Abstract
Background and Purpose
Acute ischemic lesions with restricted diffusion can resolve after early recanalization. The impact of superimposed perfusion abnormalities on the fate of acute diffusion lesions is unclear.
Methods
Data were obtained from DEFUSE, a prospective multicenter study of patients treated with IV tPA 3 to 6 hours after stroke onset. Thirty-two patients with baseline diffusion and perfusion lesions and 30 day FLAIR scans were coregistered. The acute diffusion lesion was divided into 3 regions according to the Tmax delay of the superimposed perfusion lesion: normal baseline perfusion; mild-moderately hypoperfused (2 s<Tmax≤8 s) and severely hypoper-fused (Tmax >8 s). The reversal rate was calculated as the percentage of the acute diffusion lesion that did not overlap with the final infarct on 30-day FLAIR. Diffusion reversal rates were compared based on whether a favorable clinical response occurred and whether early recanalization was achieved.
Results
On average, 54% of the acute diffusion lesion volume had normal perfusion. Diffusion reversal rates were significantly increased among cases with favorable clinical response and in patients with early recanalization, especially in regions with normal baseline perfusion. The portion of the diffusion lesion with normal perfusion had significantly higher mean apparent diffusion coefficient values and reversal rates.
Conclusion
Acute ischemic lesions with restricted diffusion are most likely to recover if reperfusion occurs within 6 hours of symptom onset, and reversibility is associated with early recanalization and favorable clinical outcome. We propose the term RADAR (Reversible Acute Diffusion lesion Already Reperfused) to describe regions of acute restricted diffusion with normal perfusion.
Keywords: brain infarction, cerebral infarct, magnetic resonance, thrombolysis
Early recanalization can reduce the extent of irreversible infarction by salvage of the ischemic penumbra.1,2 A technique that can provide fast, reliable, and accurate identification of the ischemic penumbra in acute stroke patients would facilitate identification of patients who are most likely to benefit from early recanalization. Diffusion weighted imaging (DWI) demonstrates the presence of cytotoxic ischemic injury within minutes and provides an estimate of the ischemic core;3,4 perfusion-weighted imaging (PWI) provides an assessment of cerebral hemodynamics.5 The PWI/DWI mismatch hypothesis postulates that the difference between the acute PWI and DWI lesions provides an estimate of the ischemic penumbra. Both the Echoplanar Imaging THrombolytic Evaluation Trial (EPITHET) and the Diffusion and perfusion imaging Evaluation For Understanding Stroke Evolution (DEFUSE) studies demonstrated strong associations between recanalization, reperfusion, reduced infarct growth, and favorable clinical response in patients with a PWI/DWI mismatch treated with IV tPA within 3 to 6 hours of symptom onset.6–8
Despite these findings, recent data indicate that PWI/DWI mismatch concept requires refinement to more accurately identify and quantify penumbral tissue. It has been demonstrated that PWI abnormalities often overestimate critical hypoperfusion and that this limitation can be addressed by imposing stricter thresholds on PWI lesion identification. For example, increasing the Tmax delay used to define PWI hypoperfusion in the DEFUSE and EPITHET studies from 2 seconds (s) to 4 to 6 s appears to improve the accuracy of penumbral imaging.9,10 In addition, DWI lesion have been shown to reverse, particularly in the setting of early recanalization, 11–13 suggesting that the ischemic penumbra extends into the acute DWI lesion. Two factors have been hypothesized to influence the reversal of DWI lesions: the severity of the apparent diffusion coefficient (ADC) reduction and the extent of hypoperfusion.14–16
Coregistration of acute DWI and PWI scans with follow-up FLAIR images reveals the spatial relationships between regions of abnormal diffusion and perfusion as well as the fate of these early lesions. Therefore, reversal of DWI lesions can be estimated by calculating the percentage of the acute DWI lesion that does not overlap with the final infarction on 30-day FLAIR in coregistered scans.
To clarify which regions of acute DWI lesions are most likely to represent penumbra versus core, we assessed the relationships between the reversal of baseline DWI lesions and both clinical outcome and early recanalization in the DEFUSE dataset. We hypothesized that the penumbral region of the acute DWI lesion would have the following characteristics:
Reversibility would be greater among patients with a favorable clinical outcome compared to those with unfavorable outcome.
Reversibility would be greater among patients who experienced early recanalization compared to those who did not.
In addition, we hypothesized that the DWI region that displayed the greatest degree of reversibility would have the mildest reduction in ADC values.
Methods
Patients
The inclusion criteria, study design, and primary results of the DEFUSE trial have been previously reported.6 Briefly, patients with an acute ischemic stroke with National Institute of Health Stroke Scale score (NIHSS) greater than 5 were treated with IV tPA 0.9 mg/kg 3 to 6 hours after symptom onset. Participating patients underwent an MRI of the brain before tPA treatment as well as 3 to 6 hours and 1 month afterward. Neurological deficits were evaluated before tPA therapy, at the time of the 2nd MRI scan (3 to 6 hours after tPA), and at 30 and 90 days. A favorable clinical response (FCR) was defined as a NIHSS of 0 to 1 or ≥8 points of improvement between baseline and 30 days.
MRI Protocol
Diffusion-weighted imaging was performed using a spin echo–echo-planar imaging sequence (field of view=240 mm, repetition time=5 s, echo time=minimum allowed, slice thickness=5 mm, number of slices=19, slice gap=2 mm, acquisition matrix 256×256) and lesion volumes determined using a semiautomated thresholding algorithm, which identified regions of high signal intensity that exceeded a region in the contralateral frontal lobe by more than 3 standard deviations. Dynamic susceptibility PWI was performed using gradient echo–echo-planar imaging (field of view=240 mm, repetition time=2 s, echo time=60 ms, slice thickness=7 mm, number of slices=12, slice gap=0, acquisition matrix 128×128, dynamic scans=40) and time to peak of the residue function (Tmax) maps were generated by circular deconvolution of the tissue concentration over time curve using an arterial input function from the contralateral middle cerebral artery.17,18 Baseline DWI and PWI lesions and 30-day FLAIR scans were coregistered using SPM5 (The Wellcome Department of Imaging Neuroscience, Institute of Neurology, University College London, UK).
The acute DWI lesion was divided into 3 regions: areas with no superimposed perfusion abnormality (Tmax delay <2 s), mild to moderate PWI lesions (Tmax delay 2 to 8 s), and severe delays (Tmax >8 s). DWI reversal rates were determined based on the percentage of the acute DWI lesion that did not overlap with the final infarct on 30 day FLAIR.
Magnetic Resonance Angiography
An analysis of source and maximum intensity projection images from the MRA was performed. Criteria for evaluation have been previously reported.8 Briefly, 4 vessels were evaluated at the level of the Cir of Willis: the supraclinoid internal carotid artery (ICA), the middle cerebral artery (MCA), the anterior cerebral artery (ACA), and the posterior cerebral artery (PCA). A numeric scale was used with 1=normal, 2=decreased flow, 3=occluded for the terminal ICA and the first branch of the MCA (M1). A different rating scale was used for the ACA (A1), PCA (P1), and second branch of the MCA (M2): 1=normal; 2=abnormal. The “symptomatic vessel” was identified for all patients with a baseline MRA lesion. This was defined as the vessel that corresponded with the patient’s acute DWI lesion. Recanalization was assessed only among patients with a symptomatic MRA lesion (n=24). The rate of recanalization was defined as complete (occlusion or reduced flow to normal), partial (occlusion to reduced flow), and no recanalization (no change of vessel lesion). Recanalization was dichotomized into partial and complete recanalization versus no recanalization for the analyses performed in the current study.
Study Plan/Statistics
This is a 2-part study with incremental inclusion criteria. The goal was to include as many patients as possible at each step to most accurately explore the relationships assessed.
Part 1: Investigation of the Relationship Between DWI Lesion Reversal and Clinical Outcome
Inclusion criteria included:
A technically adequate baseline DWI and PWI and 30 day FLAIR
A baseline DWI lesion and a baseline PWI lesion >10 cc.
The DWI reversal rate and ADC values were calculated for the total baseline DWI lesion volume as well as for each of the 3 regions defined by the severity of the overlying PWI deficit. Pairwise comparisons were performed between these regions for patients with FCR versus no FCR.
Part 2: Investigation of the Relationship Between DWI Lesion Reversal and Early Recanalization
Inclusion criteria included:
Meets inclusion criteria for Part 1 of the study
An MRA lesion (occluded or reduced flow) in a symptomatic vessel on the baseline scan.
The DWI reversal rate was calculated for the total baseline DWI lesion volume as well as for each of the 3 regions defined by the severity of the overlying PWI deficit based on the presence or absence of recanalization. The reversal rate of the DWI regions was also compared in the 12 patients who had early recanalization.
Statistical Analysis
We used the Mann–Whitney U test to compare groups defined by GCR and recanalization. We used Friedman and Wilcoxon Signed Ranks Tests to estimate differences based on the severity of the perfusion deficit. All probability values for multiple pairwise comparisons were adjusted in a stepwise fashion using the Bonferoni step-down method. An overall significance level was maintained at the α <0.05.
Results
Part 1
Baseline Characteristics
Thirty-two patients met the inclusion criteria (Figure 1 and Table). Their mean (±SE) age was 69 years old (±3) and the baseline median (interquartile range [IQR]) NIHSS was 13 (9 to 16). The median (IQR) time to treatment with IV tPA was 321 minutes (304 to 355) and the median (IQR) baseline DWI lesion volume was 13 cc (7 to 35). The composition of the baseline acute DWI lesion was: DWI with no superimposed perfusion abnormality mean (±SE) 54% (±4%), DWI with superimposed mild-moderate perfusion abnormality (Tmax 2 to 8 s) mean 18% (±2%), and DWI with severely hypoperfused PWI lesion (Tmax >8 s) mean 28% (±4%). The final median infarct volume on 30 day FLAIR was 26 cc (IQR, 13 to 55).
Figure 1.
Flow chart.
Table.
Baseline Characteristics
| DWI Reversal Study n=32 |
|
|---|---|
| Mean age (±SE), y | 69 (±3) |
| White, % | 85% |
| Hypertension, % | 53% |
| Diabetes, % | 28% |
| Smoking, % | 44% |
| Hyperlipidemia, % | 25% |
| Cardiac disease, % | 44% |
| History stroke/TIA, % | 13% |
| Median NIHSS baseline (IQR) | 13 (9–16) |
| Median time to first MRI (IQR), min | 284 (262–304) |
| Median time to treatment (IQR), min | 321 (304–355) |
| Median baseline DWI vol. (IQR), cc | 13 (7–35) |
| Already reperfused DWI Mean (±SE), % | 54% (±4) |
| DWI with superimposed PWI 2–8 s Mean (±SE), % | 18% (±2) |
| DWI with superimposed PWI >8 s Mean (±SE), % | 28% (±4) |
| Median final infarct (30 D FLAIR) vol. (IQR), cc | 26 (13–55) |
Reversal Rate and Clinical Outcome
The median reversal rate for the entire baseline DWI lesion was 43% (IQR=25 to 58), which varied based on the severity of the overlying perfusion deficit (P<0.001). The median DWI reversal rate was significantly higher (48%, IQR 31 to 61) in DWI regions with no PWI lesions compared with areas with Tmax 2 to 8 s (31%, IQR, 17 to 70, P=0.024) and areas with Tmax >8 s (24%, IQR 8 to 49, P<0.001). The reversal rate in the mild-moderately hypoperfused region was also significantly higher than the severely hypoperfused region, P=0.002 (Figure 2a).
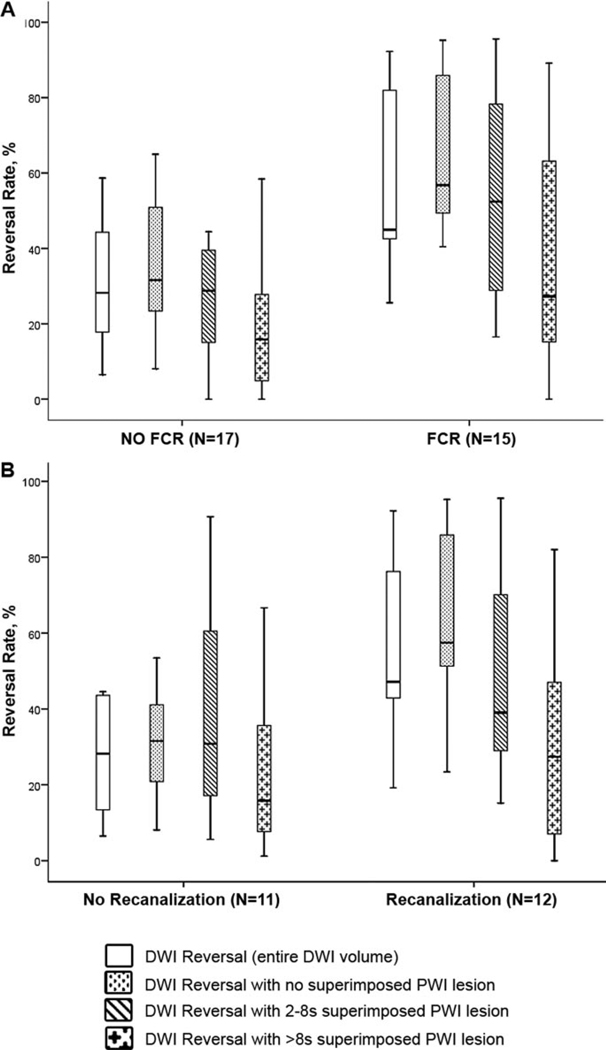
Figure 2.
a, Relationships between DWI Reversal Rate and clinical outcome. b, Relationships between DWI reversal rate and early recanalization.
There was a strong association between DWI reversibility and clinical outcome. Patients with a FCR had a median DWI reversal rate of 45% (IQR=43 to 82) versus 28% (IQR=18 to 44) in patients without FCR (P=0.006). There was also a significant difference in the DWI regions with no superimposed perfusion deficits: median reversal 57% (IQR=49 to 86) with FCR versus 32% (23 to 51) with no FCR (P=0.001), as well as a trend in DWI regions with Tmax 2 to 8 s: 52% (IQR=29 to 78) versus 28% (IQR=15 to 40; P=0.080). There was no significant difference for regions with Tmax >8 s (Figure 3).
Figure 3.
Example of DWI reversal. An 88-year-old man with a baseline NIHSS of 24 had a baseline MRI performed 270 minutes after symptom onset. After the baseline MRI he was treated with intravenous tPA, and a follow-up MRI was obtained 270 minutes after the start of treatment. The follow-up MRI scan (not shown) revealed reperfusion (PWI lesion volume was reduced from 79 cc to 12 cc) and reversal of the DWI lesion (DWI lesion volume was reduced from 42 cc to 12 cc). The final MRI was performed 27 days after symptom onset. The 30-day NIHSS was 2. Tmax Color scale: 2 s<Tmax ≤4 s (Blue); 4 s<Tmax≤6 s (Green); 6 s<Tmax≤8 s (Yellow); Tmax>8 s (Red). A, Baseline DWI: lesion volume 42 cc (normal perfusion 39%; mild-moderately hypoperfused 29% and severely hypoperfused 42%). B, Baseline PWI: lesion volumes according to Tmax delay: Tmax>2 s=79 cc; >4 s=71 cc; >6 s=59 cc and >8 s=34 cc. C, Baseline DWI with the final infarct volume from the 27-day FLAIR superimposed in pink; final infarct volume: 13 cc.
Among the 15 patients who had a FCR, the DWI reversal rate was significantly greater in the region with normal perfusion: 57% (IQR=49 to 86) compared with 52% (IQR=29 to 78) in areas with Tmax 2 to 8 s (P=0.011) and 27% (IQR=15 to 63; P=0.003) in areas with Tmax >8 s. Reversal in the DWI regions with Tmax 2 to 8 s was also significantly greater than in Tmax >8 s regions (P=0.010).
Comparisons of the Mean ADC Values of the DWI Lesion
The mean (±SE) ADC value in the DWI lesions were as follows: total DWI lesion, 632 (±0.6)×10−6 mm2/s; DWI with no superimposed PWI lesion, 665 (±0.9)×10−6 mm2/s; DWI with any super imposed PWI lesion defined by Tmax >2 s, 601 (±0.9) ×10−6 mm2/s; DWI with superimposed mild-moderate perfusion abnormality, 636 (±1.4) × 10−6 mm2/s; and DWI with superimposed severe perfusion abnormality, 582 (±1.0) × 10−6 mm2/s. These values were significantly different (P<0.001) overall. The mean ADC value was significantly higher in DWI regions with no PWI lesion compared with DWI regions with any superimposed PWI lesion (Tmax >2 s; P=0.008); as well as DWI regions with Tmax >8 s (P<0.001).The mean ADC value in DWI regions with Tmax 2 to 8 s was also significantly higher than in Tmax >8 s regions (P<0.001).
Part 2
Twenty-three patients were eligible for Part 2 of the study; 9 cases were removed from analysis: 4 had no lesion on their initial MRA, and 5 had a technically inadequate baseline (n=4) or follow-up (n=1) MRA. There were no statistically significant differences between the Part 1 and Part 2 patients regarding age, sex, baseline NIHSS, baseline DWI volume, final infarct volume, time to treatment, and time to MRI. Early recanalization occurred in 52% (n=12) of these patients in Part 2. The location of the initial symptomatic lesions were: Combined (ICA+MCA)=6, M1 segment of the MCA=14, and M2 segment of MCA=3.
Early recanalization was associated with substantial DWI reversal; patients with recanalization reversed nearly half of the baseline DWI lesion (median reversal 47% [IQR=42 to 76] versus 28% [13 to 44] in patients who did not recanalize, P=0.016). This difference in the reversal rates occurred primarily in the region of the DWI lesion that did not have a superimposed PWI deficit at baseline (57% [IQR=52 to 86] in recanalizers versus 32% [IQR=21 to 41] in nonrecanalizers, P=0.015; Figure 2b). The reversal rate of the regions with superimposed PWI lesions was not statistically different. The amount of DWI reversal among patients who recanalized was also significantly greater in regions that did not have a baseline PWI lesion than in areas with Tmax 2 to 8 s (57% versus 39%, P=0.024) or regions with Tmax >8 s (57% versus 27%, P=0.006), as well as in regions with mild-moderate versus severe PWI lesions (P=0.024).
Discussion
The results of this study demonstrate that substantial proportions of DWI lesions, imaged 3 to 6 hours after stroke onset, are potentially reversible and that the regions most likely to show reversal do not have superimposed PWI deficits and have only modest reductions in ADC values. The absence of a corresponding PWI lesion suggests that these areas of isolated DWI positivity likely experienced spontaneous early reperfusion before initial imaging, and suggests that early reperfusion mediates DWI reversibility. This assumption is supported by our findings that subsequent recanalization and milder PWI deficits were also associated with DWI reversal. Based on these findings, we propose the term RADAR (Reversible Acute Diffusion lesion Already Reperfused) to describe regions of acute restricted diffusion that do not have overlying perfusion abnormalities.
The fact that approximately half of the acute DWI lesion in stroke patients imaged 3 to 6 hours after onset appears to have undergone spontaneous reperfusion has not been previously reported. Even among the patients who had a symptomatic MRA occlusion still present (Part 2 of this study), 54% of the baseline DWI lesion did not have a superimposed perfusion deficit. This finding implies that the degree of regional hypoperfusion may fluctuate significantly during the early hours after vessel occlusion, although the mechanisms that underlie this are not clear. Possible explanations include variations in local perfusion pressure, recruitment of collaterals, or fluctuations in metabolic activity.
The observation that DWI reversal was associated with favorable clinical response suggests that DWI reversibility may be of clinical importance and supports previous studies that suggest the ischemic penumbra extents into the acute DWI lesion.11,16 Our findings imply that the “already reperfused” DWI region (RADAR) is most likely to represent part of the ischemic penumbra; however, even regions with superimposed PWI lesions were partially reversible, indicating that penumbral tissue may be diffusely present throughout acute DWI lesions. However, we found that the severely hypoperfused regions (defined by Tmax >8 s) had low ADC values and were unlikely to reverse after recanalization. Furthermore, the limited DWI reversal that did occur in these regions was not associated with favorable clinical outcome. Therefore, severely hypoperfused regions with low ADC values are likely to reflect irreversible “infarct core” when imaged at 3 to 6 hours. Assessment of the severity of hypoperfusion, ADC value, and time since symptom onset are all likely to facilitate prediction of the fate of acute DWI lesions (Figure 3).
Study Limitations
The primary limitation of our study is small sample size. Only 32 of the 74 DEFUSE patients were eligible for this substudy. The primary reason for exclusion was 18 patients did not have a DWI lesion and at least a 10 cc PWI lesion at baseline. DEFUSE included consecutive eligible patients, and those with very small baseline PWI volumes were removed from the coregistration analyses. The second most common reason for exclusion was a failure to obtain the 30-day FLAIR. This often occurred because of death before 30 days or severe disability that precluded returning to MRI scanner. Obtaining the final infarct volume at an earlier time point could help reduce this deficiency in further studies.
Data from Part 2 apply only to patients who have a visible MRA lesion 3 to 6 hours after symptom onset. These patients are likely the most appropriate target group for recanalization therapy in this time window. DEFUSE is an exploratory pilot study in which all patients were treated with tPA after the baseline MRI. Hence it is not possible to separate the effects of spontaneous versus “tPA-induced” recanalization. Finally, our data cannot verify that the regions of baseline DWI abnormality that did not have superimposed PWI lesions actually had significant perfusion deficits before initial imaging. It is conceivable that these DWI lesions were caused by an alternative mechanism such as shifts in extracellular water content related to ischemia in adjacent brain regions.
Conclusion
Coregistration of acute DWI and PWI with 30-day FLAIR images revealed that almost half of the acute DWI lesion appeared to be reperfused 3 to 6 hours after symptom onset and that this region is likely to represent penumbral tissue.
Acknowledgments
Sources of Funding
The funding for this study was provided by National Institutes of Health (NIH) grants RO1 NS39325, Principal Investigator, Gregory W. Albers; K24 NS044848, Principal Investigator, Gregory W. Albers; and K23 NS051372, Principal Investigator Maarten G. Lansberg. tPA was supplied at no charge by Genentech (U.S. and Canada sites) and Boehringer Ingelheim (Belgium site). Neither Genentech, Boehringer Ingelheim, nor the NIH played a role in the design and the conduct of the study; collection, management, analysis, and interpretation of the data; or preparation or approval of the manuscript.
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None.
References
- 1.Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- 2.Hossmann KA. Viability thresholds and the penumbra of focal ischemia [see comments] AnnNeurol. 1994;36:557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- 3.Moseley ME, Kucharczyk J, Mintorovitch J, Cohen Y, Kurhanewicz J, Derugin N, Asgari H, Norman D. Diffusion-weighted MR imaging of acute stroke: Correlation with T2-weighted and magnetic susceptibility-enhanced MR imaging in cats. AJNRAmJNeuroradiol. 1990;11:423–429. [PMC free article] [PubMed] [Google Scholar]
- 4.Hjort N, Christensen S, Solling C, Ashkanian M, Wu O, Rohl L, Gyldensted C, Andersen G, Ostergaard L. Ischemic injury detected by diffusion imaging 11 minutes after stroke. Ann Neurol. 2005;58:462–465. doi: 10.1002/ana.20595. [DOI] [PubMed] [Google Scholar]
- 5.Shih LC, Saver JL, Alger JR, Starkman S, Leary MC, Vinuela F, Duckwiler G, Gobin YP, Jahan R, Villablanca JP, Vespa PM, Kidwell CS. Perfusion-weighted magnetic resonance imaging thresholds identifying core, irreversibly infarcted tissue. Stroke. 2003;34:1425–1430. doi: 10.1161/01.STR.0000072998.70087.E9. [DOI] [PubMed] [Google Scholar]
- 6.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, Bammer R, Kakuda W, Lansberg MG, Shuaib A, Coplin W, Hamilton S, Moseley M, Marks MP. Magnetic resonance imaging profiles predict clinical response to early reperfusion: The diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 7.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, Barber PA, Bladin C, De Silva DA, Byrnes G, Chalk JB, Fink JN, Kimber TE, Schultz D, Hand PJ, Frayne J, Hankey G, Muir K, Gerraty R, Tress BM, Desmond PM. Effects of alteplase beyond 3 h after stroke in the echoplanar imaging thrombolytic evaluation trial (epithet): A placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 8.Olivot JM, Mlynash M, Thijs VN, Kemp S, Lansberg MG, Wechsler L, Schlaug G, Bammer R, Marks MP, Albers GW. Relationships between infarct growth, clinical outcome, and early recanalization in diffusion and perfusion imaging for understanding stroke evolution (DEFUSE) Stroke. 2008;39:2257–2263. doi: 10.1161/STROKEAHA.107.511535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takasawa M, Jones PS, Guadagno JV, Christensen S, Fryer TD, Harding S, Gillard JH, Williams GB, Aigbirhio FI, Warburton EA, Ostergaard L, Baron JC. How reliable is perfusion MR in acute stroke? Validation and determination of the penumbra threshold against quantitative PET. Stroke. 2008;39:870–877. doi: 10.1161/STROKEAHA.107.500090. [DOI] [PubMed] [Google Scholar]
- 10.Olivot JM, Mlynash M, Thijs V, Lansberg MG, Kemp S, Wechsler L, Bammer R, Marks M, Albers G. Optimal tmax threshold for predicting penumbral tissue in acute stroke. Stroke. 2009;40:469–475. doi: 10.1161/STROKEAHA.108.526954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kidwell CS, Saver JL, Mattiello J, Starkman S, Vinuela F, Duckwiler G, Gobin YP, Jahan R, Vespa P, Kalafut M, Alger JR. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann Neurol. 2000;47:462–469. [PubMed] [Google Scholar]
- 12.Kidwell CS, Saver JL, Starkman S, Duckwiler G, Jahan R, Vespa P, Villablanca JP, Liebeskind DS, Gobin YP, Vinuela F, Alger JR. Late secondary ischemic injury in patients receiving intraarterial thrombolysis. Ann Neurol. 2002;52:698–703. doi: 10.1002/ana.10380. [DOI] [PubMed] [Google Scholar]
- 13.Rother J, de Crespigny AJ, D’Arceuil H, Iwai K, Moseley ME. Recovery of apparent diffusion coefficient after ischemia-induced spreading depression relates to cerebral perfusion gradient. Stroke. 1996;27:980–986. doi: 10.1161/01.str.27.5.980. discussion 986–987. [DOI] [PubMed] [Google Scholar]
- 14.Oppenheim C, Grandin C, Samson Y, Smith A, Duprez T, Marsault C, Cosnard G. Is there an apparent diffusion coefficient threshold in predicting tissue viability in hyperacute stroke? Stroke. 2001;32:2486–2491. doi: 10.1161/hs1101.098331. [DOI] [PubMed] [Google Scholar]
- 15.Fiehler J, Foth M, Kucinski T, Knab R, von Bezold M, Weiller C, Zeumer H, Rother J. Severe adc decreases do not predict irreversible tissue damage in humans. Stroke. 2002;33:79–86. doi: 10.1161/hs0102.100884. [DOI] [PubMed] [Google Scholar]
- 16.Fiehler J, Knudsen K, Kucinski T, Kidwell CS, Alger JR, Thomalla G, Eckert B, Wittkugel O, Weiller C, Zeumer H, Rother J. Predictors of apparent diffusion coefficient normalization in stroke patients. Stroke. 2004;35:514–519. doi: 10.1161/01.STR.0000114873.28023.C2. [DOI] [PubMed] [Google Scholar]
- 17.Ostergaard L, Sorensen AG, Kwong KK, Weisskoff RM, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part II: Experimental comparison and preliminary results. Magn Reson Med. 1996;36:726–736. doi: 10.1002/mrm.1910360511. [DOI] [PubMed] [Google Scholar]
- 18.Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part i: Mathematical approach and statistical analysis. Magn Reson Med. 1996;36:715–725. doi: 10.1002/mrm.1910360510. [DOI] [PubMed] [Google Scholar]