Abstract
Autoantigens that contain DNA, RNA, or self-IgG are preferred targets for autoantibodies in Systemic Lupus Erythematosus (SLE). B cells promote SLE pathogenesis by: producing autoantibodies, activating autoreactive T cells, and secreting cytokines. We discuss how certain autoreactive B cells are selectively activated, with emphasis on the roles of key Toll-like receptors (TLRs). Although TLR7, which recognizes ssRNA, promotes autoimmune disease, TLR9, which recognizes DNA, unexpectedly regulates disease, despite being required for the secretion of anti-chromatin autoantibodies. We describe positive feedback loops involving B cells, T cells, DCs and soluble mediators and how these networks are regulated by TLR signals.
Introduction
Systemic autoimmune diseases, such as Systemic Lupus Erythematosus (SLE), are chronic—typically waxing and waning—syndromes that result in damage to diverse organ systems by a variety of immune mechanisms. They are undoubtedly the product of multiple and stepwise failures of immune regulation, leading to a complex scenario of established disease. Nonetheless, this does not mean that in lupus there is simply global immune activation. Rather, there is clearly specificity both in terms of lymphocyte activation, and also in the pivotal role of certain cell types and cytokines. The clues to better understanding and therapy of these diseases must come from a better understanding of the specific nature of aberrant immune activation and the temporal relationship of these events. What stimulates and what sustains autoimmunity? What specific immune circuits are dysregulated? What are the targets of self-reactivity and why?
Positive Feedback in Autoimmunity
Normally, the immune system is autoregulatory in the sense that the immune response is damped by a variety of counter-regulatory mechanisms that are induced at the time of immune activation. It is reasonable to assume that among the many genetic factors that contribute to disease are alterations in regulatory molecules or circuits, essentially reducing the brakes on (auto)immune responses [1–8]. In the context of a positive feedback loop, small changes in tuning based on genetic factors could be amplified, converting a transient response into one that is sustained and pathogenic. In addition, the immune response to foreign antigens is typically limited by the clearance of those antigens. In autoimmunity, the autoantigens are never cleared, thus driving the response indefinitely.
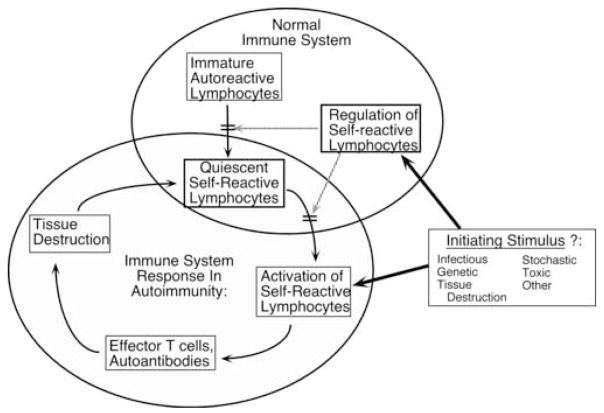
Another implication of positive feedback is that the signals and stimuli that sustain a response need not be the same as those that initiate it. Thus, an environmental stimulus, such as a toxin or an infectious agent, could initiate an anti-self response due to temporary immune dysregulation or cross-reactivity; such a response could then be sustained without the need for the initiating stimulus. Self-amplifying loops are also consistent with the waxing and waning nature of many systemic autoimmune diseases, as exemplified by the lupus “flare”. A fundamental aspect of the positive feedback concept in autoimmunity is that once self-tolerance is lost and effector function is generated, subsequent tissue damage leads to release of more self-antigen, potentially in an immunogenic form. Resultant inflammation also increases the chances that this self-antigen will lead to activation of autoreactive lymphocytes. We first proposed a general version of positive feedback in autoimmunity in the mid-1980’s (Figure 1).
Figure 1. An early model of positive feedback in the genesis of systemic autoimmunity.
Normally, the immune system (upper right large ellipse) generates autoreactive lymphocytes via the stochastic rearrangement of receptors, but tolerance mechanisms now known to include clonal deletion and receptor editing, prevent the maturation of some of these. However, some of these autoreactive cells will develop and mature, where they are normally held in check by a variety of peripheral tolerance mechanisms. One or more initiating stimuli or preconditions (such as genetic predisposition) can subvert either central or peripheral regulation of autoreactive cells, allowing them to be activated. Chronic autoimmunity will ensue if these lymphocytes generate effector functions, cause tissue injury and inflammation and autoantigen release that is able to promote further activation of autoreactive lymphocytes. It is envisioned that an initial insult could thus be converted to a self-sustaining autoimmune condition, with endogenous stimuli proving sufficient to provide dynamic and continuous activation of autoreactive lymphocytes.
As conceptually useful as this construct might be, however, it lacks mechanistic detail (perhaps not surprising given its 1980’s vintage). If feedback loops do in fact exist, then they have to be embodied by particular cell types and inflammatory mediators that communicate among these cells. In addition, specific antigens have to be incorporated as initiators and sustainers of the reaction. Connections, and the forces that regulate them, need to be identified at the cellular and molecular level. A better understanding of these circuits in a more detailed and accurate model of positive feedback should enable more intelligent design of specific inhibitors or modulators that will effectively dampen or interrupt disease.
B Cells are Central to SLE Pathogenesis
The discovery in the mid to late 1990’s that B cells played central roles in the pathogenesis of lupus [9–13] and other autoimmune diseases [14–16], gave some specific detail to the concept of positive feedback. In particular, T cell activation and target tissue infiltration were both decreased in lupus-prone MRL/Mp mice, either in the presence or absence of the Faslpr/lpr mutation, when B cells were eliminated by genetic means [9, 10, 13]. This elucidated one mechanism of positive feedback, in that B cells could promote T cell activation. Moreover, these data suggested a new function, beyond secretion of autoantibodies, for the role of B cells in lupus pathogenesis. Subsequent work confirmed this formally by demonstrating that B cells that could not secrete antibodies could nonetheless support spontaneous T cell activation, T cell tissue infiltration and early mortality in the MRL/Mplpr/lpr lupus model [11]. This connection has been extensively discussed in a prior review [17].
It has been widely assumed that B cells are important APCs for autoreactive T cells [15, 17–20], a concept supported by direct investigations on the potential of autoreactive B cells to activate T cells in vivo and in vitro [21–25]. However, the relative importance of B cell APC function has yet to be determined, and there may be additional roles for B cells, including secretion of cytokines and maintenance of lymphoid architecture (reviewed recently in [26]). Emerging studies in humans suggest that depletion of B cells in vivo can be an effective therapy for lupus [27–29]; analogous studies have demonstrated this clearly in the case of rheumatoid arthritis [30]. Intriguingly, in one study of B cell depletion in SLE patients, clinical response correlated with a decrease in T cell activation in blood, suggesting the existence of a T-B positive feedback loop [28].
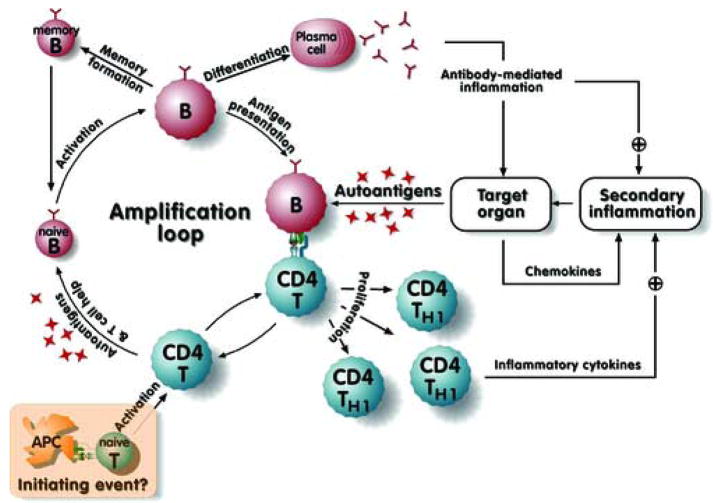
From these studies, a new view of feedback loops also emerged, which attempted to incorporate both classical pathogenesis mechanisms as well as novel roles for B cells (Figure 2, adapted from [12]). This model places B cells at the center of an amplification loop in which they activate CD4+ T cells, which in turn expand and activate additional autoreactive naïve B cells. The loop is further amplified by the inflammation and release of self-antigen that accompanies both T cell-mediated and antibody-mediated immunopathology. These results focused on the B cell as an important component, and possibly central to the regulation of autoimmunity. However, they did not shed light on the initiating factors—genetic or environmental—that activate these B cells in the first place. Nor do they distinguish overall initiating events (for example, initial activation of T cells) from subsequent amplifying events. Are B cells the initiating APC [23, 31–34] or do DCs play this role [35–38]?
Figure 2. Multiple roles of B cells in promoting positive feedback loops and pathogenesis.
This model was originally proposed in a slightly modified form in Chan et al. [12], and was kindly adapted and redrawn by T. Winkler (Erlangen, GE). A primary amplification loop occurs when B cells activate autoreactive T cells, which in turn can help autoreactive B cells proliferate, differentiate and secrete autoantibody. Whether the initiating antigen-presenting cell for autoreactive T cells is a B cell or a dendritic cell is unspecified, though recent evidence suggests B cells can perform this function as well [34]. Activated T cells can also be pathogenic via direct cytotoxicity and cytokine release, factors that were underappreciated in lupus at the time the model was suggested. Autoantigen release and target organ inflammation are proposed to provide positive feedback for further activation of self-reactive lymphocytes. In addition, antibody-mediated effector function will both damage organs and promote further inflammation.
Specificity of B Cell Autoimmune Responses
These questions are in turn linked to the issue of specificity in systemic autoimmune disease. Although systemic autoimmunity is characterized by multiple syndromes with protean clinical manifestations and different genetic predispositions, the loss of self-tolerance at the level of B cells and autoantibodies is remarkably focused on a small group of self-antigens [39, 40]. The anti-nuclear antibody response is a common feature among a number of systemic autoimmune diseases. Although in many cases, the precise nature of the in vivo immunogens is not clear, commonly targeted autoantigens can be divided into three general groups: DNA-related, RNA-related, and immune complexes (most likely containing IgG bound to DNA or RNA-related antigens) that define the rheumatoid factor (RF) specificity. These types of antigens do seem to dominate the autoimmune response, especially when one considers the myriad of self-epitopes available on all of the self-proteins of the body. One study that examined the specificity of all spontaneous hybridomas captured from the spleen of a single MRL/Mplpr/lpr mouse identified one of these three specificity types for 54% of the total, which accounted for nearly all of the expanded clones of spontaneously activated B cells (i.e. those capable of capture as hybridomas) [40]. Despite the preference for these targets, not all autoimmune individuals respond to each disease-associated autoantigen. Smith (Sm) antigen, for example, is a prototypical SLE-specific autoantigen. Yet, only about 30% of autoimmune people or mice make a detectable anti-Sm autoantibody [41–43]. Thus, 70% of individuals with lupus remain tolerant of their own Sm. Clearly, the loss of tolerance—even with regard to “immunogenic” self-antigens in genetically predisposed individuals—is a relatively rare and stochastic event. Indeed, these autoimmune responses can often be traced to large clonal expansions [44–51]. While we have already discussed in general terms how such rare events might be amplified by positive feedback circuits fueled by the continuous presence of self-antigen, the reasons for the preferred targeting have not been explored.
The explanation for dominance of certain autoantigens is not clear, though it has been the subject of considerable interest and speculation. An obvious feature of many of these autoantigens is their intracellular location, which could impair availability of self-antigen for tolerization, particularly of B cells. Although IgG is an extracellular antigen, RF B cells activated in lupus or RA appear to have an altered tolerance mechanism in that they are low affinity [52–54], not tolerized in the bone marrow or periphery [55, 56], and only elicited by polymeric IgG in the form of ICs [54, 57]. Indeed, high affinity anti-IgG antibodies of the type not typically seen in autoimmunity are eliminated in the bone marrow by deletion and receptor editing [58, 59]. Another interesting common feature of dominant autoantigens is their highly charged and polymeric nature. This is true of the nucleic acid-related antigens and also for IgGs complexed with such antigens. Highly charged and polymeric antigens may bind sufficiently to a large fraction of B cells via charge interactions, enhanced by multi-valency. While these would have some specificity, the functional preimmune repertoire for such antigens could be surprisingly high [60–63]. This high precursor frequency may explain in part the repeated targeting of these self-molecules [64]. Some have even suggested that the entire “immune response” of lupus is non-specific and that it merely reflects the outcome of polyclonal activation of the preimmune repertoire, or that at least such polyclonal stimulation is an essential or initial component of autoimmune B cell activation [65].
An important insight into potential reasons for immunogenicity was the recognition that dominant autoantigens are released from apoptotic cells and can be found in apoptotic blebs [66, 67]. Moreover, these autoantigens are processed in specific ways, both via proteolysis at specific sites as well as by degradation or preservation in specific apoptotic structures [68–72]. In some cases, processed or post-translationally modified forms of self are the preferred autoantigens. The unique conformation and concentration of such antigens, along with any potential neo-antigens generated by processing, could evade tolerance mechanisms and contribute to immunogenicity. In addition, it is possible that under some circumstances inflammation accompanying apoptosis could further enhance the chances of breaking self-tolerance [73–77].
Finally, it has been speculated for some time that there could be specific receptors for self-antigens, particularly for DNA. There are a number of such reports dating back into the 1980s but the exact nature of these putative receptors was never clarified [78–80]. Indeed, as will be discussed below in greater detail, Toll-like receptors that recognize either DNA or RNA appear to be essential for the development of anti-nuclear antibodies and most likely for RFs as well.
BCR Transgenic Models of Autoreactivity
Regardless of the reasons, the preferential targeting of certain autoantigens implied that B cells specific for these antigens would likely have unique behaviors among the universe of self-reactive B cells. In order to better study this, BCR transgenic (Tg) mice specific for these antigens, including DNA, Sm and IgG, were created by several groups [55, 81–84]. These Tg mice represented a second generation, coming after initial models that targeted “self” antigens that were not disease-related, such as (transgenically-expressed) hen egg lysozyme or alleles of MHC-I [85, 86]. These earlier models were elegant because they were designed to permit control over the autoantigen, allowing study of transgenic B cells with and without exposure to “self” antigen. These models demonstrated key basic self-tolerance mechanisms, such as anergy, deletion and receptor editing [58, 59, 86]. However, they were less useful in revealing how self-tolerance was broken in autoimmunity [87, 88].
In contrast, Tg mouse models with B cells specific for DNA/chromatin, the RNA-containing antigen Sm, and IgG (i.e. RF specificity) have been very useful in revealing the mechanisms of tolerance breakdown. DNA specific B cells, depending on their affinity, appear to undergo receptor editing or anergy [81, 89–91]. Yet, in the context of an autoimmune-prone genetic background and appropriate conditions, these B cells escape self-tolerance and differentiate into antibody-secreting cells [92–94]. A similar picture is seen for Sm-specific B cells, though they appear to be in a more immature but perhaps not anergic state in normal mice [84]. Systems specific for self-nucleic acid-associated antigens do lack one important feature of the first generation Tg mice: the autoantigen cannot be controlled. In such mice it is thus difficult to prove that autoreactive B cell activation is due to a specific autoantigen, and to define the mechanisms necessary for this activation.
The RF system, as embodied in AM14 Tg mice, has some advantages in this respect. The AM14 BCR is specific for IgG2a of the “a” allotype [55]. Thus, using IgH congenic mice, it is possible to construct AM14 Tg mice that either express (IgHa) or lack (IgHb) the autoantigen. This in turn allows direct testing of specific versus nonspecific activation and importantly allows for controlled introduction of “autoantigen” in various different forms both in vivo and in vitro. In practice, AM14 B cells were found not to be tolerized in normal mice [55, 56], yet they were specifically stimulated to undergo plasmablast differentiation, somatic mutation, affinity maturation and isotype switch outside of B cell follicles [95–97]. Overall, these model systems confirmed the unique behavior of B cells specific for dominant autoantigens—as compared to those specific for nominal or synthetic autoantigens—yet they did not explicitly clarify why these B cells were more easily activated in an autoimmune prone background.
Use of the RF Tg System to Discover a Role for Toll-like Receptors in Autoreactive B Cell Activation
Although, as of 5 years ago, in vivo studies of B cells with transgene-encoded specificity for lupus-associated autoantigens had not yet revealed the mechanisms underlying the preference for their activation in vivo, it seemed likely that these cells would be important tools in the process of elucidating this question. Indeed, studies with AM14 Tg B cells carried out in the laboratory of Dr. Ann Marshak-Rothstein in collaboration with our group turned out to be pivotal in this respect. While trying to use AM14 B cells to activate autoreactive T cells (via BCR-mediated uptake and presentation of chromatin-containing immune complexes), she noticed that anti-chromatin immune complexes were potent mitogens for RF B cells but not for other B cells [98]. Moreover, “normal” immune complexes were not mitogenic. This led us to conclude that there must be a receptor on B cells that recognizes the material complexed with the anti-chromatin antibodies (i.e. chromatin that would be released into tissue culture from dying cells). This receptor would potentiate the activating effect of BCR crosslinking by IgG2a immune complexes. However, at the time, it was unclear what that receptor might be.
With the cloning of TLR9 and the demonstration of its specificity for DNA (albeit “CpG”-containing DNA) [99], this receptor became a prime candidate, particularly given its expression in endosomal compartments of B cells. Rothstein and colleagues then generated AM14 Tg mice deficient in MyD88 (an adaptor protein required for TLR9 signaling), and demonstrated that the mitogenic activity of IgG2a anti-chromatin antibodies was abolished in MyD88-deficient B cells [100]. DNase, as well as specific inhibitors of TLR9, also inhibited AM14 B cell proliferation in response to these antibodies. This was the first evidence, in vitro, that TLRs could be important co-receptors for autoreactive B cell proliferation and that therefore, they might control the specificity of self-reactive B cell activation in vivo. Conceptually, this could apply to anti-chromatin B cells that recognize chromatin directly, as was later shown to be the case using 3H9 anti-DNA Tg mice [101]. Moreover, when the specificity of TLR7 was shown to be ssRNA [102, 103], this seemed a logical candidate to control activation of B cells specific for RNA-containing particles. Indeed, it was subsequently demonstrated that AM14 B cells could be stimulated by RNA-containing immune complexes via a TLR7-dependent mechanism [104].
Together, these in vitro studies provided a strong case for a linkage of TLR-specificity and expression to the preferred specificities of autoantibodies in systemic autoimmunity in vivo. However, at this point, the actual roles in vivo had not been tested. Nor was there any indication of how TLRs would contribute to disease. This is a potentially complicated issue, as TLR7 and TLR9 are both expressed on B cells, myeloid DCs (in mice), plasmacytoid DCs and macrophages, and thus could have multiple effects [105, 106]. Since B cells also play antibody-independent roles in disease [11], it was also not clear whether, even if TLR7 and TLR9 were to control anti-RNA and anti-DNA production, disease itself would be modulated as expected. Obviously, the answers to these questions could also have clinical implications, determining whether and how to target TLR7 and/or TLR9 for inhibition as a therapy for lupus.
Requirement for Toll-like Receptors in Autoantibody Production In Vivo
Conclusive in vivo evidence of a role for TLRs in autoimmune disease came from mice with genetic deficiency of TLR9 crossed to established models of murine lupus. We initially crossed TLR9−/− mice to Fas-deficient MRL/Mplpr/lpr mice to generate an F2 cohort of lupus-prone littermates that were either TLR9 wild-type (TLR9+/+) or TLR9−/−. In this model, we found that TLR9 was required for the generation of anti-chromatin and anti-dsDNA antibodies in vivo [107]. This effect on autoantibody formation was specific for antibodies to DNA-containing antigens, as anti-Sm and anti-cardiolipin antibodies were not decreased in TLR9−/− mice [107].
Following this initial in vivo study (and a wealth of supporting in vitro evidence), there was some controversy in the literature regarding the role of TLR9 in autoantibody formation. Wu and Peng studied a small cohort (6 mice per group) of TLR9-deficient mice more extensively backcrossed to the MRL/Mp strain, with or without Fas-deficiency. These investigators found no impairment in anti-DNA antibody formation, and even reported increased titers of these antibodies in TLR9−/− mice [108]. These findings were then challenged by a third report demonstrating the importance of TLR9 in anti-DNA autoantibody production. Using a model of autoimmunity induced by an anti-DNA BCR transgene and homozygous deficiency of the inhibitory Fcγ receptor IIB, Ehlers et al found that TLR9 and the adaptor molecule MyD88 were required for the generation of class-switched anti-DNA antibodies in autoimmune mice [109].
In order to resolve this controversy, we studied the formation of anti-dsDNA and anti-chromatin autoantibodies in TLR9−/− mice fully backcrossed to the MRL/Mplpr/lpr strain. Confirming our initial findings in hybrid TLR9−/− mice, we found that the formation of anti-chromatin and anti-dsDNA autoantibodies was indeed impaired in the absence of TLR9 [110]. The fact that this phenotype was observed in the context of a well-defined and homogenous genetic background makes confounding by unknown genetic loci unlikely. Instead, the discrepancy with the report by Wu and Peng appears to be due to the assays used to detect specific autoantibodies. These investigators used an ELISA-based assay to determine anti-DNA titers, despite the documented lack of specificity of such assays in the context of SLE [111, 112]. Indeed, we found that a standard anti-DNA ELISA was significantly correlated with serum levels of non-specific IgG, and did not agree with more specific, immunofluorescence-based assays for antibodies to DNA-containing antigens [110]. Moreover, there appeared to be a more stringent requirement for TLR9 in the generation of anti-chromatin and anti-nucleosome antibodies than for the generation of antibodies to naked dsDNA. This was also observed in an independent study of C57BL/6lpr/lpr mice, in which the genetic absence of TLR9 prevented the formation of anti-nucleosome antibodies, but had inconsistent effects on the titer of anti-dsDNA antibodies depending on the assay used [113]. These findings suggest that chromatin, rather than dsDNA, is the physiologic autoantigen in lupus, and is consistent with the demonstration that many autoantibodies in SLE preferentially bind to continuous DNA-histone epitopes rather than isolated dsDNA [114]. Nevertheless, it is clear that TLR9 is required for the generation of high-titer autoantibodies to DNA-containing antigens in murine lupus.
TLR9 signaling could have multiple different effects on autoantibody production in vivo. TLR9 is one of several TLRs expressed by human and murine B cells, and ligation of this receptor within B cells provides a potent stimulus for proliferation and differentiation to antibody-secreting plasma cells [106, 115, 116]. Indeed, B cell-intrinsic TLR expression is required to mount an effective humoral immune response to foreign antigens [117]. TLR9 is also expressed by plasmacytoid and myeloid DCs, which may be activated by endogenous TLR9 ligands in the context of autoimmunity [105, 118, 119]. Thus, it remained unclear whether B cell-intrinsic TLR9 activation was required for the generation of anti-chromatin antibodies in vivo, or whether ligation of TLR9 within DC subsets could provide the necessary costimulatory signals for autoantibody production. We therefore created lupus-prone chimeric mice by mixed bone marrow reconstitution, in which TLR9-deficiency was restricted to the B cell lineage. These mice failed to generate anti-chromatin antibodies when TLR9 was absent in B cells, even in the presence of TLR9-expressing macrophages and DCs (Christensen, et al, manuscript in preparation). We further demonstrated, using mice transgenic for the 3H9 anti-DNA BCR, that the primary effect of TLR9 on anti-DNA B cells in vivo is to facilitate their differentiation to autoantibody-secreting plasmablasts. The effect of TLR9 on B cell development was negligible in these experiments (Christensen, et al, manuscript in preparation). Thus, TLR9 promotes the activation and differentiation of anti-DNA plasmablasts in murine lupus via a B cell-intrinsic mechanism.
DNA/chromatin autoantibodies are only one class of dominant autoantibodies in human and murine lupus; antibodies to RNA-containing antigens such as Sm and RNP are another [120, 121]. Based on convincing data with TLR9 in autoimmunity, it was expected that autoantibodies to RNA antigens would also be controlled by TLRs. TLR3 was the first TLR shown to be activated by RNA motifs [122]. We therefore examined the role of this receptor in autoantibody production, but found no effect of TLR3-deficiency on autoantibodies to either RNA- or DNA-containing antigens in lupus-prone mice [107]. As discussed above, a more promising candidate was the ssRNA receptor, TLR7. Like TLR9 (and unlike TLR3), TLR7 is expressed in endosomal compartments in B cells and pDCs, and signals exclusively through a MyD88-dependent pathway [123]. The fact that MyD88-deficient lupus-prone mice exhibited a complete absence of antinuclear antibodies emphasized the importance of this pathway for autoantibodies to both DNA and RNA antigens [104]. Further support for TLR7 as the co-receptor for RNA autoantigens came from genetic analysis of the Y-linked autoimmune accelerator (Yaa) locus, which was found to be composed of a duplication of a 4 Mb region of the X chromosome containing TLR7, as well as several other immune response genes [124, 125]. Genetic transplantation of this locus to normal mice led to autoantibody production, particularly to RNA antigens in the nucleolus. A recent study by Berland, et al. described a new BCR knockin mouse with specificity for a variety of nuclear antigens including RNA [126]. Although most of the B cells appeared to undergo anergy, there was a mechanism of escape leading to the production of Tg-encoded autoantibodies. They went on to show that this escape required expression of TLR7 in the mouse. Finally, we showed that TLR7 was required for the generation of canonical autoantibodies to endogenous RNA-containing antigens, as TLR7-deficient lupus-prone mice failed to generate anti-Sm antibodies in vivo [110]. Taken together, these data indicate that TLR7 is both necessary and sufficient for anti-RNA autoantibody production in murine lupus.
Functional Consequences of TLR Activation in SLE
In addition to their parallel effects on autoantibody production, TLR7 and TLR9 also affect the development of clinical autoimmune disease. Surprisingly, however, these two receptors appear to have divergent effects. In MRL/Mplpr/lpr mice, the absence of TLR7 led to decreased lymphadenopathy and decreased markers of T and B cell activation. TLR9-deficiency, in contrast, led to increased lymphadenopathy, the accumulation of activated lymphocytes, and increased levels of circulating IgG [107, 110]. These contrasting effects were also observed in pDCs, as the absence of TLR7 led to decreased pDC activation, while increased pDC activation and increased serum IFN-α were observed in the absence of TLR9. The opposing effects of TLR7 and TLR9 on global immune activation had significant consequences for disease as well: TLR9-deficient mice developed exacerbated kidney disease with accelerated mortality, while TLR7-deficient mice developed less severe renal disease. The contrasting inflammatory and regulatory roles that we observed for TLR7 and TLR9, respectively, in autoimmune MRL/Mplpr/lpr mice were similarly suggested in other murine models [108, 113, 125].
The mechanisms mediating exacerbated or ameliorated clinical disease in lupus-prone mice deficient in TLR9 or TLR7 is unclear, given that these receptors share similar expression patterns and downstream signaling pathways [123, 127–129]. Competition for shared adaptor molecules could allow cross-inhibition or “tolerance” to endogenous nucleic acids when both receptors are ligated in the same cell [130]. In addition, multiple mechanisms for down-regulating TLR-induced signals have now been described [131], including the inhibitory adaptor proteins IRAK-M and interferon regulatory factor 4 (IRF-4) [132, 133]. Differential induction of these regulatory mechanisms by TLR7 or TLR9 may contribute to the disparate knockout phenotypes in lupus-prone mice.
It may be, however, that TLR7 and TLR9 are equally stimulatory within a given cell, and that the differential effects of these two receptors in autoimmune disease arise from functional interactions between different cell types in the immune network. Signaling through TLR9, for example, appears to activate different effector mechanisms in different cells [134]. An integrated view of the immune system may allow for understanding of the mechanisms leading to the divergent phenotypes of TLR7 and TLR9 knockouts in murine lupus. An important difference between the two receptors, in relation to global immune function, appears to be the autoantibody repertoire induced by activation of these receptors within B cells. Subtle differences between the effects of anti-DNA antibodies induced by TLR9, and anti-RNA antibodies induced by TLR7 may be amplified in the positive feedback cycles of autoimmunity, thus leading to dramatic—or even opposing—effects on chronic immune activation and disease progression.
B Cell-Intrinsic TLR Activation by Endogenous Nuclear Antigens
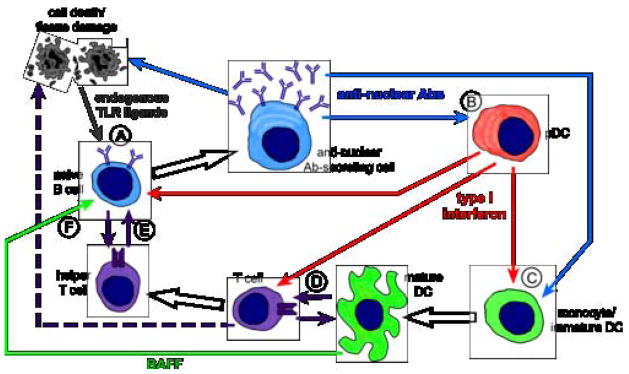
An early event in systemic autoimmunity appears to be the release of endogenous nucleic acid antigens from apoptotic or necrotic cells. An increased predisposition to autoimmune disease has been documented in multiple experimental systems wherein clearance of apoptotic or necrotic debris is impaired [135–137], and also in humans lacking complement pathways for apoptotic clearance [138]. Autoreactive B cells are uniquely stimulated by these nuclear antigens, released from dying cells, because they express receptors of both the innate (germline-encoded TLR7 and TLR9) and the adaptive immune system (somatically rearranged BCR specific for RNA- or DNA-containing antigens). As discussed above, co-ligation of the BCR and TLRs induces proliferation and plasmablast differentiation [106, 115–117]. This dual stimulation may be sufficient to overcome the regulatory mechanisms that normally prevent activation of autoreactive B cells in the periphery [101, 139]. Thus, the recognition of endogenous nuclear antigens by TLRs within endosomal compartments of autoreactive B cells is necessary for autoantibody production, and facilitates the generation of anti-nuclear antibody-secreting cells (Figure 3A).
Figure 3. B cells at the center of immune activation cycles in SLE.
(A) Endogenous DNA- and RNA-containing antigens (gray arrow) released by apoptotic and necrotic cells activate TLRs within autoreactive B cells, allowing differentiation to anti-nuclear antibody-secreting cells. Autoantibodies produced by these cells (blue arrows) can have direct pathogenic effects, releasing additional endogenous TLR ligands and promoting autoreactive B cell activation (B cell autonomous activation cycle). (B) Autoantibody immune complexes activate pDCs, inducing production of IFN-I (red arrows). IFN-I then acts upon B cells to promote antibody secretion (B cell-pDC activation cycle). (C) Autoantibody immune complexes induce maturation of monocytes or immature DCs into effective antigen presenting cells. IFN-I produced by pDCs can also facilitate myeloid DC activation. (D) Mature DCs activate cognate T cells (purple arrows) to produce autoreactive effector T cells, and IFN-I promotes T cell polarization to inflammatory TH1 effectors. Activated T cells may have direct cytotoxic effects on host tissues (dashed arrow). (E) Activated helper T cells provide additional stimulation to autoreactive B cells (purple arrows) for efficient antibody production (B cell-DC-T cell activation cycle). B cells can also reciprocally present antigen to cognate T cells (B cell-T cell activation cycle). (F) Cytokines such as BAFF produced by activated dendritic cells (green arrow) promote autoreactive B cell survival and antibody secretion (B cell-DC activation cycle).
Autoantibodies produced in SLE are thought to have direct pathogenic effects, and may promote ongoing tissue damage and cell death in target organs. This release of additional endogenous TLR ligands from necrotic or apoptotic cells could then promote further activation and expansion of autoreactive B cells, creating a B cell-autonomous activation cycle (Figure 3A). B cells with specificity for DNA or RNA-containing antigens may not be equal in this regard, however. Recent evidence suggests that, rather than mediating injury to viable cells, certain anti-DNA antibodies may promote the clearance of necrotic cell debris, and thereby reduce the availability of endogenous inflammatory mediators. The protective effects of IgM anti-DNA antibodies have been clearly demonstrated in the NZB/W model of lupus [140], and may have a regulatory function in other murine models as well [141]. Autoantibodies induced by TLR7 may therefore be more prone to activate this positive feedback activation cycle than those induced by TLR9.
Autoantibody Immune Complexes Induce IFN-I Secretion by pDCs
Type I interferons (IFN-I) encompass a family of more than twenty related gene products, of which the several interferon-alpha (IFN-α) genes and the single interferon-beta (IFN-β) gene, are most pertinent for the function of the immune system [142]. IFN-I have a panoply of effects on nearly every cell type in the immune system, and have been increasingly implicated in the pathogenesis of SLE. It was first noted in 1979 that human lupus patients have elevated circulating interferon titers, and that levels of serum interferon directly correlate with disease activity [143]. More recently, gene expression analysis has identified characteristic gene expression patterns in blood cells of lupus patients, and these expression patterns were found to overlap with those genes induced by IFN-I [144, 145]. As with the original observation of circulating IFN-I, the level of expression of interferon-inducible genes in these studies correlated with disease severity. Genetic knockouts of the type I interferon receptor in mouse models have also highlighted the importance of these cytokines in autoimmunity. Inability to respond to IFN-α/β dramatically decreased the severity of autoimmune disease in two different lupus-prone mouse strains [146, 147], although one conflicting report observed a negligible effect on disease in MRL/Mp mice [148]. These observations are supported by the fact that exogenous interferon can induce symptoms and laboratory findings of autoimmune disease in patients treated with IFN-α for unrelated conditions [149, 150].
Plasmacytoid DCs in humans and mice express TLR7 and TLR9, rapidly secrete IFN-I in response to ligation of these receptors, and are now recognized as the central interferon-producing cells of the immune system [129, 151, 152]. Given the association of IFN-I with autoimmune disease, activation of nucleic acid-sensing TLRs in pDCs by endogenous ligands is likely to occur in the context of SLE. Unlike autoreactive B cells, however, pDCs lack a specific antigen receptor capable of delivering these ligands to appropriate endosomal compartments, and are thus unlikely to be the primary sensors of nuclear antigens. Instead, pDCs appear to be stimulated “downstream” of B cell activation and autoantibody production. Immune complexes of anti-nuclear antibodies and nucleic acids released from dying cells are potent stimulators of IFN-I production [153, 154]. Analogous to B cell stimulation, this is a dual activation signal initiated at the cell surface by the FcγRIIa (in humans), and propagated in endosomal compartments by ligation of either TLR7 or TLR9 [119, 155]. Thus, following the production of anti-nuclear antibodies by autoreactive B cells, autoantibody immune complexes can induce IFN-I production by pDCs (Figure 3B).
An important effect of IFN-I is the promotion of B cell activation. IFN-I facilitates antibody secretion, particularly of the inflammatory immunoglobulin isotypes IgG2a and IgG3 in the mouse [156, 157]. Interestingly, these are the two isotypes most profoundly affected by the absence of TLR7 or TLR9 in autoimmune MRL/Mplpr/lpr mice, suggesting an interaction between B cells, pDCs, and IFN-I [110]. Moreover, in the presence of concurrent BCR and TLR stimulation (as is predicted to occur in B cells with anti-nuclear specificity), IFN-α can induce differentiation to antibody-secreting plasma cells in the absence of T cell costimulation [158]. This allows for a B cell-pDC activation cycle in SLE, whereby autoantibodies and IFN-I provide positive feedback to pDCs and autoreactive B cells, respectively (Figure 3B).
The positive feedback cycle between pDCs and autoreactive B cells appears to be somewhat different for TLR7 and TLR9. First, although autoantibodies to both DNA- and RNA-containing antigens can induce IFN-I production by pDCs, anti-RNA antibodies may be more potent in this regard [154, 159]. Moreover, antibodies to RNA-containing antigens (generated via TLR7 activation) have been correlated with increased IFN-I production and exacerbated disease in human lupus [160], and in lupus-prone mice [110]. Second, signaling through TLR7 in B cells is enhanced following exposure to IFN-I, while TLR9 signaling is not dependent upon IFN-I [161]. Thus, both at the level of B cell autoantibody production and pDC IFN-I production, TLR7 may be a more potent stimulator of this activation loop.
Activation of Myeloid DCs and Autoreactive T Cells
In mice, TLR7 and TLR9 are also expressed by subsets of myeloid dendritic cells [105]. TLR signaling in these cells results in cytokine production and maturation into efficient antigen-presenting cells (APCs) capable of activating naïve T cells [123, 162]. Importantly, DNA-containing immune complexes can ligate Fc receptors on myeloid DCs, leading to activation of TLR9 within endosomal compartments [118]. Acting in concert with TLR ligation, IFN-I is also a potent activator of monocytes and immature dendritic cells [142]. Immature blood-derived monocytes assume the activated phenotype of mature DCs and acquire robust T cell stimulatory capacity when cultured in the presence of IFN-I [163]. In the context of autoimmunity, circulating monocytes from lupus patients have an intrinsically high efficiency of antigen presentation, and this elevated APC activity is dependent upon IFN-α [164]. Thus, myeloid DCs can be activated by two TLR- and autoantibody-dependent mechanisms: immune complexes can directly activate TLRs in myeloid DCs, and these cells can be further stimulated by IFN-I produced by pDCs (Figure 3C).
Although the significance of TLR signaling within T cells is a matter of debate, it is clear that the provision of TLR agonists results in T cell activation [106, 123, 165, 166]. As discussed above, activation of TLRs within monocytes and myeloid DCs induces maturation and allows for efficient priming of naïve T cells (Figure 3D). Moreover, IFN-I can polarize the T cell response toward the inflammatory Th1 type [156, 163], and, by virtue of STAT4 activation, can directly prime T cells for production of the prototypical Th1 cytokine, gamma interferon [167]. Polarized CD4+ T cells can then provide cognate help to autoreactive B cells, completing an activation cycle between B cells, DCs, and T cells (Figure 3E). Indeed, the provision of T cell help may be the rate-limiting step in the transition from initial B cell activation to florid autoimmunity and autoantibody production [168, 169]. Cognate T-B interactions are also prone to positive feedback cycles, as activated B cells serve as potent APCs [17]. Effector T cells may also mediate direct tissue damage in lupus, either through cytotoxic activity or the secretion of inflammatory cytokines (Figure 3D).
Lastly, there is accumulating evidence that myeloid DCs can interact with B cells to promote their activation and antibody secretion. In a model of systemic bacterial infection, splenic B cells directly interacted with maturing CD11clo DCs, and required the presence of BAFF/APRIL ligands produced by these DCs for continued survival and antibody secretion [170]. BAFF (B Cell Activating Factor, also known as BlyS, or B Lymphocyte Stimulator) and APRIL (A Proliferation-Inducing Ligand) are TNF family members produced by macrophages, myeloid DCs, and stromal cells, and are critical for B cell development and survival [171]. These molecules may also play a role in the generation of T-independent humoral responses [172], and facilitate immunoglobulin class switching in the absence of T cell help [173, 174]. Importantly, myeloid DCs stimulated with chromatin-containing immune complexes secrete BAFF [118], and excess levels of BAFF have been implicated in systemic autoimmune disease [175, 176]. A final activation cycle thus exists, between B cells and myeloid DCs, mediated by autoantibody immune complexes and cytokines such as BAFF (Figure 3F).
The positive feedback cycles described here all share two important factors. First, they all depend upon recognition of endogenous nucleic acid ligands by TLR7 and TLR9 in endosomal compartments. Expression of TLRs by multiple different cell types allows for an array of functional outcomes, which work in concert to promote (or potentially regulate) immune activation. Second, following the initial activation of anti-nuclear B cells by endogenous TLR ligands, all subsequent activation cycles require the presence of autoantibody immune complexes. This places autoreactive B cells in a position of particular importance. By virtue of the integration of signals from receptors of the innate and adaptive immune system, and by the ability to coordinate the actions of both innate and adaptive effector cells, B cells reside at the center of multiple activation loops in the etiology and pathogenesis of SLE.
Concluding Remarks
Studies on the role of TLRs in systemic autoimmunity are likely to reveal novel therapeutic avenues for the treatment of autoimmune diseases. The clear requirement for TLRs in the generation of anti-nuclear antibodies, as well as the amelioration of immune activation and clinical disease in TLR7-deficient lupus-prone mice, highlights these receptors as potential candidates for pharmacologic inhibition in SLE. However, the exacerbation of disease in TLR9-deficient mice provides a note of caution. In fact, our studies on TLR7- and TLR9-deficient mice suggest that synthetic oligodeoxynucleotide inhibitors of TLR9 may actually mediate their beneficial effects through non-specific inhibition of TLR7 [159, 177]. In addition, the role of TLRs and autoantibody immune complexes in the induction of IFN-I may also be a therapeutic target. Inhibition of Fc receptor signaling, internalization, or endosomal processing may provide novel mechanisms of reducing the pathogenic effects of IFN-I in lupus.
Although TLRs have been the focus of intense research into the mechanisms of innate immune recognition, recent work has identified TLR-independent pathways of innate recognition as well. These pathways appear to be initiated by intracellular proteins that recognize RNA and DNA ligands in the cytoplasm. The RNA helicases RIG-I and Mda5 activate IFN-I secretion in response to cytosolic dsRNA [178–180], and an unknown receptor appears to mediate a similar response to cytoplasmic dsDNA [181, 182]. Like TLRs, these receptors may be inappropriately activated by endogenous nucleic acids under certain conditions. Indeed, the recognition of undigested genomic DNA in hematopoietic cells of DNaseII-deficient mice appears to occur via a TLR-independent mechanism [183]. Other innate sensors of infection may also modulate autoimmune disease, even in the absence of nucleic acid recognition. The NOD (nucleotide-binding oligomerization domain) proteins NOD1 and NOD2 are intracellular sensors of bacterial cell wall components, and have been implicated in the pathogenesis of inflammatory bowel disease [184].
Investigation of the roles of TLRs (and potentially other innate immune receptors) in systemic autoimmunity holds great promise for the understanding and treatment of autoimmune disease. In addition to providing novel therapeutic targets in SLE, the study of TLRs in autoimmunity will broaden our understanding of the fundamental distinction between self and non-self in the immune system. Elucidation of the mechanisms of disease pathogenesis may also reveal unappreciated effects of TLR signaling in different cell types, as well as emergent properties arising from the interaction of different cell types in vivo. Finally, genetic deletion of TLR7 or TLR9 can now provide a system to selectively eliminate certain autoantibody specificities in murine lupus, and thereby study the contribution of the autoantibody repertoire to the pathogenesis of autoimmune disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nature Reviews Immunology. 2003;3:253–7. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 2.von Herrath MG, Harrison LC. Antigen-induced regulatory T cells in autoimmunity. Nature Reviews Immunology. 2003;3:223–32. doi: 10.1038/nri1029. [DOI] [PubMed] [Google Scholar]
- 3.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176:705–10. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 4.Johansson M, Arlestig L, Moller B, Rantapaa-Dahlqvist S. Association of a PDCD1 polymorphism with renal manifestations in systemic lupus erythematosus. Arthritis Rheum. 2005;52:1665–9. doi: 10.1002/art.21058. [DOI] [PubMed] [Google Scholar]
- 5.Lauwerys BR, Wakeland EK. Genetics of lupus nephritis. Lupus. 2005;14:2–12. doi: 10.1191/0961203305lu2052oa. [DOI] [PubMed] [Google Scholar]
- 6.Sharif S, Arreaza GA, Zucker P, Mi QS, Delovitch TL. Regulation of autoimmune disease by natural killer T cells. Journal of Molecular Medicine. 2002;80:290–300. doi: 10.1007/s00109-002-0332-8. [DOI] [PubMed] [Google Scholar]
- 7.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–52. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 8.Nistico L, Buzzetti R, Pritchard LE, Van der Auwera B, Giovannini C, Bosi E, et al. The CTLA-4 gene region of chromosome 2q33 is linked to, and associated with, type 1 diabetes. Belgian Diabetes Registry. Hum Mol Genet. 1996;5:1075–80. doi: 10.1093/hmg/5.7.1075. [DOI] [PubMed] [Google Scholar]
- 9.Shlomchik MJ, Madaio MP, Ni D, Trounstine M, Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. J Exp Med. 1994;180:1295–306. doi: 10.1084/jem.180.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan O, Shlomchik MJ. A new role for B cells in systemic autoimmunity: B cells promote spontaneous T cell activation in MRL-lpr/lpr mice. J Immunol. 1998;160:51–9. [PubMed] [Google Scholar]
- 11.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–48. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan OTM, Madaio MP, Shlomchik MJ. The central and multiple roles of B cells in lupus pathogenesis. Immunol Rev. 1999;169:107–21. doi: 10.1111/j.1600-065x.1999.tb01310.x. [DOI] [PubMed] [Google Scholar]
- 13.Chan OT, Madaio MP, Shlomchik MJ. B cells are required for lupus nephritis in the polygenic, Fas-intact MRL model of systemic autoimmunity. J Immunol. 1999;163:3592–6. [PubMed] [Google Scholar]
- 14.Akashi T, Nagafuchi S, Anzai K, Kondo S, Kitamura D, Wakana S, et al. Direct evidence for the contribution of B cells to the progression of insulitis and the development of diabetes in non-obese diabetic mice. Int Immunol. 1997;9:1159–64. doi: 10.1093/intimm/9.8.1159. [DOI] [PubMed] [Google Scholar]
- 15.Serreze DV, Chapman HD, Varnum DS, Hanson MS, Reifsnyder PC, Richard SD, et al. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD.Ig mu null mice. J Exp Med. 1996;184:2049–53. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svensson L, Jirholt J, Holmdahl R, Jansson L. B cell-deficient mice do not develop type II collagen-induced arthritis (CIA) Clin Exp Immunol. 1998;111:521–6. doi: 10.1046/j.1365-2249.1998.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: positive feedback in systemic autoimmune disease. Nat Rev Immunol. 2001;1:147–53. doi: 10.1038/35100573. [DOI] [PubMed] [Google Scholar]
- 18.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol. 1998;161:3912–18. [PubMed] [Google Scholar]
- 19.Gray D, Siepmann K, van Essen D, Poudrier J, Wykes M, Jainandunsing S, et al. B-T lymphocyte interactions in the generation and survival of memory cells. Immunol Rev. 1996;150:45–61. doi: 10.1111/j.1600-065x.1996.tb00695.x. [DOI] [PubMed] [Google Scholar]
- 20.Stockinger B, Zal T, Zal A, Gray D. B cells solicit their own help from T cells. J Exp Med. 1996;183:891–9. doi: 10.1084/jem.183.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin R-H, Mamula MJ, Hardin JA, Janeway CA., Jr Induction of autoreactive B cells allows priming of autoreactive T cells. J Exp Med. 1991;173:1433–39. doi: 10.1084/jem.173.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mamula MJ, Lin R-H, Janeway CA, Jr, Hardin JA. Breaking T cell tolerance with foreign and self co-immunogens: a study of autoimmune B and T cell epitopes of cytochrome c. J Immunol. 1992;149:789–95. [PubMed] [Google Scholar]
- 23.Mamula MJ, Fatenejad S, Craft J. B cells process and present lupus autoantigens that initiate autoimmune T cell responses. J Immunol. 1994;152:1453–61. [PubMed] [Google Scholar]
- 24.Fatenejad S, Mamula MJ, Craft J. Role of intermolecular/intrastructural B- and T-cell determinants in the diversification of autoantibodies to ribonucleoprotein particles. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:12010–4. doi: 10.1073/pnas.90.24.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mamula MJ, Janeway CA., Jr Do B cells drive the diversification of immune responses? Immunol Today. 1993;14:151–2. doi: 10.1016/0167-5699(93)90274-O. discussion 3–4. [DOI] [PubMed] [Google Scholar]
- 26.Browning JL. B cells move to centre stage: novel opportunities for autoimmune disease treatment. Nat Rev Drug Discov. 2006;5:564–76. doi: 10.1038/nrd2085. [DOI] [PubMed] [Google Scholar]
- 27.Looney RJ. B cells as a therapeutic target in autoimmune diseases other than rheumatoid arthritis. Rheumatology (Oxford) 2005;44(Suppl 2):ii13–ii7. doi: 10.1093/rheumatology/keh618. [DOI] [PubMed] [Google Scholar]
- 28.Sfikakis PP, Boletis JN, Lionaki S, Vigklis V, Fragiadaki KG, Iniotaki A, et al. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: an open-label trial. Arthritis Rheum. 2005;52:501–13. doi: 10.1002/art.20858. [DOI] [PubMed] [Google Scholar]
- 29.Sfikakis PP, Boletis JN, Tsokos GC. Rituximab anti-B-cell therapy in systemic lupus erythematosus: pointing to the future. Curr Opin Rheumatol. 2005;17:550–7. doi: 10.1097/01.bor.0000172798.26249.fc. [DOI] [PubMed] [Google Scholar]
- 30.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–81. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 31.Tony H-P, Phillips NE, Parker DC. Role of membrane immunoglobulin (Ig) crosslinking in membrane Ig-mediated, major histocompatibility-restricted T cell-B cell cooperation. J Exp Med. 1985;162:1695–708. doi: 10.1084/jem.162.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gosselin EJ, Tony H-P, Parker DC. Characterization of antigen processing and presentation by resting B lymphocytes. J Immunol. 1988;140:1408–13. [PubMed] [Google Scholar]
- 33.Lichtman AH, Tony H-P, Parker DC, Abbas AK. Antigen presentation by hapten-specific B lymphocytes. J Immunol. 1987;138:2822–25. [PubMed] [Google Scholar]
- 34.Yan J, Harvey BP, Gee RJ, Shlomchik MJ, Mamula MJ. B cells drive early T cell autoimmunity in vivo prior to dendritic cell-mediated autoantigen presentation. J Immunol. 2006;177:4481–7. doi: 10.4049/jimmunol.177.7.4481. [DOI] [PubMed] [Google Scholar]
- 35.Ronchese F, Hausmann B. B lymphocytes in vivo fail to prime naive T cells but can stimulate antigen-experienced T lymphocytes. J Exp Med. 1993;177:679–90. doi: 10.1084/jem.177.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lassila O, Vainio O, Matzinger P. Can B cells turn on virgin T cells. Nature. 1988;334:253–5. doi: 10.1038/334253a0. [DOI] [PubMed] [Google Scholar]
- 37.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell [see comments] Nature. 1998;393:474–8. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 38.Epstein MM, Di Rosa F, Jankovic D, Sher A, Matzinger P. Successful T cell priming in B cell-deficient mice. J Exp Med. 1995;182:915–22. doi: 10.1084/jem.182.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan EM. Antinuclear antibodies: Diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- 40.Shan H, Shlomchik MJ, Marshak-Rothstein A, Pisetsky DS, Litwin S, Weigert MG. The mechanism of autoantibody production in an autoimmune MRL/lpr mouse. J Immunol. 1994;153:5104–20. [PubMed] [Google Scholar]
- 41.Hardin JA, Craft JE. Patterns of autoimmunity to nucleoproteins in patients with systemic lupus erythematosus. Rheumatic Diseases Clinics of North America. 1987;13:37–46. [PubMed] [Google Scholar]
- 42.Eisenberg RA, Craven SY, Warren RW, Cohen PL. Stochastic control of anti-Sm autoantibodies in MRL/Mp-lpr/lpr mice. J Clin Invest. 1987;80:691–7. doi: 10.1172/JCI113123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Migliorini P, Baldini C, Rocchi V, Bombardieri S. Anti-Sm and anti-RNP antibodies. Autoimmunity. 2005;38:47–54. doi: 10.1080/08916930400022715. [DOI] [PubMed] [Google Scholar]
- 44.Bloom DD, Davignon J-L, Retter MW, Shlomchik MJ, Pisetsky DS, Cohen PL, et al. V region gene analysis of anti-Sm hybridomas from MRL/Mp-lpr/lpr. The Journal of Immunology. 1993;150:1591–610. [PubMed] [Google Scholar]
- 45.Shlomchik MJ, Mascelli MA, Shan H, Radic MZ, Pisetsky D, Marshak-Rothstein A, et al. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J Exp Med. 1990;171:265. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shlomchik MJ, Marshak-Rothstein A, Wolfowicz CB, Rothstein TL, Weigert MG. The role of clonal selection and somatic mutation in autoimmunity. Nature. 1987;328:805. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- 47.Shlomchik MJ, Aucoin AH, Pisetsky DS, Weigert MG. Structure and function af anti-DNA antibodies derived from a single autoimmune mouse. Proc Natl Acad Sci. 1987;84:9150–4. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marion TN, Bothwell ALM, Briles DE, Janeway CA. IgG anti-DNA antoantibodies within an individual autoimmune mouse are the products of clonal selection. The Journal of Immunology. 1989;142:4269–74. [PubMed] [Google Scholar]
- 49.Kurth J, Perniok A, Schmitz R, Iking-Konert C, Chiorazzi N, Thompson KM, et al. Lack of deleterious somatic mutations in the CD95 gene of plasmablasts from systemic lupus erythematosus patients and autoantibody-producing cell lines. Eur J Immunol. 2002;32:3785–92. doi: 10.1002/1521-4141(200212)32:12<3785::AID-IMMU3785>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 50.Randen I, Brown D, Thompson KM, Hughes-Jones N, Pascual V, Victor K, et al. Clonally related IgM rheumatoid factors undergo affinity maturation in the rheumatoid synovial tissue. The Journal of Immunology. 1992;148:3296–301. [PubMed] [Google Scholar]
- 51.Randen I, Thompson KM, Thorpe SJ, Forre O, Natvig JB. Human monoclonal IgG rheumatoid factors from the synovial tissue of patients with rheumatoid arthritis. Scand J Immunol. 1993;37:668–72. doi: 10.1111/j.1365-3083.1993.tb01681.x. [DOI] [PubMed] [Google Scholar]
- 52.Jacobson BA, Sharon J, Shan H, Shlomchik M, Weigert MG, Marshak-Rothstein A. An isotype switched and somatically mutated rheumatoid factor clone isolated from a MRL-lpr/lpr mouse exhibits limited intraclonal affinity maturation. J Immunol. 1994;152:4489–99. [PubMed] [Google Scholar]
- 53.Eisenberg R. The specificity and polyvalency of binding of a monoclonal rheumatoid factor. Immunochemistry. 1976;13:355–9. doi: 10.1016/0019-2791(76)90347-5. [DOI] [PubMed] [Google Scholar]
- 54.Nemazee D. Immune complexes can trigger specific, T cell-dependent, autoanti-IgG antibody production in mice. J Exp Med. 1985;161:242–56. doi: 10.1084/jem.161.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shlomchik MJ, Zharhary D, Saunders T, Camper S, Weigert M. A Rheumatoid factor transgenic mouse model of autoantibody regulation. Int Immunol. 1993;5:1329–41. doi: 10.1093/intimm/5.10.1329. [DOI] [PubMed] [Google Scholar]
- 56.Hannum LG, Ni D, Haberman AM, Weigert MG, Shlomchik MJ. A disease-related RF autoantibody is not tolerized in a normal mouse: implications for the origins of autoantibodies in autoimmune disease. J Exp Med. 1996;184:1269–78. doi: 10.1084/jem.184.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coulie PG, Van Snick J. Rheumatoid factor (RF) production during anamnestic immune responses in the mouse. III. Activation of RF precursor cells is induced by their interaction with immune complexes and carrier-specific helper T cells. Journal Experimental Medicine. 1985;161:88–97. doi: 10.1084/jem.161.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–20. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nemazee D, Russell D, Arnold B, Haemmerling G, Allison J, Miller JFAP, et al. Clonal deletion of autospecific B lymphocytes. Immunological Rev. 1991;122:117–32. doi: 10.1111/j.1600-065x.1991.tb00600.x. [DOI] [PubMed] [Google Scholar]
- 60.Van Snick J, Coulie P. Monoclonal anti-IgG autoantibodies from lipopolysaccharide-activated spleen cells of 129/Sv mice. J Exp Med. 1982;155:219–30. doi: 10.1084/jem.155.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Snick JL, Masson PL. Incidence and specificities of IgA and IgM anti-IgG autoantibodies in various mouse strains and colonies. Journal Experimental Medicine. 1980;151:45–55. doi: 10.1084/jem.151.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dighiero G, Lymberi P, Holmberg D, Lundquist I, Coutinho A, Avrameas S. High frequency of natural autoantibodies in normal newborn mice. J Immunol. 1985;134:765–71. [PubMed] [Google Scholar]
- 63.Souroujon M, White SM, Andreschwartz J, Gefter ML, Schwartz RS. Preferential autoantibody reactivity of the preimmune B cell repertoire in normal mice. J Immunol. 1988;140:4173–9. [PubMed] [Google Scholar]
- 64.Shlomchik MJ, Nemazee DA, Sato VL, Van Snick J, Carson DA, Weigert MG. Variable region sequences of murine IgM anti-IgG monoclonal antibodies (Rheumatoid Factors) J Exp Med. 1986;164:407. doi: 10.1084/jem.164.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klinman DM, Steinberg AD. Systemic autoimmune disease arises from polyclonal B cell activation. J Exp Med. 1987;165:1755–60. doi: 10.1084/jem.165.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosen A, Casciola-Rosen L, Ahearn J. Novel packages of viral and self-antigens are generated during apoptosis. J Exp Med. 1995;181:1557–61. doi: 10.1084/jem.181.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Casciola-Rosen L, Andrade F, Ulanet D, Wong WB, Rosen A. Cleavage by granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity. J Exp Med. 1999;190:815–26. doi: 10.1084/jem.190.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Casciola-Rosen LA, Miller DK, Anhalt GJ, Rosen A. Specific cleavage of the 70-kDa protein component of the U1 small nuclear ribonucleoprotein is a characteristic biochemical feature of apoptotic cell death. J Biol Chem. 1994;269:30757–60. [PubMed] [Google Scholar]
- 70.Greidinger EL, Casciola-Rosen L, Morris SM, Hoffman RW, Rosen A. Autoantibody recognition of distinctly modified forms of the U1-70-kd antigen is associated with different clinical disease manifestations. Arthritis Rheum. 2000;43:881–8. doi: 10.1002/1529-0131(200004)43:4<881::AID-ANR20>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 71.Cocca BA, Cline AM, Radic MZ. Blebs and apoptotic bodies are B cell autoantigens. Journal of Immunology. 2002;169:159–66. doi: 10.4049/jimmunol.169.1.159. [DOI] [PubMed] [Google Scholar]
- 72.Radic M, Marion T, Monestier M. Nucleosomes are exposed at the cell surface in apoptosis. J Immunol. 2004;172:6692–700. doi: 10.4049/jimmunol.172.11.6692. [DOI] [PubMed] [Google Scholar]
- 73.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, et al. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–68. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Inaba K, Turley S, Yamaide F, Iyoda T, Mahnke K, Inaba M, et al. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J Exp Med. 1998;188:2163–73. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–75. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 76.Ma L, Chan KW, Trendell-Smith NJ, Wu A, Tian L, Lam AC, et al. Systemic autoimmune disease induced by dendritic cells that have captured necrotic but not apoptotic cells in susceptible mouse strains. Eur J Immunol. 2005;35:3364–75. doi: 10.1002/eji.200535192. [DOI] [PubMed] [Google Scholar]
- 77.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306–11. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 78.Bennett RM, Gabor GT, Merritt MM. DNA binding to human leukocytes. Evidence for a receptor-mediated association, internalization, and degradation of DNA. J Clin Invest. 1985;76:2182–90. doi: 10.1172/JCI112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Emlen W, Holers VM, Arend WP, Kotzin B. Regulation of nuclear antigen expression on the cell surface of human monocytes. The Journal of Immunology. 1992;148:3042–8. [PubMed] [Google Scholar]
- 80.Bennett RM, Kotzin BL, Merritt MJ. DNA receptor dysfunction in systemic lupus erythematosus and kindred disorders. J Exp Med. 1987;166:850–63. doi: 10.1084/jem.166.4.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Erikson J, Radic MZ, Camper SA, Hardy RR, Weigert MG. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 1991;349:331–34. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- 82.Offen D, Spatz L, Escowitz H, Factor S, Diamond B. Induction of tolerance to an IgG autoantibody. Proc Natl Acad Sci USA. 1992;89:8332–36. doi: 10.1073/pnas.89.17.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsao BP, Ohnishi K, Cheoutre H, Mitchell B, Teitell M, Mixter P, et al. Failed self-tolerance and autoimmunity in IgG anti-DNA transgenic mice. J Immunol. 1992;149:350–58. [PubMed] [Google Scholar]
- 84.Santulli-Marotto S, Retter MW, Gee R, Mamula MJ, Clarke SH. Autoreactive B cell regulation: peripheral induction of developmental arrest by lupus-associated autoantigens. Immunity. 1998;8:209–19. doi: 10.1016/s1074-7613(00)80473-2. [DOI] [PubMed] [Google Scholar]
- 85.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class-I antibody genes. Nature. 1989;337:562–66. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 86.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–82. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 87.Rubio CF, Kench J, Russell DM, Yawger R, Nemazee D. Analysis of central B cell tolerance in autoimmune-prone MRL/lpr mice bearing autoantibody transgenes. J Immunol. 1996;157:65–71. [PubMed] [Google Scholar]
- 88.Rathmell JC, Goodnow CC. Effects of the lpr mutation on elimination and inactivation of self-reactive B cells. J Immunol. 1994;153:2831–42. [PubMed] [Google Scholar]
- 89.Chen C, Radic MZ, Erikson J, Camper SA, Litwin S, Hardy RR, et al. Deletion and editing of B cells that express antibodies to DNA. J Immunol. 1994;152:1970–82. [PubMed] [Google Scholar]
- 90.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: An approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roark JH, Bui A, Nguyen K-AT, Mandik L, Erikson J. Persistence of functionally compromised anti-double-stranded DNA B cells in the periphery of non-autoimmune mice. Int Immunol. 1997;9:1615–629. doi: 10.1093/intimm/9.11.1615. [DOI] [PubMed] [Google Scholar]
- 92.Roark JH, Kuntz CL, Nguyen KA, Caton AJ, Erikson J. Breakdown of B cell tolerance in a mouse model of systemic lupus erythematosus. J Exp Med. 1995;181:1157–67. doi: 10.1084/jem.181.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mandik-Nayak L, Seo SJ, Sokol C, Potts KM, Bui A, Erikson J. MRL-lpr/lpr mice exhibit a defect in maintaining developmental arrest and follicular exclusion of anti-double-stranded DNA B cells. J Exp Med. 1999;189:1799–814. doi: 10.1084/jem.189.11.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen C, Li H, Tian Q, Beardall M, Xu Y, Casanova N, et al. Selection of anti-double-stranded DNA B cells in autoimmune MRL-lpr/lpr mice. J Immunol. 2006;176:5183–90. doi: 10.4049/jimmunol.176.9.5183. [DOI] [PubMed] [Google Scholar]
- 95.William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–70. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 96.William J, Euler C, Leadbetter E, Marshak-Rothstein A, Shlomchik MJ. Visualizing the onset and evolution of an autoantibody response in systemic autoimmunity. J Immunol. 2005;174:6872–8. doi: 10.4049/jimmunol.174.11.6872. [DOI] [PubMed] [Google Scholar]
- 97.William J, Euler C, Shlomchik MJ. Short-lived plasmablasts dominate the early spontaneous rheumatoid factor response: differentiation pathways, hypermutating cell types, and affinity maturation outside the germinal center. J Immunol. 2005;174:6879–87. doi: 10.4049/jimmunol.174.11.6879. [DOI] [PubMed] [Google Scholar]
- 98.Rifkin IR, Leadbetter EA, Beaudette BC, Kiani C, Monestier M, Shlomchik MJ, et al. Immune complexes present in the sera of autoimmune mice activate rheumatoid factor B cells [In Process Citation] J Immunol. 2000;165:1626–33. doi: 10.4049/jimmunol.165.3.1626. [DOI] [PubMed] [Google Scholar]
- 99.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 100.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–7. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 101.Viglianti GA, Lau CM, Hanley TM, Miko BA, Shlomchik MJ, Marshak-Rothstein A. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–47. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 102.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate Antiviral Responses by Means of TLR7-Mediated Recognition of Single-Stranded RNA. Science. 2004;303:1529–31. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 103.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-Specific Recognition of Single-Stranded RNA via Toll-like Receptor 7 and 8. Science. 2004;303:1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 104.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–7. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, et al. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol. 2003;33:827–33. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- 106.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, et al. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–7. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 107.Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–31. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu X, Peng SL. Toll-like receptor 9 signaling protects against murine lupus. Arthritis Rheum. 2006;54:336–42. doi: 10.1002/art.21553. [DOI] [PubMed] [Google Scholar]
- 109.Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J Exp Med. 2006;203:553–61. doi: 10.1084/jem.20052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–28. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 111.Emlen W, O’Neill L. Clinical significance of antinuclear antibodies: comparison of detection with immunofluorescence and enzyme-linked immunosorbent assays. Arthritis Rheum. 1997;40:1612–8. doi: 10.1002/art.1780400910. [DOI] [PubMed] [Google Scholar]
- 112.Isenberg DA, Dudeney C, Williams W, Addison I, Charles S, Clarke J, et al. Measurement of anti-DNA antibodies: a reappraisal using five different methods. Ann Rheum Dis. 1987;46:448–56. doi: 10.1136/ard.46.6.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lartigue A, Courville P, Auquit I, Francois A, Arnoult C, Tron F, et al. Role of TLR9 in anti-nucleosome and anti-DNA antibody production in lpr mutation-induced murine lupus. J Immunol. 2006;177:1349–54. doi: 10.4049/jimmunol.177.2.1349. [DOI] [PubMed] [Google Scholar]
- 114.Losman MJ, Fasy TM, Novick KE, Monestier M. Monoclonal autoantibodies to subnucleosomes from a MRL/Mp(−)+/+ mouse. Oligoclonality of the antibody response and recognition of a determinant composed of histones H2A, H2B, and DNA. J Immunol. 1992;148:1561–9. [PubMed] [Google Scholar]
- 115.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–9. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 116.Jung J, Yi AK, Zhang X, Choe J, Li L, Choi YS. Distinct response of human B cell subpopulations in recognition of an innate immune signal, CpG DNA. J Immunol. 2002;169:2368–73. doi: 10.4049/jimmunol.169.5.2368. [DOI] [PubMed] [Google Scholar]
- 117.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–8. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 118.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199:1631–40. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest. 2005;115:407–17. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Egner W. The use of laboratory tests in the diagnosis of SLE. J Clin Pathol. 2000;53:424–32. doi: 10.1136/jcp.53.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Muro Y. Antinuclear antibodies. Autoimmunity. 2005;38:3–9. doi: 10.1080/08916930400024612. [DOI] [PubMed] [Google Scholar]
- 122.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 123.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 124.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B Cell Responses to RNA-Related Antigens Due to TLR7 Gene Duplication. Science. 2006;312:1669–72. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 125.Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, et al. From the Cover: A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A. 2006;103:9970–5. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Berland R, Fernandez L, Kari E, Han JH, Lomakin I, Akira S, et al. Toll-like receptor 7-dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity. 2006;25:429–40. doi: 10.1016/j.immuni.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 127.Akira S. Toll-like receptor signaling. J Biol Chem. 2003;278:38105–8. doi: 10.1074/jbc.R300028200. [DOI] [PubMed] [Google Scholar]
- 128.Kawai T, Sato S, Ishii KJ, Coban C, Hemmi H, Yamamoto M, et al. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol. 2004;5:1061–8. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- 129.Uematsu S, Sato S, Yamamoto M, Hirotani T, Kato H, Takeshita F, et al. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-{alpha} induction. J Exp Med. 2005;201:915–23. doi: 10.1084/jem.20042372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sato S, Takeuchi O, Fujita T, Tomizawa H, Takeda K, Akira S. A variety of microbial components induce tolerance to lipopolysaccharide by differentially affecting MyD88-dependent and -independent pathways. Int Immunol. 2002;14:783–91. doi: 10.1093/intimm/dxf046. [DOI] [PubMed] [Google Scholar]
- 131.Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–58. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 132.Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 133.Negishi H, Ohba Y, Yanai H, Takaoka A, Honma K, Yui K, et al. Negative regulation of Toll-like-receptor signaling by IRF-4. Proc Natl Acad Sci U S A. 2005;102:15989–94. doi: 10.1073/pnas.0508327102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Honda K, Ohba Y, Yanai H, Negishi H, Mizutani T, Takaoka A, et al. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434:1035–40. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 135.Bickerstaff MC, Botto M, Hutchinson WL, Herbert J, Tennent GA, Bybee A, et al. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat Med. 1999;5:694–7. doi: 10.1038/9544. [DOI] [PubMed] [Google Scholar]
- 136.Napirei M, Karsunky H, Zevnik B, Stephan H, Mannherz HG, Moroy T. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat Genet. 2000;25:177–81. doi: 10.1038/76032. [DOI] [PubMed] [Google Scholar]
- 137.Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RA, et al. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196:135–40. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Manderson AP, Botto M, Walport MJ. The role of complement in the development of systemic lupus erythematosus. Annu Rev Immunol. 2004;22:431–56. doi: 10.1146/annurev.immunol.22.012703.104549. [DOI] [PubMed] [Google Scholar]
- 139.Rui L, Vinuesa CG, Blasioli J, Goodnow CC. Resistance to CpG DNA-induced autoimmunity through tolerogenic B cell antigen receptor ERK signaling. Nat Immunol. 2003;4:594–600. doi: 10.1038/ni924. [DOI] [PubMed] [Google Scholar]
- 140.Werwitzke S, Trick D, Kamino K, Matthias T, Kniesch K, Schlegelberger B, et al. Inhibition of lupus disease by anti-double-stranded DNA antibodies of the IgM isotype in the (NZB × NZW)F1 mouse. Arthritis Rheum. 2005;52:3629–38. doi: 10.1002/art.21379. [DOI] [PubMed] [Google Scholar]
- 141.Boes M, Schmidt T, Linkemann K, Beaudette BC, Marshak-Rothstein A, Chen J. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc Natl Acad Sci U S A. 2000;97:1184–9. doi: 10.1073/pnas.97.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–36. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 143.Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med. 1979;301:5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- 144.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100:2610–5. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–23. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Santiago-Raber ML, Baccala R, Haraldsson KM, Choubey D, Stewart TA, Kono DH, et al. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197:777–88. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Braun D, Geraldes P, Demengeot J. Type I Interferon controls the onset and severity of autoimmune manifestations in lpr mice. J Autoimmun. 2003;20:15–25. doi: 10.1016/s0896-8411(02)00109-9. [DOI] [PubMed] [Google Scholar]
- 148.Hron JD, Peng SL. Type I IFN protects against murine lupus. J Immunol. 2004;173:2134–42. doi: 10.4049/jimmunol.173.3.2134. [DOI] [PubMed] [Google Scholar]
- 149.Ronnblom LE, Alm GV, Oberg KE. Autoimmunity after alpha-interferon therapy for malignant carcinoid tumors. Ann Intern Med. 1991;115:178–83. doi: 10.7326/0003-4819-115-3-178. [DOI] [PubMed] [Google Scholar]
- 150.Gota C, Calabrese L. Induction of clinical autoimmune disease by therapeutic interferon-alpha. Autoimmunity. 2003;36:511–8. doi: 10.1080/08916930310001605873. [DOI] [PubMed] [Google Scholar]
- 151.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–20. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 153.Bave U, Alm GV, Ronnblom L. The combination of apoptotic U937 cells and lupus IgG is a potent IFN-alpha inducer. J Immunol. 2000;165:3519–26. doi: 10.4049/jimmunol.165.6.3519. [DOI] [PubMed] [Google Scholar]
- 154.Lovgren T, Eloranta ML, Bave U, Alm GV, Ronnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50:1861–72. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 155.Savarese E, Chae OW, Trowitzsch S, Weber G, Kastner B, Akira S, et al. U1 small nuclear ribonucleoprotein immune complexes induce type I interferon in plasmacytoid dendritic cells through TLR7. Blood. 2006;107:3229–34. doi: 10.1182/blood-2005-07-2650. [DOI] [PubMed] [Google Scholar]
- 156.Le Bon A, Schiavoni G, D’Agostino G, Gresser I, Belardelli F, Tough DF. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–70. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 157.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–34. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 158.Poeck H, Wagner M, Battiany J, Rothenfusser S, Wellisch D, Hornung V, et al. Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood. 2004;103:3058–64. doi: 10.1182/blood-2003-08-2972. [DOI] [PubMed] [Google Scholar]
- 159.Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S, et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202:1131–9. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 161.Bekeredjian-Ding IB, Wagner M, Hornung V, Giese T, Schnurr M, Endres S, et al. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J Immunol. 2005;174:4043–50. doi: 10.4049/jimmunol.174.7.4043. [DOI] [PubMed] [Google Scholar]
- 162.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 163.Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T, et al. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–88. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–3. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 165.Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–4. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 166.Suzuki N, Suzuki S, Millar DG, Unno M, Hara H, Calzascia T, et al. A critical role for the innate immune signaling molecule IRAK-4 in T cell activation. Science. 2006;311:1927–32. doi: 10.1126/science.1124256. [DOI] [PubMed] [Google Scholar]
- 167.Nguyen KB, Watford WT, Salomon R, Hofmann SR, Pien GC, Morinobu A, et al. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science. 2002;297:2063–6. doi: 10.1126/science.1074900. [DOI] [PubMed] [Google Scholar]
- 168.Sobel ES, Kakkanaiah VN, Kakkanaiah M, Cheek RL, Cohen PL, Eisenberg RA. T-B collaboration for autoantibody production in lpr mice is cognate and MHC-restricted. J Immunol. 1994;152:6011–6. [PubMed] [Google Scholar]
- 169.Seo SJ, Fields ML, Buckler JL, Reed AJ, Mandik-Nayak L, Nish SA, et al. The impact of T helper and T regulatory cells on the regulation of anti- double-stranded DNA B cells. Immunity. 2002;16:535–46. doi: 10.1016/s1074-7613(02)00298-4. [DOI] [PubMed] [Google Scholar]
- 170.Balazs M, Martin F, Zhou T, Kearney J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–52. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 171.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: A Tutorial on B Cell Survival. Annu Rev Immunol. 2003;21:231–64. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 172.von Bulow GU, van Deursen JM, Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14:573–82. doi: 10.1016/s1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 173.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–9. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–9. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Groom J, Kalled SL, Cutler AH, Olson C, Woodcock SA, Schneider P, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren’s syndrome. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Roschke V, Sosnovtseva S, Ward CD, Hong JS, Smith R, Albert V, et al. BLyS and APRIL form biologically active heterotrimers that are expressed in patients with systemic immune-based rheumatic diseases. J Immunol. 2002;169:4314–21. doi: 10.4049/jimmunol.169.8.4314. [DOI] [PubMed] [Google Scholar]
- 177.Dong L, Ito S, Ishii KJ, Klinman DM. Suppressive oligodeoxynucleotides delay the onset of glomerulonephritis and prolong survival in lupus-prone NZB × NZW mice. Arthritis Rheum. 2005;52:651–8. doi: 10.1002/art.20810. [DOI] [PubMed] [Google Scholar]
- 178.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–7. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 179.Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 180.Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–8. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 181.Ishii KJ, Coban C, Kato H, Takahashi K, Torii Y, Takeshita F, et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7:40–8. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 182.Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 183.Okabe Y, Kawane K, Akira S, Taniguchi T, Nagata S. Toll-like receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. J Exp Med. 2005;202:1333–9. doi: 10.1084/jem.20051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]