Abstract
This review of the disposition of methamphetamine in oral fluid, plasma, and urine is based on a comprehensive controlled dosing study involving five healthy, drug-free research volunteers who resided on a closed clinical ward for 12 weeks. Subjects were administered four low (10 mg) and high (20 mg) daily oral doses of methamphetamine in two separate sessions. Near-simultaneous collections of oral fluid and plasma were performed on the first day of each low- and high-dose session. Thereafter, oral fluid was provided on each day of dosing by different oral fluid collection methods. All urine specimens were collected on an ad libitum basis throughout the study. Specimens were analyzed by gas-chromatography mass spectrometry for methamphetamine and the metabolite, amphetamine, with a limit of quantification of 2.5 ng/mL for each analyte. Methamphetamine and metabolite concentrations in oral fluid appeared to follow a similar time course in oral fluid as in plasma and were dose-proportional, but oral fluid concentrations exceeded plasma concentrations. Urine drug concentrations were substantially higher than those in oral fluid. Some drug accumulation was noted with daily dosing, but generally did not markedly influence detection times or detection rates of oral fluid tests. Detection times and detection rates for oral fluid and urine were determined at cessation of 4 days of dosing. Generally, detection times and rates for urine were longer than those observed for oral fluid at conventional cutoff concentrations. When contemplating selection of oral fluid as a test matrix, the advantages of oral fluid collection should be weighed against its shorter time of detection compared to that of urine.
Keywords: methamphetamine, collection, oral fluid, saliva, urine, plasma
INTRODUCTION
Dextromethamphetamine is a powerful central nervous stimulant with a high potential for abuse. Currently, there are only a few accepted medical conditions (attention deficit disorder and short-term treatment of obesity) for pharmaceutically derived d-methamphetamine. Illicitly derived methamphetamine is widely available at relatively low cost because of its ease of manufacture in clandestine laboratories. Most synthetic procedures for illicit manufacture generally lead to production of relatively pure d-methamphetamine although some methods result in d/l-mixtures. The l-isomer (levmetamfetamine; l-desoxyephedrine) is considerably less potent and is sold over-the-counter as a nasal inhaler decongestant product. Abuse of illicit methamphetamine has increased substantially over the past decade and dominates drug use trends in many parts of the world. In 2004 there were a reported 583,000 current methamphetamine users in the United States.1
The effects of d-methamphetamine last from 6 to 12 h. After the initial “rush,” many individuals remain hyperactive, restless, and irritable. Some users are reported to be prone to aggression and violence.2 Illicit methamphetamine is used by a variety of routes including smoking and intravenous, intranasal (“snorted”), and oral administration. Methamphetamine is rapidly absorbed by all routes, but time to onset of effects is fastest by intravenous and smoked routes. The primary site of metabolism is in the liver by aromatic hydroxylation, N-demethylation (to form the metabolite amphetamine), and deamination. The basic nitrogen moiety in methamphetamine’s chemical structure (pKa = 10.1), its relatively high lipophilicity, and low plasma–protein binding (10–20%) influences distribution and excretion processes. Acidic urine enhances methamphetamine excretion and shortens its half-life in the body, whereas basic urine slows excretion and prolongs residence time.3 Transfer across membranes and excretion in saliva is also facilitated by methamphetamine’s lipid nature, low protein-binding, and ion-trapping in saliva. Typically, salivary fluid is more acidic than is plasma, thus producing higher concentrations of methamphetamine in saliva compared to plasma.4
Oral fluid testing for methamphetamine and other drugs of abuse has emerged as an important alternative to urine testing in workplace and drug treatment programs. The primary advantage of oral fluid testing is its ease of collection. Oral fluid can be noninvasively collected under direct observation, thereby reducing or eliminating problems such as adulteration and substitution that are often associated with urine collection. Disadvantages include shorter drug detection windows for oral fluid than for urine and a need for greater assay sensitivity.
Understanding methamphetamine disposition in oral fluid, blood, and urine is essential to the development and use of oral fluid tests. How closely does oral fluid parallel plasma drug concentrations? What are the influences of different oral fluid collection devices on concentration? Is there a dose–concentration relationship for drug in oral fluid similar to that found for plasma? What is the effect of repeated dosing on oral fluid concentrations? What are the detection times and detection rates for drug in oral fluid compared to plasma and urine? These are fundamental questions of vital importance to the use and interpretation of oral fluid tests. This review addresses these issues by providing a detailed summary of methamphetamine disposition in oral fluid, with comparisons to its disposition in plasma, and urine. Data for this review are derived from recent controlled dosing studies performed by Huestis and coworkers.4–6
METHAMPHETAMINE STUDY PROTOCOL
Methamphetamine was administered to five drug-free, stimulant-experienced subjects who resided on a closed research ward for the duration of the study. The protocol was approved by an Institutional Review Board, and each subject provided written informed consent. Each subject participated in two dosing sessions. The first consisted of administration of single 10-mg oral doses of methamphetamine (10 mg METH) daily for 4 days. Generally, dosing was administered on four consecutive days, but occasionally, dosing days were separated by 48–72 h. Following a 3-week washout period, each subject participated in a second dosing session and received single 20-mg oral doses of methamphetamine (20 mg METH) on four subsequent days. Blood specimens were collected at timed intervals on the first day of each dose administration, but only limited sampling was conducted on subsequent days. Oral fluid specimens were collected at near-simultaneous times as plasma specimens on the first day of dosing. Subsequent oral fluid collections on days 2–4 were made with the same sampling schedule as day 1. Different oral fluid collection methods were employed for assessment of the effect of different devices on drug concentration. Oral fluid was collected on days 1 and 2 (low and high doses) by stimulation with a citric acid sourball candy (Brach’s Confections Inc., Dallas, TX, USA), designated as “sour candy,” on day 3 of each session with a cotton swab treated with 20 mg of citric acid (Salivette® cotton swab citric acid preparation; Sarstedt, Newton, NC, USA) designated as “CAC 3,” and on day 4 of each session with cotton swabs without citric acid (Salivette cotton swab without preparation; Sarstedt), designated as “SAL 4.” Complete urine collections (ad libitum) were conducted throughout the study. No attempt was made to control water intake or urine pH during dosing and subsequent specimen collections. All specimens were analyzed for methamphetamine and amphetamine by gas-chromatography mass spectrometry (GC-MS) with a limit of quantification (LOQ) of 2.5 ng/mL for each analyte. All data reported in this review represent drug concentrations determined by GC-MS. Details of the study design and pharmacokinetic analyses have been reported.4,5
Disposition of Methamphetamine and Amphetamine in Oral Fluid, Plasma, and Urine after Administration of Single Oral Doses of Methamphetamine
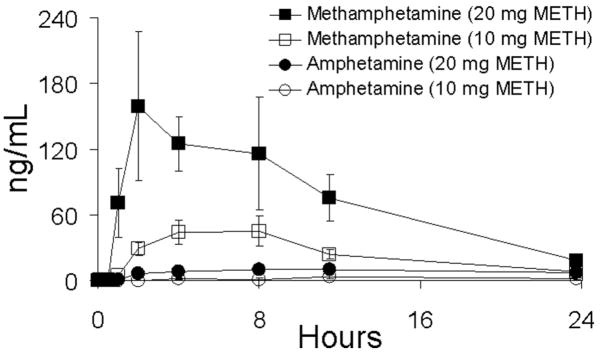
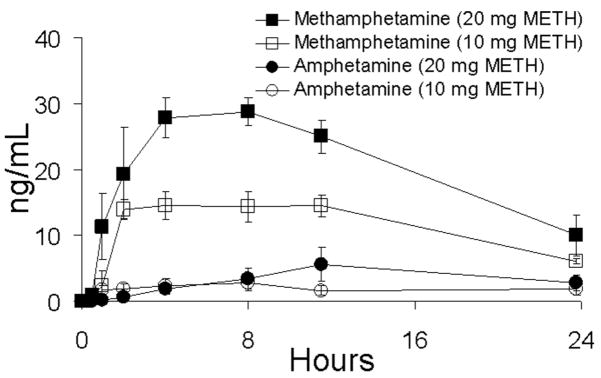
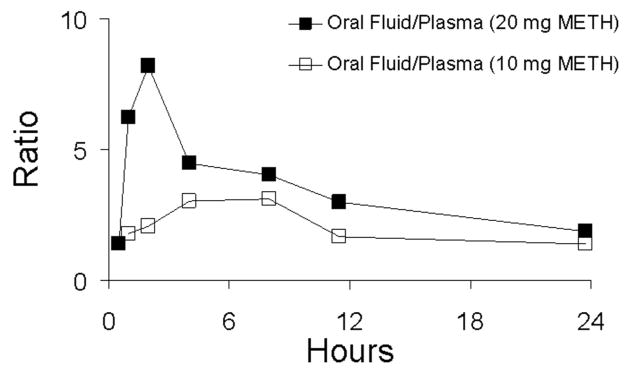
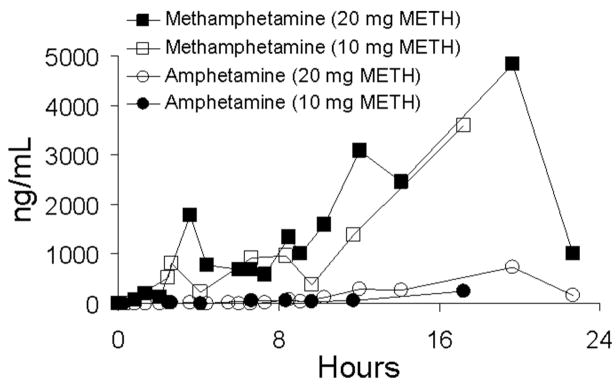
Following first-day administration of single oral doses of 10 and 20 mg of methamphetamine, drug was detected in concentrations >2.5 ng/mL in oral fluid and plasma within 0.5 to 2 h and reached maximal concentration from 2–11.5 h. Figures 1 and 2 illustrate mean data for methamphetamine and amphetamine in oral fluid and plasma, respectively, on day 1 of the dosing sessions. First detection of amphetamine occurred in the range of 1.0–23.8 h and was slightly delayed relative to methamphetamine in oral fluid and plasma. Maximal concentrations occurred from 2.0–23.8 h. A summary of Cmax, Tmax, and time to first positive methamphetamine and metabolite (amphetamine) specimens in oral fluid, plasma, and urine is shown in Table 1. Approximate proportional increases in drug concentration were observed between the low- and high-dose conditions for both oral fluid and plasma. Concentrations of methamphetamine in oral fluid ranged from 1.4 to 8.2 times higher than in plasma. Figure 3 illustrates mean oral fluid/plasma ratios over the first-day dosing sessions.
FIGURE 1.
Mean oral fluid concentrations of methamphetamine (METH) and amphetamine in five subjects following single-dose oral administration of 10 and 20 mg methamphetamine. Oral fluid was collected by stimulation with sour candy. Error bars represent standard error of the mean.
FIGURE 2.
Mean plasma concentrations of methamphetamine (METH) and amphetamine in five subjects following single-dose oral administration of 10 and 20 mg of methamphetamine. Error bars represent standard error of the mean.
TABLE 1.
Comparison of methamphetamine and amphetamine Cmax, Tmax, and time to first positive specimen at the LOQ (2.5 ng/mL) in oral fluid, plasma, and urine following a single dose (first day, mean data) of 10 and 20 mg of methamphetamine administered to five subjects. Oral fluid was collected by stimulation with sour candy
| Meth dose (mg) | Analyte | Specimen | N | Cmax ± SEM, ng/mL (range) | Tmax ± SEM, h (range) | Time to first positive (LOQ) ± SEM, h (range) |
|---|---|---|---|---|---|---|
| 10 | Meth | Oral fluid | 5 | 57.1 ± 12.9 (24.7–90.2) |
5.6 ± 1.0 (4.0–8.0) |
1.6 ± 0.2 (1.0–2.0) |
| Amp | Oral fluid | 3 | 6.5 ± 1.5 (4.6–9.4) |
10.3 ± 1.2 (8.0–11.5) |
6.5 ± 2.5 (4.0–11.5) |
|
| 20 | Meth | Oral fluid | 5 | 192.2 ± 54.0 (75.3–321.7) |
4.7 ± 1.8 (2.0–11.5) |
0.8 ± 0.1 (0.5–1.0) |
| Amp | Oral fluid | 5 | 14.3 ± 3.0 (2.8–20.2) |
8.2 ± 1.7 (2.0–11.5) |
4.1 ± 1.9 (1.0–11.5) |
|
| 10 | Meth | Plasma | 5 | 17.4 ± 1.1 (14.5–20.3) |
5.2 ± 1.2 (2.0–8.0) |
1.7 ± 0.3 (0.5–2.0) |
| Amp | Plasma | 4 | 4.2 ± 1.5 (2.0–8.4) |
15.9 ± 4.5 (8.0–23.8) |
10.1 ± 5.1 (1.0–23.8) |
|
| 20 | Meth | Plasma | 5 | 32.4 ± 3.5 (26.2–44.3) |
7.5 ± 1.5 (2.0–11.5) |
1.1 ± 0.2 (0.5–2.0) |
| Amp | Plasma | 5 | 5.6 ± 1.4 (2.9–10.6) |
13.9 ± 2.5 (11.5–23.8) |
7.4 ± 1.9 (2.0–11.5) |
|
| 10 | Meth | Urine | 5 | 2969.7 ± 607.6 (1573.7–4844.6) |
15.0 ± 1.6 (11.4–19.6) |
5.0 ± 2.2 (0.7–11.3) |
| Amp | Urine | 5 | 354.5 ± 33.8 (258.7–435.3) |
15.0 ± 1.6 (11.4–19.6) |
5.8 ± 1.9 (1.4–11.3) |
|
| 20 | Meth | Urine | 5 | 4038.4 ± 700.9 (1871.4–6003.5) |
14.0 ± 3.2 (4.8–19.7) |
3.3 ± 1.4 (0.9–8.8) |
| Amp | Urine | 5 | 737.7 ± 226.3 (266.7–1556.1) |
17.0 ± 1.4 (13.1–19.7) |
4.3 ± 1.3 (1.2–8.8) |
Cmax = maximum observed drug concentration; Tmax = time to maximum drug concentration; Meth = methamphetamine; Amp = amphetamine.
FIGURE 3.
Mean oral fluid/plasma ratios of methamphetamine (METH) in five subjects following single-dose oral administration of 10 and 20 mg of methamphetamine. Oral fluid was collected by use of sour candy.
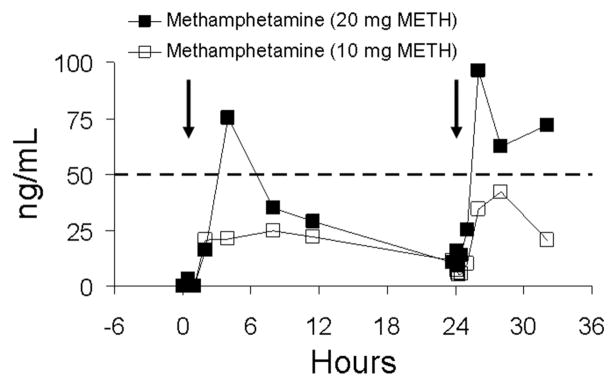
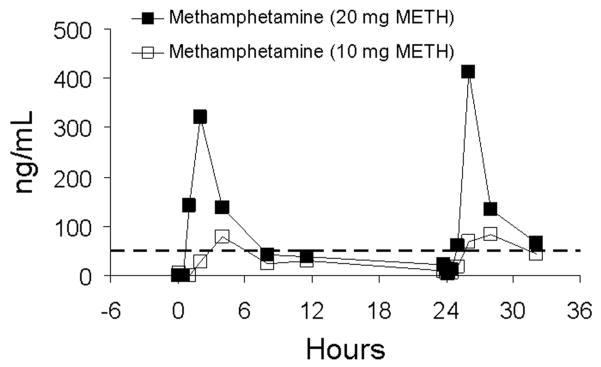
Reproducibility of methamphetamine concentrations on days 1 and 2 of the low- and high-dose session are illustrated in Figures 4 and 5 for two subjects (subjects AA and BB). Oral fluid specimen collections were made on these days by the same collection method (stimulation with sour candy). This afforded the opportunity to compare methamphetamine concentrations within the same subjects over the course of two sequential days. Day 2 concentrations for both subjects were slightly higher than day 1 as a result of carryover from the first doses. Given this consideration, responses on day 2 were relatively similar to those of day 1. Of greater note, in Figures 4 and 5 is the large intersubject variability in drug concentrations. Methamphetamine concentrations in oral fluid specimens from subject BB were approximately threefold higher than for subject AA.
FIGURE 4.
Oral fluid concentrations of methamphetamine (METH) for subject AA following single-dose oral administration of 10 and 20 mg of methamphetamine on two sequential days. Oral fluid was collected by use of sour candy. The arrows represent time of methamphetamine administration, and the dotted line represents the recommended DHHS cutoff concentration of 50 ng/mL for methamphetamine in oral fluid.
FIGURE 5.
Oral fluid concentrations of methamphetamine (METH) for subject BB following single-dose oral administration of 10 and 20 mg of methamphetamine on two sequential days. Oral fluid was collected by use of sour candy. The arrows represent time of methamphetamine administration and the dotted line represents the recommended DHHS cutoff concentration of 50 ng/mL for methamphetamine in oral fluid.
Appearance of the metabolite, amphetamine, in oral fluid, plasma, and urine was highly variable and occurred in the range of 1–23.5 h in considerably lower concentrations compared to the parent drug, methamphetamine. Figure 6 illustrates first-day urine concentrations of methamphetamine and amphetamine for a typical subject (subject AA).
FIGURE 6.
Urine concentrations of methamphetamine (METH) and amphetamine for subject AA following single-dose oral administration of 10 and 20 mg of methamphetamine.
Detection of Methamphetamine and Amphetamine in Oral Fluid and Urine at Administrative Cutoff Concentrations following First-Day Administration of Single Oral Doses of Methamphetamine
Detection rates for methamphetamine in oral fluid (10 specimens collected after dosing at timed intervals of 0.08, 0.17, 0.25, 0.5, 1, 2, 4, 8, 11.5, and 23.5 h) at the proposed Department of Health and Human Services (DHHS) administrative cutoff of 50 ng/mL (accompanied by amphetamine metabolite at assay limit of detection [LOD])7 were low to moderate following 10- and 20-mg doses of methamphetamine. Following the low dose, two subjects produced a single positive result at the DHHS cutoff concentrations for specimens collected at 4 h (4% detection rate over 24 h). A total of 25 specimens were positive for methamphetamine at 2.5 ng/mL (LOQ). After the high dose, one subject failed to produce a positive specimen at the DHHS cutoff concentration (on account of the absence of amphetamine) and four subjects produced a total of 15 positives (28.6% detection rate over 24 h). A total of 49 specimens was positive for methamphetamine at 2.5 ng/mL (LOQ). Positive specimens were generally produced over the 1–24 h period post dose. Because oral dosing was utilized in this study, the delay in absorption and metabolism of methamphetamine generally led to negative specimens collected over the first hour of specimen collection. It should be noted that oral fluid collections made during the first hour were a factor in reducing the detection rates.
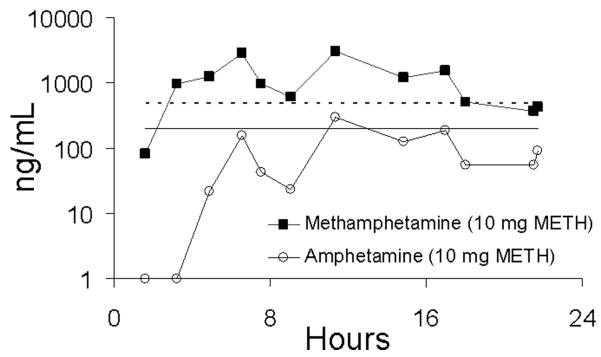
Following administration of the first dose (day 1), methamphetamine was first detected in urine at the current DHHS administration confirmation cutoff concentration of 500 ng/mL8 at mean times of 5.5 and 4.3 h, respectively, for the low and high doses of methamphetamine. Appearance of the metabolite, amphetamine, at the required cutoff concentration of 200 ng/mL was delayed relative to methamphetamine and occurred at mean times of 14.5 and 14.2 h, respectively. At the proposed lower DHHS confirmation cutoff concentrations of 250 and 100 for methamphetamine and amphetamine, first detection of methamphetamine was approximately equivalent to that observed for the higher cutoff concentration; however, amphetamine was first detected at mean times of 9.9 and 9.1 h, respectively. The data for detection of the first positive specimen are summarized in Table 2. It should be noted that DHHS criteria for a “positive” methamphetamine require that methamphetamine be accompanied by amphetamine at 200 ng/mL. It is evident from detection time data (Table 2) that many methamphetamine positives did not meet DHHS criteria as “positive” because of low amphetamine concentrations. This is illustrated in Figure 7 for one subject (subject BB) following administration of a 10-mg dose of methamphetamine. Although methamphetamine exceeded the 500 ng/mL confirmation cutoff concentration for the majority of time over the first 24 h, amphetamine concentrations were below the required concentration of 200 ng/mL in all but one specimen.
TABLE 2.
Time to detection of the first positive specimen of methamphetamine and amphetamine in urine at different DHHS confirmation cutoff concentrations following a single dose (first day, mean data) of 10 and 20 mg of methamphetamine administered to five subjects
| Meth dose (mg) | Analyte | N | Cutoff concentration (ng/mL) | Time to first positive ± SEM, h (range) |
|---|---|---|---|---|
| 10 | Meth | 5 | 500 | 5.5 ± 2.0 (1.4–11.3) |
| Amp | 5 | 200 | 14.5 ± 1.9 (9.3–19.6) | |
| 20 | Meth | 5 | 500 | 4.3 ± 1.3 (1.2–8.8) |
| Amp | 5 | 200 | 14.2 ± 2.1 (7.9–19.6) | |
| 10 | Meth | 5 | 250 | 5.5 ± 2.0 (1.4–11.3) |
| Amp | 5 | 100 | 9.9 ± 2.2 (4.2–17.2) | |
| 20 | Meth | 5 | 250 | 3.9 ± 1.3 (1.2–8.8) |
| Amp | 5 | 100 | 9.1 ± 1.6 (3.3–13.1) |
Meth = methamphetamine; Amp = amphetamine.
FIGURE 7.
Methamphetamine (METH) and amphetamine excretion in urine of one subject (subject BB) following administration of a single dose (first day) of 10 mg methamphetamine. The dotted line illustrates the 500 ng/mL DHHS confirmation cutoff concentration for methamphetamine and the solid line illustrates the 200 ng/mL DHHS confirmation cutoff concentration for amphetamine.
Detection rates for methamphetamine in urine (collected ad libitum) over the first 24 h (postdosing, day 1) at the current DHHS administrative confirmation cutoff concentration of 500 ng/mL (accompanied by 200 ng/mL of amphetamine metabolite) for the low and high doses were 31.0% (range = 7.7–66.7%) and 39.4% (range = 9.1–75.0%), respectively. Lowering the cutoff concentration requirement increased detection rates. Detection rates at the proposed confirmation cutoff concentration of 250 ng/mL (accompanied by 100 ng/mL of amphetamine) were 52.6% (range = 11.1–100.0%) and 63.5% (range = 27.3–100.0%), respectively. As noted earlier, with both cutoff concentration requirements, approximately 75% or more of false negative tests were produced as a result of amphetamine concentrations not meeting the required concentration and 25% or less were produced by both methamphetamine and amphetamine not meeting the required concentrations. Detection rates for methamphetamine at 2.5 ng/mL (LOQ) were >96% for both low- and high-dosing conditions.
Effect of Repeated Dosing on Methamphetamine Disposition
Although much of the information on drug disposition in oral fluid, plasma, and urine have been based on single-dose studies, recreational misuse and abuse frequently involves repeated daily self-administration. The design of the current study offered a unique opportunity to evaluate drug disposition in oral fluid and urine following repeated sequential and nonsequential daily dosing. Four doses of methamphetamine were administered in two sessions (low- and high-dose conditions). Oral fluid and urine collections were made throughout the study; however, plasma collections were primarily made only on the first day of each session because of blood-volume limitations. Oral fluid collections in both low- and high-dosing sessions were made by stimulation with sour candy on days 1 and 2, on day 3 with CAC 3, and on day 4 with SAL 4. Hence, it should be noted that oral fluid collections were conducted differently on days 3 and 4 and may have influenced concentrations. Drug was generally administered on a sequential daily basis, and thus, for most subjects, sessions were completed over a 4-day period. However, administration of some doses was delayed from 1 to 2 days. In particular, day 4 dosing was delayed for 2 days for four subjects (subjects S, Y, AA, low dose; subject W, high dose) because of the intervention of a weekend during which time dosing did not occur. A few other sessions had delays of 1 day. Any delays in dosing would be expected to produce less drug accumulation.
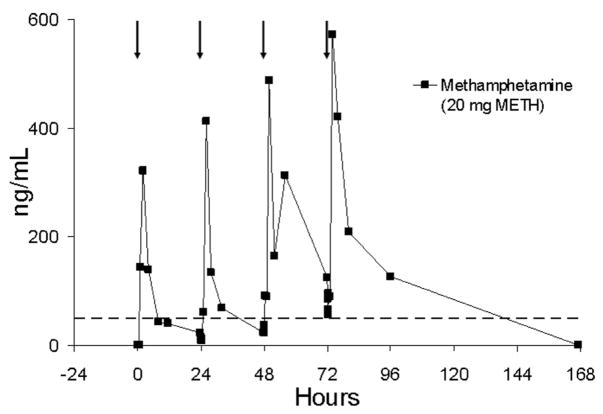
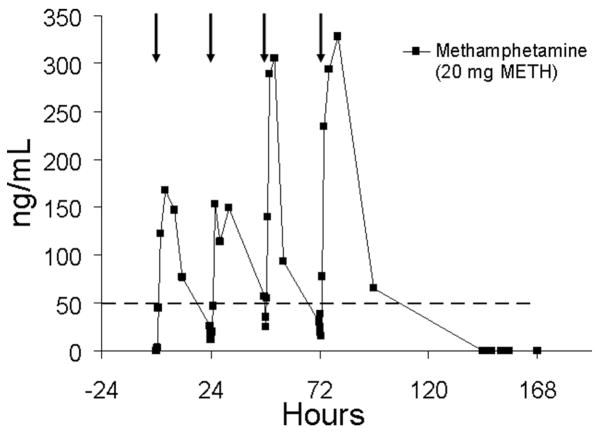
It is instructive to evaluate the effect of different daily dosing patterns. The relatively long half-life of methamphetamine4 (approximately 9–11 h) suggests that some drug accumulation should result from sequential daily dosing. Figure 8 illustrates oral fluid methamphetamine concentrations during sequential daily dosing without delay over 4 days for one subject (subject BB). There is clear indication of accumulation of methamphetamine in oral fluid from day-to-day. Note that by day 4, substantial methamphetamine accumulation occurred, and that oral fluid specimens collected prior to dosing were positive at the 50 ng/mL confirmation cutoff. Specimens for this subject continued to test positive for approximately 24 h following the fourth dose.
FIGURE 8.
Oral fluid concentrations of methamphetamine (METH) in subject BB following sequential daily single-dose oral administration of 20 mg methamphetamine on different days. Oral fluid was collected by use of sour candy on days 1 and 2, Salivette with citric acid (CAC 3) on day 3, and Salivette without citric acid (SAL 4) on day 4. The arrows represent time of methamphetamine administration and the dotted line represents the recommended DHHS cutoff concentration of 50 ng/mL for methamphetamine in oral fluid.
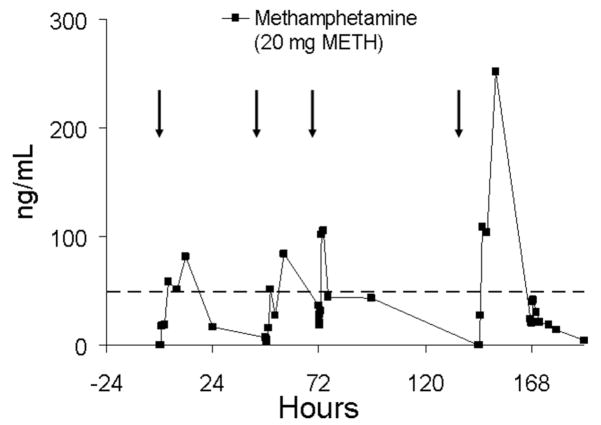
Figure 9 illustrates methamphetamine oral fluid concentrations during sequential daily dosing over 4 days for subject Y. Some accumulation is apparent from day-to-day, but not sufficient for the subject to test positive at the beginning of dosing on day 4. Specimens continued to test positive for approximately 24 h following the fourth dose.
FIGURE 9.
Oral fluid concentrations of methamphetamine (METH) in subject Y following single-dose oral administration of 20 mg methamphetamine on different days. Oral fluid was collected by use of sour candy on days 1 and 2, Salivette with citric acid (CAC 3) on day 3, and Salivette without citric acid (SAL 4) on day 4. The arrows represent time of methamphetamine administration and the dotted line represents the recommended DHHS cutoff concentration of 50 ng/mL for methamphetamine in oral fluid.
Figure 10 illustrates methamphetamine oral fluid concentrations during nonsequential daily dosing over 4 days for another subject (subject W), in which two delays in dosing occurred during the high-dose methamphetamine session. There was a 1-day delay in daily dosing between days 1 and 2, and a 2-day delay in daily dosing between days 3 and 4. No accumulation was apparent on day 4 as a result of administration of the three earlier doses. In this example, the subject tested positive for approximately 8 h following the fourth dose.
FIGURE 10.
Oral fluid concentrations of methamphetamine (METH) in subject W following single-dose oral administration of 20 mg methamphetamine on different days. Oral fluid was collected by use of sour candy on days 1 and 2, Salivette with citric acid (CAC 3) on day 3, and Salivette without citric acid (SAL 4) on day 4. The arrows represent time of methamphetamine administration and the dotted line represents the recommended DHHS cutoff concentration of 50 ng/mL for methamphetamine in oral fluid.
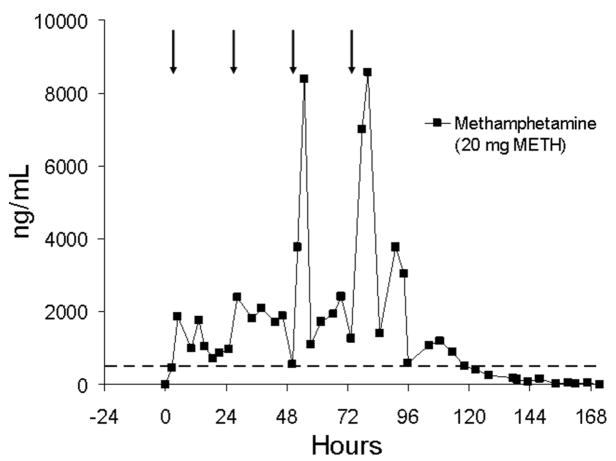
The effect of multiple dosing across four sequential days on methamphetamine urine concentrations is illustrated in Figure 11 for one subject (subject BB). There was indication of accumulation and carryover to the next dose on a day-to-day basis. Considerably higher methamphetamine concentrations occurred on days 3 and 4 of dosing. As a result of drug accumulation, most urine specimens for this subject were positive at the DHHS confirmation cutoff concentration throughout the 4 days of dosing and for approximately 46 h following the last dose.
FIGURE 11.
Urine concentrations of methamphetamine (METH) in subject BB following sequential daily single-dose oral administration of 20 mg methamphetamine on different days. The arrows represent time of methamphetamine administration and the dotted line represents the DHHS cutoff concentration of 500 ng/mL for methamphetamine in urine.
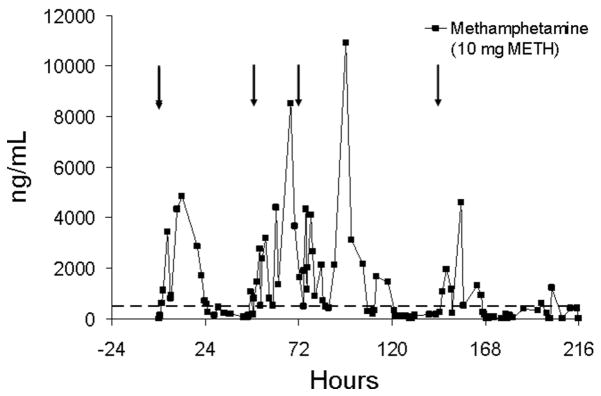
Figure 12 illustrates methamphetamine urine concentrations during nonsequential daily dosing over 4 days for another subject (subject S), in which two delays in dosing occurred during the high-dose methamphetamine session. There was a 1-day delay in daily dosing between day 1 and day 2 and a 2-day delay in daily dosing between days 3 and 4. Urine concentrations fell below the DHHS confirmation cutoff during the 1-day delay following the first dose and during the 2-day delay in dosing following the third dose. Following the fourth dose, positive specimens were intermixed with negative specimens over the remaining collection period.
FIGURE 12.
Urine concentrations of methamphetamine (METH) in subject S following nonsequential daily single-dose oral administration of 10 mg methamphetamine on different days. The arrows represent time of methamphetamine administration and the dotted line represents the DHHS cutoff concentration of 500 ng/mL for methamphetamine in urine.
Detection Times and Rates in Oral Fluid and Urine Following Repeated Dosing
Detection times or “windows of detection” are terms often used to describe how long after drug administration a test will be positive. Many factors go into determining detection times including dosage, pattern of usage, specimen type, choice of analyte(s), and assay characteristics (sensitivity, specificity, cutoff concentration). Percent detection rates are a measure of the “efficiency” of the test over a specified period (defined as the total number of positive specimens divided by the total number of specimens tested and multiplied by 100). For example, if a methamphetamine test has an average detection window of 48 h, one might find that the detection rate over the first 24 h was 100%, that is, all specimens test positive, but over the entire period of collection, the detection rate could be considerably less than 100%. Thus, both detection times and detection rates are useful in interpretation of drug tests. Detection times in urine are often also complicated by the occurrence of negative specimens interspersed with positive specimens. As drug concentration in the body diminishes as a result of metabolism and excretion, sequential collections may result in positive tests followed by negative tests and occasional reappearance of positive specimens. In addition, excess water intake can dilute urine and drug concentration and produce negative tests, whereas dehydration can concentrate urine. Although methamphetamine concentrations in oral fluid should be less influenced by these factors, drug concentration is influenced by saliva pH, degree of stimulation, and device characteristics. Thus, two approaches were adopted in this review for the analysis of detection times. Detection times are reported in terms of: (1) detection times for sequentially positive specimens (how long specimens remained positive) and (2) detection time to the last positive (how long a positive specimen could be detected).
In the current study, detection times for methamphetamine in oral fluid and urine were determined on the basis of specimens collected immediately following administration of the fourth dose in the low- and high-dose sessions (day 4). Detection times are summarized in Table 3. For oral fluid, mean detection times for consecutive positives at the recommended DHHS confirmation cutoff concentration of 50 ng/mL of methamphetamine (accompanied by amphetamine at LOD = 2.5 ng/mL) were 0.02 and 4.8 h (timed from start of dosing on the fourth day) for the low- and high-dose conditions, respectively. It should be noted that without accumulation, consecutive positives for oral fluid would not be expected following oral dosing due to the delay in absorption. During the high-dosing sessions, accumulated methamphetamine was sufficient to produce consecutive positives over a period of 24 h following the fourth dose for one subject (subject BB). At the lower cutoff concentration of 2.5 ng/mL, mean detection times for consecutive positive specimens following low- and high-dose sessions were 5.6 and 29.5 h, respectively. Oral fluid detection times to the last positive specimen at the recommended DHHS confirmation cutoff concentration were 4.0 and 20.6 h for the low- and high-dose conditions, respectively, and were 24.6 and 74.1 h at the lower cutoff concentration of 2.5 ng/mL.
TABLE 3.
Methamphetamine detection times to last consecutive positive and time to last positive in oral fluid and urine at different confirmation cutoff concentrations following a series of four single daily doses (fourth day, mean data) of 10 and 20 mg of methamphetamine administered to five subjects. Detection times are based on time of administration of fourth dose. Oral fluid was collected on day 4 with SAL 4
| Meth dose (mg) | Specimen | Cutoff concentration for methamphetamine/amphetamine (ng/mL) | Time to last consecutive positive ± SEM h (range) | Time to last positive ± SEM, h (range) |
|---|---|---|---|---|
| 10 | OF | 50/2.5 | 0.02 ± 0.02 (0.0–0.1) | 4.0 ± 1.8 (0.0–8.0) |
| 10 | OF | 2.5(methamphetamine) | 5.6 ± 4.6 (0.0–23.8) | 24.6 ± 9.9 (4.0–56.0) |
| 20 | OF | 50/2.5 | 4.8 ± 4.8 (0.0–23.8) | 20.6 ± 3.2 (8.0–23.8) |
| 20 | OF | 2.5(methamphetamine) | 29.5 ± 12.5 (0.0–76.0) | 74.1 ± 17.0 (23.8–126.9) |
| 10a | Urine | 500/200 | 10.8 ± 9.5 (0.0–48.7) | 43.6 ± 7.5 (24.6–59.5) |
| 10a | Urine | 250/100 | 21.0 ± 10.9 (0.8–48.7) | 59.5 ± 5.7 (44.3–73.0) |
| 20 | Urine | 500/200 | 32.9 ± 11.0 (6.1–59.5) | 66.9 ± 7.5 (45.9–91.7) |
| 20 | Urine | 250/100 | 57.0 ± 7.7 (31.6–76.8) | 79.7 ± 8.8 (50.8–95.5) |
Meth = methamphetamine; OF = oral fluid.
Urine detection times for the 10-mg dose condition for one subject (subject AA) were based on three daily doses (fourth-day data missing). All other detection times were based on four daily doses.
For urine, mean detection times for consecutive positives at the DHHS confirmation cutoff concentration of 500 ng/mL of methamphetamine (accompanied by amphetamine at 200 ng/mL) were 10.8 and 32.9 h for the low- and high-dose conditions, respectively. Lowering the cutoff concentration to the recommended cutoff concentrations (250/100) increased mean detection times for consecutive positives to 21.0 and 57.0 h, respectively. Drug accumulation from prior doses appears to have been a greater factor in the production of consecutive positives in urine than was observed for oral fluid.
Mean urine detection times to the last positive at the DHHS confirmation cutoff concentration of 500/200 were 43.6 and 66.9 h for the low- and high-dose conditions, respectively. Lowering the cutoff concentration to the recommended levels (250/100) increased mean detection times to the last positive to 59.5 and 79.7 h, respectively.
Detection rates for methamphetamine in oral fluid and urine also were determined on the basis of specimens collected immediately following administration of the fourth dose in the low- and high-dose sessions (day 4). In this study, oral fluid was typically collected following the fourth dose for 8–24 h, whereas urine specimens were typically collected for a minimum of 72 h. Detection rates for oral fluid and urine are summarized in Table 4. For oral fluid, mean detection rates for methamphetamine at the recommended DHHS confirmation cutoff concentration (accompanied by amphetamine at LOD = 2.5 ng/mL) were 24.6% and 56.4% (timed from start of dosing on the fourth day) for the low- and high-dose conditions, respectively. As expected, higher detection rates were observed at the lower cutoff concentration of 2.5 ng/mL (75.0% and 87.5% for the low and high doses). Again, it is noted that the detection rates for oral fluid are highly influenced by accumulation (leading to higher rates) and numerous early specimen collections (leading to lower rates as a result of delay in drug absorption from oral dosing).
TABLE 4.
Methamphetamine detection rates in oral fluid and urine at different confirmation cutoff concentrations following a series of four single daily doses (fourth day, mean data) of 10 and 20 mg of methamphetamine administered to five subjects. Detection times are based on time of administration of fourth dose. Oral fluid was collected on day 4 with the SAL 4
| Meth dose (mg) | Specimena | Time interval(h)b | Cutoff concentration for methamphetamine/amphetamine (ng/mL) | %Detection rate ± SEM, h (range) |
|---|---|---|---|---|
| 10 | OF | 0–24 | 50/2.5 | 24.6 ± 8.1 (0.0–44.4) |
| 10 | OF | 0–24 | 2.5 (methamphetamine) | 75.0 13.1 ± (37.5–100.0) |
| 20 | OF | 0–24 | 50/2.5 | 56.4 ± 11.8 (33.3–100.0) |
| 20 | OF | 0–24 | 2.5 (methamphetamine) | 87.5 ± 12.5 (37.5–100.0) |
| 10 | Urine | 0–24 | 500/200 | 48.1 ± 13.7 (25.0–100.0) |
| 10 | Urine | 0–48 | 500/200 | 46.7 ± 16.8 (13.6–100.0) |
| 10 | Urine | 0–72a | 500/200 | 37.4 ± 21.0 (12.9–100.0) |
| 20 | Urine | 0–24 | 250/100 | 75.5 ± 11.9 (41.7–100.0) |
| 20 | Urine | 0–48 | 250/100 | 69.5 ± 13.9 (27.3–100.0) |
| 20 | Urine | 0–72a | 250/100 | 55.3 ± 15.2 (32.4–100.0) |
| 10 | Urine | 0–24 | 2.5 (methamphetamine) | 98.3 ± 1.7 (91.7–100.0) |
| 10 | Urine | 0–48 | 2.5 (methamphetamine) | 95.5 ± 4.5 (77.3–100.0) |
| 10 | Urine | 0–72a | 2.5 (methamphetamine) | 92.6 ± 7.4 (70.6–100.0) |
| 20 | Urine | 0–24 | 500/200 | 87.7 ± 8.1 (60.0–100.0) |
| 20 | Urine | 0–48 | 500/200 | 87.8 ± 7.5 (69.2–100.0) |
| 20 | Urine | 0–72 | 500/200 | 72.8 ± 5.7 (56.3–88.9) |
| 20 | Urine | 0–24 | 250/100 | 100.0 ± 0.0 (NA) |
| 20 | Urine | 0–48 | 250/100 | 98.3 ± 1.7 (91.3–100.0) |
| 20 | Urine | 0–72 | 250/100 | 86.7 ± 4.5 (75.0–100.0) |
| 20 | Urine | 0–24 | 2.5 (methamphetamine) | 100.0 ± 0.0 (NA) |
| 20 | Urine | 0–48 | 2.5 (methamphetamine) | 100.0 ± 0.0 (NA) |
| 20 | Urine | 0–72 | 2.5 (methamphetamine) | 100.0 ± 0.0 (NA) |
Meth = methamphetamine; OF = oral fluid.
Detection rates for the 10-mg dose period 0–72 h are based on an N = 4 because of missing specimens from 48–72 h.
Time interval for oral fluid collection for three subjects (S, Y, AA) was 0–8 h for the 10-mg dose and for one subject (W) at the 20-mg dose due to missing specimens or a change in type of oral fluid collection device. The remaining subjects completed the 0–24 h collection period with the same oral fluid collection device (SAL 4).
Detection rates for methamphetamine/amphetamine in urine at the DHHS confirmation cutoff concentration of 500/200 were higher during the 0–24 h period (48.1% and 87.7% for the low and high doses) than observed for oral fluid. Extending the detection periods to 0–48 and 0–72 h tended to diminish rates. At the proposed DHHS confirmation cutoff concentration of 250/100, detection rates for the 0–24 h period increased to 75.5% and 100.0% for the low and high doses and diminished only slightly for the 0–48 and 0–72 h periods. At a cutoff concentration of 2.5 ng/mL, detection rates were consistently >70% for all subjects at the low-dose and were 100% for the high-dose condition.
CONCLUSIONS
The disposition of methamphetamine and its metabolite, amphetamine, in oral fluid followed a similar time course as plasma. Proportional increases in concentration were apparent under low- and high-dosing conditions for both specimens. Higher concentrations in oral fluid were produced relative to plasma, likely as a result of the higher acidity of saliva relative to plasma. Upon repeated daily dosing, accumulation was apparent in oral fluid specimens and urine. Intrasubject variability in methamphetamine concentrations for oral fluid specimens under identical dosing and collection conditions (days 1 and 2) appeared to be relatively low, but high intersubject variability was noted. Methamphetamine detection in oral fluid and urine at conventional cutoff concentrations was assessed at the end of 4-day repeated oral dosing sessions of low- and high-dose methamphetamine. Urine testing characteristically provided relatively high detection rates for up to 3 days following drug cessation. In contrast, oral fluid testing typically provided moderate detection rates for 24 h. Detection at lower concentrations generally extended detection times for both oral fluid and urine. In general, it was observed that the use of oral fluid as a specimen for methamphetamine testing provided a shorter “window of detection.” The choice between the use of oral fluid and urine in drug testing programs should include considerations of inherent differences in time course of detection, advantage of observed oral fluid collection, and program goals.
Acknowledgments
E. J. Cone is a consultant to OraSure Diagnostics, a company that manufactures oral fluid diagnostic products.
References
- 1.Substance Abuse and Mental Health Services Administration. Results from the 2004 National Survey on Drug Use and Health: National Findings. NSDUH Series H-28, DHHS Publication No. SMA 05-4062, 1–292. 2005. Rockville, MD, Office of Applied Studies.
- 2.Maxwell JC. Emerging research on methamphetamine. Curr Opin Psychiatry. 2005;18:235–242. doi: 10.1097/01.yco.0000165592.52811.84. [DOI] [PubMed] [Google Scholar]
- 3.Beckett AH, Salmon JA, Mitchard M. The relation between blood levels and urinary excretion of amphetamine under controlled acidic and under fluctuating urinary pH values using [14C] amphetamine. J Pharm Pharmacol. 1969;21:251–258. doi: 10.1111/j.2042-7158.1969.tb08241.x. [DOI] [PubMed] [Google Scholar]
- 4.Schepers RJ, Oyler JM, Joseph RE, Jr, et al. Methamphetamine and amphetamine pharmacokinetics in oral fluid and plasma after controlled oral methamphetamine administration to human volunteers. Clin Chem. 2003;49:121–132. doi: 10.1373/49.1.121. [DOI] [PubMed] [Google Scholar]
- 5.Kim I, Oyler JM, Moolchan ET, et al. Urinary pharmacokinetics of methamphetamine and its metabolite, amphetamine following controlled oral administration to humans. Ther Drug Monit. 2004;26:664–672. doi: 10.1097/00007691-200412000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Oyler JM, Cone EJ, Joseph RE, Jr, et al. Duration of detectable methamphetamine and amphetamine excretion in urine after controlled oral administration of methamphetamine to humans. Clin Chem. 2002;48:1703–1714. [PubMed] [Google Scholar]
- 7.DHHS. Proposed revisions to mandatory guidelines for federal workplace drug testing programs. Fed Register. 2004;69:19673–19732. [Google Scholar]
- 8.DHHS. Mandatory guidelines for federal workplace drug testing programs. Fed Register. 1993;58:6062–6072. [Google Scholar]