Abstract
Inflammatory bowel disease, including ulcerative colitis and Crohn’s disease, substantially increases the risk of colorectal cancer. However, mechanisms linking mucosal inflammation to the sequence of dysplasia are incompletely understood. While studies have demonstrated oxidative damage to the colon, this study tests whether genotoxicity is elicited systemically by acute and chronic intestinal inflammation. In this study, genotoxic endpoints were assessed in peripheral leukocytes (DNA single and double strand breaks and oxidative DNA damage) and normochromatic erythrocytes (micronuclei) during chemical or immune-mediated colitis. During three consecutive cycles of intestinal inflammation induced by dextran sulfate sodium (DSS) administration, genotoxicity to peripheral leukocytes and erythroblasts was detected in both acute and chronic phases of DSS-induced inflammation. Reactive oxygen species mediated oxidative stress and DNA damage was confirmed with positive 8-oxoguanine and nitrotyrosine staining in peripheral leukocytes. Levels of DNA damage generally decreased during remission and increased during treatment, correlating with clinical symptoms and systemic inflammatory cytokine levels. In Gαi2−/− and IL-10−/− transgenic mice susceptible to immune-mediated colitis and inflammation-associated adenocarcinoma, similar levels of peripheral leukocyte and erythroblast genotoxicity were also observed. Moreover, this systemic genotoxicity was observed in mice with subclinical inflammation, which was further elevated in those with severe mucosal inflammation. We propose that mucosal inflammation, by eliciting substantial and ongoing systemic DNA damage, contributes early on to genetic instability necessary for progression to IBD-associated dysplasia and the development of cancer.
Keywords: ulcerative colitis, Crohn’s disease, dextran sulfate sodium, DNA damage, IL-10, G-alphai2
INTRODUCTION
As many as 1.4 million people in the United States and 2.2 million people in Europe currently suffer from inflammatory bowel diseases (IBD) such as ulcerative colitis (UC) and Crohn’s disease, and the incidence is continuously increasing across the modernizing world (1). Patients afflicted with these diseases also carry an increased risk for development of colorectal cancer, most notably due to the cumulative effects of chronic colonic inflammation (2). In addition to colorectal cancer, hepatobiliary carcinoma and hematopoetic cancers, such as colonic lymphoma, are observed in some patients with long standing UC (3).
A variety of mouse models of chronic colitis have been developed to study the host genetic traits and environmental factors (resident enteric microbiota or mucosal metabolic stressors), many of which demonstrate the association of chronic intestinal inflammation with colonic adenocarcinoma as in human IBD. Perhaps most studied are genetic traits which augment inflammation by impairing metabolic stress mechanisms (4, 5), the mucin barrier (6), innate immune signaling (7), or immune regulation (8–11). These foregoing studies reflect the interplay of such host factors with a cancer-promoting role of resident enteric bacteria, and this interaction has been confirmed by interventional studies (12–15). However, the mechanisms linking metabolic stress, inflammation, and cancer remain incompletely understood.
In addition to genetic models of chronic colitis, an important experimental model of colitis and colitis-associated neoplasia is administration of dextran sulfate sodium (DSS), a non-genotoxic sulfated polysaccharide (16, 17). Acute and chronic colonic inflammation can be induced eventually leading to dysplasia followed by colorectal cancer. Penetrance correlates to duration of inflammation produced either by recurrent administration alone (17, 18), or by brief exposure in mice with genetic traits that amplify and perpetuate colitis (6, 7, 11). DSS-induced colitis has been attributed to direct metabolic epithelial toxicity, augmented by mucosal intrusion of enteric microbiota and their products, with resultant inflammation predominantly driven by innate rather than adaptive immune cell types (6, 12, 16, 19).
Several studies have addressed potential carcinogenesis-promoting mechanisms in colitis, including repair and regeneration of the epithelial barrier, the innate immune response, role of cytokines, and oxidative DNA damage at the local sites of inflammation in the colon (19–22). However, we are unaware of studies that have determined whether intestinal inflammation affects the level of systemic genotoxicity, such as in the peripheral blood. Accordingly, this study was developed to characterize and quantify the association of systemic DNA damage in acute and chronic colitis, by both DSS administration and in models of spontaneous chronic immune colitis.
MATERIALS AND METHODS
Animals
C57BL/6Jpun/pun (3 to 4 months), Gαi2−/− (B6/129Sv background, 3 months) (9)and IL-10−/− (C3H/HeJBir background, 3 or 6 months) were housed in the UCLA Department of Laboratory and Animal Medicine under specific pathogen free conditions, autoclaved bedding and food, with standard rodent chow diet, acidified drinking water, and 12:12 light:dark cycle. All mice were bred at UCLA except IL-10−/− and C3H/HeJ which were purchased from Jackson Laboratory (Bar Harbor, ME).
Induction of chemical colitis
Experimental colitis was induced with 3% (w/v) DSS (Fisher Scientific, MW 40,000) dissolved in acidified drinking water (changed daily) ad libitum for 3 cycles. One cycle consisted of 7 days of treated water followed by 14 days of normal drinking water. Acute colitis was defined as a 7 day treatment, and chronic colitis as any further treatment including remission periods. Control animals received sterile acidified water only. Symptoms (weight loss, stool consistency, gross bleeding) were recorded daily for calculation of disease activity index (23).
Blood collection
Peripheral blood was collected from experimental mice via the facial/mandibular vein with a 5mm lancet (Braintree Scientific, Braintree, MA) into EDTA coated collection tubes (Braintree Scientific). For the comet assay, blood was immediately diluted 1:1 in PBS/10% DMSO and frozen at −80°C until further analysis. Freshly collected blood was immediately processed for all other assays. Identical blood samples were used for genotoxic endpoints as well as for cytokine expression.
Alkaline comet assay
To detect single and double strand breaks, as well as alkali labile sites in DNA, the alkaline comet assay was performed as described previously (24). Frozen blood was further diluted 1:15 in PBS before further preparation. After lysis and electrophoresis, gels were stained with SYBR Gold (Molecular Probes) and visualized under a fluorescent microscope (Olympus Ax70, Tokyo, Japan) at 10x magnification. Comet images were analyzed with the CASP image analysis program (http://casp.sourceforge.net). The olive tail moment, which represents both tail length and fraction of DNA in the tail, was used for data collection and analysis, in which apoptotic cells were excluded under previously proposed criteria (24).
Determination of oxidative DNA damage
The enzyme hOgg1-modified comet assay was used for determination of oxidative DNA damage (25). Following lysis, samples were washed in an enzyme wash buffer (40mM HEPES, 0.1M KCl, 0.5mM EDTA, 0.2mg/ml BSA, pH 8.0) then incubated at 37°C for 10 min in either control (buffer with no hOGG1) or enzyme treated (buffer with hOGG1) solutions according to the manufacturer’s recommendations.(New England Biolabs, Ipswich, MA). Both control and enzyme treated gels were then placed in electrophoresis buffer and processed identically to the alkaline comet assay.
Immunofluorescence
Peripheral blood was incubated in Buffer EL (Qiagen, Valencia, CA) on ice to remove erythrocytes. Samples were then processed on coverslips essentially as described elsewhere (26). Briefly, after fixation, permeabilization, and blocking, cells were incubated with mouse anti-phospho-Histone H2A.X S139(P) at 1:400, mouse anti-8-oxoguanine clone 413.5 at 1:250, or rabbit anti-nitrotyrosine at 1:200 (all from Upstate, Temecula, CA) followed by FITC-conjugated anti-mouse IgG or Rhodamine-conjugated anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA) at 1:200. Coverslips were mounted with VECTASHIELD with 4,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA). Images were captured with CytoVision® (Applied Imaging Corporation, San Jose, CA) connected to a Zeiss Axioplan 2 microscope. At least 125 cells were counted and cells with more than four distinct foci in the nucleus were considered positive for γH2AX(26). Apoptotic cells, which are distinguishable due to presence of 10-fold the number of nuclear foci in damaged cells (27), were not included in analyses.
Paraffin sections (5µm) of colons from IL-10−/− and wildtype controls were microwaved in 10mM citrate buffer (pH 6) for 10min for antigen retrieval, blocked, then incubated with anti-8-oxoguanine or anti-nitrotyrosine followed by secondary antibodies identical to the procedures described above.
In vivo micronucleus assay
Micronuclei (MN) formation was determined in peripheral blood erythrocytes to assess chromosomal instability. Similar to a previously proposed method (28), 3 µl of whole blood was spread on a microscope slide and stained in Modified Wright-Giemsa solution (Sigma-Aldrich, St. Louis, MO). MN were counted and scored with an Olympus Ax70 (Tokyo, Japan) at 100x following previously proposed criteria (29). At least 4000 mature erythrocytes were counted per mouse, and the frequency of MN formation was calculated as number of micronucleated erythrocytes per 1000 normochromatic erythrocytes.
RNA Isolation and Quantitative Real-Time PCR
Total RNA was isolated using QiaAmp RNA Blood Mini Kit (Qiagen) according to manufacturer’s instructions. 25ng/µl of total RNA was used for reverse transcription using OligodT (Invitrogen) and Superscript III Reverse Transcriptase (Invitrogen). 10ng/µl of cDNA was used for quantitative real time PCR using Taqman Gene Expression Assays (Applied Biosystems, Foster City, CA p/n 4331182) for Tbp (TATA binding protein), TNF-α (tumor necrosis factor α), MCP-1 (monocyte chemoattractant protein 1, also known as CC chemokine ligand 2, CCL2), IFN-γ (interferon γ)TGF-β (tumor growth factor β) and Taqman Gene Expression Master Mix according to manufacturer’s instructions on the ABI Prism 7500 sequence detection system (Applied Biosystems). Tbp was chosen as the endogenous control due to its low variability and low to medium relative abundance in terms of expression in blood (30). Each measurement was performed in triplicate and results were analyzed using SDS 2.2.1 software (Applied Biosystems). Gene expression was determined using the relative standard curve method normalized to Tbp expression.
Statistical Analyses
Results are expressed as mean ± standard error of the mean. Statistical significance was determined by nonparametric one way ANOVAs with Dunn’s multiple comparison post test or a paired Student’s t-tests with log-transformed data for time point comparisons, and defined as p<0.05. ANOVAs of linear regression models were used as appropriate. Calculations were performed with the statistical analysis software GraphPad Instat version 3.00 (GraphPad Software, San Diego, CA) or R: A language and environment for statistical computing. (R Development Core Team (2007). R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org).
RESULTS
Evaluation of Experimental Colitis
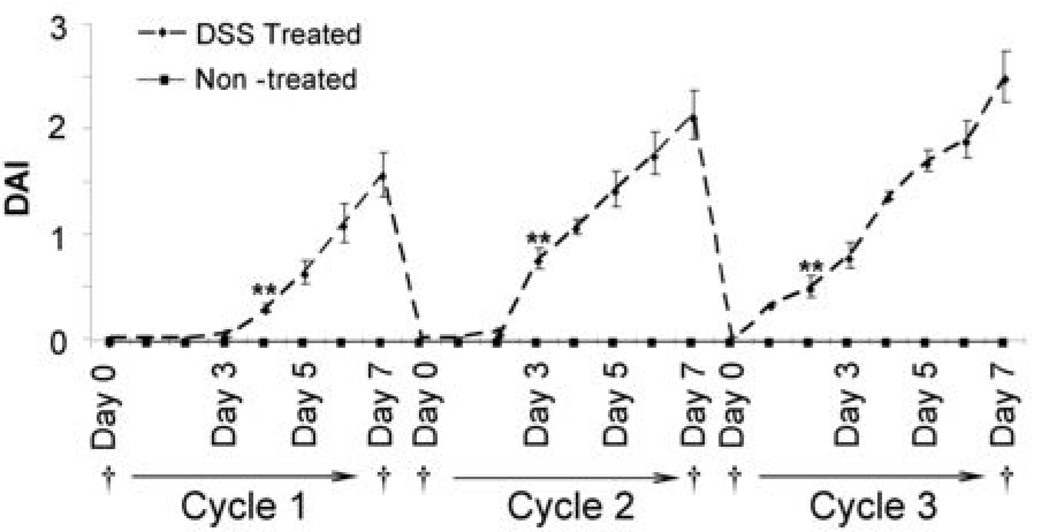
The disease activity index (DAI) is the average combined score of weight loss (0–4), stool consistency (0–4), and bleeding (0–4), used to score clinical symptoms (23). DSS-treated mice demonstrated rectal bleeding starting day 4 in cycle 1, represented by the increase in the DAI compared to non-treated animals (Figure 1). However, the onset of severe symptoms came earlier in the second and third cycles of treatment due to chronic inflammation, even after 14 day remission periods. Bleeding and diarrhea ceased as soon as treatment was stopped during remission and no mortalities were observed after three cycles of treatment. Food intake was also not affected throughout the study and significant weight loss was only apparent during the end of the second and third cycle.
Fig. 1. Disease Activity Index (DAI) of DSS Treated vs. Non-Treated Mice.
DSS treated mice (n=10) demonstrated significantly higher disease activity indices everyday after Day 4 of Cycle 1 (p<0.001), Day 3 of Cycle 2 (p<0.001), and Day 2 of Cycle 3 (p<u0.001) compared to non-treated mice (n=10). Data are represented as mean ± standard error of the mean (SEM). † : Blood collection points.
DSS Treatment Causes DNA Single and Double Strand Breaks in Peripheral Leukocytes
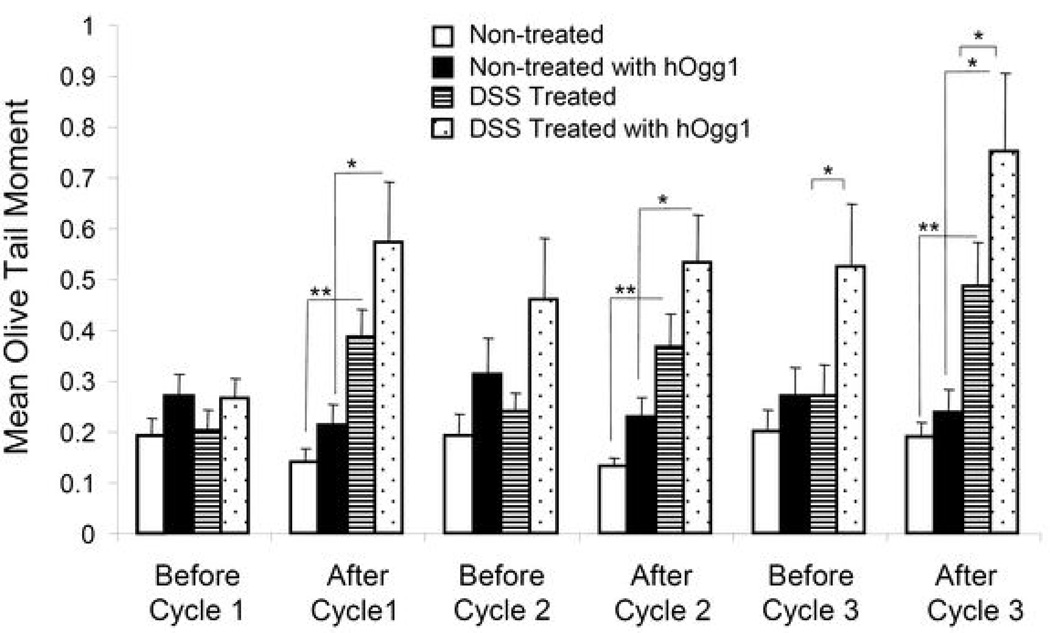
Single and double strand breaks as well as alkali-labile sites in DNA of peripheral leukocytes were measured in terms of the mean olive tail moment with the alkaline comet assay (Figure 2). While mean olive tail moments of non-treated mice remained low throughout each cycle of treatment, DSS treated mice demonstrated significantly higher olive tail moments at the end of each cycle (p<0.01). After each remission period of 14 days, levels of DNA damage decreased most likely due to DNA repair. The hOgg1 modified alkaline comet assay was also used to detect oxidative base damage. Ogg1 primarily recognizes and removes 8-oxoguanine through a base excision repair pathway, as well as 8-oxoadenine, fapy-guanine, and methyl-fapy-guanine (31). Mean olive tail moments were higher when incubated with hOgg1 after treatment cycles in treated mice compared to hOgg1 incubated non-treated mice (p<0.05), indicating presence of oxidized base damage. When compared to levels before cycle 1, hOgg1 incubated DNA from DSS-treated mice were significantly higher at every following time point (p<0.01, not shown). Levels of DNA damage increased with each treatment cycle especially when including oxidative base damage, indicating damaging effects of acute and more significantly, chronic inflammation. A small number of apoptotic cells with extensive DNA fragmentation were apparent after treatment cycles, however were not included in calculation of mean olive tail moments.
Fig. 2. Mean Olive Tail Moments.
At least 150 “comets” were scored per mouse in the DSS treated group (n=10) and in the non-treated group (n=10). Data were log transformed before applying statistical tests, and are represented as mean ± SEM. *: p<0.05, **: p<0.01.
Presence of DNA double strand breaks alone was confirmed with immunofluorescence of γ–H2AX (Figure 3A). In response to double strand breaks, histone 2AX is phosphorylated (γ-H2AX) in a 2-Mbp region flanking the double strand break within 15 minutes (27). Percentage of cells positive for nuclear foci increased dramatically in the DSS treated group after the first 7 day acute treatment (p<0.01) compared to non-treated animals. Although not as dramatic, percent positive cells remained elevated over non-treated animals until end of treatment (p<0.05). Efficient DNA double strand break repair may be activated, decreasing the presence of foci in chronic inflammation due to the severely damaging nature of double strand breaks compared to oxidative base damage or single strand breaks.
Fig. 3. γ-H2AX foci and Micronucleus Induction.
A.Percent positive cells for γ-H2AX foci in peripheral leukocytes. Presence of double strand breaks was confirmed by immunofluorescence of γ-H2AX. Positive cells contained >4 distinct nuclear foci. Image caption: Positive and negative cell for nuclear foci, 100X magnification. At least 125 cells were analyzed per sample. Data are represented as mean ± SEM, n=10 per treatment group. **: p<0.01, *: p<0.05 B. Micronucleus induction in peripheral normochromatic erythrocytes. At least 4000 normochromatic erythrocytes were counted and scored for presence of micronuclei. Data are represented as mean ± SEM of micronucleated normochromatic erythrocytes (MN-NCE) per 1000 NCEs. Image caption: MN-NCEs and NCEs, 100X magnification. **: p<0.01, *: p<0.05, by nonparametric one way ANOVA with Dunn’s multiple comparison test. ANOVA of normal linear regression showed effect of treatment, cycle of treatment and interaction of effect of treatment and cycle of treatment to be significant (p<0.01).
DSS-Induced Inflammation is Clastogenic to Erythroblasts
The in vivo micronucleus (MN) assay was carried out in mature normochromatic erythrocytes circulating in the peripheral blood to determine chromosomal damage to erythroblasts (Figure 3B). The incidence of micronuclei is commonly used as an index of cytogenetic damage, including chromosome breaks, spindle abnormalities, or structurally abnormal chromosomes; most frequently in erythroblasts/erythrocytes from peripheral blood or bone marrow (29). Mature micronucleated normochromatic erythrocytes represent the final developmental stage of erythroblasts containing micronuclei stemming in the bone marrow, and thus permit the study of both the generation and elimination of micronucleated erythrocytes (32).
Micronucleus formation was significantly induced after the first cycle of treatment in DSS treated animals (p<0.01) compared to non-treated animals, and was further induced after the second and third cycles compared to both non-treated animals, and levels before cycle 1 (p<0.01). Similar to patterns seen in the results of the alkaline comet assay, micronuclei formation decreased after remission periods, and increased after each cycle of treatment. This indicates clearance of micronucleated erythrocytes by the spleen followed by induction during treatment periods. Starting at the before cycle 3 time point, micronucleated erythrocyte levels were slightly elevated even in non-treated animals, most likely due to the effects of repeated blood draws and consequentially high rate of erythopoiesis.
DSS Treatment Modulates mRNA Expression of Cytokines in Peripheral Blood
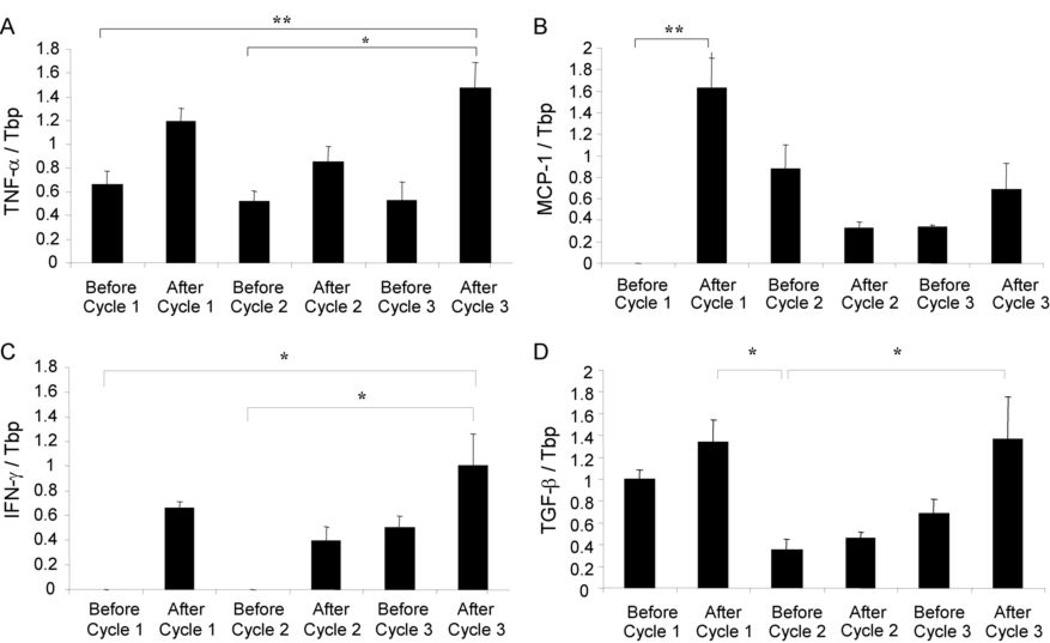
Systemic inflammation due to DSS treatment was demonstrated by cytokine gene expression in the peripheral blood of treated animals. Leukocytes circulating in the periphery mounted a strong Th1 response characterized by up-regulation of TNF-α, MCP-1 (CCL2), and IFN-γ particularly after the first cycle of treatment (Figure 4). TNF-α transcript levels followed DNA damage patterns of increasing after each 7 day treatment cycle, then decreasing after each 14 day remission period. MCP-1 and IFN-γ transcript levels increased after the first cycle, then decreased after the remission period, where they remained low until rising once again in the third cycle; demonstrating a delayed secondary induction compared to TNF-α. TGF-β, an anti-inflammatory cytokine, was also modulated similarly to MCP-1 and IFN-γ. DSS treatment induces both a Th1 response as well as an anti-inflammatory response over the acute and chronic phases of treatment in the peripheral blood.
Fig. 4. Quantitative Real Time-PCR of Cytokines in Peripheral Blood.
Expression levels of cytokines were determined only in DSS treated mice (n=10). Data are represented as mean ± SEM of gene expression divided by Tbp expression. A. Transcript levels of TNF-α divided by Tbp. B. Transcript levels of MCP-1 divided by Tbp. C. Transcript levels of IFN-γ divided by Tbp. D. Transcript levels of TGF-β divided by Tbp. Statistical significance was determined by non-parametric one-way ANOVAs with Dunn’s multiple comparison test. *: p<0.05, **: p<0.01
DNA Damage is Observed in Genetic Models of Mucosal Inflammation
In order to further determine whether systemic genotoxicity is a general consequence of colitis, we measured DNA damage in two genetic models of mucosal inflammation without the use of DSS. We examined Gαi2−/− mice at age 3 months (chronic active inflammation with neoplastic changes in colon), and IL-10−/− at age 3 and 6 months (in which mice have subclinical disease with minimal histologic inflammation, and active disease and inflammation with mild epithelial hyperplasia, respectively) (Figure 5A). Single and double DNA strand breaks were significantly higher (p<0.01) in both Gαi2 −/− mice compared to age-matched Gαi2 +/− mice without clinical symptoms and in IL-10−/− mice with sub-clinical inflammation compared to age-matched IL-10+/+ mice using the comet assay (Figure 5B). We then hypothesized that IL-10−/− mice with severe mucosal inflammation would have greater DNA damage than those with sub-clinical inflammation. These mice indeed demonstrated higher levels of strand breaks than IL-10−/− mice with sub-clinical inflammation (p<0.01), comparable to those seen in Gαi2 −/− mice. Oxidative base damage, however, seemed only apparent in Gαi2 mice as measured by hOgg1 incubation. DNA double strand breaks measured by γ-H2AX immunofluorescence (Figure 5C) were also elevated in both Gαi2 −/− and IL-10−/− compared to Gαi2 +/− and IL-10+/+ mice, respectively, though only statistically significant in Gαi2 −/− mice (p<0.05), and in IL-10−/− mice with severe mucosal inflammation (p<0.05). Finally, micronucleus induction in erythroblasts were also significantly elevated in Gαi2 −/− mice compared to Gαi2 +/− mice (p<0.01), and elevated but not statistically significantly elevated in IL-10−/− versus IL-10+/+ mice (Figure 5D). Systemic genotoxicity can therefore be incurred by several modes of inflammation, independent of DSS administration.
Fig. 5. Systemic Genotoxicity in Mouse Models of Mucosal Inflammation.
Blood was sampled from Gαi2−/−, IL-10−/−, and control IL-10+/+ mice for genotoxicity assays at age 3 months; in addition, IL-10−/− mice were sampled at age 6 months, when colitis in this genetic background has progressed to greater clinical activity. A. Representative colon histology (hematoxylin and eosin staining at indicated magnifications) from Gαi2 and IL-10 mice, both at age 3 months. B. Alkaline comet assay with and without hOgg1 incubation was carried out in peripheral leukocytes. Error bars are SEM, n=6 per group. *: p<0.05, **: p<0.01 by Student’s unpaired t-test. C. Percent positive cells for γ-H2AX foci in peripheral leukocytes. Error bars are SEM. *:p<0.05 by Student’s unpaired t-test. D. MN-NCEs per 1000 NCEs in peripheral blood. Error bars are SEM. **: p<0.01 by Student’s unpaired t-test.
Intestinal Inflammation Induces ROS Mediated Oxidative Stress and DNA Damage
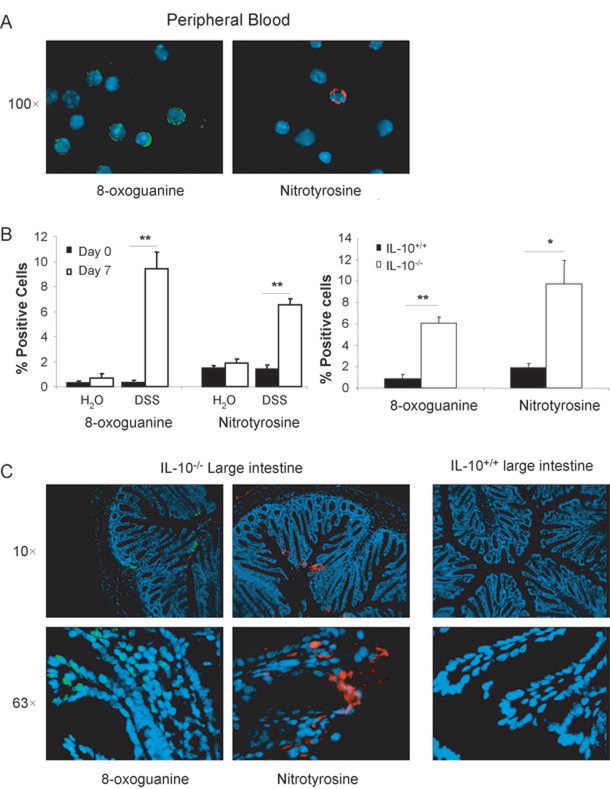
To determine potentially causative species of oxidative stress due to intestinal inflammation, peripheral leukocytes from DSS treated mice (7 days, 3% w/v) and IL-10−/− mice (6 months) were isolated and stained for 8-oxoguanine or nitrotyrosine (Figures 6A and B). 8-oxoguanine is an oxidative DNA lesion formed by reaction of hydroxyl radicals, metal hyrdroperoxides, or peroxynitrite with DNA, causing G:C to T:A transversions during replication (33). Nitrotyrosine is a biochemical marker for NO-induced peroxynitrite formation involving reactions with reactive oxygen and nitrogen species resulting in nitrative damage to proteins (34). DSS-induced inflammation caused a significant increase in both 8-oxoguanine and nitrotyrosine (p<0.01) in peripheral leukocytes, as did those isolated from IL-10−/− mice (p<0.05). Colon sections from IL-10−/− mice also demonstrated 8-oxoguanine residues mostly in the nuclei of the surface epithelial cells as well as in infiltrating inflammatory cells within or near the lamina propria as “focus” like or pan-nuclear staining (Figure 6C). Nitrotyrosine residues were also present in the cytoplasm of epithelial cells and inflammatory cells, whereas no immunoreactivity was observed in wildtype mice.
Fig. 6. 8-oxoguanine and Nitrotyrosine Formation in Peripheral Leukocytes and the Colon.
A. Representative images of positive staining for 8-oxoguanine (green, left) and nitrotyrosine (red, right) in leukocytes of DSS-treated wildtype (7 days) and IL-10−/−mice (6 months). B. Percent positive cells for 8-oxoguanine and nitrotyrosine staining before and after DSS treated mice (7 days), n=6 per group (LEFT) and in IL-10−/− mice (6 months), n=4 per group (RIGHT). *: p<0.05, **: p<0.01 by Student’s unpaired t-test. C. Representative images of 8-oxoguanine (green) and nitrotyrosine (red) in colon sections of IL-10−/− mice (6 months) and wildtype mice.
DISCUSSION
Previous studies have established the role of inflammation-derived oxidative DNA damage to inflammatory and surrounding epithelial cells only at the localized sites of inflammation in the colon. Our study demonstrates for the first time that this damage extends beyond the site of inflammation to circulating leukocytes and erythroblasts in the bone marrow, manifesting a systemic effect, and correlating to oxidative damage found in inflammatory tissue. Genotoxicity to peripheral leukocytes was evident in terms of both single and double strand breaks to DNA accompanied by oxidative base damage while chromosomal aberrations took place in erythroblasts. Such findings were observed both in acute and chronic phases of chemical colitis induced by DSS administration, and in untreated Gαi2−/− and IL-10−/− mice undergoing spontaneous immune colitis. Moreover, in IL-10−/− mice, which are notable for a delayed onset of colitis, genotoxicity was further elevated in mice which had proceeded to a state of clinically active colitis versus those with sub-clinical inflammation. Markers of reactive oxygen species (ROS) derived oxidative stress demonstrated presence of 8-oxoguanine and nitrotyrosine in peripheral leukocytes of DSS treated mice and IL-10−/− mice, representing possible mechanisms of genotoxicity and correlating to oxidative damage seen in the colon. Accordingly, the present study reveals that systemic genotoxicity is a prevalent feature of subclinical, acute, and chronic colitis.
In DSS-treated mice, repair of DNA damage was observed during remission periods, represented by a decrease in damage markers. However, the extent of repair appeared slightly less in the last remission due to increasing severity of chronic inflammation. Despite increasing severity of inflammation, double strand breaks remained only slightly elevated over non-treated animals, which may imply efficient repair in comparison to single strand breaks and oxidative damage. DSS administration also induced systemic distribution of cytokines, as evidenced by modulation of transcript levels in peripheral blood. Interestingly, TNF-α was up-regulated during treatment, and down-regulated during remission, mirroring patterns seen in genotoxicity to leukocytes. Similar to previous cytokine studies in the colons of DSS treated mice (20), features of both Th1 and Th2 activity were observed systemically in the peripheral blood, leading to chronic activation of immune cells. The decrease in MCP-1 and IFN-γ expression after the first cycle of treatment may be explained by a shift towards higher expression of Th2 cytokines and a decrease in selective Th1 cytokines, as recently documented (35) in DSS treated mice. Chronic DSS treatment mimics IBD with similar cytokine profiles demonstrating dysregulated and imbalanced immunologic responses to commensal bacterial antigens. Dysregulated and polarized cytokine production play key roles in enhancing chronic inflammation and tumorigenesis through signaling release of pro-tumor mediators (36).
The present study shows that both chemical and genetic/immune models of inflammation-mediated carcinogenesis not only parallel the inflammation to dysplasia to cancer sequence of human IBD, but also manifest inflammation-associated oxidative stress in the colon as seen in UC and Crohn’s disease. Unlike other colitis-associated neoplasia models utilizing genotoxic colon carcinogens as initiators of neoplasia (azoxymethane or 1,2-dimethylhydrazine), DSS itself is not a mutagen nor genotoxic (37). However, it has been shown to both directly and indirectly activate macrophages and other inflammatory cells (16, 38), a central feature of genetic models of immune colitis (8–11). Thus, carcinogenesis arising in these settings is solely a manifestation of chronic inflammation. The prominent mucosal and systemic activation of macrophages, neutrophils, eosinophils, and other effectors in DSS-induced colitis, genetic immune colitis (and in active disease of patients with IBD) is a potential source of oxidative stress. This may cause oxidative and nitrative damage locally through oxidative burst, and through release of cytokines that induce receptor-mediated reactive oxidative species production by target cells. Microsatellite instability was identified in tumors in colons of DSS-treated wildtype mice, and more so in Msh2−/− mice (39). DSS treatment also induced 8-oxoguanine residues in mouse colonic mucosa (22), suggesting oxidative damage directly at the site of inflammation. Notably, this observed systemic genotoxicity is a secondary effect of DSS treatment, namely the consequence of systemic inflammation and inflammation-associated oxidative stress. In agreement with these findings, we have demonstrated 8-oxoguanine and nitrotyrosine formation in the surface epithelium and inflammatory infiltrate of IL-10−/− colons as well as in peripheral blood of IL-10−/− and DSS treated wildtype mice, indicating systemic presence of peroxynitrite and reactive oxygen and nitrogen species.
We envision two, non-exclusive processes linking local inflammation and systemic genotoxicity. First, locally activated innate immune cells may release reactive species inducing formation of other reactive species such as hydroxyl radicals and NO-derived peroxynitrite, damaging emigrating resident leukocytes, that then circulate into the periphery. Alternatively, inflammatory cytokines achieve biologically significant systemic levels, upon which they induce autonomous, cytokine-receptor mediated production of free radicals (and genotoxic damage) in remote leukocyte populations. Both scenarios are possible, as we observed pro-inflammatory cytokines throughout DSS treatment in the peripheral blood, and oxidative DNA damage and nitrotyrosine formation in circulating leukocytes. Similarly, micronucleus formation in the erythroblasts of the bone marrow in our study may have been a result of activated T-cells that are part of the normal recirculating lymphocyte pool circulating into the bone marrow, and leading to oxidative damage. Accumulation of single and double strand breaks can sequentially lead to chromosome breaks and micronuclei formation (40).
In addition, biologic processes affected by inflammation may also determine the fate of cells bearing genotoxic damage. Since inflammatory mediators elicit both epithelial cell proliferation and anti-apoptotic signals, epithelial cells in chronic inflammation are at particular risk to DNA damage leading to fixation of mutations that may not be properly repaired and removed (22). In DSS colitis, oxidative DNA damage was positively correlated with apoptosis in the small intestine but not the large intestine (41). This biologic difference may contribute to the relative susceptibility to cancer progression in the large intestine. While the mechanism of this differential induction of apoptosis is uncertain, genotoxic stress induces expression of ligands for the NKG2D receptor (42). This receptor is differentially expressed on resident CD8+ T cells and natural killer cells of the small versus large intestine, and is a potent inducer of anti-epithelial cytotoxicity in this intestinal region (43). Finally, the possibility of reciprocal regulation of inflammation and DNA repair pathway elements is an emerging area of investigation (44).
In summary, intestinal inflammation is associated with systemic genotoxicity through single and double DNA strand breaks, oxidative DNA damage, protein nitration, and micronucleus formation. We propose that elements of the inflammatory response including ROS derived oxidative stress are responsible for the observed systemic genotoxicity, although the exact cell types and inflammatory products responsible remain to be defined. Previous studies have observed oxidative base damage, microsatellite instability, and gene mutations directly in the colonic mucosa of both human IBD and experimental murine colitis. Here, we highlight that systemic DNA damage accompanied by systemic inflammation is an early event involved in the promotion of genetic instability. Such systemic genotoxicity may be a biologically relevant and sensitive biomarker of one process contributing to inflammation-associated carcinogenesis.
ACKNOWLEDGEMENTS
Supported by NIH grants ES09519 (RS), DK46763 (JB), CA016042 (Jonsson Comprehensive Cancer Center), the Jonsson Comprehensive Cancer Center Foundation (RS and JB), and the Crohn’s and Colitis Foundation of America (JB). We would like to thank Nagesh Rao for technical assistance with the micronucleus assay.
Footnotes
No disclosures of conflict of interest.
REFERENCES
- 1.Loftus EV. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323(18):1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 3.Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008;14(3):378–389. doi: 10.3748/wjg.14.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu FF, Esworthy RS, Chu PG, et al. Bacteria-induced intestinal cancer in mice with disrupted Gpx1 and Gpx2 genes. Cancer Res. 2004;64(3):962–968. doi: 10.1158/0008-5472.can-03-2272. [DOI] [PubMed] [Google Scholar]
- 5.Maggio-Price L, Bielefeldt-Ohmann H, Treuting P, et al. Dual infection with Helicobacter bilis and Helicobacter hepaticus in p-glycoprotein-deficient mdr1a−/−mice results in colitis that progresses to dysplasia. Am J Pathol. 2005;166(6):1793–1806. doi: 10.1016/S0002-9440(10)62489-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An G, Wei B, Xia B, et al. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med. 2007;204(6):1417–1429. doi: 10.1084/jem.20061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greten FR, Eckmann L, Greten TF, et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118(3):285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Beatty PL, Plevy SE, Sepulveda AR, Finn OJ. Cutting edge: transgenic expression of human MUC1 in IL-10−/−mice accelerates inflammatory bowel disease and progression to colon cancer. J Immunol. 2007;179(2):735–739. doi: 10.4049/jimmunol.179.2.735. [DOI] [PubMed] [Google Scholar]
- 9.Rudolph U, Finegold MJ, Rich SS, et al. Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nat Genet. 1995;10(2):143–150. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- 10.McPherson M, Wei B, Turovskaya O, Fujiwara D, Brewer S, Braun J. Colitis immunoregulation by CD8+ T cell requires T cell cytotoxicity and B cell peptide antigen presentation. Am J Physiol Gastrointest Liver Physiol. 2008 doi: 10.1152/ajpgi.90221.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nemetz N, Abad C, Lawson G, et al. Induction of colitis and rapid development of colorectal tumors in mice deficient in the neuropeptide PACAP. Int J Cancer. 2008;122(8):1803–1809. doi: 10.1002/ijc.23308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popivanova BK, Kitamura K, Wu Y, et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118(2):560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Mahony L, Feeney M, O'Halloran S, et al. Probiotic impact on microbial flora, inflammation and tumour development in IL-10 knockout mice. Aliment Pharmacol Ther. 2001;15(8):1219–1225. doi: 10.1046/j.1365-2036.2001.01027.x. [DOI] [PubMed] [Google Scholar]
- 14.Erdman SE, Poutahidis T, Tomczak M, et al. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol. 2003;162(2):691–702. doi: 10.1016/S0002-9440(10)63863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takedatsu H, Michelsen KS, Wei B, et al. TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T-Helper 1 and T-Helper 17 activation. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98(3):694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 17.Cooper HS, Murthy S, Kido K, Yoshitake H, Flanigan A. Dysplasia and cancer in the dextran sulfate sodium mouse colitis model. Relevance to colitis-associated neoplasia in the human: a study of histopathology, B-catenin and p53 expression and the role of inflammation. Carcinogenesis. 2000;21(4):757–768. doi: 10.1093/carcin/21.4.757. [DOI] [PubMed] [Google Scholar]
- 18.Lennard-Jones JE, Melville DM, Morson BC, Ritchie JK, Williams CB. Precancer and cancer in extensive ulcerative colitis: findings among 401 patients over 22 years. Gut. 1990;31(7):800–806. doi: 10.1136/gut.31.7.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107(6):1643–1652. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 20.Dieleman LA, Akol H, Bloemena E, PeÑA AS, Meuwissen SGM, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114(3):385. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohkusa T, Okayasu I, Tokoi S, Araki A, Ozaki Y. Changes in bacterial phagocytosis of macrophages in experimental ulcerative colitis. Digestion. 1995;56(2):159–164. doi: 10.1159/000201236. [DOI] [PubMed] [Google Scholar]
- 22.Meira LB, Bugni JM, Green SL, et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest. 2008;118(7):2516. doi: 10.1172/JCI35073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murthy SN, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;38(9):1722–1734. doi: 10.1007/BF01303184. [DOI] [PubMed] [Google Scholar]
- 24.Olive PL, Banath JP. The comet assay: a method to measure DNA damage in individual cells. Nat Protocols. 2006;1(1):23–29. doi: 10.1038/nprot.2006.5. [DOI] [PubMed] [Google Scholar]
- 25.Smith CC, O'Donovan MR, Martin EA. hOGG1 recognizes oxidative damage using the comet assay with greater specificity than FPG or ENDOIII. Mutagenesis. 2006;21(3):185–190. doi: 10.1093/mutage/gel019. [DOI] [PubMed] [Google Scholar]
- 26.Goldstine JV, Nahas S, Gamo K, et al. Constitutive phosphorylation of ATM in lymphoblastoid cell lines from patients with ICF syndrome without downstream kinase activity. DNA Repair. 2006;5(4):432–443. doi: 10.1016/j.dnarep.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Muslimovic A, Ismail IH, Gao Y, Hammarsten O. An optimized method for measurement of gamma-H2AX in blood mononuclear and cultured cells. Nat Protocols. 2008;3(7):1187. doi: 10.1038/nprot.2008.93. [DOI] [PubMed] [Google Scholar]
- 28.Schmid W. Chemical mutagens Principles and methods for their detection. New York: Plenum; 1976. The micronucleus test for cytogenetic analysis; pp. 31–53. [Google Scholar]
- 29.Hayashi M, Tice RR, MacGregor JT, et al. In vivo rodent erythrocyte micronucleus assay. Mutat Res. 1994;312(3):293–304. doi: 10.1016/0165-1161(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 30.Lossos IS, Czerwinski DK, Wechser MA, Levy R. Optimization of quantitative real-time RT-PCR parameters for the study of lymphoid malignancies. Leukemia. 17(4):789–795. doi: 10.1038/sj.leu.2402880. [DOI] [PubMed] [Google Scholar]
- 31.Bjoras M, Luna L, Johnsen B, et al. Opposite base-dependent reactions of a human base excision repair enzyme on DNA containing 7, 8-dihydro-8-oxoguanine and abasic sites. EMBO J. 1997;16(20):6314–6322. doi: 10.1093/emboj/16.20.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinheider G, Neth R, Marquardt H. Evaluation of nongenotoxic and genotoxic factors modulating the frequency of micronucleated erythrocytes in the peripheral blood of mice. Cell Biol Toxicol. 1986;2(1):197–211. doi: 10.1007/BF00117712. [DOI] [PubMed] [Google Scholar]
- 33.Pinlaor S, Ma N, Hiraku Y, et al. Repeated infection with Opisthorchis viverrini induces accumulation of 8-nitroguanine and 8-oxo-7,8-dihydro-2'-deoxyguanine in the bile duct of hamsters via inducible nitric oxide synthase. Carcinogenesis. 2004;25(8):1535–1542. doi: 10.1093/carcin/bgh157. [DOI] [PubMed] [Google Scholar]
- 34.Liu JS, Zhao ML, Brosnan CF, Lee SC. Expression of inducible nitric oxide synthase and nitrotyrosine in multiple sclerosis lesions. Am J Pathol. 2001;158(6):2057. doi: 10.1016/S0002-9440(10)64677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alex P, Zachos NC, Nguyen T, et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15(3):341–352. doi: 10.1002/ibd.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johansson M, Denardo DG, Coussens LM. Polarized immune responses differentially regulate cancer development. Immunol Rev. 2008;222(1):145–154. doi: 10.1111/j.1600-065X.2008.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mori H, Ohbayashi F, Hirono I, Shimada T, Williams GM. Absence of genotoxicity of the carcinogenic sulfated polysaccharides carrageenan and dextran sulfate in mammalian DNA repair and bacterial mutagenicity assays. Nutr Cancer. 1984;6(2):92–97. doi: 10.1080/01635588509513812. [DOI] [PubMed] [Google Scholar]
- 38.Shintani N, Nakajima T, Sugiura M, et al. Proliferative effect of dextran sulfate sodium (DSS)-pulsed macrophages on T cells from mice with DSS-induced colitis and inhibition of effect by IgG. Scand J Immunol. 1997;46(6):581–586. doi: 10.1046/j.1365-3083.1997.d01-169.x. [DOI] [PubMed] [Google Scholar]
- 39.Kohonen-Corish MRJ, Daniel JJ, te Riele H, Buffinton GD, Dahlstrom JE. Susceptibility of Msh2-deficient mice to inflammation-associated colorectal tumors. Cancer Res. 2002;62(7):2092–2097. [PubMed] [Google Scholar]
- 40.Obe G, Pfeiffer P, Savage JRK, et al. Chromosomal aberrations: formation, identification and distribution. Mutat Res. 2002;504(1–2):17–36. doi: 10.1016/s0027-5107(02)00076-3. [DOI] [PubMed] [Google Scholar]
- 41.Hong MY, Turner ND, Carroll RJ, Chapkin RS, Lupton JR. Differential response to DNA damage may explain different cancer susceptibility between small and large intestine. Exp Biol Med. 2005;230(7):464–471. doi: 10.1177/153537020523000704. [DOI] [PubMed] [Google Scholar]
- 42.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436(7054):1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meresse B, Curran SA, Ciszewski C, et al. Reprogramming of CTLs into natural killer-like cells in celiac disease. J Exp Med. 2006;203(5):1343. doi: 10.1084/jem.20060028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coscoy L, Raulet DH. DNA mismanagement leads to immune system oversight. Cell. 2007;131(5):836–838. doi: 10.1016/j.cell.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]