Abstract
BACKGROUND & AIMS
LIGHT (lymphotoxin-like inducible protein that competes with glycoprotein D for herpes virus entry on T cells) is a TNF core family member that regulates T cell activation and causes experimental inflammatory bowel disease. Additional data suggest that LIGHT may be involved in the pathogenesis of human inflammatory bowel disease. The aim of this study was to determine if LIGHT was capable of signaling directly to intestinal epithelia and to define the mechanisms and consequences of such signaling.
METHODS
The effects of LIGHT and interferon-γ (IFN-γ) on barrier function, cytoskeletal regulation, and tight junction structure were assessed in mice and intestinal epithelial monolayers.
RESULTS
LIGHT induced barrier loss in cultured epithelia via myosin II regulatory light chain (MLC) phosphorylation; both barrier loss and MLC phosphorylation were reversed by MLC kinase (MLCK) inhibition. IFN-γ pretreatment, which induced lymphotoxin β receptor (LTβR) expression, was required for these effects and neither barrier dysfunction nor intestinal epithelial MLC phosphorylation occurred in LTβR-knockout mice. In cultured monolayers, endocytosis of the tight junction protein occludin correlated with barrier loss. Internalized occludin co-localized with caveolin-1. LIGHT-induced occludin endocytosis and barrier loss were both prevented by inhibition of caveolar endocytosis.
CONCLUSIONS
T cell-derived LIGHT activates intestinal epithelial LTβR to disrupt barrier function. This requires MLCK activation and caveolar endocytosis. These data suggest a novel role for LIGHT in disease pathogenesis and suggest that inhibition of MLCK-dependent caveolar endocytosis may represent an approach to restoring barrier function in inflammatory bowel disease.
Keywords: tight junction, interferon-γ, tumor necrosis factor, LIGHT, lymphotoxin, endocytosis, cytoskeleton, myosin, inflammatory bowel disease
INTRODUCTION
LIGHT (lymphotoxin-like inducible protein that competes with glycoprotein D for herpes virus entry on T cells) is a member of the tumor necrosis factor (TNF) core family involved in T cell regulation during innate and adaptive immune responses. Although related, it is notable that LIGHT and TNF activate distinct, non-overlapping surface receptors. Transgenic overexpression of LIGHT in murine T cells leads to development of experimental inflammatory bowel disease (IBD) 1, 2. The pattern of disease in these transgenic mice is similar to Crohn’s disease and, like Crohn’s disease, is ameliorated by TNF neutralization 1, 2. While a specific role for LIGHT in human IBD has not yet been demonstrated, it is notable that LIGHT expression is markedly increased in mucosal biopsies from patients with active IBD 3 and that LIGHT maps to 19p13.3 4, a known IBD susceptibility locus 5. Moreover, LIGHT enhances TNF and IFN-γ release from T cells 3, 6, consistent with the known contributions of these cytokines to human disease. Thus, available data suggest that LIGHT, which is released by T cells, participates in IBD pathogenesis primarily via T cell regulation 6–8.
Intestinal epithelial barrier defects are well-recognized in IBD 9–11. While a primary barrier defect may be present in IBD kindreds 12–14, it is also clear that epithelial barrier defects can be induced by inflammatory cytokines 15–24. Recent work has demonstrated that barrier dysfunction induced by inflammatory processes can be due to epithelial damage as well as non-apoptotic regulation of tight junction permeability 19, 22, 25–28. In vitro and in vivo studies have shown that TNF signals directly to intestinal epithelia to regulate barrier function via myosin light chain kinase (MLCK) activation 20, 29–33. We recently reported that acute LIGHT administration also causes MLCK-dependent intestinal epithelial barrier dysfunction in vivo 20. However, due to the complexities of the in vivo system used, these data could not discriminate between direct effects of LIGHT on intestinal epithelia and those mediated by intermediates, such as TNF or immune cells. Thus, although some reports suggest that LIGHT may be capable of signaling to epithelial-derived cancer cells 34, direct LIGHT signaling to epithelia has not been explored or considered in intestinal disease.
The aim of this study was to determine if LIGHT is capable of signaling directly to intestinal epithelia and to define the mechanisms and consequences of such signaling. The data show that LIGHT signals directly to intestinal epithelia via the lymphotoxin β receptor LTβR). This induces both transcriptional and enzymatic MLCK activation and results in caveolar endocytosis of tight junction components, including occludin. In addition to demonstrating LIGHT-mediated barrier regulation, these data are the first to demonstrate a functional requirement for endocytosis during cytokine-induced barrier dysfunction.
MATERIALS AND METHODS
Monolayer preparation and transepithelial electrical resistance measurement
Caco-2BBE cell 35, 36 cultures were grown as monolayers on collagen-coated polycarbonate membrane Transwell supports (Corning, Cambridge, MA) with 0.4 µm pores for 17–20 days after confluence, as described previously 30. Transwell supports with 0.33- and 5-cm2 surface areas were used for electrophysiological and biochemical studies, respectively. Cytokines (R&D Systems, Minneapolis, MN), were added to the basal chamber without manipulating the apical media unless otherwise specified. Sulfasalazine (MP Biochemicals, Aurora, OH), curcumin (Calbiochem, San Diego, CA), BAY 11-7085 (Calbiochem), MG132 (Calbiochem), chlorpromazine (Sigma, St. Louis, MO), amiloride (Sigma), methyl-β-cyclodextrin (Sigma), and monodansyl cadaverine (Sigma) were added to apical and basal chambers. Transepithelial resistance (TER) was measured with an epithelial voltohmmeter under open circuit conditions (World Precision Instruments, Sarasota, FL) as described previously 30. TER averaged 250 Ω·cm2, after subtraction of a blank that includes filter and fluid resistances, prior to cytokine treatment. To facilitate comparisons between experiments, the TER of all monolayers was normalized to that of control monolayers in the same experiment.
SDS-PAGE and immunoblot
Monolayers were scraped directly into 0.5 ml SDS-PAGE sample buffer, sonicated, separated on SDS-PAGE gels (Cambrex, Rockland, ME), and transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA)). Lysates of isolated colonocytes were processed similarly 33. Immunoblots were performed using antibodies specific for MLCK (clone K36, Sigma), total MLC 33, phosphorylated MLC 37, ZO-1 (Invitrogen, Carlsbad, CA) occludin (Invitrogen), claudin-1 (Invitrogen), caspase-3 (Cell Signaling Technology, Beverly, MA), caspase-8 (Cell Signaling Technology), HVEM (R&D Systems), and LTβR (R&D Systems). After incubation with peroxidase-conjugated secondary antibodies (Cell Signaling Technology), blots were visualized by enhanced chemiluminescence using Super Signal West Pico Reagents (Pierce Biotechnology Inc, Rockford, IL). Quantitative analysis was performed using Metamorph 6.2 (Molecular Devices Corp, Downingtown, PA).
Real Time RT-PCR
Monolayers were scraped directly into TRIzol and sonicated. RNA was extracted and further purified as described previously 38. Long (epithelial) MLCK mRNA expression was determined by SYBR green real-time PCR using the MyiQ Real-Time PCR Detection System (Bio-Rad Laboratories), as described previously 38. GAPDH was used as an internal standard for normalization.
In vivo studies
Seven- to ten-week-old wild type, HVEM−/− 39, and LTβR−/− 40 mice on C57BL/6 genetic background, as described previously39, 40, were used for all studies. Knockout mice were generously provided by Klaus Pfeffer (Technical University of Munich, Munich, Germany). Genotypes were confirmed by PCR, as described39, 40. Animal experiments were carried out in accordance with National Institutes of Health guidelines under protocols approved by the Institutional Animal Care and Use Committee at the University of Chicago.
Mice were injected intraperitoneally with either 5µg TNF or 5µg LIGHT in 250µl PBS or with PBS alone, as described previously 20. To determine paracellular permeability, 250 µl of 1 mg/ml Alexa 488 conjugated BSA (Invitrogen) was injected intravenously 33. A ~5 cm loop of jejunum was cannulated and perfused from 1.5 to 3.5 hours after cytokine injection. BSA clearance was calculated as described previously 20:
To isolate intestinal epithelial cells, a fresh section of jejunum was opened lengthwise, washed in 4°C Ca2+- and Mg+- free Hank’s buffered saline solution (CMF-HBSS), transferred to CMF-HBSS containing 10mM dithiothreitol and 50nM calyculin A (Calbiochem), and incubated for 30 minutes at 4°C. After incubation, the tube was shaken briefly and the tissue transferred to a fresh tube containing CMF-HBSS with 1mM EDTA and 50nM calyculin A. After 1 hour, epithelial cells were dislodged by vigorous shaking and large pieces of tissue were removed from the tube and discarded. Epithelial cells were harvested by centrifugation at 500 × g for 10 minutes and pellets were resuspended in SDS-PAGE sample buffer.
Immunofluorescence
Cultured monolayers were fixed with 1% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.4, with 1 mM CaCl2 for 30 minutes at room temperature. After three washes in PBS and a 10 minute incubation in PBS with 50 mM NH4Cl, cells were permeablized in PBS with 3% BSA and 0.05% saponin (wash buffer) in five 5 minute incubations. Monolayers were then incubated with anti-claudin-1, anti-ZO-1, or anti-occludin antibodies in wash buffer for 2 hours, washed 5 times, and incubated for 1 hour with appropriate Alexa Fluor 488- or 594-conjugated secondary antibodies (Invitrogen) and Hoechst 33342 (Invitrogen). After 5 washes, monolayers were rinsed in water and mounted in Prolong Gold (Invitrogen).
For colocalization studies monolayers were fixed in methanol overnight at −20°C, air-dried, rehydrated with 100 µM bis(sulfosuccinimidyl)suberate (BS3) in PBS with 0.1% n-octylglutaraldehyde (PBS+) for 30 minutes, washed in PBS+, quenched in 100 mM ethylenediamine, pH 7.5, and washed once more in PBS+. Monolayers were then blocked in 1% nonfat dry milk, 1% fish gelatin, and 1% normal donkey serum in PBS+ for 1 hours, incubated with mouse anti-occludin and either rabbit anti-caveolin-1 (Santa Cruz Biotechnology, Santa Cruz, CA) or rabbit anti-clathrin heavy chain antibodies (Santa Cruz Biotechnology) for 2 hours, washed, and incubated with Alexa Fluor-conjugated secondary antibodies for 1 hour. After five washes, monolayers were rinsed in water and mounted in Prolong Gold.
For immunofluorescence of mouse jejunum, tissues were snap-frozen in OCT and stored at −80°C. Frozen sections (5µm) were collected on coated slides, fixed in 1% PFA, washed three times with PBS, and blocked and quenched for 30 minutes in PBS with 10% normal goat serum and 50mM NH4Cl. After incubation for 2 hours with rabbit anti-occludin (Invitrogen), sections were washed and incubated for 1 hour with Alexa 594-conjugated goat anti-rabbit Ig and Alexa 488-conjugated phalloidin (Invitrogen). Stained sections were mounted using Prolong Gold antifade reagent (Invitrogen, Eugene, OR).
Samples were imaged using a Leica DMLB epifluorescence microscope equipped with 63X and 100X PL-APO objectives, an 88000 filter set (Chroma Technology, Brattleboro, VT), and a Retiga EXi camera (Q Imaging, Burnaby, BC, Canada) controlled by MetaMorph. Monolayers were imaged as z-stacks at 0.2 µm intervals and deconvolved using Autodeblur 9.3 (Media Cybernetics, Silver Spring, MD) for 10 iterations.
Morphometry
Deconvolved z-stacks were merged, after pseudocolor assignment, using MetaMorph. Vesicles were defined as round or oval structures present in three or more z-planes. The number of vesicles in a single cell was counted over the full height of the cell. Signals were considered to colocalize if there was ≥80% overlap between channels. For each measurement, 15 randomly chosen average-shaped and -sized cells were counted.
Statistical analysis
All data are presented as mean ± SE. All experiments were performed with triplicate or greater samples, and data shown are representative of three or more independent studies. P value was determined by two-tailed Student's t test and was considered to be significant if less than 0.05.
RESULTS
LIGHT causes epithelial barrier dysfunction
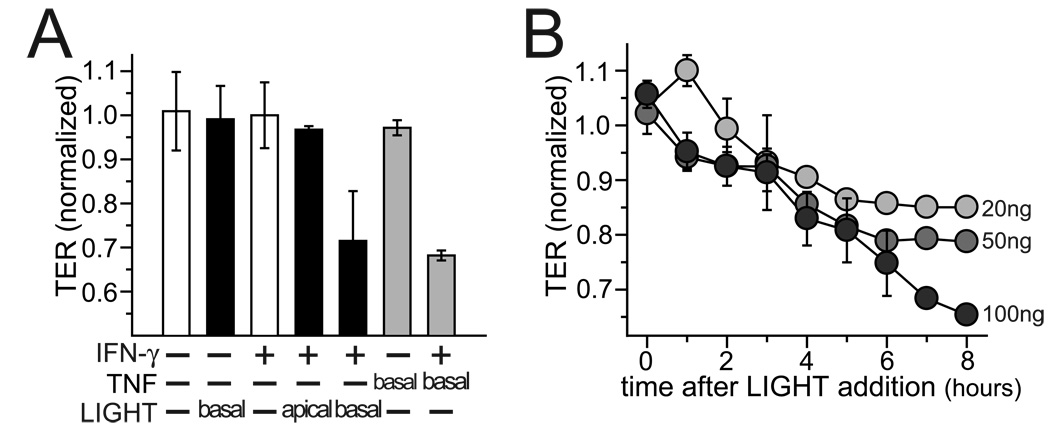
We have recently used an in vivo mouse model to demonstrate that intraperitoneal LIGHT injection causes intestinal epithelial barrier disruption 20. This barrier disruption was similar in magnitude and mechanism to that induced by intraperitoneal TNF injection. However, complexities of the in vivo system make it difficult to determine if the effects of systemic LIGHT administration on intestinal epithelial barrier function are the direct result of LIGHT signaling or occur indirectly via T cell activation. To determine if LIGHT can signal directly to intestinal epithelial cells we treated Caco-2 monolayers with LIGHT. Even at high concentrations, up to 500 ng/ml, LIGHT was unable to reduce transepithelial resistance (TER), a sensitive measure of epithelial barrier function. Although this negative result could suggest that LIGHT cannot signal directly to epithelia, we chose to ask if IFN-γ priming could sensitize Caco-2 monolayers to LIGHT. LIGHT treatment caused TER to fall by 28±5% in monolayers primed by incubation with 10ng/ml IFN-γ in the basal media for 24 hours (Fig. 1A). This response was highly polarized; only basal, but not apical, LIGHT was able to disrupt barrier function (Fig. 1A), and was dose-dependent (Fig. 1B). These data therefore demonstrate that LIGHT is able to signal directly to intestinal epithelia to reduce barrier function in a polarized, dose-dependent manner.
Figure 1. LIGHT causes polarized, dose-responsive, and IFN-γ-dependent epithelial barrier dysfunction.
A. Well-differentiated Caco-2 cells monolayers were pre-treated with or without IFN-γ (10 ng/ml for 24 hours) in the basal chamber prior to transfer to fresh media, without IFN-γ, either with or without TNF (5 ng/ml) or LIGHT (50 ng/ml) added to apical or basal chambers, as indicated. TER was measured 8 hours after transfer. TER data were normalized to control monolayers handled identically without cytokines. Mean ± SE of triplicate monolayers, representative of more than 10 independent experiments, are shown. P < 0.01 for TNF or LIGHT (after IFN-γ pre-treatment) vs. all other conditions.
B. Caco-2 monolayers pre-treated with IFN-γ as above were transferred to fresh media, without IFN-γ, but with LIGHT, at indicated doses, at t=0. TER data were normalized to control monolayers pre-treated with IFN-γ but transferred to media without LIGHT. Mean ± SE of triplicate monolayers, representative of more than 6 independent experiments, are shown.
LIGHT-induced barrier loss occurs independently of apoptosis, tight junction protein degradation, or NFκB signaling
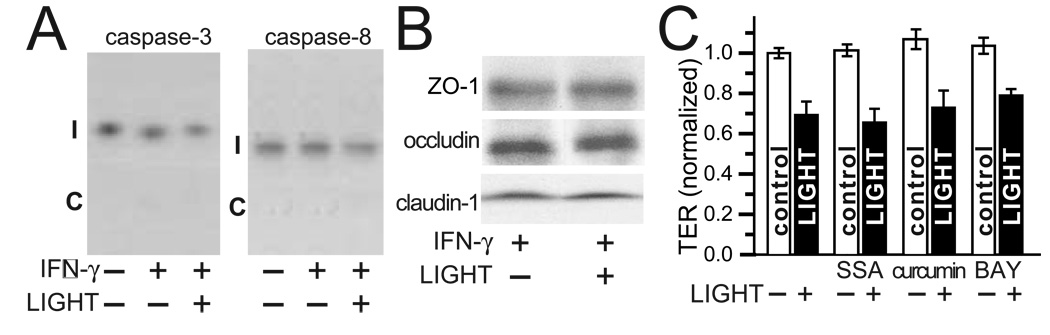
Although this is the first report of direct LIGHT effects on intestinal barrier function, many studies have examined mechanisms of TNF-induced barrier loss. Roles for epithelial apoptosis 26, reduced tight junction protein expression 41, NFκB-dependent and NFκB-independent processes 30, 31, 38, 42, and MLCK activation 20, 30, 32, 33 have been suggested in TNF-induced barrier loss. We therefore sought to determine the contributions of these processes to LIGHT-induced barrier loss. Fluorescent microscopic examination of nuclear DNA failed to detect increased numbers of fragmented, i.e. apoptotic, nuclei in IFN-γ primed, LIGHT-treated monolayers (data not shown). In addition, LIGHT treatment of IFN-γ primed monolayers did not cause cleavage of caspase-3 or caspase-8 (Fig. 2A). Thus, we conclude that LIGHT does not cause barrier dysfunction via induction of epithelial apoptosis.
Figure 2. LIGHT-induced barrier loss does not require apoptosis, tight junction protein degradation, or NFκB signaling.
A. Caco-2 monolayers treated sequentially with IFN-γ (10 ng/ml for 24 hours) and/or LIGHT (50 ng/ml for 8 hours), as described above and indicated in the figure, before harvest for SDS-PAGE and immunoblot analysis. Intact (I) caspase-3 and caspase-8 are readily detected, but cleavage products (C), are not. Data are representative of 3 independent experiments.
B. Caco-2 monolayers treated with IFN-γ (10 ng/ml for 24 hours) followed by LIGHT (50 ng/ml for 8 hours), as indicated, were harvested for SDS-PAGE and immunoblot analysis. No changes in cellular content of the tight junction proteins ZO-1, occludin, or claudin-1 was detected. Data are representative of 4 independent experiments.
C. Caco-2 monolayers were pre-treated with IFN-γ, as above, followed by transfer to media with or without LIGHT (50 ng/ml) and NFκB inhibitors; 2 mM sulfasalazine (SSA); 5 µM curcumin, or 10 µM BAY 11-7085 (BAY). Mean ± SE of TER, normalized to monolayers pre-treated with IFN-γ only, is shown 8 hours after transfer. This experiment, which is representative of 4 similar studies, was performed in triplicate. P < 0.05 for LIGHT vs. control with each drug.
We also considered the possibility that the observed barrier loss could be a result of tight junction protein degradation. Epithelial cell lysates from IFN-γ primed monolayers were compared prior to and after LIGHT treatment. SDS-PAGE immunoblot analysis showed that expression of ZO-1, occludin, and claudin-1 was not affected by LIGHT (Fig. 2B). Thus, LIGHT does not exert its effect on barrier function by altering tight junction protein expression. Although results of studies evaluating the participation of NFκB in TNF-dependent barrier dysfunction have been inconsistent 30, 38, 42, it is well-documented that LIGHT can activate NFκB. However, three separate well-established NFκB inhibitors, sulfasalazine, curcumin, and BAY 11-7085, each failed to prevent LIGHT-induced barrier loss (Fig. 2C). Thus, NFκB activation is not required for LIGHT-induced barrier loss. These data show that the decrease in TER induced by LIGHT is non-apoptotic, is not the result of tight junction protein degradation, and does not require NFκB activation.
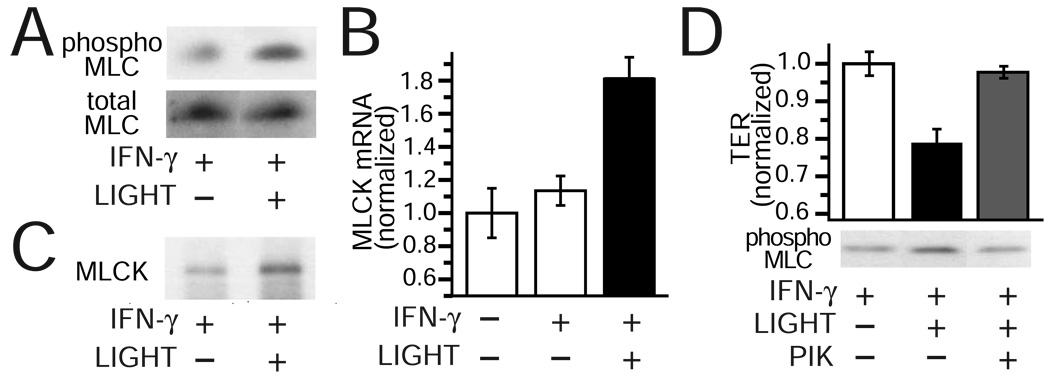
LIGHT induces barrier dysfunction via transcriptional and enzymatic MLCK activation
We have previously shown that transcriptional and enzymatic MLCK activation are necessary for TNF-induced barrier loss 30, 31. We therefore asked if LIGHT-induced barrier loss involved MLCK activation. LIGHT treatment of IFN-γ primed monolayers causes a marked increase in MLC phosphorylation (Fig. 3A). This was associated with increased MLCK transcription (Fig. 3B) as well as increases in total MLCK protein expression (Fig. 3C). Thus, LIGHT treatment of IFN-γ primed monolayers causes both transcriptional and enzymatic MLCK activation. To determine if increased MLCK activity was necessary for LIGHT-induced barrier loss, monolayers were treated with the highly-specific MLCK inhibitor PIK 32, 43. This completely reversed the effects of LIGHT on barrier function and MLC phosphorylation (Fig. 3D). Thus, like TNF, LIGHT induces intestinal epithelial barrier loss by activating MLCK.
Figure 3. LIGHT induces barrier dysfunction via transcriptional and enzymatic MLCK activation.
A. Total and phosphorylated (phospho) MLC were assessed by SDS-PAGE and immunoblot in IFN-γ pre-treated Caco-2 monolayers treated with or without LIGHT (50 ng/ml for 8 hours). Data are representative of 3 independent experiments. P < 0.01 for IFN-γ and LIGHT vs. other conditions.
B. MLCK mRNA content was assessed by quantitative real time PCR was isolated from monolayers after IFN-γ pre-treatment with or without LIGHT (50 ng/ml for 4 hours). Data are representative of 4 independent experiments.
C. MLCK protein content was assessed by SDS-PAGE and immunoblot in IFN-γ pretreated Caco-2 monolayers treated with or without LIGHT (50 ng/ml for 8 hours). Total MLC was used as a loading control (see panel A). Data are representative of 3 independent experiments.
D. Caco-2 monolayers pre-treated with IFN-γ as above were transferred to fresh media, without IFN-γ, but with LIGHT. After 8 hours, PIK (250 µM) was added to the apical media. TER was measured 30 minutes later. Data were normalized to control monolayers pre-treated with IFN-γ but transferred to media without LIGHT and reported as mean ± SE of triplicate monolayers. Results are representative of 4 independent experiments. P < 0.05 for PIK addition to LIGHT-treated monolayers.
LIGHT regulates intestinal epithelial barrier function via lymphotoxin beta receptor
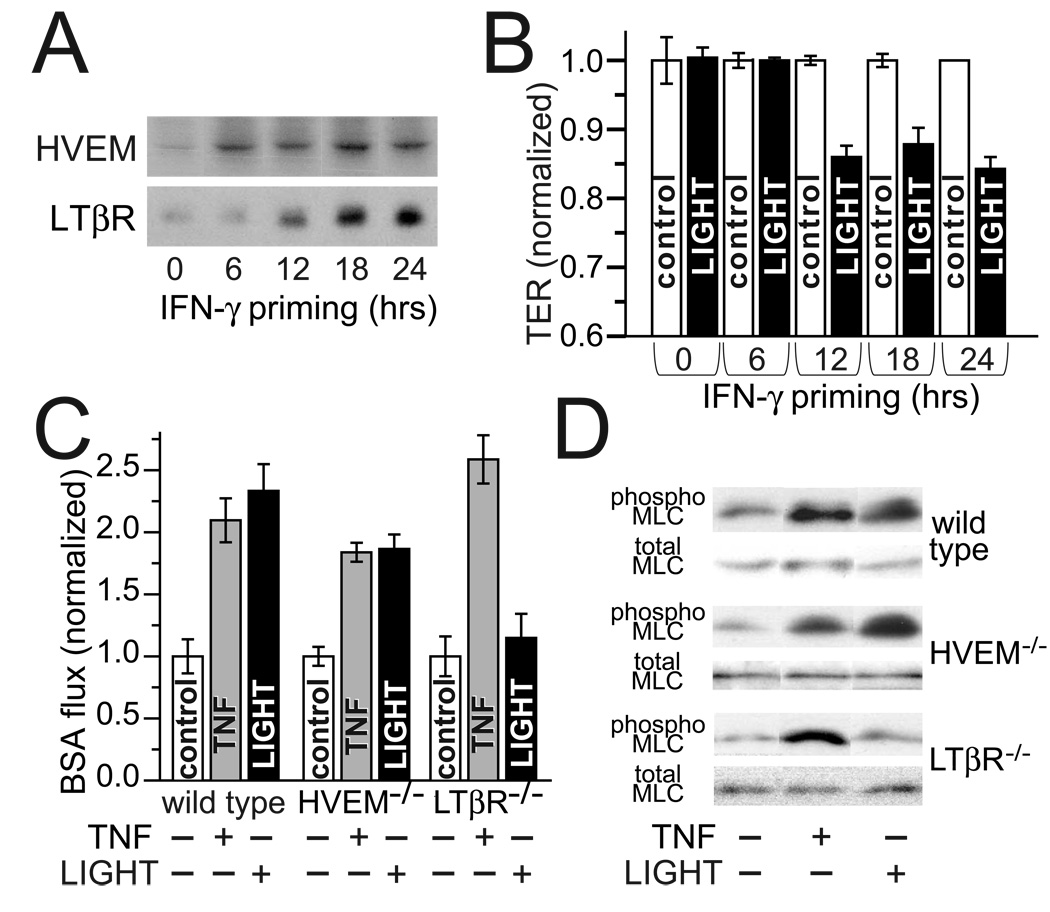
The data above suggest that LIGHT regulates intestinal epithelial barrier function by mechanisms similar to those used by TNF. These include the central role of MLCK and the requirement that monolayers be pretreated with IFN-γ. While TNF causes MLCK activation and barrier function regulation by activation of TNF receptor 2, with the principal role of IFN-γ priming being upregulation of TNF receptor 2 expression 31, LIGHT does not signal through TNF receptors, but can activate two other cell surface receptors; lymphotoxin beta receptor (LTβR) and herpes virus entry mediator (HVEM). No data are available to address which LIGHT receptor is expressed in intestinal epithelia or which receptor mediates LIGHT-induced barrier loss. We therefore asked if IFN-γ priming increased expression of either LTβR or HVEM. Expression of both receptors was upregulated by culture of monolayers with basolateral IFN-γ (Fig. 4A). HVEM expression was increased with 6 hours of IFN-γ treatment while increased expression of LTβR was not apparent for 12 hours (Fig. 4A). As effective antibodies to selectively block HVEM or LTβR function are not available, we took advantage of the differential kinetics of receptor induction by IFN-γ to help determine which receptor mediates the epithelial barrier response to LIGHT. Monolayers were incubated with IFN-γ for variable intervals to induce expression of HVEM alone or HVEM and LTβR. After IFN-γ washout monolayers were transferred to media with or without basolateral LIGHT. A minimum of 12 hours of IFN-γ priming was necessary before monolayers were responsive to LIGHT (Fig. 4B). This interval matched the minimum time necessary for induction of LTβR expression. These data therefore suggest that the effect of LIGHT on intestinal epithelial barrier function is mediated by LTβR. However, these in vitro data cannot exclude the possibility that the actual role of IFN-γ in priming cultured monolayers to respond to LIGHT is mediated by a mechanism other than induction of LTβR expression.
Figure 4. IFN-γ induces LTβR expression which, in turn, mediates LIGHT-induced barrier dysfunction.
A. Caco-2 monolayers treated with IFN-γ (10 ng/ml) in the basal chamber for indicated intervals were harvested for SDS-PAGE and immunoblot analysis. HVEM upregulation was detected within 6 hours, while LTβR expression required at least 12 hours of IFN-γ treatment. Data are representative of 3 independent experiments and are from monolayers handled in parallel to those used in panel B.
B. Caco-2 monolayers pre-treated with IFN-γ for varying intervals, as in part A, prior to transfer to fresh media, without IFN-γ, with or without LIGHT. TER was measured after 8 hours. Data were normalized to control monolayers that were not exposed to IFN-γ or LIGHT and are reported as mean ± SE of triplicate monolayers. Results are representative of 3 independent experiments. P < 0.01 for control vs. LIGHT after 12, 18, or 24 hours of IFN-γ pre-treatment.
C. Perfusion assays were used to determine if LTβR also mediated LIGHT-induced barrier dysfunction in vivo. As reported previously, both TNF and LIGHT (5µg injected i.p.) caused large increases in BSA flux across the jejunal epithelium of wild type mice, consistent with increased paracellular permeability. Similar responses were seen in HVEM−/− mice. In contrast, LIGHT was unable to increase BSA flux in LTβR−/− mice. This was not due to a global defect in these mice, as the response to TNF was maintained in LTβR−/− mice. Results are representative of 3 independent experiments. P < 0.05 for TNF vs. control in all mice and LIGHT vs. control in wild type and HVEM−/− mice.
D. Jejunal epithelia were isolated from wild type, HVEM−/−, and LTβR−/− mice 3 hours after injection of TNF or LIGHT. As reported previously, both LIGHT and TNF induced epithelial MLC phosphorylation in wild type mice. Similar results were obtained with HVEM−/− mice. Although LTβR−/− mice responded normally to TNF, they did not increase jejunal epithelial MLC phosphorylation in response to LIGHT. Results are representative of 3 independent experiments.
As an alternative to specific LTβR blocking antibodies, we took advantage of the availability of LTβR and HVEM knockout mice. These were studied using an in vivo model of cytokine-induced barrier dysfunction 20. Following injection of purified recombinant LIGHT, barrier function was assessed by measuring flux of tagged serum albumin from the blood stream into the jejunal lumen 20, 33. LIGHT induced marked increases in albumin flux, i.e. reductions in barrier function, in both wild type and HVEM knockout mice (Fig. 4C). In contrast, LIGHT did not affect albumin flux in LTβR knockout mice (Fig. 4C). Since wild type, HVEM knockout, and LTβR knockout mice were all able to decrease barrier function in response to TNF (Fig. 4C), these data demonstrate that LTβR is a critical intermediate in LIGHT signaling to regulate intestinal epithelial barrier function in vivo. Consistent with the in vitro data suggesting that epithelial MLC phosphorylation is necessary for LIGHT-induced barrier loss, MLC phosphorylation was increased by LIGHT in wild type and HVEM knockout, but not LTβR knockout, mice (Fig. 4D). Together with the in vitro data, these in vivo data indicate that LIGHT signals to intestinal epithelia through LTβR to increase MLC phosphorylation and decrease epithelial barrier function.
LIGHT-induced barrier loss is associated with tight junction protein endocytosis
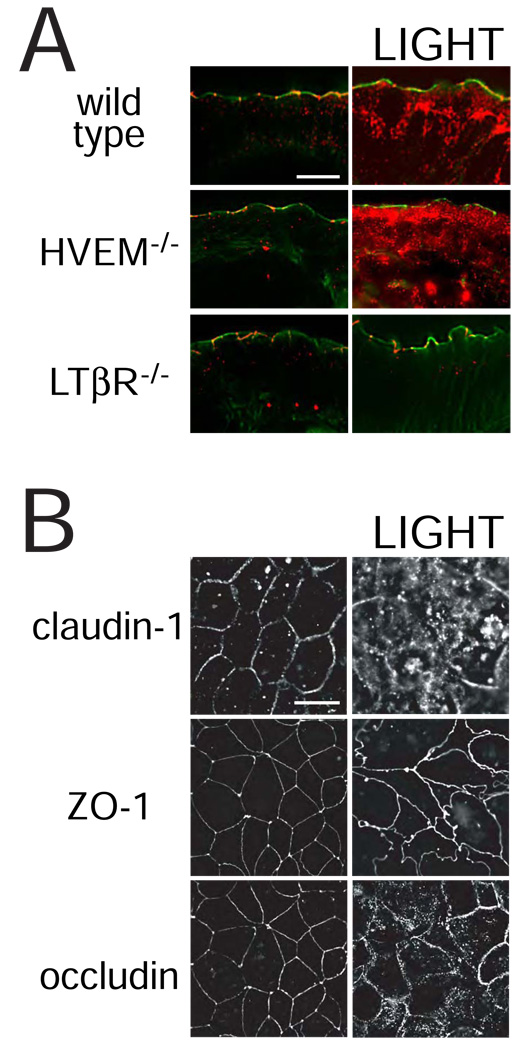
We have previously shown that LIGHT causes endocytosis of the tight junction protein occludin in murine jejunal enterocytes 20. To determine if this too requires LTβR signaling, occludin distribution was assessed in wild type, HVEM knockout, and LTβR knockout mice (Fig. 5A). In the absence of cytokine injection, occludin within jejunal enterocytes of all mice was primarily at tight junctions and occasionally within intracellular vesicles. TNF induced occludin internalization in all mice (data not shown), but LIGHT only enhanced endocytosis in wild type and HVEM knockout mice (Fig. 5A). No increase in occludin internalization was seen in LIGHT-treated LTβR knockout mice (Fig. 5A). Similarly, LIGHT induced endocytosis of claudin-1 and occludin in IFN-γ primed intestinal epithelial monolayers (Fig. 5B). ZO-1 internalization was not seen, but the normally smooth arc-like ZO-1 profiles were transformed into a complex series of irregular undulations (Fig. 5B) similar to those induced by cytokine-independent MLCK activation 44.
Figure 5. LIGHT-induced barrier dysfunction is accompanied by tight junction protein endocytosis.
A. Jejunal mucosa from wild type, HVEM−/−, and LTβR−/− mice were snap-frozen 3 hours after injection of LIGHT. Sections were stained for occludin (red) and F-actin (green). In control mice occludin is primarily localized to the tight junction, where it punctuates the perijunctional actomyosin ring. LIGHT induces the formation of large intracellular occludin stores in jejunal epithelia of wild type and HVEM−/− mice. In contrast, LTβR−/− mice did not internalize occludin in response to LIGHT. Results are representative of 3 independent experiments. Bar = 10 µm.
B. Caco-2 monolayers were pre-treated with IFN-γ followed by transfer to media with or without LIGHT. After 8 hours the monolayers were fixed and immunostained for claudin-1, ZO-1, and occludin. Obvious claudin-1 and occludin internalization is induced by LIGHT. Vesicular ZO-1 deposits are not formed, but the distribution at the tight junction is markedly disrupted. Results are representative of 5 independent experiments. Bar = 20 µm.
LIGHT-induced barrier loss correlates with occludin internalization
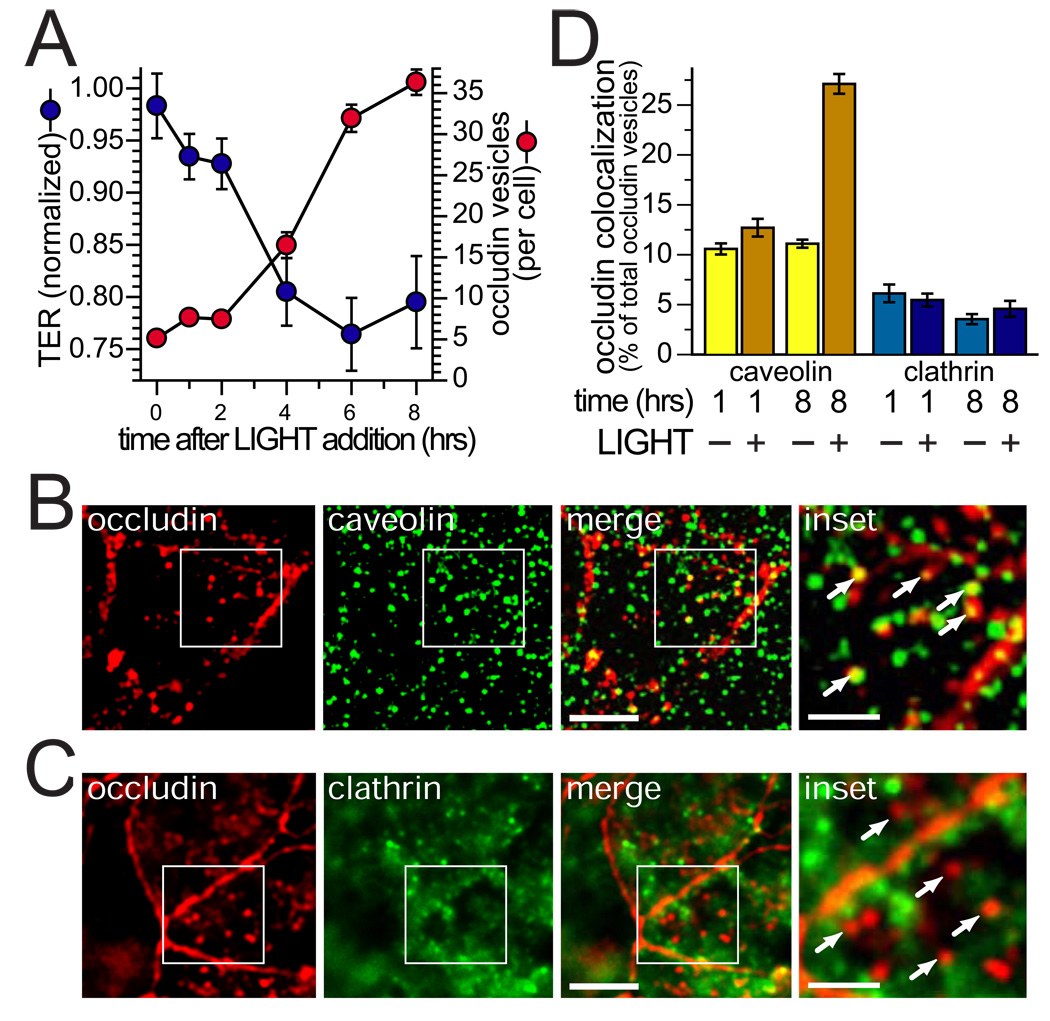
The data above show that occludin is internalized following LTβR activation. To begin to characterize the contribution of this endocytic process to barrier loss, we first correlated the number of occludin-positive vesicles per cell with barrier function during treatment of IFN-γ primed intestinal epithelial monolayers with LIGHT. Between 2 and 4 hours after LIGHT addition, TER fell by 12% and the number of occludin-positive vesicles more than doubled to 16 per cell (Fig. 6A).
Figure 6. Occludin and caveolin-1 endocytosis accompany LIGHT-induced barrier dysfunction.
A. Caco-2 monolayers were pre-treated with IFN-γ were transferred to media with LIGHT for indicated times before fixation. Morphometric analysis was performed after immunostaining for occludin. The number of intracellular occludin vesicles was counted for 15 cells at each time point. Results are representative of 3 independent experiments.
B. Caco-2 monolayers were pre-treated with IFN-γ and transferred to media with LIGHT for 8 hours. Many of the newly-formed occludin vesicles also contained caveolin-1 (arrows in the inset). In contrast, most occludin-containing vesicles were negative for clathrin heavy chain (arrows in the inset). Bars = 10 µm and 2 µm (inset).
C. Caco-2 monolayers were pre-treated with IFN-γ and transferred to media with LIGHT for 8 hours. Most newly-formed occludin-containing vesicles were negative for clathrin heavy chain (arrows in the inset). Bars = 10 µm and 2 µm (inset).
D. Caco-2 monolayers were pre-treated with IFN-γ were transferred to media with or without LIGHT for 1 or 8 hours, as indicated times. These were then labeled for occludin and either caveolin-1 or clathrin heavy chain, as in panels B and C. Morphometric analysis of the frequency with which occludin vesicles were also positive for caveolin-1 or clathrin heavy chain is shown based on analysis of 15 cells examined for each condition. Results are representative of 3 independent experiments. P < 0.01 for caveolin-occludin colocalization 8 hours after LIGHT addition vs. all other conditions.
Caveolae-mediated endocytosis, clathrin-mediated endocytosis, and macropinocytosis have all been implicated in tight junction protein internalization in response to various stimuli 45–47. To preliminarily determine the route of LIGHT-induced occludin endocytosis, monolayers were immunostained for occludin and either caveolin-1 (Fig. 6B) or clathrin heavy chain (Fig. 6C). The fraction of occludin-containing vesicles that also contained caveolin-1 more than doubled 8 hours after LIGHT treatment (Fig. 6D). In contrast, the fraction of occludin-containing vesicles that also contained clathrin heavy chain was not changed by LIGHT. These data show that occludin is internalized into caveolin-1-containing compartments during LIGHT-induced barrier loss.
Caveolar endocytosis is required for LIGHT-induced claudin-1 and occludin internalization and barrier loss
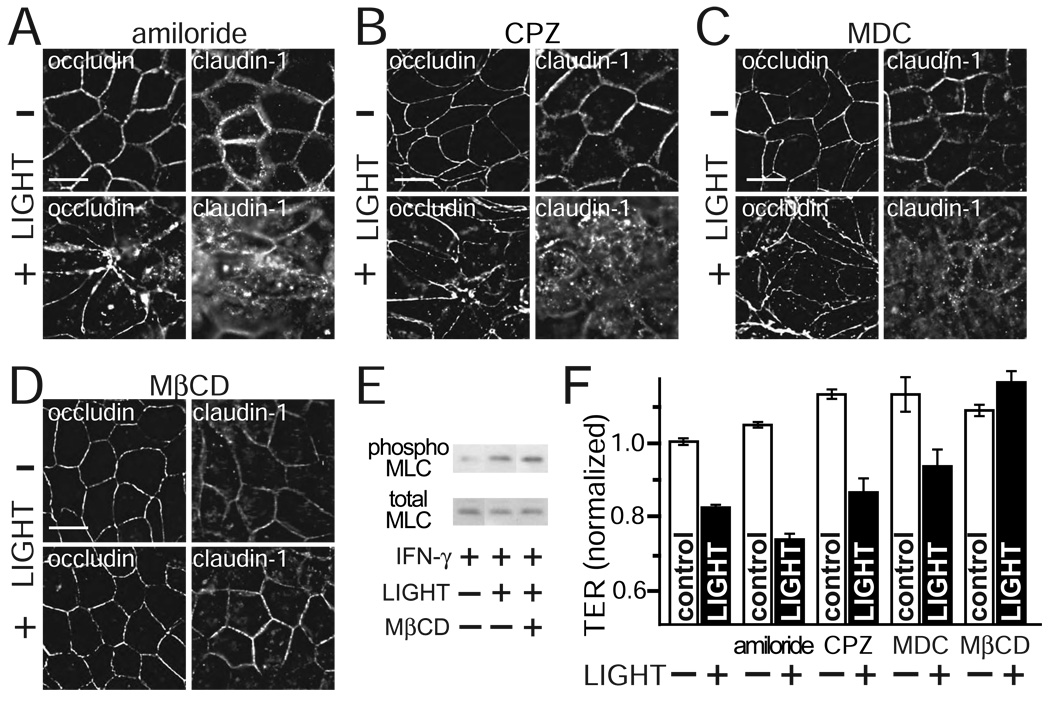
While the data above show that LIGHT causes occludin to be internalized into caveolin-1-containing vesicles, they do not directly demonstrate the endocytosis occurs via caveolae. To determine the specific pathway of tight junction protein endocytosis, monolayers were treated with inhibitors of each pathway. Amiloride, which inhibits Na+/H+ exchange and selectively blocks macropinocytosis without affecting clathrin-mediated endocytosis 48, did not prevent LIGHT-induced internalization of occludin or claudin-1 (Fig. 7A). Similarly, two separate agents that prevent clathrin-mediated endocytosis, chlorpromazine 49 and monodansyl cadaverine 50 did not block occludin or claudin-1 endocytosis (Fig. 7B, C). In contrast, the cholesterol binding drug methyl-β-cyclodextrin, which interferes with caveolar endocytosis, completely prevented occludin and claudin-1 internalization following LIGHT treatment (Fig. 7D). This was not due to disruption of LIGHT signaling by methyl-β-cyclodextrin, as MLC phosphorylation was still induced by LIGHT in methyl-β-cyclodextrin-treated monolayers (Fig. 7E). Thus, LIGHT-induced tight junction protein internalization occurs via caveolar endocytosis.
Figure 7. Caveolar endocytosis is required for LIGHT-induced occludin and claudin-1 internalization and barrier dysfunction.
A. Caco-2 monolayers were pre-treated with IFN-γ were transferred to media with 200 µM amiloride and with or without LIGHT for 8 hours, as indicated. Representative images of occludin and claudin-1 distributions are shown. Results are typical of 3 independent experiments. Bar = 20 µm.
B. Caco-2 monolayers were pre-treated with IFN-γ were transferred to media with 60 µM chlorpromazine (CPZ) and with or without LIGHT for 8 hours, as indicated. Representative images of occludin and claudin-1 distributions are shown. Results are typical of 3 independent experiments. Bar = 20 µm.
C. Caco-2 monolayers were pre-treated with IFN-γ were transferred to media with 200 µM monodansyl cadaverine (MDC) and with or without LIGHT for 8 hours, as indicated. Representative images of occludin and claudin-1 distributions are shown. Results are typical of 3 independent experiments. Bar = 20 µm.
D. Caco-2 monolayers were pre-treated with IFN-γ were transferred to media with 2.5 mM methyl-β-cyclodextrin (MβCD) and with or without LIGHT for 8 hours, as indicated. Representative images of occludin and claudin-1 distributions are shown. Results are typical of 3 independent experiments. Bar = 20 µm.
E. Total and phosphorylated (phospho) MLC were assessed by SDS-PAGE and immunoblot in IFN-γ pre-treated Caco-2 monolayers treated with or without LIGHT and MβCD for 8 hours. Data are representative of 3 independent experiments.
F. Caco-2 monolayers were pre-treated with IFN-γ followed by transfer to media with or without LIGHT and endocytosis inhibitors. Mean ± SE of TER, normalized to monolayers pre-treated with IFN-γ only, is shown 8 hours after transfer. This triplicate experiment is representative of 4 similar studies. P < 0.05 for LIGHT vs. control with each drug except methyl-β-cyclodextrin.
To assess the functional relevance of tight junction protein internalization on LIGHT-induced barrier loss, TER was measured in monolayers treated with the same endocytosis inhibitors. Macropinocytosis and clathrin-mediated endocytosis inhibitors were not able to protect against LIGHT-induced barrier loss (Fig. 7F). In contrast, the caveolar endocytosis inhibitor methyl-β-cyclodextrin completely prevented LIGHT-induced barrier loss (Fig. 7F). These data therefore show that caveolar endocytosis is necessary for LIGHT-induced barrier loss.
DISCUSSION
Human and animals studies have shown that barrier defects can precede IBD development and reactivation, but can also be elicited by inflammatory activity 10–13, 16, 28, 33, 51–54. Despite this, the pathogenic roles of intestinal epithelial tight junction dysfunction in IBD development remain undefined. In part, this reflects our incomplete understanding of the stimuli and mediators that cause tight junction dysfunction. The goals of this study were to determine if, separate from T cell activation, LIGHT was able to regulate tight junction permeability and to determine the cellular mechanisms responsible for such regulation.
Our preliminary experiments suggested that LIGHT was not able to signal directly to intestinal epithelia. However, by analogy with TNF signaling, in which IFN-γ-dependent induction of TNF receptor expression allows well-differentiated epithelial monolayers to respond to TNF, we hypothesized that IFN-γ might enhance responsiveness to LIGHT. This was correct, as IFN-γ-primed monolayers responded to LIGHT in a polarized manner. Both the requirement for IFN-γ-pretreatment and the polarized response to basolateral LIGHT are explained by the data showing that LTβR, which is expressed basolaterally in intestinal epithelia, is the receptor that mediates the effects of LIGHT on barrier function. Since these data demonstrate conclusively that LIGHT induces barrier dysfunction independent of TNF signaling, it is tempting to speculate that LIGHT may contribute to the TNF-independent barrier hyperresponsiveness present in a subset of Crohn’s disease patients 14. One could also hypothesize that IFN-γ plays a critical permissive role in vivo, similar to that demonstrated in these in vitro studies. This is consistent with the in vivo observation that IFN-γ neutralization prevents experimental colitis only when administered prior to development of overt disease 55. Whether this early requirement for IFN-γ in vivo reflects a role in inducing epithelial TNF core family member receptors, as we have demonstrated in vitro, awaits further study.
Recent studies have suggested that cytokines modulate intestinal epithelial tight junction barrier properties by modifying claudin protein expression 17, 18, 56 or MLCK activation 20, 28–33, 57. Together with data shown that LIGHT increases MLCK expression and activity, the restoration of barrier function following specific MLCK inhibition demonstrated MLCK activation to be the primary mechanism of LIGHT-induced tight junction regulation. This is also consistent with the relatively rapid kinetics of the barrier response to LIGHT 57.
To further dissect the effects of LIGHT on the tight junction, immunofluorescent studies of LIGHT-treated monolayers were performed and showed that LIGHT caused tight junction reorganization. Notably, occludin endocytosis correlated directly with barrier loss. We therefore sought to determine the mechanism of occludin endocytosis. The data show that occludin internalized following LIGHT exposure colocalizes with caveolin-1, but not clathrin heavy chain. This suggested that LIGHT triggers caveolar occludin endocytosis, which was confirmed by demonstrating that LIGHT-induced occludin endocytosis was blocked by disruption of caveolae with MβCD. Interestingly, this contrasts sharply with the role of macropinocytosis in IFN-γ-induced tight junction protein internalization 45, despite the essential contribution of MLC phosphorylation to IFN-γ-, TNF-, LIGHT-induced tight junction protein internalization 22, 30, 33. Perhaps more importantly, blockade of LIGHT-induced occludin endocytosis by disruption of caveolae was able to completely prevent barrier dysfunction. Thus, these data “close the loop” by demonstrating that occludin endocytosis is mediated by caveolae and that caveolar endocytosis is required for LIGHT-induced barrier dysfunction to occur. This is a critical distinction from work in which various pathways of endocytosis have been defined morphologically but not shown to be necessary for regulation of tight junction barrier function.
These data show that LIGHT-induced MLC phosphorylation triggers caveolar endocytosis of occludin. This all the more remarkable because occludin endocytosis has been identified as a morphologic correlate of cytoskeletal tight junction regulation following TNF-induced MLCK activation, rho activation, and actin depolymerization 30, 33, 47, 58. In the case of pharmacological actin depolymerization, occludin endocytosis requires caveolar function 47. Thus, it may be that caveolar endocytosis is a common mechanism of rapid, cytoskeletally-mediated tight junction regulation. However, while these data demonstrate that occludin endocytosis accompanies cytoskeletally-mediated tight junction regulation, they do not implicate occludin as the critical functional protein removed from the tight junction. This is particularly important to emphasize as, despite numerous in vitro studies suggesting important functional roles for occludin 59–68, the occludin knockout mouse has normal intestinal barrier function without any recognizable intestinal disease 69, 70. Unfortunately, as no studies of intestinal barrier function in response to stressors, such as cytokines, have been reported in the occludin knockout mouse, it is presently impossible to be decisive regarding the functional importance of intestinal epithelial occludin. Regardless of the specific role of occludin, the present data clearly show that caveolar endocytosis is required for LIGHT-induced barrier loss and, by inference, suggest that this mechanism may also apply to TNF-induced barrier loss.
In summary, these data demonstrate that LIGHT signals directly to intestinal epithelial cells via LTβR. Since LIGHT is primarily expressed by activated T cells, this reveals a previously unrecognized mechanism by which T cells can disrupt intestinal barrier function in IBD. LIGHT induces both transcriptional and enzymatic activation of intestinal epithelial MLCK and stimulates occludin endocytosis through a caveolar pathway, all of which are required for LIGHT-induced barrier loss. Thus, inhibition of both MLCK and caveolar endocytosis represent potential therapeutic targets for restoration of barrier function in IBD.
Acknowledgements
This work was supported by National Institutes of Health grants (DK61931, DK68271, DK58897, and AI62026) and The University of Chicago Cancer Research Center (P30 CA14599). D.R.C. is a fellow of the National Institutes of Health in The University of Chicago Medical Scientist Training Program (T32 GM07281).
Abbreviations
- HVEM
herpes virus entry mediator
- IFN-γ
interferon-γ
- IBD
inflammatory bowel disease
- LIGHT
lymphotoxin-like inducible protein that competes with glycoprotein D for herpes virus entry on T cells
- LTβR
lymphotoxin β receptor
- MLC
myosin II regulatory light chain
- MLCK
myosin light chain kinase
- TER
transepithelial resistance
- TNF
tumor necrosis factor
Footnotes
No conflicts of interest exist.
REFERENCES
- 1.Wang J, Anders RA, Wu Q, Peng D, Cho JH, Sun Y, Karaliukas R, Kang HS, Turner JR, Fu YX. Dysregulated LIGHT expression on T cells mediates intestinal inflammation and contributes to IgA nephropathy. J Clin Invest. 2004;113:826–835. doi: 10.1172/JCI20096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaikh RB, Santee S, Granger SW, Butrovich K, Cheung T, Kronenberg M, Cheroutre H, Ware CF. Constitutive expression of LIGHT on T cells leads to lymphocyte activation, inflammation, and tissue destruction. J Immunol. 2001;167:6330–6337. doi: 10.4049/jimmunol.167.11.6330. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Anders RA, Wang Y, Turner JR, Abraham C, Pfeffer K, Fu YX. The Critical Role of LIGHT in Promoting Intestinal Inflammation and Crohn's Disease. J Immunol. 2005;174:8173–8182. doi: 10.4049/jimmunol.174.12.8173. [DOI] [PubMed] [Google Scholar]
- 4.Granger SW, Butrovich KD, Houshmand P, Edwards WR, Ware CF. Genomic characterization of LIGHT reveals linkage to an immune response locus on chromosome 19p13.3 and distinct isoforms generated by alternate splicing or proteolysis. J Immunol. 2001;167:5122–5128. doi: 10.4049/jimmunol.167.9.5122. [DOI] [PubMed] [Google Scholar]
- 5.Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, McLeod RS, Griffiths AM, Green T, Brettin TS, Stone V, Bull SB, Bitton A, Williams CN, Greenberg GR, Cohen Z, Lander ES, Hudson TJ, Siminovitch KA. Genomewide search in Canadian families with inflammatory bowel disease reveals two novel susceptibility loci. Am J Hum Genet. 2000;66:1863–1870. doi: 10.1086/302913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohavy O, Zhou J, Granger SW, Ware CF, Targan SR. LIGHT expression by mucosal T cells may regulate IFN-gamma expression in the intestine. J Immunol. 2004;173:251–258. doi: 10.4049/jimmunol.173.1.251. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Fu YX. Tumor necrosis factor family members and inflammatory bowel disease. Immunol Rev. 2005;204:144–155. doi: 10.1111/j.0105-2896.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- 8.Mackay F, Browning JL, Lawton P, Shah SA, Comiskey M, Bhan AK, Mizoguchi E, Terhorst C, Simpson SJ. Both the lymphotoxin and tumor necrosis factor pathways are involved in experimental murine models of colitis. Gastroenterology. 1998;115:1464–1475. doi: 10.1016/s0016-5085(98)70025-3. [DOI] [PubMed] [Google Scholar]
- 9.Hollander D. The intestinal permeability barrier. A hypothesis as to its regulation and involvement in Crohn's disease. Scand J Gastroenterol. 1992;27:721–726. doi: 10.3109/00365529209011172. [DOI] [PubMed] [Google Scholar]
- 10.Yacyshyn BR, Meddings JB. CD45RO expression on circulating CD19+ B cells in Crohn's disease correlates with intestinal permeability. Gastroenterology. 1995;108:132–137. doi: 10.1016/0016-5085(95)90017-9. [DOI] [PubMed] [Google Scholar]
- 11.Wyatt J, Vogelsang H, Hubl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet. 1993;341:1437–1439. doi: 10.1016/0140-6736(93)90882-h. [DOI] [PubMed] [Google Scholar]
- 12.Hollander D. Permeability in Crohn's disease: Altered barrier functions in healthy relatives? Gastroenterology. 1993;104:1848–1851. doi: 10.1016/0016-5085(93)90668-3. [DOI] [PubMed] [Google Scholar]
- 13.Buhner S, Buning C, Genschel J, Kling K, Herrmann D, Dignass A, Kuechler I, Krueger S, Schmidt HH, Lochs H. Genetic basis for increased intestinal permeability in families with Crohn's disease: role of CARD15 3020insC mutation? Gut. 2006;55:342–347. doi: 10.1136/gut.2005.065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suenaert P, Bulteel V, Vermeire S, Noman M, Van Assche G, Rutgeerts P. Hyperresponsiveness of the mucosal barrier in Crohn's disease is not tumor necrosis factor-dependent. Inflamm Bowel Dis. 2005;11:667–673. doi: 10.1097/01.mib.0000168371.87283.4b. [DOI] [PubMed] [Google Scholar]
- 15.Hollander D. Crohn's disease, TNF-alpha, and the leaky gut. The chicken or the egg? Am J Gastroenterol. 2002;97:1867–1868. doi: 10.1111/j.1572-0241.2002.05895.x. [DOI] [PubMed] [Google Scholar]
- 16.Suenaert P, Bulteel V, Lemmens L, Noman M, Geypens B, Van Assche G, Geboes K, Ceuppens JL, Rutgeerts P. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn's disease. Am J Gastroenterol. 2002;97:2000–2004. doi: 10.1111/j.1572-0241.2002.05914.x. [DOI] [PubMed] [Google Scholar]
- 17.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, Collins JE. Inflammatory processes have differential effects on Claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest. 2005;85:1139–1162. doi: 10.1038/labinvest.3700316. [DOI] [PubMed] [Google Scholar]
- 19.Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- 20.Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest. 2006;116:2682–2694. doi: 10.1172/JCI29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nusrat A, Turner JR, Madara JL. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G851–G857. doi: 10.1152/ajpgi.2000.279.5.G851. [DOI] [PubMed] [Google Scholar]
- 22.Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: Myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell. 2005;16:5040–5052. doi: 10.1091/mbc.E05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullin JM, Snock KV. Effect of tumor necrosis factor on epithelial tight junctions and transepithelial permeability. Cancer. Res. 1990;50:2172–2176. [PubMed] [Google Scholar]
- 24.Taylor CT, Dzus AL, Colgan SP. Autocrine regulation of epithelial permeability by hypoxia: role for polarized release of tumor necrosis factor alpha. Gastroenterology. 1998;114:657–668. doi: 10.1016/s0016-5085(98)70579-7. [DOI] [PubMed] [Google Scholar]
- 25.Abreu MT, Palladino AA, Arnold ET, Kwon RS, McRoberts JA. Modulation of barrier function during Fas-mediated apoptosis in human intestinal epithelial cells. Gastroenterology. 2000;119:1524–1536. doi: 10.1053/gast.2000.20232. [DOI] [PubMed] [Google Scholar]
- 26.Gitter AH, Bendfeldt K, Schulzke JD, Fromm M. Leaks in the epithelial barrier caused by spontaneous and TNF-alpha-induced single-cell apoptosis. Faseb J. 2000;14:1749–1753. doi: 10.1096/fj.99-0898com. [DOI] [PubMed] [Google Scholar]
- 27.Gitter AH, Wullstein F, Fromm M, Schulzke JD. Epithelial barrier defects in ulcerative colitis: characterization and quantification by electrophysiological imaging. Gastroenterology. 2001;121:1320–1328. doi: 10.1053/gast.2001.29694. [DOI] [PubMed] [Google Scholar]
- 28.Blair SA, Kane SV, Clayburgh DR, Turner JR. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest. 2006;86:191–201. doi: 10.1038/labinvest.3700373. [DOI] [PubMed] [Google Scholar]
- 29.Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005;288:G422–G430. doi: 10.1152/ajpgi.00412.2004. [DOI] [PubMed] [Google Scholar]
- 30.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang F, Schwarz BT, Graham WV, Wang Y, Su L, Clayburgh DR, Abraham C, Turner JR. IFN-gamma-induced TNFR2 upregulation is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology. 2006;131:1153–1163. doi: 10.1053/j.gastro.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, Mrsny RJ, Turner JR. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology. 2002;123:163–172. doi: 10.1053/gast.2002.34235. [DOI] [PubMed] [Google Scholar]
- 33.Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, Clarke LL, Mrsny RJ, Turner JR. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115:2702–2715. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang M, Guo R, Zhai Y, Yang D. LIGHT sensitizes IFNgamma-mediated apoptosis of MDA-MB-231 breast cancer cells leading to down-regulation of anti-apoptosis Bcl-2 family members. Cancer Lett. 2003;195:201–210. doi: 10.1016/s0304-3835(03)00148-4. [DOI] [PubMed] [Google Scholar]
- 35.Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997;273:C1378–C1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- 36.Peterson MD, Mooseker MS. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J Cell Sci. 1992;102:581–600. doi: 10.1242/jcs.102.3.581. [DOI] [PubMed] [Google Scholar]
- 37.Berglund JJ, Riegler M, Zolotarevsky Y, Wenzl E, Turner JR. Regulation of human jejunal transmucosal resistance and MLC phosphorylation by Na+-glucose cotransport. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1487–G1493. doi: 10.1152/ajpgi.2001.281.6.G1487. [DOI] [PubMed] [Google Scholar]
- 38.Graham WV, Wang F, Clayburgh DR, Cheng JX, Yoon B, Wang Y, Lin A, Turner JR. Tumor necrosis factor-induced long myosin light chain kinase transcription is regulated by differentiation-dependent signaling events. Characterization of the human long myosin light chain kinase promoter. J Biol Chem. 2006;281:26205–26215. doi: 10.1074/jbc.M602164200. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Subudhi SK, Anders RA, Lo J, Sun Y, Blink S, Wang J, Liu X, Mink K, Degrandi D, Pfeffer K, Fu YX. The role of herpesvirus entry mediator as a negative regulator of T cell-mediated responses. J Clin Invest. 2005;115:711–717. doi: 10.1172/JCI200522982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 41.Mankertz J, Tavalali S, Schmitz H, Mankertz A, Riecken EO, Fromm M, Schulzke JD. Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J Cell Sci. 2000;113:2085–2090. doi: 10.1242/jcs.113.11.2085. [DOI] [PubMed] [Google Scholar]
- 42.Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367–G376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- 43.Owens DW, Wilson NJ, Hill AJ, Rugg EL, Porter RM, Hutcheson AM, Quinlan RA, van Heel D, Parkes M, Jewell DP, Campbell SS, Ghosh S, Satsangi J, Lane EB. Human keratin 8 mutations that disturb filament assembly observed in inflammatory bowel disease patients. J Cell Sci. 2004;117:1989–1999. doi: 10.1242/jcs.01043. [DOI] [PubMed] [Google Scholar]
- 44.Shen L, Black ED, Witkowski ED, Lencer WI, Guerriero V, Schneeberger EE, Turner JR. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci. 2006;119:2095–2106. doi: 10.1242/jcs.02915. [DOI] [PubMed] [Google Scholar]
- 45.Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. Faseb J. 2005;19:923–933. doi: 10.1096/fj.04-3260com. [DOI] [PubMed] [Google Scholar]
- 46.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell. 2004;15:176–188. doi: 10.1091/mbc.E03-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol Biol Cell. 2005;16:3919–3936. doi: 10.1091/mbc.E04-12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.West MA, Bretscher MS, Watts C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J Cell Biol. 1989;109:2731–2739. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang LH, Rothberg KG, Anderson RG. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123:1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davies PJ, Davies DR, Levitzki A, Maxfield FR, Milhaud P, Willingham MC, Pastan IH. Transglutaminase is essential in receptor-mediated endocytosis of alpha 2-macroglobulin and polypeptide hormones. Nature. 1980;283:162–167. doi: 10.1038/283162a0. [DOI] [PubMed] [Google Scholar]
- 51.Olson TS, Reuter BK, Scott KG, Morris MA, Wang XM, Hancock LN, Burcin TL, Cohn SM, Ernst PB, Cominelli F, Meddings JB, Ley K, Pizarro TT. The primary defect in experimental ileitis originates from a nonhematopoietic source. J Exp Med. 2006;203:541–552. doi: 10.1084/jem.20050407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Irvine EJ, Marshall JK. IncreasedintestinalpermeabilityprecedestheonsetofCrohn'sdiseaseinasubject with familial risk. Gastroenterology. 2000;119:1740–1744. doi: 10.1053/gast.2000.20231. [DOI] [PubMed] [Google Scholar]
- 53.Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis. 1999;5:262–270. doi: 10.1097/00054725-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Ferrier L, Mazelin L, Cenac N, Desreumaux P, Janin A, Emilie D, Colombel JF, Garcia-Villar R, Fioramonti J, Bueno L. Stress-induced disruption of colonic epithelial barrier: Role of interferon-gamma and myosin light chain kinase in mice. Gastroenterology. 2003;125:795–804. doi: 10.1016/s0016-5085(03)01057-6. [DOI] [PubMed] [Google Scholar]
- 55.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–662. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 56.Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weber CR, Turner JR. Inflammatory bowel disease: is it really just another break in the wall? Gut. 2007;56:6–8. doi: 10.1136/gut.2006.104182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hopkins AM, Walsh SV, Verkade P, Boquet P, Nusrat A. Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J Cell Sci. 2003;116:725–742. doi: 10.1242/jcs.00300. [DOI] [PubMed] [Google Scholar]
- 59.Furuse M, Fujimoto K, Sato N, Hirase T, Tsukita S. Overexpression of occludin, a tight junction-associated integral membrane protein, induces the formation of intracellular multilamellar bodies bearing tight junction-like structures. J Cell Sci. 1996;109:429–435. doi: 10.1242/jcs.109.2.429. [DOI] [PubMed] [Google Scholar]
- 60.McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Sci. 1996;109:2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- 61.Chen Y, Merzdorf C, Paul DL, Goodenough DA. COOH terminus of occludin is required for tight junction barrier function in early Xenopus embryos. J Cell Biol. 1997;138:891–899. doi: 10.1083/jcb.138.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wong V, Gumbiner BM. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol. 1997;136:399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huber D, Balda MS, Matter K. Occludin modulates transepithelial migration of neutrophils. J Biol Chem. 2000;275:5773–5778. doi: 10.1074/jbc.275.8.5773. [DOI] [PubMed] [Google Scholar]
- 64.Li D, Mrsny RJ. Oncogenic Raf-1 disrupts epithelial tight junctions via downregulation of occludin. J Cell Biol. 2000;148:791–800. doi: 10.1083/jcb.148.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murata M, Kojima T, Yamamoto T, Go M, Takano K, Osanai M, Chiba H, Sawada N. Down-regulation of survival signaling through MAPK and Akt in occludin-deficient mouse hepatocytes in vitro. Exp Cell Res. 2005;310:140–151. doi: 10.1016/j.yexcr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 66.Van Itallie CM, Anderson JM. Occludin confers adhesiveness when expressed in fibroblasts. J Cell Sci. 1997;110:1113–1121. doi: 10.1242/jcs.110.9.1113. [DOI] [PubMed] [Google Scholar]
- 67.Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol. 2005;288:C1231–C1241. doi: 10.1152/ajpcell.00581.2004. [DOI] [PubMed] [Google Scholar]
- 68.Rajasekaran SA, Barwe SP, Gopal J, Ryazantsev S, Schneeberger EE, Rajasekaran AK. Na,K-ATPase regulates tight junction permeability through occludin phosphorylation in pancreatic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2006 doi: 10.1152/ajpgi.00297.2006. [DOI] [PubMed] [Google Scholar]
- 69.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schulzke JD, Gitter AH, Mankertz J, Spiegel S, Seidler U, Amasheh S, Saitou M, Tsukita S, Fromm M. Epithelial transport and barrier function in occludin-deficient mice. Biochim Biophys Acta. 2005;1669:34–42. doi: 10.1016/j.bbamem.2005.01.008. [DOI] [PubMed] [Google Scholar]