Abstract
Objectives
Amyloid senile plaques and tau neurofibrillary tangles are neuropathological hallmarks of Alzheimer's disease that may be associated with mild cognitive impairment or mood and anxiety symptoms years before the dementia diagnosis. To address this issue, we obtained positron emission tomography (PET) scans after intravenous injections of 2-(1-{6-[(2-[F18]fluoroethyl)(methyl)amino]-2-naphthyl} ethylidene) malononitrile (FDDNP), a molecule that binds to amyloid plaques and neurofibrillary tangles, to determine whether symptoms of depression and anxiety in non-demented subjects were associated with increased FDDNP-PET binding values.
Methods
Forty-three middle-aged and elderly volunteers received clinical and FDDNPPET assessments. Subjects were non-demented - 23 of them were diagnosed with MCI, and 20 were cognitively normal. Subjects with a diagnosis of major depression or an anxiety disorder were excluded. Correlations between standardized measures of depressive and anxiety symptoms and regional FDDNP binding values were calculated.
Results
The MCI and comparison subjects did not differ by the depression and anxiety scores. In the MCI group, depression correlated with lateral temporal, and trait anxiety correlated with posterior cingulate FDDNP binding. In the comparison group, depression correlated with medial temporal, and trait anxiety scores correlated with medial temporal , and frontal FDDNP binding.
Discussion
This is the first report to demonstrate a relationship between the severity of depression and anxiety symptoms and FDDNP binding values in non-demented middle age and older individuals. The results suggest a relationship between relatively mild mood symptoms and biomarkers of cerebral amyloid and tau deposition and vary according to degree of cognitive impairment. The presence of MCI may signify different pathophysiological mechanisms underlying mood and anxiety symptoms.
Keywords: Positron Emission Tomography (FDDNP-PET), Depression, State/Trait Anxiety, Mild Cognitive Impairment, Middle-aged and Older adults
Early detection of Alzheimer's disease (AD) is important for identifying candidates for therapeutic interventions that can delay the onset and slow the progression of AD. One approach to early detection is to identify individuals at greater risk for cognitive decline. Among the consistently identified risk factors for AD are mild cognitive impairment (MCI), a transitional stage between normal aging and AD (1,2), as well as anxiety and depression preceding the onset of AD that occur most often later in life with the onset after 60 years of age (3-9).
There is accumulating evidence that late-life depression and anxiety are risk factors for MCI and AD (10-12). Late-life depression (LLD) is typically associated with impairment in executive functions, including perseverative responses and disturbances in initiation of behavior and in inhibition of inappropriate responses (13-18). Interestingly, recent reports of late-life anxiety identified predominant memory deficits (19). Another recent report demonstrated that the presence of anxiety increased the rate of conversion from MCI to AD by two-fold (20). There are also converging neuropathological data linking mood disorders and MCI to AD (21-26).
Neuropathological studies indicate that two abnormal proteins, beta-amyloid (in senile plaques) and tau (neurofibrillary tangles), accumulate in a predictable spatial pattern in aging and AD (21,22). These changes increase in prevalence with age. Autopsy studies indicate that the level of AD pathology in MCI is intermediate to that of persons with normal cognition and those with dementia (23,24). These neuropathological changes may be associated with mood and anxiety symptoms that precede the diagnosis of dementia by several years and put subjects at an additional risk for dementia. Recent publications on the neuropathological correlates of late-onset major depression (4) and increased plasma Aβ42 and Aβ42/40 ratios in geriatric depression (25,26) provide observations supporting this link between increased amyloid burden and late-life mood disorders for increasing the risk of MCI and AD.
Recent developments of noninvasive PET molecular imaging probes for imaging amyloid (27) or amyloid and tau (28) show promise for detecting homogeneous groups of persons at risk for AD according to underlying neuropathology. Our group developed a small molecule, 2-(1-{6-[(2-[F-18]fluoroethyl)(methyl)amino]-2-naphthyl}ethylidene)malononitrile (FDDNP), for use as an in vivo chemical marker of cerebral aggregates of amyloid and tau proteins (28). Both FDDNP and its parent molecule, DDNP, are fluorescent and provide clear in vitro visualizations of plaques and tangles in Alzheimer's brain specimens examined under a confocal fluorescence microscope (29). We recently reported (30) that FDDNP-PET scanning differentiated patients with MCI from patients with AD and from cognitively normal older adults. Global FDDNP-PET signal was highest in patients with AD, intermediate in those with MCI, and lowest in normal comparison subjects.
In the present study, we determined whether symptoms of depression and anxiety in subjects with MCI or healthy comparison subjects were associated with increased regional FDDNP-PET binding. We hypothesized that the severity of depression and anxiety will be associated with increased regional FDDNP binding, particularly in the frontal and temporal regions in both cognitive groups.
Methods
Subjects were drawn from a larger, longitudinal study of AD, detailed elsewhere (30). Briefly, baseline cognitive assessments and PET with FDDNP were performed in 43 middle-aged or older volunteers who completed depression and anxiety assessments. Volunteers were recruited through advertisements of a study of mild memory impairment, media coverage of the study, and referrals by physicians and families. Members of the research staff screened potential volunteers via telephone interviews.
All subjects underwent clinical and cognitive assessments and FDDNP-PET scans. Depression and anxiety were evaluated using the Geriatric Depression Scale (GDS) (31) and the State-Trait Anxiety Inventory (STAI) (32). Both instruments have been used in our ongoing studies to screen for depression and anxiety. The GDS, a self-report of depressive symptoms (31), consists of 30 items. A cut-off of 10 items has been suggested to differentiate patients with mild depression from normal comparison subjects and 20 or more items to indicate significant depression. The State-Trait Anxiety Inventory (STAI) provides a reliable measure of both temporary and dispositional anxiety in adults (32). The STAI consists of two subscales: state anxiety and trait anxiety. The scale's first 20 items measure state anxiety by asking subjects how they feel “right now,” and subjects rate their feelings on a four-point intensity scale. The next 20 items measure trait anxiety by asking subjects how they “generally” feel, and subjects rate themselves on a four-point frequency scale. It is the most widely used measure of anxiety in both clinical and research settings. Volunteers with a diagnosis of major depression or anxiety disorders were excluded.
All subjects received magnetic resonance imaging (MRI) scans that were co-registered to PET scans for determination of regions of interest, including medial and lateral temporal, posterior cingulate, parietal, frontal, and global (overall average) regions. Subjects with evidence of stroke on MRI were excluded from the study.
To diagnose MCI, we used modified diagnostic criteria (2,33), which include (1) patient awareness of memory decline, preferably confirmed by another person; (2) measurable, greater-than-normal cognitive impairment detected with standard assessment tests; (3) ability to perform normal daily activities, and (4) an absence of dementia.
Imaging Methods
As described elsewhere, FDDNP was prepared at very high specific activities (>37 GBq/mol) (34,35). All scans were performed with the ECAT HR or EXACT HR+ tomograph (Siemens-CTI, Knoxville, TN) with subjects supine with the imaging plane parallel to the orbito-meatal line. A bolus of FDDNP (320 - 550 MBq) was injected via an in-dwelling venous catheter and consecutive dynamic PET scans were performed for 2 hours. Scans were decay corrected and reconstructed using filtered back-projection (Hann filter, 5.5 mm FWHM) with scatter and measured attenuation correction. The resulting images contained 47 contiguous slices with plane separation of 3.37 mm (ECAT HR) or 63 contiguous slices with plane separation of 2.42 mm (EXACT HR+). Results did not differ significantly according to the scanner used. We conducted t-tests within MCI and comparison groups separately, to determine if there were any differences in FDDNP signal in ROIs related to the use of two PET scanners. Data from these analyses indicate no significant differences [t=0.36 to 1.35; p=0.2 to 0.7]. To quantify FDDNP binding, we performed Logan graphical analysis with cerebellum as the reference region for time points between 30 and 125 minutes (36). The slope of the linear portion of the Logan plot is the relative distribution volume (DVR), which is equal to the distribution volume of the tracer in a region of interest divided by that in the reference region. We generated DVR parametric images and analyzed them using regions of interest drawn manually on the co-registered MRI scans, or on an image obtained in the first 5 minutes after injection (perfusion image), bilaterally on parietal, medial temporal (limbic regions, including hippocampus, parahippocampal areas, and entorhinal cortex), lateral temporal, posterior cingulate, parietal and frontal regions, as previously described (34). Each regional DVR or binding value was expressed as an average of left and right regions. Rules for region of interest drawing were based on the standard identification of gyral and sulcal landmarks with respect to the atlas of Talairach and Tournoux (37). The atlas provided a visual guide and reference for identifying the important landmarks needed in delineating the ROI. Region of interest determinations were performed by individuals blind to the clinical assessments.
Anatomical brain MRI scans were obtained using a 3-Tesla magnet (General Electric-Signa, Milwaukee, WI) scanner. Fifty-four transverse planes were collected throughout the brain, superior to the cerebellum, using a double-echo, fast-spin echo series with a 24-cm field of view and 256 x 256 matrix with 3 mm/0 gap (TR = 6000 [3T] and 2000 [1.5T]; TE = 17/85 [3T] and 30/90 [1.5T]).
Data Analysis
Descriptive statistics were obtained for the MCI and comparison groups separately. Student's t-tests were used to compare the groups on continuous variables, and chi-square tests were used for categorical variables. Spearman correlation coefficients between GDS, state anxiety, trait anxiety and regional FDDNP binding values were estimated to determine whether severity of depression and anxiety were associated with amyloid plaque and tangle binding in the brain within each cognitive group. A significance level of .05 was used for all inferences.
Results
Demographic variables for the subjects are presented in Table 1. All 43 subjects (22 women, 21 men; age range 40-87 years) were non-demented (mean [SD] Mini-Mental State Exam scores = 28.4 [1.5]), well-educated (mean [SD] education years = 17.1 [3.1]), middle-aged and older (mean [SD] age = 66.1 [12.4]) volunteers, and 23 were diagnosed with MCI. The MCI and comparison subjects did not differ significantly in age, education, gender, or AD family history, nor did they differ in the severity of depression as measured by the GDS and their state and trait anxiety scores. The MCI group showed significantly greater FDDNP-binding compared with comparison subjects in all regions except posterior cingulate (Table 2). Our earlier report (30), which included a slightly larger sample (some of the subjects included in that study did not receive the STAI) showed significant differences in all regions.
Table 1.
Demographic and Clinical Characteristics of the Sample.
| Variables | MCI group Mean (SD), Range | Comparison subjects Mean (SD), Range | Statistics* t(41)/χ2(1);p-value |
|---|---|---|---|
| Gender (F:M) | 14:9 | 8:12 | 1.9,0.2 |
| AD family history (Y:N) | 8:15 | 10:10 | 1.0; 0.3 |
| Age (yrs) | 68.2 (12.2), 49-87 | 63.7 (12.5), 40-87 | 1.2;0.2 |
| Education (yrs) | 16.6 (3.4), 12-24 | 17.7 (2.7), 13-22 | -1.1;0.3 |
| MMSE | 27.6 (1.4), 25-30 | 29.4 (0.9), 27-30 | -4.8;0.0001 |
| GDS | 5.7 (4.4), 1-15 | 5.3 (3.7), 0-15 | 0.4;0.7 |
| State Anxiety | 36.5 (10.0), 20-57 | 31.7 (8.8), 20-52 | 1.7;0.1 |
| Trait Anxiety | 35.2 (9.1), 20-49 | 30.9 (10.9), 15-58 | 1.4;0.2 |
For all continuous variables student's t-tests were conducted; for gender and AD family history, the ratio between the groups was compared using Chi-square test. MMSE- Mini-Mental State Examination; GDS- Geriatric Depression Scale, State Anxiety and Trait Anxiety- are Sub-scale scores of the State-Trait Anxiety Inventory.
Table 2.
Regional FDDNP-Binding Values in MCI and Comparison Subjects.
| Subjects/Regions | MCI group Mean (SD) | Comparisons Mean (SD) | Statistics* t(41); p-value |
|---|---|---|---|
| Global | 1.11 (0.03) | 1.08 (0.03) | 3.29;0.002 |
| Medial Temporal | 1.15 (0.04) | 1.11 (0.04) | 2.86;0.007 |
| Lateral Temporal | 1.12 (0.04) | 1.08 (0.04) | 3.22;0.002 |
| Parietal | 1.08 (0.04) | 1.06 (0.03) | 2.2;0.03 |
| Posterior Cingulate | 1.12 (0.06) | 1.09 (0.04) | 1.6;0.12 |
| Frontal | 1.06 (0.03) | 1.04 (0.04) | 2.1;0.04 |
For all measures, student's t-tests were conducted.
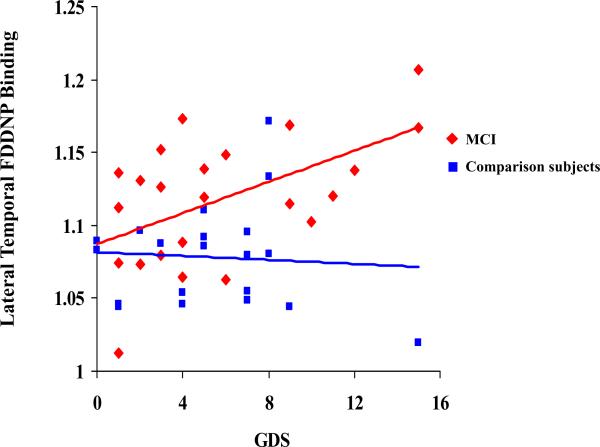
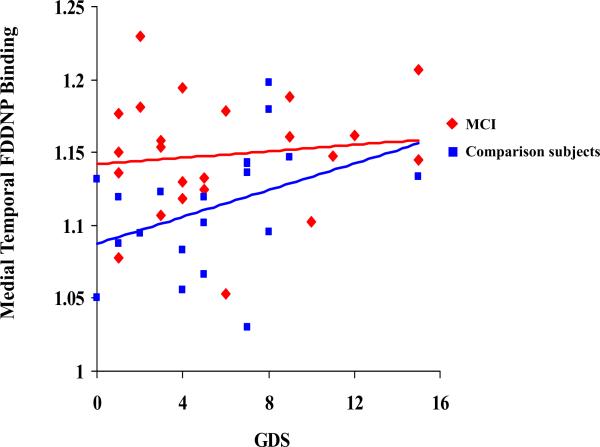
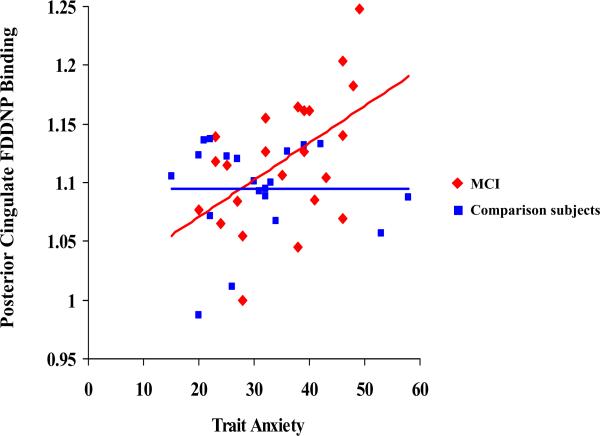
Table 3 and Figures 1, 2 and 3 demonstrate the correlations between depression and trait anxiety scores and the regional FDDNP binding. In the MCI group, depression scores correlated with lateral temporal FDDNP binding values. The severity of the trait anxiety scores correlated with posterior cingulate FDDNP binding values. In the comparison group, depression scores correlated with medial temporal FDDNP binding. The severity of the trait anxiety scores correlated with medial temporal and frontal FDDNP binding values. The state anxiety scores did not correlate with any of the regional FDDNP binding values in either of the cognitive groups. All correlations remained significant after controlling for age. We compared the groups on the significance of the differences in correlations between the FDDNP regional binding values and the GDS and the trait anxiety scores. The difference between the groups in correlation between the GDS scores and the medial temporal lobe FDDNP binding achieved the level of significance (80% C.I.[-0.75;-0.13]; z=-1.7; p=0.04). Other correlations showed only a trend toward significant differences (p>=0.1), which may be due to a small sample size and the mild severity of symptoms.
Table 3.
Correlations Between Depression (GDS) and Trait Anxiety Scores and regional FDDNP Binding.
| Measure | Depression | Trait Anxiety | ||
|---|---|---|---|---|
| Subjects / Regions | MCI (N=23) r* (P) | Comparison subjects (N=20) r* (P) | MCI (N=23) r* (P) | Comparison subjects (N=20) r* (P) |
| Medial Temporal | 0.06 (0.79) | 0.55 (0.01) | 0.29 (0.17) | 0.59 (0.005) |
| Lateral Temporal | 0.44 (0.03) | -0.04 (0.86) | 0.35 (0.09) | -0.10 (0.65) |
| Parietal | 0.11 (0.60) | 0.13 (0.57) | 0.004 (0.98) | 0.22 (0.34) |
| Posterior Cingulate | 0.28 (0.18) | 0.02 (0.91) | 0.49 (0.02) | -0.09 (0.68) |
| Frontal | 0.20 (0.35) | 0.34 (0.14) | 0.14 (0.51) | 0.47 ( 0.04) |
r- Spearman correlation coefficient P values are included in parentheses. Significant correlations are bolded.
Figure 1.
Correlations between the depression (GDS) scores and the FDDNP Binding in the Lateral Temporal region in the two cognitive groups.
Figure 2.
Correlations between the depression (GDS) scores and the FDDNP Binding in the Medial Temporal region in the two cognitive groups.
Figure 3.
Correlations between the Trait Anxiety scores and the FDDNP Binding in the Posterior Cingulate region in the two cognitive groups.
Discussion
This is the first report to demonstrate a relationship between severity of depressive and anxiety symptoms and FDDNP binding values in non-demented middle age and older people. Our findings support previous work suggesting a relationship between more severe forms of geriatric depression and brain amyloid deposition (4,25). We also report the patterns of associations in the two groups that underscores the importance of the MCI diagnosis in relation to the underlying pathophysiology of mood and anxiety symptoms. Surprisingly, the depression scores showed correlation only with the lateral temporal FDDNP binding in the MCI group, while the medial temporal FDDNP binding values correlated with the severity of depression in the comparison subjects. However, the severity of anxiety symptoms correlated with the FDDNP binding in posterior cingulate region in the MCI group and with frontal and medial temporal regions in the comparison subjects. Our preliminary findings suggest differences in the neural circuitry involvement in the pathogenesis of mood and anxiety symptoms in relation to the cognitive status. In other words, mood and anxiety states with and without MCI may represent two endophenotypes with different underlying neural mechanisms and gene expression. Although at this time we have only limited evidence to support this hypothesis, we plan to explore the relationships between such clinical phenotypes of mood and anxiety disorders with and without MCI to the FDDNP-PET and genetic markers in future prospective studies that will include larger samples.
Although major depression can cause cognitive symptoms in younger and older adults, the relationship between late-life major depression and cognitive impairment is particularly complex. For example, not all studies find the neuropathological link between depression and dementia (39). Significant cognitive impairment occurs in more than half of elderly depressed patients during an episode of depression (40). Impaired cognition does not completely normalize with successful treatment of depression, especially in the areas of memory, executive function and information-processing speed (41,42). Nearly 50% of those with cognitive impairment during a depressive episode or “pseudodementia” go on to develop dementia in the next 5 years (43).
A number of studies have found an association between late-life major depression and increased risk for clinically diagnosed AD (7-10; 43-49). Thus, a diagnosis of late-life major depression similar to the MCI diagnosis may serve to identify a high-risk group that would benefit from initiation of therapies with the goal of delaying or preventing the onset of dementia (42, 40). Sweet and colleagues recently (2004) demonstrated a neuropathological link in 10 subjects initially diagnosed with major depression who were followed in a longitudinal study. Seven (70%) subjects had evidence of onset of a dementia prior to death. Major depression with dementia was significantly associated with a neuropathological diagnosis of AD. Therefore, current evidence suggests that AD is the predominant neuropathological condition in geriatric major depression subjects with dementia. In search of antemortem surrogate diagnostic markers of risk for dementia, Pomara et al (26) analyzed plasma amyloid beta 1-42 (Aβ42) levels because elevated plasma amyloid beta 1-42 (Aβ42) level has been linked to increased risk for incident AD in cognitively-intact elderly. Results indicated that plasma Aβ42 levels and the Aβ42/40 ratios were elevated in the depressed group relative to control subjects, suggesting that increased plasma Aβ42 and Aβ42/40 ratios are present in geriatric depression.
Our results presented in Figure 1 suggest that the relationship between depression symptoms and FDDNP binding is mainly driven by subjects with GDS scores above the cutoff for mild depression. This finding is supported by the work of Sweet et al (2004) (4) showing an increase in amyloid load in patients with geriatric depression, as well as the reports by Rapp et al (2006, 2008) (50,51) showing an increase in plaques and tangles in Alzheimer's disease patients with depression co-morbidity (50). Rapp et al (2008) (51) demonstrated in patients with AD that the presence of depression comorbidity corresponds to increases in AD-related neuropathologic changes with accumulation of neurofibrillary tangles beyond age, gender, level of education, and cognitive status, suggesting an interaction between depression and the neuropathologic processes in AD. In another report, a life time prevalence of major depression was associated with increased hippocampal plaques and tangles in patients with Alzheimer disease (50).
Although much less is known about the link between generalized anxiety and AD, recent studies indicate that the relationship may be even stronger than that with major depression (19,6), and anxiety may represent an additional risk for progression from MCI to AD (20). Interestingly, in patients with MCI, anxiety but not depressive symptoms were associated with conversion to AD, while in cognitively normal subjects depression was associated with AD diagnosis at follow up (52). No other surrogate biological markers or autopsy data have been used to support this link. Similarly, there are no studies linking less severe forms of depression or anxiety to dementia and cognitive impairment, particularly, using biological markers.
Autopsy confirmation is seen as the “gold standard” for a diagnosis of AD; however, in most clinical research settings, a clinical diagnosis of AD is a reasonable proxy, with autopsy confirmation rates generally reported between 85 and 100% (53-56). As has been demonstrated recently, the use of biological markers and in vivo neuroimaging can further improve the accuracy of early detection of dementia in these high-risk populations (27, 28, 57-64).
We are not able to compare our results to other studies because of the unique design and methodology of our study. The differences that we observed in the correlations between the regional FDDNP binding and depression and anxiety scores in the two cognitive groups may be important as predictors of future development of mood and anxiety disorders, or dementia. This will need to be tested in a longitudinal follow up. However, several recent conceptual papers indicated the importance of application of integrative approaches combining genetics and in-vivo neuroimaging methods using biological markers to understand such complex issues as the potential overlap between depression and dementia, especially for the development of early diagnosis tools and preventive interventions (65-67).
With regard to the localization of the FDDNP binding and potential neurocircuits involved, our findings are consistent with the findings in the literature identifying neurocircuitry of mood and anxiety disorders, evidenced by the association of neuroticism and anticipatory anxiety with smaller anterior and posterior cingulate volumes (68), and negative association with brain activity on fMRI in the posterior cingulate gyrus in younger adults (69). A magnetization transfer (MTR) study of geriatric depression reported reduced myelin integrity in specific aspects of frontostriatal and limbic networks that indicate decreased organization of white matter fibers in specific frontal and temporal regions including dorsolateral prefrontal, anterior and posterior cingulate regions (70). However, a recent study demonstrated the relationship between depressive symptoms and medial temporal lobe atrophy to conversion to Alzheimer's disease in a prospective study (71). Atrophy of the medial temporal lobe and left lateral temporal were predictors of conversion from MCI to Dementia (72, 73). A recent SPECT study demonstrated different patterns of blood flow in subjects with AD compared to subjects with depression. Depressed subjects had reductions in blood flow in the lateral frontal, left thalamus and bilateral medial frontal regions, and AD subjects had reduced blood flow to the lateral parietal, lateral temporal, bilateral precuneus and bilateral posterior cingulate gyrus (74). However, no prior studies demonstrated that the pathophysiolgy of mood and anxiety symptoms in older adults is affected by the accumulation of amyloid and tau-protein in the regions of interest using PET imaging. It is possible that long-standing trait anxiety or mood disorders would contribute to the neural damage and overproduction of amyloid and tau in the structures that are involved in the pathogenesis of anxiety and depression. For example, our recent report identified such a link by showing changes in 5-HT1A receptor densities in the living brain of AD patients (ADs). Mild cognitive impairment patients (MCI) were also correlated with global and regional measures of glucose metabolic activity, with the extent and spatial distribution of the NFT/ -amyloid senile plaque deposition (75). However, we need to be careful about overinterpreting this data due to a relatively small sample size, and expect replication of the findings in future studies. This particularly refers to our finding of the different association between the GDS scores and the FDDNP binding in the medial temporal in the MCI and comparison subjects that was found to be statistically significant (Figure 2). It may have to do with the accumulation of plaques and tangles initially in the medial temporal cortex and spreading to the lateral temporal cortex in the MCI subjects compared to normal subjects who may only show the association in the medial temporal cortex (76), or this may be entirely due to a relatively small sample size.
Our findings suggest that this relationship between mood and anxiety symptoms and biomarkers of cerebral amyloid and tau deposition may vary on the basis of comorbid mild cognitive impairment. However, the major limitations of the study are its exploratory nature that may result in a type 1 error, a relatively small sample size and mild severity of depression and anxiety. Future studies should assess the presence of biomarkers of cerebral amyloid and tau deposition in more severe forms of mood and anxiety disorders in middle-aged and older individuals with and without cognitive impairment.
ACKNOWLEDGMENTS AND DISCLOSURES
Supported by NIH grants MH077650; AT003480; P01-AG024831, AG13308, P50 AG 16570, MH/AG58156, MH52453; AG10123; M01-RR00865, DOE contract DE-FC03-87-ER60615, GCRC Program, the Larry L. Hillblom Foundation, Rotary CART Fund; Alzheimer's Association, Alzheimer's Disease Research Center Pilot Award; Turken Foundation, Fran and Ray Stark Foundation Fund for Alzheimer's Disease Research; Ahmanson Foundation; Lovelace Foundation, Judith Olenick Elgart Fund for Research on Brain Aging, John D. French Foundation for Alzheimer's Research, and the Tamkin Foundation.
The authors also thank Ms. Andrea Kaplan, Ms. Debbie Dorsey, Ms. Teresann Crow-Lear, Mr. Sharone Trifskin, Dr Jeanne Kim, and Dr. Achinoam Socher for help in subject recruitment, data management, and study coordination.
Footnotes
The University of California, Los Angeles, owns the U.S. patent (6,274,119) entitled “Methods for Labeling beta-Amyloid Plaques and Neurofibrillary Tangles,” which has been licensed Siemens. Drs. Small, Huang, and Barrio are among the inventors. Dr. Small reports having served as a consultant and received lecture fees from Abbott, Eisai, Forest, Novartis, Ortho-McNeil, Pfizer, Siemens, Servier, and VerusMed. Dr. Lavretsky reports having received lecture fees from Eisai, Jannsen, and Pfizer, consulting fees from Forest Laboratories and Myriad Pharmaceuticals, and received a research grant from Forest Laboratories. Dr. Barrio reports having served as a consultant and received lecture fees from Nihon Medi-Physics Co, Bristol-Meyer Squibb, PETNet Pharmaceuticals, and Siemens.
References
- 1.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Int Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 3.Archer N, Brown RG, Reeves SJ, Boothby H, Nicholas H, Foy C, Williams J, Lovestone S. Premorbid personality and behavioral and psychological symptoms in probable Alzheimer disease. Am J Geriatr Psychiatry. 2007;15:202–13. doi: 10.1097/01.JGP.0000232510.77213.10. [DOI] [PubMed] [Google Scholar]
- 4.Sweet RA, Hamilton RL, Butters MA, Mulsant BH, Pollock BG, Lewis DA, Lopez LO, DeKosky ST, Reynolds CF., 3rd Neuropathologic correlates of late onset major depression. Neuropsychopharm. 2004;29:2242–2250. doi: 10.1038/sj.npp.1300554. [DOI] [PubMed] [Google Scholar]
- 5.Galvin JE, Powlishta KK, Wilkins K, McKeel DW, Xiong C, Grant E, Storandt M, Morris JC. Predictors of preclinical Alzheimer's Disease and dementia. Arch Neurol. 2005;62:758–765. doi: 10.1001/archneur.62.5.758. [DOI] [PubMed] [Google Scholar]
- 6.DeLuca AK, Lenze EJ, Mulsant BH, Butters MA, Karp JF, Dew MA, Pollock BG, Shear MK, Houck PR, Reynolds CF., 3rd Comorbid anxiety disorder in late life depression: association with memory decline over four years. Int J Geriatr Psychiatry. 2005;20:848–54. doi: 10.1002/gps.1366. [DOI] [PubMed] [Google Scholar]
- 7.Wilson RS, Barnes LL, Mendes de Leon CF, Aggarwal NT, Schneider JS, Bach J, Pilat J, Beckett LA, Arnold SE, Evans DA, Bennett DA. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59:364–370. doi: 10.1212/wnl.59.3.364. [DOI] [PubMed] [Google Scholar]
- 8.Yaffe K, Blackwell T, Gore R, Sands L, Reus V, Browner WS. Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry. 1999;56:425–430. doi: 10.1001/archpsyc.56.5.425. [DOI] [PubMed] [Google Scholar]
- 9.Paterniti S, Verdier-Taillefer MH, Dufouil C, Alperovitch A. Depressive symptoms and cognitive decline in elderly people: longitudinal study. Br J Psychiatry. 2002;181:406–410. doi: 10.1192/bjp.181.5.406. [DOI] [PubMed] [Google Scholar]
- 10.Devanand DP, Sano M, Tang MX, Taylor S, Gurland BJ, Wilder D, Stern Y, Mayeux R. Depressed mood and the incidence of Alzheimer's disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53:175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- 11.Jorm AF. History of depression as a risk factor for dementia: an updated review. Aust N Z J Psychiatry. 2001;35:776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- 12.Lavretsky H, Ercoli L, Siddarth P, Bookheimer S, Miller K, Small G. Apolipoprotein epsilon4 allele status, depressive symptoms, and cognitive decline in middle-aged and elderly persons without dementia. Am J Geriatr Psychiatry. 2003;11:667–73. doi: 10.1176/appi.ajgp.11.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexopoulos GS, Schultz SK, Lebowitz BD. Late-life depression: a model for medical classification. Biol Psychiatry. 2005;58:283–9. doi: 10.1016/j.biopsych.2005.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexopoulos GS. Role of executive function in late-life depression. J Clin Psychiatry. 2003;64(Suppl 14):18–23. [PubMed] [Google Scholar]
- 15.Elderkin-Thompson V, Kumar A, Bilker WB, Dunkin JJ, Mintz J, Moberg PJ, Mesholam RI, Gur RE. Neuropsychological deficits among patients with late-onset minor and major depression. Arch Clin Neuropsychol. 2003;18:529–549. doi: 10.1016/s0887-6177(03)00022-2. [DOI] [PubMed] [Google Scholar]
- 16.Elderkin-Thompson V, Mintz J, Haroon E, Lavretsky H, Kumar A. Executive dysfunction and memory in older patients with major and minor depression. Arch Clin Neuropsychol. 2006;21:669–676. doi: 10.1016/j.acn.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. Am J Psychiatry. 2002;159:1119–1126. doi: 10.1176/appi.ajp.159.7.1119. [DOI] [PubMed] [Google Scholar]
- 18.Nebes RD, Butters MA, Houck PR, Zmuda MD, Aizenstein H, Pollock BG, Mulsant BH, Reynolds CF., 3rd Dual-task performance in depressed geriatric patients. Psychiatry Res. 2001;102:139–151. doi: 10.1016/s0165-1781(01)00244-x. [DOI] [PubMed] [Google Scholar]
- 19.Mantella RC, Butters MA, Dew MA, Mulsant BH, Begley AE, Tracey B, Shear MK, Reynolds CF, 3rd, Lenze EJ. Cognitive impairment in late-life generalized anxiety disorder. Am J Geriatr Psychiatry. 2007;15:673–679. doi: 10.1097/JGP.0b013e31803111f2. [DOI] [PubMed] [Google Scholar]
- 20.Palmer K, Berger AK, Monastero R, Winblad B, Backman L, Fratiglioni L. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology. 2007;68:1596–602. doi: 10.1212/01.wnl.0000260968.92345.3f. [DOI] [PubMed] [Google Scholar]
- 21.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 22.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 23.Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, Boeve BF, Jicha GA, Ivnik RJ, Smith GE, Tangalos EG, Braak H, Kokmen E. Neuropathology of amnestic mild cognitive impairment. Arch Neurol. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 24.Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 25.Pomara N, Willoughby L, Sidtis JJ, Mehta PD. Selective reductions in Aβ 1-42 in healthy elderly subjects during longitudinal follow-up: a preliminary report. Am J Geriatr Psychiatry. 2005;13:914–917. doi: 10.1176/appi.ajgp.13.10.914. [DOI] [PubMed] [Google Scholar]
- 26.Pomara N, Doraiswamy PM, Willoughby LM, Roth AE, Mulsant BH, Sidtis JJ, Mehta PD, Reynolds CF, 3rd, Pollock BG. Elevation in plasma Abeta42 in geriatric depression: a pilot study. Neurochem Res. 2006;31:341–349. doi: 10.1007/s11064-005-9029-z. [DOI] [PubMed] [Google Scholar]
- 27.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, 2, Savitcheva I, Huang G, Estrada S, Ausén B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Långström B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 28.Shoghi-Jadid K, Small GW, Agdeppa ED, Kepe V, Ercoli LM, Siddarth P, Read S, Satyamurthy N, Petric A, Huang S, Barrio JR. Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer's disease. Am J Geriatr Psychiatry. 2002;10:24–35. [PubMed] [Google Scholar]
- 29.Agdeppa ED, Kepe V, Liu J, Flores-Torres S, Satyamurthy N, Petric A, Cole GM, Small GW, Huang S, Barrio JR. Binding characteristics of radiofluorinated 6-dialkylamino-2-napthylethylidene derivatives as PET imaging probes β-amyloid plaques in Alzheimer's disease. J Neurosci. 2001;21(RC189):1–5. doi: 10.1523/JNEUROSCI.21-24-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Small GW, Kepe V, Ercoli LM, Siddarth P, Bookheimer SY, Miller KJ, Lavretsky H, Burggren AC, Cole GM, Vinters HV, Thompson PM, Huang S, Satyamurthy N, Phelps ME, Barrio JR. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med. 2006;355:2652–2663. doi: 10.1056/NEJMoa054625. [DOI] [PubMed] [Google Scholar]
- 31.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 19821983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 32.Spielberger CD. Manual for the State-Trait Anxiety Inventory (STAI) Consulting Psychologists Press; Palo Alto, CA: 1983. pp. 70–86. [Google Scholar]
- 33.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund L, Nordberg A, Bäckman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment: beyond controversies, towards a consensus. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 34.Kepe V, Barrio JR, Huang S, Ercoli L, Siddarth P, Shoghi-Jadid K, Cole GM, Satyamurthy N, Cummings JL, Small GW, Phelps ME. Serotonin 1A receptors in the living brain of Alzheimer's disease patients. Proc Natl Acad Sci USA. 2006;103:702–707. doi: 10.1073/pnas.0510237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Kepe V, Zabjec A, Žabjek A, Petrič A, Padgett HC, Satyamurthy N, Barrio JR. High yield, automated radiosynthesis of 2-(1-{6-[(2-[18F]Fluoro-ethyl)(methyl)amino]-2-naphthyl}ethylidene) malononitrile ([18F]FDDNP) ready for animal or human administration. Mol Imaging Biol. 2007;9:6–16. doi: 10.1007/s11307-006-0061-4. [DOI] [PubMed] [Google Scholar]
- 36.Logan J, Fowler J, Volkow N, Logan J, Fowler JS, Volkow ND, Wang G, Ding Y, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Talairach J, Tournoux P. Three-Dimensional Proportional System: An Approach to Cerebral Imaging. Thieme Medical Publishers, Inc.; New York, NY: 1988. Coplanar Stereotaxic Atlas of the Human Brain; pp. 1–22. [Google Scholar]
- 38.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 39.O'Brien J, Thomas A, Ballard C, Brown A, Ferrier N, Jaros E, Perry R. Cognitive impairment in depression is not associated with neuropathologic evidence of increased vascular or Alzheimer-type pathology. Biol Psychiatry. 2001;49:130–136. doi: 10.1016/s0006-3223(00)00944-6. [DOI] [PubMed] [Google Scholar]
- 40.Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, Zmuda MD, Bhalla R, Meltzer CC, Pollock BG, Reynolds CF, 3rd, Becker JT. The nature and determinants of neuropsychological functioning in late life depression. Arch Gen Psychiatry. 2004;6:587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- 41.Butters MA, Becker JT, Nebes RD, Zmuda MD, Mulsant BH, Pollock BG, Reynolds CF., 3rd Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000;157:1949–1954. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- 42.Nebes RD, Pollock BG, Houck PR, Butters MA, Mulsant BH, Zmuda MD, Reynolds CF., 3rd Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. J Psychiatr Res. 2003;37:99–108. doi: 10.1016/s0022-3956(02)00085-7. [DOI] [PubMed] [Google Scholar]
- 43.Alexopoulos GS, Meyers BS, Young RC, Mattis S, Kakuma T. The course of geriatric depression with 'reversible dementia': a controlled study. Am J Psychiatry. 1993;150:1693–1699. doi: 10.1176/ajp.150.11.1693. [DOI] [PubMed] [Google Scholar]
- 44.Kral VA, Emery OB. Long-term follow-up of depressive pseudodementia of the aged. Can J Psychiatry. 1989;34:445–446. doi: 10.1177/070674378903400515. [DOI] [PubMed] [Google Scholar]
- 45.Jorm AF, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, Heyman A, Kokmen E, Kondo K, Mortimer JA, Rocca WA. Psychiatric history and related exposures as risk factors for Alzheimer's disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20(Suppl 2):S43–S47. doi: 10.1093/ije/20.supplement_2.s43. [DOI] [PubMed] [Google Scholar]
- 46.Kokmen E, Beard CM, Chandra V, Offord KP, Schoenberg BS, Ballard DJ. Clinical risk factors for Alzheimer's disease: a population-based case control study. Neurology. 1991;41:1393–1397. doi: 10.1212/wnl.41.9.1393. [DOI] [PubMed] [Google Scholar]
- 47.van Duijn CM, Clayton DG, Chandra V, Fratiglioni L, Graves AB, Heyman A, Jorm AF, Kokmen E, Kondo K, Mortimer JA, Rocca WA, Shalat SL, Soininen H, Hofman A. EURODEM Risk Factors Research Group: Interaction between genetic and environmental risk factors for Alzheimer's disease: a reanalysis of casecontrol studies. Genet Epidemiol. 1994;11:539–551. doi: 10.1002/gepi.1370110609. [DOI] [PubMed] [Google Scholar]
- 48.Speck CE, Kukull WA, Brenner DE, Bowen JD, McCormick WC, Teri L, Pfanschmidt ML, Thompson JD, Larson EB. History of depression as a risk factor for Alzheimer's disease. Epidemiology. 1995;6:366–369. doi: 10.1097/00001648-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Green RC, Cupples LA, Kurz A, Auerbach S, Go R, Sadovnick D, Duara R, Kukull WA, Chui H, Edeki T, Griffith PA, Friedland RP, Bachman D, Farrer L. Depression as a risk factor for Alzheimer disease: the MIRAGE study. Arch Neurol. 2003;60:753–759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- 50.Rapp MA, Schaider-Beeri M, Grossman HT, Sano M, Perl DP, Purohit DP, Gorman JM, Haroutunian V. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Arch Gen Psychaitry. 2006;63:1616–167. doi: 10.1001/archpsyc.63.2.161. [DOI] [PubMed] [Google Scholar]
- 51.Rapp MA, Schaider-Beeri M, Purohit DP, Perl DP, Haroutunian V, Sano M. Increased neurofibrillary tangles in patients with Alzheimer disease with comorbid depression. Am J Geriatr Psychiatry. 2008;16:168–174. doi: 10.1097/JGP.0b013e31816029ec. [DOI] [PubMed] [Google Scholar]
- 52.Palmer K, Berger AK, Monastero R, Winblad B, Backman L, Fratiglioni L. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology. 2007;68:1596–1602. doi: 10.1212/01.wnl.0000260968.92345.3f. [DOI] [PubMed] [Google Scholar]
- 53.Becker JT, Boller F, Lopez OL, Saxton J, McGonigle KL. The natural history of Alzheimer's disease: description of study cohort and accuracy of diagnosis. Arch Neurol. 1994;51:585–594. doi: 10.1001/archneur.1994.00540180063015. [DOI] [PubMed] [Google Scholar]
- 54.Gearing M, Mirra SS, Hedreen JC, Sumi SM, Hansen LA, Heyman A. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part X. Neuropathology confirmation of the clinical diagnosis of Alzheimer's disease. Neurology. 1995;45:461–466. doi: 10.1212/wnl.45.3.461. [DOI] [PubMed] [Google Scholar]
- 55.Lopez OL, Becker JT, Klunk WE, Saxton J, Hamilton RL, Kaufer DI, Sweet RA, Cidis Meltzer C, Wisniewski S, Kamboh MI, DeKosky ST. Research evaluation and diagnosis of possible Alzheimer's disease over the last two decades. II. Neurology. 2000a;55:1863–1869. doi: 10.1212/wnl.55.12.1863. [DOI] [PubMed] [Google Scholar]
- 56.Lopez OL, Becker JT, Klunk WE, Saxton J, Hamilton RL, Kaufer DI, Sweet RA, Cidis Meltzer C, Wisniewski S, Kamboh MI, DeKosky ST. Research evaluation and diagnosis of possible Alzheimer's disease over the last two decades. I. Neurology. 2000b;55:1854–1862. doi: 10.1212/wnl.55.12.1854. [DOI] [PubMed] [Google Scholar]
- 57.Small GW, Agdeppa ED, Kepe V, Satyamurthy N, Huang SC, Barrio JR. In vivo brain imaging of tangle burden in humans. J Mol Neurosci. 2002;19:323–327. doi: 10.1385/jmn:19:3:321. [DOI] [PubMed] [Google Scholar]
- 58.Silverman DH. Brain 18F-FDG PET in the diagnosis of neurodegenerative dementias: comparison with perfusion SPECT and with clinical evaluations lacking nuclear imaging. J Nucl Med. 2004;45:594–607. [PubMed] [Google Scholar]
- 59.Verhoeff NP, Wilson AA, Takeshita S, Trop L, Hussey D, Singh K, Kung HF, Kung M, Houle S. In-vivo imaging of Alzheimer disease beta-amyloid with [11C]SB-13 PET. Am J Geriatr Psychiatry. 2004;12:584–595. doi: 10.1176/appi.ajgp.12.6.584. [DOI] [PubMed] [Google Scholar]
- 60.de Leon MJ, Klunk W. Biomarkers for the early diagnosis of Alzheimer's disease. Lancet Neurol. 2006;5:198–199. doi: 10.1016/S1474-4422(06)70357-X. [DOI] [PubMed] [Google Scholar]
- 61.Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 62.Mayeux R, Tang MX, Jacobs DM, Manly J, Bell K, Merchant C, Small SA, Stern Y, Wisniewski HM, Mehta PD. Plasma amyloid beta-peptide 1-42 and incipient Alzheimer's disease. Ann Neurol. 1999;46:412–416. doi: 10.1002/1531-8249(199909)46:3<412::aid-ana19>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 63.Kung M, Skovronsky DM, Hou C, Zhuang Z, Gur TL, Shang B, Trojanowski JQ, Lee VMY, Kung HF. Detection of amyloid plaques by radioligands for Aβ40 and Aβ42. J Mol Neurosci. 2003;20:15–23. doi: 10.1385/JMN:20:1:15. [DOI] [PubMed] [Google Scholar]
- 64.Bussiere T, Friend PD, Sadeghi N, Wicinski B, Lin GI, Bouras C, Gianakopoulos P,N, Robakis K, Morrison JH, Perl DP, Hof PR. Stereologic assessment of the total cortical volume occupied by amyloid deposits and its relationship with cognitive status in aging and Alzheimer's disease. Neuroscience. 2002;112:75–91. doi: 10.1016/s0306-4522(02)00056-8. [DOI] [PubMed] [Google Scholar]
- 65.Smith GS, Gunning-Dixon FM, Lotrich FE, Taylor WD, Evans JD. Translational research in late-life mood disorders: implications for future intervention and prevention research. Neuropsychopharmacology. 2007 Sep;32(9):1857–75. doi: 10.1038/sj.npp.1301333. [DOI] [PubMed] [Google Scholar]
- 66.Kumar A, Ajilore O, Kepe V, Barrio JR, Small G. Mood, cognition and in vivo protein imaging: the emerging nexus in clinica neuroscience. Int J Geriatr Psychiatry. 2007 Nov 29; doi: 10.1002/gps.1941. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steffens DC, Otey E, Alexopoulos GS, Butters MA, Cuthbert B, Ganguli M, Geda YE, Hendrie HC, Krishnan RR, Kumar A, Lopez OL, Lyketsos CG, Mast BT, Morris JC, Norton MC, Peavy GM, Petersen RC, Reynolds CF, Salloway S, Welsh-Bohmer KA, Yesavage J. Perspectives on depression, mild cognitive impairment, and cognitive decline. Arch Gen Psychiatry. 2006;63(2):130–8. doi: 10.1001/archpsyc.63.2.130. [DOI] [PubMed] [Google Scholar]
- 68.Caetano SC, Kaur S, Brambilla P, Nicoletti M, Hatch JP, Sassi RB, Mallinger AG, Keshavan MS, Kupfer DJ, Frank E, Soares JC. Smaller cingulate volumes in unipolar depressed patients. Biol Psychiatry. 2006;59:702–706. doi: 10.1016/j.biopsych.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 69.Kumari V, Ffytche DH, Das M, Wilson GD, Goswami S, Sharma T. Neuroticism and brain responses to anticipatory fear. Behav Neurosci. 2007;121:643–652. doi: 10.1037/0735-7044.121.4.643. [DOI] [PubMed] [Google Scholar]
- 70.Gunning-Dixon FM, Hoptman MJ, Lim KO, Murphy CF, Klimstra S, Latoussakis V, Majcher-Tascio M, Hrabe J, Ardekani BA, Alexopoulos GS. Macromolecular white matter abnormalities in geriatric depression: a magnetization transfer imaging study. Am J Geriatr Psychiatry. 2008;16:255–262. doi: 10.1097/JGP.0b013e3181602a66. [DOI] [PubMed] [Google Scholar]
- 71.Geerlings MI, den Heijer T, Koudstaal PJ, Hofman A, Breteler MM. History of depression, depressive symptoms, and medial temporal lobe atrophy and the risk of Alzheimer disease. Neurology. 2008;70:1258–64. doi: 10.1212/01.wnl.0000308937.30473.d1. [DOI] [PubMed] [Google Scholar]
- 72.Korf ES, Wahlund LO, Visser PJ, Scheltens P. Medial temporal lobe atrophy on MRI predicts dementia in patients with mild cognitive impairment. Neurology. 2004;63:94–100. doi: 10.1212/01.wnl.0000133114.92694.93. [DOI] [PubMed] [Google Scholar]
- 73.Karas G, Sluimer J, Goekoop R, van der Flier W, Rombouts SA, Vrenken H, Scheltens P, Fox N, Barkhof F. Amnestic mild cognitive impairment: structural MR imaging findings predictive of conversion to Alzheimer disease. Am J Neuroradiol. 2008;29:944–499. doi: 10.3174/ajnr.A0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hanada K, Hosono M, Kudo T, Hitomi Y, Yagyu Y, Kirime E, Komeya Y, Tsujii N, Hitomi K, Nishimura Y. Regional cerebral blood flow in the assessment of major depression and Alzheimer's disease in the early elderly. Nucl Med Commun. 2006;27:535–41. doi: 10.1097/00006231-200606000-00010. [DOI] [PubMed] [Google Scholar]
- 75.Kepe V, Barrio JR, Huang S-C, et al. Serotonin 1A receptors in the living brain of Alzheimer's disease patients. Proc Natl Acad Sci U S A. 2006;103:702–707. doi: 10.1073/pnas.0510237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]