Summary
In both mammals and insects, neurons involved in learning are strongly modulated by the inhibitory neurotransmitter GABA. The GABAA receptor, Resistance to dieldrin (Rdl), is highly expressed in the Drosophila mushroom bodies (MBs), a group of neurons playing essential roles in insect olfactory learning. Flies with increased or decreased expression of Rdl in the MBs were generated. Olfactory associative learning tests showed that Rdl over-expression impaired memory acquisition but not memory stability. This learning defect was due to disrupting the physiological state of the adult MB neurons rather than causing developmental abnormalities. Remarkably, Rdl knockdown enhanced memory acquisition but not memory stability. Functional cellular imaging experiments showed that Rdl over-expression abolished the normal calcium responses of the MBs to odors while Rdl knock-down increased these responses. Together, these data suggest that RDL negatively modulates olfactory associative learning, possibly by gating the input of olfactory information into the MBs.
Introduction
Neurons comprising the neural circuits that mediate they are modulated by the inhibitory neurotransmitter gamma-amino butyric acid (GABA). For instance, the hippocampus, which is involved in the formation of multiple types of memories in mammalian organisms, is densely innervated by GABAergic interneurons (Freund et al., 1996). The insect mushroom bodies (MBs), which similarly are involved in the formation of multiple types of memories, are also subject to GABAergic modulation (Perez-Orive et al., 2002; Yasuyama et al., 2002). These and other similar observations make clear that a deep understanding of the molecular and systems neuroscience properties that underlie memory formation will not emerge until a detailed knowledge of when and where GABAergic modulation occurs and how this modulation alters the function of the cells and networks that mediate memory formation.
GABAA receptors are GABA gated chloride channels. Accumulating pharmacological and genetic evidence suggests that GABAA receptors participate in the cellular and circuit mechanisms underlying learning and memory, but the current information is inconsistent and lacks depth. Several prior studies have used intraperitoneal or intracerebroventricular injection of GABAA receptor agonists or antagonists and monitored effects on behavior (McNamara et al., 1993; Anglade et al., 1994; Chambers et al; 2003; Zarrindast et al., 2004). However, the widespread effects caused by this approach make it impossible to assign behavioral changes to any specific population of neurons. Better spatial resolution for the pharmacological effects has been achieved by injecting drugs into specific brain regions either before or after training or just prior to testing and in several cases, receptor agonists have inhibited behavioral performance and antagonists have facilitated it (Jasnow and Huhman, 2001; Zarrindast et al; 2002; Huff et al., 2005; Van Nobelen and Kokkinidis et al., 2006). However, these studies fail to provide information regarding the specific cell type affected within the targeted region and the identity of the targeted GABA receptor. Furthermore, they provide no information about how the pharmacological agents affect the information processing relevant to learning mechanisms by the neurons. Moreover, the simplistic idea that GABAA receptor agonists and antagonists/inverse agonists may decrease and increase behavioral performance, respectively, remains controversial because of reports to the contrary (Chrobak and Napier, 1992; Morón et al., 2002).
Genetic dissections of GABAA receptor function using viable knockouts have provided more specific information regarding the receptor type involved, but lack information about how information processing is altered, the neurons involved in the behavior being tested, and whether the behavioral results are due to a physiological disruption of GABAA function or a developmental insult secondary to the developmental loss of the receptor. Moreover, the controversies regarding the direction of behavioral change (improve vs impair) with decreased receptor function remain. For instance, DeLorey et al (1998) reported that GABAAβ3 knock-out mice showed impaired performance several days after training in a step-through passive avoidance task and contextual fear conditioning, but Collinson et al (2002) and Crestani et al (2002) reported GABAAα5 mutant mice to have enhanced performance in a match-to-place version of the water maze test and in trace fear conditioning, respectively. Although genetic dissections point to the inadequacy of pharmacological manipulations by emphasizing receptor-specific functions, the use of whole animal knockouts fails to offer reliable conclusions about where and how GABAA receptors influence the complex neural circuitry underlying learning and memory.
We chose to probe the role of GABAergic modulation using Drosophila olfactory learning as a model because of the ability to bidirectionally alter the expression of specific GABAA receptors in identified populations of neurons of the adult and to probe how these modulations alter the information processing capabilities of the neurons. In Drosophila, at least three genes are thought to encode GABAA receptors: Rdl (resistance to dieldrin), Grd (GABA and glycine-like receptor of Drosophila) and Lcch3 (ligand-gated chloride channel homologue 3). Rdl is by far the best characterized of the three molecularly and through functional expression experiments (Hosie et al., 1997; Buckingham et al., 2005). RDL also has an important role in insecticide resistance (ffrench-Constant et al., 2004). This receptor is highly expressed in the Drosophila antennal lobes (ALs) and the MBs (Harrison et al., 1996), both of which are essential structures required for the acquisition, storage and retrieval of olfactory memory (Davis, 2004; Davis, 2005; Liu and Davis, 2006; Krashes et al., 2007).
One attractive idea for the role of GABAergic inhibition of neurons involved in learning is that the inhibition serves to sparsen sensory representations to make learning easier and recall faster (Perez-Orive et al., 2002; Olshausen and Field, 2004). The projection neurons in the insect AL receive information about odors from olfactory receptor neurons in the antennae and transmit this information to higher-order structures including the MBs and the lateral horn (LH). The AL projection neurons exhibit robust firing when the animal senses an odor, but the robustness of the response in the postsynaptic MB neurons is sparsened because of postulated feed-forward GABAergic inhibition received from the LH (Perez-Orive et al., 2002). Unfortunately, there remains no direct experimental evidence in favor of or against this hypothesis.
In this study, we generated flies with elevated or decreased expression of Rdl in the MBs and assayed the learning performance of these flies along with the calcium responses in the MBs produced by odor and electric shock stimulation. Our results indicate that the level of memory acquisition is inversely related to the level of RDL expression in the MB neurons, indicating that RDL in the MBs inhibits olfactory learning. This inhibition of learning is due to the expression level of Rdl at the time of learning, rather than to developmental alterations that may occur in the neural circuit from perturbing Rdl expression during development. Furthermore, the calcium response of MB neurons to odor stimulation is also inversely related to the level of Rdl expression in these neurons, indicating that the expression level of Rdl gates the receipt of information about the conditioned stimulus during olfactory learning.
Results
Rdl Over-expression and Knock-down in the Drosophila MBs
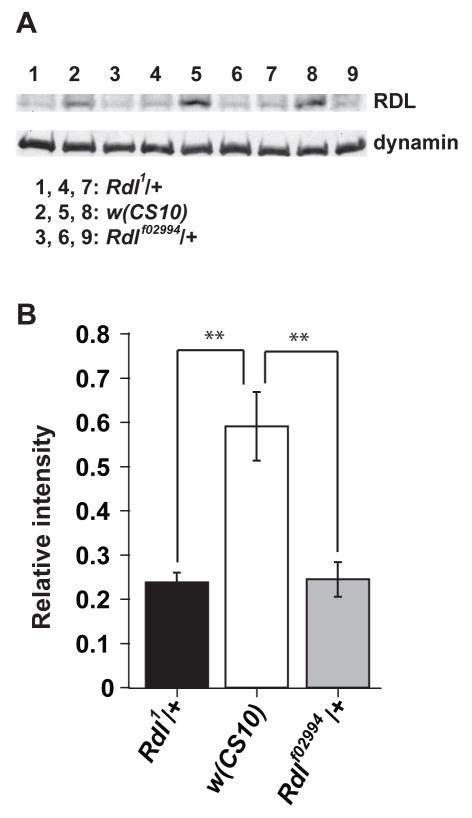
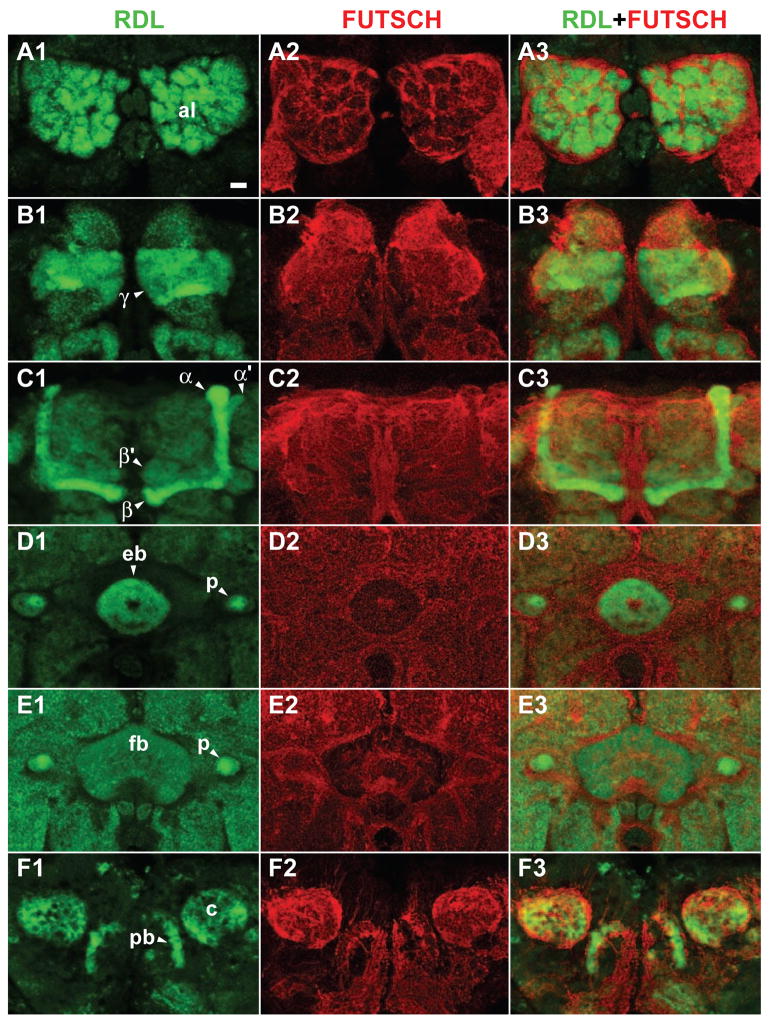
We began by verifying the reported expression pattern (Harrison et al., 1996) of Rdl in the adult Drosophila brain. We developed a polyclonal antibody that recognizes RDL protein by immunoblotting (Figure 1). The abundance of this protein was reduced in flies heterozygous for two different null and homozygous lethal alleles of Rdl, Rdl1 and Rdlf02994, confirming the ability of this antibody to detect the RDL protein. We then carefully characterized the expression pattern of Rdl in the central brain by immunohistochemistry. The RDL protein was detected throughout the ALs, the MBs and the central complex (Figure 2). In the MBs, RDL was detected both in the dendrites (calyces, Figure 2E) and the axons (α, α′, β, β′, γ lobes and peduncles, Figure 2B–D), but no RDL signal was observed in the cell bodies of MB neurons.
Figure 1. Anti-RDL antiserum recognized RDL protein on Western blots.
(A) Western blots showing anti-RDL signals (about 65 kD) and anti-dynamin loading controls. A total protein extract from 3 fly heads was loaded in each lane, with 3 independent samples (n=3) for each genotype. Lanes 1,4,7: Rdl1/+. Lanes 2, 5, 8: w(CS10). Lanes 3, 6, 9: Rdlf02994/+.
(B) Quantification of the relative Rdl expression level using the grayscale intensity of the anti-RDL signal normalized to the anti-dynamin signal for each genotype. Means ± SEM are shown. **: P<0.01.
Figure 2. Rdl expression in the brain.
(A1)-(F3) Frontal confocal images of the brain from anterior to posterior. Panels A1 through F1 show anti-RDL immunostaining. Panels A2 through F2 show counter-staining with anti-FUTSCH (22C10). Panels A3 through F3 show merged images. Scale bar = 10 μm.
(A1) Anti-RDL immunostaining of antennal lobe glomeruli (al).
(B1) Anti-RDL immunostaining of the axons of the γ MB neurons (γ lobes).
(C1) Anti-RDL immunostaining of the axon branches of the α/β and α′/β′ MB neurons (α/β and α′/β′ lobes, respectively).
(D1) Anti-RDL immunostaining of the axons of MB neurons in the peduncles (p) and of processes in the ellipsoid body (eb) of the central complex.
(E1) Anti-RDL immunostaining of MB axons in the peduncles (p) and of processes in the fan-shaped body (fb) of the central complex.
(F1) Anti-RDL immunostaining of the calyces of the MBs, which house the dendrites of these neurons, and of processes in the protocerebral bridge (pb) of the central complex.
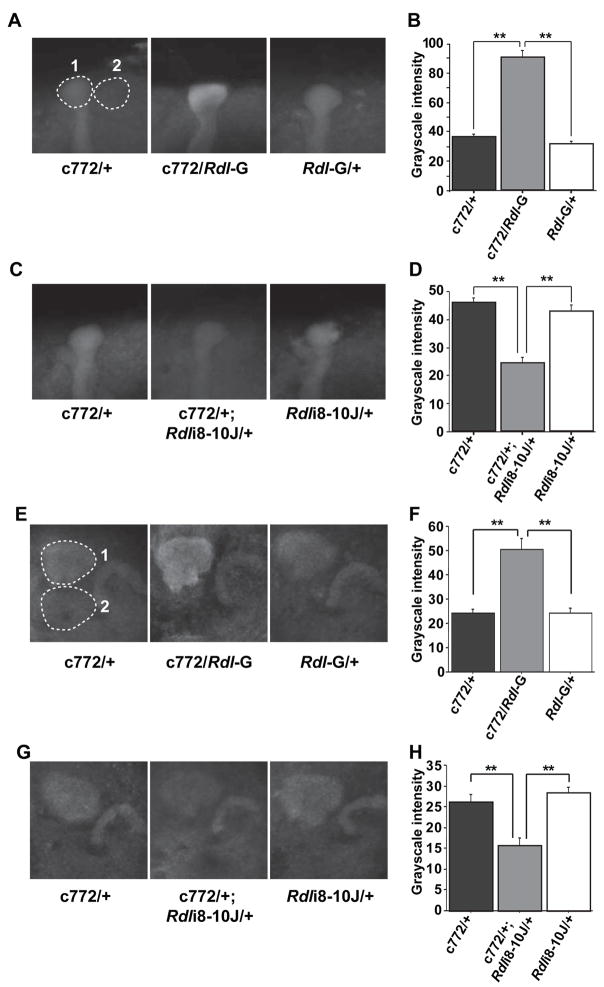
Since Rdl is expressed in the MBs, we decided to alter the Rdl expression levels in the MBs using the Gal4-UAS binary expression system in Drosophila (Duffy, 2002) with expression of transgenes for Rdl or RNA interference (RNAi) constructs to Rdl. RDL has been reported to form functional homo-oligomeric GABA gated chloride channels when expressed in Xenopus oocytes (Buckingham et al., 1994) and in an insect cell line (Lee et al., 1993). Transgenic flies carrying the full length Rdl cDNA down stream of UAS sequences were constructed. In total, seven transgenic lines carrying UAS-Rdl constructs inserted at different genomic locations were generated. We randomly selected two lines, Rdl-G (inserted on the second chromosome) and Rdl-K (inserted on the X chromosome), and crossed them to 11 different Gal4 lines, most of which drive gene expression preferentially in the adult MBs. Among these Gal4 lines tested (see Experimental Procedures), only one line, c772-Gal4 (Zars et al., 2000), produced viable adult progeny when combined with the UAS-Rdl transgene. It is likely that the viability of the c772-Gal4/UAS-Rdl progeny is due to the late developmental onset of expression of the c772-Gal4 driver compared to other MB Gal4 transgenes (Armstrong et al., 1998). Quantification of the RDL expression level by immunohistochemistry and confocal microscopy of the tips of the α lobes of the MBs (Figure 3A) showed that flies carrying both c772-Gal4 and UAS-Rdl have a 2.5 fold increase in the RDL signal (Figure 3B) compared with the two control lines carrying only c772-Gal4 or UAS-Rdl. A similar result was obtained after quantifying expression in the calyces of the MBs (Figure 3E, 3F).
Figure 3. Quantification of Rdl over-expression and knock-down in the MBs using the c772-Gal4 driver.
(A) Representative grayscale images of anti-RDL immunofluorescence in Rdl over-expressing flies (c772/Rdl-G) and in control flies carrying only the c772-Gal4 driver (c772/+) or the UAS-Rdl transgene (Rdl-G/+). Each image is an average projection image through the tip of the α lobes, such that the signal intensity represents the 3-dimensional volume of the region. The Rdl expression level was calculated as the signal intensity of the α lobe tip (dashed circle #1) after subtracting the signal intensity of a nearby background region (dashed circle #2) of the same size and shape.
(B) Quantification of Rdl over-expression in the α lobe tips. Twenty-four samples of each genotype (n=24) were quantified as described in (A) using the same confocal settings. The average grayscale intensity for all three groups is plotted in (B). Means ± SEM are shown.
(C) Representative images of anti-RDL immunofluorescence in the Rdl knock-down flies (c772/+; Rdli8-10J/+) and in control flies carrying only the c772-Gal4 driver (c772/+) or the UAS-RdlRNAi transgene (Rdli8-10J/+).
(D) Quantification of Rdl knock-down in the α lobe tips using twenty-four samples of each genotype (n=24).
(E) Representative grayscale images of anti-RDL immunofluorescence in Rdl over-expressing flies (c772/Rdl-G) and in control flies carrying only the c772-Gal4 driver (c772/+) or the UAS-Rdl transgene (Rdl-G/+). Each image is an average projection image through the calyces. The Rdl expression level was calculated as the signal intensity of the calyx (dashed circle #1) after subtracting the signal intensity of a nearby background region (dashed circle #2) of the same size and shape. Note that in the two control flies, there was comparable signal intensity in the calyx and the protocerebral bridge but in the over-expression group, there was an elevated signal for RDL in the calyx compared to the protocerebral bridge. This served as an internal control for MB specific over-expression of Rdl. The converse was true for the knock-down flies, where there was a reduced signal in the calyx compared to the protocerebral bridge (G).
(F) Quantification of Rdl over-expression in the calyces using twenty-four samples of each genotype (n=24).
(G) Representative images of anti-RDL immunofluorescence in the Rdl knock-down flies (c772/+; Rdli8-10J/+) and in control flies carrying only the c772-Gal4 driver (c772/+) or the UAS-RdlRNAi transgene (Rdli8-10J/+).
(H) Quantification of Rdl knock-down in the calyces using twenty-four samples of each genotype (n=24). **: P<0.01. Data for panels (B) and (D), (F) and (G) were obtained from two separate experiments.
We also used RNAi to knock-down the expression of Rdl in the MBs. We generated multiple transgenic lines carrying one of three different RNAi constructs (Rdli4-5, Rdli2-7 or Rdli8-10, “i” for “interference” and numbers indicate targeted exon ranges) inserted at different chromosome locations. Thirty-seven independent fly lines carrying one of these RNAi constructs were generated. These flies were crossed to the pan-neuronal driver ELAV-Gal4 and screened for RNAi efficiency by immunoblotting. Thirteen of the lines exhibited significant reductions of RDL protein (data not shown). We then narrowed the selection again by crossing these 13 lines to a stronger pan-neuronal Gal4 line, c155-Gal4 line. One of the 13 lines, Rdli8-10J, produced lethality in combination with c155-Gal4, mimicking the homozygous lethal phenotype of Rdl null alleles. We therefore focused our experiments principally on this RNAi line. When c772-Gal4 was used to drive Rdli8-10J, a 50% decrease in RDL immunoreactivity in the tips of the α lobes (Figure 3C, 3D) and calyces (Figure 3G, 3H) of the MBs was observed.
Over-expression of Rdl in the MBs Impairs Memory Acquisition
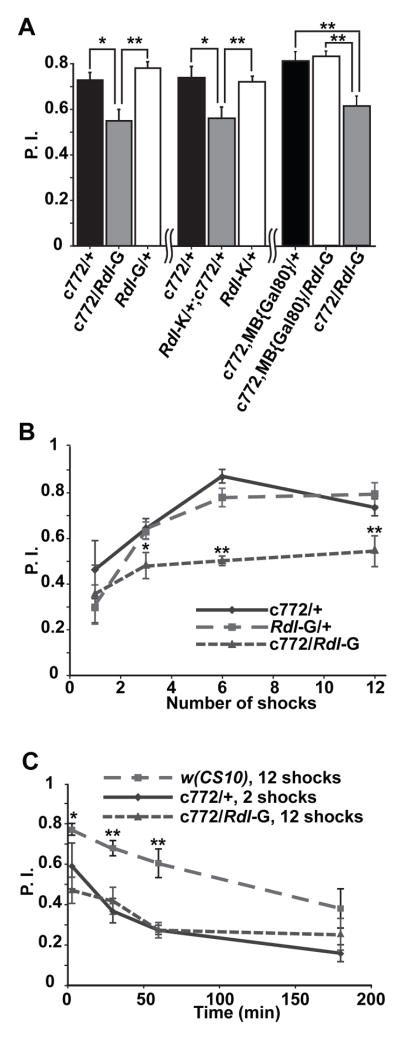
We tested the olfactory associative learning of flies over-expressing Rdl in the MBs with the c772-Gal4 driver. For these tests, flies were trained with a one min presentation of an odor used as a conditioned stimulus (CS+) coincident with 12 × 1.25 sec electric shock pulses delivered every 5 sec during the odor presentation. Rdl over-expressing flies showed approximately a 30% reduction in performance score compared to that of the two control groups when tested immediately after training (Figure 4A). This reduction was observed with two different UAS-Rdl lines carrying the transgene insertion on different chromosomes (Rdl-G and Rdl-K), indicating that the disruption of performance was not a dominant effect of gene disruption at the site of transgene insertion (Figure 4A). Odor and shock avoidance control experiments showed no statistically significant difference among all three groups (Table 1), suggesting that over-expression disrupted the flies’ ability to associate odor and shock information rather than altering the perception of these stimuli.
Figure 4. Impaired olfactory learning with over-expression of Rdl in the MBs.
(A) Over-expression of RDL impaired olfactory learning tested 3 min after training. The c772-Gal4 flies were crossed to two lines carrying independent insertions of UAS-Rdl: Rdl-G (inserted on the 2nd chromosome; left) and Rdl-K (inserted on the X chromosome; middle). Over-expression of either UAS-Rdl transgene with c772-Gal4 impaired performance relative to the control groups carrying only the c772-Gal4 driver or the UAS-Rdl transgene. Over-expression of Rdl with the combined c772-Gal4, MB{Gal80} driver did not affect learning while over-expression with the original c772-Gal4 driver impaired learning (right).
(B) Over-expression of Rdl impaired the acquisition of olfactory memory. Flies were trained using 1, 3, 6 or 12 pulses of electric shock during a CS+ odor presentation of 1 min. Both control groups (c772/+ and Rdl-G/+) exhibited increased performance with shock number, reaching the maximum at 6 shocks. The Rdl over-expressing flies exhibited poorer performance, which did not increase significantly with shock number.
(C) The Rdl over-expressing flies exhibited normal memory stability. The Rdl over-expressing flies and control flies carrying only c772-Gal4 were trained with a different number of electric shock pulses to normalize their initial performance and then tested at several time points after training. The performance scores of these two groups were indistinguishable at all time points tested. The memory stability curves of both groups were parallel over time with the curve obtained for wild type w(CS10) flies trained with a saturating number (12) of electric shock pulses. For all panels, n = 6 for each group. Means ± SEM are shown. *:p<0.05, **: p<0.01.
Table 1. Odor and shock avoidance controls for the genotypes assayed for learning.
There were no statistically significant differences among any of the groups for each of the individual experiments (separated by table boundaries). n = 8 for each group.
| Genotype | BEN avoidance | OCT avoidance | Shock avoidance |
|---|---|---|---|
| c772/+ | 0.887±0.041 | 0.788±0.029 | 0.730±0.050 |
| Rdl-G/+ | 0.758±0.067 | 0.813±0.022 | 0.785±0.064 |
| c772/Rdl-G | 0.817±0.043 | 0.769±0.036 | 0.754±0.046 |
|
| |||
| c772/+ | 0.832±0.064 | 0.838±0.033 | / |
| Rdl-K/+ | 0.824±0.053 | 0.857±0.021 | / |
| Rdl-K/+; c772/+ | 0.832±0.030 | 0.858±0.042 | / |
|
| |||
| c772/+ | 0.813±0.033 | 0.848±0.065 | 0.828±0.062 |
| Rdli8-10J/+ | 0.816±0.051 | 0.849±0.043 | 0.845±0.020 |
| c772/+; Rdli8-10J/+ | 0.751±0.044 | 0.830±0.073 | 0.786±0.030 |
|
| |||
| c772/+ | 0.757±0.090 | 0.788±0.052 | 0.770±0.073 |
| 4XRdli4-5/+ | 0.747±0.032 | 0.791±0.058 | 0.821±0.053 |
| c772/+; 4XRdli4-5/+ | 0.734±0.078 | 0.750±0.045 | 0.777±0.047 |
|
| |||
| c739/+ | 0.821±0.058 | 0.864±0.048 | 0.835±0.040 |
| Rdli8-10J/+ | 0.856±0.057 | 0.838±0.034 | 0.786±0.051 |
| c739/+; Rdli8-10J/+ | 0.725±0.071 | 0.801±0.049 | 0.887±0.045 |
Since the c772-Gal4 driver promotes expression in brain regions besides the MBs, such as the ALs and the ellipsoid body, we wanted to confirm that over-expression of Rdl in the MBs rather than these other sites of expression produced the learning phenotype. We introduced a transgene expressing Gal80, an inhibitor of Gal4, from a MB-specific promoter (MB{Gal80}, Krashes et al., 2007), into the chromosome carrying c772-Gal4 by recombination. The resulting c772-Gal4, MB{Gal80} combined driver eliminated the expression from the MBs but left intact the Gal4 expression in other regions such as the ALs (Figure S1). Flies carrying the combined driver along with UAS-Rdl exhibited a performance score that was indistinguishable from the control group carrying the combined drive alone, demonstrating that the impairment of learning observed previously was due to Rdl over-expression in the MBs (Figure 4A).
To further dissect the operational role of Rdl in memory formation, we assayed memory acquisition and memory stability in flies over-expressing this receptor. For memory acquisition tests, we trained flies by varying the number of electric shock pulses delivered with the odor in order to measure performance as a function of the intensity of training. The two control groups exhibited gradually improving performance with an increasing number of shock pulses paired with odor, reaching a ceiling level of performance with 6 shocks. The Rdl over-expression group, in contrast, exhibited poor performance independent of the number of shocks, suggestive of a defect in memory acquisition (Figure 4B). To probe the possibility that the Rdl over-expressing flies were acquiring memory normally but forgetting very rapidly, we measured memory stability after normalizing the initial performance with differential training. For this experiment, the c772-Gal4/+ control flies received 2 shocks paired with odor while c772-Gal4/UAS-Rdl-G flies received 12 shocks to normalize the initial performance of both groups. After such training, the Rdl over-expression group exhibited the same memory decay dynamics as the control group, indicating that memories formed in the Rdl over-expressing flies had the same stability as control animals (Figure 4C). The combined data suggested that Rdl over-expression disrupted acquisition and not memory stability.
Rdl Over-expression Impairs Learning Physiologically Rather than Developmentally
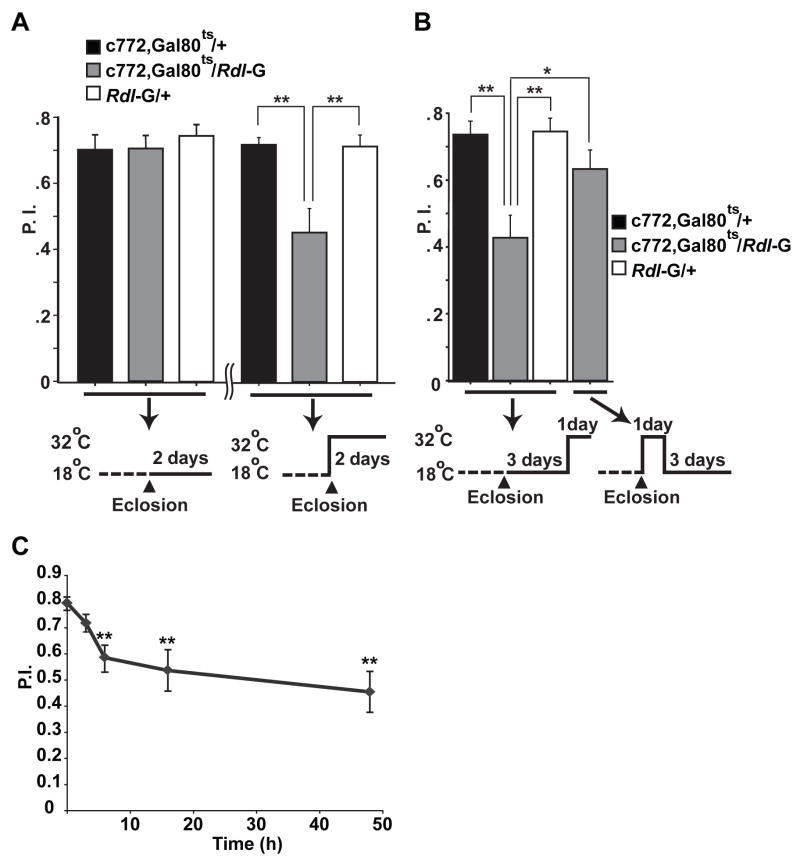
Rdl null flies are early embryonic lethal (ffrench-Constant et al., 1991) and Rdl over-expression with most Gal4 drivers we tested caused lethality, indicating that the expression level of Rdl is critical for proper development. This fact confounds the interpretation of the experiments showing that over-expression impaired learning. Rdl over-expression could potentially disrupt the development and thus the structure of the adult MBs to cause the learning phenotype, or alternatively, Rdl over-expression could acutely disrupt the normal physiological state of the neurons post-developmentally thus affecting learning processes directly. The classical Gal4-UAS system provides limited temporal control of transgene expression, since UAS-transgenes are expressed at developmental times determined by the tissue specific promoter driving Gal4 expression (McGuire et al., 2004a). To ascertain whether Rdl over-expression causes a learning phenotype by disrupting normal developmental or physiological processes, we used the TARGET system (McGuire et al., 2003; 2004b) to control Rdl over-expression in both time and space.
Flies carrying transgenes for over-expressing Rdl and ubiquitously expressing Gal80ts (tub-Gal80ts), a temperature-sensitive inhibitor of Gal4, were raised at 18 °C. As flies eclosed from the pupae and became adults, they were shifted to 32 °C, a temperature at which the Gal80ts protein becomes inactivated to de-repress the c772-Gal4 driver for Rdl over-expression. Flies carrying c772-Gal4, UAS- Rdl, and Gal80ts showed a significantly reduced performance after shifting to high temperature compared to the two control groups, while flies of the same genotype kept at 18 °C performed at control levels in learning tests (Figure 5A). These data indicated that over-expression of Rdl during adulthood alone was sufficient to impair learning. Flies shifted to 32 °C after adulthood for one day and then returned to 18 °C for a three-day recovery period exhibited normal performance, indicating that the defect caused by Rdl over-expression was reversible (Figure 5B). The memory impairment produced by Rdl over-expression was observed as early as 3–6 hr after the flies were shifted to high temperature (Figure 5C), providing additional evidence contrary to the developmental hypothesis for the learning impairment. Together these data suggested that Rdl over-expression impaired learning by disrupting the normal physiological state of the MB neurons rather than causing irreversible structural or developmental defects. Thus RDL participates during the learning process per se.
Figure 5. Impaired olfactory learning with over-expression of Rdl in adult MBs.
(A) Flies over-expressing RDL in the MBs during adulthood were impaired for olfactory learning performance. The experimental flies carried the c772-Gal4 driver, the UAS-Rdl-G transgene, and a temperature sensitive Gal80 (Gal80ts) transgene in which Gal80ts was driven by the ubiquitously expressed tubulin promoter. All the flies were raised at 18 °C to inhibit Gal4 activity with the active form of the Gal80ts repressor. No impairment was observed when the flies were raised to adulthood and trained and tested at 18 °C (left). When the flies were shifted to 32 °C for two days after eclosion to de-repress Gal4 and allow for over-expression of Rdl, this adulthood over-expression produced a learning defect when compared with the two control groups of flies carrying only the c772-Gal4 driver or the UAS-Rdl-G transgene alone (right).
(B) The impairment of learning produced by Rdl over-expression was reversible. The same three groups of flies as in (A) were raised at 18 °C and maintained at 18 °C for three days after eclosion. They were then shifted to 32°C for one day prior to training. The over-expression group showed decreased performance after one-day of over-expression of Rdl in adulthood. A separate group of c772-Gal4, Gal80ts; UAS-Rdl-G flies were raised at 18°C and shifted to 32°C for one day after eclosion to de-repress Gal4 activity. Then they were shifted back to 18°C for three more days to re-repress Gal4 activity. This recovery treatment restored learning performance to a level indistinguishable from the control groups. n = 6 for each group. *: P<0.05; **: P<0.01.
(C) Time course of the effect of Rdl over-expression on learning. Flies carrying the c772-Gal4 driver, the UAS-Rdl-G transgene and Gal80ts were raised at 18 °C and shifted to 32 °C for 0, 3, 6, 16 and 48 hours after eclosion to induce the over-expression of Rdl. The performance index at 3 min after training is shown for each group. For all panels, n = 6 for each group. Means ± SEM are shown. **: P<0.01, compared with 0 h.
Knock-down of Rdl in the MBs Enhances Memory Acquisition
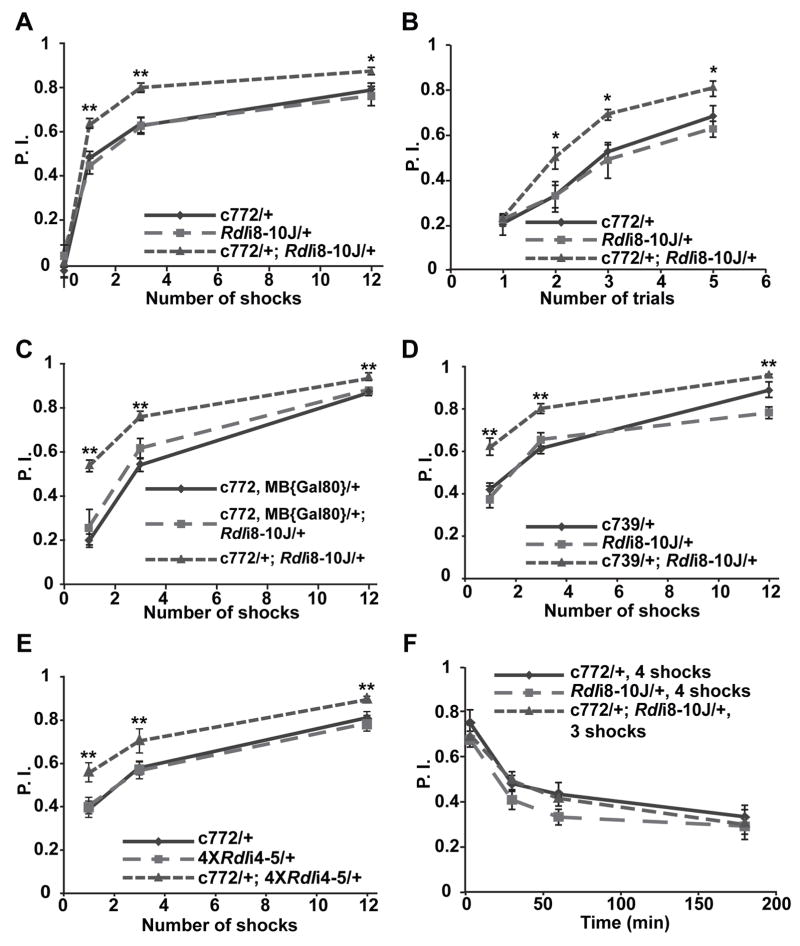
We tested flies with reduced Rdl expression in the MBs to see if they had impaired, normal, or enhanced learning. Surprisingly, the c772-Gal4/Rdli8-10J flies exhibited significantly enhanced performance in memory acquisition tests. When trained with a one min odor presentation along with 1, 3, or 12 electric shock pairings, the flies with reduced expression of Rdl exhibited a 10–20% increase in performance index relative to control flies (Figure 6A). To extend these results and to rule out the possibility that the enhanced performance might be influenced by alterations in the time interval between the shock pulses during training, we used a more stringent training protocol which employs a 10 sec odor presentation along with a single electric shock pulse delivered at the end of the odor presentation (Beck et al., 2000). When trained with 2, 3, or 5 of these “short program” training trials presented in succession, the Rdl knock-down group again exhibited a similar enhanced performance, although this performance enhancement was not evident after only 1 trial (Figure 6B). To confirm that the enhanced performance was due to a reduction of Rdl in the MBs, we knocked-down the expression of Rdl using the c772-Gal4 driver and compared this to flies with Rdl knockdown by the driver line carrying both c772-Gal4 and MB{Gal80}. No enhancement of acquisition was observed using the combined c772-Gal4, MB{Gal80} driver while the c772-Gal4 driver alone still enhanced acquisition when driving Rdli8-10J (Figure 6C). This result strongly suggested that the enhancement of memory acquisition was due to reduced Rdl expression in intrinsic MB neurons. To further narrow the subset of MB neurons that mediate this effect, we performed the same memory acquisition tests using the c739-Gal4 driver. The c739-Gal4 driver has a more restricted expression pattern, exhibiting a selectively high level of expression in the α/β neurons of the MBs and a very low level of expression in the ALs (Stocker et al., 1997; Akalal et al., 2006), whereas the c772-Gal4 driver promotes expression in the α/β, α′/β′, and γ MB neurons as well as the ALs (Zars et al., 2000; Figure S2A,B). When the c739-Gal4 was used to drive the expression of Rdli8-10J, an enhanced performance similar to that observed with c772-Gal4 was observed (Figure 6D). Moreover, in order to rule out the possibility that the performance enhancement was due to an insertional effect of the Rdli8-10J transgene or potential off target effects of the construct Rdli8-10, we tested another Rdl RNAi line, the 4XRdli4-5 line, which carries four copies of a different Rdl RNAi construct (Rdli4-5) inserted at four different chromosomal locations. Again, an enhanced performance similar to that observed using the Rdli8-10J line was observed (Figure 6E). Finally, we also tested the memory stability of flies with reduced expression of Rdl by normalizing their initial performance to that of the control groups with differential training. The memory decay curves constructed from this experiment for flies with reduced Rdl expression in the MBs and the controls were nearly identical (Figure 6F). The odor and shock avoidance for all experimental groups showed no difference from control groups (Table 1).
Figure 6. Enhanced olfactory learning with Rdl knock-down in the MBs.
(A) The Rdl knock-down flies carrying the c772-Gal4 driver and the UAS-RdlRNAi construct Rdli8-10J exhibited enhanced olfactory learning using 1, 3 or 12 electric shock pulses presented within a 1 min exposure to the conditioned odor.
(B) Flies carrying the c772-Gal4 driver and the UAS-RdlRNAi construct Rdli8-10J exhibited a similar enhancement of olfactory learning after multiple trials consisting of a 10 sec odor presentation along with a single electric shock pulse.
(C) Knock-down of Rdl by the combined c772-Gal4,MB{Gal80} driver did not affect acquisition while knock-down by the original c772-Gal4 driver enhanced acquisition.
(D) The Rdl knock-down flies carrying the c739-Gal4 driver and the UAS-RdlRNAi construct Rdli8-10J exhibited enhanced olfactory learning using 1, 3 or 12 electric shock pulses presented within a 1 min exposure to the conditioned odor.
(E) The Rdl knock-down flies carrying the c772-Gal4 driver and the UAS-RdlRNAi constructs 4XRdli4-5 show enhanced olfactory learning using 1, 3 or 12 shock pulses presented within a 1 min odor exposure.
(F) The Rdl knock-down flies exhibited normal memory decay. The Rdl knock-down flies and the two control groups were trained to a similar initial performance level by using a different number of electric shock pulses along with a 1 min presentation of the conditioned odor. There was no significant difference in performance among the three groups at any of the time points tested. For all panels, n = 6 for each group. Means ± SEM are shown. *: P<0.05; **: P<0.01.
The combined data lead to the inescapable conclusion that the enhanced performance in memory acquisition was caused by a reduction of Rdl expression in the α/β MB neurons. To our knowledge, this is the first enhancement of short-term memory or learning reported in Drosophila. Altogether, the results indicated that knock-down of Rdl in the MBs enhances memory acquisition but not memory stability, nicely consistent with the conclusions from Rdl over-expression experiments.
RDL Inhibits Calcium Responses in the MBs Evoked by Odors But Not Electric Shock
To help understand the underlying neuronal mechanisms of the changes in memory formation produced by Rdl over-expression or knock-down in the MBs, we assayed the response properties of the MB neurons using functional imaging while odors or electric shock pulses were presented to the fly. G-CaMP is a calcium sensitive fluorescent protein that can be used to monitor neuronal activity in the living animals (Yu et al., 2004; Yu et al., 2005; Yu et al., 2006). We constructed flies carrying the c772-Gal4 transgene driving both a G-CaMP reporter and Rdl over-expression or knock-down constructs in the MBs. Functional imaging was performed through a small window cut in heads of living flies while odors or electric shock pulses were presented to the animals.
Since the c772-Gal4 driver is expressed in both the MBs and the ALs and the ALs extend projections into the MB calyx, we needed to be certain that the responses recorded in the MB calyx were from intrinsic MB neurons rather than the presynaptic projection neurons from the ALs. We expressed G-CaMP or membrane localized mCD8-GFP using the c772-Gal4 driver and carefully analyzed confocal stacks of these fly brains. No expression of the reporters was observed in the nerve tracts that connect the ALs to the MBs (antennal cerebral tracts), indicating that the basal fluorescence in the calyx was due to expression in the dendrites of the MB neurons (Figure S2C-K). The expression of the c772-Gal4 driver in the AL must therefore be confined to neurons other than those that project to the MB calyx.
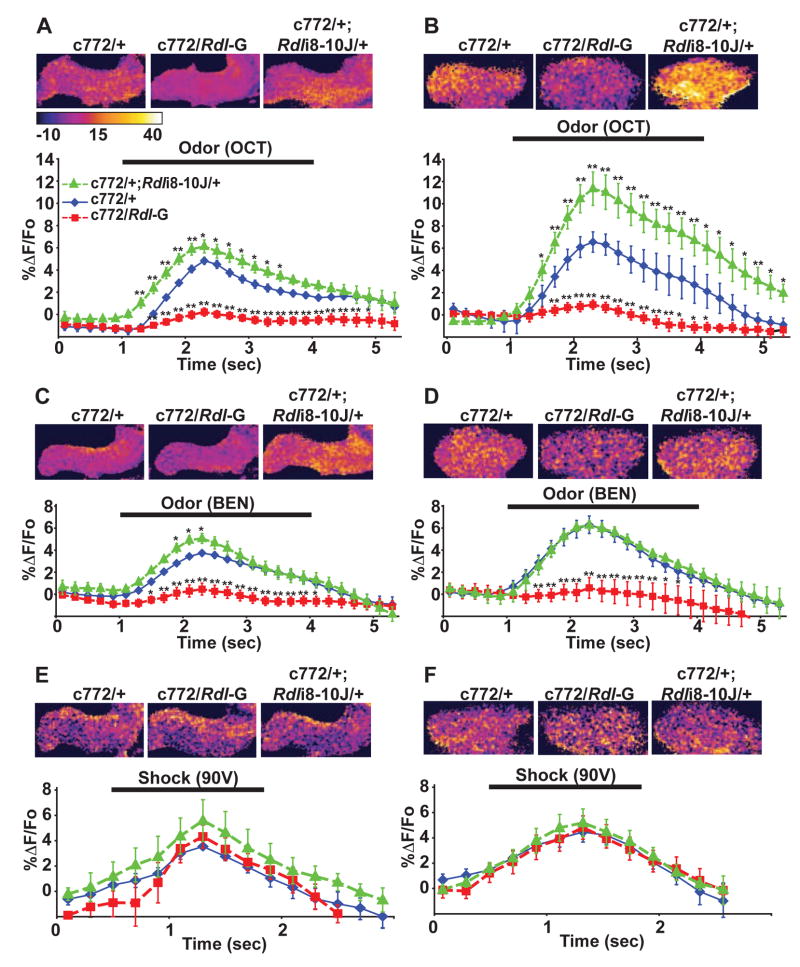
When presented with one of the odors (3-octanol) previously used for learning assays, the control flies (c772-Gal4/+) exhibited a measurable calcium response in both the horizontal lobes and calyces, indicated by increased G-CaMP fluorescence during the odor presentation. Over-expression of Rdl significantly attenuated this calcium response, while knock-down of Rdl enhanced the response in both regions (Figure 7A, B). We also tested the response of the MBs to a second odor used in the behavior tests (benzaldehyde) and observed similar results (Figure 7C, D), except that the Rdl knockdown group did not exhibit an enhanced response in the calyx compared to the control group. It may be that a ceiling level of G-CaMP response for the calyx was reached at the benzaldehyde concentration used for these experiments.
Figure 7. Calcium responses of MB neurons to odor and shock stimulation in RDL over-expression and knock-down flies.
(A)–(F) Neuronal responses to stimuli in the MB neurons were quantified as the percent change in G-CaMP fluorescence as a function of time (stimulus delivery indicated by horizontal bar in each panel). Over-expression of Rdl (c772/Rdl-G) produced a significant reduction in the amplitude of the response to 3-octanol (OCT) relative to control flies (c772/+) in the MB lobes (A) and in the calyx (B). Conversely, knock-down of Rdl (c772/+; Rdli8-10J/+) caused a significant increase in the amplitude of the response to OCT compared to control animals in both regions of the MBs. Similar effects were observed when flies were stimulated with benzaldehyde (BEN) (C) and (D), except Rdl knock-down failed to elicit a significant increase of response in the calyx. In contrast to odor stimulation, electrical shock failed to elicit a significant difference in the level of response among any of genotypes tested in the MB lobes (E) and in the calyx (F). Representative confocal images of the MBs of flies from each group were pseudocolored based on the percent change in G-CaMP fluorescence to illustrate the differences in response within the regions of the MB neurons that were analyzed (upper part of each panel). For all panels, n ≥ 6 for each group. Means ± SEM are shown. Asterisks indicate significant differences from the c772/+ control group, *: P<0.05; **: P<0.01.
Surprisingly, the level of Rdl expression in the MBs did not significantly alter the calcium response of MB neurons when electric shock stimuli were presented (Figure 7E, F). This was not due to an unknown factor that masked a difference in excitability of the MBs between different groups, because each individual fly was used for testing responses to electric shock and odor, and the expected differential responses to odor were observed (Figure S3). These results suggested that the expression level of the RDL receptor in the MBs selectively gates the magnitude of the neuronal responses to odors without altering responsiveness to electric shock.
Discussion
We used both over-expression and knock-down strategies with tissue and time-specific control to probe the role of the GABAA receptor RDL in olfactory learning. The abundant endogenous expression of Rdl in the olfactory nervous system strongly suggests a critical role in odor perception, discrimination, and learning. Our results conclusively show a physiological role for Rdl in olfactory learning. Over-expression of Rdl in the MBs impaired learning while knock-down of Rdl in the same neurons enhanced learning. Our data also show that RDL is involved in memory acquisition but not memory stability. These behavioral data along with functional imaging results indicate the GABAergic system inhibits olfactory learning, probably by gating the level of olfactory information into the MBs.
The Rdl gene exhibits extensive alternative splicing. Exons 3 and 6 of the Rdl gene have 2 alternative splice forms each, so that the Rdl gene encodes 4 different isoforms, all of which are found in RNA isolated from early embryos (ffrench-Constant and Rocheleau, 1993). When expressed in Xenopus oocytes, the proteins produced from alternative splicing show differential responses to agonists (Hosie et al., 2001), suggesting different physiological properties for the isoforms in vivo. Since the detailed temporal and spatial expression pattern of each isoform in the adult fly brain has not been reported, we designed the antigen and all RNAi constructs against sequences common to all known isoforms. We therefore cannot identify those isoform(s) that are expressed in the adult MBs and those that are responsible for inhibiting olfactory learning.
Prior studies have shown that GABAergic inhibition shapes odor-evoked spatiotemporal activity patterns in the Drosophila ALs (Wilson and Laurent, 2005). GABA receptor function in the honeybee AL has also been shown to be required for fine, but not coarse, odor discrimination, by using picrotoxin to inhibit AL GABA receptors. These observations raise the issue of why the c772-Gal4, MB{Gal80}; UAS-Rdli flies exhibited normal olfactory learning. Although it is possible that c772-Gal4 drives expression in AL interneurons other than those involved in olfactory discrimination, which would explain the observation, the more likely explanation is that the odors used in our study are quite disparate, allowing for the normal learning of these odors. This predicts that a phenotype may emerge in tests of these flies for fine odor discrimination.
One possible role for the GABAergic inhibition of the MB neurons is to sparsen the odor representations (Perez-Orive et al., 2002). Sparsening of sensory representations has been proposed as a simplification that the nervous system makes to allow easier and faster encoding and retrieval of memories (Olshausen and Field, 2004). In its simplest form, the sparsening hypothesis for GABAergic inhibition of the MBs predicts that lessening the inhibition by reducing Rdl expression should make the representations more complex and more difficult to learn, whereas we observed enhanced acquisition with reduced Rdl expression. Rather than facilitating and enhancing memory formation by the sparsening of representations, our results are more consistent with the alternative idea that the GABAergic system inhibits learning.
What is the purpose of a neural system that inhibits learning? One possibility is that this inhibitory system may provide a necessary balance for the acquisition of different forms of memory. Extinction is an active form of learning occurring when the repeated presentation of a CS alone causes a gradual decrease in the conditioned response in a previously conditioned animal. The surface expression of the GABAA receptor and the expression level of gephyrin, a protein involved in GABAA receptor clustering, have been reported to decrease in the basolateral amygdala of the rat after fear conditioning, yet these GABAergic markers significantly increase after extinction training (Chhatwal et al., 2005), suggesting that the GABAergic system has opposing roles for conditioning and extinction. Our preliminary data also show that Rdl knock-down reduced extinction, supporting the hypothesis that the GABAergic system inhibits conditioning while enhancing extinction (unpublished data).
Second, this inhibitory system could serve as a noise filter for information transmission from the ALs to the MBs. The projection neurons of the ALs convey olfactory information to at least two, third-order olfactory areas: the MBs and the lateral horns. The MBs are required for olfactory learning and the lateral horns are thought to be involved in establishing odor identity (Tanaka et al., 2004). Recently, excitatory local neurons were discovered in the ALs (Shang et al., 2007; Olsen et al., 2007). These neurons may be involved in signal amplification by providing cross excitation to projection neurons that are innervated by olfactory receptor neurons that are not responsive to the test odor. The net result is enhanced and more generalized output from the ALs. While this signal amplification could potentially be beneficial for odor detection and discrimination in the lateral horns, it could also introduce extra noise and be detrimental to the MBs for learning about odors in a specific way relative to their importance. By reducing the activity of the MB neurons, the GABAergic system could potentially reduce the time window for coincidence detection in the MBs, thus inhibiting generalized learning and facilitating selective learning. Thus the GABAergic system may be a noise filter needed by the MBs for optimal learning.
Finally, the inhibitory system on the MBs may allow learning to occur through the mechanism of inhibiting the inhibition. There are two major questions of focus for future investigations relative to this idea. One key question is whether learning alters the abundance or function of RDL receptors in the MB neurons. This change could serve to lessen the inhibitory constraints on MB neurons post-conditioning, thus potentiating the effect of the trained odor. The surface expression of GABAA receptors has been reported to decrease in the basolateral amygdala after fear conditioning in rodents (Chhatwal et al., 2005). A related question is whether there are learning induced changes that occur in the presynaptic GABAergic extrinsic neurons that innervate the MBs. Although these neurons and potential learning-induced changes are yet to be identified in Drosophila, it has been reported that classical olfactory discrimination conditioning of the mouse alters the release of neurotransmitters in the olfactory bulb, including the release of GABA (Brennan et al., 1998). Presynaptic changes in the release of GABA due to conditioning might also have similar effects by potentiating CS responses after learning. Glutamate released by repetitive activation of the Schaffer collateral triggers a heterosynaptic and persistent depression of GABA release onto CA1 pyramidal neurons (Chevaleyre and Castillo, 2003). The appropriate paired stimulation of MB neurons by CS and US could in a related way, produce a retrograde signal to depress GABA release and thus potentiate learning mediated by the MB neurons.
Experimental Procedures
Fly Culture and Genetics
Flies were cultured on standard medium at 25°C, 60% relative humidity and a 12-hour light/dark cycle. The w(CS10) flies (Canton-S flies carrying the w1118 mutation) served as a wild-type control for some experiments. All of the transgenic or mutant flies were either generated in w(CS10) background or out-crossed into w(CS10) background for at least 6 generations by standard Drosophila genetics. Rdl1/TM3, Sb1 and w1118; Rdlf02994/TM6B, Tb1 flies originally came from the Bloomington Drosophila Stock Center. Rdl1 is a null allele of Rdl caused by γ-irradiation (ffrench-Constant et al., 1991) and Rdlf02994 is a null allele caused by insertion of a piggyBac transposable element (Thibault et al., 2004). Both alleles are homozygous lethal. Eleven Gal4 lines were tested as drivers for the over-expression of Rdl: c155, c502b, c772, c739, ELAV-Gal4, GH146, Mz717, Mz1162, Np178, OK107 and P247. Among these lines, only the c772-Gal4 line produced viable progeny when driving the over-expression of Rdl.
Transgenic Animals
The full-length Rdl cDNA construct was made from the Berkeley Drosophila Genome Project cDNA clone GH09619 from the Drosophila Genomics Resource Center. Our sequencing results showed that this clone contained a four base pair deletion in the coding region by comparison with genomic DNA. This deletion resulted in a premature stop codon and truncation of the RDL protein, predicted to be missing the majority of the 2nd intracellular loop, the 4th transmembrane domain and the C-terminal extracellular domain. We corrected this deletion by adding back the missing four base pairs using PCR and confirmed it by DNA sequencing. For the Rdl RNAi construct named Rdli4-5, the cDNA sequence containing exon 4 and 5 of Rdl was inverted and fused down stream of the genomic region with the corresponding exons and introns (Kalidas and Smith, 2002). For the Rdli2-7 construct, two copies of cDNA sequence containing exon 2 to 7 of Rdl were cloned into the pWIZ vector in a head-to-head orientation, separated by the 72 base pair white gene intron 2 from the vector (Lee and Carthew, 2003). The construct Rdli8-10 was made in the similar fashion, except it targeted the cDNA region from exon 8 to 10. Full-length Rdl cDNA and Rdli4-5 constructs were sub-cloned into the pUAST vector. These pUAST or pWIZ constructs were used to generate transgenic flies through P-element mediated germline transformation. The insertion sites in the transformants were chromosomally mapped using standard genetic methods or mapped molecularly by inverse PCR and sequencing. For reasons that remain unclear, all 7 transgenic lines carrying the full-length Rdl cDNA construct exhibited dominant male sterility, so they were maintained over balancers and only females were used for crosses in all experiments.
Polyclonal Antibody, Immunoblotting and Immunohistochemistry
To generate a polyclonal antibody against RDL, we amplified the corrected Rdl cDNA sequence corresponding to amino acid sequence 354–525 (the predicted 2nd intracellular domain) of the protein by PCR and sub-cloned this sequence in frame with GST protein coding sequences from the bacteria expression vector pGEX-KG. This fusion protein was subsequently expressed in E. coli and purified using a Glutathione Sepharose column (Amersham Biosciences). The purified protein was used to raise anti-RDL antisera from rabbits (Open Biosystems). Antisera from both animals gave identical immunostaining patterns in the Drosophila central brain using a 1:100 dilution. Immunoblotting showed that at a 1:500 dilution, antisera from animal C9345 recognized both the GST-RDL peptide fusion protein in bacterial extracts and endogenous RDL protein from fly head extracts with the expected size of 65 KDa. For immunohistochemistry, cryosections (15 μm) of w(CS10) fly heads were stained with a 1:100 dilution of anti-RDL antisera and a 1:10 dilution of 22C10 (Developmental Studies Hybridoma Bank), a monoclonal antibody against the microtubule-associated protein FUTSCH (Hummel et al., 2000). To quantify the over- or under-expression of Rdl in the MBs, whole mount brains stained with anti-RDL antisera were prepared and image stacks obtained by confocal microscopy. All the images in the same comparison group were taken using the same settings (laser power, pinhole, gain, offset, zoom, etc). Each image analyzed was the average projection of a series of confocal planes (about 20–30 sections) through the tip of the α lobe or the calyx, so that the signal intensity of any given region of interest represented the whole volume of the region rather than a single confocal plane. The average grayscale intensity of the region of interest was measured with NIH ImageJ software and calculated as described in the legend of Figure 3.
Behavioral Assays
Drosophila olfactory learning was tested using an olfactory classical conditioning paradigm (Tully and Quinn, 1985). Briefly, flies were sequentially exposed to two odors (benzaldehyde [BEN] and 3-octanol [OCT]) for one min each. The first odor (CS+) was paired with electric shock pulses (US) followed by a second odor (CS−) without shock. The flies were then loaded into a T-maze and allowed to choose between two arms, each containing one of the two odors. The flies’ avoidance of the odor paired with shock was calculated as the performance index (P.I.), which was the number of flies that responded correctly minus the number of flies that responded incorrectly, divided by total number of the flies. A P.I. = 1 indicates that all flies moved into the correct arm while a P.I. = 0 indicates a 50:50 distribution between the arms and no learning. To eliminate naïve odor bias, each trial was composed of two simultaneous half trials where one group was trained to associate BEN with shock and the other to associate OCT with shock, and the complete P.I. was the average of these two half P.I.s. For standard training, both odors were presented for 1 min each, and the first odor was paired with 12 evenly distributed shocks, each lasting for 1.25 s. We modified this by maintaining the time for odor exposure but varied the number of shocks that were applied (between 1 and 12), also the shocks were evenly distributed throughout the 1 min exposure to the CS+, with the last shock always occurring at the end of the exposure. The other modified training procedure is known as “short program” training (Beck et al., 2000). For a single training trial, flies were exposed to 10 sec of CS+ with a 1.25 s shock delivered at the 8th sec, followed by a rest of 30 sec, and a subsequent exposure to 10 sec of CS− without shock. For multiple training trials, the inter-trial-interval was 30 sec. To eliminate visual distractions, all experiments were performed inside a darkroom illuminated with dark red light, which was invisible to the flies.
Functional Imaging
Adult, female flies aged 1–3 days were used for imaging. Each fly was pushed into the end of a pipette tip, and the top and bottom of the tip were beveled to expose the dorsal surface of the fly head as well as the proboscis. A patch of cuticle spanning the area between the eyes and extending anteriorly to the ptilinal suture was removed from the top of the fly head. The tracheae were removed and the opening was covered with a small piece of plastic wrap to create an imaging window. The head was then secured to the pipette tip using myristic acid. Imaging was performed using a Leica TCS SP5 confocal microscope and a 20× objective. The G-CaMP reporter was excited with 488 nm light and the emitted light was collected between 505–535 nm. Olfactory responses were acquired in 20-sec imaging periods, during which flies received a 3 sec olfactory stimulation. Odorant cues were delivered using an olfactometer, which provided a stream of air to the antennae at a rate of 100 mL/min. Using a computerized controller, the air stream was mixed with air wafted over either mineral oil (control) or a solution of odorant, diluted to 1.0% in mineral oil. Between control and odor presentations, flies were allowed to rest for 2 min; only flies that did not respond to the mineral oil presentation were used for quantification of the odor response. Electric shock pulses of 90V and 1.25 sec duration were delivered to the legs and abdomen via a platinum electrode. Following the shock stimuli, flies were rested for 2 min, and then presented with an odor cue. Only flies that responded to both electrical and olfactory stimuli were used for quantification of the shock responses. Stimulus responses were quantified as a percent change in fluorescence of the G-CaMP reporter within the region of interest. To determine the percent change, the time series were temporally smoothed using a sliding window to bin consecutive frames. The baseline fluorescence (F0) was calculated by averaging the 10 bins just prior to odor or shock delivery. F0 was subtracted from each bin, and the difference was divided by F0. In this way, we determined the average percent change in fluorescence within a given region of interest across time. We pseudocolored the images according to the percent change on a pixel-by-pixel basis. All of these analyses were performed using a custom-made plug-in for the NIH ImageJ software. When quantifying the shock responses, we found that motion artifacts sometimes confounded changes in reporter intensity. To spatially register the slices within each time series, we used the ImageJ StackReg plug-in to apply a rigid body transformation to each data set (Thévenaz et al., 1998).
Statistics
Statistical analyses were performed using the StatView software (SAS Institute Inc.). One-way ANOVA was followed by planned pairwise comparisons between relevant groups with Fisher’s PLSD test.
Supplementary Material
Acknowledgments
We thank Y. S. Lee and R. W. Carthew for providing us the pWIZ vector. The MAb 22C10 monoclonal antibody developed by S. Benzer was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. Studies reported here were supported by NS19904 to R. L. D., the G. Harold and Leila Y. Mathers Charitable Foundation, and the R. P. Doherty-Welch Chair in Science at the Baylor College of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akalal DB, Wilson CF, Zong L, Tanaka NK, Ito K, Davis RL. Roles for Drosophila mushroom body neurons in olfactory learning and memory. Learn Mem. 2006;13:659–568. doi: 10.1101/lm.221206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglade F, Bizot JC, Dodd RH, Baudoin C, Chapouthier G. Opposite effects of cholinergic agents and benzodiazepine receptor ligands in a passive avoidance task in rats. Neurosci Lett. 1994;182:247–250. doi: 10.1016/0304-3940(94)90808-7. [DOI] [PubMed] [Google Scholar]
- Armstrong JD, deBelle JS, Wang Z, Kaiser K. Metamorphosis of the mushroom bodies; large scale rearrangements of the neural substrates for associative learning and memory in Drosophila. Learn Mem. 1998;5:102–114. [PMC free article] [PubMed] [Google Scholar]
- Beck CD, Schroeder B, Davis RL. Learning performance of normal and mutant Drosophila after repeated conditioning trials with discrete stimuli. J Neurosci. 2000;20:2944–2953. doi: 10.1523/JNEUROSCI.20-08-02944.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Schellinck HM, De La Riva C, Kendrick KM, Keverne EB. Changes in neurotransmitter release in the main olfactory bulb following an olfactory conditioning procedure in mice. Neurosci. 1998;87:583–590. doi: 10.1016/s0306-4522(98)00182-1. [DOI] [PubMed] [Google Scholar]
- Buckingham SD, Biggin PC, Sattelle BM, Brown LA, Sattelle DB. Insect GABA receptors: splicing, editing, and targeting by antiparasitics and insecticides. Molec Pharm. 2005;68:942–951. doi: 10.1124/mol.105.015313. [DOI] [PubMed] [Google Scholar]
- Buckingham SD, Hosie AM, Roush RL, Sattelle DB. Actions of agonists and convulsant antagonists on a Drosophila melanogaster GABA receptor (Rdl) homo-oligomer expressed in Xenopus oocytes. Neurosci Lett. 1994;181:137–140. doi: 10.1016/0304-3940(94)90578-9. [DOI] [PubMed] [Google Scholar]
- Chambers MS, Atack JR, Broughton HB, Collinson N, Cook S, Dawson GR, Hobbs SC, Marshall G, Maubach KA, Pillai GV, Reeve AJ, MacLeod AM. Identification of a novel, selective GABAA α5 receptor inverse agonist which enhances cognition. J Med Chem. 2003;46:2227–2240. doi: 10.1021/jm020582q. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci. 2005;25:502–506. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Napier TC. Antagonism of GABAergic transmission within the septum disrupts working/episodic memory in the rat. Neurosci. 1992;47:833–841. doi: 10.1016/0306-4522(92)90033-x. [DOI] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, et al. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the α5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc Natl Acad Sci U S A. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL. Olfactory learning. Neuron. 2004;44:31–48. doi: 10.1016/j.neuron.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Davis RL. Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu Rev Neurosci. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- DeLorey TM, Handforth A, Anagnostaras SG, Homanics GE, Minassian BA, Asatourian A, Fanselow MS, Delgado-Escueta A, Ellison GD, Olsen RW. Mice lacking the beta3 subunit of the GABAA receptor have the epilepsy phenotype and many of the behavioral characteristics of Angelman syndrome. J Neurosci. 1998;18:8505–8514. doi: 10.1523/JNEUROSCI.18-20-08505.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JB. Gal4 system in Drosophila: a fly geneticist’s Swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- ffrench-Constant RH, Mortlock DP, Shaffer CD, MacIntyre RJ, Roush RT. Molecular cloning and transformation of cyclodiene resistance in Drosophila: an invertebrate gamma-aminobutyric acid subtype A receptor locus. Proc Natl Acad Sci USA. 1991;88:7209–7213. doi: 10.1073/pnas.88.16.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ffrench-Constant RH, Rocheleau TA. Drosophila gamma-aminobutyric acid receptor gene Rdl shows extensive alternative splicing. J Neurochem. 1993;60:2323–2326. doi: 10.1111/j.1471-4159.1993.tb03523.x. [DOI] [PubMed] [Google Scholar]
- ffrench-Constant RH, Daborn PJ, Le Goff G. The genetics and genomics of insecticide resistance. Trends Genet. 2004;20:163–170. doi: 10.1016/j.tig.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Harrison JB, Chen HH, Sattelle E, Barker PJ, Huskisson NS, Rauh JJ, Bai D, Sattelle DB. Immunocytochemical mapping of a C-terminus anti-peptide antibody to the GABA receptor subunit, RDL in the nervous system in Drosophila melanogaster. Cell Tissue Res. 1996;284:269–278. doi: 10.1007/s004410050587. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Aronstein K, Sattelle DB, ffrench-Constant RH. Molecular biology of insect neuronal GABA receptors. Trends Neurosci. 1997;20:578–583. doi: 10.1016/s0166-2236(97)01127-2. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Buckingham SD, Presnail J, K and, Sattelle DB. Alternative splicing of a Drosophila GABA receptor subunit gene identifies determinants of agonist potency. Neurosci. 2001;102:709–714. doi: 10.1016/s0306-4522(00)00483-8. [DOI] [PubMed] [Google Scholar]
- Huff NC, Wright-Hardesty KJ, Higgins EA, Matus-Amat P, Rudy JW. Context pre-exposure obscures amygdala modulation of contextual-fear conditioning. Learn Mem. 2005;12:456–460. doi: 10.1101/lm.6705. [DOI] [PubMed] [Google Scholar]
- Hummel T, Krukkert K, Roos J, Davis G, Klambt C. Drosophila Futsch/22C10 is a MAP1B-like protein required for dendritic and axonal development. Neuron. 2000;26:357–370. doi: 10.1016/s0896-6273(00)81169-1. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Huhman KL. Activation of GABAA receptors in the amygdala blocks the acquisition and expression of conditioned defeat in Syrian hamsters. Brain Res. 2001;920:142–150. doi: 10.1016/s0006-8993(01)03054-2. [DOI] [PubMed] [Google Scholar]
- Kalidas S, Smith DP. Novel genomic cDNA hybrids produce effective RNA interference in adult Drosophila. Neuron. 2002;33:177–184. doi: 10.1016/s0896-6273(02)00560-3. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during Drosophila odor memory processing. Neuron. 2007;53:103–115. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Rocheleau T, Zhang HG, Jackson MB, ffrench-Constant RH. Expression of a Drosophila GABA receptor in a baculovirus insect cell system. Functional expression of insecticide susceptible and resistant GABA receptors from the cyclodiene resistance gene Rdl. FEBS Lett. 1993;335:315–318. doi: 10.1016/0014-5793(93)80409-n. [DOI] [PubMed] [Google Scholar]
- Lee YS, Carthew RW. Making a better RNAi vector for Drosophila: use of intron spacers. Methods. 2003;30:322–329. doi: 10.1016/s1046-2023(03)00051-3. [DOI] [PubMed] [Google Scholar]
- Liu X, Davis RL. Insect olfactory memory in time and space. Curr Opin Neurobiol. 2006;16:679–685. doi: 10.1016/j.conb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Roman G, Davis RL. Gene expression systems in Drosophila: a synthesis of time and space. Trends Genet. 2004a;20:384–391. doi: 10.1016/j.tig.2004.06.012. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in DrosophilaSci. STKE. 2004b;220:pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- McNamara RK, dePape GE, Skelton RW. Differential effects of benzodiazepine receptor agonists on hippocampal long-term potentiation and spatial learning in the Morris water maze. Brain Res. 1993;626:63–70. doi: 10.1016/0006-8993(93)90563-3. [DOI] [PubMed] [Google Scholar]
- Morón I, Ramirez-Lugo L, Ballesteros MA, Gutiérrez R, Miranda MI, Gallo M, Bermúdez-Rattoni R. Differential effects of bicuculline and muscimol microinjections into the nucleus basalis magnocellularis in taste and place aversive memory formation. Behav Brain Res. 2002;134:425–431. doi: 10.1016/s0166-4328(02)00056-6. [DOI] [PubMed] [Google Scholar]
- Olsen SR, Bhandawat V, Wilson RI. Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron. 2007;54:89–103. doi: 10.1016/j.neuron.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshausen BA, Field DJ. Sparse coding of sensory inputs. Curr Opin Neurobiol. 2004;14:481–487. doi: 10.1016/j.conb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297:359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- Shang Y, Claridge-Chang A, Sjulson L, Pypaert M, Miesenbock G. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell. 2007;128:601–612. doi: 10.1016/j.cell.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker RF, Heimbeck G, Gendre N, de Belle JS. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J Neurobiol. 1997;32:443–456. doi: 10.1002/(sici)1097-4695(199705)32:5<443::aid-neu1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Stopfer M, Bhagavan S, Smith BH, Laurent G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390:70–74. doi: 10.1038/36335. [DOI] [PubMed] [Google Scholar]
- Tanaka NK, Awasaki T, Shimada T, Ito K. Integration of chemosensory pathways in the Drosophila second-order olfactory centers. Curr Biol. 2004;14:449–457. doi: 10.1016/j.cub.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Thévenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Med Imaging. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Thibault ST, Singer MA, Miyazaki WY, Milash B, Dompe NA, Singh CM, Buchholz R, Demsky M, Fawcett R, Francis-Lang HL, et al. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet. 2004;36:283–287. doi: 10.1038/ng1314. [DOI] [PubMed] [Google Scholar]
- Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- Van Nobelen M, Kokkinidis L. Amygdaloid GABA, not glutamate neurotransmission or mRNA transcription controls footshock-associated fear arousal in the acoustic startle paradigm. Neurosci. 2006;137:707–716. doi: 10.1016/j.neuroscience.2005.08.061. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J Neurosci. 2005;25:9069–9079. doi: 10.1523/JNEUROSCI.2070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuyama K, Meinertzhagen IA, Schurmann FW. Synaptic organization of the mushroom body calyx in Drosophila melanogaster. J Comp Neurol. 2002;445:211–226. doi: 10.1002/cne.10155. [DOI] [PubMed] [Google Scholar]
- Yu D, Akalal DB, Davis RL. Drosophila α/β mushroom body neurons form a branch-specific, long-term cellular memory trace after spaced olfactory conditioning. Neuron. 2006;52:845–355. doi: 10.1016/j.neuron.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Keene AC, Srivatsan A, Waddell S, Davis RL. Drosophila DPM neurons form a delayed and branch-specific memory trace after olfactory classical conditioning. Cell. 2005;123:945–957. doi: 10.1016/j.cell.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Yu D, Ponomarev A, Davis RL. Altered representation of the spatial code for odors after olfactory classical conditioning. Neuron. 2004;42:437–449. doi: 10.1016/s0896-6273(04)00217-x. [DOI] [PubMed] [Google Scholar]
- Zars T, Fischer M, Schulz R, Heisenberg M. Localization of a short-term memory in Drosophila. Science. 2000;288:672–675. doi: 10.1126/science.288.5466.672. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Bakhsha A, Rostami P, Shafaghi B. Effects of intrahippocampal injection of GABAergic drugs on memory retention of passive avoidance learning in rats. J Psychopharmacol. 2002;16:313–319. doi: 10.1177/026988110201600405. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Shamsi T, Azarmina P, Rostami P, Shafaghi B. GABAergic system and imipramine-induced impairment of memory retention in rats. Eur Neuropsychopharmacol. 2004;14:59–64. doi: 10.1016/s0924-977x(03)00068-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.