Abstract
Clinical studies have reported that the widely used anti-hyperglycemic drug metformin significantly reduces cardiac risk factors and improves clinical outcomes in patients with heart failure. The mechanisms by which metformin exerts these cardioprotective effects remain unclear and may be independent of anti-hyperglycemic effects. We tested the hypothesis that chronic activation of AMPK with low-dose metformin exerts beneficial effects on cardiac function and survival in in vivo murine models of heart failure. Mice were subjected to permanent left coronary artery (LCA) occlusion or to 60 min LCA occlusion followed by reperfusion for 4 wks. High-resolution, two-dimensional echocardiography was performed at baseline and 4 wk post myocardial infarction to assess left ventricular (LV) dimensions and function. Metformin (125 μg/kg) administered to mice at ischemia and then daily, improved survival by 47% (p < 0.05 vs. vehicle) at 4 wk following permanent LCA occlusion. Additionally, metformin given at reperfusion and then daily, preserved LV dimensions and LV ejection fraction (p < 0.01 vs. vehicle) at 4 wk. The improvement in cardiac structure and function was associated with increases in AMPK and eNOS phosphorylation as well as increased PGC-1α expression in cardiac myocytes. Furthermore, metformin significantly improved myocardial cell mitochondrial respiration and ATP synthesis compared to vehicle. The cardioprotective effects of metformin were ablated in mice lacking functional AMPK or eNOS. This study demonstrates that metformin significantly improves left ventricular function and survival via activation of AMPK and its downstream mediators, eNOS and PGC-1α in a murine model of heart failure.
Keywords: myocardial ischemia, heart failure, metformin, nitric oxide
Introduction
Heart failure is the inability of the heart to meet hemodynamic demands and represents the end stage of various forms of cardiac disease. In the industrialized nations, heart failure represents a major health problem that has been increasing in prevalence and incidence. In the United States, heart failure affects more than 5 million people, with 500,000 new cases reported every year. It is responsible for almost 1 million hospital admissions and 40,000 deaths annually1. The most important cause of HF is coronary artery disease (CAD) and acute myocardial infarction leading to loss of functioning myocytes, development of myocardial fibrosis, and subsequent left ventricular (LV) remodeling, all of which contribute towards the development of LV dysfunction.
Metformin is an orally administered biguanide drug that is widely used to lower blood glucose concentrations in patients with diabetes mellitus. Metformin decreases blood glucose by mechanisms different from those of sulphonylureas or insulin and exerts its actions by enhancing insulin sensitivity, inducing greater peripheral uptake of glucose, and decreasing hepatic glucose output while lowering plasma insulin concentrations2. Additionally, blood glucose control is achieved without any weight gain especially in patients with obesity and metabolic syndrome3. Analysis of the United Kingdom Prospective Diabetes Study (UKPDS) demonstrated that improved glycemic control in overweight patients treated with metformin was associated with a decreased risk of diabetes related cardiovascular end points and all cause deaths when compared to conventional therapies that lower blood glucose to similar levels4. Therefore, the cardioprotective effects of metformin cannot be attributed to its anti-hyperglycemic effects alone and may be related to the actions of metformin on lipid metabolism, vascular smooth muscle and cardiomyocyte calcium handling, endothelial function, hypercoagulation, and platelet reactivity5.
Experimental studies suggest that the pleiotropic effects of metformin are mediated in part by activation of AMP-activated protein kinase (AMPK)6, a protein kinase that is activated in response to alterations in cellular energy levels. When activated, AMPK stimulates fatty acid oxidation, promotes glucose transport, accelerates glycolysis, and inhibits both triglyceride and protein synthesis7. Activation of AMPK has also been shown to increase the phosphorylation and activity of endothelial nitric oxide synthase (eNOS)8 as well as the expression of transcriptional coactivator and metabolic regulator, peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 α)9, both of which are important regulators of mitochondrial biogenesis and function. Activation of AMPK by metformin may account for the reduction of cardiovascular disease risk10 and improvement of vascular function in patients with type 2 diabetes11. The purpose of the present study was to investigate the potential cardioprotective effects of a chronic low dose administration of metformin on survival and cardiac function in a murine model of heart failure.
Materials and Methods
Animals
C57BL/6J mice obtained from Jackson Laboratories (Bar Harbor, ME) were utilized for the present study. Additionally, we used mice completely deficient in eNOS (eNOS-/-, maintained on a C57BL/6J background), as well as, cardiac-specific transgenic mice overexpressing a dominant-negative AMPKα2 subunit (AMPKα2). The generation of AMPKα2 dn mice has been described previously12. AMPKα2 dn Tg and non-transgenic (NTg) littermates were bred in our colony and maintained on an FVB background. All animals were used at 8-10 weeks of age and received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society of Medical Research and the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996). The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Albert Einstein College of Medicine.
Materials
Metformin (1,1-dimethylbiguanide hydrochloride) was purchased from Sigma (St. Loius, MO). It was dissolved in saline and administered at a dose of 125 μg/kg in a final volume of 100 μL as an intracardiac (i.c.) injection at the time of reperfusion once (Metformin) or once at reperfusion followed by daily intraperitoneal (i.p.) injections (Metformin QD) for 4wk. Saline was used as a vehicle at reperfusion followed by daily i.p. injections for 4 wk (vehicle). In the permanent left coronary artery occlusion (LCA) occlusion model (Figure 1A), metformin was administered once at the onset of ischemia i.c. followed by daily i.p. injections for 4 wk.
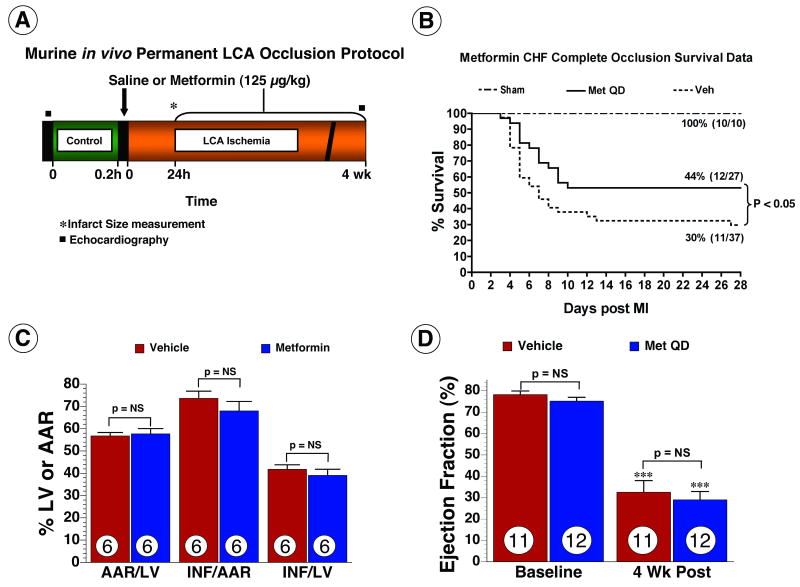
Figure 1.
Metformin improved survival following permanent occlusion of left coronary artery. (A) Murine in vivo permanent left coronary artery (LCA) occlusion protocol. (B) Kaplan-Meier survival curve following metformin therapy in mice during the 4 wk post myocardial infarction. Metformin significantly improved survival by 47% (p < 0.05) compared to vehicle treated mice. (C) Myocardial infarct size analysis in a subset of animals subjected to 24 hr of myocardial ischemia. Area-at-risk (AAR) as a percentage of the left ventricle (LV) was similar between both groups. Metformin (125 μg/kg) administration did not attenuate myocardial infarct size following permanent LCA occlusion. (D) LV function was evaluated in a subset of animals at 4 wks of myocardial ischemia. Ejection fraction at baseline and following myocardial ischemia were similar in both groups. Values are means ± S.E.M. Numbers inside bars indicate the number of animals that were investigated in each group. *** p < 0.001 vs. Baseline. Veh = vehicle, Met QD = Metformin QD.
Heart failure Protocols
Heart failure was induced by either permanent ligation of the LCA (Figure 1A) or by subjecting the mice to 60 min of LCA occlusion followed by reperfusion for up to 4 wk (Figure 2A). Surgical ligation of the LCA was performed according to methods described previously13.
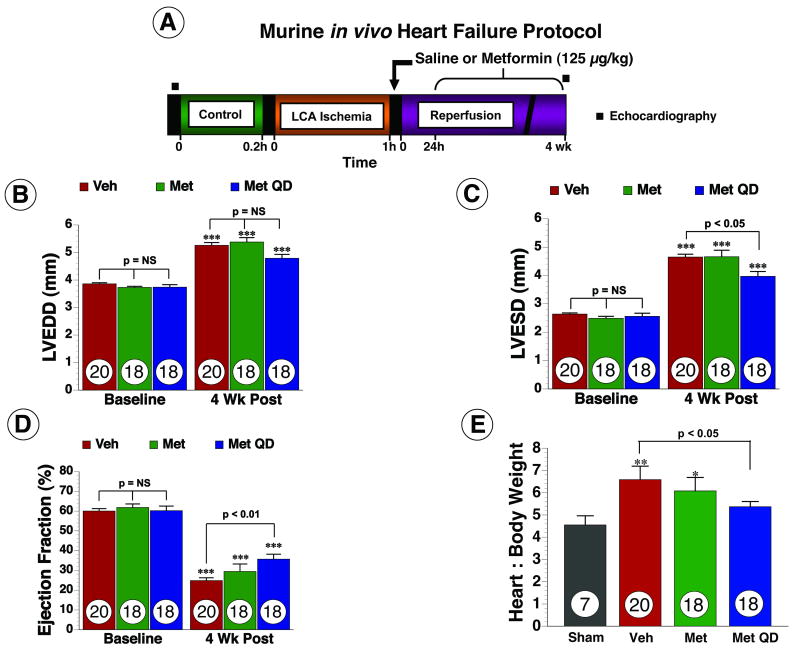
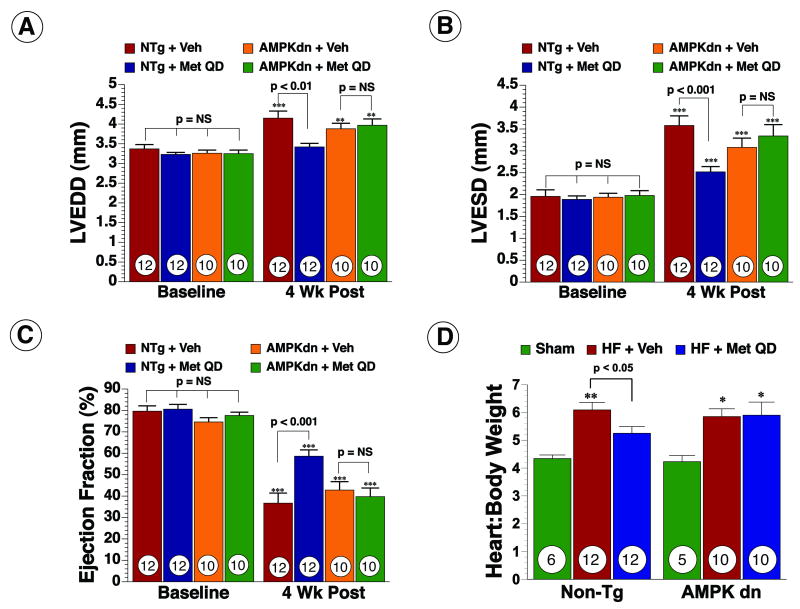
Figure 2.
Metformin improved left ventricular structure and function, as well as, cardiac hypertrophy in mice following myocardial ischemia and reperfusion. (A) Murine in vivo heart failure protocol. (B) Left ventricular end diastolic diameter (LVEDD) (C) left ventricular end systolic diameter (LVESD) and (D) Ejection fraction were calculated using two-dimensional B-mode echocardiography images at baseline and following myocardial ischemia in all groups (E) Heart to body weight (H/BW) ratio used as a measure of cardiac hypertrophy. Values are means ± S.E.M. Numbers inside bars indicate the number of animals that were investigated in each group. *** p < 0.001 vs. Baseline, ** p < 0.01 vs. Sham, * p < 0.05 vs. Sham. Veh = vehicle, Met = Metformin, Met QD = Metformin QD.
Myocardial Area-at-Risk and Infarct Size Determination
Left ventricular area-at-risk (AAR) and infarct size (Inf) determination was performed using Evans blue dye and 2,3,5-triphenyltetrazolium chloride (TTC, Sigma) staining method. All of the procedures for the AAR and Inf determination have been previously described14.
Echocardiograhic Assessment of Left Ventricular Structure and Function
Baseline echocardiography images were obtained one week prior to LCA ischemia to avoid any anesthetic effects as previously described15. After 4 wk following the myocardial infarction (4 wk Post), Echo images were obtained and analyzed once again.
Histological Analysis of Infarct Size
After the post myocardial infarction echocardiographic assessment, the hearts of mice were processed for histological assessment of infarct size as previously described14.
Western Blot Analysis
Western blot analysis was performed as previously described15.
Cardiac Mitochondria Isolation
Cardiac mitochondria were isolated from the following groups of mice as previously described16: sham operated, vehicle and Metformin QD treated mice.
Mitochondrial Respiratory Rate and ATP synthesis
Oxygen consumption and ATP synthesis of cardiac mitochondria was measured as previously described16,17.
Statistical Analysis
All the data in this study are expressed as mean ± standard error (SEM). Differences in data between the groups were compared using Prism 4 (GraphPad Software, Inc) with Student's paired 2-tailed t-test or one-way analysis of variance (ANOVA) where appropriate. For the ANOVA, if a significant variance was found, the Tukey or Bonferoni test was used as the post hoc analysis. p values less than 0.05 was considered significant.
Results
Metformin improves survival following permanent LCA occlusion
Mice were subjected to permanent occlusion of LCA and metformin or vehicle was administered prior to LCA ligation and then daily for 4 wk (Figure 1A). 2-D Echo was obtained at baseline and 4 wk to assess LV function. At 4 wk, both vehicle and metformin treated mice subjected to myocardial infarction exhibited significant mortality compared with sham-operated mice. Mice receiving metformin exhibited an overall survival rate of 44% (12/27) during the 4 wk protocol compared with mice treated with vehicle, which exhibited a 30% (11/37) survival. Metformin treatment, therefore led to a 47% improvement in survival compared with the vehicle (p < 0.05 between groups) (Figure 1B). The extent of myocardial infarction was evaluated in mice receiving either metformin (n=6) or vehicle (n=6) at 24 hr in separate groups of mice. The AAR per LV, Inf per AAR and Inf per LV were similar (p = not significant, NS) in both metformin and vehicle treated mice (Figure 1C). Following permanent LCA occlusion, at 4 wk, EF was severely reduced (p < 0.001 vs. baseline) and similar in the vehicle (n=11) and metformin (n=12) treated mice (Figure 1D). These data demonstrate that metformin treatment significantly improves survival in mice subjected to permanent occlusion of LCA and the survival benefit is independent of any effect on infarct size or left ventricular function.
Metformin improves LV structure and function and attenuates cardiac hypertrophy in heart failure
In order to study the effects of metformin in a more clinically relevant model of heart failure that mimics the effects of coronary revascularization therapy, mice were subjected to 60 min of LCA occlusion followed by reperfusion (Figure 2A). In the initial set of experiments, metformin (125 μg/kg) or vehicle were administered at the time of reperfusion and the extent of myocardial infarction was evaluated at 24 hr (Online Figure I). We found that metformin treatment decreased Inf/AAR by 29% (68.63±2.34% for vehicle vs. 48.92±2.51% for Metformin, p < 0.001) following MI compared to vehicle. To investigate if the reduction in infarct size alone was sufficient to improve LV structure and function, mice were randomized into three groups and subjected to 60 min ischemia. The first group received saline as a vehicle at reperfusion (i.c) and then daily (i.p) injections (vehicle), the second group received metformin as a single (i.c.) bolus only once at the time of reperfusion (Metformin) and the third group received metformin (i.c.) once at reperfusion and then as daily (i.p.) injections (Metformin QD). Echo was performed at baseline and 4 wk to assess LV dimensions and EF in all groups. Following the severe ischemic insult, all groups of mice developed profound ischemia-induced cardiomyopathy as evidenced by a significant increase in both LVEDD and LVESD (Figure 2B-C) and a significant decrease in EF (Figure 2D). At 4 wk following reperfusion, a single administration of metformin at reperfusion alone did not attenuate LV dilatation or improve LV function. In contrast, daily administration of metformin starting at the time of reperfusion significantly improved LVEDD and LVESD by 28% each (p < 0.05 vs. vehicle) and EF by 31% (p < 0.01 vs. vehicle) at 4 wk. In addition, cardiac hypertrophy, as measured by heart to body weight (H/BW) ratio was increased in vehicle and Metformin groups (p < 0.01) but not in the Metformin QD group compared to sham (p= NS) (Figure 2E).
We also measured the infarct area relative to the entire left ventricle at 4 wk following reperfusion (Online Figure I). For each heart, we analyzed multiple sections taken from the mid-ventricle and then averaged these numbers to obtain a single Inf/LV measurement for each animal. Vehicle-treated mice displayed a 13.62 ± 1.16% Inf/LV. Conversely, both groups treated with metformin displayed a smaller area of scar formation. Analysis from the multiple mid-ventricle sections per animal revealed that the mice in the Metformin group displayed a 9.01 ± 0.69% Inf/LV (p<0.01 vs. vehicle) and the mice in the Metformin QD group displayed a 8.16 ± 063% Inf/LV (p<0.001 vs. vehicle). The difference between the metformin treatment groups was not statistically significant (p=NS). Furthermore, no differences in blood glucose were observed following treatment with Metformin QD for 4 wks (Online Table I). Since Metformin QD caused significant improvements in LV function, LV dimension and cardiac hypertrophy, this therapeutic strategy was used in all subsequent experiments.
Metformin promotes the phosphorylation of AMPK and eNOS and increases the expression of PGC1α during heart failure
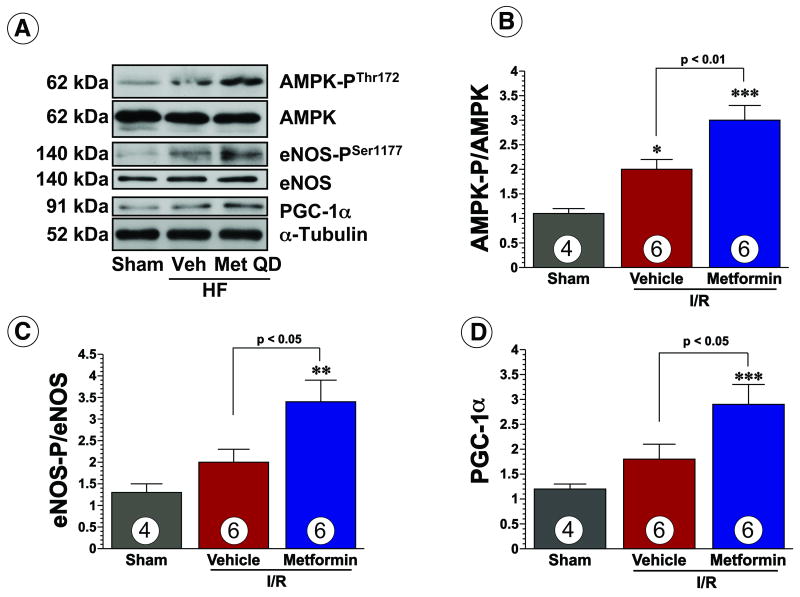
We have previously reported that a single injection of metformin phopshorylates and increases the activity of both AMPK and eNOS for up to 24hr15. In the present study, we investigated if Metformin QD resulted in sustained elevations in AMPK and eNOS phosphorylation as well as expression of PGC1α at 4 weeks following reperfusion of the ischemic myocardium (Figure 3A). Phosphorylation of AMPKThr172 was elevated in both vehicle (p < 0.05) and Metformin QD (p < 0.001) treated groups (Figure 3B). Metformin QD was found to significantly augment the ischemia-induced increase in AMPK-PThr172 (p < 0.01 vs. vehicle). Similarly, Metformin QD treatment significantly increased phosphorylation of eNOSSer1177 (p < 0.05 vs. vehicle) (Figure 3C) and expression of PGC1α (p < 0.05 vs. vehicle) (Figure 3D) compared to sham and vehicle treatment. These results suggest that the activation of AMPK and its downstream mediators eNOS and PGC1α may underlay the beneficial effects of metformin in the myocardium and protect against ischemia-induced heart failure.
Figure 3.
Chronic administration of metformin phosphorylates AMPK and eNOS and increases the expression of PGC1α at 4 weeks during heart failure (HF). (A) Representative immunoblots of AMPK phosphorylated at residue threonine 172 (AMPK-PThr172) and total AMPK eNOS phosphorylated at residue Serine 1177 (eNOS-PSer1177), total eNOS, and PGC-1α and α-tubulin following HF protocol. Densitometric analysis of (B) phosphorylated state AMPKThr172. Bars represent the ratio of phosphorylated AMPK to total AMPK. (C) phosphorylated state eNOS-PSer1177. Bars represent the ratio of phosphorylated eNOS to total eNOS (D) total PGC1α Values are means ± S.E.M. Numbers inside bars indicate the number of animals that were investigated in each group. * p < 0.05, ** p < 0.01, *** p < 0.01 compared to Sham. Veh = vehicle, Met QD = Metformin QD.
Metformin improves mitochondrial respiration and ATP synthesis during heart failure
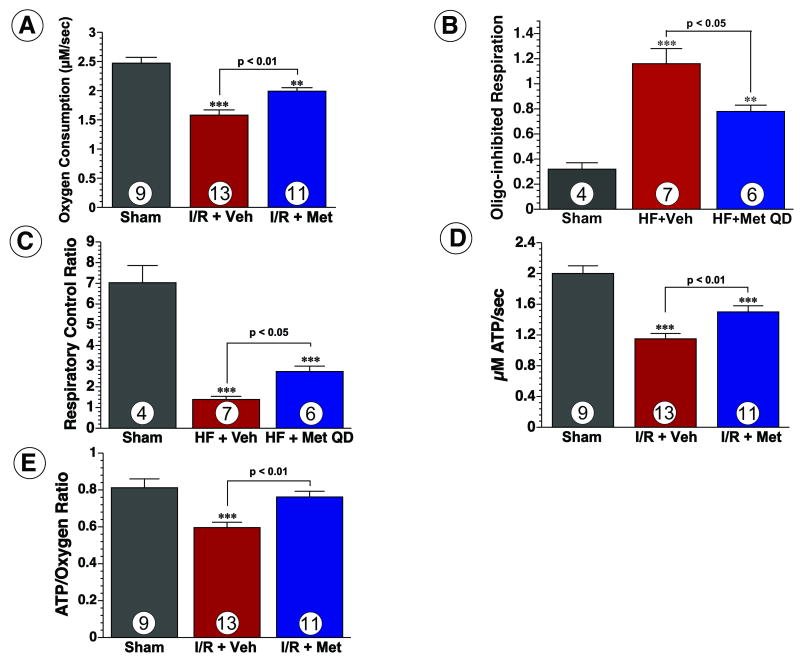
Since both, eNOS and PGC1α are important regulators of mitochondrial biogenesis and mitochondrial function, we investigated if metformin treatment had any beneficial effects on mitochondrial function and ATP synthesis. Mitochondria isolated from the hearts of vehicle-treated mice were found to have a 36% reduction in maximal ADP-stimulated (state 3) oxygen consumption as compared to sham-operated animals (Figure 4A). Mitochondria from vehicle-treated mice also had an increased oligomycin-inhibited respiration (Figure 4B) and reduced respiratory control ratio (Figure 4C), suggestive of uncoupling. Additionally, ATP synthesis rates and the ATP/oxygen consumption ratio in the mitochondria from vehicle-treated mice were significantly reduced as compared to sham-operated mice (Figure 4D-E). Conversely, mitochondria from Metformin QD-treated mice were found to have significantly greater rates of oxygen consumption, lower rates of oligomycin-inhibited respiration, higher respiratory control ratios, greater ATP synthesis rates, and higher ATP/oxygen consumption ratios as compared to vehicle-treated mice. This data indicates that the respiration of cardiac mitochondria during heart failure was inefficient, likely a result of uncoupled respiration, and that metformin treatment attenuates this dysfunction.
Figure 4.
Metformin therapy improves mitochondrial respiration and ATP synthesis. (A) Mitochondrial oxygen consumption rates, (B) oligomycin (oligo)-inhibited respiration, (C) respiratory control ratios of mitochondria isolated from sham-operated, vehicle-treated, and Metformin QD-treated mice 4 wk after myocardial infarction. Mean values (±SEM) are shown for state 3 (ADP-stimulated) respiration in the presence of succinate and glycerol-3-phospate. (D) ATP synthesis rates and (E) the ratio of ATP synthesis to maximal oxygen consumption obtained from the same isolated mitochondria shown in panel A. Values are means ± S.E.M. Numbers inside bars indicate the number of animals that were investigated in each group. *** p < 0.001 vs. Sham, ** p < 0.01 vs. Sham. Veh = vehicle, Met QD = Metformin QD.
Metformin mediated improvements in LV structure and function are abrogated in AMPK deficient mice
To investigate the role of AMPK in metformin-mediated cardioprotection, both AMPKdn and NTg mice were subjected to the ischemia-induced heart failure protocol. Metformin administration at reperfusion decreased Inf/AAR by 30% (43.2±3.6% for vehicle vs. 30.2±5.8% for Met QD, p < 0.05) in NTg mice but not in AMPKdn mice (48.1±5.7% for vehicle vs. 44±8.4% for Met QD, p = NS). Metformin QD did not result in any significant improvements in LVEDD (Figure 5A), LVESD (Figure 5B) and EF (Figure 5C) in AMPKdn mice and no differences were observed in cardiac hypertrophy as measured by H/BW ratios (Figure 5E). Metformin QD however did improve all parameters of LV structure and function in NTg mice compared to vehicle and the results were consistent with the improvement seen in C57BL/6 mice (Figure 5). These data suggest that AMPKα2 plays an important role in metformin-mediated cardioprotection against HF.
Figure 5.
AMPK mediates metformin-induced cardioprotection. (A) LVEDD, (B) LVESD and (C) Ejection Fraction (EF) and (D) H/BW ratios were measured at baseline and following myocardial ischemia in NTg and AMPKdn mice ± daily metformin therapy. Values are means ± S.E.M. Numbers inside bars indicate the number of animals that were investigated in each group. ** p < 0.01 vs. Baseline, *** p < 0.001 vs. Baseline, * p < 0.05 vs. Sham. Veh = vehicle, Met QD = Metformin QD.
Metformin mediated improvements in LV structure and function are abrogated in eNOS-/- mice
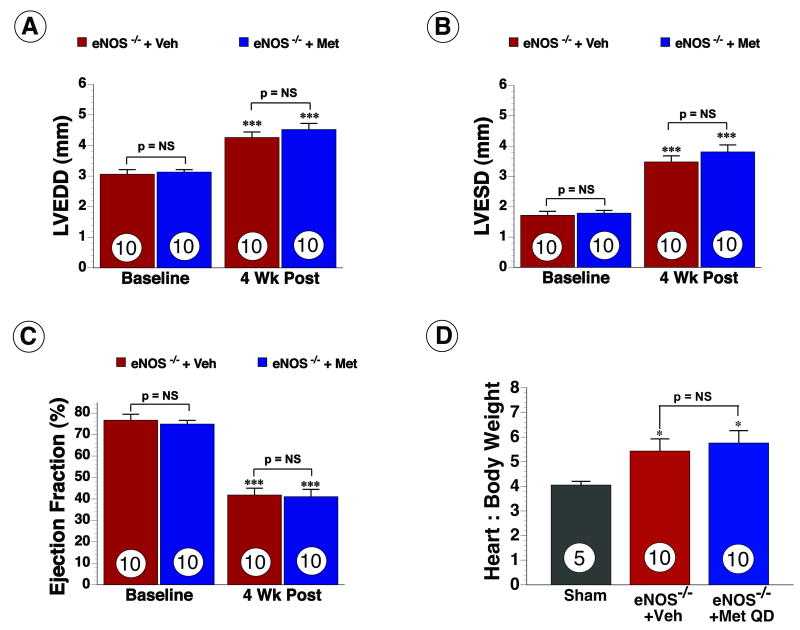
To investigate the role of eNOS in metformin-mediated cardioprotection, eNOS-/- mice were subjected to the HF protocol. Metformin administered at reperfusion did not attenuate Inf per AAR and Inf per LV (p = NS) at 24hr. At 4 wk, Echo analysis revealed LV dilation and systolic dysfunction in the hearts of both groups as evidenced by a significant increase in LVEDD (Figure 6A) and LVESD (Figure 6B) and EF (Figure 6C). Metformin QD did not result in any significant improvements in LVEDD, LVESD and EF in eNOS-/- mice and no differences were observed in cardiac hypertrophy as measured by H/BW ratios. (Figure 6E). These data suggest that eNOS and NO may also be important mediators of metformin-mediated cardioprotection.
Figure 6.
eNOS mediates metformin-induced cardioprotection. (A) LVEDD, (B) LVESD and (C) Ejection Fraction (EF) and (D) H/BW ratios was measured at baseline and following myocardial ischemia in eNOS deficient mice ± daily metformin therapy. Values are means ± S.E.M. Numbers inside bars indicate the number of animals that were investigated in each group. *** p < 0.001 vs. Baseline, * p < 0.05 vs. Sham. Veh = vehicle, Met QD = Metformin QD.
Discussion
This study demonstrates that metformin therapy significantly retards the progression of ischemic cardiomyopathy and heart failure in mice after myocardial infarction. The key findings in this study are: (1) metformin therapy improved survival by 47% after permanent occlusion of the left coronary artery compared to treatment with saline, (2) metformin therapy led to significant improvements in cardiac remodeling and function during heart failure, (3) metformin-mediated cardioprotection was associated with an increase in phosphorylation of AMPK and eNOS and an increase in expression of PGC-1α and (4) metformin therapy attenuated mitochondrial dysfunction during heart failure. These data provide additional insight into the pleiotropic effects of metformin in cardiovascular disease and its therapeutic role in ischemic induced heart failure.
The pleiotrophic actions of metformin are thought to be mediated by the activation of AMPK18,19, an important regulator of diverse cellular pathways20. Chronic activation of AMPK phosphorylates transcription factors altering gene expression21 and modulates mitochondrial biogenesis22. In vitro studies have shown that AMPK activation is a key mediator of the changes in substrate utilization during cardiac ischemia and functions to maintain energy homeostasis, cardiac function and myocardial viability23. Our data demonstrate that metformin increases AMPK phosphorylation and that the cardioprotective actions of metformin were ablated in AMPKα2 dominant negative mice. These results suggest that the chronic activation of AMPK during the development of ischemia-induced heart failure is a critical mechanism mediating the beneficial actions of metformin.
Heart failure is associated with abnormalities of mitochondrial biogenesis24 and mitochondrial injury correlates strongly with its severity25. In heart failure, there is a decrease in activity of complexes of the respiratory chain and Krebs cycle enzymes. The reduced expression of mitochondrial proteins results in decreased mitochondrial respiration efficiency and limited ATP synthesis capacity and myocardial energy production26. The decreased oxidative capacity of the failing myocardium therefore limits the ability of the heart to meet hemodynamic demands and leads to symptoms of heart failure. Both, eNOS and PGC-1α are important regulators of mitochondrial biogenesis and function and play important roles in the pathophysiology of HF27,28. For instance, targeted overexpression of the eNOS gene within the vascular endothelium has been shown to attenuate cardiac dysfunction and improve survival in ischemic cardiomyopathy29. AMPK has been shown to increase the phosphorylation of eNOS leading to an increase in eNOS activity and NO bioavailability30. Additionally, we15 and others8,30 have demonstrated that metformin increases the phosphorylation of eNOS in an AMPK-dependent manner, as evidenced by the finding that metformin fails to increase eNOS phosphorylation in the hearts of AMPKα2 dominant negative mice. The results of the current study support these previous findings, as we found that metformin therapy promotes the phosphorylation of eNOS during heart failure and that the metformin-mediated improvements in LV function were abolished in the absence of eNOS. AMPK is also an upstream activator of PGC-1α and may exert its actions by increasing the expression of PGC-1α9. PGC-1α is a member of a family of transcription coactivators that plays a central role in the regulation of cellular energy metabolism31. PGC-1α is induced in response to conditions that demand increased myocardial ATP synthesis32,33 and has been shown to drive mitochondrial biogenesis and improve mitochondrial function in cardiac myocytes and hearts of transgenic mice28. PGC-1α deficient mice have decreased expression of genes involved in mitochondrial oxidative phosphorylation and have decreased state 3 mitochondrial respiration rates27,33. In our study metformin treatment increases the expression of PGC-1α during heart failure. Furthermore, we have demonstrated that metformin improves mitochondrial oxygen consumption and ATP synthesis. These beneficial actions may be mediated by an increase in PGC1-α expression and/or eNOS phosphorylation.
The findings of the current study highlight a metformin-mediated cardioprotective signaling pathway involving AMPK, eNOS, and PGC-1α Previously, we evaluated the cardioprotective effects of a single administration of metformin in the setting of acute myocardial ischemia-reperfusion injury and found that metformin reduced infarct size when it was administered at the time of reperfusion in an AMPK-eNOS dependent fashion15. While the current study also demonstrates that metformin provides protection in a similar manner there are some important differences. First of all, the findings of the current study are significant because they demonstrate that chronic metformin therapy initiated after myocardial ischemia is beneficial for the treatment of heart failure. Importantly, we found that although a single administration of metformin at the time of reperfusion is beneficial in attenuating infarct size, this alone is not sufficient to cause a significant improvement in cardiac function after 4 wk. On the other hand, daily metformin therapy initiated at the time of reperfusion provided significant improvements in cardiac function and LV dimensions. This suggests that metformin treatment could potentially be initiated at the time of coronary artery reperfusion and then continued daily in patients undergoing myocardial ischemia to achieve a long-term improvement in cardiac function and to decrease the morbidity and mortality resulting from heart failure. These findings support several experimental and clinical studies reporting that metformin possesses significant cardioprotective actions and is safe in the setting of diabetes and HF34,35. Although previously contraindicated in heart failure due to the potential risk for development of lactic acidosis, the Food and Drug Administration (FDA), in response to the findings of several recent studies, has now updated the prescribing information for metformin to eliminate this contraindication36. A meta-analysis of controlled studies evaluating anti-diabetic agents and outcomes in patients with heart failure and diabetes found that metformin when compared to other anti-hyperglycemic therapies significantly reduced mortality and hospital admissions in treated patients despite a similar decrease in hemoglobin A1C values, suggesting that metformin may have additional cytoprotective actions beyond blood glucose lowering actions34. This observation is further supported by experimental studies demonstrating that metformin does not affect glucose values in non-diabetic rodents37, yet improves cardiac function following in vitro global ischemia38. Finally, the findings of the current study expand on our initial findings and provide data demonstrating that metformin can attenuate mitochondrial dysfunction through the activation of AMPK and the downstream signaling pathway involving eNOS and/or PGC-1α. As such, this current study is timely and provides important insights into the use of metformin treatment for cardiovascular disease in all patient populations.
The current study mainly focused on the ability of metformin to improve mitochondrial function during heart failure, however, there are certainly a number of other effects mediated by AMPK, eNOS, and PGC-1α that could account for the observed cardioprotection. In particular, the ability of metformin therapy to promote the phosphorylation of eNOS and increase NO bioavailability provides numerous potential cardioprotective actions in the setting of HF, such as vasodilation and the inhibition of oxidative stress and apoptosis39. All of these actions in addition to the effects of NO on the mitochondria could account for the improvements in LV function following metformin treatment. In particular, the effects of NO on hemodynamics could play an important role in providing prolonged changes in afterload and coronary blood flow regulation, which could then promote LV function and improve LV ejection fraction. However, in a previous study, we found that a single administration of metformin (125 μg/kg) did not alter hemodynamics in the period immediately following its administration. Nonetheless, since we have not evaluated if chronic metformin therapy could alter hemodynamics, we cannot rule out the possibility that the activation of eNOS over the period of 4 weeks can improve outcome through changes in afterload and/or coronary blood flow. Therefore, additional studies are warranted to fully understand the cardioprotective signaling mechanisms of metformin in the treatment of heart failure.
In summary, our findings demonstrate that low dose metformin administered at the time of reperfusion and daily improves survival and affords significant cardioprotection against ischemia-induced heart failure by improving mitochondrial function via activation of AMPK and the downstream signaling pathway involving eNOS and PGC-1α. These data suggest that metformin therapy should not be limited to the treatment of hyperglycemia but may rather have practical clinical use following myocardial infarction in all patient populations to reduce the morbidity and mortality from ischemia induced heart failure.
Acknowledgments
We thank Konstantinos P. Koulogiannis, M.D. and Liran Blum, M.D., for their expert technical assistance during the course of these studies.
Sources of Funding: Supported by a grant from the NIH (2 RO1 HL-060849-08) and the American Diabetes Association (7-04-RA-59) to D.J.L and by a grant from the NIH (F32 DK 077380-01) to J.W.C.
Footnotes
Disclosures: None
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.United Kingdom Prospective Diabetes Study 24: a 6-year, randomized controlled trial comparing sulfonylurea, insulin, and metformin therapy in patients with newly diagnosed type 2 diabetes that could not be controlled with diet therapy. United Kingdom Prospective Diabetes Study Group. Ann Intern Med. 1998;128:165–175. doi: 10.7326/0003-4819-128-3-199802010-00001. [DOI] [PubMed] [Google Scholar]
- 3.Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 4.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 5.Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002;137:25–33. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- 6.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 8.Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem. 2003;278:31629–31639. doi: 10.1074/jbc.M212831200. [DOI] [PubMed] [Google Scholar]
- 9.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbasi F, Chu JW, McLaughlin T, Lamendola C, Leary ET, Reaven GM. Effect of metformin treatment on multiple cardiovascular disease risk factors in patients with type 2 diabetes mellitus. Metabolism. 2004;53:159–164. doi: 10.1016/j.metabol.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Meaney E, Vela A, Samaniego V, Meaney A, Asbun J, Zempoalteca JC, Elisa ZN, Emma MN, Guzman M, Hicks J, Ceballos G. Metformin, Arterial Function, Intima-Media Thickness and Nitroxidation in Metabolic Syndrome: The Mefisto Study. Clin Exp Pharmacol Physiol. 2008 doi: 10.1111/j.1440-1681.2008.04920.x. [DOI] [PubMed] [Google Scholar]
- 12.Xing Y, Musi N, Fujii N, Zou L, Luptak I, Hirshman MF, Goodyear LJ, Tian R. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative alpha2 subunit of AMP-activated protein kinase. J Biol Chem. 2003;278:28372–28377. doi: 10.1074/jbc.M303521200. [DOI] [PubMed] [Google Scholar]
- 13.Gundewar S, Calvert JW, Elrod JW, Lefer DJ. Cytoprotective effects of N,N,N-trimethylsphingosine during ischemia- reperfusion injury are lost in the setting of obesity and diabetes. Am J Physiol Heart Circ Physiol. 2007;293:H2462–2471. doi: 10.1152/ajpheart.00392.2007. [DOI] [PubMed] [Google Scholar]
- 14.Calvert JW, Gundewar S, Yamakuchi M, Park PC, Baldwin WM, 3rd, Lefer DJ, Lowenstein CJ. Inhibition of N-ethylmaleimide-sensitive factor protects against myocardial ischemia/reperfusion injury. Circ Res. 2007;101:1247–1254. doi: 10.1161/CIRCRESAHA.107.162610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57:696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- 16.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duncan JG, Fong JL, Medeiros DM, Finck BN, Kelly DP. Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation. 2007;115:909–917. doi: 10.1161/CIRCULATIONAHA.106.662296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertrand L, Ginion A, Beauloye C, Hebert AD, Guigas B, Hue L, Vanoverschelde JL. AMPK activation restores the stimulation of glucose uptake in an in vitro model of insulin-resistant cardiomyocytes via the activation of protein kinase B. Am J Physiol Heart Circ Physiol. 2006;291:H239–250. doi: 10.1152/ajpheart.01269.2005. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, He H, Balschi JA. Metformin and phenformin activate AMP-activated protein kinase in the heart by increasing cytosolic AMP concentration. Am J Physiol Heart Circ Physiol. 2007;293:H457–466. doi: 10.1152/ajpheart.00002.2007. [DOI] [PubMed] [Google Scholar]
- 20.Hardie DG. The AMP-activated protein kinase pathway--new players upstream and downstream. J Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 21.Woods A, Azzout-Marniche D, Foretz M, Stein SC, Lemarchand P, Ferre P, Foufelle F, Carling D. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol Cell Biol. 2000;20:6704–6711. doi: 10.1128/mcb.20.18.6704-6711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell RR, 3rd, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaper J, Froede R, Hein S, Buck A, Hashizume H, Speiser B, Friedl A, Bleese N. Impairment of the myocardial ultrastructure and changes of the cytoskeleton in dilated cardiomyopathy. Circulation. 1991;83:504–514. doi: 10.1161/01.cir.83.2.504. [DOI] [PubMed] [Google Scholar]
- 25.Sabbah HN, Sharov V, Riddle JM, Kono T, Lesch M, Goldstein S. Mitochondrial abnormalities in myocardium of dogs with chronic heart failure. J Mol Cell Cardiol. 1992;24:1333–1347. doi: 10.1016/0022-2828(92)93098-5. [DOI] [PubMed] [Google Scholar]
- 26.Ning XH, Zhang J, Liu J, Ye Y, Chen SH, From AH, Bache RJ, Portman MA. Signaling and expression for mitochondrial membrane proteins during left ventricular remodeling and contractile failure after myocardial infarction. J Am Coll Cardiol. 2000;36:282–287. doi: 10.1016/s0735-1097(00)00689-6. [DOI] [PubMed] [Google Scholar]
- 27.Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L, Fiorani M, Pisconti A, Brunelli S, Cardile A, Francolini M, Cantoni O, Carruba MO, Moncada S, Clementi E. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc Natl Acad Sci U S A. 2004;101:16507–16512. doi: 10.1073/pnas.0405432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones SP, Greer JJ, van Haperen R, Duncker DJ, de Crom R, Lefer DJ. Endothelial nitric oxide synthase overexpression attenuates congestive heart failure in mice. Proc Natl Acad Sci U S A. 2003;100:4891–4896. doi: 10.1073/pnas.0837428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- 31.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finck BN, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation. 2007;115:2540–2548. doi: 10.1161/CIRCULATIONAHA.107.670588. [DOI] [PubMed] [Google Scholar]
- 33.Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, Rybkin II, Shelton JM, Manieri M, Cinti S, Schoen FJ, Bassel-Duby R, Rosenzweig A, Ingwall JS, Spiegelman BM. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Eurich DT, McAlister FA, Blackburn DF, Majumdar SR, Tsuyuki RT, Varney J, Johnson JA. Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: systematic review. Bmj. 2007;335:497. doi: 10.1136/bmj.39314.620174.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts F, Ryan GJ. The safety of metformin in heart failure. Ann Pharmacother. 2007;41:642–646. doi: 10.1345/aph.1H523. [DOI] [PubMed] [Google Scholar]
- 36.Inzucchi SE, Masoudi FA, McGuire DK. Metformin in heart failure. Diabetes Care. 2007;30:e129. doi: 10.2337/dc07-1686. [DOI] [PubMed] [Google Scholar]
- 37.Verma S, McNeill JH. Metformin improves cardiac function in isolated streptozotocin-diabetic rat hearts. Am J Physiol. 1994;266:H714–719. doi: 10.1152/ajpheart.1994.266.2.H714. [DOI] [PubMed] [Google Scholar]
- 38.Legtenberg RJ, Houston RJ, Oeseburg B, Smits P. Metformin improves cardiac functional recovery after ischemia in rats. Horm Metab Res. 2002;34:182–185. doi: 10.1055/s-2002-26705. [DOI] [PubMed] [Google Scholar]
- 39.Lefer AM. Attenuation of myocardial ischemia-reperfusion injury with nitric oxide replacement therapy. Ann Thorac Surg. 1995;60:847–851. doi: 10.1016/0003-4975(95)00423-I. [DOI] [PubMed] [Google Scholar]