Abstract

Enantioenriched pyranones are important intermediates in the synthesis of natural products and the generation of compound libraries. A one-pot method for their synthesis is outlined. Catalytic asymmetric alkylation of 2-furfurals in the presence of catalytic (−)-MIB generates enantioenriched furyl zinc alkoxides. Addition of water/THF followed by NBS results in formation of pyranones with ee’s >90% and yields between 46–77%.
Advances in organic synthesis enable chemists to prepare most natural product targets. Even with state of the art methods, however, syntheses often require many synthetic manipulations and purifications, resulting in low overall yields and generation of large amounts of chemical waste. To address these issues, increasing synthetic efficiency and reducing E-factors (which is defined as the ratio of the mass of waste produced to the mass of product), are becoming more important in designing synthetic routes.1 One approach to streamline organic synthesis is through tandem and sequential reactions that accomplish multiple steps in a single flask and minimize isolations, purifications, and solvent use.
Herein, we present a one-pot method to prepare enantioenriched pyranones from 2-furfurals. Pyranones are valuable building blocks that have been extensively used in natural product2,3,4,5 and diversity oriented syntheses.6
6-Hydroxy-(2H)-pyran-3-ones are typically prepared by the oxidative rearrangement of furyl alcohols in the Achmatowicz reaction.7 Key intermediates in the Achmatowicz reaction are illustrated in Scheme 1. An example of the Achmatowicz reaction in the synthesis of (−)-8a-epi-swainsonine2 by O’Doherty is shown in Scheme 2.
Scheme 1.
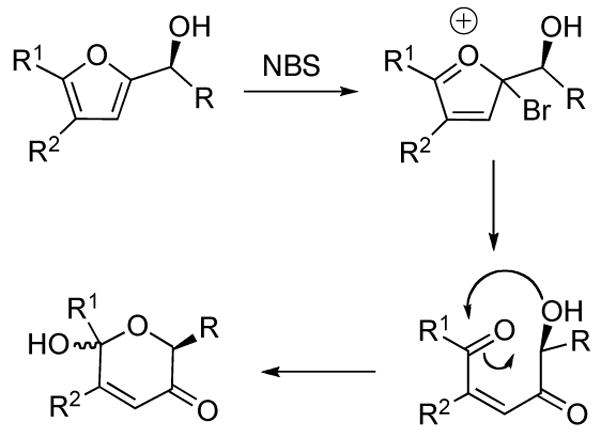
Key intermediates in the Achmatowicz reaction.7
Scheme 2.
O’Doherty synthesis of (−)-8a-epi-swainsonine featuring a ketone reduction with Noyori’s catalyst10 and the Achmatowicz reaction.
The most popular approach to enantioenriched pyranones has relied on kinetic resolution (KR) of racemic furyl alcohols,8 as illustrated in Fürstner’s elegant synthesis of ipomoeassian E (Scheme 3).9 Upon treatment of the racemic furyl alcohol under KR conditions with the Sharpless-Katsuki catalyst and TBHP, the matched enantiomer underwent the Achmatowicz reaction to generate the optically active pyranone. The unreacted enantiomerically enriched furyl alcohol was then isolated in 47% yield (>99% ee) and subjected to a second Achmatowicz reaction.9
Scheme 3.
Fürstner’s KR in the synthesis of ipomoeassin E.9
In a KR, the product ee decreases with rising conversion while the starting material ee increases.11 Thus, unless the KR selectivity factor (=kfast/kslow) is very large, it is always the enantioenriched starting material that is carried forward.
Our approach to enantioenriched pyranones is based on our enantioselective carbonyl addition/epoxidation (Scheme 4).12 These one-pot transformations generate epoxy alcohols with three contiguous stereogenic centers with high enantio- and diastereoselectivity. In applying this approach to the synthesis of enantioenriched pyranones, we focused on 2-furfurals as substrates.
Scheme 4.
Our one-pot transformations.12
Employing Nugent’s enantioenriched amino alcohol (−)-MIB (Scheme 5, 4 mol %),13,14 we first examined the asymmetric addition of diethylzinc to 2-furfural in hexanes at 0 °C. We were pleased to observe that the addition furnished the furyl alcohol in 97% enantioselectivity and 98% yield. With suitable conditions for the asymmetric addition, attention was then focused on the second half of the tandem process, the Achmatowicz reaction.
Scheme 5.

Asymmetric addition with MIB.
We initially employed reagents and conditions for the Achmatowicz reaction that were similar to those used in our asymmetric addition/diastereoselective epoxidation chemistry (Scheme 4, B and C). Thus, after completion of the asymmetric addition, TBHP (up to 5 equiv) and Ti(Oi-Pr)4 (20 mol %) were added at 0 °C and the oxidation was stirred for 12–15 h (Table 1, entry 1). Under these, and related conditions, the oxidation stalled at or below 50% conversion in all cases. Longer reaction times and addition of a second dose of Ti(Oi-Pr)4 were unsuccessful at increasing conversions. Despite extensive experimentation, we were unable to satisfactorily optimize this process.
Table 1.
Optimization of oxidation conditions
 | |||
|---|---|---|---|
| entry | additive | oxidanta | yield (%) |
| 1 | TBHP (5 equiv) | Ti(Oi-Pr)4 (20 mol %) | 50 |
| 2 | TBHP (2 equiv) | OV(acac)2 (10 mol %) | 0 |
| 3 | TBHP (3 equiv) | OV(acac)2 (40 mol %) | 50 |
| 4b | TBHP (3 equiv) | OV(acac)2(30 mol %) | 50 |
| 5 | TBHP (3 equiv) | OV(acac)2 (30 mol %) | 70 |
| 6 | TBHP (3 equiv) | OV(acac)2 (15 mol %) | 70 |
| 7b,c | THF/H2O | NBS | 73 |
Oxidants were slowly added as a solution by syringe pump unless otherwise noted. See Supporting Information for additional details.
The oxidant was added neat.
NBS was added in three portions over 30 min at rt.
We next examined the OV(acac)2/TBHP system for the Achmatowicz oxidation (Table 1, entries 2–6). Direct addition of OV(acac)2 and TBHP to the asymmetric addition reaction mixture after the aldehyde was consumed resulted in reduction of the vanadium by the residual alkylzinc species (entry 2). We therefore added the TBHP (2–3 equiv) to first quench alkyl organozinc species, followed by slow addition of OV(acac)2. With the vanadium catalyst, our best results were obtained with 15 mol % catalyst loading and 3 equiv TBHP under slow addition conditions (70% yield, entry 6). In general, OV(acac)2/TBHP systems require significantly lower catalyst loadings, but these were unsuccessful in this oxidation.
Employing more traditional Achmatowicz oxidation conditons, we found that addition of THF/H2O (4:1) to the furyl zinc alkoxide product followed by N-bromosuccinimide (NBS) in three equal portions at rt resulted in complete consumption of the furyl alcohol in about 4 h. The pyranone was isolated in 73% yield after purification as a 2.6:1 ratio of diastereomers about the anomeric carbon (Table 1, entry 7). Due to the low cost and ease of the NBS oxidation, these conditions were used to determine the substrate scope of the one-pot pyranone synthesis in Table 2.
Table 2.
Substrate scope for one-pot synthesis of pyranones
 | |||||
|---|---|---|---|---|---|
| entry | ZnR2 | SM | product | ee %a | yield % |
| 1 | ZnEt2 |

|

|
97 | 73 |
| 2 | ZnMe2 | 94 | 70 | ||
| 3 | Zn[(CH2)4OTBS)]2 | 95 | 61 | ||
| 4 | ZnEt2 |
|

|
91 | 71 |
| 5 | ZnEt2 |

|

|
99 | 72 |
| 6 | ZnEt2 |

|

|
97 | 77 |
| 7 | ZnMe2 | 97 | 76 | ||
| 8 | ZnEt2 |

|

|
95 | 63 |
| 9 | ZnMe2 | 92 | 46 | ||
Enantiomeric excess was determined after the dialkylzinc addition to the furfurals.
A series of 2-furfurals were examined in combination with dialkylzinc reagents in the one-pot pyranone synthesis (Table 2). It is noteworthy that enantioselectivities were >90% with all substrates examined. Isolated yields for the one-pot procedure ranged from 46 to 77%. Of note, the pyranone in entry 2 is an intermediate in O’Doherty’s syntheses of daumone, digitoxin, and anthrax.3
2-Furfural derivatives substituted at the 5 or 4 and 5 positions participated in the reaction to yield pyranone products (entries 3—8). Functionalized organozinc reagents generated by Knochel’s procedure15 were also employed to provide pyranone products (entry 3) in comparable yields to reactions utilizing commercially available dialkylzinc reagents. As shown in entry 4, the TBS protected alcohol was tolerated. A decrease in the yield was noted for 4,5-disubstituted 2-furfural (entries 8–9). It is possible that the cyclization onto the α-substituted enone intermediate (Scheme 1, R2≠H) is more difficult than when R2=H for steric reasons.
In summary, we have developed a one-pot protocol to prepare enantioenriched pyranones with enantioselectivities >90%. The procedure involves initial asymmetric alkylation of 2-furfurals to generate zinc furyl alkoxide intermediates. Subsequent oxidation with NBS promotes the Achmatowicz reaction to furnish the pyranone products. This method enables the one-pot synthesis of a variety of pyranones simply by varying the structure of the 2-furfural derivative and organozinc reagents employed. Our protocol represents an improvement over the kinetic resolution approach to pyranones by circumventing the need for additional purifications and leading to an increase in overall yield. Enantioenriched pyranones such as these have been used in several natural product syntheses. Given the utility of enantioenriched pyranones in synthesis, we believe that this one-pot method will be useful in the assembly of natural products.
Supplementary Material
Acknowledgments
We are grateful to the National Science Foundation (CHE-0615210) and National Institutes of Health, National Institute of General Medical Sciences (GM58101) for financial support of this work.
Footnotes
Supporting Information Available Procedures and full characterization are available (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Sheldon RA. Green Chem. 2007;9:1273–1283. [Google Scholar]
- 2.Abrams JN, Babu RS, Guo H, Le D, Le J, Osbourn JM, O’Doherty GA. J Org Chem. 2008;73:1935–1940. doi: 10.1021/jo702476q. [DOI] [PubMed] [Google Scholar]
- 3.(a) Guo H, O’Doherty GA. Org Lett. 2005;7:3921–3924. doi: 10.1021/ol051383e. [DOI] [PubMed] [Google Scholar]; (b) Zhou MQ, O’Doherty GA. J Org Chem. 2007;72:2485–2493. doi: 10.1021/jo062534+. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Guo HB, O’Doherty GA. J Org Chem. 2008;73:5211–5220. doi: 10.1021/jo800691v. [DOI] [PubMed] [Google Scholar]
- 4.(a) Leverett CA, Cassidy MP, Padwa A. J Org Chem. 2006;71:8591–8601. doi: 10.1021/jo0616714. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Harris JM, Padwa A. J Org Chem. 2003;68:4371–4381. doi: 10.1021/jo034324s. [DOI] [PubMed] [Google Scholar]
- 5.(a) Um JM, Houk KN, Phillips AJ. Org Lett. 2008;10:3679–3772. doi: 10.1021/ol801421n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Griggs ND, Phillips AJ. Org Lett. 2008;10:4955. doi: 10.1021/ol802041c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Henderson JA, Jackson KL, Phillips AJ. Org Lett. 2007;9:5299. doi: 10.1021/ol702559e. [DOI] [PubMed] [Google Scholar]; (d) Babu RS, O’Doherty GA. J Carbohydr Chem. 2005;24:169–177. [Google Scholar]; (e) Peng X, Li A, Lu J, Wang Q, Pan X, Chan ASC. Tetrahedron. 2002;58:6799–6804. [Google Scholar]; (f) Yang ZC, Jiang XB, Wang ZM, Zhou WS. J Chem Soc, Perkin Trans. 1;1997:317–321. [Google Scholar]; (g) Wender PA, Bi FC, Buschmann N, Gosselin F, Kan C, Kee JM, Ohmura H. Org Lett. 2006;8:5373–5376. doi: 10.1021/ol062234e. [DOI] [PubMed] [Google Scholar]; (h) Jackson KL, Henderson JA, Morris JC, Motoyoshi H, Phillips AJ. Tetrahedron Lett. 2008;49:2939–2941. doi: 10.1016/j.tetlet.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Couladouros EA, Strongilos AT. Angew Chem Int Ed. 2002;41:3677–3680. doi: 10.1002/1521-3773(20021004)41:19<3677::AID-ANIE3677>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]; (j) Zhou X, Wu W, Liu X, Lee CS. Org Lett. 2008;10:5525–5528. doi: 10.1021/ol8022787. [DOI] [PubMed] [Google Scholar]; (k) Babu RS, O’Doherty GA. J Am Chem Soc. 2003;125:12406–12407. doi: 10.1021/ja037097k. [DOI] [PubMed] [Google Scholar]; (l) Harris JM, O’Doherty GA. Org Lett. 2000;2:2983–2986. doi: 10.1021/ol000179i. [DOI] [PubMed] [Google Scholar]; (m) DeShong P, Waltermire RE, Ammon HL. J Am Chem Soc. 1988;110:1901–1910. [Google Scholar]; (n) Martin SF, Zinke PW. J Am Chem Soc. 1989;6:2311–2313. [Google Scholar]; (o) Yu X, O’Doherty GA. Org Lett. 2008;10:4529–4532. doi: 10.1021/ol801817f. [DOI] [PubMed] [Google Scholar]
- 6.Burke MD, Berger EM, Schreiber SL. J Am Chem Soc. 2004;126:14095–14104. doi: 10.1021/ja0457415. [DOI] [PubMed] [Google Scholar]
- 7.(a) Achmatowicz O, Bielski R. Carbohydr Res. 1977;55:165–176. doi: 10.1016/s0008-6215(00)84452-3. [DOI] [PubMed] [Google Scholar]; (b) Georgiadis MP, Couladouros EA. J Org Chem. 1986;51:2725–2727. [Google Scholar]
- 8.(a) Kametani T, Tsubuki M, Tatsuzaki Y, Honda T. J Chem Soc Perkin Trans 1. 1990:639–646. [Google Scholar]; (b) Kusakabe M, Kitano Y, Kobayashi Y, Sato F. J Org Chem. 1989;54:2085–2091. [Google Scholar]; (c) Honda T, Sano N, Kanai K. Heterocycles. 1995;41:425–429. [Google Scholar]; (d) Yang ZC, Jiang XB, Wang ZM, Zhou WS. J Chem Soc, Chem Commun. 1995:2389–2390. [Google Scholar]
- 9.Fürstner A, Nagano T. J Am Chem Soc. 2007;129:1906–1907. doi: 10.1021/ja068901g. [DOI] [PubMed] [Google Scholar]
- 10.(a) Fujii A, Hashiguchi S, Uematsu N, Ikariya T, Noyori R. J Am Chem Soc. 1996;118:2521–2522. [Google Scholar]; (b) Coral JA, Guo H, Shan M, O’Doherty GA. Heterocycles. 2009;79:521–529. [Google Scholar]
- 11.Walsh PJ, Kozlowski MC. Fundamentals of Asymmetric Catalysis. University Science Books; Sausalito, California: 2008. p. 231. Chapter 7. [Google Scholar]
- 12.(a) Kelly AR, Lurain AE, Walsh PJ. J Am Chem Soc. 2005;127:14668–14674. doi: 10.1021/ja051291k. [DOI] [PubMed] [Google Scholar]; (b) Jeon SJ, Li HM, Walsh PJ. J Am Chem Soc. 2005;127:16416–16425. doi: 10.1021/ja052200m. [DOI] [PubMed] [Google Scholar]; (c) Wooten A, Kim JG, Walsh PJ. Org Lett. 2007;9:381–384. doi: 10.1021/ol062264h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kim JG, Waltz KM, Garcia IF, Kwiatkowski D, Walsh PJ. J Am Chem Soc. 2004;126:12580–12585. doi: 10.1021/ja047758t. [DOI] [PubMed] [Google Scholar]; (e) Hussain MM, Walsh PJ. Acc Chem Res. 2008;41:883–893. doi: 10.1021/ar800006h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Nugent WA. Chem Commun. 1999:1369–1370. [Google Scholar]; (b) Chen YK, Jeon SJ, Walsh PJ. Org Synth. 2005;82:87–92. [Google Scholar]; (c) Kim JG, Walsh PJ. Angew Chem Int Ed. 2006;45:4175–4178. doi: 10.1002/anie.200600741. [DOI] [PubMed] [Google Scholar]
- 14.Kelly AR, Kerrigan MH, Walsh PJ. J Am Chem Soc. 2008;130:4097–4104. doi: 10.1021/ja710988q. [DOI] [PubMed] [Google Scholar]
- 15.Langer F, Schwink L, Devasagayaraj A, Chavant PY, Knochel P. J Org Chem. 1996;61:8229–8243. doi: 10.1021/jo961129n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





