Abstract
Invariant natural killer T (iNKT) cells recognize glycolipid antigens such as the marine sponge-derived glycosphingolipid α-galactosylceramide (αGalCer) presented by the CD1d protein. In vivo activation of iNKT cells with αGalCer results in robust cytokine production followed by the acquisition of an anergic phenotype. Here, we have investigated mechanisms responsible for the establishment of αGalCer-induced iNKT cell anergy. We found that αGalCer-activated iNKT cells rapidly upregulated expression of the inhibitory co-stimulatory receptor programmed death (PD)-1 at their cell surface, and this increased expression was retained for at least one month. Blockade of the interaction between PD-1 and its ligands, PD-L1 and PD-L2, at the time of αGalCer treatment prevented the induction iNKT cell anergy, but was unable to reverse established iNKT cell anergy. Consistently, injection of αGalCer into PD-1-deficient mice failed to induce iNKT cell anergy. However, blockade of the PD-1:PD-L pathway failed to prevent bacterial- or sulfatide-induced iNKT cell anergy, suggesting additional mechanisms of iNKT cell tolerance. Finally, we showed that blockade of PD-1:PD-L interactions enhanced the antimetastatic activities of αGalCer. Collectively, our findings reveal a critical role for the PD-1:PD-L costimulatory pathway in the αGalCer-mediated induction of iNKT cell anergy that can be targeted for the development of immunotherapies.
Introduction
Invariant natural killer T cells (iNKT)3 are a unique subset of T lymphocytes that recognize glycolipid antigens in the context of the antigen-presenting glycoprotein CD1d (1–5). iNKT cells express a semi-invariant T cell receptor (TCR) together with surface markers that are characteristic of the natural killer (NK) cell lineage (6). iNKT cells can react with glycosphingolipids derived from Sphingomonas bacteria (7–9), diacylglycerols from Borrelia burgdorferi (10), the endogenous glycolipid isoglobotrihexosylceramide (11), and the marine sponge-derived antigen α-galactosylceramide (αGalCer) (12). Glycolipid-activated iNKT cells produce copious amounts of cytokines, which endow these cells with potent immunoregulatory properties (13). Consequently, iNKT cells have been implicated in regulating a variety of immune responses (1–4). These potent immunoregulatory properties of iNKT cells are being exploited for the development of vaccine adjuvants (14), immunotherapy of cancer (15, 16) and prevention of autoimmune and inflammatory conditions (17).
In order to develop safe and effective iNKT cell-based therapies, it is critically important to obtain a thorough understanding of the in vivo response of iNKT cells to glycolipid antigen stimulation. This issue is best understood for the prototypical iNKT cell antigen αGalCer. In vivo activation of iNKT cells with αGalCer results in dynamic changes in the iNKT cell population, which is characterized by surface TCR down-modulation, robust cytokine secretion, clonal expansion, homeostatic contraction, and acquisition of an anergic phenotype (18–25). iNKT cells rendered anergic in this manner failed to protect mice against the development of metastatic B16 melanomas in the lung upon re-stimulation with αGalCer (21). Instead, αGalCer exacerbated B16 lung metastases in the αGalCer-experienced animals, raising concerns regarding the utility of repeated αGalCer therapy in a multi-dose setting (21). These findings highlight the need for effective means to avoid the induction of iNKT cell anergy and to break anergy once it has been established.
Programmed death (PD)-1 (CD279) is a member of the CD28 family of co-stimulatory molecules (26, 27). Interaction of PD-1 on T cells with its ligands, PD-L1 (B7-H1 or CD274) and PD-L2 (B7-DC or CD273) on antigen-presenting cells (APC), delivers inhibitory signals to T cells. PD-1 and its ligands have been implicated in the induction and maintenance of tolerance in conventional T cells in a variety of settings (26, 27). Consistent with the role of PD-1 in regulating T cell tolerance, PD-1-deficient mice exhibit increased susceptibility to the development of autoimmunity. In the C57BL/6 background, aged PD-1-deficient mice develop glomerulonephritis and arthritis with deposition of IgG3 and C3 in the glomeruli (28), whereas in the BALB/c background, aged knockout animals manifest cardiomyopathy due to autoantibody production against heart troponin (29, 30). A direct role for the PD-1:PD-L pathway in the induction and maintenance of anergy in conventional CD4+ and CD8+ T lymphocytes has also been documented (31–33).
We hypothesized that the PD-1:PD-L pathway plays a critical role in the induction of anergy in glycolipid-activated iNKT cells. We have found that antibody-mediated blockade of PD-1:PD-L interactions at the time of αGalCer treatment prevented the induction of iNKT cell anergy in wild-type animals. Likewise, PD-1-deficient mice were resistant to the development of αGalCer-induced iNKT cell anergy. Moreover, blockade of PD-1:PD-L interactions enhanced the antitumor activities of αGalCer in mice. However, despite its critical role in αGalCer-induced iNKT cell anergy, the PD-1:PD-L pathway did not appear to be required for bacterial- or Type II NKT cell-mediated anergy, suggesting multiple pathways for iNKT tolerance. These findings reveal a critical role of the PD-1:PD-L costimulatory pathway for glycolipid-mediated induction of iNKT cell anergy, with important implications for the development of iNKT cell-based therapies.
Materials and Methods
Mice
Female C57BL/6 (B6) and adult thymectomized B6 mice were purchased from the Jackson Laboratory. PD-1-deficient mice (34) on a B6 background were obtained from Dr. Tasuku Honjo (Kyoto University, Yoshida-Konoe, Sakyo-ku, Kyoto, Japan) via Dr. Megan Sikes (Transplantation Biology Research Center, Massachusetts General Hospital, Harvard Medical School, Boston, MA). All animal studies were approved by the Institutional Animal Care and Use Committee of Vanderbilt University.
Reagents
αGalCer (KRN7000) was obtained from Kirin Brewery Co. and sulfatide was obtained from Matreya Inc. These lipids were reconstituted in PBS containing 0.5% polysorbate-20 (Sigma-Aldrich). Biotinylated CD1d monomers were obtained from the NIAID Tetramer facility (Germantown, MD) and fluorescently labeled tetrameric CD1d molecules were loaded with αGalCer as described previously (35). Anti-TCR-β-fluorescein isothiocyanate (FITC) and -allophycocyanin, anti-NK1.1-phycoerythrin (PE) and -allophycocyanin, anti-CD11c-allophycocyanin, anti-B220-peridinin chlorophyll protein (PerCP), anti-IL-4-PE, anti-IFN-γ-FITC, and anti-CD69-FITC, were obtained from BD Biosciences-Pharmingen. Anti-PD-1-PE, -PD-L1-PE and -PD-L2-PE were obtained from eBioscience. For blocking experiments, purified hamster anti-mouse PD-1 (J43 (36)) or control hamster antibodies, rat anti-mouse PD-L1 (MIH5 (31), Riken Cell Bank, Ibaraki, Japan) and rat anti-mouse PD-L2 (TY25 (37)) or control rat antibodies were used.
Flow cytometry
Single-cell suspensions of the spleen and liver were prepared and stained with fluorescently labeled mAbs as described previously (38). In all experiments, dead cells were excluded from the analysis by electronic gating. The iNKT cell population was identified as B220−TCR-β+tetramer+ cells. For analysis of intracellular cytokines produced by iNKT in vivo, mice were injected with αGalCer or vehicle, sacrificed 1 hour later, and splenocytes or liver mononuclear cells were cultured in Golgi Plug™ (BD Biosciences-Pharmingen) for an additional 2 hours. For measurement of intracellular IFN-γ levels of NK cells, mice were injected with αGalCer or vehicle, sacrificed 6 hours later, and splenocytes were cultured in Golgi Plug™ (BD Biosciences-Pharmingen) for an additional 2 hours. Intracellular cytokine staining was performed with Cytofix/Cytoperm reagents (BD Biosciences-Pharmingen) according to the manufacturer’s protocol. For staining of DC, Fc receptors were first blocked by addition of anti-CD16/32 antibodies (BD Biosciences-Pharmingen), and DC were identified on the basis of high CD11c expression. Flow cytometry was performed using a FACSCalibur instrument (BD), and the acquired data were analyzed using FlowJo software (Tree Star Inc.).
Measurement of iNKT cell anergy
For evaluation of αGalCer-induced iNKT cell anergy, mice were injected i.p. with 2 μgαGalCer in 200 μl PBS containing 0.025% polysorbate-20 (vehicle) as described previously (21). Blocking antibodies directed against PD-1 or its ligands, or control antibodies were administered at times indicated in figures. Mice were analyzed around 1 month after the initial αGalCer injection for the measurement of iNKT cell recall responses ex vivo or in vivo. For measurement of in vitro responses, mice were sacrificed and spleen cell suspensions were plated in U-bottomed 96-well plates at 2 × 105 cells per well in RPMI medium containing 10% FCS (R-10) in the presence of titrated doses of αGalCer or vehicle. For proliferation assays, 1 μCi of [3H]thymidine (PerkinElmer Life Sciences) was added to the wells after 60 hours of culture, and cells were cultured for an additional 12 hours. Cells were then harvested, and uptake of radioactivity was measured. For measurement of cytokine secretion, supernatants were harvested after 60 hours of culture, and cytokine levels were evaluated by ELISA. For in vivo recall responses, the mice were reinjected with 2 μg of αGalCer followed by evaluation of intracellular cytokine expression by iNKT cells at 1 hour, IFN-γ production and cytotoxicity by NK cells at 6 hours, serum IFN-γ production at 1 day and in vivo expansion at 3 days. For evaluation of bacteria-induced iNKT cell anergy, mice were injected i.v. with heat-killed E. coli (ATCC, catalog no. 25922) (0.5 ×109 CFU in 200 μl PBS) as described (39). Mice were tested for ex vivo and in vivo responses to αGalCer stimulation three weeks later. For evaluation of sulfatide-induced iNKT cell anergy, mice were injected i.p. with 20 μg sulfatide and analyzed for ex vivo responses to αGalCer 24 hours later as described (40), or injected with αGalCer for evaluation of iNKT cell proliferation and IFN-γ production as described above.
ELISA
A standard sandwich ELISA was performed to measure mouse IFN-γ and IL-4 levels. IFN-γ- and IL-4-paired antibodies and cytokine standards were obtained from BD Biosciences-Pharmingen. For detection, streptavidin-HRP conjugate (Zymed Laboratories Inc.) was used, and the color was developed with the substrate 3,3′,5,5′-tetramethylbenzidine (Dako Corp.) in the presence of H2O2.
CFSE dilution analysis
Liver mononuclear cells were labeled with 5 mM CFSE (Invitrogen) for 10 minutes at 37°C in PBS containing 0.1% BSA and washed twice with complete RPMI medium. Labeled cells (3 ×105 cells/well) were then stimulated with αGalCer for 96 hours in complete RPMI. At the end of the culture, cells were harvested, stained with anti-TCR-β and CD1d-tetramers and analyzed by flow cytometry.
Determination of B16 melanoma lung metastases
Mice were injected i.v. with 5 × 105 B16 melanoma cells suspended in PBS. Mice were treated with αGalCer or vehicle at 0, 3, and 7 days. In some experiments, αGalCer was injected at 5, 8 and 12 days after melanoma injection. After 15–17 days of challenge with melanoma cells, mice were sacrificed, lungs were removed, and the number of metastatic nodules was counted as described previously (21, 24).
NK cell cytotoxicity assay
NK cell cytotoxicity was evaluated against the NK cell targetcell line YAC-1, in a standard 51Cr release assay. Data arepresented as the percent specific lysis, which was calculatedas follows: 100 × ((experimental release − spontaneousrelease)/(maximum release − spontaneous release)).
Statistics
Statistical significance between multiple groups was determined by application of ANOVA followed by Bonferroni post-hoc test. The samples were first subjected to Kolmogorov-Smirnov normality test with Dallal-Wilkinson-Lillie for P value. When samples did not follow normal distribution, Kruskal-Wallis followed by Dunn’s post-hoc test was used instead. A P value less than 0.05 was considered significant for the multiple comparison tests. All the statistical analyses were performed using Graphpad Prism software (La Jolla, CA).
Results
αGalCer treatment preferentially upregulates PD-1 on iNKT cells
Consistent with its expression in conventional T cells (36), resting iNKT cells expressed very low levels of PD-1 (Fig. 1). However, αGalCer treatment resulted in the rapid induction of PD-1 on iNKT cells in liver and spleen (Fig. 1). Profound PD-1 induction was detected within 12 hours of αGalCer treatment and its expression was maintained at high levels during the expansion (day 3) and contraction phases (days 7–15) of the iNKT cell population. Interestingly, PD-1 expression on iNKT cells was maintained during the anergic phase of αGalCer-experienced iNKT cells, and at least up to 30 days after the initial αGalCer treatment. Similar results were obtained in thymectomized animals (Supplementary Fig. 1), indicating that the induction and maintenance of PD-1 expression on iNKT cells was thymus-independent. Furthermore, similar induction of PD-1 expression following αGalCer treatment was not detected on conventional T cells (Fig. 1), suggesting that its expression was specific to iNKT cells. Expression of CTLA-4, another member of the CD28 family of co-stimulatory molecules that delivers inhibitory signals to T cells (41), remained largely unaltered on iNKT cells after αGalCer treatment (Supplementary Fig. 2).
Figure 1.
Expression of PD-1 on iNKT cells following αGalCer stimulation in vivo. Mice were injected with 2 μg of αGalCer, spleen and liver mononuclear cells were prepared at the indicated time points, and cells were stained with anti-TCR-β-FITC, tetramer-allophycocyanin, anti-B220-PerCP, and PD-1-PE or PE-labeled hamster isotype control and analyzed by flow cytometry. Numbers indicate the percentage of TCR-β+tetramer+ cells among B220− cells. A representative of 5 separate experiments is shown.
αGalCer treatment transiently induces the expression of PD-1 ligands on antigen presenting cells
Because PD-1 interacts with PD-L1 and PD-L2 on APC (26, 27), we investigated the expression profiles of PD-L1 and PD-L2 on dendritic cells (DC) and B cells. Consistent with prior studies (37, 42), DC and B cells constitutively expressed PD-L1 but expressed very low levels of PD-L2 (Fig. 2). Upon treatment with αGalCer, PD-L1 became upregulated on both types of APC between 12 hours and 3 days but returned to pre-injection levels by 7 days. αGalCer similarly induced a transient expression of PD-L2 on DC, whereas its expression of B cells remained unaltered. Thus, activation of iNKT cells by αGalCer resulted in transient induction of PD-1 ligands on DC and B cells.
Figure 2.
Expression of PD-L1 and PD-L2 on APC following αGalCer injection in vivo. Mice were treated as in Figure 1 and spleen cells were stained with anti-B220-PerCP, anti-CD11c-APC, and anti-PD-L1-PE, anti-PD-L2-PE or isotype control anti-rat IgG2a-PE. A representative of 3 separate experiments is shown.
Blockade of PD-1:PD-L interactions prevents the induction of iNKT cell anergy mediated by αGalCer
To investigate the role of the PD-1:PD-L pathway in the induction and maintenance of iNKT cell anergy, we tested the effects of PD-1:PD-L blockade on the development of iNKT cell anergy. For this purpose, we employed anti-PD-1 blocking antibodies or a combination of blocking antibodies directed against PD-L1 and PD-L2. In a first set of experiments mice were treated with blocking or control antibodies every 3–4 days after αGalCer injection for a period of one month (Fig. 3A). iNKT cells were then re-stimulated either ex vivo or in vivo with αGalCer and iNKT cell proliferative and cytokine responses were measured (Fig. 3, B and C). Consistent with prior results, splenocytes from vehicle-treated mice proliferated and produced cytokines in response toαGalCer stimulation ex vivo, whereas splenocytes from mice treated with αGalCer or with αGalCer and control antibody demonstrated a blunted response to ex vivo restimulation with αGalCer. In sharp contrast, splenocytes from mice treated with αGalCer and anti-PD-L1/L2 antibodies retained their capacity to respond to the subsequent αGalCer stimulation. Nevertheless, this abrogation of iNKT cell anergy was not complete. A similar but less drastic effect was observed for mice treated with αGalCer and anti-PD-1 antibodies, likely because the anti-PD-1 antibody employed is not as effective in blocking PD-1:PD-L interactions (36, 43, 44). Similar results were obtained when the secondary response of iNKT cells to αGalCer was investigated in vivo. iNKT cells from mice treated with vehicle expanded profoundly in response to in vivo injection with αGalCer, whereas mice treated with αGalCer in the absence or presence of control antibody were resistant to αGalCer-induced expansion. However, iNKT cells from mice treated with αGalCer and anti-PD-L1/L2 antibodies expanded substantially in response to αGalCer re-injection. Consistent with the in vitro studies, anti-PD-1 antibodies had little effect on the capacity of αGalCer-experienced iNKT cells to respond to the secondary stimulation with this antigen.
Figure 3.
PD-1:PD-L blockade prevents the induction of iNKT cell anergy mediated by αGalCer. (A) Treatment scheme. Mice were injected with 2 μg of αGalCer or its vehicle together with anti-PD-1 (300 μg), anti-PD-L1 plus anti-PD-L2 (300 μg each) or normal hamster or rat IgG antibodies (300 μg). Thereafter, antibodies (200 μg/antibody) were repeatedly administered every 3–4 days as indicated. Mice were analyzed for αGalCer recall responses ex vivo or in vivo at 32–35 days after the initial αGalCer injection, on the day of the last antibody treatment. (B) Mice were treated as illustrated in A. Splenocytes (2 × 105 per well) were cultured with graded doses of αGalCer and after 3 days, proliferation was assessed by [3H]thymidine incorporation, and culture supernatants were evaluated for IL-4 and IFN-γ levels by ELISA. Proliferation results represent the mean ± SEM of 3 mice in each group. Representative data of 2 individual experiments are shown. (C) The mice were treated as shown in A and groups of mice were injected in vivo with 2μg of αGalCer or vehicle control to measure iNKT cell expansion. After 3 days, mice were sacrificed and splenocytes were stained with anti-TCR-β-FITC, tetramer-allophycocyanin and anti-B220-PerCP. Data shown are representative of an experiment with 3 mice per group, from a total of 2 individual experiments.
Next, we determined whether the PD-1:PD-L pathway was critical for the induction and/or maintenance of iNKT cell anergy. When antibody treatment was initiated 10 days after the initial αGalCer injection, iNKT cells retained their anergic phenotype (Fig. 4), suggesting that the PD-1:PD-L pathway was dispensable for maintaining iNKT cell anergy once it had been established. Consistent with this finding, treatment of splenocyte cultures from αGalCer-experienced mice with anti-PD-L1/L2 antibodies was unable to overcome iNKT cell anergy (data not shown). In sharp contrast, antibody treatment during the first week following the initial αGalCer injection (treatment at day 0, 3 and 7) rendered iNKT cells fully reactive to the secondary stimulation with αGalCer (Fig. 5). Interestingly, however, this antibody treatment regimen did not impact PD-1 expression on iNKT cells during the course of the experiment (data not shown), suggesting that the rescue of iNKT cell function was not due to a blockade in PD-1 induction.
Figure 4.
Blockade of PD-1:PD-L interactions cannot reverse iNKT cell anergy once it has been established. Mice were injected with 2 μg of αGalCer or its vehicle together with the indicated antibodies, starting at day 10 after αGalCer injection. Anti-PD-1 (300μg), anti-PD-L1 plus anti-PD-L2 (300 μg each), or normal hamster or rat IgG antibodies were injected the first time and 200 μg of each antibody were used for repeated injections every 3–4 days as shown (A). The mice were analyzed for αGalCer recall responses ex vivo (B) or in vivo (C) at 32–35 days after the initial αGalCer injection, on the day of the last antibody treatment. Mice were analyzed as in Figure 3. Data shown are representative of an experiment with 3 mice per group, from a total of 2 individual experiments.
Figure 5.
Blockade of PD-1:PD-L interactions at the time of αGalCer priming prevents the induction of iNKT cell anergy. Mice were injected with 2 μg of αGalCer or its vehicle along with antibody treatment given three times at day 0, 3 and 7 (A). 300 μg of the respective antibodies were injected the first time and subsequently at 200 μg. The mice were analyzed for αGalCer recall responses ex vivo (B) or in vivo (C–E) at 32–35 days after the initial αGalCer injection. Mice were analyzed as in Figure 3. (D, E) Spleen cells (D) and liver mononuclear cells (E) were surface stained with B220-PerCP and tetramer-allophycocyanin followed by intracellular staining with anti-IL-4-PE and -IFN-γ-FITC. Data shown are representative of an experiment with 2 mice per group, from a total of 3 individual experiments.
Collectively, our results indicated a critical role for PD-1:PD-L interactions in the induction of iNKT cell anergy, but that disruption of these interactions did not reverse already established anergy.
Blockade of PD-1:PD-L interactions during initial αGalCer treatment restores the antimetastatic activities of αGalCer-activated iNKT cells
αGalCer exhibits potent antimetastatic activities in mice (1). We have previously shown that the anergic phenotype of iNKT cells has a profound impact on the therapeutic activities of αGalCer, resulting in exacerbation of cancer metastasis (21), thus raising concerns regarding the utility of repeated αGalCer treatment during cancer therapy. We therefore tested the effects of PD-1:PD-L blockade during the initial αGalCer treatment on the subsequent capacity of αGalCer to protect mice against B16 tumor metastases. Mice were treated with αGalCer in the presence or absence of PD-1:PD-L blockade, and one month after the primary αGalCer injection, mice were challenged with B16 melanoma cells and treated with αGalCer. Consistent with our prior studies (21), we found that αGalCer was effective in clearing tumors from vehicle-treated mice but exacerbated tumor development in αGalCer-experienced mice (Fig. 6). In sharp contrast, iNKT cells from mice treated with anti-PD-L1/L2 antibodies at the time of primary αGalCer injection retained their capacity to clear B16 tumor cells. Because the anti-metastatic activities of αGalCer involve IFN-γ secretion and NK cell transactivation (45, 46), we also investigated whether PD-1:PD-L blockade was able to restore these aspects of iNKT cell function. Results showed that iNKT cells from mice previously treated with αGalCer in the presence of anti-PD-L1/L2 antibodies, upon re-injection with αGalCer, were able to induce serum IFN-γ levels and transactivate NK cells to produce IFN-γ and exhibit cytotoxicity against YAC-1 target cells (Supplementary Fig. 3).
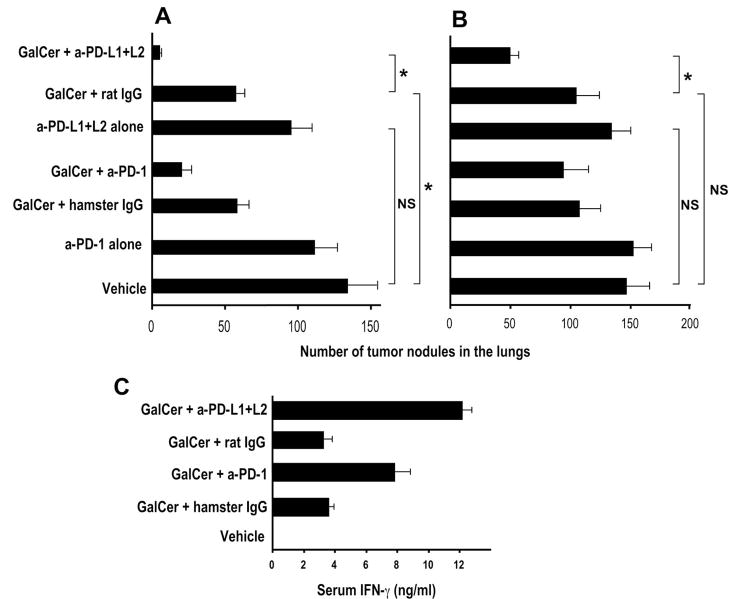
Figure 6.
Blockade of PD-1:PD-L interactions during initial αGalCer treatment restores the antimetastatic activities of αGalCer-activated iNKT cells. B6 mice were injected with 2 μg of αGalCer or its vehicle along with the indicated antibodies at 0, 3, and 7 days (300, 200 and 200 μg of each antibody, respectively). One month later, mice were challenged i.v. with 3 × 105 B16 melanoma cells and treated with αGalCer (2μg/injection) or vehicle at 0, 3, and 7 days after the tumor challenge. Mice were sacrificed 15 days after tumor challenge and the numbers of metastatic nodules were counted in the lungs. Results shown are the mean ± SEM of 2 experiments with a total of 8–9 mice in each group. *P<0.05.
PD-1-deficient mice are resistant to αGalCer-induced iNKT cell anergy
To further define the role of PD-1 in the induction of iNKT cell anergy by αGalCer, we employed PD-1-deficient mice (34). These animals displayed a modest reduction in iNKT cell numbers in spleen and liver as compared with wild-type mice (Fig. 7A) and iNKT cells from these animals exhibited a modestly increased response to αGalCer stimulation (Fig. 7A-C and data not shown). To assay iNKT cell anergy, we injected PD-1-deficient and wild-type control mice with vehicle or αGalCer, and one month later, we rechallenged mice in vivo with αGalCer followed by evaluation of iNKT cell expansion and cytokine production. Unlike iNKT cells from αGalCer-treated wild-type mice, iNKT cells from αGalCer-treated PD-1-deficient mice responded robustly to αGalCer restimulation, with respect to their capacity to expand (Fig. 7, A and B) and produce cytokines (Fig. 7C). Nevertheless, our data suggested that αGalCer-treated PD-1-deficient mice still had a modest reduction in their response to αGalCer rechallenge (Fig. 7A–C), suggesting that additional pathways contribute to the induction of iNKT cell anergy.
Figure 7.
αGalCer fails to induce iNKT cell anergy in PD-1-deficient mice. PD-1−/− and wild-type B6 mice were injected with αGalCer (2 μg/mouse, i.p.) or vehicle control. (A) One month later groups of mice were re-injected with αGalCer or vehicle. Three days later, single-cell suspensions of the spleen were prepared, stained with anti-TCR-β-FITC, anti-B220-PerCP and tetramer-allophycocyanin, and analyzed by flow cytometry. Numbers indicate the percentage of TCR-β+tetramer+ cells among B220− cells. (B) Data from A presented as absolute numbers of iNKT cells. The data shown represent the mean ± SEM of 4 mice pooled from two experiments. (C) One month after αGalCer or vehicle treatment, mice were re-injected with αGalCer or vehicle. Mice were sacrificed 1 hour later and tetramer+B220− cells from spleen and liver were analyzed for intracellular production of IL-4 and IFN-γ. Results shown are representative of 3 individual experiments. (D) One month after αGalCer or vehicle treatment mice were challenged i.v. with 3 × 105 B16 melanoma cells and treated with αGalCer (2 μg/injection) or vehicle at 0, 3, and 7 days after the tumor challenge. Mice were sacrificed 15 days after tumor challenge, and the numbers of metastatic nodules were counted in the lungs. Results shown are the mean ± SEM of 2 experiments with a total of 10–13 mice in each group. *P<0.05; NS, not significant.
Next, we determined the effects of αGalCer pre-treatment on the antimetastatic activities of iNKT cells in PD-1-deficient mice. Similar to wild-type animals, αGalCer treatment protected naive PD-1-deficient mice against B16 tumor metastases (Fig. 7D). However, in αGalCer pre-treated animals, αGalCer retained its therapeutic activities against B16 melanoma cells in PD-1-deficient mice, but exacerbated metastasis formation in wild-type mice. Protection against the development of tumor metastases in PD-1-deficient animals correlated with restored IFN-γ levels in the serum and the capacity of NK cells to synthesize intracellular IFN-γ and lyse YAC-1 target cells (Supplementary Fig. 4).
PD-1:PD-L blockade enhances the antimetastatic activities of αGalCer
We tested the therapeutic potential of combined treatment with αGalCer and PD-1:PD-L blockade against metastatic tumors. In a first set of experiments, mice were injected with B16 tumor cells and treated with αGalCer and blocking antibodies at 0, 3 and 7 days, followed by evaluation of tumor metastases at 15 days. Results demonstrated that blockade of PD-1:PD-L interactions enhanced the antimetastatic activities of αGalCer (Fig. 8A). In contrast, treatment with the blocking antibodies in the absence of αGalCer did not affect tumor development. In a second set of experiments, we initiated combinedαGalCer and antibody treatment 5 days after B16 tumor challenge, mimicking a treatment regimen of established tumors. Again, anti-PD-L1/L2 antibodies (but not anti-PD-1 antibodies) enhanced the antimetastatic activities of αGalCer (Fig. 8B). Consistent with these findings, we found that combined treatment with αGalCer and PD-1:PD-L blockade enhanced serum IFN-γ levels (Fig. 8C).
Figure 8.
Blockade of PD-1:PD-L interactions enhances the antimetastatic activities of αGalCer. (A) B6 mice were challenged i.v. with 5 × 105 B16 melanoma cells and treated with αGalCer (2 μg/injection) or vehicle at 0, 3, and 7 days after the tumor challenge. The indicated antibodies were administered at 0, 3 and 7 days (300, 200 and 200 μg of each antibody, respectively). Mice were sacrificed after 15 days, and the numbers of metastatic nodules were counted in the lungs. Results shown are the mean ± SEM of 2 experiments with a total of 9 mice in each group. (B) Alternatively, B6 mice were challenged with tumor cells as above, and injected with αGalCer (2 μg/injection) or vehicle along with the indicated antibodies at 5, 8, and 12 days after the tumor challenge. Mice were sacrificed 17 days after the initial tumor challenge, and the numbers of metastatic nodules were counted in the lungs. Results shown are the mean ± SEM of 2 experiments with a total of 10 mice in each group. (C) B6 mice were injected with αGalCer (2 μg/injection) or vehicle along with 300 μg of the indicated antibodies. The mice were bled 24 hours later and serum IFN-γ levels were measured by ELISA. Data shown represent the mean ± SEM of 4 mice per group pooled from two experiments. * P<0.05; NS, not significant.
PD-1:PD-L interactions are not required for induction of iNKT cell anergy mediated by bacteria or sulfatide
We (39) and others (47, 48) have recently demonstrated that multiple bacterial microorganisms can induce anergy in iNKT cells. We therefore tested the role of the PD-1:PD-L pathway in the induction of anergy by heat-inactivated Escherichia coli. E. coli microorganisms induced a sustained upregulation of PD-1 expression on iNKT cells (Fig. 9A), although this was much less pronounced than the levels of PD-1 induced by αGalCer (compare Fig. 9A with Fig. 1). Further, these organisms retained their capacity to induce iNKT cell anergy in PD-1-deficient mice (Fig. 9B).
Figure 9.
Role of the PD-1:PD-L pathway in iNKT cell hyporesponsiveness induced by bacteria or sulfatide. (A and B) E. coli-induced iNKT cell hyporesponsiveness. PD-1−/− or wild-type B6 mice were injected with 0.5 × 109 heat-killed E. coli (i.v.), αGalCer or PBS. (A) Mice were sacrificed at 0, 3 or 21 days, spleen cells were prepared and stained with anti-TCR-β-FITC, anti-PD-1-PE, anti-B220-PerCP, and tetramer-allophycocyanin and analyzed by flow cytometry. (B) Mice were challenged 3 weeks later with vehicle or αGalCer for 3 days to measure in vivo iNKT cell expansion, or for 1 hour to measure iNKT cell intracellular IFN-γ and IL-4 levels. Results shown are representative of 2 individual experiments. (C and D) Mice were treated with sulfatide (20 μg/mouse) in the presence or absence of the indicated antibodies. (C) Animals were sacrificed 24 hours later. Liver mononuclear cells were labeled with CFSE and then stimulated with αGalCer for 96 hours, followed by staining and flow cytometry. Numbers indicate the percentage of positive cells that proliferated. Results are representative of an experiment with 3 mice per group, from a total of 2 experiments. (D) Alternatively, mice were injected with αGalCer for 3 days to measure in vivo iNKT cell expansion, or for 1 hour to measure iNKT cell intracellular IFN-γ levels. Results shown are representative of 4 mice per group, from a total of 2 experiments.
In recent studies, we have shown that sulfatide, a ligand for a subset of CD1d-restricted NKT cells (Type II NKT cells) expressing diverse TCRs, can also induce hyporesponsiveness in iNKT cells (40). Again, similar to bacteria-induced iNKT cell anergy, blockade of PD-1:PD-L interactions was unable to prevent the induction of iNKT cell anergy mediated by sulfatide (Fig. 9C and D).
Discussion
Anergy is a physiological state induced by antigen in T lymphocytes that is characterized by impaired proliferative and cytokine responses to subsequent exposure to cognate antigen (33, 49). The induction of anergy plays a critical role in maintaining self-tolerance and its loss can result in autoimmunity. Understanding the mechanisms by which anergy is induced is important because it permits investigators to manipulate T cell responses. While anergy in conventional T cells is induced only in certain conditions, such as during situations of incomplete or chronic T cell stimulation (49, 50), anergy appears to be the physiological response of iNKT cells to cognate glycolipid antigens (21–23). Expression of a semi-invariant TCR by iNKT cells permits the host to generate an effective response to glycolipid antigens. Because of their innate characteristics, the response of iNKT cells is fast and robust, with production of cytokines that are potentially toxic to the host. For example αGalCer can induce liver toxicity in mice and, in older animals, even death (51). As such, the activity of iNKT cells requires tight regulation, to avoid exuberant inflammation and its associated pathology. Consequently, anergy might be a means to avoid such adverse outcomes of iNKT cell activation. While iNKT cell anergy might be beneficial to the host under normal conditions, it poses a problem with regard to the therapeutic activities of iNKT cells. Our finding that the PD-1:PD-L pathway plays a critical role in mediating αGalCer-induced iNKT cell anergy opens up the possibility to manipulate iNKT cell function during glycolipid therapy. Our findings are in general agreement with a recent study (52), published after submission of the present manuscript, demonstrating a critical role for the PD-1:PD-L pathway in αGalCer-induced iNKT cell anergy.
Prior studies have provided evidence that PD-1:PD-L interactions contribute not only to the induction but also to maintenance of anergy in conventional T cells, at least in the systems tested (31–33). In addition, blockade of PD-1:PD-L interactions was able to reverse the exhausted phenotype of CD8+ and CD4+ lymphocytes during chronic infections (44, 53, 54). We found that blockade of PD-1:PD-L interactions at the time of αGalCer treatment was able to prevent induction of iNKT cell anergy, but had little effect on the anergic phenotype once it was established. This outcome was surprising, as anergic iNKT cells expressed high surface levels of PD-1 (Fig. 1). Thus, other intercellular interactions might play a role in maintaining iNKT cell anergy. Although CTLA-4 has been implicated in the induction of anergy in conventional CD4+ T cells (55, 56), it was not induced at significant levels on αGalCer-activated iNKT cells (Supplementary Fig. 2). In addition, we found that αGalCer was able to induce iNKT cell anergy in mice with a combined deficiency in the ligands of CTLA-4 (B7-1 and B7-2) (Supplementary Fig. 5B), arguing against a role for CTLA-4 in αGalCer-induced iNKT cell anergy.
Recent studies have shown that PD-L1 can bind with B7-1 and that this interaction delivers an inhibitory signal to T cells and inhibits proliferation (57). Therefore, it is possible that the effects of PD-L1 on iNKT cell anergy were mediated, at least in part, through its interaction with B7-1. However, our finding that PD-1-deficient mice are resistant to induction of iNKT cell anergy by αGalCer argues for a critical role of PD-1. In addition, we found that iNKT cells from B7-1-deficient mice can be rendered anergic following treatment of these animals with αGalCer (Supplementary Fig. 5A). Based on these findings we favor the notion that interactions between PD-1 and its ligands PD-L1 and PD-L2 are critical for the induction of iNKT cell anergy.
We found that antibodies directed against PD-L1 and PD-L2 were more effective than antibodies against PD-1 to prevent anergy induction (Fig. 3 and 5). One likely reason is that the anti-PD-1 antibody employed is not as effective in blocking PD-1:PD-L interactions (36, 43, 44). The effects of the anti-PD-1 antibody on anergy induction also depended on the parameter investigated, with few effects observed when iNKT cell anergy was analyzed by total proliferative and cytokine responses in spleen cell cultures (Fig. 3B and 5B) or by investigating iNKT cell expansion in vivo (Fig. 3C and 5C), but more profound effects were observed when investigating intracellular cytokine production by iNKT cells (Fig. 5D and E), serum IFN-γ production (Supplementary Fig. 3A) or intracellular IFN-γ production by NK cells (Supplementary Fig. 3B).
Although our data clearly demonstrate a critical role for the PD-1:PD-L pathway in anergy induction, blockade of this interaction, or PD-1-deficiency, did not completely overcome iNKT cell hyporesponsiveness induced by αGalCer (Fig. 3, 5, and 7). These findings suggest that additional mechanisms contribute to αGalCer-induced iNKT cell anergy.
In addition to αGalCer, several other conditions can result in the induction of iNKT cell hyporesponsiveness. Multiple bacterial microorganisms and microbial products induce a hyporesponsive phenotype in iNKT cells (39, 47, 48). Although some bacteria contain glycolipid antigens that can activate iNKT cells, most bacteria activate these cells in an indirect manner, via microbial products that can activate toll-like receptors on DC (58, 59). DC activated in this manner elaborate cytokines such as IL-12 and IL-18 that can activate iNKT cells. In some, but not all cases, this mode of iNKT cell activation requires CD1d expression on the DC. Sulfatide, a ligand of a subset of CD1d-restricted T cells referred to as Type II NKT cells that express more diverse TCRs than iNKT cells, also induces iNKT cell anergy, in a manner that requires interactions with DC (40). The phenotype of iNKT cells rendered hyporesponsive by bacterial microorganisms or sulfatide bears many similarities with iNKT cells that became anergic in response to αGalCer. It was therefore surprising that the PD-1:PD-L pathway did not appear to be critical for the induction of iNKT cell hyporesponsiveness by E. coli bacteria or sulfatide (Fig. 9). Interestingly, we have previously shown that the induction of iNKT cell hyporesponsiveness by E. coli or sulfatide requires IL-12 production whereas IL-12 is dispensable for αGalCer-induced iNKT cell anergy (39, 40). These findings suggest multiple pathways and mechanisms for the induction of peripheral iNKT cell tolerance.
iNKT cells hold substantial promise for the development of immunotherapies (45). We have previously shown that iNKT cell anergy induced by αGalCer or bacterial microorganisms impairs the therapeutic activities of iNKT cells against metastatic cancers (21, 39). Here we have shown that blockade of PD-1:PD-L interactions at the time of αGalCer treatment prevents the induction of iNKT cell anergy, preserving the therapeutic activities of these cells against B16 tumors (Fig. 6). Likewise, iNKT cells in αGalCer-treated PD-1-deficient mice retained their anti-metastatic activities (Fig. 7D). In addition to preventing the induction of iNKT cell anergy, we further found that PD-1:PD-L blockade enhanced the antimetastatic activities of αGalCer (Fig. 8). Therefore, our findings suggest that combined therapy with αGalCer and PD-1:PD-L blockade is superior to αGalCer therapy alone. Furthermore, because this protocol prevents the induction of iNKT cell anergy, it should be effective when employed repeatedly, such as during disease relapse.
Supplementary Material
Acknowledgments
We thank Kirin Brewery Co. for providing αGalCer, Drs. Tasuku Honjo and Megan Sykes for providing PD-1-deficient mice, Tiffaney Vincent for technical assistance, and Dr. Sebastian Joyce for helpful discussions.
Footnotes
This work was supported by NIH grants AI070305 (to L.V.K.), HL089667 (to L.V.K.) and CA100660 (to V.K.), a pilot project from the Diabetes Research and Training Center at Vanderbilt (to L.W.), and a postdoctoral fellowship from the National Multiple Sclerosis Society of America (to V.V.P.).
Abbreviations used in this paper: B6, C57BL/6; αGalCer, α-galactosylceramide; iNKT, invariant natural killer T; PD, programmed death.
References
- 1.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Vα14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 2.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 3.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;26:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 4.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 5.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, Rossjohn J. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 6.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 7.Mattner J, DeBord KL, Ismail N, Goff RD, Cantu CI, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Buetler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 8.Kinjo Y, Wu DY, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 9.Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligand for NKT cells. Eur J Immunol. 2005;35:1692–1701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- 10.Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia M, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, Khurana A, Hoebe K, Behar SM, Buetler B, Wilson IA, Tsuji M, Sellati TJ, Wong CH, Kronenberg M. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 11.Zhou D, Mattner J, Cantu C, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu Y, Yamashita T, Teneberg S, Wang D, Proia R, Levery SB, Savage PB, Teyton L, Bendelac A. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 12.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 13.Parekh VV, Wilson MT, Van Kaer L. iNKT-cell responses to glycolipids. Crit Rev Immunol. 2005;25:183–213. doi: 10.1615/critrevimmunol.v25.i3.20. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, Lalani S, Parekh VV, Wu L, Van Kaer L. Glycolipid ligands of invariant natural killer T cells as vaccine adjuvants. Expert Rev Vaccine. 2008;7:1519–1532. doi: 10.1586/14760584.7.10.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seino K, Motohashi S, Fujisawa T, Nakayama T, Taniguchi M. Natural killer T cell-mediated antitumor immune responses and their clinical applications. Cancer Sci. 2006;97:807–812. doi: 10.1111/j.1349-7006.2006.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujii S. Exploiting dendritic cells and natural killer T cells in immunotherapy against malignancies. Trends Immunol. 2008;29:242–249. doi: 10.1016/j.it.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Van Kaer L. α-Galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat Rev Immunol. 2005;5:31–42. doi: 10.1038/nri1531. [DOI] [PubMed] [Google Scholar]
- 18.Wilson MT, Johansson C, Olivares-Villagomez D, Singh AK, Stanic AK, Wang CR, Joyce S, Wick MJ, Van Kaer L. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci USA. 2003;100:10913–10918. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowe NY, Uldrich AP, Kyparissoudis K, Hammond KJL, Hayakawa K, Sidobre S, Keating R, Kronenberg M, Smyth MJ, Godfrey DI. Glycolipid antigen drives rapid expansion and sustained cytokine production by NKT cells. J Immunol. 2003;171:4020–4027. doi: 10.4049/jimmunol.171.8.4020. [DOI] [PubMed] [Google Scholar]
- 20.Harada M, Seino KI, Wakao H, Sakata S, Ishizuka Y, Ito T, Kojo S, Nakayama T, Taniguchi M. Down-regulation of the invariant Vα14 antigen receptor in NKT cells upon activation. Int Immunol. 2004;16:241–247. doi: 10.1093/intimm/dxh023. [DOI] [PubMed] [Google Scholar]
- 21.Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ulrich AP, Crowe NY, Kyparissoudis K, Pellicci DG, Zhan Y, Lew AM, Bouillet P, Strasser A, Smyth MJ, Godfrey DI. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J Immunol. 2005;175:3092–3101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikarashi Y, Iizuka A, Koshidaka Y, Heike Y, Takaue Y, Yoshida M, Kronenberg M, Wakasugi H. Phenotypical and functional alterations during the expansion phase of invariant Vα14 natural killer T (Vα14i NKT) cells in mice primed with α-galactosylceramide. Immunology. 2005;116:30–37. doi: 10.1111/j.1365-2567.2005.02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-γ-producing NKT response induced with α-galactosylceramide-loaded DCs. Nat Immunol. 2002;3:867–874. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 25.Parekh VV, Lalani S, Van Kaer L. The in vivo response of invariant natural killer T cells to glycolipid antigens. Int Rev Immunol. 2006;26:31–48. doi: 10.1080/08830180601070179. [DOI] [PubMed] [Google Scholar]
- 26.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 28.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 30.Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, Ishida M, Hiai H, Matsumori A, Minato N, Honjo T. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9:1477–1483. doi: 10.1038/nm955. [DOI] [PubMed] [Google Scholar]
- 31.Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, Yagita H, Azuma M, Sayegh MH, Bluestone JA. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203:2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsushima F, Yao S, Shin T, Flies A, Flies S, Xu H, Tamada K, Pardoll DM, Chen L. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood. 2007;110:180–185. doi: 10.1182/blood-2006-11-060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saibil SD, Deenick EK, Ohashi PS. The sound of silence: modulating anergy in T lymphocytes. Curr Opin Immunol. 2007;19:658–664. doi: 10.1016/j.coi.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int Immunol. 1998;10:1563–1572. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- 35.Bezbradica JS, Stanic AK, Joyce S. Characterization and functional analysis of mouse invariant natural T (iNKT) cells. Curr Prot Immunol. 2006:14.13.1–14.13.27. doi: 10.1002/0471142735.im1413s73. [DOI] [PubMed] [Google Scholar]
- 36.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 37.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, Azuma M, Yagita H. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 38.Parekh VV, Singh AK, Wilson MT, Olivares-Villagomez D, Bezbradica JS, Inazawa H, Ehara H, Sakai T, Serizawa I, Wu L, Wang CR, Joyce S, Van Kaer L. Quantitative and qualitative differences in the in vivo response of NKT cells to distinct α- and β-anomeric glycolipids. J Immunol. 2004;173:3693–3706. doi: 10.4049/jimmunol.173.6.3693. [DOI] [PubMed] [Google Scholar]
- 39.Kim S, Lalani S, Parekh VV, Vincent TL, Wu L, Van Kaer L. Impact of bacteria on the phenotype, functions and therapeutic activities of iNKT cells in mice. J Clin Invest. 2008;118:2301–2315. doi: 10.1172/JCI33071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest. 2007;117:2302–2312. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 42.Zhong X, Tumang JR, Gao W, Bai C, Rothstein TL. PD-L2 expression extends beyond dendritic cells/macrophages to B1 cells enriched for V(H)11/V(H)12 and phosphatidylcholine binding. Eur J Immunol. 2007;37:2405–2410. doi: 10.1002/eji.200737461. [DOI] [PubMed] [Google Scholar]
- 43.Ito T, Ueno T, Clarkson MR, Yuan X, Jurewicz MM, Yagita H, Azuma M, Sharpe AH, Auchincloss H, Jr, Sayegh MH, Najafian N. Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo. J Immunol. 2005;174:6648–6656. doi: 10.4049/jimmunol.174.11.6648. [DOI] [PubMed] [Google Scholar]
- 44.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 45.Wilson MT, Singh AK, Van Kaer L. Immunotherapy with ligands of natural killer T cells. Trends Mol Med. 2002;8:225–231. doi: 10.1016/s1471-4914(02)02325-0. [DOI] [PubMed] [Google Scholar]
- 46.Smyth MJ, Crowe NY, Pellicci DG, Kyparissoudis K, Kelly JM, Takeda K, Yagita H, Godfrey DI. Sequential production of interferon-γ by NK1.1+ T cells and natural killer cells is essential for the antimetastatic effect of α-galactosylceramide. Blood. 2002;99:1259–1266. doi: 10.1182/blood.v99.4.1259. [DOI] [PubMed] [Google Scholar]
- 47.Chiba A, Dascher CC, Besra GS, Brenner MB. Rapid NKT cell responses are self-terminating during the course of microbial infection. J Immunol. 2008;181:2292–2302. doi: 10.4049/jimmunol.181.4.2292. [DOI] [PubMed] [Google Scholar]
- 48.Choi HJ, Xu H, Geng Y, Colmone A, Cho H, Wang CR. Bacterial infection alters the kinetics and function of iNKT cell responses. Journal of leukocyte biology. 2008;84:1462–1471. doi: 10.1189/jlb.0108038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 50.Macian F, Im SH, Garcia-Cozar FJ, Rao A. T-cell anergy. Curr Opin Immunol. 2004;16:209–216. doi: 10.1016/j.coi.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 51.Osman Y, Kawamura T, Naito T, Takeda K, Van Kaer L, Okumura K, Abo T. Activation of hepatic NKT cells and subsequent liver injury following administration of α-galactosylceramide. Eur J Immunol. 2000;30:1919–1928. doi: 10.1002/1521-4141(200007)30:7<1919::AID-IMMU1919>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 52.Chang WS, Kim JY, Kim YJ, Kim YS, Lee JM, Azuma M, Yagita H, Kang CY. Cutting edge: Programmed death-1/programmed death ligand 1 interaction regulates the induction and maintenance of invariant NKT cell anergy. J Immunol. 2008;181:6707–6710. doi: 10.4049/jimmunol.181.10.6707. [DOI] [PubMed] [Google Scholar]
- 53.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 54.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 55.Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–417. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 56.Greenwald RJ, V, Boussiotis A, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145–155. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 57.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tupin E, Kinjo T, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. 2007;5:405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 59.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ’Swiss-Army knife’ of the immune system. Curr Opin Immunol. 2008;20:358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.