Abstract
Activated mast cells are a major source of the eicosanoids, prostaglandin (PG)D2 and leukotriene (LT)C4, which contribute to allergic responses. These eicosanoids are produced following the ERK1/2-dependent activation of cytosolic phospholipase A2 (cPLA2), thus liberating arachidonic acid which is subsequently metabolized by the actions of 5-lipoxygenase (5-LO) and cyclooxygenase (COX) to form LTC4 and PGD2 respectively. These pathways also generate reactive oxygen species (ROS) which have been proposed to contribute to FcɛRI-mediated signaling in mast cells. Here we demonstrate that, in addition to ERK1/2-dependent pathways, ERK1/2-independent pathways also regulate FcɛRI-mediated eicosanoid and ROS production in mast cells. A role for the Tec kinase, Btk, in the ERK1/2-independent regulatory pathway was revealed by the significantly attenuated FcɛRI-dependent PGD2, LTC4 and ROS production in mast cells derived from the bone marrow (BMMCs) of Btk−/− mice. The FcɛRI-dependent activation of Btk; and eicosanoid and ROS generation in BMMCs and human mast cells were similarly blocked by the phosphoinositide 3-kinase (PI3K) inhibitors, Wortmannin and LY294002, indicating that Btk-regulated eicosanoid and ROS production occurs downstream of PI3K. In contrast to ERK1/2, the PI3K/Btk pathway does not regulate cPLA2 phosphorylation, but rather appears to regulate the generation of ROS, LTC4 and PGD2 by contributing to the necessary Ca2+ signal for the production of these molecules. These data demonstrate that strategies to decrease mast cell production of ROS and eicosanoids would have to target both ERK1/2-depedent and PI3K/Btk dependent pathways.
Keywords: Mast cells, FcɛRI, Btk, LTC4, PGD2, ROS
INTRODUCTION
Mast cell-derived mediators play a central role in the initiation of the inflammatory reactions associated with atopic asthma and other allergic disorders (1, 2). These mediators, which are released following antigen-dependent aggregation of IgE-occupied high affinity IgE receptors (FcɛRI) on the mast cell surface (1, 3, 4), are broadly grouped into three main categories: granule-associated mediators; chemokines and cytokines; and eicosanoids. Much is known regarding the signaling events leading to the release of granule-associated mediators and, to a certain extent, cytokine production (reviewed in (3, 5)). However, the signaling events mediating eicosanoid generation are less well defined.
The eicosanoids generated in activated mast cells are primarily represented by leukotriene (LT)C4 and prostaglandin (PG)D2 (1, 3, 6–8). Multiple processes are required for the generation of these mediators, however, the major initiating step is the liberation of arachidonic acid from membrane lipids, primarily, 1-acyl, 2-arachidoyl-phosphatidylcholine, following hydrolysis catalysed by cytosolic phospholipase (cPL)A2 (9, 10). The liberated arachidonic acid is subsequently metabolized to form LTC4 and PGD2 by the actions of 5-lipoxygenase (5-LO) and LTC4 synthase (11, 12), and cyclooxgenase (COX) respectively (8, 13). Reactive oxygen species (ROS) including hydroperoxides, hydrogen peroxide, and superoxide, are formed during the generation of both LTC4 and PGD2 (14, 15). ROS have been proposed to regulate mast cell responses, however, these conclusions remain controversial. In this respect, although it has been suggested that ROS are involved in the signals leading to degranulation and cytokine secretion in mast cells (16, 17), other studies have concluded that FcɛRI-dependent degranulation and cytokine production is independent of ROS production (14).
The ability of cPLA2 to generate free arachidonic acid requires cPLA2 to be phosphorylated, thus activated, and for it to be translocated to the membrane, allowing access to its phospholipid substrate(s) (9, 11, 18). The phosphorylation of cPLA2 appears to be mediated by the MAP kinases, ERK1/2, whereas its translocation is dependent on Ca2+ (19–22). ROS production is also a Ca2+-dependent processes (17) but the upstream events required for ROS production and cPLA2 activation have not been fully delineated.
In human mast cells, we have previously demonstrated that both phosphoinositide 3-kinase (PI3K)–dependent and PI3K-independent pathways contribute to the increase in cytosolic Ca2+ concentrations required for mast cell degranulation (23). The PI3K-dependent pathway appears to be mediated through the Tec kinase, Btk. We (24), and others (25, 26), have shown that Btk enhances FcɛRI-mediated PLCγ1 activation in a PI3K-dependent manner. Therefore, it is possible that Btk may similarly contribute to the regulation of the Ca2+ signal required for eicosanoid generation and ROS production in activated mast cells. Here we show that both ERK1/2-dependent and ERK1/2-independent pathways regulate FcɛRI-mediated LTC4 and PGD2 generation and ROS production. The generation of these products was substantially reduced in parallel with the Ca2+ signal in Btk-deficient mast cells and in wild type mast cells treated with PI3K inhibitors, providing the first evidence that activation of a PI3K-Btk-PLC pathway is required for the Ca2+ -dependent production of eicosanoids and ROS in FcɛRI-mediated activated cells. The recognition of the complementary signaling pathways required for FcɛRI-mediated eicosanoid and ROS generation implies that therapeutic approaches to block the generation of these products requires a coordinated strategy targeting these pathways.
MATERIALS AND METHODS
Cell isolation and mast cell culture
The Btk knock-out, kindly provided by Dr. Anne B. Satterthwaite, University of Texas Southwestern Medical Center and the wild type (WT) mice used in this study have been described (24). Mice were backcrossed with C57BL/6J (The Jackson Laboratory, Bar Harbor, ME) over 6 generations. The wild type mice were derived from the same parental lines as the knock-out mice. The genotype of these mice was confirmed by reverse transcription-PCR of tail biopsies (data not shown). Mouse bone marrow-derived mast cells (BMMCs) were obtained by flushing bone marrow progenitors from the femurs of the mice then culturing the cells for 4–6 weeks in RPMI 1640 medium containing IL-3 (30 ng/ml) (Peprotech, Rocky Hill, NJ) as described (23, 27)
Primary human mast cells (HuMCs) were obtained from CD34+ peripheral blood progenitor cells grown in StemPro-34 culture media containing recombinant human IL-3 (30 ng/ml) (first week only), IL-6 (100 ng/ml), and SCF (100 ng/ml) (Peprotech) as described (23, 28). Experiments were conducted on these cells 7–10 weeks after the initiation of culture, at which point, the population was greater than 99% mast cells as assessed by toluidine blue staining.
Cell activation
HuMCs or BMMCs were sensitized overnight with an optimal concentration (100 ng/ml) of biotinylated human IgE or mouse SPE-7 (IgE anti-DNP) (Sigma, St. Louis, MO) respectively in cytokine free medium. The following day, the cells were washed with HEPES buffer (10 mM HEPES [pH 7.4], 137 mM NaCl, 2.7 mM KCl, 0.4 mM Na2HPO4·7H2O, 5.6 mM glucose, 1.8 mM CaCl2·2H2O, 1.3 mM MgSO4·7H2O) containing 0.04% BSA (Sigma) to remove excess IgE then the cells resuspended in this buffer at the required cell density for a specific assay. The BMMCs or HuMCs were stimulated with DNP-HSA (10 ng/ml) or with streptavidin (SA, 10 ng/ml) at 37°C respectively.
Intracellular ROS detection by microfluorimetry
Intracellular ROS were measured as described (14). Briefly, sensitized mast cells were preincubated with or without indicated inhibitors at 4°C for 10–20 min. After centrifugation, cells were incubated with DCF-diacetate (20 µM) (EMD Biosciences, Gibbstown, NJ) in cell culture medium for 20 min at 4°C. Cells were then washed with HEPES buffer containing 0.04% BSA and seeded at 2 × 105 cells per well in a black opaque 96-well microplate in the presence or absence of inhibitors and/or antigen. DCF fluorescence was monitored at 37 °C in 1 min intervals for 20–30 min using a GENios fluorescent plate reader (ReTiSoft, Ontario, Canada) with an excitation wavelength of 492 nm and emission wavelength of 535 nm. Fluorescence was expressed as relative fluorescent units.
LTC4 and PGD2 measurements
The release of LTC4 and PGD2 from antigen-triggered cells was measured as described (14). Briefly, IgE-sensitized HuMCs or BMMCs were preincubated with or without indicated inhibitors for 20 min prior to add DNP-HSA (10 ng/ml, BMMCs) or SA (10 ng/ml, HuMCs) for 20 min, and then cell-free supernatants were analyzed for LTC4 and PGD2 by competitive enzyme immunoassay (Cayman Chemicals, Ann Arbor, MI), according to the manufacturer’s instructions. For comparison, the results are proportionally represented to ng/ml in 100,000 cells/100 µl.
Immunoblotting
For immunoblot analyses, mast cell lysates were prepared as described (27, 29) and proteins separated by electrophoresis on 4–12% NuPAGE BisTris gels (Invitrogen, Carlsbad, CA). Following membrane transfer, proteins were probed utilizing the following antibodies: anti-β-actin mAB (clone AC-15) (Sigma); anti-phospho-PLCγ2 (Tyr(P)-759) pAb, anti-phospho-AKT (Ser(P)-473) pAb, anti-phospho-ERK1/2 (Thr(P)-202, Tyr(P)-204) pAb, anti-phospho-cPLA2 (Ser(P)-505) pAb, anti-cPLA2 pAB, anti-ERK pAb, anti-AKT (Cell Signaling, Beverly, MA); anti-phospho-PLCγ1 (Tyr(P)-783) pAb (BIOSOURCE, Invitrogen), anti-phospho-Btk (Tyr(P)-551) mAb (BD bioscience, San Jose, CA), anti-PLCγ1 pAb, and anti-PLCγ2 pAb Santa Cruz Biotechnology, Santa Cruz, CA). To normalize protein loading, membranes were stripped and probed for β-actin, or alternatively identically loaded samples were probed for β-actin. Changes in protein phosphorylation, were determined by scanning the ECL films using a Quantity One scanner (Bio-Rad, Hercules, CA).
Intracellular Ca2+ Determination
Sensitized BMMCs were incubated with Fura 2-AM (2 µM) (Molecular Probes, Eugene, OR) for 30 min at 37 °C, washed, and resuspended in HEPES buffer containing 0.04% BSA and sulfinpyrazone (0.3 mM) (Sigma), and then Ca2+ flux was measured as described (23). Fluorescence was measured at two excitation wavelengths (340 and 380 nm) and an emission wavelength of 510 nm. The ratio of the fluorescence readings was calculated following subtraction of the fluorescence of the cells that had not been loaded with Fura 2-AM.
IP3 Assay
Sensitized wild type or Btk−/− BMMCs (2×106) were stimulated with DNP-HSA (10 ng/ml) for 30 sec and then cellular IP3 concentrations were determined as described (27) using a commercially available kit (GE Healthcare, Uppsala Sweden) according to the manufacturer’s instructions. The results are expressed as picomoles of IP3 per 2×106 cells.
Statistical Analysis
Data are represented as the mean ±S.E. The statistical analyses were performed by unpaired Student’s t-test. Differences were considered significant when p<0.05. The n values represent experiments from multiple preparations.
RESULTS
ERK1/2-dependent cPLA2 activation and eicosanoid and ROS generation in primary cultured human and mouse mast cells
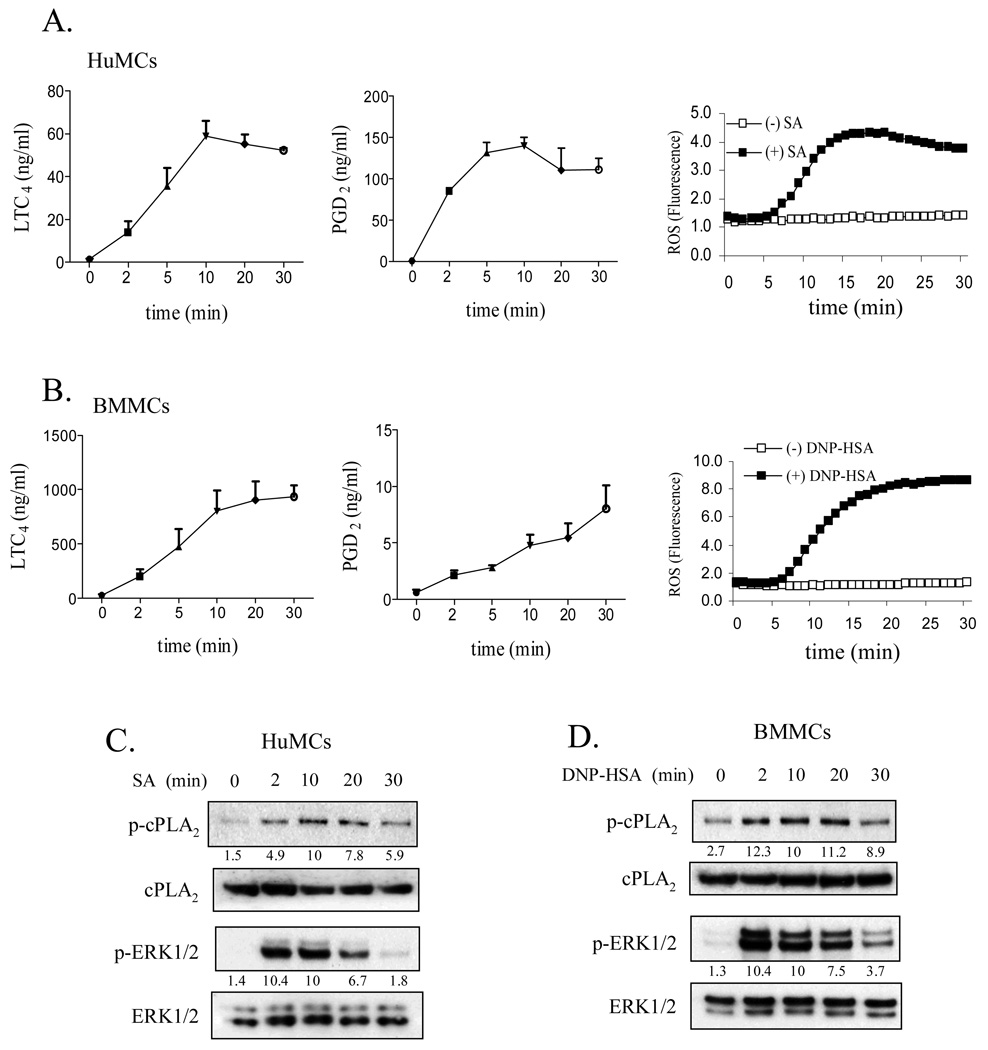
Since it has been demonstrated that FcɛRI-mediated cPLA2 activation is dependent on phosphorylation of cPLA2 by ERK in the RBL 2H3 rat mast cell line (20), we examined whether cPLA2 mediated eicosanoid and ROS generation were similarly regulated in an ERK1/2-dependent manner in primary cultured human and mouse mast cells. SA- or DNP-HSA-induced FcɛRI aggregation, as shown in figure 1A and 1B, respectively resulted in a rapid increase in PGD2 and LTC4 release and ROS production in both HuMCs and mouse BMMCs. As discussed in the figure legend, the apparent delay in ROS production represented a technical artifact due to the time required for the cells to reach 37 °C. In HuMCs, PGD2 was the predominant eicosanoid produced, whereas, in the mouse BMMCs, LTC4 was the predominant form. Except for PGD2 release in BMMCs, maximal release in each case was observed within 10 minutes of cell activation. These observations are consistent with the kinetics observed in RBL 2H3 cells (20). Over this time frame, the phosphorylation of ERK1/2 and cPLA2 was similarly observed to increase following FcɛRI aggregation in HuMCs (Fig. 1C) and mouse BMMCs (Fig. 1D). Maximum ERK1/2 phosphorylation appeared to slightly precede the maximum increase in cPLA2 phosphorylation.
Figure 1.
The generation of eicoanoids and ROS (A, B) and phosphorylation of cPLA2 and ERK1/2 (C, D) in antigen-challenged human (A, C) and mouse (B, D) mast cells. IgE-sensitized HuMCs and BMMCs were stimulated with streptavidin (SA, 10 ng/ml) or DNP-HSA (10 ng/ml) respectively for the indicated times, then cell-free supernatants were analyzed for LTC4 and PGD2 content (A, B). ROS generation was monitored by DCF fluorescence at 37 °C for up to 30 min at 1 min intervals (A, B). cPLA2 and ERK1/2 phosphorylation was determined by immunoblot analysis as discussed in Materials and Methods. Experiments not shown revealed that the apparent delay in the ROS production shown in this and subsequent figures was a technical artifact reflecting the time required for the cells to reach optimal temperature (37°) (C, D). Protein levels were normalized to cPLA2 or ERK then to antigen responses at 10 min to determine the relative intensities presented under each blot. In A and B, results are means ± S.E. of 3 separate experiments performed in duplicate for LTC4 and PGD2 generation and kinetic graphs representative of 3 separate experiments for ROS production. In C and D, the blots are representative of three independent experiments.
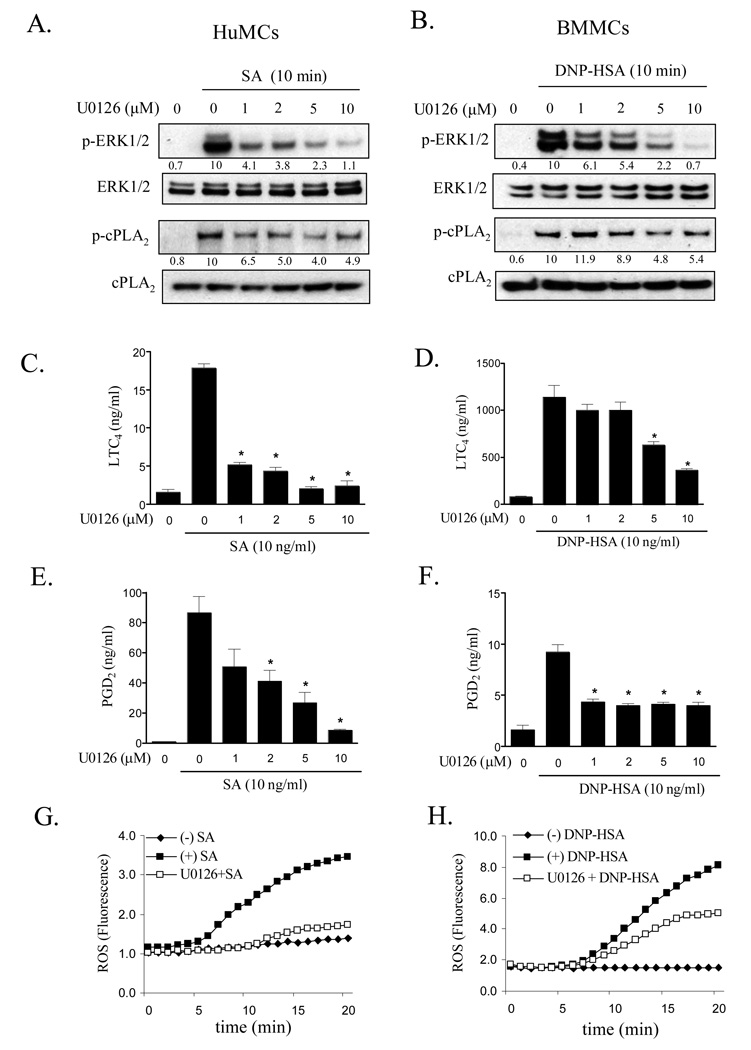
To determine whether ERK1/2 participated in cPLA2 activation and eicosanoid and ROS generation in primary cultures of mouse and human mast cells, we next examined the ability of the MEK1/2 inhibitor, U0126, to block these responses. As expected, U0126 markedly inhibited FcɛRI-dependent ERK1/2 phosphorylation in both HuMCs (Fig. 2A) and BMMCs (Fig. 2B) in a concentration-dependent manner between 1 and 10 µM, while having minimal effect on the phosphorylation of the MAPKs, p38 and JNK over this concentration range (data not shown). Similarly, U0126 (10 µM) significantly inhibited FcɛRI-mediated cPLA2 phosphorylation (HuMCs: Fig. 2A; BMMCs: Fig. 2B), LTC4 (HuMCs: Fig. 2C; BMMCs: Fig. 2D), and PGD2 (HuMCs: Fig. 2E; BMMCs: Fig. 2F) generation. ROS production was also markedly inhibited by U0126 (HuMCs: Fig. 2G, BMMCs: Fig. 2H) suggesting that ROS production in these cells was also regulated by ERK. HuMCs appeared to be more sensitive to the effects of U0126 on ROS production compared to BMMCs. Thus both the production of eicosanoids and ROS in primary cultures of mouse and human mast cells is, in part regulated by an ERK1/2-dependent pathway.
Figure 2.
The effect of the ERK1/2 inhibitor on ERK1/2 and cPLA2 phosphorylation (A, B) and eicosanoid (C–F) and ROS generation (G,H) in antigen-challenged human (A, C, E, G) and mouse (B, D, F, H) mast cells. HuMCs and BMMCs were pretreated with or without U0126 for 15–20 min prior to stimulation with streptavidin (SA, 10 ng/ml) or DNP-HSA (10 ng/ml) respectively for the indicated times. cPLA2 and ERK1/2 phosphorylation (A, B) was determined by immunoblot analysis as described for Fig. 1. Protein levels were normalized to cPLA2 or ERK then to the antigen response in the absence of inhibitor to determine the relative intensities presented under each blot. For eicosanoid release, HuMCs (C, E) or BMMCs (D, F) were preincubated with or without U0126 for 15–20 min prior to stimulation with antigen for 20 min and cell-free supernatants were analyzed for LTC4 and PGD2. ROS generation (G, H) was monitored, as described for Fig. 1, at 37°C at indicated times. The blots (A, B) are representative of three independent experiments. When compared at the 10 µM concentration, U0126 significantly inhibited ERK phosphorylation and cPLA2 phosphorylation in both HuMCs and BMMCs (p < 0.05). Results are for LTC4 and PGD2 release (C–F) are means ± S.E. of 3 separate experiments, and ROS data (G, H) are representative of 3 separate experiments. *, p < 0.05 for comparison with antigen alone by Student’s t-test.
The degree of inhibition of eicosanoid production (LTC4 87%, PGD2 90% inhibition in HuMCs; LTC4 68%, PGD2 57% inhibition in BMMCs, both at 10 µM of U0126), however, was greater than that observed for cPLA2 phosphorylation (52% in both HuMCs and BMMCs at 10µM of U0126). In addition, the titration curves for the effects of U0126 on LTC4 and PGD2 production in either HuMCs or BMMCs (Fig. 2 C–F) only partially correlated with that for cPLA2 phosphorylation. These data led us to investigate whether other pathways contribute to the regulation of cPLA2 activation leading to eicosanoid generation in activated mast cells.
PI3K- and PLCγ-regulated FcɛRI-mediated eicosanoid and ROS generation
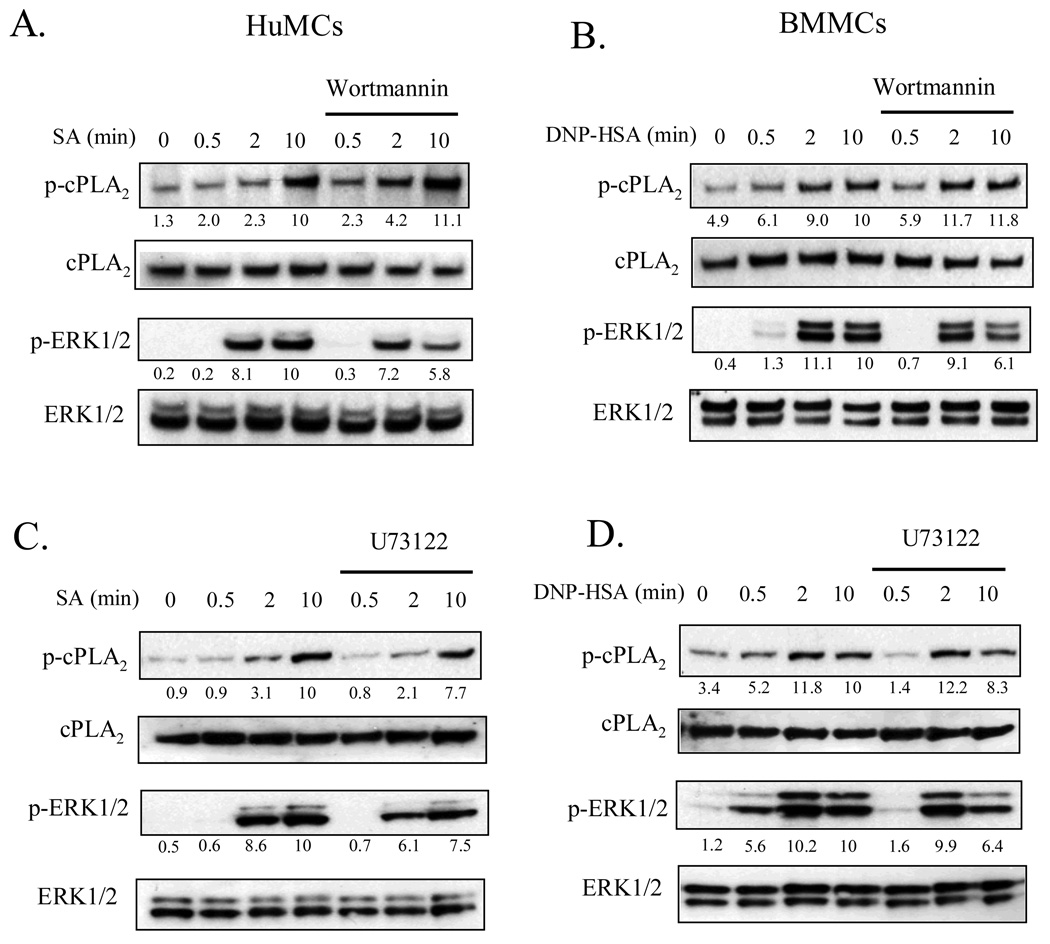
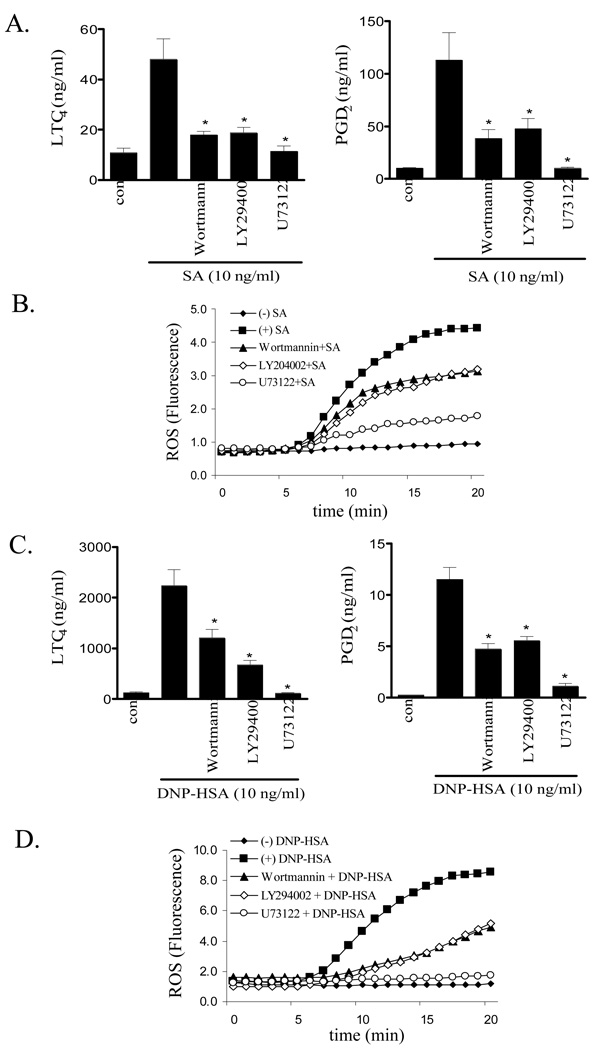
PI3K and PLCγ1 regulate two key intermediary signaling pathways for antigen-mediated mast cell degranulation (23). The effects of respective inhibitors of theses enzymes, wortmannin and U73122, on cPLA2 activation and eicosanoid and ROS generation were thus examined. As shown in Figure 3, both compounds inhibited (∼250-40%) FcɛRI-mediated of ERK1/2 phosphorylation 10 min after stimulation of both HuMCs (Fig. 3A and C) and BMMCs (Fig. 3B and D) However, neither wortmannin nor U73122 significantly inhibited cPLA2 phosphorylation. These data show that although ERK is partially regulated by PI3K, FcɛRI-mediated cPLA2 phosphorylation is independent of PI3K activation. However, these inhibitors as well as LY294002, another PI3K inhibitor, significantly reduced FcɛRI-mediated LTC4 and PGD2 release and ROS production (HuMCs: Fig. 4A and B, BMMCs: Fig. 4C and D). However at 100 nM and 10 µM respectively, wortmannin and LY294002 only partially inhibited these responses. As previously shown (27), and as discussed later, these concentrations completely block PI3K-dependent Akt phosphorylation in mast cells. This is consistent with the conclusion that, although PLCγ is absolutely required for FcɛRI-dependent eicosanoid generation, a PI3K-independent pathway is involved in addition to a PI3K-dependent pathway.
Figure 3.
The effects of the PI3K inhibitor, wortmannin (A, B), and the PLC inhibitor, U73122 (C, D), on cPLA2 and ERK1/2 phosphorylation in human (A, C) and mouse (B, D) mast cells. IgE-sensitized HuMCs or BMMCs were pretreated with or without indicated inhibitors (Wortmannin 100 nM, U73122 1 µM) for 15 min prior to stimulation with streptavidin (SA, 10 ng/ml) or DNP-HSA (10 ng/ml) respectively for the indicated times. cPLA2 and ERK1/2 phosphorylation was determined by immunoblot analysis as described for Fig. 1. Protein levels were normalized to cPLA2 or ERK then to the antigen response responses at 10 min to determine the relative intensities presented under each blot. The blots are representative of three independent experiments.
Figure 4.
The effect of PI3K and PLC inhibitors on eicosanoid (A, C) and ROS generation (B, D) in human (A, B) and mouse (C, D) mast cells. IgE-sensitized HuMCs and BMMCs were pretreated with or without indicated inhibitors (Wortmannin 100 nM, LY294002 10 µM, U73122 1 µM) for 15 min. Following treatment with streptavidin (SA, 10 ng/ml) or antigen (10 ng/ml), cell-free supernatants were analyzed for LTC4 and PGD2 content and cellular ROS generation was monitored as described in Materials and Methods at indicated time points. Results are means ± S.E. of 3 separate experiments performed in duplicate for LTC4 and PGD2 generation and representative of 3 separate experiments for ROS production. *, p < 0.05 for comparison with antigen alone by Student’s t-test.
The role of Btk in PI3K and PLCγ-dependent eicosanoid and ROS generation
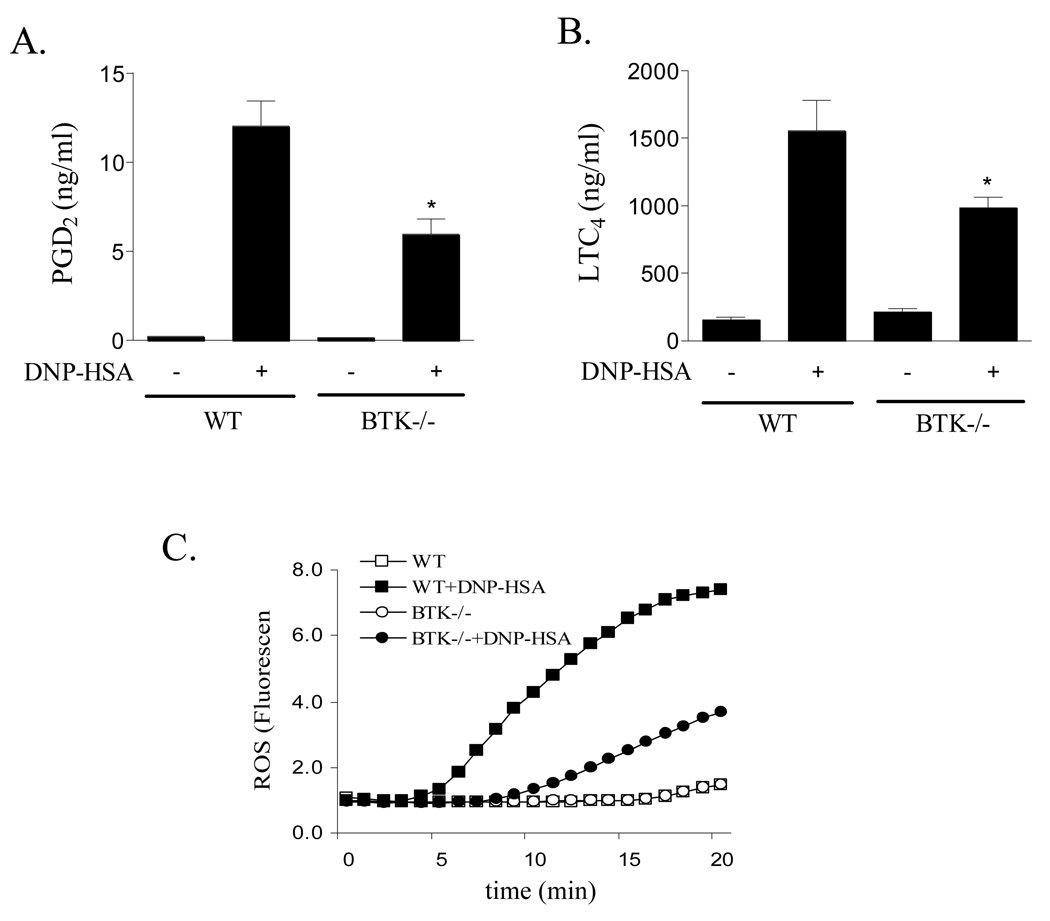
Since Btk is a possible intermediary in the PI3K dependent regulation of PLCγ1, (30) we next examined whether Btk played a similar role in the regulation of eicosanoid generation following FcɛRI aggregation. For these studies we utilized BMMCs derived from the bone marrow of Btk−/− and Btk+/+ (wild type) mice. Figure 5 shows that there was a partial attenuation of FcɛRI-mediated PGD2, LTC4 and ROS generation in the Btk−/− BMMCs. The degree of inhibition mimicked the partial inhibition of PGD2 and LTC4 production observed in the cells treated with PI3K inhibitors, but was less than that achieved in cells treated with the PLCγ inhibitor, U73122.
Figure 5.
Eicosanoid release (A, B) and ROS generation (C) in Btk−/− and wild type (WT) BMMCs. WT and Btk−/− BMMCs were sensitized and treated with antigen (10 ng/ml) for 20 min and cell-free supernatants were analyzed for LTC4 and PGD2 release and ROS generation as described for Fig. 1. The results are means ± S.E. of 3 separate experiments performed in duplicate for LTC4 and PGD2 generation and representative of 3 separate experiments for ROS production. *, p < 0.05 for comparison with antigen alone by Student’s t-test.
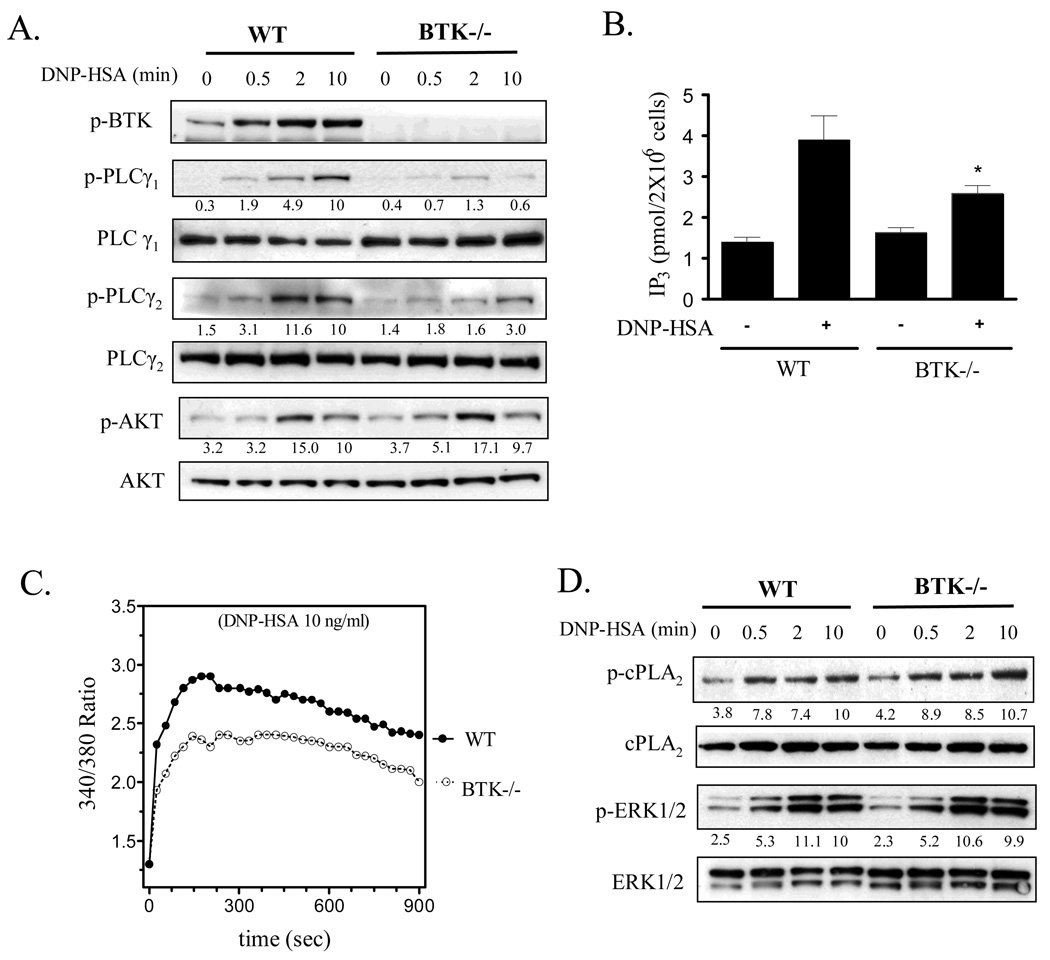
In the Btk−/− BMMCs there was a significant inhibition of PLCγ1 and PLCγ2 but not AKT phosphorylation (Fig 6A). Similarly PLCγ-dependent IP3 production (Fig. 6B) and in agreement with previous observations (24, 26), Ca2+ mobilization (Fig. 6C) was also attenuated in the Btk−/− BMMCs. The defect in Ca2+ mobilization in the Btk−/− BMMCs mirrored a similar decrease observed in wild type BMMCs treated with the PI3K inhibitors, wortmannin and LY 294002 (data not shown). No defects were observed in FcɛRI-mediated ERK1/2 and cPLA2 phosphorylation in the Btk−/− BMMCs (Fig. 6D).
Figure 6.
PLCγ activation (A, B), calcium mobilization (C), and cPLA2 phosphorylation (D) in Btk−/− and wild type (WT) BMMCs. (A, D) IgE-sensitized WT BMMCs and Btk−/− BMMCs were treated with antigen (10 ng/ml) for the indicated times. Btk, PLCγ and AKT phosphorylation was determined by immunoblot analysis as described for Fig.1. Protein levels were normalized to the non-phosphorylated proteins then to antigen responses at 10 min to determine the relative intensities presented under each blot. (B) Sensitized BMMCs from WT or Btk−/− were stimulated with DNP-HSA (10 ng/ml) for 30 seconds, then IP3 levels determined as described in “Materials and Methods”. (C) BMMCs from WT or Btk−/− BMMCs were loaded with Fura 2- AM then changes in intracellular Ca2+ concentrations in response to DNP-HSA 10 ng/ml were determined at the indicated time points. The protein blots (A, D) are representative of three independent experiments. When compared to the wild type controls at 10 min, PLCγ1 phosphorylation and PLCγ2 phosphorylation were significantly lower in the BTK−/− BMMCs (p < 0.05). The data (B) (n=4) are presented as means ± S.E. of separate experiments conducted in duplicate. The Ca2+ data are representative of n=3 experiments conducted in duplicate. For calcium results, when compared at the 300 s point, and for indicted values in other graphs, *: p < 0.05 for comparison with antigen response in WT by Student’s t-test.
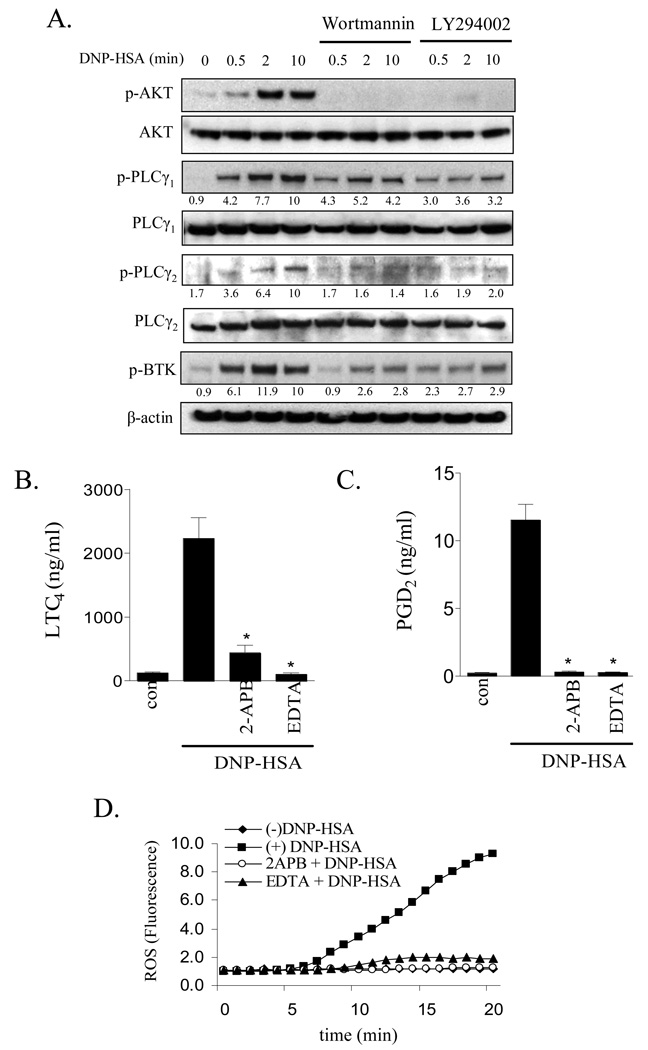
The above data suggested that Btk regulated cPLA2 activation as well as eicosanoid and ROS generation upstream of the PLCγ-mediated Ca2+ signal. The unimpaired AKT phosphorylation in Btk−/− BMMCs indicated that Btk was downstream of PI3K. This conclusion is supported by the fact that phosphorylation of Btk, PLCγ1 and PLCγ2 was reduced following treatment of wild type BMMCs with the PI3K inhibitors (Fig. 7A).
Figure 7.
The effect of PI3K inhibitors on PLCγ and BTK phosphorylation (A), and the effect of inhibitors of the calcium signal on eicosanoid release (B, C) and ROS generation (D) in BMMCs. (A) IgE-sensitized BMMCs were pretreated with or without indicated inhibitors (Wortmannin 100 nM, LY294002 10 µM) for 15 min followed by treatment with antigen (10 ng/ml) for the indicated times. Protein levels were normalized to non-phosphorylated proteins then to antigen responses at 10 min to determine the relative intensities presented under each blot. (B, C) BMMCs were pretreated with or without indicated inhibitors (2-APB 50 µM, EDTA 5 mM) for 15 min. Following treatment with DNP-HSA (10 ng/ml), cell-free supernatants were analyzed for LTC4 and PGD2. (D) ROS generation was monitored as described for Fig.1. The blots (A) are representative of three independent experiments. Wortmannin and LY294002 significantly inhibited AKT, PLCγ1 and γ2, and BTK phosphorylation (p < 0.05). Results (B, C) are means ± S.E. of 3 separate experiments performed in duplicate for LTC4 and PGD2 generation and the ROS generation (D) is representative of 3 separate experiments. *, p < 0.05 for comparison with antigen alone by Student’s t-test.
To determine whether the observed defect in Ca2+ mobilization observed in the Btk−/− BMMCs and wild type BMMCs treated with the PI3K or PLCγ inhibitor accounts for the inhibition of eicosanoid and ROS generation, we employed the Ca2+ chelator, EDTA, and the IP3 receptor antagonist 2-APB. Both ablated the FcɛRI-mediated Ca2+ signal in BMMCs (data not shown). These agents also blocked FcɛRI-mediated PGD2, LTC4 and ROS production to a similar extent to that observed following treatment of the cells with the PLCγ inhibitor, U73122 (Fig. 4). These data demonstrated that all three responses were dependent on Ca2+. Therefore, it is reasonable to conclude that Btk regulates eicosanoid and ROS generation in part through its regulation of the Ca2+ signal.
DISCUSSION
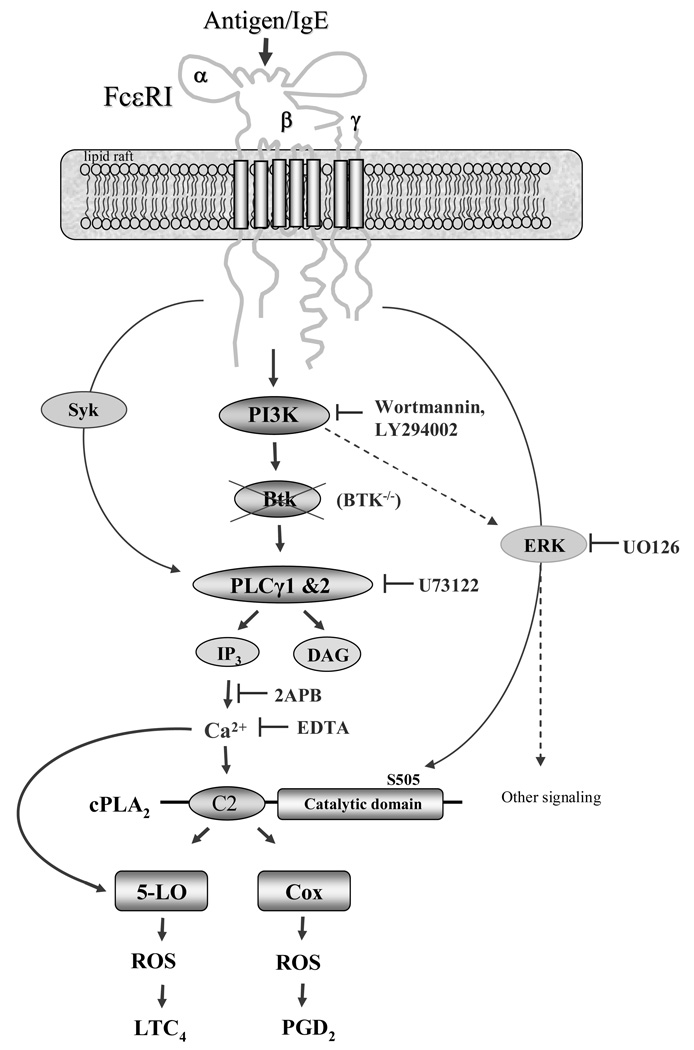
In this study, and as summarized in figure 8, we have demonstrated that PI3K and Btk is required for optimal FcɛRI-mediated cPLA2 activation leading to COX- and 5-LO-mediated generation of eicosanoids and ROS (Fig. 1) in mast cells. The PI3K-Btk axis appears to regulate these responses by the latent regulation of the PLCγ-induced calcium signal which is essential for cPLA2 activation and which likely also contributes to the regulation of COX and 5-LO. Rather than leading to cPLA2 phosphorylation, which is regulated to a certain extent by an ERK1/2-dependent mechanism, the calcium-dependent regulation of eisosanoid and ROS generation by PI3K and Btk may lead to the binding of Ca2+ to the C2 domain of cPLA2 which regulates membrane targeting.
Figure 8.
Proposed mechanism of FcɛRI-induced eicosanoid and ROS generation. FcɛRI aggregation induces Btk activation which is downstream of PI3K. Activated Btk contributes to the PLCγ-induced Ca2+ signal which is essential for cPLA2, COX, and 5-LO activation. Whereas activated ERK1/2 leads to cPLA2 activation through its phosphorylation, the Ca2+ provided by the PI3K-Btk likely activates cPLA2 by binding to the C2 domain. The sites of action of the inhibitors and gene knock outs used in this study are indicated in the figure.
The role of ERK1/2 in cPLA2 phosphorylation leading to eicosanoid generation previously reported in RBL 2H3 cells (20) was confirmed in human and mouse mast cells by the concurrent antigen-dependent increase in ERK1/2 and cPLA2 phosphorylation in HuMCs and BMMCs (Fig.1) and, more specifically, by the ability of the MEK1/2 inhibitor U0126 to attenuate these responses in parallel with the attenuation of PGD2 and LTC4 release (Fig. 2A–F). These studies also revealed a role of ERK1/2 in the production of ROS (Fig. 2G, H). Nevertheless, the effects of U0126 on the aforementioned responses were partial, suggesting that other pathways are involved in these responses.
The role of PI3K and Btk in the ERK-independent regulation of eicosanoid and ROS generation was indicated by the ability of PI3K inhibitors to reverse FcɛRI-mediated PGD2 and LTC4 release and ROS production in both HuMCs and BMMCs (Fig. 4); and by the substantial reduction of these responses in Btk−/− BMMCs. (Fig. 5). These data obtained from the Btk−/− BMMCs, however, is in contrast to a previous report of unimpaired FcɛRI-mediated LT release in these cells (26). The reason for the disparity is currently unclear. However, the ability of the PI3K inhibitors to block Btk activation (Fig. 7A) and mimic the Btk−/− phenotype (Fig. 4, Fig. 5), indicates that PI3K contributes to the control of eicosanoid and ROS generation by regulating the activity of Btk. It is possible, however, that PI3K also acts independently of Btk. For example, it has been reported that PI3K/Rac/PKCδ acts upstream of ERK and cPLA2 to regulate FcɛRI-mediated cysteinyl leukotriene synthesis in RBL 2H3 cells (31). Regardless, the small differences noted in phosphorylation of cPLA2 (Fig.3A–B and Fig. 6D), implies that PI3K and Btk were regulating eicosanoid and ROS generation other than through control of ERK1/2-mediated cPLA2 phosphorylation. This conclusion was further supported by the lack of difference in the phosphorylation of ERK1/2 in the Btk−/− BMMCs (Fig. 6D).
Although the PI3K inhibitors produced a slight reduction in FcɛRI-mediated ERK1/2 phosphorylation (Fig. 3A and B), this did not translate to reduced cPLA2 phosphorylation. It may be argued that the degree of inhibition of ERK1/2 phosphorylation produced by the PI3K inhibitors (42 % in HuMCs, 39 % in BMMCs at 10 min of stimulation) was not of sufficient magnitude to reduce the phosphorylation of cPLA2. However, it is also possible that the inhibitors of the ERK1/2 pathway used in this and a previous study (19) may be also targeting other kinase pathways leading to cPLA2 phosphorylation.
The ability of the Ca2+-chelator, EDTA, and the IP3 receptor antagonist, 2-APB, to block antigen-induced eicosanoid generation and ROS production (Fig. 7B–D), demonstrates the requirement for an increase in cytosolic Ca2+ concentrations for the liberation of free arachidonic acid, and the subsequent production of PGD2 and LTC4, as well as ROS, in activated mast cells. In addition to phosphorylation, cPLA2 activation requires translocation from the cytosol to the perinuclear membrane. This translocation is induced in response to an increase in intracellular Ca2+, and subsequent binding of Ca2+ to the C2 domain of cPLA2 resulting in increased affinity of cPLA2 for membranes (21, 22). Both COX and 5-LO also require Ca2+ for activity (Fig. 7B and C) and translocation to the perinuclear membrane. Thus, the results of the experiments conducted with EDTA and 2-APB can be explained by an inhibition of cPLA2 activation through reducing the effective concentrations of Ca2+ which interact with the C2 domain of cPLA2.
In this (Fig. 6C) and in previous studies (24) we have demonstrated that PI3K and Btk contribute to the Ca2+ signal induced upon FcɛRI aggregation in mast cells. Therefore, it is likely that PI3K and Btk regulate eicosanoid and ROS generation by this means. This pathway likely involves a latent signal that allows the maintenance of PLCγ activation which is induced upon FcɛRI aggregation. In agreement with previous reports (24, 26), we accordingly observed that FcɛRI-mediated PLCγ phosphorylation and PLCγ-dependent IP3 production were partially attenuated in Btk−/− cells (Fig. 6) and in BMMCs treated with the PI3K inhibitors (Fig. 7A). This level of inhibition was similar to the degree of attenuation of FcɛRI-mediated eicosanoid and ROS generation observed under similar experimental conditions. The results obtained under Ca2+-free conditions and with the IP3 binding inhibitor, 2-APB, however, demonstrate that there is an absolute requirement for both the PLCγ-mediated generation of IP3 and Ca2+ for FcɛRI-mediated eicosanoid and ROS generation in mast cells (Fig. 7B–D). The residual calcium signal and eicosanoid and ROS generation observed in Btk−/− (Fig. 6C) cells and wild type BMMCs treated with the PI3K inhibitors (data not shown) therefore, suggests that other, as yet unidentified. signals may also contribute to the aforementioned responses.
In summary, the results of this study demonstrate that ERK1/2- and PI3K-Btk-dependent pathway (Fig. 8), independently contribute to antigen-mediated generation of eicosanoids and ROS in mast cells. Whereas ERK1/2 regulates cPLA2 phosphorylation, the PI3K-BTK pathway contributes to the required Ca2+ signal for cPLA2, COX and 5-LO activation. Therefore, effective therapeutic suppression of eicosanoid and ROS production in activated mast cells may require a combination of strategies to block both ERK- and PI3K/Btk-regulated pathways.
ACKNOWLEDGEMENTS
We thank Dr. Anne B. Satterthwaite, University of Texas Southwestern Medical Center, for kindly providing Btk−/− mice.
Footnotes
Research in the authors’ laboratories is supported by the NIAID and NHLBI Divisions of Intramural Research within the National Institutes of Health, USA.
REFERENCES
- 1.Brown JM, Wilson TM, Metcalfe DD. The mast cell and allergic diseases: role in pathogenesis and implications for therapy. Clin Exp Allergy. 2008;38:4–18. doi: 10.1111/j.1365-2222.2007.02886.x. [DOI] [PubMed] [Google Scholar]
- 2.Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787–799. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- 3.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 4.Metzger H. The receptor with high affinity for IgE. Immunol Rev. 1992;125:37–48. doi: 10.1111/j.1600-065x.1992.tb00624.x. [DOI] [PubMed] [Google Scholar]
- 5.Rivera J. Molecular adapters in FcɛRI signaling and the allergic response. Curr Opin Immunol. 2002;14:688–693. doi: 10.1016/s0952-7915(02)00396-5. [DOI] [PubMed] [Google Scholar]
- 6.Mekori YA, Metcalfe DD. Mast cells in innate immunity. Immunol Rev. 2000;173:131–140. doi: 10.1034/j.1600-065x.2000.917305.x. [DOI] [PubMed] [Google Scholar]
- 7.Boyce JA. Mast cells and eicosanoid mediators: a system of reciprocal paracrine and autocrine regulation. Immunol Rev. 2007;217:168–185. doi: 10.1111/j.1600-065X.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 8.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 9.Gijon MA, Leslie CC. Regulation of arachidonic acid release and cytosolic phospholipase A2 activation. J Leukoc Biol. 1999;65:330–336. doi: 10.1002/jlb.65.3.330. [DOI] [PubMed] [Google Scholar]
- 10.Serhan CN, Haeggstrom JZ, Leslie CC. Lipid mediator networks in cell signaling: update and impact of cytokines. Faseb J. 1996;10:1147–1158. doi: 10.1096/fasebj.10.10.8751717. [DOI] [PubMed] [Google Scholar]
- 11.Murphy RC, Gijon MA. Biosynthesis and metabolism of leukotrienes. Biochem J. 2007;405:379–395. doi: 10.1042/BJ20070289. [DOI] [PubMed] [Google Scholar]
- 12.Werz O, Steinhilber D. Therapeutic options for 5-lipoxygenase inhibitors. Pharmacol Ther. 2006;112:701–718. doi: 10.1016/j.pharmthera.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 14.Swindle EJ, Coleman JW, DeLeo FR, Metcalfe DD. FcɛRI- and Fcγ receptor-mediated production of reactive oxygen species by mast cells is lipoxygenase- and cyclooxygenase-dependent and NADPH oxidase-independent. J Immunol. 2007;179:7059–7071. doi: 10.4049/jimmunol.179.10.7059. [DOI] [PubMed] [Google Scholar]
- 15.Nanda BL, Nataraju A, Rajesh R, Rangappa KS, Shekar MA, Vishwanath BS. PLA2 mediated arachidonate free radicals: PLA2 inhibition and neutralization of free radicals by anti-oxidants–a new role as anti-inflammatory molecule. Curr Top Med Chem. 2007;7:765–777. doi: 10.2174/156802607780487623. [DOI] [PubMed] [Google Scholar]
- 16.Swindle EJ, Metcalfe DD. The role of reactive oxygen species and nitric oxide in mast cell-dependent inflammatory processes. Immunol Rev. 2007;217:186–205. doi: 10.1111/j.1600-065X.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- 17.Inoue T, Suzuki Y, Yoshimaru T, Ra C. Reactive oxygen species produced up- or downstream of calcium influx regulate proinflammatory mediator release from mast cells: Role of NADPH oxidase and mitochondria. Biochim Biophys Acta. 2008;1783:789–802. doi: 10.1016/j.bbamcr.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh M, Tucker DE, Burchett SA, Leslie CC. Properties of the Group IV phospholipase A2 family. Prog Lipid Res. 2006;45:487–510. doi: 10.1016/j.plipres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Kimata M, Inagaki N, Kato T, Miura T, Serizawa I, Nagai H. Roles of mitogen-activated protein kinase pathways for mediator release from human cultured mast cells. Biochem Pharmacol. 2000;60:589–594. doi: 10.1016/s0006-2952(00)00354-3. [DOI] [PubMed] [Google Scholar]
- 20.Hirasawa N, Santini F, Beaven MA. Activation of the mitogen-activated protein kinase/cytosolic phospholipase A2 pathway in a rat mast cell line. Indications of different pathways for release of arachidonic acid and secretory granules. J Immunol. 1995;154:5391–5402. [PubMed] [Google Scholar]
- 21.Kramer RM, Sharp JD. Structure, function and regulation of Ca2+-sensitive cytosolic phospholipase A2 (cPLA2) FEBS Lett. 1997;410:49–53. doi: 10.1016/s0014-5793(97)00322-0. [DOI] [PubMed] [Google Scholar]
- 22.Clark JD, Lin LL, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, Milona N, Knopf JL. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca2+-dependent translocation domain with homology to PKC and GAP. Cell. 1991;65:1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- 23.Tkaczyk C, Beaven MA, Brachman SM, Metcalfe DD, Gilfillan AM. The phospholipase Cγ1-dependent pathway of FcɛRI-mediated mast cell activation is regulated independently of phosphatidylinositol 3-kinase. J Biol Chem. 2003;278:48474–48484. doi: 10.1074/jbc.M301350200. [DOI] [PubMed] [Google Scholar]
- 24.Iwaki S, Tkaczyk C, Satterthwaite AB, Halcomb K, Beaven MA, Metcalfe DD, Gilfillan AM. Btk plays a crucial role in the amplification of FcɛRI-mediated mast cell activation by kit. J Biol Chem. 2005;280:40261–40270. doi: 10.1074/jbc.M506063200. [DOI] [PubMed] [Google Scholar]
- 25.Felices M, Falk M, Kosaka Y, Berg LJ. Tec kinases in T cell and mast cell signaling. Adv Immunol. 2007;93:145–184. doi: 10.1016/S0065-2776(06)93004-1. [DOI] [PubMed] [Google Scholar]
- 26.Kawakami Y, Kitaura J, Satterthwaite AB, Kato RM, Asai K, Hartman SE, Maeda-Yamamoto M, Lowell CA, Rawlings DJ, Witte ON, Kawakami T. Redundant and opposing functions of two tyrosine kinases, Btk and Lyn, in mast cell activation. J Immunol. 2000;165:1210–1219. doi: 10.4049/jimmunol.165.3.1210. [DOI] [PubMed] [Google Scholar]
- 27.Kuehn HS, Beaven MA, Ma HT, Kim MS, Metcalfe DD, Gilfillan AM. Synergistic activation of phospholipases Cγ and Cβ: a novel mechanism for PI3K-independent enhancement of FcɛRI-induced mast cell mediator release. Cell Signal. 2008;20:625–636. doi: 10.1016/j.cellsig.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirshenbaum AS, Goff JP, Semere T, Foster B, Scott LM, Metcalfe DD. Demonstration that human mast cells arise from a progenitor cell population that is CD34+, c-kit+, and expresses aminopeptidase N (CD13) Blood. 1999;94:2333–2342. [PubMed] [Google Scholar]
- 29.Tkaczyk C, Metcalfe DD, Gilfillan AM. Determination of protein phosphorylation in FcɛRI-activated human mast cells by immunoblot analysis requires protein extraction under denaturing conditions. J Immunol Methods. 2002;268:239–243. doi: 10.1016/s0022-1759(02)00210-7. [DOI] [PubMed] [Google Scholar]
- 30.Saito K, Scharenberg AM, Kinet JP. Interaction between the Btk PH domain and phosphatidylinositol-3,4,5-trisphosphate directly regulates Btk. J Biol Chem. 2001;276:16201–16206. doi: 10.1074/jbc.M100873200. [DOI] [PubMed] [Google Scholar]
- 31.Cho SH, You HJ, Woo CH, Yoo YJ, Kim JH. Rac and protein kinase C-δ regulate ERKs and cytosolic phospholipase A2 in FcɛRI signaling to cysteinyl leukotriene synthesis in mast cells. J Immunol. 2004;173:624–631. doi: 10.4049/jimmunol.173.1.624. [DOI] [PubMed] [Google Scholar]