester, a member of the air monitoring staff instructed the woman in the use of the personal monitor, which is lightweight, quiet and is worn in a backpack. The woman was asked to wear the monitor during the daytime hours for 2 consecutive days and to place the monitor near the bed at night. During the morning of the second day, the air monitoring staff-person and interviewer visited the woman’s home to change the battery-pack and administer the full questionnaire. They also checked to see that the monitor has been running continuously and that there have been no technical or operating failures. A staff-member returned to the woman’s home on the morning of the third day to pick up the equipment.
A Personal Environmental Monitoring Sampler (PEMS) was used to measure particle mass. The PEMS is designed to achieve the particle target size of ≤ 2.5 μm at a flow rate of 4.0 liters per minute (LPM) with an array of 10 impactor nozzles. Flow rates were calibrated (with filters in place) using a bubble meter prior to the monitoring, and are checked again with a change of the battery pack on the second day and at the conclusion of the monitoring. Pumps operated continuously at 2 LPM over the 48-hour period. Particles were collected on Teflon membrane filter (37 mm Teflo™, Gelman Sciences). The combination of low pressure drop (permitting use of a low power sampling pump), low hygroscopicity (minimizing bound water interference in mass measurements), and low trace element background (improving analytical sensitivity) of these filters make them highly appropriate for personal particle sampling.
Statistical Analysis
The purpose of the statistical analysis was to assess the impact of prenatal environmental risk factors on the occurrence of wheezing phenotypes and severity of respiratory symptoms recorded in the first two years of life of children. To identify potential confounders, associations between population characteristics and outcome variables were investigated. Association between wheezing phenotypes and independent variables was analyzed by the multinomial logit model (30). The multinomial logit model is a generalization of logistic regression to more than two outcomes. As mentioned we coded wheezing phenotypes from 0 – 3, and the outcome 0 was used as the base reference group. The multinomial model assumes r equations for the r outcomes. One equation sets β coefficient to zero so the associated outcome is the base reference group. Since the model is fitted using one of the outcomes as a base reference (never wheezers), the probabilities that we calculated are relative to that base group. Since interpretation of regression coefficients are difficult because of nonlinearity of the link function and the incorporation of the base reference group, we transformed beta coefficients in relative risk ratios (RRR).
The effect of environmental exposure on the severity of respiratory symptoms in infants measured over 24 months of age was assessed by incidence rate ratios (IRRs) estimated by the Zero-inflated Poisson regression model (ZIP), which better fits the overdispersed count Poisson data with excess of observed zeros than the traditional Poisson regression model (30). Dependent variables were counts of observed total number of days a given symptom present in the follow-up period (0, 1, 2, 3, 4, etc). As in the multinomial models, in the regression models a set of potential confounders or modifiers (gender of child, maternal education, parity, maternal atopy, prenatal ETS, prenatal exposure to fine particles and moldy/damp household) were taken into consideration. As distribution of PM2.5 concentrations was markedly skew, in all statistical analyses the prenatal PM2.5 exposure was log-transformed to the basis 2. This makes IRRs and RRs easily interpretable as the relative changes by doubling the exposure concentration. Statistical analyses were performed with STATA 10 version software for Windows (31 – 32)
Results
In the total study sample, 126 children (26.9%) had at least one wheezing episode in the first two years of life. The onset of wheezing in the first year of life was recorded in 84 infants (17.9%) and new wheezing beyond the first year of age was observed in 42 children (9.0%). Out of 84 infants for whom onset of wheezing has been reported in the first 12 months of age, 30 children (6.4%) had it still through the second year of life.
Table 1 presents the characteristics of the study sample and the occurrence of respiratory symptoms in children with reference to the wheezing phenotypes. While there was about the same proportion of boys and girls in the total study sample, there was more boys than girls among persistent wheezers (63.3% vs. 36.7). Wheezers appeared to have more often atopic mothers and more often lived in moldy/damp houses, were exposed to prenatal or postnatal ETS and showed an excessive severity of respiratory illness measured by the number of days with coughing, difficult breathing, runny/stuffy nose or pharyngitis/tonsillitis.
Table 1.
Characteristics of the study sample by the wheezing phenotype and the occurrence of respiratory symptoms in the two-year follow-up study
| Wheezing phenotypes | ||||||
|---|---|---|---|---|---|---|
| Variables | 0 (N=342) | 1 (N=54) | 2 (N=42) | 3 (N=30) | Total (468) | |
| Gender: | Boys n (%) | 164 (48.0) | 31 (57.4) | 21 (50.0) | 19 (63.3) | 235 (50.2)* |
| Girls n (%) | 178 (52.0) | 23 (42.6) | 21 (50.0) | 11 (36.7) | 233 (49.8) | |
|
| ||||||
| Birth weight (g): | Mean | 3413.5 | 3415.9 | 3344.3 | 3394.3 | 3406.4* |
| SD | 473.3 | 424.3 | 543.0 | 551.9 | 478.7 | |
|
| ||||||
| Length at birth(cm): | Mean | 54.64 | 54.96 | 54.05 | 53.80 | 54.57* |
| SD | 2.81 | 2.47 | 3.54 | 2.79 | 2.87 | |
|
| ||||||
| Gestational age (weeks): | ||||||
| Mean | 39.398 | 39.241 | 39.262 | 38.933 | 39.338* | |
| SD | 1.445 | 1.373 | 1.976 | 1.911 | 1.524 | |
|
| ||||||
| Parity: | 1 n (%) | 223 (65.2) | 34 (63.0) | 22 (52.4) | 12 (40.0) | 291 (62.2)** |
| ≥2 n (%) | 119 (34.8) | 20 (37.0) | 20 (47.6) | 18 (60.0) | 177 (37.8) | |
|
| ||||||
| Maternal age (years): | ≤ 26 | 114 (33.3) | 18 (33.3) | 15 (35.7) | 11 (36.7) | 158 (33.8)* |
| 27 – 29 | 122 (35.7) | 18 (33.3) | 14 (33.3) | 13 (43.3) | 167 (35.7) | |
| > 29 | 106 (31.0) | 18 (33.3) | 13 (31.0) | 6 (20.0) | 143 (30.6) | |
|
| ||||||
| Maternal educat.: | elementary | 29 (8.5) | 3 (5.6) | 5 (11.9) | 7 (23.3) | 44 (9.4)** |
| medium | 90 (26.3) | 13 (24.1) | 5 (11.9) | 7 (23.3) | 115 (24.6) | |
| higher | 223 (65.2) | 38 (70.4) | 32 (76.2) | 16 (53.3) | 309 (66.0) | |
|
| ||||||
| Maternal atopy (+): | n (%) | 80 (23.4) | 15 (27.8) | 8 (19.0) | 13 (43.3) | 116 (24.8)** |
|
| ||||||
| Prenatal ETS (+) | n (%) | 80 (23.4) | 15 (27.8) | 14 (33.3) | 15 (50%) | 124 (26.5)** |
|
| ||||||
| Postnatal ETS (+): | n (%) | 51 (14.9) | 8 (14.8) | 10 (23.8) | 11 (36.7) | 80 (17.1) ** |
|
| ||||||
| Moulds (+): | n (%) | 37 (10.8) | 9 (16.7) | 6 (14.3) | 9 (30.0) | 61 (13.0) ** |
|
| ||||||
| Dampness (+): | n (%) | 51 (14.9) | 10 (18.5) | 6 (14.3) | 11 (36.7) | 78 (16.7) ** |
|
| ||||||
| Prenatal PM25 (μg/m3) | ||||||
| Median | 35.0 | 35.6 | 36.9 | 37.6 | 35.2* | |
| Interquartile range | 22.6–51.2 | 21.8–47.5 | 23.2–53.6 | 22.4–58.6 | 14.8 | |
| Missing | 2 | 0 | 0 | 1 | 3 | |
|
| ||||||
| Number of days with cough | ||||||
| Median | 19.0 | 29.0 | 34.5 | 54.0 | 22.0** | |
| Interquartile range | 14.0 | 21.4 | 20.6 | 27.9 | 15.5 | |
|
| ||||||
| Number of days with difficult breathing: | ||||||
| Median | 0.0 | 3.0 | 6.0 | 13.5 | 0.0** | |
| Interquartile range | 1.5 | 7.0 | 7.1 | 10.3 | 3.5 | |
|
| ||||||
| Number of days with runny or stuffy nose: | Median | 36.5 | 53.0 | 58.5 | 87.0 | 41.5** |
| Interquartile range | 19.5 | 34.4 | 25.8 | 37.4 | 22.9 | |
|
| ||||||
| Number of days with tonsillitis/pharyngitis: | Median | 5.5 | 4.5 | 8.5 | 8.0 | 6.0* |
| Interquartile range | 7.0 | 8.0 | 10.1 | 11.0 | 7.0 | |
p > 0.05;
p ≤ 0.05
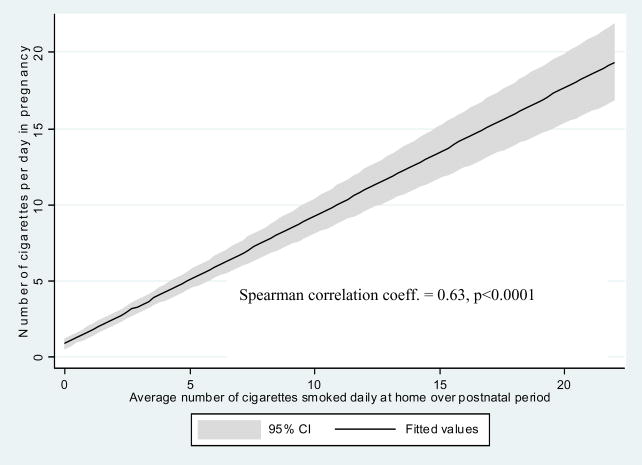
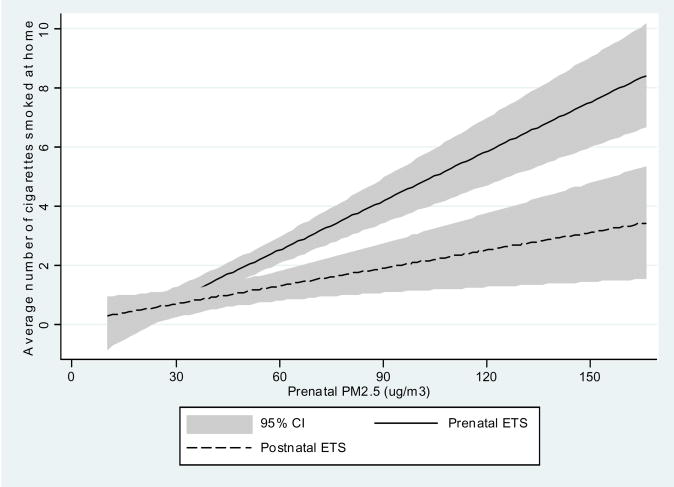
Personal measurements of prenatal daily exposure to PM2.5 particles in the study population were within a wide range of 10.3 μg/m3 – 294.9 μg/m3 with the median of 35.3 μg/m3. Prevalence of the self-reported prenatal ETS exposure was given in Table 1 as 26.5% while postnatal ETS exposure was recorded in 17.1% of children, house dampness in 16.7%, and presence of moulds/dampness in 13.0% of households. There was a significant correlation between self-reported ETS over pregnancy and ETS recorded in the postnatal period (Figure 1). On the other side, there was a significant interrelationship between PM2.5 and both prenatal ETS (Spearman r = 0.34, p <0.001) and postnatal ETS levels measured by the number of cigarettes smoked daily at home (Spearman r = 0.28, p <0.001) (Figure 2).
Figure 1.
Correlation between average number of cigarettes smoked daily at home in the prenatal and postnatal periods.
Figure 2.
Correlation between PM2.5 and average number of cigarettes smoked daily at home in the prenatal and postnatal periods.
The multinomial logit regression model was used to establish the pattern of risk factors for each wheezing phenotype (Table 2). Only persistent wheezing was significantly associated with maternal atopy (RRR = 3.05; 95%CI: 1.30 – 7.15), parity (RRR = 3.05; 95%CI: 1.67 – 5.58), and prenatal ETS exposure (RRR= 1.13; 95%CI: 1.04 – 1.23). The prenatal exposure to fine particles (PM2.5) was dropped from the regression models since it failed to explain better the variation between dependent and independent variables. The relative risk estimates for persistent wheezing were inversely related to length of the baby at birth (RRR = 0.88; 95%CI: 0.77 – 1.01).
Table 2.
Multinomial logit regression model of wheezing phenotypes as health outcomes and environmental exposure variables and potential modifiers
| Wheezing phenotypes | RRR | p-value | [95% Conf. | Interval] |
|---|---|---|---|---|
| Transient early wheezing | ||||
| Age groups | 1.015 | 0.941 | 0.672 | 1.533 |
| Education level | 1.329 | 0.292 | 0.783 | 2.255 |
| Maternal atopy | 1.272 | 0.474 | 0.657 | 2.464 |
| Girls | 0.724 | 0.294 | 0.395 | 1.324 |
| Older siblings | 1.173 | 0.537 | 0.706 | 1.950 |
| Length at birth (cm) | 1.026 | 0.638 | 0.920 | 1.145 |
| Moldy/damp house | 1.315 | 0.106 | 0.943 | 1.835 |
| Prenatal ETS | 0.984 | 0.759 | 0.894 | 1.084 |
| Postnatal ETS | 1.069 | 0.061 | 0.997 | 1.146 |
| Transient late wheezing | ||||
| Age groups | 0.736 | 0.209 | 0.456 | 1.187 |
| Education level | 1.599 | 0.121 | 0.884 | 2.893 |
| Maternal atopy | 0.799 | 0.597 | 0.349 | 1.830 |
| Girls | 0.889 | 0.730 | 0.456 | 1.732 |
| Older siblings | 2.188 | 0.003 | 1.307 | 3.663 |
| Length at birth | 0.916 | 0.122 | 0.820 | 1.023 |
| Moldy/damp house | 0.966 | 0.890 | 0.594 | 1.571 |
| Prenatal ETS | 1.057 | 0.219 | 0.967 | 1.157 |
| Postnatal ETS | 1.007 | 0.885 | 0.912 | 1.111 |
| Persistent wheezing | ||||
| Age | 0.644 | 0.155 | 0.351 | 1.181 |
| Education | 1.019 | 0.952 | 0.539 | 1.928 |
| Maternal atopy | 3.046 | 0.010 | 1.298 | 7.150 |
| Girls | 0.475 | 0.088 | 0.202 | 1.118 |
| Older siblings | 3.053 | 0.000 | 1.670 | 5.580 |
| Length at birth | 0.878 | 0.063 | 0.766 | 1.007 |
| Moldy/damp house | 1.342 | 0.150 | 0.898 | 2.005 |
| Prenatal ETS | 1.131 | 0.004 | 1.040 | 1.231 |
| Postnatal ETS | 0.945 | 0.483 | 0.809 | 1.104 |
(Children without wheezing during the first two years are the reference group)
Age groups: <26 yrs, 27 – 29 yrs, >29 yrs)
Education level (elementary/medium/high)
Maternal atopy (Y/No)
Older siblings (number of older siblings: 0, 1, 2= two or more siblings)
Length at birth (cm)
Moldy/damp house (Y/No)
Prenatal ETS (number of cigarettes smoked daily at home over pregnancy)
Postnatal ETS (number of cigarettes smoked daily at home in the postnatal period)
Severity of individual respiratory symptoms assessed by incidence rate ratios (IRRs) estimated in the ZIP models were adjusted for wheezing phenotypes, prenatal PM2.5 exposure and other potential confounders (tables 3 – 6). Tables in the upper part contain the Poisson portion on incidence risk ratios (IRR) and in the lower part the logistic portion which is based on zero days for a given symptom and reports the odds ratios (OR). Due to significant colinearity between both prenatal/postnatal ETS variables and PM2.5, the ETS variables were dropped from the regression models. Adding both ETS variables into the models failed to explain better the amount of variability of symptoms than PM2.5 itself.
Table 3.
Zero-inflated Poisson model for number of days with coughing in two-years olds due to prenatal air quality and other potential risk factors
| Poisson portion | IRR | p-value | [95% Conf. | Interval] |
|---|---|---|---|---|
| Age groups | ||||
| Age <=26 | Reference | |||
| Age_27 – 29 | 0.967 | 0.106 | 0.929 | 1.007 |
| Age_>29 | 0.791 | 0.000 | 0.754 | 0.830 |
| Maternal education | ||||
| Elementary | Reference | |||
| Medium | 0.943 | 0.038 | 0.892 | 1.000 |
| Higher | 0.767 | 0.000 | 0.728 | 0.809 |
| Maternal atopy | 1.132 | 0.000 | 1.091 | 1.175 |
| Girls | 1.026 | 0.111 | 0.993 | 1.061 |
| Older siblings | 1.259 | 0.000 | 1.227 | 1.291 |
| Wheezing phenotypes | ||||
| No wheezing | Reference | |||
| Transient early wheeze | 1.612 | 0.000 | 1.540 | 1.688 |
| Transient late wheeze | 1.514 | 0.000 | 1.438 | 1.593 |
| Persistent wheezing | 2.137 | 0.000 | 2.032 | 2.248 |
| Prenatal PM2.5 | 1.032 | 0.001 | 1.012 | 1.052 |
| Moldy/damp house | 1.088 | 0.000 | 1.068 | 1.108 |
| Logistic portion | OR | p-value | [95% Conf. | Interval] |
| Age groups | −0.429 | 0.358 | −1.344 | 0.485 |
| Education levels | −0.169 | 0.799 | −1.470 | 1.132 |
| Maternal atopy | 0.185 | 0.630 | −0.567 | 0.937 |
| Girls | 0.172 | 0.603 | −0.476 | 0.819 |
| Older siblings | −0.379 | 0.240 | −1.011 | 0.252 |
| Wheezing phenotypes | −1.279 | 0.012 | −2.279 | −0.280 |
| Prenatal PM2.5 | −0.286 | 0.172 | −0.696 | 0.124 |
| Moldy/damp house | −0.238 | 0.403 | −0.795 | 0.319 |
| _cons | −0.286 | 0.837 | −3.001 | 2.429 |
Age groups: <26 yrs, 27 – 29 yrs, >29 yrs)
Education level (elementary/medium/high)
Maternal atopy (Y/No)
Older siblings (number of older siblings: 0, 1, 2= two or more siblings)
Length at birth (cm)
Prenatal PM2.5 (μg/m3) log-transformed to the basis 2.
Moldy/damp house (Y/No)
Prenatal ETS (number of cigarettes smoked daily at home over pregnancy)
Postnatal ETS (number of cigarettes smoked daily at home in the postnatal period)
Table 6.
Zero-inflated Poisson model for number of days with runny or stuffy nose in two- years olds due to prenatal air quality and other potential risk factors
| Poisson portion | IRR | p-value | [95% Conf. | Interval] |
|---|---|---|---|---|
| Age groups | ||||
| Age <=26 yrs | Reference | |||
| Age_27 – 29 yrs | 0.919 | 0.000 | 0.891 | 0.948 |
| Age_>29 yrs | 0.775 | 0.000 | 0.747 | 0.804 |
| Maternal education | ||||
| Elementary | Reference | |||
| Medium | 1.096 | 0.000 | 1.045 | 1.150 |
| Higher | 1.108 | 0.000 | 1.060 | 1.159 |
| Maternal atopy | 1.188 | 0.000 | 1.155 | 1.222 |
| Girls | 1.007 | 0.548 | 0.982 | 1.033 |
| Older siblings | 1.382 | 0.000 | 1.356 | 1.409 |
| Wheezing phenotypes | ||||
| No wheezing | Reference | |||
| Transient early wheeze | 1.461 | 0.000 | 1.410 | 1.513 |
| Transient late wheeze | 1.203 | 0.000 | 1.154 | 1.255 |
| Persistent wheezing | 1.803 | 0.000 | 1.730 | 1.878 |
| Prenatal PM2.5 | 1.009 | 0.201 | 0.994 | 1.025 |
| Moldy/damp house | 1.030 | 0.000 | 1.015 | 1.046 |
| Logistic portion | OR | p-value | [95% Conf. | Interval] |
| Age groups | 1.117 | 0.004 | 0.358 | 1.878 |
| Education levels | −0.604 | 0.136 | −1.398 | 0.190 |
| Maternal atopy | 0.535 | 0.425 | −0.779 | 1.850 |
| Girls | 0.258 | 0.656 | −0.879 | 1.396 |
| Older siblings | −1.833 | 0.023 | −3.416 | −0.251 |
| Wheezing phenotypes | −8.180 | 0.998 | −12.360 | 12.324 |
| Prenatal PM2.5 | −0.264 | 0.466 | −0.976 | 0.446 |
| Moldy/damp house | −0.076 | 0.850 | −0.877 | 0.723 |
| _cons | −0.040 | 0.987 | −5.021 | 4.941 |
Age groups: <26 yrs, 27 – 29 yrs, >29 yrs)
Education level (elementary/medium/high)
Maternal atopy (Y/No)
Older siblings (number of older siblings: 0, 1, 2= two or more siblings)
Length at birth (cm)
Prenatal PM2.5 (μg/m3) log-transformed to the basis 2
Moldy/damp house (Y/No)
Prenatal ETS (number of cigarettes smoked daily at home over pregnancy)
Postnatal ETS (number of cigarettes smoked daily at home in the postnatal period)
Coughing duration (overall number of days in the first two years of life) adjusted for wheezing phenotypes were positively associated with prenatal PM2.5 exposure expressed in log units (IRR = 1.03; 95%CI: 1.01 – 1.05), moldy/damp house (IRR = 1.09; 95%CI: 1.07 – 1.11), maternal atopy (IRR = 1.13; 95%CI: 1.09 – 1.18) and older siblings (IRR = 1.26; 95%CI: 1.23 – 1.29). All wheezing phenotypes had the pronounced effect on the occurrence of coughing symptoms. While the transient wheezing increased the severity of coughing by about 50 – 60% the persistent wheezing doubled the risk (IRR = 2.14; 95%CI: 2.03 – 2.25). Better maternal education and older maternal age at delivery were inversely associated with the IRR for coughing days. The number of zero events was only inversely associated with wheezing phenotypes (Table 3).
The adjusted IRR of difficult breathing was also significantly associated with prenatal PM2.5 exposure (IRR = 1.17; 95%CI: 1.12 – 1.23), moldy/damp house (IRR = 1.13; 95%CI: 1.08 – 1.17) and maternal atopy (IRR = 1.45; 95%CI: 1.36 – 1.59). The estimated IRR was lower in girls than in boys. All wheezing phenotypes significantly increased the severity of difficult breathing, but better maternal education and older maternal age at delivery were inversely associated with the IRR. The number of zero events was only inversely associated with wheezing phenotypes (table 4).
Table 4.
Zero-inflated Poisson model for number of days with difficult breathing in two- years olds due to prenatal air quality and other potential risk factors
| Poisson portion | IRR | p-value | [95% Conf. | Interval] |
|---|---|---|---|---|
| Age groups | ||||
| Age <=26 yrs | Reference | |||
| Age_27 – 29 yrs | 0.923 | 0.096 | 0.840 | 1.014 |
| Age_>29 yrs | 0.681 | 0.000 | 0.608 | 0.764 |
| Maternal education | ||||
| Elementary | Reference | |||
| Medium | 0.654 | 0.000 | 0.577 | 0.743 |
| Higher | 0.831 | 0.002 | 0.740 | 0.932 |
| Maternal atopy | 1.467 | 0.000 | 1.356 | 1.588 |
| Girls | 0.632 | 0.000 | 0.584 | 0.684 |
| Older siblings | 1.300 | 0.000 | 1.232 | 1.371 |
| Wheezing phenotypes | ||||
| No wheezing | Reference | |||
| Transient early wheeze | 1.602 | 0.000 | 1.455 | 1.764 |
| Transient late wheeze | 1.193 | 0.002 | 1.065 | 1.337 |
| Persistent wheezing | 1.325 | 0.000 | 1.188 | 1.477 |
| Prenatal PM2.5 | 1.174 | 0.000 | 1.123 | 1.227 |
| Moldy/damp house | 1.126 | 0.000 | 1.082 | 1.172 |
| Logistic portion | OR | p-value | [95% Conf. | Interval] |
| Age groups | −0.093 | 0.510 | −0.370 | 0.183 |
| Education levels | 0.052 | 0.746 | −0.263 | 0.367 |
| Maternal atopy | −.0.317 | 0.179 | −0.781 | 0.145 |
| Girls | −0.169 | 0.407 | −0.569 | 0.231 |
| Older siblings | −0.265 | 0.138 | −0.617 | 0.085 |
| Wheezing phenotypes | −0.847 | 0.000 | −1.107 | −0.585 |
| Prenatal PM2.5 | −0.072 | 0.559 | −0.314 | 0.170 |
| Moldy/damp house | −0.075 | 0.576 | −0.342 | 0.190 |
| _cons | 1.745 | 0.035 | 0.120 | 3.370 |
Age groups: <26 yrs, 27 – 29 yrs, >29 yrs)
Education level (elementary/medium/high)
Maternal atopy (Y/No)
Older siblings (number of older siblings: 0, 1, 2= two or more siblings)
Length at birth (cm)
Prenatal PM2.5 ((μg/m3) log-transformed to the basis 2
Moldy/damp house (Y/No)
Prenatal ETS (number of cigarettes smoked daily at home over pregnancy)
Postnatal ETS (number of cigarettes smoked daily at home in the postnatal period)
The adjusted IRRs of pharyngitis/tonsillitis were also significantly associated with prenatal PM2.5 exposure (IRR = 1.11; 95%CI: 1.07 – 1.14), moldy/damp house (IRR = 1.12; 95%CI: 1.08 – 1.45) and maternal atopy (IRR = 1.19; 95%CI: 1.12 – 1.27), but better maternal education and older maternal age at delivery were inversely associated with the IRR. The number of zero events was inversely associated with older siblings (Table 5).
Table 5.
Zero-inflated Poisson model for number of days with pharyngitis/tonsillitis in two- years olds due to prenatal air quality and other potential risk factors
| Poisson portion | IRR | p-value | [95% Conf. | Interval] |
|---|---|---|---|---|
| Age groups | ||||
| Age <=26 yrs | Reference | |||
| Age_27 – 29 yrs | 1.099 | 0.007 | 1.026 | 1.178 |
| Age_>29 yrs | 0.861 | 0.001 | 0.790 | 0.938 |
| Maternal education | ||||
| Elementary | Reference | |||
| Medium | 0.784 | 0.000 | 0.712 | 0.864 |
| Higher | 0.757 | 0.000 | 0.693 | 0.828 |
| Maternal atopy | 1.194 | 0.000 | 1.120 | 1.273 |
| Girls | 0.893 | 0.000 | 0.843 | 0.946 |
| Older siblings | 1.213 | 0.000 | 1.159 | 1.269 |
| Wheezing phenotypes | ||||
| No wheezing | Reference | |||
| Transient early wheeze | 1.036 | 0.445 | 0.945 | 1.135 |
| Transient late wheeze | 1.225 | 0.000 | 1.118 | 1.342 |
| Persistent wheezing | 1.085 | 0.113 | 0.980 | 1.201 |
| Prenatal PM2.5 | 1.105 | 0.000 | 1.068 | 1.143 |
| Moldy/damp house | 1.116 | 0.000 | 1.084 | 1.149 |
| Logistic portion | OR | p-value | [95% Conf. | Interval] |
| Age groups | 0.46 | 0.275 | −0.116 | 0.354 |
| Education levels | −0.044 | 0.771 | −0.343 | 0.620 |
| Maternal atopy | −0.158 | 0.488 | −0.604 | 0.288 |
| Girls | −0.272 | 0.159 | −0.652 | 0.107 |
| Older siblings | −0.355 | 0.039 | −0.692 | −0.018 |
| Wheezing phenotypes | −0.080 | 0.470 | −0.298 | 0.138 |
| Prenatal PM2.5 | −0.097 | 0.402 | −0.326 | 0.131 |
| Moldy/damp house | −0.218 | 0.120 | −0.492 | 0.056 |
| _cons | 1.055 | 0.178 | −0.480 | 2.592 |
Age groups: <26 yrs, 27 – 29 yrs, >29 yrs)
Education level (elementary/medium/high)
Maternal atopy (Y/No)
Older siblings (number of older siblings: 0, 1, 2= two or more siblings)
Length at birth (cm)
Prenatal PM2.5 ((μg/m3) log-transformed to the basis 2
Moldy/damp house (Y/No)
Prenatal ETS (number of cigarettes smoked daily at home over pregnancy)
Postnatal ETS (number of cigarettes smoked daily at home in the postnatal period)
The IRRs of runny or stuffy nose correlated significantly with moldy/damp house (IRR = 1.03; 95%CI: 1.02 – 1.05), maternal atopy (IRR = 1.19; 95%CI: 1.16 – 1.22) and older siblings (IRR = 1.38; 95%CI: 1.36 – 1.41). All wheezing phenotypes significantly increased the severity of difficult breathing but better maternal education and older maternal age at delivery were inversely associated with severity of these symptoms. The number of zero events was only inversely associated with older siblings (Table 6).
DISCUSSION
Approximately one third of the children (26.9%) in our study experienced wheezing symptoms in the first two years of life and in about two third of cases the symptoms developed already in the first years of life. Wheezing observed in the first 12 months of age appeared to be easily reversible and in about 70% of wheezing infants the symptoms were not confirmed in the second year of the follow-up.
Our study demonstrated a set of risk factors for the onset of persistent wheezing and its likelihood increased with maternal atopy, older siblings in the household and prenatal exposure to ETS, but inversely correlated with the size of newborns at delivery. The data showed a strong link between severity of respiratory illness and early wheezing and documented a set of other risk factors related to the severity of respiratory illness such as prenatal PM2.5 exposure, moldy/damp house, maternal atopy and presence of older siblings. The adjusted incidence risk ratios (IRR) of coughing, difficult breathing, runny/stuffy nose and pharyngitis/tonsillitis significantly were associated inversely with maternal age and education level.
Although prenatal exposure to prenatal PM2.5 particulate matter was not directly associated with the onset of early wheezing, however, this exposure could have had indirect effect on the occurrence of early wheezing through its effects on size of newborns and intrauterine lung growth. Observed in our study an inverse relationship between infants’ length at birth and persistent wheezing could indicate that a shorter length of newborns might be a proxy measure of slower biological maturation of respiratory tract, which obviously depends on many factors such as maturation of target lung cell population, their relevant enzyme and detoxication systems (33 – 37). Animal studies already documented that both intrauterine and postnatal exposure to pollutants could lead to impaired lung growth (38). In this context, we may also refer to our earlier findings, which showed that prenatal exposure to PM2.5 was significantly associated with lower birthweight and shorter length of babies at birth (39).
The observed inverse relationship between length at birth and the occurrence of persistent wheezing in early childhood provides a support for the fetal origins hypothesis (40). The hypothesis proposes that the fetal growth changes with altered nutrient or oxygen supply and intrauterine exposure to environmental hazards may affect growing organs and hormonal and metabolic pathways and lead to impairment of fetus growth and specific organs such as lungs (42, 43). There are known reports documenting the relationship between size of newborns and respiratory health in adult life. For instance, the recent study of over 2000 British women found a positive association between birthweight and lung function at age 60 –79 years (43). Other studies also reported an association between birthweight and respiratory health or lung function measured in adulthood (44 – 47).
Very strong association of persistent wheezing with severity of respiratory infections found in our study may have its origin in the fact that wheezing in early infancy is a surrogate marker of the pulmonary immune immaturity, which is manifested by an enhanced susceptibility to the effects of respiratory viruses. Frequent recurrent insults to the airway epithelium in the course of respiratory infections in wheezers may result in various inflammatory processes that induce structural airway changes (48). The importance of early wheezing for the respiratory health of young children was emphasized by the findings that most cases of persistent wheezing and asthma, which begin in early life are often associated with reduced infant lung function. For instance, the recent study in preschool children in the Manchester Asthma and Allergy Study (49) has shown that both transient and persistent wheezers have reduced lung function compared with nonwheezing children, but the deficit was considerably greater in persistent wheezers. It is possible that the deficits in lung function in persistent and transient wheezers may occur at a much younger age.
Although postnatal ETS is assumed to be a risk factor for both susceptibility and severity of respiratory infections, but the role intrauterine exposure to ETS is less clear (50) In our study we could only confirm the significant impact of prenatal ETS on the onset of persistent wheezing, but we were not able to assess separate effects of ETS and PM2.5 on the severity of respiratory symptoms. There was a significant interrelationship between PM2.5 and both prenatal ETS (Spearman r = 0.34, p <0.001) and postnatal ETS (Spearman r = 0.28, p <0.001). This interrelationship creates colinearity in regression models and difficulties in separating the effect of ETS on the health outcomes from that attributed to fine particles. Stepwise regression indicated that adding both ETS variables into the models did not better explain the amount of variability in severity of respiratory symptoms than PM2.5 itself. After infancy and very early childhood is over, then prenatal effect of PM2.5 exposure upon health of babies may gradually be attenuated and postnatal environment starts to play a leading part. The harmful impact of ETS confirmed in some previous studies may result from its interrelationship with fine particles and in studies where the environmental impact on health outcomes was not controlled for PM2.5 level, the effect of ETS could be demonstrated.
In our study the prenatal exposure to PM2.5 particulate matter had a moderate but significant impact on severity of respiratory illness in postnatal early life. The biological mechanisms whereby prenatal PM2.5 exposure might cause adverse health outcomes in children are yet unclear. PM2.5 is a proxy measure of a whole complex of toxic agents present in the environment – including PAHs - that could adversely affect growth and maturation of lung in early childhood. Fine particles are usually a product of combustion processes that generate other toxic agents (51), which may interact at the molecular level with DNA (52). Prenatal exposure to immunotoxic fine particles may impair the immune function of the fetus and subsequently may be responsible for an increased susceptibility of newborns and young infants to respiratory infections.
Our observations on the importance of older siblings for respiratory health of children are in very good agreement with the Tucson Children’s Respiratory Study (53), which has shown that children with more exposure to other children at home or at day care were more likely to have frequent wheezing at age of 2 years than children with little or no exposure. In the birth cohort COAST study (54) day care attendance and/or the presence of siblings significantly increased the likelihood of contracting viral infections (1.5 to 2.1 fold increase) during infancy. The higher risk of respiratory infections in children having older siblings is assumed to be related to the fact that older siblings introduce bacterial or viral infections into the family circle.
A number of earlier studies have also found significant association between respiratory infections and family history of asthma or atopy. For example, Gurwitz and coworkers found that children hospitalized with RSV had a higher proportion of first-degree relatives with bronchial hyperactivity (55). Similarly, Trefny et al. (56) found that infants hospitalized with RSV bronchiolitis were more likely to have a family history of asthma. The role of family history of atopy in the occurrence of respiratory infections is not fully understood as yet. It may be a proxy of intrinsic genetic susceptibility, cytokine dysregulation, lung development altered antiviral immunity or increased inflammatory response (19, 33, 57). The influence of family history is likely to be clarified by ongoing genetic studies taking into consideration gene-environment interactions.
At present there is no convincing explanation for the inverse association between maternal age and respiratory illnesses. Maternal age may be a proxy for some unknown social factors not considered in the analysis. Maybe younger mothers are not as responsive as older mothers to their infants’ needs or present some less favorable behavior during early infancy of children. The way in which mothering skills may affect the young child and respiratory health problems is also unknown. Interestingly, the effects of maternal education showed a similar impact as that found for maternal age and this again might indicate that some important mothering skills in caring for newborns and infants related to maternal education may be important for respiratory health of babies. Educational level of mothers is not only a proxy of socioeconomic status of the family, but it may be related to other relevant factors such as maternal life style and dietary habits before and during pregnancy or feeding practices of infants and young children. In this respect the results of our study calls for more research efforts aiming at explanation of the other factors hidden behind proxy measures of quality of maternal care of babies.
The weakness of our study results from the fact that we could not unmistakably distinguish the effect of prenatal PM2.5 exposure from that of the postnatal exposure since the PM2.5 postnatal measurements were not repeated in the same way. Postnatal air indoor quality was based on questionnaire data regarding passive smoking and the presence of dampness/moulds in the households. Therefore, we are not certain whether our findings represent delayed effects of prenatal PM2.5 exposure on infants, or more immediate effects of postnatal PM2.5 exposure over the first two years of life. On the other hand, we have to underline strength of the study. The important potential confounders of the relationship between prenatal ambient risk factors and the respiratory outcomes of infants such as chronic diseases or active tobacco smoking by mothers have been removed through entry criteria. Other risk factors that are thought to affect the probability of respiratory diseases in infants such as maternal atopy, postnatal indoor air quality (passive smoking, presence of dampness/moulds in the households) have been taken into consideration. A significant feature of our study is the personal monitoring of ambient PM2.5 exposure, which is a highly relevant measure of individual exposure. Previous studies have attempted to quantify the concentration of outdoor air pollutants measured in the residence area, and assign exposure values to the study subjects or use the area-based exposures to approximate individual exposures. Another strong point of our study is very careful monitoring data of respiratory health outcomes in children performed by trained interviewers over 8 time points over the follow-up.
Summing up, the results of our study confirmed that the likelihood of persistent wheezing increased with prenatal exposure to ETS, presence of dampness/moulds in the house, maternal atopy but was inversely correlated with the size of the baby at delivery. Moreover, the severity of respiratory illness in early childhood was particularly higher in wheezers and also depended on prenatal exposure PM2.5, dampness/molds in the households, and maternal atopy. Interestingly, the study showed a clear inverse association between maternal age or maternal education and respiratory illnesses and calls for more research efforts aiming at explanation of factors hidden behind proxy measures of maternal care of babies. The data support the hypothesis that burden of respiratory symptoms in early childhood and possibly in later life may be programmed already in prenatal period when the respiratory system is completing its growth and maturation.
Acknowledgments
This is part of an ongoing comparative longitudinal investigation on the health impact of prenatal exposure to outdoor/indoor air pollution in infants and children being conducted in New York City and Krakow. The study received funding from an RO1 grant entitled, “Vulnerability of the Fetus/Infant to PAH, PM2.5 and ETS” (5 RO1 ES10165 NIEHS; 02/01/00 - 01/31/04) and from the NIEHS (RO1 ES010165-0451) the Lundin Foundation and the Gladys T. and Roland Harriman Foundation. Principal investigator: Prof. FP Perera.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burr ML, Butland BK, King S, Vaughan_Williams E. Changes in asthma prevalence: two surveys 15 years apart. Arch Dis Child. 1989;64:1452–56. doi: 10.1136/adc.64.10.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yunginger J, Reed CE, O’Connel EJ, Melton LJ, O’Fallon WM, Silverstaein MD. A community-based study of the epidemiology of asthma. Incidence rates, 1964 – 1983. Am Rev Respir Dis. 1992;146:888–94. doi: 10.1164/ajrccm/146.4.888. [DOI] [PubMed] [Google Scholar]
- 3.Woolcock A, Peat JK. Evidence for the increase in asthma worldwide. In: Chadwick DJ, Cardew G, editors. The Rising trends in Asthma. Ciba Foundation, J. Wiley and sons; 1997. pp. 122–134. [DOI] [PubMed] [Google Scholar]
- 4.Steering committee of the International Study on Asthma and Allergies in Childhood (ISAAC) Worldwide variations in the prevalence of asthma symptoms: the international study of asthma and allergies in childhood (ISAAC) Eur Respir J. 1998;12:315–335. doi: 10.1183/09031936.98.12020315. [DOI] [PubMed] [Google Scholar]
- 5.Sly PD, Willet K. Developmental physiology. In: Silverman M, editor. Childhood Asthma and Other Wheezing Disorders. Chapman & Hall; London: 1995. pp. 55–66. [Google Scholar]
- 6.Fergusson DM, Horwood LJ, Shannon FT, Taylor B. Parental smoking and lower respiratory illness in the first three years of life. J Epidemiol Comm Health. 1981;35:180–184. doi: 10.1136/jech.35.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gortmaker SL, Walker DK, Jacobs FH, Ruch-Ross H. Parental smoking and the risk of childhood asthma. Am J Publ Health. 1982;72:574–579. doi: 10.2105/ajph.72.6.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedreira FA, Guandolo VL, Feroli EJ, Mella GW, Weiss IP. Involuntary smoking and incidence of respiratory illness during the first year of life. Pediatrics. 1985;75:594–597. [PubMed] [Google Scholar]
- 9.McConnochie KM, Roghmann KJ. Parental smoking, presence of older siblings, and family history of asthma increase risk of bronchiolitis. Am J Dis Child. 1986;140(8):806–812. doi: 10.1001/archpedi.1986.02140220088039. [DOI] [PubMed] [Google Scholar]
- 10.Neuspiel DR, Rush D, Butler NR, Golding J, Bijur PE, Kurzon M. Parental smoking and post-infancy wheezing in children: a prospective study. Am J Publ Health. 1989;79:168–171. doi: 10.2105/ajph.79.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forastiere F, Corbo GM, Michelozzi P, Pistelli R, Agabiti N, Brancato G, Ciappi G, Perucci CA. Effects of environment and passive smoking on the respiratory health of children. Int J Epidemiol. 1992;21:66–73. doi: 10.1093/ije/21.1.66. [DOI] [PubMed] [Google Scholar]
- 12.Martinez FD, Cline M, Burrows B. Increased incidence of asthma in children of smoking mothers. Pediatrics. 1992;89:21–26. [PubMed] [Google Scholar]
- 13.Ronchetti R, Bonci E, Cutrera R, de Castro G, Indinnimeo L, Midulla F, Tancredi G, Martinez FD. Enhanced allergic sensitization related to parental smoking. Arch Dis Child. 1992;67:496–500. doi: 10.1136/adc.67.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chilmonczyk BA, Salmun LM, Megathlin KN, Neveux LM, Palomaki GE, Knight GJ, Pulkkinen AJ, Haddow JE. Association between exposure to environmental tobacco smoke and exacerbations of asthma in children. New Engl J Med. 1993;328:1665–1669. doi: 10.1056/NEJM199306103282303. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Rennie DC, Dosman JA. Influence of environmental tobacco smoke on asthma in nonallergic and allergic children. Epidemiology. 1996;7:536–539. [PubMed] [Google Scholar]
- 16.Cook DG, Strachan DP. Summary of effects of parental smoking on the respiratory health of children and implications for research. Thorax. 1999;54:357–366. doi: 10.1136/thx.54.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jedrychowski W, Maugeri U, Jedrychowska I. In search for epidemiologic evidence on air quality and health in children and adults. Center for Research and studies in Biomedicine Luxembourg. 2000:89–102. [Google Scholar]
- 18.Mannino DM, Moorman JE, Kingsley B, Rose D, Repace J. Health effects related to environmental tobacco smoke exposure in children in the United States: data from the third National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2001;155:36–41. doi: 10.1001/archpedi.155.1.36. [DOI] [PubMed] [Google Scholar]
- 19.Dockery DW, Skerret PJ, Walters D, Gilliland F. Effects of Air Pollution on Children’Health and develoment. Review of evidence. WHO Regional Office for Europe; 2005. Development of lung function; pp. 108–134. [Google Scholar]
- 20.Taylor B, Wadsworth J. Maternal smoking during pregnancy and lower respiratory tract illness in early life. Arch Dis Child. 1987;62:766–791. doi: 10.1136/adc.62.8.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanrahan JP, Tager IB, Segal MR, et al. The effects of maternal smoking during pregnancy on early lung function. Am Rev Respir Dis. 1992;145:1129–1135. doi: 10.1164/ajrccm/145.5.1129. [DOI] [PubMed] [Google Scholar]
- 22.Tager IB, Hanrahan JP, Tosteson TD, Castile RG, Brown RW, Weiss ST, Speizer FE. Lung function, pre- and post-natal smoke exposure, and wheezing in the first year of life. Am Rev Respir Dis. 1993;147:811–817. doi: 10.1164/ajrccm/147.4.811. [DOI] [PubMed] [Google Scholar]
- 23.Stick SM, Burton PR, Gurrin L, Sly PD, LeSouef PN. Effects of maternal smoking during pregnancy and a family history of asthma on respiratory function in newborn infants. Lancet. 1996;348:1060–1064. doi: 10.1016/s0140-6736(96)04446-7. [DOI] [PubMed] [Google Scholar]
- 24.DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics. 2004;113:1007–1015. [PubMed] [Google Scholar]
- 25.Miller RI, Garfinkel R, Horton M, Camann, Perera FP, Whyatt R, Kinney PL. Polycyclic aromatic hydrocarbons, environmental tobacco smoke, and respiratory symptoms in an inner-city birth cohort. Chest. 2004;126:1071–1078. doi: 10.1378/chest.126.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jedrychowski W, Galas A, Flak E, Jacek R, Penar A, Spengler J, Perera FP. Increased burden of respiratory disease in the first six months of life due to prenatal environmental tobacco smoke: Krakow birth cohort study. Early Child Development and Care. 2007;177:369–381. [Google Scholar]
- 27.Barker DJP. Fetal and infant origins of adult disease. London: BMJ Publishing; 1992. [Google Scholar]
- 28.Stick S. Pediatric origins of adult lung disease. The contribution of airway development to paediatric and adult lung disease. Thorax. 2000;55:587–594. doi: 10.1136/thorax.55.7.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jedrychowski W, Whyatt R, Camann D, Bawle U, Peiki K, Spengler J, Dumyahn T, Perera F. Effect of prenatal PAH exposure on birth outcomes and neurocognitive development among a cohort of Polish mothers and newborns. Study design and preliminary ambient data. Int J Occup Med Environ Hlth. 2003;16:21–29. [PubMed] [Google Scholar]
- 30.Hardin JW, Hilbe JM. Generalized linear models and extensions. 2. Stata Press Publication; Stata Corp LP, Texas: 2007. [Google Scholar]
- 31.STATA software for windows, release 10,.StaCorp, Texas, 2007.
- 32.Kohler U, Kreuter F. Data analysis using STATA. Stata Press Publication; College Station, Texas: 2005. [Google Scholar]
- 33.Holgate S. WHO momograph: Effects of Air Pollution on Children’Health and Develoment. Review of evidence. WHO Regional Office for Europe; 2005. Mechanisms by which air pollution injures the child’s respiratory system; pp. 29–43. [Google Scholar]
- 34.Hislop AA, Wigglesworth JS, Desai R. Alveolar development in the human fetus and infant. Early Human Develop. 1986;13:1–11. doi: 10.1016/0378-3782(86)90092-7. [DOI] [PubMed] [Google Scholar]
- 35.Zeltmer TB, Burri PH. The postnatal development and growth of the human lung. II Morphology. Respiration Physiology. 1986;67:269–282. doi: 10.1016/0034-5687(87)90058-2. [DOI] [PubMed] [Google Scholar]
- 36.Lieberman E, Torday J, Barbieri R, et al. Association of intrauterine cigarette smoke exposure with indices of fetal lung maturation. Obstet Gynecol. 1992;79:564–70. [PubMed] [Google Scholar]
- 37.Dezateux C, Stocks J, Wade AM, Dundas I, Fletcher ME. Airway function at one year: association with premorbid airway function, wheezing and maternal smoking. Thorax. 2001;56:680–686. doi: 10.1136/thorax.56.9.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinkerton KE, Joad JP. The mammalian respiratory system and critical windows of exposure for children’s health. Environ Health Perspect. 2000;108(suppl 3):457–462. doi: 10.1289/ehp.00108s3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jedrychowski W, Bendkowska I, Flak E, Penar A, Jacek R, Kaim I, Spengler JD, Camann D, Perera FP. Estimated risk for altered fetal growth resulting from exposure to fine particles during pregnancy: an epidemiologic prospective cohort study in Poland. Environ Health Perspect. 2004;112:1398–402. doi: 10.1289/ehp.7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lucas A, Fewtrell MS, Cole TJ. Fetal origins of adult disease: the hypothesis revisited. BMJ. 1999;319:245–9. doi: 10.1136/bmj.319.7204.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barker DJP, Godfrey KM, Fall C, Osmond C, Winter PD, Shaheen SO. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ. 1991;303:671–5. doi: 10.1136/bmj.303.6804.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips BCD. Fetal origins of adult disease: epidemiology and mechanisms. J Clin Path. 2000;53:822–828. doi: 10.1136/jcp.53.11.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawlor DA, Ebrahim S, Smith GD. Association of birth weight with adult lung function: findings from the British women’s heart and health study and meta-analysis. Thorax. 2005;60:851–858. doi: 10.1136/thx.2005.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan KN, Noble-Jamieson CM, Elliman AEM, Bryan EM, Silverman M. Lung function in children of low birth weight. Arch Dis Child. 1989;64:1284–93. doi: 10.1136/adc.64.9.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rona RJ, Gulliford MC, Chinn S. Effects of prematurity and intrauterine growth on respiratory health and lung function in childhood. BMJ. 1993;306:817–20. doi: 10.1136/bmj.306.6881.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaheen SO, Sterne JA, Tucker JS, Florey V du C. Birth weight, childhood lower respiratory tract infection, and adult lung function. Thorax. 1998;53:549–53. doi: 10.1136/thx.53.7.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edwards CA, Osman LM, Godden DJ, Campbell DM, Douglas JG. Relationship between birth weight and adult lung function: controlling for maternal factors. Thorax. 2003;58:1061–1065. doi: 10.1136/thorax.58.12.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Erlevyn-Lajeunesse MDS, Hunt LP, Pohunek P, Dobson SJ, Kochnar P, Warner JA, Warner JO. Broncoalveolar lavage MMP-9 and TIM-1 in preschool wheezers and their relationship to persistent wheeze. Pediatr Res. 2008;64:194–199. doi: 10.1203/PDR.0b013e318175dd2d. [DOI] [PubMed] [Google Scholar]
- 49.Lowe LA, Simpson A, Woodcock A, Morris J, Murray C, Custovic A. Wheeze phenotypes and lung function in preschool children. Am J Respir Crit Care Med. 2005;171:231–237. doi: 10.1164/rccm.200406-695OC. [DOI] [PubMed] [Google Scholar]
- 50.Bradley JP, Bacharier LB, Bonfiglio J, Schechtman KB, Strunk R, Storch G, Castro M. Severity of respiratory syncythial virus bronchiololitis is affected by cigarette smoke exposure and atopy. Pediatrics. 2005;115:e7–e14. doi: 10.1542/peds.2004-0059. [DOI] [PubMed] [Google Scholar]
- 51.Spengler JD, Samet JM, McCarthy JF. Chapter 9: Air cleaning-particles. Vol. 28 2001. Indoor Air Quality Handbook; pp. 9.1–9. [Google Scholar]; Chapter 26: Multiple chemical intolerance and indoor air quality. pp. 26.1–26.27. [Google Scholar]; Chapter 70: Risk analysis framework. McGraw-Hill; pp. 70.3–70.38. [Google Scholar]
- 52.Perera FP, Rauh V, Whyatt RM, Tsai WY, Bernert JT, Tu YH, et al. Molecular evidence of an interaction between prenatal environmental exposures and birth outcomes in a multiethnic population. Environ Hlth Perspect. 2004;112:626–630. doi: 10.1289/ehp.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ball TM, Catro-Rodrigez JA, Griffith KA, Holberg CJ, Martinez ED, Wright AL. Siblings day-care attendance, and risk of asthma and wheezing during childhood. NEJM. 2000;343:538–543. doi: 10.1056/NEJM200008243430803. [DOI] [PubMed] [Google Scholar]
- 54.Lemanske RF., Jr The child origins of ASTma (COAST) study. Pediatr Allergy Immunol. 2002;13:38–43. doi: 10.1034/j.1399-3038.13.s.15.8.x. [DOI] [PubMed] [Google Scholar]
- 55.Gurwitz D, Mindorff, Levison H. Increased incidence of bronchial reactivity in children with a history of bronchiolitis. J Pediatr. 1981;98:551–555. doi: 10.1016/s0022-3476(81)80758-5. [DOI] [PubMed] [Google Scholar]
- 56.Trefny P, Stricker T, Baerlocher C, Sennhauser FH. Family history of atpy and clinical course of RSV infection in ambulatory and hospitalized infants. Pediatr Pulmonol. 2000;30:302–306. doi: 10.1002/1099-0496(200010)30:4<302::aid-ppul5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 57.Singh AM, Moore PE, Gern JE, Lemanske RF, Hartert TV. Bronchiolotis to asthma. A review and call for studies of gene-virus interactions in asthma causation. Am J Resp Crit Care med. 2007;175:108–119. doi: 10.1164/rccm.200603-435PP. [DOI] [PubMed] [Google Scholar]