Abstract
The present experiment examined the influence of insular cortex (IC) lesions on the intake of a taste stimulus in a consummatory procedure that used morphine as the unconditioned stimulus. In normal rats, morphine caused a rapid reduction in saccharin intake when the taste was novel but not when it was familiar. Irrespective of stimulus novelty, morphine had little influence on the saccharin consumption of IC-lesioned rats. The results are discussed in terms of a lesion-induced disruption of (i) a reward comparison mechanism and (ii) the perception of taste novelty.
Keywords: Insular cortex, taste, morphine, relative value, novelty perception, rat
A number of lesion-behavior studies have implicated the insular cortex (IC) in the acquisition of conditioned taste aversions (CTAs; e.g., Bermudez-Rattoni & McGaugh, 1991; Braun, Slick, & Lorden, 1972; Cubero, Thiele, & Bernstein, 1999; Fresquet, Angst, & Sandner, 2004; Gallo, Roldan, & Bures, 1992; Nerad, Ramirez-Amaya, Ormsby & Bermudez-Rattoni, 1996). However, since these studies employed a procedure with a single pairing of the taste conditioned stimulus (CS) and the lithium chloride (LiCl) unconditioned stimulus (US), it is difficult to determine whether IC lesions attenuate or abolish CTA acquisition. Recent work in our laboratory, using a multiple conditioning trial design, found that IC-lesioned (ICX) rats are capable of acquiring a CTA but that they do so at a slower rate than neurologically intact subjects (Roman, Nebieridze, Sastre, & Reilly, 2006). The attenuation of CTA acquisition seems best attributed to an inability on the part of ICX rats to accurately recognize the novelty of the unfamiliar taste stimulus rather than of an associative deficit or a disruption in the processing of the toxic US. A failure to detect taste novelty (and thereby to mistakenly treat the taste CS as if it were familiar) would be expected to attenuate CTA acquisition because of a latent inhibition-like effect. Consistent with this analysis, IC lesions disrupt CTA acquisition when the taste CS is novel but not when the taste CS is familiar (Kiefer & Braun, 1977; Roman & Reilly, 2007).
Suppression of CS intake can be induced with a wide variety of USs, ranging from toxins (e.g., LiCl) to X-rays to drugs of abuse (see, for example, Gamzu, Vincent Boff, 1985; Riley & Tuck, 1985). Psychoactive drugs have received a great deal of attention in recent years because of the problem of drug addiction, and this has extended into the realm of CTA research. To our knowledge, only two studies have examined the influence of IC lesions on CTAs induced with a psychoactive drug (Mackey, Keller & van der Kooy, 1986; Zito, Bechera, Greenwood & van der Kooy, 1988). Although both studies found that IC lesions disrupted morphine-induced CTAs (assessed with a two-bottle, saccharin vs. water, test trial), it is difficult to determine the nature of the deficit because data from the conditioning trials was not included in the reports. However, since the ICX rats in each study showed normal acquisition of a morphine-induced conditioned place preference, it seems highly unlikely that the CTA deficit can be attributed to a lesion-induced disruption in the processing of the morphine drug state.
Deficits in morphine-induced CS suppression have also been reported in rats with lesions of the gustatory thalamus (GT; Grigson, Lyuboslavsky, & Tanase, 2000; Reilly & Trifunovic, 1999a). The GT is connected reciprocally with the IC (Cechetto & Saper, 1987; Kosar, Grill, & Norgren, 1986; Norgren & Wolf, 1975; Wolf, 1968), and there is therefore reason to anticipate a similar deficit consequent to lesions of the IC. To afford comparability with the aforementioned studies that examined GT-lesioned (GTX) rats in morphine-induced CS suppression, the design of the present experiment utilized a 5 min CS access period during each conditioning trial. This is a departure from CTA studies, which typically permit 15 or more min CS access. The use of a shorter CS access period derives from Grigson’s (1997) analysis that drug-induced CS suppression is analogous to a consummatory contrast effect in which ingestion of a relatively low-value reward (e.g., saccharin) is suppressed in anticipation of a more highly valued reward (e.g., sucrose; for a review of the consummatory contrast literature see Flaherty, 1996). Evidence is accumulating in supports of the interpretation that psychoactive drug-induced CS suppression is the result of a comparison of the relative reward values of the CS and the US drug (for a review see Grigson, Twining, Freet, Wheeler, & Geddes, 2009). According to this “reward comparison hypothesis,” the positive attributes of a CS are greatly exceeded by the pleasurable properties of the US drug state (as with sucrose in the traditional paradigm) and so consumption of the CS is reduced. By Grigson’s analysis, then, self-administered drugs of abuse support taste CS suppression because of their high reward value not because of their supposed aversive properties.
The experimental design included both acquisition and reversal trials. During acquisition rats were injected with either the morphine US or the saline vehicle following the saccharin CS. In reversal, the contingencies were switched such that the rats given the morphine US during acquisition were injected with saline and vice versa. The results obtained from the reversal trials are informative for at least two reasons. First, the non-reinforced trials permit an examination of extinction performance following the taste-drug pairings. Second, data from the rats that receive morphine during reversal training allows some evaluation of the effect of prior non-reinforced exposure of the taste CS on later CS-US learning. This effect has not, to our knowledge, been investigated in this paradigm.
Method
Subjects
Forty-two male CD-IGS rats purchased from Charles River Laboratories (Portage, MI) were used in these experiments. All rats were individually housed in hanging stainless steel cages in a room maintained on a 12-hour light/dark cycle; ad libitum food and water were available except where noted otherwise. The animals weighed ~300 g at the time of surgery, and were treated according to the guidelines recommended by the National Institutes of Health, the American Psychological Association, and the Institutional Animal Care and Use Committee at the University of Illinois at Chicago.
Surgery
Rats were anesthetized using sodium pentobarbital at 50 mg/kg, IP. Once unconscious, each rat received one of three surgical treatments: bilateral N-Methyl-D-aspartic acid (NMDA) lesions of the IC (Group ICX), ICX surgery without the NMDA infusion, or placement in the stereotaxic instrument and no subsequent surgery. Rats in the latter two treatment conditions served as control subjects (Group SHAM).
Surgery began by fixing the rat in a stereotaxic apparatus (ASI; Warren, MI) with nontraumatic ear bars, shaving the scalp, and applying a 10% iodine antiseptic to the surgical site. Body temperature was monitored and maintained at 37°C using a rectal probe and heating pad (Harvard Apparatus, Holliston, MA). An anteroposterior incision was then made along the midline of the head, and the skin and membrane retracted to expose the skull. The head was leveled between bregma and lambda, and trephine holes drilled in the skull above the surgical sites. For rats in the ICX group, glass capillary pipettes (tip ~75 µm diameter) were backfilled with 0.15 M NMDA (Sigma, St. Louis, MO) and lowered into two infusion sites: site 1, AP +1.2 mm, ML ±5.2 mm, DV − 5.0 mm; site 2, AP +1.2 mm, ML ±5.2 mm, DV −4.3 mm. The NMDA was then iontophoretically infused into these target locations with a Midgard precision current source (Stoelting, Wood Dale, IL) using a current of −10 µA for 10 min at site 1 and 6 min at site 2. The infusions were repeated in the other hemisphere, and then the incision was closed with wound clips and the rat placed under a heat source until recovered from the anesthesia. The rat was then returned to its home cage and allowed to recover for at least 7 days before behavioral experimentation began.
Apparatus
All behavioral testing was conducted in the rats’ home cages. While on water restriction, the rats were given fluids from an inverted 100 ml Nalgene graduated cylinder attached to the front of the cage, with a stainless steel spout and silicone stopper. Fluid intake from these bottles was recorded to the nearest 0.5 ml.
Procedure
Following recovery from surgery, all rats were put on a deprivation schedule allowing 5 min access to water in the morning, and 60 min access 5 hours later in the afternoon. Once water intake on this schedule stabilized, the rats were divided into four groups according to their surgical condition (SHAM or ICX) and whether they were to receive injections of physiological saline (S) or 15 mg/kg morphine sulfate (M; Henry Schein, Indianapolis, IN) during acquisition and reversal. The groups were thus: SHAM-SM, SHAM-MS, ICX-SM, and ICX-MS.
On the morning after these groups were assigned, all rats received 5 min access to a 0.15% (w/v) sodium saccharin solution instead of water. The saccharin exposure was followed 5 min later by an IP injection of either saline or morphine depending upon group assignment. This was repeated on alternating days for 7 trials, with the usual 5 min morning water sessions on intervening days. After the seventh conditioning trial, the contingencies were reversed so that the rats that had been receiving morphine were given saline, and vice versa. This reversal training continued for a total of 9 trials. The 60 min afternoon water sessions took place every day regardless of earlier treatments.
Histology
After the behavioral testing was completed, the rats were deeply anesthetized with sodium pentobarbital (100 mg/ml) and perfused transcardially with physiological saline and 4% buffered formalin. The brains were extracted and stored in 4% buffered formalin followed by 20% sucrose for at least 2 days each. The brains were frozen and sliced in a cryostat at 50 µm, stained with cresyl violet, and evaluated under a light microscope.
Results
Anatomical
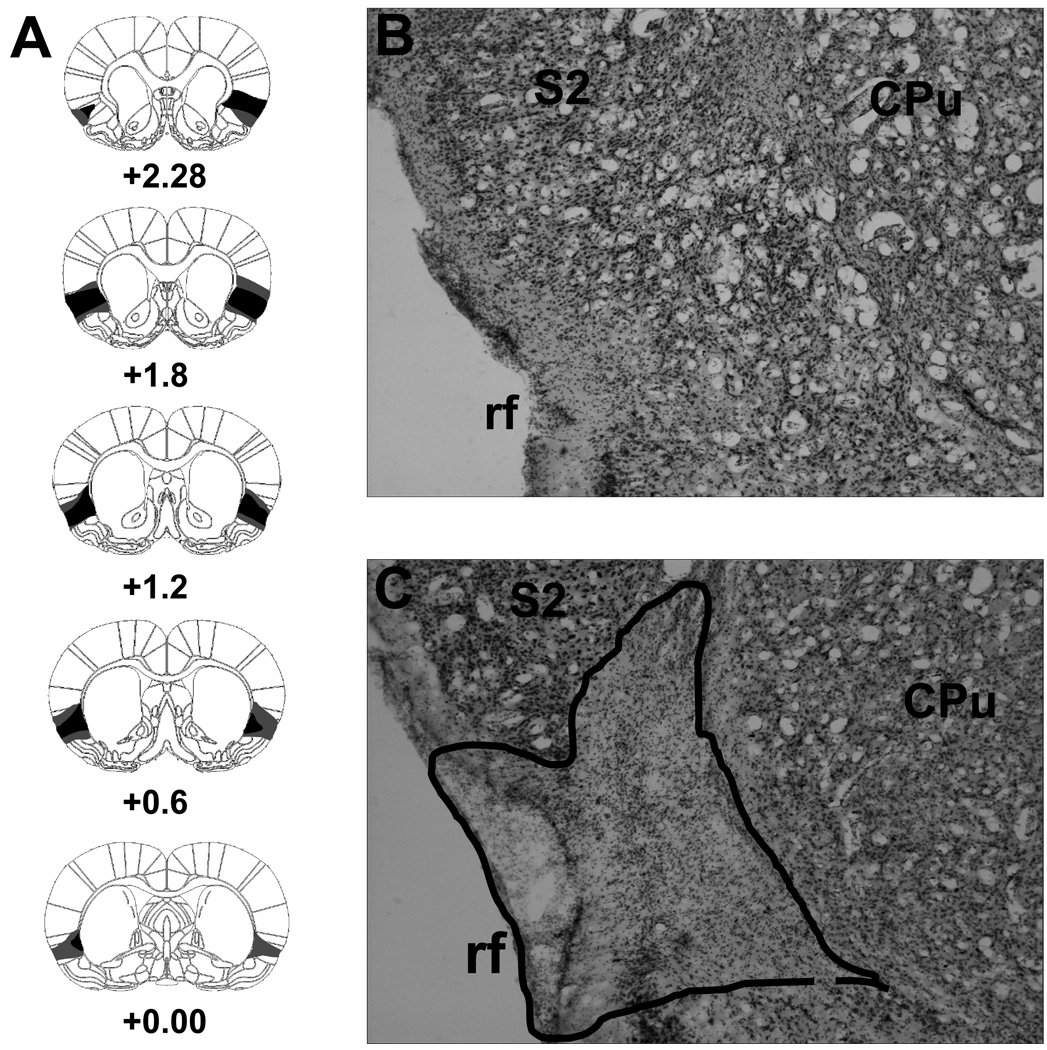
The gustatory region of the IC as described by Kosar and colleagues (1986), extends for about 1.0 mm both anterior and posterior to the point of intersection between the rhinal fissure and the middle cerebral artery (i.e., approximately +0.8 mm relative to bregma; Paxinos & Watson, 2005). The ICX subjects that remained in the final analysis had damage to the majority of this tissue from ~0.0 mm (bregma) to 2.2 mm anterior to bregma. Figure 1A shows serial reconstructions of the largest and smallest lesions included in the final results. A representative photomicrograph of a neurologically intact IC is shown in Figure 1B and an IC lesion can be seen in Figure 1C. Rats with IC lesions that were either subtotal (n = 4) or unilateral (n = 1) were dropped from the study. Additionally, two rats (1 SHAM and 1 ICX) became ill and were unable to complete the experiment; their data were also excluded from the final analyses. The final number of subjects in each group was: 9 SHAM-SM, 10 SHAM-MS, 8 ICX-SM, and 8 ICX-MS.
Figure 1.
Serial reconstructions (Panel A) of the smallest (black) and largest (gray) lesions of the insular cortex at five coronal levels (+2.28, +1.80, +1.20, +0.60, 0.00 relative to bregma). Representative digital photomicrographs of a cresyl violet-stained coronal section (approximately +1.0 mm relative to bregma) through the insular cortex of a neurologically intact subject (Panel B) and a rat with a neurotoxic lesion (Panel C). The extent of cell loss is outlined with a dashed line. CPu = caudate putamen; rf = rhinal fissure; S2 = secondary somatosensory cortex. The diagrams in Panel A are adapted with permission from (Paxinos and Watson 2005).
Behavioral
Analysis of data from the surgical and non-surgical control rats found no significant behavioral differences (Fs < 1). Therefore, in all subsequent analyses data from these two groups were collapsed into a single control group, Group SHAM.
Before the first conditioning trial, average consumption was calculated for each group across the three most recent 5-min water trials, with the following results (mean ±SE): SHAM-SM = 10.70 ±0.50 ml; SHAM-MS = 10.47 ±0.65 ml; ICX-SM = 10.08 ±0.57 ml; ICX-MS = 9.83 ±0.72 ml. An analysis of variance (ANOVA) was conducted on the data from which these means were derived and found no significant main effect of Lesion (SHAM vs. ICX; p > .30) or of Condition (S vs. M, a pseudofactor at this point; F < 1), and no Lesion X Condition interaction (F < 1). Thus, IC lesions had no influence on water intake prior to the start of the experiment.
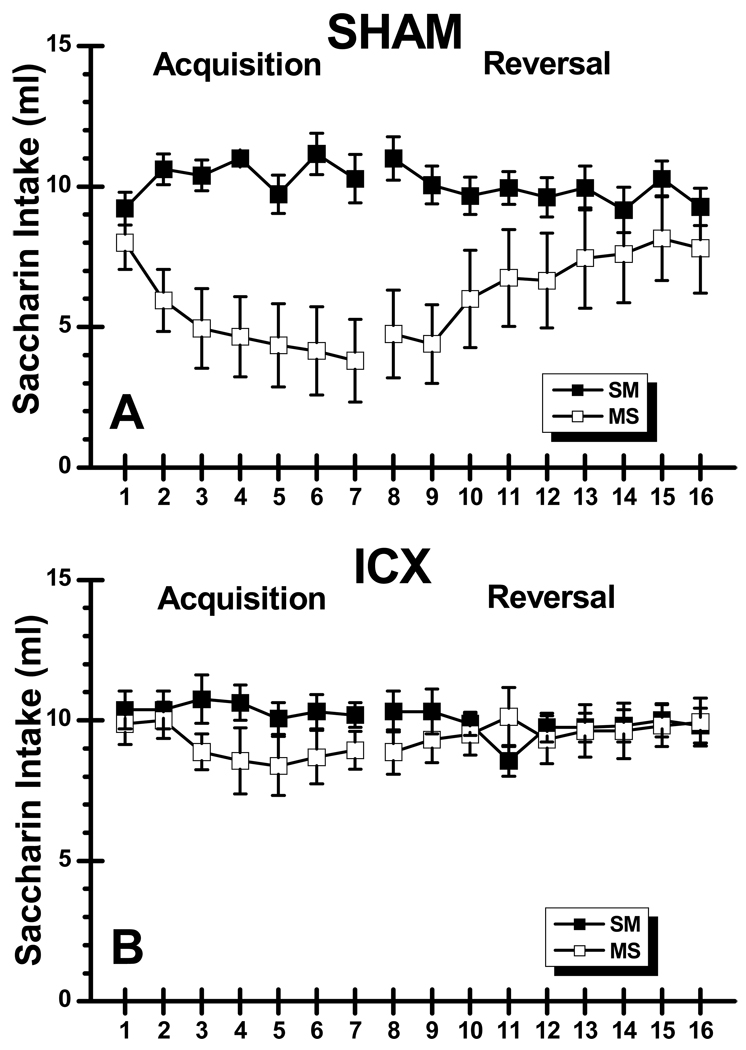
Saccharin consumption data during acquisition and reversal are shown in Figure 2. Inspection of the acquisition phase of the graph shows that SHAM-MS rats quickly decreased saccharin intake relative to the saline injected SHAM subjects whereas the morphine-treated ICX rats showed no such effect relative to the ICX control group. An ANOVA performed on the acquisition data found significant main effects of Lesion, F(1, 31) = 5.37, p < .05, Condition, F(1, 31) = 14.68, p < .05, and Trials, F(6, 186) = 2.97, p < .05. These main effects were the result of ICX rats consuming more saccharin overall than SHAM subjects, saline rats drinking more than morphine subjects, and total consumption decreasing across trials, respectively. The same ANOVA also revealed a Lesion X Condition X Trials interaction, F(6,186) = 2.19, p < .05, with SHAM-MS subjects drinking less saccharin across trials compared to the other three groups, which separate ANOVAs found did not differ from each other (Fs < 1).
Figure 2.
Mean (±SE) saccharin intake (ml) across 7 acquisition trials and 9 reversal trials in normal (SHAM; Panel A) subjects and rats with neurotoxic lesions of the insular cortex (ICX; Panel B) that were injected with saline during acquisition and morphine during reversal (SM) or vice versa (MS).
Comparisons between SHAM groups across acquisition trials revealed that there was no significant intake difference on the first trial (p > .05) but morphine injected SHAM rats consumed significantly less saccharin on trials 2–7 than the saline-injected SHAM subjects (ps < .001). In summary, there were no differences between SHAM-SM, ICX-SM, and ICX-MS groups during acquisition, while SHAM-MS rats drank significantly less than their counterparts in the other three groups. These data indicate that morphine induced saccharin suppression in SHAM, but not ICX, subjects during the seven acquisition trials.
After the contingencies were switched during reversal training (trials 8–16), it is clear that group SHAM-MS increased consumption of the saccharin CS after it was no longer followed by morphine (see Figure 2). In addition, SHAM-SM subjects showed little suppression during CS-drug pairings. As in the acquisition phase of this experiment, neither group of ICX rats decreased saccharin intake. An ANOVA conducted on the data from the nine reversal trials found a significant Lesion X Condition X Trials interaction, F(8, 248) = 2.15, p < .05. To follow up this interaction, we conducted two sets of analyses: one comparing data between SHAM groups on each trial, and the other between the ICX groups. Despite the increase in consumption by group SHAM-MS during reversal, those subjects drank significantly less saccharin than their SHAM-SM counterparts on all nine reversal trials (ps < .05). Extinction of the suppression learned during acquisition did occur in SHAM-MS rats, as they drank more saccharin on the final reversal trial than they did on the first, F(1, 248) = 23.73, p < .001. While SHAM-MS animals drank more saccharin in the later trials, reflecting the extinction of the morphine conditioning, SHAM-SM rats showed a small (less than 2 ml), but significant, amount of suppression between the first and final reversal trial, F(1, 248) = 6.81, p < .05. It is worth noting, however, that the amount consumed on the final reversal trial by the SHAM-SM rats (9.22 ±0.58 ml) is essentially identical to the amount consumed on the first acquisition trial (9.28 ±0.66 ml). The two ICX groups only differed from each other on reversal trials 8 and 11, with ICX-SM animals consuming more saccharin on the trial 8, F(1, 248) = 4.22, p < .05, and ICX-MS rats drinking more on trial 11, F(1, 248) = 4.98, p < .05. The overall pattern of results in the reversal phase show that SHAM-MS rats increased saccharin intake, and that the SHAM-SM rats showed a small amount of saccharin suppression to a level equivalent to their initial intake on trial 1 of the experiment. Finally, there is no indication that ICX rats learned any information about the relationship between saccharin and morphine in this experiment.
Discussion
In the acquisition phase of the experiment, SHAM-MS animals quickly (i.e., after a single CS-US pairing) learned to suppress consumption of saccharin when it was followed by morphine, a finding that is consistent with previous results using experimentally naïve rats (e.g., Grigson, 1997, Experiment 3; Grigson et al., 2000; Experiment 1). Unlike their SHAM counterparts, ICX-MS rats did not decrease their consumption of the saccharin solution, even after repeated pairings with the morphine US. Thus, the acquisition data indicate that IC lesions have a profound influence on morphine-induced suppression of saccharin intake. The behavioral deficit in ICX rats mirrors the effect of GT lesions in this paradigm (Grigson et al., 2000; Reilly & Trifunovic, 1999a). It might be noted that GTX rats also fail to acquire sucrose-induced suppression of a saccharin CS in the anticipatory negative contrast paradigm (Reilly, Bornovalova & Trifunovic, 2004; Reilly & Pritchard, 1996; Schroy, Wheeler, Davidson, Scalera, Twining & Grigson, 2005) as well as successive negative contrast in which a low-value reward (i.e., saccharin) is substituted for an expected high-value reward (i.e., sucrose; Reilly & Trifunovic, 1999b, 2003). The performance of GTX animals in these tasks has been interpreted as a lesion-induced impairment of the reward comparison mechanism that governs the ability to accurately compare the reward values of appetitive stimuli across sessions (e.g., Reilly 2005a, 2005b). In other words, GTX rats respond based upon the absolute value of appetitive stimuli rather than the reward value relative to each other. Since morphine treated ICX rats failed to decrease saccharin intake in the present experiment, the explanation forwarded for the behavior of GTX rats may also be applicable to ICX rats. Indeed, as noted in the Introduction, the GT is reciprocally connected with the IC and similar behavioral deficits suggest that these two structures serve an inter-dependent function in the brain circuit underlying reward comparison.
There are, however, reasons to question whether this analysis is complete with regard to the role of the IC in morphine-induced suppression of saccharin intake. We have recently obtained evidence that IC lesions disrupt the initial neophobic response to a novel taste (but not to a novel aqueous odor or a novel trigeminal stimulus; Lin, Roman, St. Andre & Reilly, under review). These findings suggest that ICX rats may fail to recognize the novelty of taste stimuli or perhaps IC lesions disrupt the fear response that is triggered by the detection of a novel taste. ICX rats also show impaired CTA acquisition when the taste CS is novel but not when it is familiar in a latent inhibition procedure (Roman & Reilly, 2007). This pattern of impaired and spared functions encourages the view that IC lesions disrupt the perception of taste novelty. If familiarity with the taste CS delays the suppression of saccharin (as seen in the SHAM subjects switched from saline injections in acquisition to the morphine US during the reversal phase of the present experiment), and ICX subjects respond to the novel CS as if it was familiar, then the failure of the lesioned rats to decrease saccharin intake across trials may represent a latent inhibition-like retardation of learning.
There are, then, two plausible explanations for the failure of morphine to suppress saccharin intake in rats with IC lesions. It is possible, of course, that both types of deficit (reward comparison and novelty perception) contribute to the abnormal performance of the ICX rats in the present experiment. However, if IC lesions disrupt incentive learning in the same all-or-none manner that appears to be the case with GT lesions (Grigson et al., 2000; Reilly & Trifunovic, 1999a), then one would predict that ICX rats, with or without prior experience of the taste cue, would never learn to suppress saccharin intake. On the other hand, if IC lesions disrupt the perception of taste novelty, then ICX rats should, eventually, learn to suppress intake of both a novel and a familiar CS given sufficient saccharin-morphine conditioning trials as occurs in latent inhibition studies involving saccharin-LiCl pairings (e.g., Roman & Reilly, 2007).
Overall, the pattern of results obtained from the present experiments revealed two important new findings regarding morphine-induced CS suppression. First, over 7 acquisition trials, IC lesions prevented the acquisition of saccharin suppression induced by morphine, an effect that is readily acquired by normal animals. Second, neurologically intact rats failed to learn to suppress the same saccharin solution if they have prior nonreinforced exposure to saccharin (i.e., latent inhibition seems to occur in the CS-drug paradigm). Together these first two findings encourage the view that IC lesions disrupt a reward comparison mechanism and/or the perception of taste novelty.
Acknowledgments
We thank Jian-You Lin for assistance at all stages of this research which was supported by grants DC04341 and DC06456 from the National Institute of Deafness and Other Communication Disorders. Portions of the data in this article were presented at the 37th Annual Meeting of the Society for Neuroscience, San Diego, CA, November 2007.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/bne.
References
- Bermudez-Rattoni F, McGaugh JL. Insular cortex and amygdala lesions differentially affect acquisition on inhibitory avoidance and conditioned taste aversion. Brain Research. 1991;549:165–170. doi: 10.1016/0006-8993(91)90616-4. [DOI] [PubMed] [Google Scholar]
- Braun JJ, Slick TB, Lorden JF. Involvement of gustatory neocortex in the learning of taste aversions. Physiology & Behavior. 1972;9:637–641. doi: 10.1016/0031-9384(72)90023-6. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representations in the cortex and thalamus in the rat. Journal of Comparative Neurology. 1987;262:27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- Cubero I, Thiele TE, Bernstein IL. Insular cortex lesions and taste aversion learning: effects of conditioning method and timing of lesion. Brain Research. 1991;839:323–330. doi: 10.1016/s0006-8993(99)01745-x. [DOI] [PubMed] [Google Scholar]
- Flaherty CF. Incentive relativity. Cambridge, England: Cambridge University Press; 1996. [Google Scholar]
- Fresquet N, Angst MJ, Sandner G. Insular cortex lesions alter conditioned taste avoidance in rats differentially when using two methods of sucrose. Behavioural Brain Research. 2004;153:357–365. doi: 10.1016/j.bbr.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Gallo M, Roldan G, Bures J. Differential involvement of gustatory cortex and amygdala in the acquisition and retrieval of conditioned taste aversion in rats. Behavioural Brain Research. 1992;52:91–97. doi: 10.1016/s0166-4328(05)80328-6. [DOI] [PubMed] [Google Scholar]
- Gamzu E, Vincent G, Boff E. A pharmacological perspective of drugs used in establishing conditioned food aversions. Annals of the New York Academy of Sciences. 1985;443:231–249. doi: 10.1111/j.1749-6632.1985.tb27077.x. [DOI] [PubMed] [Google Scholar]
- Grigson PS. Conditioned taste aversions and drugs of abuse: A reinterpretation. Behavioral Neuroscience. 1997;111:129–136. [PubMed] [Google Scholar]
- Grigson PS, Lyuboslavsky P, Tanase D. Bilateral lesions of the gustatory thalamus disrupt morphine, but not LiCl-induced conditioned taste aversions in rats: Evidence for the reward comparison hypothesis. Brain Research. 2000;858:327–337. doi: 10.1016/s0006-8993(00)01939-9. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Twining RC, Freet CS, Wheeler RA, Geddes RI. Drug-induced suppression of CS intake: Reward, aversion, and addiction. In: Reilly S, Schachtman TR, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. New York: Oxford University Press; 2009. pp. 74–91. [Google Scholar]
- Kiefer SW, Braun JJ. Absence of differential associative responses to novel and familiar taste stimuli in rats lacking gustatory neocortex. Journal of Comparative Physiology and Psychology. 1977;91:498–507. doi: 10.1037/h0077347. [DOI] [PubMed] [Google Scholar]
- Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. I. Physiological properties and cytoarchitecture. Brain Research. 1986;379:329–341. doi: 10.1016/0006-8993(86)90787-0. [DOI] [PubMed] [Google Scholar]
- Lin JY, Roman C, St. Andre J, Reilly S. Taste, olfactory and trigeminal neophobia in rats with forebrain lesions. doi: 10.1016/j.brainres.2008.11.040. (Under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubow RE. Latent inhibition and conditioned attention theory. Cambridge: Cambridge University Press; 1989. [Google Scholar]
- Lubow RE. Conditioned taste aversion and latent inhibition A review. In: Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and neural processes. New York: Oxford University Press; 2009. pp. 37–57. [Google Scholar]
- Mackey WB, Keller J, van der Kooy D. Visceral cortex lesions block conditioned taste aversions induced by morphine. Pharmacology Biochemistry and Behavior. 1986;24:71–78. doi: 10.1016/0091-3057(86)90047-x. [DOI] [PubMed] [Google Scholar]
- Nerad L, Ramirez-Amaya V, Ormsby CE, Bermudez-Rattoni F. Differential effects of anterior and posterior insular cortex lesions on the acquisition of conditioned taste aversion and spatial learning. Neurobiology of Learning and Memory. 1996;66:44–50. doi: 10.1006/nlme.1996.0042. [DOI] [PubMed] [Google Scholar]
- Norgren R, Wolf G. Projections of thalamic gustatory and lingual areas in the rat. Brain Research. 1975;92:123–129. doi: 10.1016/0006-8993(75)90531-4. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed. San Diego, CA: Academic Press; 2005. [Google Scholar]
- Reilly S. Gustatory thalamus and consummatory contrast effects. Boston, MA: Invited talk at the Eastern Psychological Association; 2005a. [Google Scholar]
- Reilly S. Gustatory thalamus and incentive relativity. Washington, DC: Invited address at the American Psychological Association; 2005b. [Google Scholar]
- Reilly S, Bornovalova M, Trifunovic R. Excitotoxic lesions of the gustatory thalamus spare simultaneous contrast effects but eliminate anticipatory negative contrast: Evidence against a memory deficit. Behavioral Neuroscience. 2004;118:365–376. doi: 10.1037/0735-7044.118.2.365. [DOI] [PubMed] [Google Scholar]
- Reilly S, Pritchard TC. Gustatory thalamus lesions in the rat: II. Aversive and appetitive taste conditioning. Behavioral Neuroscience. 1996;110:746–759. [PubMed] [Google Scholar]
- Reilly S, Trifunovic R. Progressive ratio performance in rats with gustatory thalamus lesions. Behavioral Neuroscience. 1999a;113:1008–1019. doi: 10.1037//0735-7044.113.5.1008. [DOI] [PubMed] [Google Scholar]
- Reilly S, Trifunovic R. Gustatory thalamus lesions eliminate successive negative contrast in the rat. Behavioral Neuroscience. 1999b;113:1242–1248. [PubMed] [Google Scholar]
- Reilly S, Trifunovic R. Gustatory thalamus lesions disrupt successive negative contrast: Evidence against a memory deficit. Behavioral Neuroscience. 2003;117:606–615. doi: 10.1037/0735-7044.117.3.606. [DOI] [PubMed] [Google Scholar]
- Riley AL, Tuck DL. Conditioned taste aversions: A behavioral index of toxicity. Annals of the New York Academy of Sciences. 1985;443:272–292. doi: 10.1111/j.1749-6632.1985.tb27079.x. [DOI] [PubMed] [Google Scholar]
- Roman C, Nebieridze N, Sastre A, Reilly S. Effects of lesions of the bed nucleus of the stria terminalis, lateral hypothalamus, or insular cortex on conditioned taste aversion and conditioned odor aversion. Behavioral Neuroscience. 2006;120:1257–1267. doi: 10.1037/0735-7044.120.6.1257. [DOI] [PubMed] [Google Scholar]
- Roman C, Reilly S. Effects of insular cortex lesions on conditioned taste aversion and latent inhibition. European Journal of Neuroscience. 2007;26:2627–2632. doi: 10.1111/j.1460-9568.2007.05872.x. [DOI] [PubMed] [Google Scholar]
- Schroy PL, Wheeler RA, Davidson C, Scalera G, Twinning RC, Grigson PS. The role of the gustatory thalamus in the anticipation and comparison of rewards over time in rats. American Journal of Physiology. 2005;288:R966–R980. doi: 10.1152/ajpregu.00292.2004. [DOI] [PubMed] [Google Scholar]
- Wolf G. Projections of thalamic and cortical gustatory areas in the rat. Journal of Comparative Neurology. 1968;132:519–530. doi: 10.1002/cne.901320403. [DOI] [PubMed] [Google Scholar]
- Zito KA, Bechera A, Greenwood C, van der Kooy D. The dopamine innervation of the visceral cortex mediates the aversive effects of opiates. Pharmacology Biochemistry and Behavior. 1988;30:693–699. doi: 10.1016/0091-3057(88)90086-x. [DOI] [PubMed] [Google Scholar]