Abstract
Background
The quality of nursing home (NH) care for residents with advanced dementia has been described as suboptimal. One relatively understudied factor in the provision of NH care is the role of private oversight and monitoring by family members and friends.
Objective
To examine the association between private oversight and the quality of end-of-life care for NH residents with advanced dementia.
Research Design
This study employed longitudinal data on 323 NH residents with advanced dementia living in 22 Boston-area facilities. Using bivariate and multivariate methods, we analyzed the association between visit time by the resident’s health care proxy (HCP) and measures of quality of end-of-life care.
Results
The relationship between visit time and quality was nonlinear. Residents who were visited 1–7 hours/weekly had less pain, fewer pressure ulcers, less dyspnea and fewer hospital transfers compared to residents who had no visits or who were visited > 7 hours/week. After adjusting for covariates, residents who were visited >7 hour/week had more pressure ulcers, more pain, greater dyspnea, fewer DNH orders, and lower HCP satisfaction with care compared with residents who were visited 1–7 hours/week.
Conclusions
Several measures of quality of NH care for end-stage dementia exhibit a nonlinear relationship with the degree of HCP oversight, such that no visiting or very high levels of visiting are associated with worse quality. Future research will need to address whether families with greater oversight tend to make decisions that promote worse quality of care, or whether worse quality of care promotes greater family oversight.
Keywords: nursing homes, family, quality of care, dementia, end-of-life care
Introduction
The nursing home (NH) sector is one of the most heavily regulated industries within the U.S. economy.(1) In spite of the high degree of government involvement within the industry, quality has generally been perceived to be substandard over the past three decades.(2) One relatively understudied factor in the provision of NH care is the role of private oversight and monitoring by family members and friends.
Monitoring of care can be difficult for NH residents. This is especially true for residents with advanced dementia. Given that these residents cannot monitor their own care, they depend on oversight from their designated health care proxies (HCPs), who are most often their family members. Over 70% of the 5 million Americans with dementia die in NHs (3, 4). Recent data suggest that the quality of care provided to these residents is not optimal.(5–11) Compared to other terminally ill NH residents, those with dementia receive less adequate pain control,(5–7) suffer from greater neglect,(12) have fewer advance directives limiting aggressive care,(8) undergo more burdensome interventions,(8) and are less likely to be referred to hospice.(9–11). To our knowledge, the role of private oversight by HCPs in the quality of end-of-life care provided to NH residents with advanced dementia has never been studied.
The conventional wisdom is that greater care oversight should improve quality of care. Previous research has tested this relationship using two general strategies. The first approach measures the degree of consumer oversight with a variable measuring “confusion” on the part of the resident. Using 1983 data from Wisconsin cost reports, Nyman found that a higher percentage of cognitively impaired residents within in a NH was not associated with the number of violations of the Medicaid certification code.(13) However, this approach does not address the individual’s degree of oversight from a family member or other legal guardian. A second approach measures the degree of family contact directly. Using data from the National Long-Term Care Survey merged with Medicare claims, Chou found that NH residents were less likely to experience death, pressure ulcers, dehydration, or urinary tract infections when a family member or friend visits them in the first month of their stay.(14) A limitation with this approach is that unobserved individual or facility attributes may be correlated with both the amount of family contact and the quality of care. Furthermore, visitation frequency by family members to NH residents changes over time,(15, 16) and therefore needs to be followed beyond the initial admission period when the residents’ health status may be relatively unstable.
In order to address the aforementioned methodological limitations of prior studies of family oversight, and to examine the role of HCP oversight in the quality of care provided to advanced dementia patients, we employed data from the CASCADE study (Choices, Attitudes, Strategies, and Care for Advanced Dementia at the End-of-Life). The study followed a cohort of 323 NH residents with advanced dementia living in 22 Boston-area and their HCPs prospectively for 18 months or until the resident’s death. The main objective was to examine the association between the duration of weekly HCP visits (i.e., oversight) to the resident and measures indicative of quality end-of-life care.
METHODS
Data
The study population was comprised of both NH residents with advanced dementia and their HCPs. From February 1, 2003, until September 30, 2006, NH residents with advanced dementia living in 22 Boston-area facilities were recruited as part of the ongoing prospective cohort Choices, Attitudes and Strategies for Care of Advanced Dementia at the End-of-Live (CASCADE) study.(17) The number of residents recruited from each NH ranged from 1 to 49 with an average of 14.7 residents per home. Relative to all U.S. NHs, the CASCADE facilities were more likely to be larger, nonprofit-owned and have a special care dementia unit, but they were similar in terms of demographics, staffing and quality-of-care.(17) The overarching goal of the CASCADE study was to describe multiple dimensions of the end-of-life experience for NH residents with advanced dementia and their families. Data available for this report were derived from the final cohort of 323 recruited residents and their HCPs, however follow-up data collection remains ongoing for a small subset of residents (N < 30).
With the objective of identifying a cohort with advanced dementia, residents’ most recent Minimum Data Set assessments were used to identify those with a Cognitive Performance Scale score of 5 or 6, indicating severe to very severe cognitive impairment.(18) Once identified, the charts of these residents underwent screening for full eligibility. Criteria included [i] being age of 60 years or older, [ii] a length of stay of 30 days or longer, [iii] cognitive impairment due to dementia, [iv] Global Deterioration Scale score of 7,(19) and [v] an appointed HCP who could communicate in English. The diagnosis of dementia was confirmed with the resident’s physician if it was ambiguous in the record. At a Global Deterioration Scale of 7, residents with dementia are characterized by very severe cognitive decline, minimal to no verbal communication, dependence in eating and toileting, incontinence of urine and stool, and loss of the ability to walk.(19) Residents were excluded if they were in a subacute or short-term rehabilitative unit; had cognitive impairment due to stroke, traumatic brain injury, tumor, or a chronic psychiatric condition; or were in a coma. Finally, participant facilities were required to have at least 60 beds and be located within a 60-mile radius of Boston.
An information sheet describing the study was mailed to the HCPs of eligible residents. Unless the HCPs requested no further contact, they were telephoned one week later to obtain informed consent. HCPs provided consent for both themselves and for the residents with advanced dementia.
Resident assessments involved a chart review, a nursing interview, and a brief clinical examination, and were conducted at baseline and quarterly thereafter for up to 18 months or until death. Resident assessments and HCP interviews were also conducted following the death of those residents who died during the follow-up period. Information collected in these assessments pertained only to the very last week of life. The immediate dying process is unique both in terms of the intensity of HCP visitation and quality of care variables (e.g., pain), and therefore, not generalizable to “usual” circumstances. Thus, the post-death resident assessments and HCP interviews were excluded from these analyses.
Variables
Baseline resident characteristics obtained from the chart included: age, gender, and race. General health status and disease severity was quantified during a nursing interview using the Bedford Alzheimer’s Nursing Severity-Subscale (BANS-S) (range, 7–28 with higher scores indicating worse health status and greater disease severity).(20) HCP characteristics measured at the baseline interview included: age, gender, marital status (married vs. not married), educational level (high school or greater vs. less than high school, and relationship to the resident (child vs. other).
As a measure of family oversight, the number of hours a week that the HCP spent visiting the resident at the NH was ascertained from the HCP at baseline and at each follow-up assessment. The response categories were: no visits, 1–7 hours/week of visits and greater than 7 hours/week of visits.
From the CASCADE dataset, we selected outcome variables that have been endorsed as measures of the quality of end-of-life care for older persons (21), and specifically for those with end-stage dementia (22). These measures can be placed into the following categories: physical symptoms, advance care planning, medical treatments, locus of care, and family satisfaction (22). In the area of physical discomfort, whether or not the resident experienced pain (at least 5 days/month), or dyspnea (at least 5 days/month) was determined from a review of the medical record. Existing pressure ulcers of a stage 2 or higher were ascertained from a nurse interview.(23) The documentation of a do-not-hospitalize (DNH) order was used as a measure of advance care planning. In the area of medical treatments, the use of antipsychotics was determined from the medication administration record. Whether or not the resident experienced a hospital transfer (hospitalization or emergency room visit) was used as a measure of locus of care. Satisfaction with care was measured at each HCP interview using the Satisfaction with Care at the End-of-Life in Dementia Scale (SWC-EOLD) (24, 25); a validated scale that ranges form 10–40, with higher scores indicating greater satisfaction. Finally, whether or not the resident was ever referred to hospice was ascertained from the medical record.
Certain variables were point-in-time measures determined at the baseline assessment and all other time periods (pressure ulcers, DNH order, and the SWC-EOLD). Other measures reflected events that occurred in the 3 months since the prior assessment (pain, dyspnea, hospice referral, hospital transfer, antipsychotic use), and therefore were not measured at baseline.
Statistical Analysis
Baseline resident and HCP characteristics were described using means for continuous variables and proportions for categorical variables for the 323 residents and HCPs. For the remaining analyses, the unit of analyses was the multiple assessments conducted during the entire 18-month follow-up period, with the baseline subject characteristics carried forward. Descriptive analyses were also conducted to present the amount of HCP visit time (hours per week), and occurrence of the quality of care measures.
Bar graphs were used to display the occurrence of the quality of care measures stratified by weekly HCP visit time as follows: none, 1–7 hours and greater than 7 hours. Multivariate models were constructed for each individual quality of care measure, using 1–7 hours as the referent group. All models were adjusted for the resident and HCP baseline characteristics contained in Table 1. We included person-level random effects to account for any unobserved factors at the resident level and clustering of multiple observations within individuals. We also controlled for facility and year dummies to account for any unobserved facility factors and time trends in our outcomes of interest. For example, the facility dummies might account for the presence of an active family council in a given facility, while the time dummies might account for changes in nursing home policy over time. The satisfaction model was estimated using least squares, while the other models with binary outcomes were estimated using logistic regression.
Table 1.
Baseline resident and HCP characteristics (N = 323)
| Variable | Mean | Std. Dev. |
|---|---|---|
| Nursing home resident characteristics | ||
| Female | 0.854 | 0.354 |
| Age (years) | 85.3 | 7.5 |
| White | 0.894 | 0.308 |
| BANS-S | 21.0 | 2.3 |
| Health care proxy characteristics | ||
| Female | 0.640 | 0.481 |
| Child of nursing home resident | 0.675 | 0.470 |
| Age (years) | 60.0 | 11.6 |
| Education above high school | 0.755 | 0.431 |
Notes: BANS-S = Bedford Alzheimer Nursing Scale – Severity Subscale (possible scores 7–28; higher scores indicate greater functional disability)
RESULTS
Among the 1,763 NH residents screened for the study, 570 (32.3%) residents met eligibility criteria. Among those eligible, 323 (56.7%) residents with advanced dementia and their HCPs were recruited into the study and included in these analyses. This recruitment rate is slightly higher than previous studies involving interviews of family members about the end-of-life experience of a loved one.(26–28) Importantly, non-recruited eligible residents did not differ significantly from those recruited with respect to mean age or gender. In addition to the 323 baseline assessments, there were 1,126 follow-up assessments during the course of the study, of which 149 death assessments were excluded, for a total of 1,300 assessments available for analysis. Owing to intermittent quarter interviews when the HCP could not be contacted, missing data on HCP visits caused us to exclude another 175 “follow-up” assessments for a final sample of 1,125 assessments across 323 resident-HCP dyads.
The baseline resident sample was predominantly female (85.4%) and white (89.4%) with a mean age of 85.3 years (see Table 1). The average BANS-S score was 21.0 (SD = 2.3), reflecting a high level of functional disability. The HCPs were approximately two-thirds female, with the majority having some education beyond high school (75.5%) and a mean age of 60.0 years. The majority of HCPs (67.4%) were the children of the nursing home residents.
Across the 1,125 baseline and follow-up interviews, HCPs indicated that they spent the following amount of time visiting the residents each week: none, 11.6%; 1–7 hours, 70.2%; and, greater than 7 hours, 18.1% (see Table 2). Across the baseline and follow-up assessments, the rate of pressure ulcers (stage 2 or higher) was 9.7% and the frequency of a DNH order was 52.5%. The average satisfaction score on the SWC-EOLD scale was 31.7 (median=31; SD = 4.6), and was normally distributed. The occurrence of other end-of-life outcomes on the follow-up assessments were as follows, pain, 7.7%; dyspnea, 5.9%; hospice use, 6.6%; hospital transfer, 6.7%; and antipsychotic use, 19.9%.
Table 2.
Duration of weekly visits by health care proxies (HCPs) and palliative care quality measures for nursing residents with advanced dementia (N=1,125 assessments)
| Variable | Mean | Std. Dev. |
|---|---|---|
| Duration of HCP visits per week | ||
| None | 0.116 | 0.321 |
| 1–7 hours | 0.702 | 0.457 |
| >7 hours | 0.181 | 0.385 |
| Nursing home quality measures | ||
| Pressure ulcer (stage 2 or higher) at time of survey | 0.097 | 0.296 |
| Pain (5 days/month) since previous survey | 0.077 | 0.267 |
| Dyspnea (5 days/month) since previous survey | 0.059 | 0.235 |
| Hospice use since previous survey | 0.066 | 0.248 |
| Hospital transfer since previous survey | 0.067 | 0.251 |
| Do-not-hospitalize order at time of survey | 0.525 | 0.500 |
| HCP Satisfaction (SWC-EOLD scale) at time of survey | 31.7 | 4.6 |
| Antipsychotic use since previous survey | 0.199 | 0.400 |
Notes: SWC-EOLD=Satisfaction with Care at the End-of-Life in Dementia Scale (ranges form 10–40, with higher scores indicating greater satisfaction).
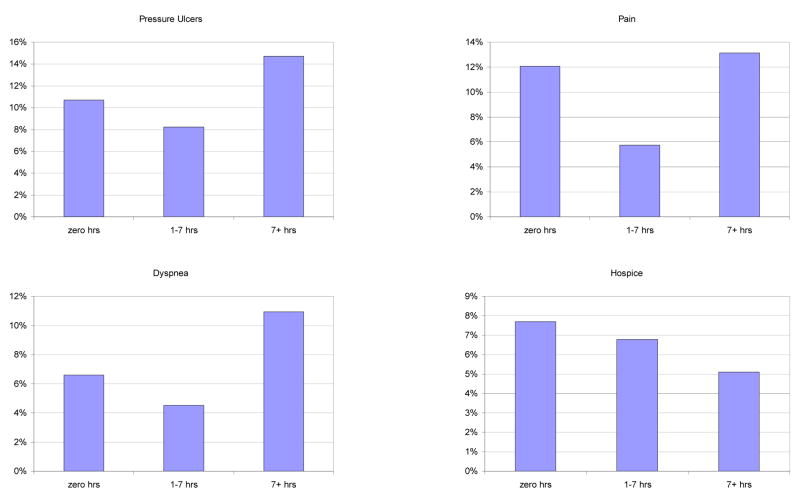
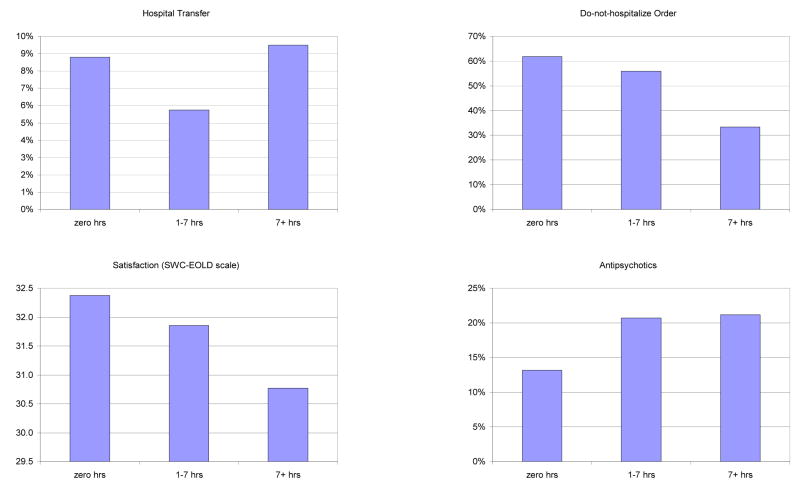
The bar graphs in Figure 1 displays the unadjusted associations between HCP visit time and each quality of care measure. For four of the outcomes, this was ‘J-shaped’. Compared to residents who received no visits or residents with > 7 hours/week of visits, residents with 1–7 hours of visits a week had better quality of care as measured by fewer pressure ulcers, less daily pain, less dyspnea, and lower likelihood of a hospital transfer. Lower visit time was monotonically associated with worse quality for other four outcomes: lower hospice use, fewer DNH orders, lower HCP satisfaction with care, and greater use of antipsychotics. That is, relative to residents with no visits, residents had somewhat worse quality with 1–7 hours/week of visits and even worse quality with >7 hours/week of visits.
Figure 1.
Intensity of weekly healthcare proxy visits and quality of nursing home care
Table 2 presents the results of the multivariate analysis for all eight quality measures. Given the J-shaped distribution for certain outcomes in Figure 1, the reference category in the presentation of the HCP visit results is having 1–7 hours/week of visits from the HCP. Once again, these analyses incorporate random effects at the person level and fixed effects at the facility level to account for unobserved factors. There were no significant differences in end-of-life outcomes between individuals receiving no visits and individuals receiving 1–7 hours/week of visits from the HCP. However, quality was significantly (p<0.05) worse for individuals receiving greater than 7 hours of visits/week relative to individuals receiving 1–7 hours/week across 5 of the 8 quality measures. Specifically, individuals with greater than 7 hours/week of visits were 2.0 times as likely to experience a pressure ulcer, 2.9 times as likely to experience pain, 5.0 times as likely to experience dyspnea, and 0.34 times as likely to have a DNH order in place. Moreover, HCPs who visited >7 hours/week provided a 1.01 (or 3.2%) lower satisfaction score relative to HCPs who visited 1–7 hours/week.
DISCUSSION
Oversight by family members and friends is an important, but understudied, aspect of the U.S. health care system. In this study of NH residents with advanced dementia, quality of care across several measures was highest in our bivariate analyses for those residents whose HCPs visited 1–7 hours per week, compared to those were never visited or who received over 7 hours of weekly visits. In our multivariate models, there were no outcome variables for which HCP visits > 7 hours/week was associated with better quality of care. Most notably, HCPs who visited the most each week were the least satisfied with care and the NH residents they visited were more likely to have pressure ulcers, pain, dyspnea and no DNH order. One possible explanation for these results is that both minimal and excessive family oversight contributes to worse quality of care for advanced dementia residents. Alternatively, it is possible that poor quality and hence, more dissatisfaction with care, promotes the need for greater family oversight. Regardless of the direction of causality, our results suggest that high levels of HCP visit time can serve as a potential marker for quality of care problems, which may be useful to government surveyors, ombudspersons and facility management.
To our knowledge, this is the first study to examine the association between the amount of family visits and the quality of care in advanced dementia. Therefore, comparisons with the literature are limited. Nonetheless, the distribution of HCP visits was similar to that reported in other studies from the NH setting.(16, 29) At one extreme, our bivariate finding that no visit from the HCP was associated with worse quality of care is consistent with other studies in the general NH population.(14) Regardless of HCP oversight, there is no justifiable explanation for NHs not to provide comparable treatment to all residents aimed at minimizing physical discomfort (i.e., pain, dyspnea, pressure ulcers).
No prior reports of which we are aware have found that excessive family oversight is associated with reduced satisfaction with care, worse quality of physical care (i.e., pressure ulcers, pain, dyspnea), or more aggressive treatment decisions (i.e., no DNH order). There are two possible explanations for these findings. First, HCPs who visit very often may be inherently more dissatisfied, opt for more aggressive care, and promote worse quality of care. In support of this notion, Kidder and colleagues suggest that there may be a threshold at which family involvement ceases to be constructive and may even become counter-productive.(30) Families with excessive oversight and complaints may lead to avoidance behavior by staff, and consequently lead to worse care. In extreme cases, Kidder and colleagues further postulate that excessive family involvement may reflect psychological problems in the family member or family system.
The second possible explanation is that HCPs with more concerns that care is suboptimal are compelled to provide greater oversight. Family members view monitoring care as one of the main factors motivating their involvement with NH residents.(31) Thus, it is not surprising that greater family anxiety is reported to be associated with higher visitation frequency.(29) Inadequate communication is a major source of dissatisfaction among family members in the NH setting.(27) As such, greater visitation frequent may reflect the HCPs’ attempt to improve communication.
Unfortunately, the current study does not allow us to clarify the direction of causality between greater HCP visits and NH quality. A randomized trial of family oversight is not feasible. However, qualitative studies involving in-depth interviews with HCPs is one approach to better understand the association between family involvement and the quality of NH care.(29, 31) Another idea is to examine the timing of changes in HCP visits relative to the timing of changes in quality. Depending on the quality measure, our data supported both a change in quality preceding a change in HCP visits and a change in HCP visits preceding a change in quality. Moving forward, instrumental variables is a statistical approach that has been used to address similar issues of endogeniety.(32) An example of an instrumental variable is the distance from the HCP’s residence to the NH. However, in the CASCADE study, 90% of HCPs lived within one hour of the facility, and thus, it was not a suitable instrument in this context, but it may be considered for future research on this issue.
This paper is limited in several ways. First, visit duration was determined from HCP self-reports, and therefore subject to recall bias. Prior work suggests that estimates of family visitation are higher when reported by family members compared to staff.(33) Second, HCP visits may not provide an accurate measure of the degree of family involvement as family members may provide oversight through other means (e.g., telephone conversations with the staff), and data were not collected regarding oversight by individuals other than the HCPs (i.e., other relatives or friends). Third, the results of this study are limited to NH residents with severe dementia. Although, these individuals provide an ideal population in which to isolate the role of informal oversight, the results may not extend to other NH residents, particularly to those with intact cognition. Finally, our sample is drawn from 22 Boston-area NHs, focusing on a predominantly white sample. Thus, our findings may not be generalizable to other regions of the country or residents from other racial groups.
In sum, the visitation of NH residents with advanced dementia by their HCPs varies, and this variability is associated with the quality of care that these residents receive. Most notably, our multivariate results suggest that high levels of family visits are associated with worse quality of care. Future research needs to clarify the association between excessive family involvement and poor quality measures by addressing potential endogeneity into study designs. In the meanwhile, efforts should continue by NH providers and policy-makers to encourage the positive aspects of family involvement and constructive collaborations between families and staff towards the goal of providing the highest quality care for residents with advanced dementia.(34)
Table 3.
Multivariate analysis: Association between health care proxy visits and quality of care measures among nursing residents with advanced dementia
| Pressure Ulcer ≥ stage 2 | Pain > 5 days/month | Dyspnea >5 days/month | Hospice | Hospital Transfer | DNH order | Antipsychotics | SWC-EOLD Scale | |
|---|---|---|---|---|---|---|---|---|
| No visits | 0.69 (0.34) | 1.73 (0.89) | 1.90 (1.34) | 0.12 (0.20) | 1.29 (0.74) | 0.96 (0.36) | 1.30 (0.92) | 0.39 (0.46) |
| 1–7 hours/week | --- | --- | --- | --- | --- | --- | --- | --- |
| > 7 hours/week | 2.02* (0.64) | 2.89** (1.16) | 5.04** (2.73) | 0.94 (1.02) | 1.04 (0.47) | 0.34** (0.10) | 2.65 (2.26) | −1.01** (0.35) |
|
| ||||||||
| N | 1,115 | 796 | 796 | 796 | 796 | 1,115 | 796 | 1,115 |
Notes: Estimates based on regressions controlling for the resident and health care proxy covariates in Table 1, a random effect at the person level, and facility and year fixed effects. All models were estimated using logistic regression except for the satisfaction model, which was estimated using least squares. The logistic regression results are presented as odds ratios. Standard errors are presented in parentheses. DNH = do-not-hospitalize; SWC-EOLD=Satisfaction with Care at the End-of-Life in Dementia Scale (ranges form 10–40, with higher scores indicating greater satisfaction).
= statistically significant at 5% level;
= statistically significant at 1% level.
References
- 1.Walshe K. Regulating U.S. nursing homes: are we learning from experience? Health Aff (Millwood) 2001;20:128–144. doi: 10.1377/hlthaff.20.6.128. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine. Improving the quality of long-term care. Washington, D.C: National Academy Press; 2001. [Google Scholar]
- 3.Mitchell SL, Teno JM, Miller SC, et al. A national study of the location of death for older persons with dementia. J Am Geriatr Soc. 2005;53:299–305. doi: 10.1111/j.1532-5415.2005.53118.x. [DOI] [PubMed] [Google Scholar]
- 4.Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 5.Won A, Lapane K, Gambassi G, et al. Correlates and management of nonmalignant pain in the nursing home. SAGE Study Group. Systematic Assessment of Geriatric drug use via Epidemiology. J Am Geriatr Soc. 1999;47:936–942. doi: 10.1111/j.1532-5415.1999.tb01287.x. [DOI] [PubMed] [Google Scholar]
- 6.Bernabei R, Gambassi G, Lapane K, et al. Management of pain in elderly patients with cancer. SAGE Study Group. Systematic Assessment of Geriatric Drug Use via Epidemiology. Jama. 1998;279:1877–1882. doi: 10.1001/jama.279.23.1877. [DOI] [PubMed] [Google Scholar]
- 7.Miller SC, Mor V, Wu N, et al. Does receipt of hospice care in nursing homes improve the management of pain at the end of life? J Am Geriatr Soc. 2002;50:507–515. doi: 10.1046/j.1532-5415.2002.50118.x. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell SL, Kiely DK, Hamel MB. Dying with advanced dementia in the nursing home. Arch Intern Med. 2004;164:321–326. doi: 10.1001/archinte.164.3.321. [DOI] [PubMed] [Google Scholar]
- 9.Casarett DJ, Hirschman KB, Henry MR. Does hospice have a role in nursing home care at the end of life? J Am Geriatr Soc. 2001;49:1493–1498. doi: 10.1046/j.1532-5415.2001.4911242.x. [DOI] [PubMed] [Google Scholar]
- 10.Hanrahan P, Luchins DJ. Access to hospice programs in end-stage dementia: a national survey of hospice programs. J Am Geriatr Soc. 1995;43:56–59. doi: 10.1111/j.1532-5415.1995.tb06243.x. [DOI] [PubMed] [Google Scholar]
- 11.Christakis NA, Escarce JJ. Survival of Medicare patients after enrollment in hospice programs. N Engl J Med. 1996;335:172–178. doi: 10.1056/NEJM199607183350306. [DOI] [PubMed] [Google Scholar]
- 12.Kayser-Jones J. The experience of dying: an ethnographic nursing home study. Gerontologist. 2002;42(Spec No 3):11–19. doi: 10.1093/geront/42.suppl_3.11. [DOI] [PubMed] [Google Scholar]
- 13.Nyman JA. Excess demand, consumer rationality, and the quality of care in regulated nursing homes. Health services research. 1989;24:105–127. [PMC free article] [PubMed] [Google Scholar]
- 14.Chou SY. Asymmetric information, ownership and quality of care: an empirical analysis of nursing homes. J Health Econ. 2002;21:293–311. doi: 10.1016/s0167-6296(01)00123-0. [DOI] [PubMed] [Google Scholar]
- 15.Gaugler JE, Zarit SH, Pearlin LI. Family involvement following institutionalization: modeling nursing home visits over time. Int J Aging Hum Dev. 2003;57:91–117. doi: 10.2190/8MNF-QMA3-A5TX-6QQ3. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto-Mitani N, Aneshensel CS, Levy-Storms L. Patterns of family visiting with institutionalized elders: the case of dementia. J Gerontol B Psychol Sci Soc Sci. 2002;57:S234–246. doi: 10.1093/geronb/57.4.s234. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell SL, Kiely DK, Jones RN, et al. Advanced dementia research in the nursing home: the CASCADE study. Alzheimer Dis Assoc Disord. 2006;20:166–175. doi: 10.1097/00002093-200607000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris JN, Fries BE, Mehr DR, et al. MDS Cognitive Performance Scale. J Gerontol. 1994;49:M174–M182. doi: 10.1093/geronj/49.4.m174. [DOI] [PubMed] [Google Scholar]
- 19.Reisberg B, Ferris SH, de Leon MJ, et al. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 20.Volicer L, Hurley AC, Lathi DC, et al. Measurement of severity in advanced Alzheimer’s disease. J Gerontol. 1994;49:M223–226. doi: 10.1093/geronj/49.5.m223. [DOI] [PubMed] [Google Scholar]
- 21.Lynn J. Measuring quality of care at the end of life: a statement of principles. J Am Geriatr Soc. 1997;45:526–527. doi: 10.1111/j.1532-5415.1997.tb05184.x. [DOI] [PubMed] [Google Scholar]
- 22.Teno JM, Landrum K, Lynn J. Defining and measuring outcomes in end-stage dementia. Alzheimer Dis Assoc Disord. 1997;11 (Suppl 6):25–29. [PubMed] [Google Scholar]
- 23.National Pressure Ulcer Advisory Panel. Pressure ulcers prevalence, cost and risk assessment: consensus development conference statement. Decubitus. 1989;2:24–28. [PubMed] [Google Scholar]
- 24.Volicer L, Hurley AC, Blasi ZV. Scales for evaluation of End-of-Life Care in Dementia. Alzheimer Dis Assoc Disord. 2001;15:194–200. doi: 10.1097/00002093-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Kiely DK, Volicer L, Teno J, et al. The validity and reliability of scales for the evaluation of end-of-life care in advanced dementia. Alzheimer Dis Assoc Disord. 2006;20:176–181. doi: 10.1097/00002093-200607000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinhauser KE, Christakis NA, Clipp EC, et al. Factors considered important at the end of life by patients, family, physicians, and other care providers. Jama. 2000;284:2476–2482. doi: 10.1001/jama.284.19.2476. [DOI] [PubMed] [Google Scholar]
- 27.Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. Jama. 2004;291:88–93. doi: 10.1001/jama.291.1.88. [DOI] [PubMed] [Google Scholar]
- 28.Prigerson HG, Frank E, Kasl SV, et al. Complicated grief and bereavement-related depression as distinct disorders: preliminary empirical validation in elderly bereaved spouses. Am J Psychiatry. 1995;152:22–30. doi: 10.1176/ajp.152.1.22. [DOI] [PubMed] [Google Scholar]
- 29.Lindman Port C. Identifying changeable barriers to family involvement in the nursing home for cognitively impaired residents. Gerontologist. 2004;44:770–778. doi: 10.1093/geront/44.6.770. [DOI] [PubMed] [Google Scholar]
- 30.Kidder SW, Smith DA. Is there a conflicted surrogate syndrome affecting quality of care in nursing homes? J Am Med Dir Assoc. 2006;7:168–172. doi: 10.1016/j.jamda.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Davies S, Nolan M. ‘Making it better’: self-perceived roles of family caregivers of older people living in care homes: a qualitative study. Int J Nurs Stud. 2006;43:281–291. doi: 10.1016/j.ijnurstu.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Grootendorst P. A review of instrumental variables estimation of treatment effects in the applied health sciences. Health Services and Outcomes Research Methodology. 2007;7:159–179. [Google Scholar]
- 33.Port CL, Hebel JR, Gruber-Baldini AL, et al. Measuring the frequency of contact between nursing home residents and their family and friends. Nurs Res. 2003;52:52–56. doi: 10.1097/00006199-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Lau WY, Shyu YI, Lin LC, et al. Institutionalized elders with dementia: collaboration between family caregivers and nursing home staff in Taiwan. J Clin Nurs. 2008;17:482–490. doi: 10.1111/j.1365-2702.2007.01955.x. [DOI] [PubMed] [Google Scholar]