Abstract
The goal was to compare incidental and intentional spatial sequence learning in youth-onset psychosis and ADHD. We tested 8- to 19-year-olds with psychosis or ADHD and healthy controls on a serial reaction time (RT) task and used manual and oculomotor measures to examine learning. Participants were also administered a block in which they were explicitly instructed to learn a sequence. As in our previous studies with healthy adults and children, oculomotor anticipations and RTs showed learning effects similar to those in the manual modality. Results showed intact sequence-specific learning but fewer oculomotor anticipations in both clinical groups during incidental learning. In intentional learning, only the psychosis group showed impairments compared to controls. There were no interactions between age and diagnosis. Thus, the psychosis group showed relatively preserved incidental learning despite impairments in intentional learning. Additionally, both clinical groups showed impairments in the ability to search for, extract, and anticipate regularities (whether the regularities were there or not), but not in the ability to respond to these regularities when they were there.
Keywords: youth-onset psychosis, ADHD, sequence learning, oculomotor learning, anticipations
Schizophrenia and ADHD
There are intriguing areas of overlap between schizophrenia and ADHD. For example, both disorders are associated with impairments in the prefrontal and cingulate cortices, corpus callosum, basal ganglia, and cerebellum (e.g., Ellison-Wright, Glahn, Laird, Thelen, & Bullmore, 2008; Harrison, 1999; Kieling, Goncalves, Tannock, & Castellanos, 2008; Seidman, Valera & Makris, 2005), and dysfunction of the catecholamine systems (e.g., Carlsson, Hansson, Waters & Carlsson, 1997; Pliszka, 2005). Furthermore, both disorders involve deficits in cognitive domains such as attention, strategic thinking, working memory, and inhibition, and in motor control (e.g., Barr, 2001; Karatekin, 2001), suggesting that disruptions in these cognitive domains may involve overlapping circuits.
Yet, there have been relatively few direct comparisons between psychosis and ADHD. Almost all of these comparisons have involved youth-onset schizophrenia or “multidimensionally impaired” (MDI) children with psychotic symptoms (McKenna, Gordon, Lenane, Kaysen, Fahey, & Rapaport, 1994). Youth-onset schizophrenia is a rare, severe and more genetically loaded form of the disorder that does not differ qualitatively from the adult-onset form on most of the dimensions examined (e.g., Asarnow et al., 2002; Nicolson et al., 2003; Vourdas, Pipe, Corrigall, & Frangou, 2003). In the current study, we compared children and adolescents with psychosis or ADHD on skill learning, a cognitive process that affects many domains of cognitive and social functioning and appears to be impaired in both disorders. To our knowledge, there have not yet been any studies directly comparing either incidental or intentional skill learning between these disorders.
Sequence Learning
We examined incidental and intentional skill learning on the Serial Reaction Time (SRT) task (Nissen & Bullemer, 1987). On this task, series of stimuli are displayed briefly in one of several (typically 3 or 4) locations arranged horizontally on a computer screen. Participants respond by pressing keys corresponding to the locations. Although participants are not informed, the stimulus locations sometimes follow a sequence—typically 10- or 12-items in length—repeated across blocks. Learning is inferred if manual response times (RTs) (a) decrease across repeated exposure to the sequences (initial learning), and (b) increase for intervening random stimuli (interference). The initial decrease in RTs reflects a combination of general visual-motor learning and sequence-specific learning. Therefore, the interference effects on random stimuli provide a clearer index of sequence-specific learning (Knopman & Nissen, 1987). Because participants usually report little sequence-specific knowledge on direct recall or recognition measures, the SRT task has been interpreted as a form of learning without explicit awareness.
The neural substrates of incidental sequence learning on the SRT task include areas believed to be impaired in both schizophrenia and ADHD. Depending on the nature of the task and the stage of learning, these substrates include the prefrontal cortex; the premotor, supplementary motor and primary motor cortices; parietal, occipital and medial temporal cortices; the anterior cingulate; the basal ganglia; and the cerebellum (reviewed in Ashe, Lungu, Basford, & Lu, 2006; Tanji, 2001).
Behavioral studies show a distinction between the cognitive architectures of incidental and intentional learning on the SRT task (e.g., Gebauer & Mackintosh, 2007; Jiménez, Vaquero, & Lupiáñez, 2006; Unsworth & Engle, 2005), although there are dissenting views on this distinction (Wilkinson & Shanks, 2004). Electrophysiological studies indicate that the amplitude of the P300 and N200 components are larger in intentional than in incidental learning (Rüsseler, Henninghausen, Münte, & Rösler, 2003). Functional neuroimaging studies reveal overlap between the neural structures supporting incidental versus intentional learning; however, there are some distinctions (e.g., Aizenstein et al., 2004; Ashe et al., 2006; Fletcher et al., 2005; Willingham, Salidis, & Gabrieli, 2002). For example, direct comparisons of the two forms of learning consistently show greater activity in the intentional compared to the incidental condition in the prefrontal cortex, with some studies also showing greater activity in the parietal cortices, the cerebellum, the anterior and middle cingulate regions, the caudate, the brainstem, and the fusiform gyrus (e.g., Aizenstein et al., 2004; Eliassen, Souza, & Sanes, 2001; Willingham et al., 2002).
Sequence Learning in Schizophrenia
There have been a number of studies that specifically examined performance on variants of the SRT task in schizophrenia. All of these studies were conducted on adults with the disorder compared to healthy controls, and all studies measured perceptual learning through manual responses. The results have been conflicting. Six studies have found no impairment in sequence learning in individuals with schizophrenia (Foerde et al., 2008; Perry, Light, Davis, & Braff, 2000; Reiss et al., 2006; Zedkova, Woodward, Harding, Tibbo, & Purdon, 2006) or schizotypal traits (Ferraro & Okerlund, 1995; Pedersen & Rist, 2001). In contrast, six studies have found either diminished (Green, Kern, Williams, McGurk, & Kee, 1997; Marvel, Schwartz, Howard, & Howard, 2005; Pedersen et al., 2008; Schwartz, Howard, Howard, Hovaguimian, 2003) or no sequence learning at all in schizophrenia (Exner, Weniger, Schmidt-Samoa, & Irle, 2006b; Kumari et al., 2002). The results of one study pointed to two subgroups in schizophrenia, one that shows normal sequence learning but may rely on explicit learning strategies to perform the task, and another that fails to show sequence-specific learning (Marvel et al., 2007). Finally, in a comparison of six adults with schizophrenia with healthy controls, Dominey and Georgieff (1997) found that individuals with schizophrenia could learn the surface structure of a 12-step sequence, but not the abstract structure (i.e., the relationships between the steps), although they were explicitly informed in advance about the existence of an abstract structure.
One factor that might explain the discrepancies in the behavioral results across studies is the stage of illness at which participants are tested: impairments may be greater during the acute stage than at later stages (Exner, Boucsein, Degner, & Irle, 2006a). If the participants are currently on typical neuroleptics, which block dopamine receptors to a greater extent on average than do atypical neuroleptics, sequence-learning deficits are also more likely to be observed (Green et al., 1997; Kumari et al., 1997, 2002; Pedersen et al., 2008; Reiss et al., 2006; Stevens et al., 2002). Nevertheless, treatment with typical neuroleptics does not completely account for SRT impairments in schizophrenia.
In contrast, there is clear evidence that individuals with schizophrenia are impaired on intentional learning and working memory tasks (e.g., reviewed in Reichenberg & Harvey, 2007), and that these memory deficits extend to youth-onset schizophrenia (e.g., Frangou, Hadjulis, & Vourdas, 2008; Rhinewine et al., 2005; Roofeh et al., 2006; White, Ho, Ward, O'Leary, & Andreasen, 2006). In the only SRT study in which incidental and intentional sequence learning were directly compared in schizophrenia (Pedersen et al., 2008), the schizophrenia group was impaired on both the incidental and intentional tasks. The failure of the schizophrenia sample in the study by Dominey and Georgieff (1997) to learn the surface, but not the abstract, structure of sequences, was also interpreted as evidence for impairments in explicit learning in the presence of intact implicit processing.
Sequence Learning in ADHD
Although there are no published studies of the SRT task in children or adults with ADHD, there is reason to believe that sequence learning may be impaired in this disorder. For example, in a functional MRI study of self-paced sequential finger tapping in children with ADHD and healthy controls, Mostofsky and colleagues (2006) found no group differences in tapping speed but decreased activation in the primary motor and parietal cortices in ADHD. The researchers concluded that the ADHD group was “less able to recruit posterior parietal systems important for motor imagery necessary to guide the correct sequence of finger movements”(p. 55). In another study using behavioral and electrophysiological measures, children with ADHD were found to be less sensitive to violations of regularity in 2- to 3- stimulus sequences than controls (Klorman et al., 2002). According to retrospective maternal reports, children with ADHD also have trouble learning motor skills, such as closing their buttons and zippers, tying their shoes, and printing letters (Karatekin, Markiewicz, & Siegel, 2003).
There is a stronger base of evidence to support the hypothesis that participants with ADHD might be impaired in intentional learning. Prior studies report impairments in ADHD on verbal and spatial working memory tasks (reviewed in Frazier, Demaree, & Youngstrom, 2004; Harvey et al., 2004; Martinussen, Hayden, Hogg-Johnson, & Tannock, 2005). In addition, children with ADHD have difficulty performing a simple visual RT task when they also have to perform a digit span task or even simply count aloud from 1 to 9 (Karatekin, 2004).
Finally, there is robust evidence of impairments in ADHD in the neural substrates supporting both incidental and intentional sequence learning, including the prefrontal cortex, the basal ganglia, and the cerebellum (e.g., Valera, Faraone, Murray, & Seidman, 2007).
Direct Comparisons of Learning and Memory between Schizophrenia and ADHD
There have been only a few direct comparisons of learning and memory between schizophrenia and ADHD. All of these studies have involved intentional learning tasks. On the Digit Span, Caplan and colleagues (2001) found better performance in ADHD than in childhood-onset schizophrenia. Although results were in the same direction in Karatekin and Asarnow (1998), McCarthy et al. (2005) and Øie et al. (1999), differences between the schizophrenia and ADHD groups did not reach significance. Øie and colleagues (1999) further found that adolescents with schizophrenia were more impaired than adolescents with ADHD on a visual memory task, whereas the groups did not differ on a verbal list-learning task.
Precursors of the Current Study
Before examining the clinical groups, we conducted two studies to delineate normative patterns of performance in healthy young adults and children on the SRT task. In addition, anticipating that the psychosis and ADHD groups might have difficulty with musculoskeletal responses, we recorded eye movements to the stimuli during the task. We included an intentional learning condition to compare performance on incidental versus intentional forms of sequence learning. In the first study with adults (Marcus, Karatekin, & Markiewicz, 2006), both manual and oculomotor RTs decreased with increased exposure to the sequence and increased on the pseudo-random blocks. In addition, participants spontaneously made anticipatory eye movements to the correct box on a third to half of all the trials, and the frequency of these eye movements showed the same learning and interference effects as manual and oculomotor RTs. We interpreted the results as indicating that participants overtly shifted visual-spatial attention to likely target locations prior to stimulus onset. Following intentional learning instructions, these shifts probably reflected, at least to some extent, conscious hypothesis-testing strategies. On the sequence and pseudo-random blocks, however, they seemed to reflect an obligatory search for spatial regularities in the environment, whether these regularities existed or not. Thus, participants appeared to constantly anticipate stimulus locations from the beginning, and learning in the sequence blocks consisted largely of improvements in the speed and accuracy of these oculomotor anticipations.
In a second study (Karatekin, Marcus, & White, 2007), we examined age-related changes on the task. Participants, including those who took part in the current study, were divided into four age groups. Results indicated that the search for regularities and the ability to rapidly learn a sequence under incidental conditions are mature by ages 8-10. In contrast, the ability to learn a sequence intentionally, which requires cognitive resources and strategies, appeared to continue to develop through adolescence.
Goals and Predictions of the Current Study
In the current study, we administered the same tasks to children and adolescents with psychosis or ADHD. Participants in the psychosis group were all clinically stable outpatients, and only one was on a typical neuroleptic. The first question concerned indices of sequence-specific learning. On the one hand, neural substrates of incidental sequence learning overlap with regions implicated in both disorders. Thus, it would be reasonable to predict impairments in both disorders. On the other hand, approximately half the SRT studies in adults with schizophrenia, including studies of clinically stable patients who were not predominantly on typical neuroleptics, have found no deficits in incidental learning. Thus, we did not make predictions regarding sequence-specific learning in either group. However, in none of the SRT studies in schizophrenia in which initial learning was analyzed did the researchers find any impairments in the schizophrenia-spectrum groups compared to controls. Therefore, we predicted no impairments in initial learning in at least the psychosis group. Second, we wished to determine whether there would be evidence of sequence learning in both the manual and oculomotor modalities. Third, we examined whether the clinical groups would show any impairments in anticipatory eye movements, for either general learning or sequence-specific learning. Based on previous evidence of impairments in anticipatory processing in both schizophrenia (Exner et al., 2006b ; Posada, Franck, Georgieff, & Jeannerod, 2001 and ADHD (Hurks et al., 2005; Perchet, Revol, Fourneret, Mauguière, & Garcia-Larrea, 2001), we expected at least some impairment on this measure in both clinical groups. Fourth, given the studies mentioned earlier regarding memory deficits in schizophrenia and ADHD, we predicted that both clinical groups would show impairments in the intentional learning condition. Finally, we examined whether there would be any interactions between diagnosis and age-related changes in either incidental or intentional learning.
Method
Participants
Table 1 lists participants' demographic and clinical characteristics. Potential participants were excluded if they were not fluent in English or were color blind, if they had been premature by more than four weeks, had a history of significant neurological conditions, or an IQ of lower than 70. Potential participants were excluded from the ADHD and control groups if they had been adopted, or had first-degree biological relatives with schizophrenia. Potential participants were excluded from the ADHD group if they had been diagnosed with or suspected of having a pervasive developmental disorder, or if they had never met criteria for the Combined subtype. We included two adolescents who met criteria for the Inattentive subtype, but who had previously met criteria for the Combined subtype and scored above 60 on the Attention Problems scale of the Achenbach Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001). Potential controls were excluded if they had ever taken psychoactive medications, been diagnosed with a major psychiatric disorder or met criteria for a current disorder, had attention problems for which they had sought help, or had first-degree biological relatives with ADHD.
Table 1.
Demographic and Clinical Characteristics of the Participants
| Control | Psychosis | ADHD | Diagnosis Effects | Post-Hoc Tests | |
|---|---|---|---|---|---|
| N | 58 | 29 | 33 | ||
| M:F, % | 45:55 | 52:48 | 79:21 | X22 = 10.1, p = .006, φ = .29 | ADHD ≠ (Psychosis, C) |
| Age in years (SD) | 12.8 (2.8) | 14.9 (3.4) | 12.8 (2.7) | F2,117 = 5.46, p = .005, η2p = .09 | Psychosis > (ADHD = C) |
| Range | 8.8-20.1 | 8.4-20.1 | 8.8-18.9 | ||
| Parental socioeconomic statusa | 53 (10) | 42 (14) | 50 (9) | F2,108 = 9.23, p < .001 η2p = .15 | Psychosis < (ADHD = C) |
| Estimated IQb (SD) | 114 (13) | 97 (16) | 108 (14) | F2,108 = 12.69, p < .001, η2p = .19 | Psychosis < (ADHD = C) |
| Range | 77-144 | 74-132 | 85-141 | ||
| WIATc | |||||
| Reading | 110 (10) | 97 (16) | 103 (13) | F2,112 = 11.49, p < .001, η2p> = .17 | (Psychosis = ADHD) < C |
| Spelling | 109 (11) | 98 (15) | 98 (13) | F2,112 = 10.57, p < .001, η2p = .16 | (Psychosis = ADHD) < C |
| Handedness | 6.4 (1.7) | 5.5 (2.5) | 6.2 (1.8) | ns | |
| SANS/SAPSd,e | |||||
| Negative symptoms | 2.8 (1.0) | ||||
| Positive symptoms | 1.8 (1.0) | ||||
| Psychotic symptoms | 2.5 (1.2) | ||||
| Medications, % | |||||
| Antipsychotic | 17 (59%) | 0 | |||
| Psychostimulant | 4 (14%) | 20 (61%) | |||
| Antidepressant | 9 (31%) | 1 (3%) | |||
| Mood stabilizer | 8 (28%) | 0 | |||
| Alpha-adrenergic | 2 (7%) | 2 (6%) | |||
| Anti-histamine | 3 (10%) | 0 | |||
| Benzodiazepine | 3 (10%) | 0 | |||
| Ethnicity | X82 = 18.3,p = .019,ϕ = .39 | ||||
| Caucasian | 50 (86%) | 17 (59%) | 28 (85%) | ||
| African-American | 1 (2 %) | 3 (10%) | 4 (12%) | ||
| Asian | 1 (2 %) | 3 (10%) | 0 | ||
| Hispanic | 1 (3%) | 0 | |||
| Mixed/Other | 6 (10%) | 5 (17%) | 1 (3%) |
Notes. C = Control. ns = not significant. SANS/SAPS = Scales for the Assessment of Negative/Positive Symptoms. WIAT = Wechsler Individual Achievement Test. Medications taken by fewer than 5% of participants are not reported. Handedness was measured by asking children to pick up seven objects with their preferred hand; 1 point was awarded for each object picked up with the right hand.
IQ was estimated from the Vocabulary and Block Design subtests from the Wechsler Intelligence Scale, 3rd ed. (WISC-III; Wechsler, 1991), the WISC-IV (Wechsler, 2003), or the Wechsler Adult Intelligence Scale, 3rd ed. (Wechsler, 1997).
The values for the SANS and SAPS were calculated as the mean of the global measures within each instrument (O'Leary, Flaum, Kesler, Flashman, Arndt, & Andreasen, 2000).
Diagnoses are listed in Table 2. In the psychosis group, average age of onset of psychotic symptoms was 12.9 years (SD = 3.1, range = 7-17). We included participants with Psychosis NOS only if they had first- or second-degree biological relatives with schizophrenia. Additional details of the recruitment and diagnostic procedures can be found in Karatekin, White, & Bingham (2008).
Table 2.
Frequency (and Percentage) of Major Lifetime Diagnoses in Each Diagnostic Group
| Control | Psychosis | ADHD | |
|---|---|---|---|
| Schizophrenia | 16 (55%) | ||
| Schizophreniform | 2 (7%) | ||
| Schizoaffective | 6 (21%) | ||
| Psychosis Not Otherwise Specified | 5 (17%) | ||
| ADHD | |||
| Combined | 31 (94%) | ||
| Inattentive (with history of Combined) | 2 (6%) | ||
| Mood Disorders | 2 (3%) | 4 (14%) | 7 (21%) |
| Anxiety Disorders | 2 (3%) | 3 (10%) | 4 (12%) |
| Oppositional Defiant Disorder/Conduct Disorder | 0 | 6 (21%) | 12 (36%) |
| Substance Use/Abuse | 0 | 2 (7%) | 2 (6%) |
| Tic disorder | 0 | 0 | 2 (6%) |
Note. All diagnoses in the control group refer to past diagnoses.
Participants were excluded from the ADHD group if they were taking psychoactive medications other than psychostimulants. Participants were also asked to refrain from taking psychostimulants for at least 24 hours prior to cognitive testing, and they were excluded if they or their parents were not willing to discontinue medications for this period of time. However, on the day of testing, one participant in the ADHD and two in the psychosis group reported having taken a psychostimulant despite our instructions. In addition, two participants with ADHD reported having taken antidepressants or alpha-adrenergic medications within the 48 hours prior to testing. We decided to include these participants to avoid reducing the sample size1. In the psychosis group, only one participant on antipsychotic medications was taking a typical neuroleptic (haloperidol). The other participants were all on atypical neuroleptics.
One 14-year-old control boy was excluded because he developed an unusually explicit and rapid awareness of the sequence on the implicit blocks and performed far above the level of the other participants on the Recognition (17/20) and Prediction (17/20) tasks. Three boys with ADHD (aged 10, 12, and 13) were excluded due to behavior problems during testing.
Procedure
The current study was part of a larger study of youth-onset psychosis and ADHD. As part of this larger study, most participants were administered other tasks over two sessions, and some underwent neuroimaging (11 control and 9 ADHD participants were included in Karatekin, 2006; 11 control and 9 psychosis participants were in White et al., 2007; 56 controls were in Karatekin et al., 2007; 33 psychosis, 14 ADHD, and 14 control participants were in Karatekin et al., 2008). The task described in the current paper was administered on the first day of testing. Most of the participants were also administered divided attention (Karatekin et al., 2008) and inhibition tasks on the first day. Each of these tasks took 30-40 min. The divided attention task was always presented first, and the SRT and the inhibition tasks were presented in counterbalanced order.
As shown in Figure 1(b), the SRT task consisted of 5 blocks of incidental learning trials, followed by three direct measurements of sequence awareness (verbal response, recognition, and prediction). Finally, participants completed one block of trials in an intentional learning condition in which they were explicitly directed to learn a sequence. The apparatus, experimental procedure, and analysis of eye movements were identical to those in Karatekin et al. (2007). Please refer to this manuscript for additional details on the procedure.
Figure 1.
(a) Depiction of events in a single trial. (b) Summary of the experimental tasks. All participants were administered the tasks in the order listed in the figure.
Incidental Learning
Four boxes were displayed horizontally in the center of the screen [(see Figure 1(a)]. The stimulus, a colored image of a butterfly, was displayed in the center of one of the four boxes for 1000 ms followed by a 500 ms inter-trial interval. Participants were instructed to look at the butterfly and to press one of four buttons corresponding to the boxes as quickly as they could without making mistakes.
Each block of trials consisted of 100 presentations of the butterfly. In the sequence blocks (2nd, 3rd, and 5th blocks), a 10-item sequence of locations was presented 10 times, with no gaps between sequences. The order of the sequence was 3-2-4-3-1-4-2-3-4-1 (based on Beldarrain, Grafman, Pascual-Leone, & Garcia-Monco, 1999), where the numbers correspond to the boxes from left to right in which the butterfly appeared. In the pseudo-random blocks (1st and 4th blocks), the stimuli were presented in a pseudorandom order that was constructed so that the overall frequency of locations matched that of the sequence trials. In addition, for every 20 trials (1-20, 21-40, etc.) of the pseudorandom blocks, the frequency of 1st order transitions matched that of the sequence block. The same 100-trial order was used for the pseudorandom blocks. The first block was preceded by 10 practice trials. Participants were not informed whether a sequence was present.
Sequence Awareness
After the 5th block, participants were asked: “Did you notice anything about the order in which the butterflies appeared?” If they responded positively, they were asked to describe what they had noticed. Regardless of their response, they were informed that the stimuli had sometimes appeared in a repeating sequence. They were then shown series of four stimuli and asked to judge if these were part of the sequence they had seen (Recognition). Twenty series were shown, 10 of which were from the sequence. In the next task (Prediction), a stimulus was displayed in one of the boxes and participants verbally predicted the box in which they thought the next stimulus would appear. They were instructed to refer to the boxes as 1, 2, 3, or 4 (from left to right). Twenty trials were administered, consisting of two repetitions of the 10-item sequence, with no gaps between the repetitions.
Intentional Sequence Learning
After the awareness tasks, participants were administered a final block of 100 trials (Block 6). They were informed that this block would contain a new repeating sequence, and that the stimulus would be a picture of a crab. They were instructed to look at the stimulus and to press the corresponding button as quickly as they could but without making mistakes. They were instructed to try to learn the sequence, and were informed that they would be asked to describe it at the end of the block. They were again asked to label the boxes as 1 through 4. At the conclusion of this block, participants were asked to describe the pattern and what they did to try to learn it. The new 10-item sequence (2-1-4-3-1-3-2-1-2-4) was repeated 10 times. Thus, it was matched to the sequence in the incidental condition in that it also contained a 10-step ambiguous deterministic sequence.
Dependent Variables
Manual Responses
A response was counted as accurate if the correct button was pressed, independent of eye movements. Mean manual RT for correct trials in each block was calculated. Anticipatory responses were defined as responses initiated prior to, or within 100 ms after, stimulus onset.
Oculomotor Responses
To measure oculomotor responding, we calculated the time at which the participant's gaze was directed at the correct target location. Oculomotor anticipations were defined as oculomotor responses with a negative RT. We added the stipulation that an anticipation could not occur more than 1350 ms prior to stimulus onset, as these might be confused with responses to the previous trial. Thus, oculomotor RTs reflect the speed with which participants correctly anticipated/responded to the stimuli, and oculomotor anticipations reflect the proportion of oculomotor RTs that occurred prior to stimulus onset. Mean oculomotor RTs and proportion of oculomotor anticipations (i.e., number of trials with anticipations divided by the number of trials on which a valid oculomotor response to the correct box was recorded) for each block were used as dependent variables.
Measures of Incidental Sequence Learning
Two simultaneous planned comparisons were used to evaluate expected patterns of learning on manual and oculomotor RTs and oculomotor anticipations. The first, which assesses initial learning, was the difference between Blocks 1 (random) and 3 (sequence); this difference reflects motor facilitation and learning after the first 20 repetitions of the sequence. The second comparison involved the difference between Block 4 (random) and the average of Blocks 3 and 5 (sequence). This worsening of performance on Block 4 relative to the adjacent blocks measures the degree of interference due to the pseudorandom trials.
Sequence Awareness Tasks
Responses to the initial verbal query were coded dichotomously based on whether participants spontaneously reported any awareness of a pattern. The score for the recognition task was the number of correct judgments out of 20 trials. The scores for the prediction task were the longest string of responses matching any part of the sequence, including predictions across the boundary between two presentations of the sequence, and the number of correct responses out of 20 trials.
Intentional Sequence Learning (Block 6)
Recording and calculation of manual and oculomotor measures are described above. The learning score for the verbal report after Block 6 was the length of the longest string of recalled responses matching any part of the 10-item sequence (including responses that crossed the boundary between repetitions of the sequence).
Statistical Analysis
Statistical analyses were conducted with SPSS 14.0 and MacAnova 5.06 (an open-source crossplatform statistics program for Windows, Macintosh and Linux at http://www.stat.umn.edu/macanova/).
Transformations of responses to achieve normality and constant variance were sought among the Box-Cox family of distributions. These are equivalent to power transformations y → yP, except that y → log(y) when p = 1. Power was selected to be close to the maximum likelihood estimate of p using a graphical procedure (Box & Cox, 1964). With this procedure, manual RTs and anticholinergic equivalents of antipsychotic medications were log transformed, and chlorpromazine equivalents of antipsychotics were transformed by taking their square root. Oculomotor RTs were transformed with the formula log10(250-RT). Accuracy of manual responses was transformed with the formula -ln (1-proportion correct); when accuracy was perfect, it was replaced with .995. This transformation yielded constant variance across groups and minimized outliers, while preserving the order of low and high values.
Demographic variables were analyzed with univariate ANOVAs, and significant findings were followed up with Tukey tests. Categorical variables were analyzed with X2 tests. T tests were used to compare performance to chance level on the Recognition task. Correlations were calculated using Pearson correlation coefficients.
Repeated-measures Type III ANCOVAs, with age as the covariate, were used to examine effects of subject variables. The covariate was modified by subtracting the mean age of all participants from each participant's age. Each ANCOVA tested linear and quadratic trends for age. Models were selected by backward elimination of non-significant terms involving age, starting with the highest order interactions. When the quadratic trend on age was significant, the linear trend was not reported. IQ was not used as a covariate because controlling for it would have reduced variance due to the disorders. In addition, there does not appear to be a relation between IQ and performance on the SRT task and other tests of implicit learning (Gebauer & Mackintosh, 2007). Huynh-Feldt adjustments to dfs were used to compute F-statistic p values, and Huynh-Feldt-adjusted dfs were reported where applicable.
For manual RTs and oculomotor RTs and anticipations in Blocks 1-5, we used planned comparisons to compare consecutive pairs of blocks and Blocks 3 and 5. Thus, ANCOVA results on block effects reflect these planned comparisons and are not omnibus tests on all blocks. Simultaneous planned comparisons were used to examine initial learning from Block 1 to Block 3 and interference on Block 4. Post-hoc analyses of ANCOVAs were conducted using custom macros for MacAnova. Main effects and interactions were generally not followed up when there were higher-order interactions involving the same variables. Tests of between- and within-subject contrasts and slopes were based on appropriate t statistics. To protect against multiple testing, p values were Bonferroni corrected, that is, multiplied by the number of simultaneous tests. When the contrast involved a between-subjects contrast, Tukey-Kramer p values based on the Studentized range were computed and then, where appropriate, Bonferroni corrected by the number of intra-subject contrasts being considered simultaneously. Because the planned comparisons involving initial learning and interference were analyzed simultaneously, p values were Bonferroni-corrected. Reported p values are Bonferroni corrected.
Partial η2 (η 2p) was used to calculate effect sizes for ANCOVAs, and φ for X2 tests. To calculate the effect sizes of pairwise group differences presented in Table 3, we used a measure similar to Cohen's d but that took into account the age differences among groups. Specifically, we divided the difference of the group means (age adjusted as appropriate) by the square root of the MSe term for the between-subjects analysis section of the ANCOVA. In cases where there was an interaction between group and age, the value reflects the effect size at the average age for the whole sample. When the two groups are similar in age, as in the ADHD-control comparisons, our measure of effect size and Cohen's d yield similar results.
Table 3.
Performance on Sequence Awareness Tasks and on Verbal Report in Intentional Learning
| Effect Size of Pairwise Group Differences | ||||||
|---|---|---|---|---|---|---|
| Control | Psychosis | ADHD | C vs. Psychosis | C vs. ADHD | Psychosis vs. ADHD | |
| Verbal Report of Awareness | 62% | 64% | 73% | |||
| Blocks 1-5: Longest stringa | 2.7 (0.9) | 3.7 (0.8) | 3.4 (0.9) | 1.06 | 0.55 | 0.51 |
| Recognition: Total correctb | 10.8 (2.1) | 11.3 (1.9) | 11.0 (1.5) | 0.30 | 0.12 | 0.18 |
| Prediction: Total correctb | 9.9 (2.8) | 8.4 (2.7) | 10.4 (2.7) | 0.54 | 0.19 | 0.73 |
| Prediction: Longest stringa | 3.7 (1.7) | 3.1 (1.2) | 3.9 (1.7) | 0.39 | 0.09 | 0.47 |
| Block 6: Longest stringa | 5.1 (2.5) | 3.3 (2.2) | 4.3 (2.8) | 0.90 | 0.30 | 0.60 |
= Out of 10
= Out of 20.
Notes. Numbers in parentheses refer to standard deviations. C = Control. Effect size was calculated by dividing the difference between age-adjusted group means by the square root of the MSe term for the between-subjects analysis section of the ANCOVA. In cases where there was an interaction between group and age, the value reflects the size of the effect at the average age for the whole sample. When the two groups are similar in age, as in the ADHD-control comparisons, our measure of effect size and Cohen's d yield similar results.
Findings are reported as significant if α ≤ .050.
Result
Incidental Learning (Blocks 1-5)
Accuracy of manual responses
Accuracy of manual responses, averaged across blocks, ranged from 90% to 97% across groups. A 3 (diagnosis) x 5 (block) repeated-measures ANCOVA yielded a block effect, F (3.9, 457.3) = 5.35, p < .001, η2p = .04. Accuracy was lower in Block 4 than in Block 5. There was a large diagnosis effect, F (2, 116) = 17.92, p < .001, η2p = .24; controls had more accurate trials than both clinical groups, who did not differ from each other. We also found a linear increase in accuracy with age, F (1, 116) = 33.36, p < .001, η2p = .22.. ANCOVAs on initial learning and interference yielded only an interference effect, F (1, 117) = 18.55, p < .001, η2p = .14.
Manual RTs
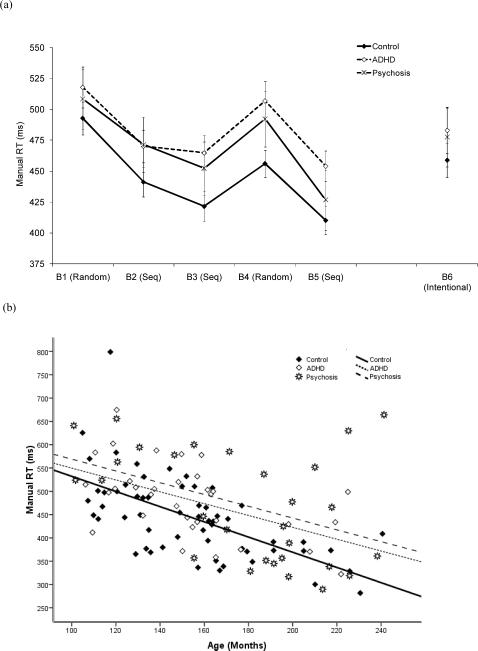
As can be seen in Figure 2(a), all groups showed a learning effect from Block 1 to 3 and an interference effect on Block 4. The ANCOVA showed a linear decrease in RTs with age, F (1, 116) = 49.66, p < .001, η2p = .30. There was a block effect, F (3.5, 402.6) = 81.11, p < .001, η2p = .41: each block differed significantly from the previous one, and RTs were shorter in Block 5 than in Block 3.
Figure 2.
(a) Mean manual RTs (ms) as a function of diagnosis and block. (b) Manual RTs (ms), averaged across Blocks 1-5, as a function of age and diagnosis. In all figures, the error bars are 95% confidence intervals, and the lines represent the quadratic trend with age in each diagnostic group. Seq = Sequence.
There was a modest diagnosis effect, F (2, 116) = 5.16, p = .007, η2p = .08, and a block by diagnosis interaction, F (6.9, 402.6) = 2.07, p = .046, η2p = .03. Overall RTs were shorter in the control than in the psychosis group, and RTs on Block 4 were shorter in the control than in the psychosis group.. The ADHD-control difference approached significance, p = .065.
There was a large decrease in RTs from Block 1 to 3, F (1, 116) = 147.86, p < .001, η2p = .56, and a large interference effect on Block 4, F (1, 116) = 161.93, p < .001, η2p = .58. In addition, the magnitude of the improvement from Block 1 to 3 increased linearly with age, F (1, 116) = 7.84, p = .020, η2p = .06. Diagnosis did not interact with either initial learning or interference.
Frequency of manual anticipations
Manual anticipations were rare, with average frequency ranging between 0 to 2 across blocks and diagnostic groups. Therefore, they were not analyzed further.
Oculomotor RTs
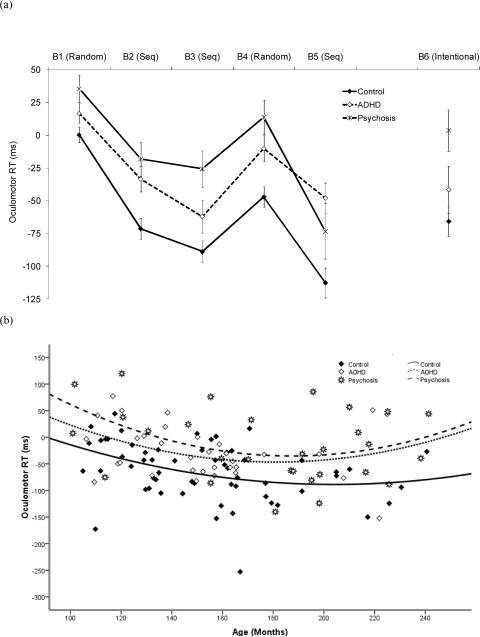
As shown in Figure 3(a), oculomotor RTs paralleled the pattern of manual RTs, showing both learning and interference effects in all age groups. Figure 3(b) displays oculomotor RTs as a function of age in all groups. There was a quadratic trend for age, F (1, 115) = 7.34, p = .008, η2p = .06, with RTs first decreasing, then increasing slightly (perhaps due to the psychosis participants), a large diagnosis effect, F (2, 115) = 13.15, p < .001, η2p = .19, a block effect, F (3.4, 390.0) = 51.05, p < .001, η2p = .31, and a diagnosis by block interaction, F (6.8, 390.0) = 2.56, p = .015, η2p =.04. Overall RTs were longer in the clinical groups than in the control group, and RTs in the psychosis group were longer than those in controls on Blocks 1-4. RTs in the ADHD group were longer than those in controls only on Block 5.
Figure 3.
(a) Mean oculomotor RTs (ms) as a function of diagnosis and block. (b) Mean oculomotor RTs, (ms) averaged across Blocks 1-5, as a function of age and diagnosis. Seq = Sequence.
As with manual RTs, there was both a large initial learning effect from Block 1 to 3, F (1, 117) = 146.85, p < .001, η2p = .56, and a large interference effect on Block 4, F (1, 117) = 82.50, p < .001, η2p = .41. Again, there was no evidence of an interaction with either age or diagnosis.
Frequency of oculomotor anticipations
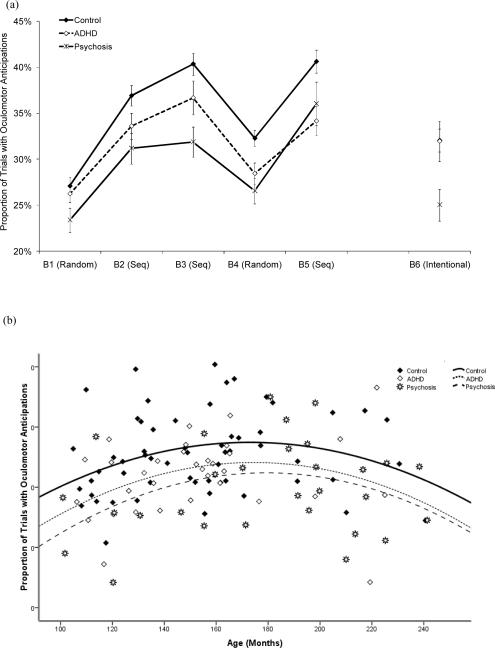
As shown in Figure 4(a), the proportion of trials with anticipations (oculomotor RT < 0 ms) followed similar patterns across blocks as RTs, increasing with repeated exposure to the sequence, and decreasing on the pseudo-random block. The ANCOVA showed a quadratic trend for age, F (1, 115) = 10.86, p < .001, η2p = .09, first increasing and then decreasing slightly (see Figure 4b). There was a diagnosis effect, F (2, 115) = 7.24, p = .001, η2p = .11, a block effect, F (3.7, 428.8) = 48.00, p < .001, η2p = .29, and an interaction between diagnosis and block, F (7.4, 428.8) = 2.23, p = .028, η2p =.04. Overall, the controls made more anticipatory eye movements than both clinical groups, who did not differ. Post-hoc tests of the diagnosis by block interaction indicated that the psychosis group made fewer anticipations than controls on Blocks 3 and 4..
Figure 4.
(a) Proportion of trials with oculomotor anticipations as a function of diagnosis and block. (b) Proportion of trials with oculomotor anticipations, averaged across Blocks 1-5, as a function of age and diagnosis. Seq = Sequence.
There was a large increase in anticipations from Block 1 to 3, F (1, 117) = 159.78, p < .001, η2p = .58, and a substantial interference effect on Block 4, F (1, 117) = 117.59, p < .001, η2p = .50. Neither effect interacted with diagnosis.
Sequence Awareness
Verbal Response
When asked whether they noticed anything about the order of the stimuli, most of the participants indicated that there was some regularity (Table 3). A χ2 test showed that the proportion of participants indicating some awareness of a pattern did not differ across groups. When asked to elaborate on what they noticed, most participants could not describe the sequence extensively. The longest string described correctly ranged from 2 to 6 across groups; median values were 3 in the control and ADHD groups and 4 in the psychosis group. Thus, although many participants developed some awareness that the stimuli followed a pattern, they did not gain explicit knowledge of the elements of that sequence beyond 3-4 steps. A one-way ANCOVA on the longest string recalled showed a diagnosis effect, F (2, 53) = 4.98, p = .010, η2p =.16, with the psychosis group recalling more than the control group.
Recognition Task
A one-way ANCOVA on correct responses did not yield any significant results. Performance was better than chance (10/20) in the control, t (57) = 2.82, p = .007, ADHD, t (32) = 3.73, p = .001, and psychosis groups, t (28) = 3.86, p = .001. As shown in Table 3, however, scores averaged around 11/20 in all groups and effect sizes of group differences were small to moderate.
Prediction Task
Average scores ranged from approximately 8 to 10 out of 20 on this test (Table 3). Assuming that participants noticed that the stimulus never appeared in the same location twice in a row, performance at chance level would be 6.7/20. As with Recognition, t tests showed that all groups performed better than chance, all ps ≤ .001. A one-way ANCOVA on number of correct responses showed a modest diagnosis effect, F (2, 117) = 4.42, p = .014, η2p = .07. Performance was worse in the psychosis than in the ADHD group and marginally worse than in the control group, p = .051.. The average length of the longest string of correct responses ranged from 3.1 to 3.9 across groups. The ANCOVA on this measure did not yield any significant results.
Role of Explicit Awareness in Incidental Sequence Learning
To make sure that results were not due to explicit awareness, we repeated the analyses on manual and oculomotor RTs and oculomotor anticipations after excluding all participants who scored 4 or above on the initial verbal probe (3 control, 5 ADHD and 7 psychosis participants). Contrary to what we found earlier, the diagnosis by block interaction did not reach significance for any of the measures. No other result involving diagnosis differed from what we reported above. .
Intentional Learning (Block 6)
On Block 6, participants were instructed to learn the sequence. On the verbal report of the sequence (see Table 3), accuracy increased linearly with age, F (1, 116) = 8.45, p = .004, η2p = .07. There was also a fairly large diagnosis effect, F (2, 116) = 7.25, p = .001, η2p =.11: the psychosis group recalled less of the sequence than the control group.
A one-way ANCOVA on accuracy of manual responses showed a linear increase with age, F (1, 116) = 23.42, p < .001, η2p = .17, and a large diagnosis effect, F (2, 116) = 14.89, p < .001, η2p = .20. The controls were more accurate than the clinical groups, who did not differ from each other.
RTs and anticipations on Block 6 are displayed in Figures 2-4. Manual RTs decreased linearly with age, F (1, 116) = 58.68, p < .001, η2p =.34. There was a modest diagnosis effect, F (1, 116) = 3.55, p = .032 η2p = .06, with longer RTs in the psychosis than in the control group.
Manual anticipations were slightly more common on this block than in Blocks 1-5. Although the modal value was 0 in all groups, anticipations ranged from 0 to 34 out of 100 trials across participants. These data were not analyzed due to floor effects.
Oculomotor RTs showed a quadratic trend with age, F (1, 115) = 5.97, p = .016, η2p = .05, first decreasing then increasing slightly. There was a large diagnosis effect, F (2, 115) = 14.28, p < .001, η2p = .20: the psychosis group had longer RTs than both the control and ADHD groups, who did not differ.
Oculomotor anticipations also showed a quadratic trend with age, F (1, 115) = 4.77, p = .031, η2p = .04, first increasing, then decreasing slightly. There was a diagnosis effect, F (2, 115) = 8.35, p < .001, η2p = .13. The psychosis group made fewer anticipations than the other two groups, who did not differ.
Medication Effects
As many psychiatric medications have anticholinergic properties that can influence memory, an anticholinergic equivalent measure was calculated for each patient based on anticholinergic receptor binding affinity (Chew et al., 2006; de Leon, Canuso, White, & Simpson, 1994; Minzenberg, Poole, Benton, & Vinogradov, 2004). Correlations between logarithms of anticholinergic equivalents and manual accuracy, manual and oculomotor RTs, oculomotor anticipations in Blocks 1-5 (averaged across blocks) and Block 6, and verbal memory measures on Blocks 1-5 and 6 did not yield any significant results.
In addition, each participant's current dose of antipsychotic medications was converted to a chlorpromazine equivalent, which provides an estimate of D2 blocking activity (Woods, 2003). There were no correlations between antipsychotic dose equivalents and manual accuracy, RTs, oculomotor anticipations, or verbal memory measures in Blocks 1-5 (averaged across blocks) and Block 6.
We also compared participants in the psychosis group who were (N = 17) or were not (N = 12) taking antipsychotic medications. None of the analyses listed above yielded main effects of medication or interactions between medication status and other variables.,
Discussion
Summary of Results
The goal of the study was to examine incidental versus intentional learning in youth with psychosis or ADHD. Although accuracy of manual responses on the incidental learning blocks was lower in the clinical groups than in the control group, average accuracy was nevertheless over 90% across all blocks and groups. As sequence-specific learning appeared to be intact in both clinical groups, results suggest that the slightly lower accuracy rates did not hinder this type of learning.
In general, results were similar across manual and oculomotor modalities. Both clinical groups had longer manual and oculomotor RTs and fewer oculomotor anticipations than controls. Although the psychosis group was quantitatively more impaired than the ADHD group on all three measures, group differences did not reach significance. All groups showed initial learning effects from Block 1 to 3 on all measures, and the extent of this learning did not differ between the control and clinical groups.
Importantly, there was no evidence that either clinical group had any impairment in sequence-specific learning. Given the constraints imposed on the sequence and pseudo-random blocks, results indicate that both clinical groups were able to learn at least second-order transitions as well as controls.
Explicit awareness was low in all groups, although all groups performed better than chance on Recognition and Prediction. Thus, all groups appeared to have had some awareness that there was a regularity to the sequence, but could not articulate this regularity beyond about 3-4 steps. None of the results for initial learning or interference changed when we excluded participants who may have gained explicit knowledge of the sequence during task performance.
On intentional sequence-learning, the psychosis group performed worse than the controls in terms of verbal report of the sequence, oculomotor RTs and anticipations, and worse than the control group only on accuracy of manual RTs. Contrary to expectations, the only difference between the control and ADHD groups was that the ADHD group had lower manual accuracy.
Incidental Sequence Learning
The results extend the results of previous studies finding no incidental sequence-specific learning impairment in schizophrenia to outpatients with youth-onset psychosis, almost none of whom were taking typical neuroleptics. As in previous studies of schizophrenia, group differences in explicit sequence knowledge could not explain these findings. Results did not change when those with greater explicit awareness were excluded from analyses, and the impairment of the psychosis group on intentional learning makes it unlikely that they were using explicit strategies to perform the incidental learning task.
The finding of no sequence-specific impairment in ADHD was unexpected, given the few previous studies in ADHD on related tasks. The discrepancy could be related to the fact that cognitive processes assessed on previous tasks are different from the type of learning assessed on the SRT task.
Results also indicate that the kind of attentional and working memory impairments observed in youth-onset psychosis and ADHD do not impair sequence-specific learning, lending support to the hypothesis that sequence-specific learning does not rely heavily on attention or working memory.
Despite intact sequence-specific learning, however, both clinical groups made fewer anticipatory eye movements and were slower to make these eye movements than controls. Since learning and interference effects did not interact with diagnosis, it is unlikely that the reduced frequency of anticipations was attributable to sequence-specific processes. Fatigue, poor motivation, and general lack of attentiveness are also unlikely explanations for the group difference, as accuracy of manual responses remained high throughout the task.
There are several possible explanations for the group differences in oculomotor anticipations. The simplest is that they might be attributable to a slow rate of processing information and anticipating stimuli. Another possibility, particularly given the fact that inter-stimulus interval was constant, is that they might have been due to impairments in processing temporal information. If this impairment involved a systematic overestimation of the time at which the next stimulus would appear, it could have led to delayed oculomotor responses, resulting in fewer anticipations. Indeed, there is evidence of impairments in time perception in both schizophrenia (e.g., Haggard, Martin, Taylor-Clarke, Jeannerod, & Franck, 2003) and ADHD (e.g., Toplak & Tannock, 2005). However, individuals with schizophrenia tend to underestimate the duration between their action and its consequences for short durations (250 ms; Haggard et al., 2003). Individuals with ADHD also underestimate target durations for 3- to 17-s intervals (Kerns, McInerney, & Wilde, 2001), or show no impairments for intervals between 2 and 10 s (Mullins, Bellgrove, Gill, & Robertson, 2005; Smith, Taylor, Rogers, Newman, & Rubia, 2002).
Therefore, another possibility is that the reduced frequency of anticipations was reflecting a deficit in anticipatory processing. As noted in the Introduction, other studies have found deficits in anticipatory processing in both disorders. In our study as well, both groups showed an impairment in the ability to search for, extract, and/or anticipate regularities in the environment (whether the regularities are there or not) despite a relatively intact ability to respond to these regularities when they are there.
What might be the neural correlates of anticipatory processing? A functional neuroimaging study by Huettel and colleagues (2002) provides clues to this question. In this study, healthy adults were shown 1,800 stimuli one at a time and instructed to press one of two buttons depending on the stimulus. The order of the stimuli was random, and participants were informed of this beforehand. The researchers later extracted patterns that occurred by chance (2 to 8 consecutive runs of alternating or repeating stimuli) and found increased and differential brain activity in response to violations of these “local” patterns. The prefrontal cortex was sensitive to violations of both repeating and alternating patterns, whereas the caudate and putamen were sensitive to violations of repeating patterns only. This difference was interpreted as indicating that the basal ganglia are more involved in shifting response modes, whereas the prefrontal cortex is involved in detecting more complex patterns. The researchers concluded that this type of pattern recognition is “an [automatic,] obligatory, dynamic process that includes the extraction of local structure from even random sequences” (p. 489) and that activity in the prefrontal-striatal regions might have reflected the “moment-to-moment updating of mental models for pattern” (p. 488). Shanks and colleagues (2005) have also speculated that “it may be an intrinsic property of the learning system that it always makes an effortful attempt to learn about contingencies, even when those contingencies are random” (p. 380). It has also been proposed that learning information about general regularities in the stimuli (e.g., absolute frequencies) is dissociable from learning transitional probabilities between stimuli (Lungu, Wächter, Liu, & Willingham, 2004). In our study, anticipations may have been more closely tied to the first than to the second type of learning.
Based on the results of the current study, we propose that the neural substrates of the obligatory search for patterns in general and sequence-specific learning of the kind measured on the SRT task are separable and that the abnormalities in anticipations observed in the clinical groups (more so in psychosis than in ADHD) are due to abnormalities in regions of the prefrontal cortex supporting extraction of regularities from random or semi-random patterns.
Intentional Sequence Learning
The psychosis group was impaired in intentional learning, consistent with the findings of other studies on memory in schizophrenia. Contrary to expectations, however, the ADHD group did not show impairments in this condition, except for reduced accuracy of manual responses. This failure to find intentional learning deficits in the ADHD group could be due to the fact that the task was not sensitive or taxing enough to detect deficits in this group.
The intentional condition did not differentiate between general learning and sequence-specific learning. With this caveat in mind, relatively preserved incidental learning despite impairments in intentional learning in the psychosis group adds to evidence that the two forms of learning are dissociable.
Intentional learning relies more on strategic processes and working memory than incidental learning (e.g., Jiménez et al., 2006; Unsworth & Engle, 2005), and differences between the psychosis and ADHD groups were likely related to greater impairment in these processes in the psychosis group. It has also been suggested that incidental and intentional learning tasks result in the learning of response versus stimulus locations, respectively (Knee, Thomason, Ashe, & Willingham, 2007). Thus, it is possible that individuals with schizophrenia are also impaired in the learning of stimulus, but not response, locations.
Age-Related Trends in Sequence Learning
We examined normative developmental trends in the current sample of controls in a previous study (Karatekin et al., 2007). Therefore, normative findings will not be discussed in detail. It should be mentioned that we treated age as a categorical variable in our previous study and as a continuous variable in the current study. Therefore, we were better able to characterize the nature of age-related changes in the current study. Despite this difference in the method of analysis, we reached essentially the same conclusions. There was no indication of age-related changes in incidental sequence-specific learning, despite clear evidence of such changes in intentional learning.
None of the analyses yielded a significant interaction between diagnosis and the linear or quadratic trends for age. With the caveat that this was not a longitudinal study, results suggest that the groups did not differ in terms of the maturation of the cognitive processes assessed in this study.
Limitations
The temporal resolution of the eye monitor, and consequently that of individual RTs, was somewhat low (60 Hz). Therefore, some of the negative findings regarding RTs may have become significant with higher temporal resolution.
We could not assess the effects of comorbid conditions in the clinical groups adequately due to small sample sizes. Results are limited to the Combined subtype of ADHD. Additionally, we had few girls in the ADHD group, as it was difficult to find girls who met criteria for the Combined subtype.
Because there was no Bonferroni correction across terms in the ANCOVAs, some of the results, especially higher-order interactions, close to a p value of .05 may have been spurious.
Suggestions for Future Research
Although there are several studies of incidental learning in schizophrenia and ADHD, we focused on the SRT task as a measure of skill learning in this study. Incidental/implicit learning is not a unitary phenomenon (e.g., Gebauer & Mackintosh, 2007), and it is difficult to make inferences from the results of one implicit learning task to another. Therefore, it is necessary to continue to examine and compare different types of skill learning in both disorders. The importance of this is underscored by a recent study (Foerde et al., 2008) in which the performance of adults with schizophrenia was intact on the SRT task but impaired on a more cognitive measure of skill learning hypothesized to be mediated by a different corticostriatal loop than the SRT task. Even within the SRT task, the nature of the task (e.g., the type of sequence used, the stimulus and response modalities) matters for performance. So the current results should not be taken to indicate that incidental learning in general is intact in both psychosis and ADHD.
It is well-established that the cognitive architecture and neural bases of skill learning change with time, so it is also important to establish the nature of these changes in psychosis and ADHD. To our knowledge, there is only one study in which the time-course of skill learning was examined in either disorder. In this study, individuals with schizophrenia showed no impairment in initial learning of a finger tapping motor sequence task, but failed to demonstrate normal sleep-dependent improvement in this skill 24 hours later (Manoach, Cain, Vangel, Khurana, Goff, & Stickgold, 2004).
Studies of skill consolidation are also relevant for cognitive and motor treatment protocols in both disorders. Our results suggest that individuals with youth-onset psychosis and ADHD can learn at least some skills normally, and this area of preserved functioning can be incorporated into treatment regimens. However, even if they can acquire certain skills normally, can they retain these skills in the long run?
Finally, it would be worthwhile to examine anticipations further in both disorders. Our results indicate that in both disorders, there may be a fundamental impairment in searching for/anticipating forthcoming events, even in situations where few regularities exist and where they are not expected or instructed to search for regularities. Whether the groups were simply slow to anticipate or made fewer anticipations, results point to an impairment in top-down guidance of visual-spatial attention in a predictive manner. This impairment was evident in both incidental and intentional learning in the psychosis group, but only in incidental learning in the ADHD group. Although the impairment is manifested more clearly in eye movements than in manual responses, it may not be restricted to visual-spatial attenion. Although this would not be considered an “executive” process in the conventional sense and is not likely to take up a lot of cognitive resources, a deficit in anticipatory processes still has important implications for a wide range of cognitive and social processes in both disorders.
Acknowledgments
We thank the families and teachers for participating in the study; Afshan Anjum, Angie Guimaraes, Bonnie Houg, Cacy Miranda, Kathryn McGraw-Schuchman, and Marie Gabrielle Reed for helping with diagnostic assessments; Clay Collins, Nicholas Davenport, Anita Fuglestad, David Marcus, and Marcus Schmidt for helping with data collection and analyses; and research assistants for helping with data entry and organization. Funding was provided by NIMH (1RO3-MH063150; K08-MH068540), National Alliance for Research on Schizophrenia and Depression (NARSAD), the Essel Foundation, and the University of Minnesota Center for Neurobehavioral Development.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/neu.
We re-analyzed the results after excluding these five participants, and re-examined main effects of diagnosis, interactions with diagnosis, and post-hoc tests on main effects of, or interactions with, diagnosis. ANCOVAs on accuracy of manual responses, manual and oculomotor RTs, and frequency of oculomotor anticipations; and tests on all indices of sequence awareness on Blocks 1-6 yielded only three results that differed from the main analyses: (1) For manual RTs on Blocks 1-5, the block by diagnosis interaction no longer reached significance, p = .072. (2) The difference in manual RTs of the ADHD and control groups on Block 2 became significant, with longer RTs in the ADHD group. (3) For oculomotor anticipations on Block 6, the main effect of diagnosis no longer reached significance, p = .078. None of these results changes the main conclusions of the study.
References
- Achenbach TM, Rescorla L. Manual for the ASEBA school-age forms & profiles: An integrated system of multi-informant assessment. ASEBA; Burlington, VT: 2001. [Google Scholar]
- Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. University of Vermont; Burlington, VT: 1991a. [Google Scholar]
- Achenbach TM. Manual for the Teacher's Report Form and 1991 Profile. University of Vermont; Burlington, VT: 1991b. [Google Scholar]
- Aizenstein HJ, Stenger VA, Cochran J, Clark K, Johnson M, Nebes RD, et al. Regional brain activation during concurrent implicit and explicit sequence learning. Cerebral Cortex. 2004;14:199–208. doi: 10.1093/cercor/bhg119. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The scale for the assessment of negative symptoms (SANS) University of Iowa; Iowa City, IA: 1983. [Google Scholar]
- Andreasen NC. The scale for the assessment of positive symptoms (SAPS) University of Iowa; Iowa City, IA: 1984. [Google Scholar]
- Asarnow RF, Nuechterlein KH, Subotnik KL, Fogelson DL, Torquato RD, Payne DL, et al. Neurocognitive impairments in nonpsychotic parents of children with schizophrenia and attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2002;59:1053–1060. doi: 10.1001/archpsyc.59.11.1053. [DOI] [PubMed] [Google Scholar]
- Ashe J, Lungu OV, Basford AT, Lu X. Cortical control of motor sequences. Current Opinion in Neurobiology. 2006;16:213–221. doi: 10.1016/j.conb.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Barr WB. Schizophrenia and attention deficit disorder: Two complex disorders of attention. Annals of the New York Academy of Sciences. 2001;931:239–250. doi: 10.1111/j.1749-6632.2001.tb05782.x. [DOI] [PubMed] [Google Scholar]
- Beldarrain MG, Grafman J, Pascual-Leone A, Garcia-Monco JC. Procedural learning is impaired in patients with prefrontal lesions. Neurology. 1999;52:1853–1860. doi: 10.1212/wnl.52.9.1853. [DOI] [PubMed] [Google Scholar]
- Box GEP, Cox DR. An analysis of transformations. (Series B).Journal of the Royal Statistical Society. 1964;26:211–252. [Google Scholar]
- Caplan R, Guthrie D, Tang B, Nuechterlein KH, Asarnow RF. Thought disorder in Attention-Deficit Hyperactivity Disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:965–972. doi: 10.1097/00004583-200108000-00019. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Hansson LO, Waters N, Carlsson ML. Neurotransmitter aberrations in schizophrenia: New perspectives and therapeutic implications. Life Sciences. 1997;61:75–94. doi: 10.1016/s0024-3205(97)00228-2. [DOI] [PubMed] [Google Scholar]
- Chew M, Mulsant B, Pollock B, Lehman M, Greenspan A, Kirshner M, et al. A model of anticholinergic activity of atypical antipsychotic medications. Schizophrenia Research. 2006;88:63–72. doi: 10.1016/j.schres.2006.07.011. [DOI] [PubMed] [Google Scholar]
- De Leon J, Canuso C, White AO, Simpson GM. A pilot effort to determine benzotropine equivalents of anticholinergic medications. Hospital and Community Psychiatry. 1994;45:606–607. doi: 10.1176/ps.45.6.606. [DOI] [PubMed] [Google Scholar]
- Dominey PF, Georgieff N. Schizophrenics learn surface but not abstract structure in a serial reaction time task. NeuroReport. 1997;8:2877–2882. doi: 10.1097/00001756-199709080-00015. [DOI] [PubMed] [Google Scholar]
- Eliassen JC, Souza T, Sanes JN. Human brain activation accompanying explicitly directed movement sequence learning. Experimental Brain Research. 2001;141:269–280. doi: 10.1007/s002210100822. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: An anatomical likelihood estimation meta-analysis. American Journal of Psychiatry. 2008;165:1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner C, Boucsein K, Degner D, Irle E. State-dependent implicit learning deficit in schizophrenia: Evidence from 20-month follow-up. Psychiatry Research. 2006a;142:39–52. doi: 10.1016/j.psychres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Exner C, Weniger G, Schmidt-Samoa C, Irle E. Reduced size of the pre-supplementary motor cortex and impaired motor sequence learning in first-episode schizophrenia. Schizophrenia Research. 2006b;84:386–396. doi: 10.1016/j.schres.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Ferraro FR, Okerlund M. Implicit memory of schizotypal individuals. Perceptual and Motor Skills. 1995;80:371–376. doi: 10.2466/pms.1995.80.2.371. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Zafiris O, Frith CD, Honey RAE, Corlett PR, Zilles K, et al. On the benefits of not trying: Brain activity and connectivity reflecting the interactions of explicit and implicit sequence learning. Cerebral Cortex. 2004;15:1002–1015. doi: 10.1093/cercor/bhh201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerde K, Poldrack RA, Knowlton BJ, Sabb FW, Bookheimer SY, Bilder RM, et al. Selective corticostriatal dysfunction in schizophrenia: Examination of motor and cognitive skill learning. Neuropsychology. 2008;22:100–109. doi: 10.1037/0894-4105.22.1.100. [DOI] [PubMed] [Google Scholar]
- Frangou S, Hadjulis M, Vourdas A. The Maudsley early onset schizophrenia study: Cognitive function over a 4-year follow-up period. Schizophrenia Bulletin. 2008;34:52–59. doi: 10.1093/schbul/sbm124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18:543–555. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- Gebauer GF, Mackintosh NJ. Psychometric intelligence dissociates implicit and explicit learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2007;33:34–54. doi: 10.1037/0278-7393.33.1.34. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Williams O, McGurk S, Kee K. Procedural learning in schizophrenia: Evidence from serial reaction time. Cognitive Neuropsychiatry. 1997;2:123–134. doi: 10.1080/135468097396360. [DOI] [PubMed] [Google Scholar]
- Haggard P, Martin F, Taylor-Clarke M, Jeannerod M, Franck N. Awareness of action in schizophrenia. NeuroReport. 2003;14:1081–1085. doi: 10.1097/01.wnr.0000073684.00308.c0. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia: A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Harvey AS, Epstein JN, Curry JF. Neuropsychology of adults with attention-deficit/hyperactivity disorder: A meta-analytic review. Neuropsychology. 2004;18:485–503. doi: 10.1037/0894-4105.18.3.485. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Yale University; New Haven, CT: 1975. [Google Scholar]
- Huettel SA, Mack PB, McCarthy G. Perceiving patterns in random series: Dynamic processing of sequence in prefrontal cortex. Nature Neuroscience. 2002;5:485–490. doi: 10.1038/nn841. [DOI] [PubMed] [Google Scholar]
- Hurks PPM, Adam JJ, Hendriksen JGM, Vles JSH, Feron FJM, Kalff AC, et al. Controlled visuomotor preparation deficits in attention-deficit/hyperactivity disorder. Neuropsychology. 2005;19:66–76. doi: 10.1037/0894-4105.19.1.66. [DOI] [PubMed] [Google Scholar]
- Jiménez L, Vaquero JMM, Lupiáñez J. Qualitative differences between implicit and explicit sequence learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2006;32:475–490. doi: 10.1037/0278-7393.32.3.475. [DOI] [PubMed] [Google Scholar]
- Karatekin C. Developmental disorders of attention. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. MIT Press; Cambridge, MA: 2001. pp. 561–576. [Google Scholar]
- Karatekin C. A test of the integrity of the components of Baddeley's model of working memory in Attention-Deficit/Hyperactivity Disorder (ADHD) Journal of Child Psychology and Psychiatry. 2004;45:912–926. doi: 10.1111/j.1469-7610.2004.t01-1-00285.x. [DOI] [PubMed] [Google Scholar]
- Karatekin C. Improving antisaccade performance in adolescents with Attention- Deficit/Hyperactivity Disorder (ADHD) Experimental Brain Research. 2006;174:324–341. doi: 10.1007/s00221-006-0467-x. [DOI] [PubMed] [Google Scholar]
- Karatekin C, Asarnow RF. Working memory in Childhood-Onset Schizophrenia and Attention-Deficit/Hyperactivity Disorder (ADHD) Psychiatry Research. 1998;80:165–176. doi: 10.1016/s0165-1781(98)00061-4. [DOI] [PubMed] [Google Scholar]
- Karatekin C, Marcus DJ, White TJ. Oculomotor and manual indices of incidental and intentional spatial sequence learning in middle childhood and adolescence. Journal of Experimental Child Psychology. 2007;96:107–130. doi: 10.1016/j.jecp.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Karatekin C, Markiewicz SW, Siegel MA. A preliminary study of motor problems in children with Attention-Deficit/Hyperactivity Disorder (ADHD) Perceptual and Motor Skills. 2003;97:1267–1280. doi: 10.2466/pms.2003.97.3f.1267. [DOI] [PubMed] [Google Scholar]
- Karatekin C, White TJ, Bingham C. Divided attention in youth-onset psychosis and Attention-Deficit/Hyperactivity Disorder (ADHD) Journal of Abnormal Psychology. 2008;117:881–895. doi: 10.1037/a0013446. [DOI] [PubMed] [Google Scholar]
- Kerns KA, McInerney RJ, Wilde NJ. Time reproduction, working memory, and behavioral inhibition in children with ADHD. Child Neuropsychology. 2001;7:21–31. doi: 10.1076/chin.7.1.21.3149. [DOI] [PubMed] [Google Scholar]
- Kieling C, Goncalves RR, Tannock R, Castellanos FX. Neurobiology of Attention Deficit Hyperactivity Disorder. Child and Adolescent Psychiatric Clinics of North America. 2008;17:285–307. doi: 10.1016/j.chc.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Klorman R, Thatcher JE, Shaywitz SE, Fletcher JM, Marchione KE, Holahan JM, et al. Effects of event probability and sequence on children with attention-deficit/hyperactivity, reading, and math disorder. Biological Psychiatry. 2002;52:795–804. doi: 10.1016/s0006-3223(02)01415-4. [DOI] [PubMed] [Google Scholar]
- Knee R, Thomason S, Ashe J, Willingham DT. The representation of explicit motor sequence knowledge. Memory & Cognition. 2007;35:326–333. doi: 10.3758/bf03193453. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Nissen MJ. Procedural learning is impaired in Huntington's disease: Evidence from the serial reaction time task. Neuropsychologia. 1987;29:245–254. doi: 10.1016/0028-3932(91)90085-m. [DOI] [PubMed] [Google Scholar]
- Kumari V, Corr PJ, Mulligan OF, Cotter PA, Checkley SA, Gray JA. Effects of acute administration of d-amphetamine and haloperidol on procedural learning in man. Psychopharmacology. 1997;129:271–276. doi: 10.1007/s002130050190. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA, Honey GD, Soni W, Bullmore ET, Williams SCR, et al. Procedural learning in schizophrenia: a functional magnetic resonance imaging investigation. Schizophrenia Research. 2002;57:97–107. doi: 10.1016/s0920-9964(01)00270-5. [DOI] [PubMed] [Google Scholar]
- Lungu OV, Wächter T, Liu T, Willingham DT. Probability detection mechanisms and motor learning. Experimental Brain Research. 2004;159:135–150. doi: 10.1007/s00221-004-1945-7. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Cain MS, Vangel MG, Khurana A, Goff DC, Stickgold R. A failure of sleep-dependent procedural learning in chronic, medicated schizophrenia. Biological Psychiatry. 2004;56:951–956. doi: 10.1016/j.biopsych.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Marcus DJ, Karatekin C, Markiewicz S. Oculomotor evidence of sequence learning on the serial reaction time (SRT) task. Memory & Cognition. 2006;34:420–432. doi: 10.3758/bf03193419. [DOI] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- Marvel CL, Schwartz BL, Howard DV, Howard JH. Implicit learning of nonspatial sequences in schizophrenia. Journal of the International Neuropsychological Society. 2005;11:659–667. doi: 10.1017/S1355617705050861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel CL, Turner BM, O'Leary DS, Johnson HJ, Pierson RK, Ponto LLB, et al. The neural correlates of implicit sequence learning in schizophrenia. Neuropsychology. 2007;21:761–777. doi: 10.1037/0894-4105.21.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J, Kraseski K, Schvartz I, Mercado V, Daisy N, Tobing L, et al. Sustained attention, visual processing speed, and IQ in children and adolescents with schizophrenia spectrum disorder and psychosis not otherwise specified. Perceptual and Motor Skills. 2005;100:1097–1106. doi: 10.2466/pms.100.3c.1097-1106. [DOI] [PubMed] [Google Scholar]
- McKenna K, Gordon C, Lenane M, Kaysen D, Fahey K, Rapaport J. Looking for childhood-onset schizophrenia: The first 71 cases screened. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33:636–644. doi: 10.1097/00004583-199406000-00003. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Poole JH, Benton C, Vinogradov S. Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. American Journal of Psychiatry. 2004;161:116–124. doi: 10.1176/appi.ajp.161.1.116. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Rimrodt SL, Schafer JGB, Boyce A, Goldberg MC, Pekar JJ, et al. Atypical motor and sensory cortex activation in Attention-Deficit/Hyperactivity Disorder: A functional magnetic resonance imaging study of simple sequential finger tapping. Biological Psychiatry. 2006;59:48–56. doi: 10.1016/j.biopsych.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Mullins C, Bellgrove MA, Gill M, Robertson IH. Variability in time reproduction: Difference in ADHD Combined and Inattentive subtypes. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:169–176. doi: 10.1097/00004583-200502000-00009. [DOI] [PubMed] [Google Scholar]
- Nicolson R, Brookner FB, Lenane M, Gochman P, Ingraham LJ, Egan MF, et al. Parental schizophrenia spectrum disorders in childhood-onset and adult-onset schizophrenia. American Journal of Psychiatry. 2003;160:490–495. doi: 10.1176/appi.ajp.160.3.490. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: Evidence from performance measures. Cognitive Psychology. 1987;19:1–32. [Google Scholar]
- Øie M, Sundet K, Rund BR. Contrasts in memory functions between adolescents with schizophrenia or ADHD. Neuropsychologia. 1999;37:1351–1358. doi: 10.1016/s0028-3932(99)00043-3. [DOI] [PubMed] [Google Scholar]
- O'Leary DS, Flaum M, Kesler ML, Flashman LA, Arndt S, Andreasen NC. Cognitive correlates of the negative, disorganized, and psychotic symptom dimensions of schizophrenia. Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12:4–15. doi: 10.1176/jnp.12.1.4. [DOI] [PubMed] [Google Scholar]
- Pedersen A, Rist F. Implicit memory in schizotypal subjects and normal controls Effects of a secondary task on sequence learning. Perceptual and Motor Skills. 2001;92:349–367. doi: 10.2466/pms.2001.92.2.349. [DOI] [PubMed] [Google Scholar]
- Pedersen A, Siegmund A, Ohrmann P, Rist F, Rothermundt M, Suslow T, et al. Reduced implicit and explicit sequence learning in first-episode schizophrenia. Neuropsychologia. 2008;46:186–195. doi: 10.1016/j.neuropsychologia.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Perchet C, Revol O, Fourneret P, Mauguière F, Garcia-Larrea L. Attention shifts and anticipatory mechanisms in hyperactive children : An ERP study using the Posner paradigm. Biological Psychiatry. 2001;50:44–57. doi: 10.1016/s0006-3223(00)01119-7. [DOI] [PubMed] [Google Scholar]
- Perry W, Light GA, Davis H, Braff DL. Schizophrenia patients demonstrate a dissociation on declarative and non-declarative memory tests. Schizophrenia Research. 2000;46:167–174. doi: 10.1016/s0920-9964(99)00229-7. [DOI] [PubMed] [Google Scholar]
- Pliszka SR. The neuropsychopharmacology of Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2005;57:1385–1390. doi: 10.1016/j.biopsych.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Posada A, Franck N, Georgieff N, Jeannerod M. Anticipating incoming events: An impaired cognitive process in schizophrenia. Cognition. 2001;81:209–225. doi: 10.1016/s0010-0277(01)00133-0. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: Integration of performance based and brain imaging findings. Psychological Bulletin. 2007;133:833–858. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- Reiss JP, Campbell DW, Leslie WD, Paulus MP, Ryner LN, Polimeni JO, et al. Deficit in schizophrenia to recruit the striatum in implicit learning: A functional magnetic resonance imaging investigation. Schizophrenia Research. 2006;87:127–137. doi: 10.1016/j.schres.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Rhinewine JP, Lencz T, Thaden EP, Cervellione KL, Burdick KE, Henderson I, et al. Neurocognitive profile in adolescents with early-onset schizophrenia: Clinical correlates. Biological Psychiatry. 2005;58:705–712. doi: 10.1016/j.biopsych.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Roofeh D, Cottone J, Burdick KE, Lencz T, Gyato K, Cervellione KL, et al. Deficits in memory strategy use are related to verbal memory impairments in adolescents with schizophrenia-spectrum disorders. Schizophrenia Research. 2006;85:201–212. doi: 10.1016/j.schres.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Rüsseler J, Henninghausen E, Münte TF, Rösler F. Differences in incidental and intentional learning of sensorimotor sequences as revealed by event-related brain potentials. Cognitive Brain Research. 2003;15:116–126. doi: 10.1016/s0926-6410(02)00145-3. [DOI] [PubMed] [Google Scholar]
- Schwartz BL, Howard DV, Howard JH, Hovaguimian A, Deutsch SI. Implicit learning of visuospatial sequences in schizophrenia. Neuropsychology. 2003;17:517–533. doi: 10.1037/0894-4105.17.3.517. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N. Structural brain imaging of Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2005;57:1263–1272. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Shanks DR, Rowland LA, Ranger MS. Attentional load and implicit sequence learning. Psychological Research. 2005;69:369–382. doi: 10.1007/s00426-004-0211-8. [DOI] [PubMed] [Google Scholar]
- Smith A, Taylor E, Rogers JW, Newman S, Rubia K. Evidence for a pure time perception deficit in children with ADHD. Journal of Child Psychology and Psychiatry. 2002;43:529–542. doi: 10.1111/1469-7610.00043. [DOI] [PubMed] [Google Scholar]
- Stevens A, Schwarz J, Schwarz B, Ruf I, Kolter T, Czekalla J. Implicit and explicit learning in schizophrenics treated with olanzapine and with classic neuroleptics. Psychopharmacology. 2002;160:299–306. doi: 10.1007/s00213-001-0974-1. [DOI] [PubMed] [Google Scholar]
- Swanson J. School based assessments and interventions for ADD students. KC Publishing; Irvine, CA: 1992. [Google Scholar]
- Tanji J. Sequential organization of multiple movements: Involvement of cortical motor areas. Annual Review of Neuroscience. 2001;24:631–651. doi: 10.1146/annurev.neuro.24.1.631. [DOI] [PubMed] [Google Scholar]
- Toplak ME, Tannock R. Time perception: Modality and duration effects in Attention-Deficit/Hyperactivity Disorder (ADHD) Journal of Abnormal Child Psychology. 2005;33:639–654. doi: 10.1007/s10802-005-6743-6. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. Individual differences in working memory capacity and learning: Evidence from the serial reaction time task. Memory & Cognition. 2005;33:213–220. doi: 10.3758/bf03195310. [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Vourdas A, Pipe R, Corrigall R, Frangou S. Increased developmental deviance and premorbid dysfunction in early onset schizophrenia. Schizophrenia Research. 2003;62:13–22. doi: 10.1016/s0920-9964(02)00429-2. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3rd ed Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 4th ed Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- Wechsler Individual Achievement Test (WIAT) Psychological Corporation; San Antonio, TX: 1992. [Google Scholar]
- White T, Ho B-C, Ward J, O'Leary D, Andreasen NC. Neuropsychological performance in first-episode adolescents with schizophrenia: A comparison with first-episode adults and adolescent control subjects. Biological Psychiatry. 2006;60:463–471. doi: 10.1016/j.biopsych.2006.01.002. [DOI] [PubMed] [Google Scholar]
- White T, Kendi ATK, Lehericy S, Kendi M, Karatekin C, Guimaraes A, et al. Disruption of hippocampal connectivity in children and adolescents with schizophrenia—a voxel-based diffusion tensor imaging study. Schizophrenia Research. 2007;90:302–307. doi: 10.1016/j.schres.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Wilkinson L, Shanks DR. Intentional control and implicit sequence learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2004;30:354–369. doi: 10.1037/0278-7393.30.2.354. [DOI] [PubMed] [Google Scholar]
- Willingham DB, Salidis J, Gabrieli JDE. Direct comparison of neural systems mediating conscious and unconscious skill learning. Journal of Neurophysiology. 2002;88:1451–1460. doi: 10.1152/jn.2002.88.3.1451. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. Journal of Clinical Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Zedkova L, Woodward ND, Harding I, Tibbo PG, Purdon SE. Procedural learning in schizophrenia investigated with functional magnetic resonance imaging. Schizophrenia Research. 2006;88:198–207. doi: 10.1016/j.schres.2006.06.039. [DOI] [PubMed] [Google Scholar]