Abstract
Members of the Bcl-2 family play a major role in the pathobiology of head and neck cancer. We have shown that Bcl-2 orchestrates a crosstalk between tumor cells and endothelial cells that have a direct impact on the progression of head and neck squamous cell carcinoma (HNSCC). Notably, Bcl-2 is significantly upregulated in the tumor associated endothelial cells as compared to the endothelial cells of normal oral mucosa in patients with HNSCC. Here, we evaluated the effect of TW-37, a small molecule inhibitor of Bcl-2, on the cell cycle and survival of endothelial cells and HNSCC and on the progression of xenografted tumors. TW-37 has an IC50 of 1.1 µM for primary human endothelial cells and averaged 0.3 µM for head and neck cancer cells (OSCC3, UM-SCC-1, UM-SCC-74A). Combination of TW-37 and cisplatin showed enhanced cytotoxic effects for endothelial cells and HNSCC in vitro, as compared with single drug treatment . Notably, while cisplatin led to an expected G2/M cell cycle arrest, TW-37 mediated an “S” phase cell cycle arrest in endothelial cells and in HNSCC. In vivo, TW-37 inhibited tumor angiogenesis and induced tumor apoptosis without significant systemic toxicities. Combination of TW-37 and cisplatin enhanced the time to tumor failure (i.e. 4-fold increase in tumor volume), as compared to either drug given separately. Collectively, these data reveal that therapeutic inhibition of Bcl-2 function with TW-37 is sufficient to arrest endothelial cells and HNSCC in the “S” phase of cell cycle, and to inhibit head and neck tumor angiogenesis.
Keywords: apoptosis, neovascularization, cisplatin, cancer
Introduction
The long-term prognosis of patients with advanced head and neck squamous cell carcinoma (HNSCC) has shown modest improvement over the last three decades (1, 2). The treatment of choice for these patients depends on the stage and the site of the tumor, but in general it consists of a combination of surgery, chemotherapy, and radiation therapy (3). Cisplatin is the most commonly used conventional chemotherapeutic drug for the treatment of locally advanced head and neck cancer (4, 5). The contribution of chemotherapeutic agents in the clinical outcome of patients with advanced HNSCC is becoming increasingly well understood. Studies have demonstrated that chemotherapy improves larynx preservation rates when combined with radiation (6–9). Intensification of combination chemotherapy regimens with taxanes, platinum-based compounds and 5-Fluorouracil has shown improvement of survival of HNSCC patients (10–15). These results suggest that the combination of drugs might yield better results than single drug therapies. However, these combination regimens have increased normal tissue toxicities demonstrated by weight loss requiring feeding tube placement, failure to complete the treatment course, and even deaths due to therapy. Combination therapies involving cisplatin and molecularly targeted agents, particularly inhibitors of EGF signaling, have been used to reduce the toxicity of combined regimens described above but have also shown modest results (16). Considering the critical role of Bcl-2 family proteins in the pathobiology of squamous cell carcinomas (17), therapeutic inhibition of Bcl-2 function might improve the survival of patients with head and neck cancer.
Bcl-2 family proteins are key regulators of cell survival (18). Interestingly, while germline Bcl-2 knockout is lethal (19), conditional knockout mice appear to be healthy and have normal survival upon Bcl-2 downregulation (20). These data demonstrate that Bcl-2 is required during development, but does not appear to play a critical role in the homeostasis of adult tissues. Together, these studies may explain the lack of significant systemic toxicities observed when Bcl-2 is inhibited systemically with a small molecule inhibitor (21).
Pro-survival proteins, such as Bcl-xl and Bcl-2, are upregulated in many cancers and contribute to resistance to therapy (18, 22). The use of adjuvant agents that target anti-apoptotic proteins in HNSCC may overcome chemotherapeutic resistance. Notably, (-)-gossypol was shown to decrease cisplatin resistance in head and neck cancer cells (23–25). TW-37 belongs to a novel class of targeted drugs that has been developed by structure-based design (26). TW-37 binds to the BH3 (Bcl-2 homology domain 3) binding groove of Bcl-2 and competes with pro-apoptotic proteins (such as Bid, Bim and Bad) preventing their heterodimerization with Bcl-2, and therefore allowing these proteins to induce apoptosis (26). TW-37 binds to Bcl-2 with a Ki of 290 nmol/L (26, 27). In addition, TW-37 also binds to Bcl-xL and Mcl-1 with a Ki of 1,110 and 260 nmol/L, respectively (26, 27). This small molecule has shown anti-tumor effects in lymphoma and pancreatic cancer models as monotherapy (27, 28). In addition, we have shown that inhibition of Bcl-2 function with sub-apoptotic concentrations of TW-37 are sufficient to induce a significant decrease the angiogenic phenotype of endothelial cells in vitro (21). Here, we performed experiments to test the hypothesis that TW-37 inhibits head and neck tumor angiogenesis and slows down tumor progression.
Materials and Methods
Cell culture
Primary human dermal microvascular endothelial cells (HDMEC; Lonza, Allendale, NJ, USA) were cultured in endothelial cell growth medium (EGM2-MV; Lonza). Oral squamous cell carcinoma-3 (OSCC-3; gift from M. Lingen, University of Chicago); UM-SCC-1, UM-SCC-74A (gift from T. Carey, University of Michigan, Ann Arbor, MI) were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% Fetal Bovine Serum, 200 mM L-Glutamine, 125 units/ml Penicillin, and 125 µg/mL Streptomycin in a humidified CO2 incubator at 37°C.
Cytotoxicity assays
Sulforhodamine B (SRB) cytotoxicity assays were performed as described (21). Briefly, optimal cell density for cytotoxicity assays was determined by growth curve analysis. HDMEC were seeded at 2 × 103 cells per well of 96-well plates and allowed to adhere overnight. Drug or vehicle control was diluted in EGM2-MV and used to treat cells for 72 or 96 hours. Cells were fixed onto the plates by addition of 10% cold trichloroacetic acid (final concentration) for 1 hour at 4°C. Cellular protein was stained by addition of 0.4% Sulforhodamine B (Sigma, St. Louis, MO, USA) in 1% acetic acid and incubation at room temperature for 30 minutes. Unbound SRB was removed by washing with 1% acetic acid and plates were air-dried. Bound SRB was resolubilized in 10 mmol/L unbuffered Tris-base and absorbance was determined on a microplate reader at 560 nm (Genios Tecan, Tecan, Graz, Austria). Test results were normalized against initial plating density and drug-free controls. Data were obtained from triplicate wells per condition and representative of at least three independent experiments.
Western blot analysis
HDMEC, UM-SCC-1, or OSCC3 were exposed to 0–2.2 µM TW37 and/or 0–4 µM cisplatin. Whole cell lysates were resolved by electrophoresis and membranes were probed overnight at 4°C with a 1:1,000 dilution of hamster anti-human Bcl-2 monoclonal antibody (BD Bioscience, San Jose, CA, USA) for 1 hour at room temperature. Alternatively, HDMEC were exposed to 0–10 µM TW37 fro 24 hours. Whole cell lysates were resolved by electrophoresis and membranes were probed overnight at 4°C with a 1:1,000 dilution of rabbit anti-human Chk1 and phosphor-Chk1 (Cell Signaling, Danvers, MA, USA), rabbit anti-human Cyclin D1 or mouse anti-human CDK4 antibody (Santa Cruz, Santa Cruz, CA, USA). Then, membranes were exposed to appropriate peroxidase-coupled secondary antibodies and proteins were visualized with ECL (Amersham, Sunnyvale, CA, USA).
Flow cytometry
Cells were seeded at 5 × 104 per well in a six-well plate and allowed to adhere overnight. Medium was aspirated, and drug or controls was diluted in EGM2-MV medium and added to the cells. Cells were incubated for 72 hours and assessed for apoptosis by hypotonic lysis and staining of DNA with propidium iodide (PI), as described (29). Apoptotic levels were determined by flow cytometry and cell cycle analysis of sub-G1 fractions. Data were obtained from triplicate wells per condition and are representative of at least three independent experiments.
SCID mouse model of human tumor angiogenesis
Xenograft human tumors vascularized with human blood vessels were generated, as described (30, 31). Briefly, highly porous poly-L(lactic) acid scaffolds were prepared and seeded with 9 × 105 HDMEC plus 1 × 105 OSCC-3 cells. Male 5- to 7-week-old SCID mice (CB.17.SCID; Taconic, Germantown, NY, USA) were anesthetized with ketamine and xylazine, and two scaffolds were implanted in the subcutaneous space of the dorsal region of each mouse. Eighteen days after implantation, mice were randomized into 4 groups (seven mice per group) and adjusted to equalize the mean tumor volume in each group. For tumor progression studies, mice were injected intraperitonealy with either two doses of 5 mg/kg cisplatin (5 days apart); or with 15 mg/kg TW-37 for 10 consecutive days; or combination of the two drug regimens. Vehicle alone (i.e. PBS/Tween 80/ethanol) was injected intraperitonealy for 10 consecutive days in the control group. Tumor volume was calculated using the formula volume (mm3) = L × W2 / 2 (L: length, mm; W: width, mm). At the termination of the experiment, mice were euthanized, tumors were retrieved and fixed overnight in 10% buffered formalin at 4°C. Kaplan-Meier curves were generated using as criteria for failure the time when tumor volume reached a 4-fold increase as compared to pre-treatment volume. Alternatively, for tumor angiogenesis studies mice either received two doses of 5 mg/kg cisplatin 5 days apart or 15 mg/kg TW-37 for 6 consecutive days or combination treatment for 6 days. The following day, mice were euthanized, tumors were harvested, fixed, and processed for standard immunohistochemistry. Histological sections were incubated in antigen retrieval solution (Dako, Carpinteria, CA, USA) for 30 min at 90°C, followed by incubation with polyclonal anti-human factor VIII antibody (1:500 dilution; Lab Vision, Fremont, CA, USA) overnight at 4°C, as previously described (32). The number of microvessels in 6 random fields per scaffold was counted in eight scaffolds per experimental condition under a light microscope at 200× magnification. The care and treatment of experimental animals was in accordance with University of Michigan institutional guidelines. At least three independent experiments were performed to verify reproducibility of results.
In Situ TUNEL
Tissues were permeabilized by incubation with 0.1% Triton-X100, 0.1% sodium citrate solution for 8 minutes. Subsequently, tissues were incubated with TdT and fluorescein-dUTP (In Situ Cell Death Detection Kit Fluorescein; Roche, Basel, Switzerland), according to manufacture’s instructions. The number of TUNEL-positive cells was quantified under fluorescence microscopy (Leica DM 5,000B) with the Image J software (NIH). Confocal images were done using a Zeiss 510 META laser scanning confocal microscope. Laser excitation was 364 for DAPI and 488 for FITC. Zeiss software provided the scanned images, which were incorporated into Photoshop CS2 (Adobe) for producing the final configurations presented here.
Statistical analyses
Statistical significance was determined by one-way ANOVA followed by post-hoc tests, using the SigmaStat 2.0 software (SPSS; Chicago, IL, USA). The analysis of the data from the Kaplan-Meyer curves was performed with the Gehan-Breslow-Wilcoxon test using the GraphPad software (GraphPad Software, La Jolla, CA, USA). The combinatorial index (CI) was calculated by CalcuSyn software (Biosoft, Cambridge, United Kingdom).
Results
Comparative analysis of the cytotoxicity of TW-37 and cisplatin in endothelial cells and head and neck cancer cells
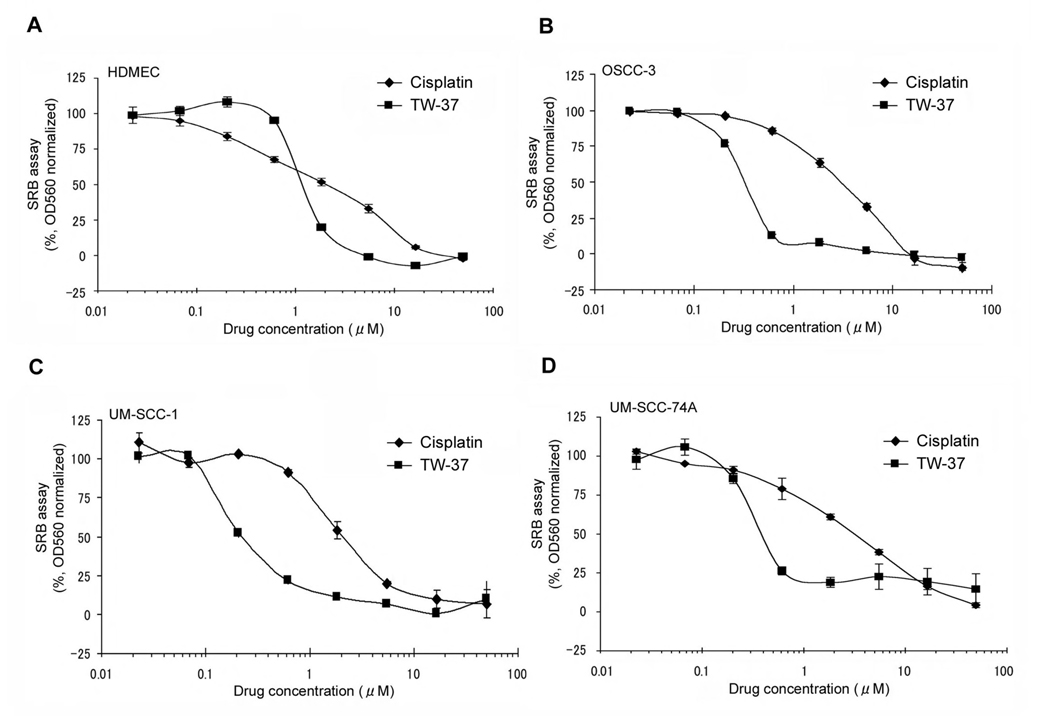
The initial screening of the effect of cisplatin and TW-37 on primary human endothelial cells and several head and neck squamous cell carcinoma cell lines was performed using the SRB cytotoxicity assay. Pilot studies demonstrated that seeding 2,000 endothelial cells per well or 2,000 tumor cells per well for 72 hours allows for evaluation of the effect of the drugs while cells were still in linear phase of proliferation (data not shown). The IC50 for TW-37 was 1.1 µmol/L in HDMEC (Fig. 1A), and approximately 0.3 µmol/L in the three head and neck cancer cell lines (i.e. OSCC-3, UM-SCC-1, and UM-SCC-74A) evaluated here (Fig. 1B–D). For cisplatin, the IC50 for all cell lines tested here was approximately 2.0 µmol/L (Fig. 1A–D). Together, these results demonstrate that TW-37 is more cytotoxic on an equimolar basis than cisplatin in endothelial cells and head and neck cancer cells in vitro.
Figure 1.
Cytotoxic activity of cisplatin and TW-37, a small molecule inhibitor of Bcl-2, in endothelial cells and head and neck cancer cells. A–D, The cytotoxicity of cisplatin and TW-37 on human dermal microvascular endothelial cells (HDMEC), oral squamous cell carcinoma-3 (OSCC3), and two University of Michigan squamous cell carcinoma cell lines (UM-SCC-1 and UM-SCC-74A) was determined by SRB assay. Cells were exposed to 0–50 µM cisplatin or TW-37 for 72 hours. Results are normalized against vehicle control and initial plating density. Experiments were performed in triplicate wells per condition, and graph is representative of three independent experiments.
Combination of TW-37 and cisplatin showed enhanced cytotoxic effects for endothelial cells and head and neck cancer cells as compared with single drug treatment
TW-37 was discovered using structure-based database screening for molecules that interacted with Bcl-2 with high affinity and prevented its interaction with proteins of the Bcl-2 family, such as Bax, Bim, Bad, and Bid (26, 27). Therefore, it is not expected that TW-37 would affect Bcl-2 expression levels. However, the effect of combination TW-37 and cisplatin on Bcl-2 expression in endothelial cells and in head and neck cancer cells is not known. Here, we observed that concentrations of TW-37 and/or cisplatin that inhibit cell growth do not affect the expression of Bcl-2 in the endothelial cells or in the head and neck tumor cells (Suppl. Fig. 1).
For combination therapy studies in endothelial cells, we selected three concentrations of cisplatin (1 µM, 2 µM, 3 µM) and three concentrations of TW-37 (0.7 µM, 1.1 µM, 1.5 µM). These concentrations were selected as representatives of the IC50 in endothelial cells for each drug (Fig. 1) plus one concentration above and one below the IC50. Combination treatment with TW-37 at IC50, or higher concentration, resulted in higher cytotoxicity to endothelial cells than single drug treatment (Fig. 2A). When cisplatin was combined with 1.1 µM TW-37, the Combinatorial Index (CI) was between 0.9 and 1.1, which indicated additive effect of the drugs. However, when cisplatin was combined with higher concentration of TW-37 (i.e. 1.5 µM), the CI was less than 0.9, which demonstrated synergy between the two drugs (Fig. 2A).
Figure 2.
Cytotoxic effect of the combination of cisplatin and TW-37 on endothelial cells and head and neck tumor cells. A–D, The cytotoxicity of cisplatin and/or TW-37 on human dermal microvascular endothelial cells (HDMEC), oral squamous cell carcinoma-3 (OSCC3), and two University of Michigan squamous cell carcinoma cell lines (UM-SCC-1 and UM-SCC-74A) was determined by SRB assay. Cells were exposed to the IC50, or to one lower and one higher, concentration of cisplatin and/or TW-37 for 72 hours. Combinatorial Index (CI) was calculated in various drug combinations by CalcuSyn software (Biosoft). A synergistic effect, i.e. CI<0.9, is depicted by (■) and an additive effect, i.e. 0.9≤CI≤1.1 is depicted by (▲). In all cases, results are normalized against control and initial plating density. Each panel is representative of at least three independent experiments, performed in triplicate wells per condition.
We also investigated the effect of combination treatment on three head and neck cancer cell lines. Here, the concentrations of TW-37 were lowered to 0.1 µM, 0.3 µM, and 0.5 µM to reflect a range above and below the average IC50 of this drug (i.e. 0.3 µM) for the head and neck cancer cell lines tested here. The concentrations of cisplatin were maintained at 1 µM, 2 µM and 3 µM (as above) because these values remained within the active range of cisplatin in the head and neck tumor cell lines. Combination treatment had significantly higher cytotoxic effect than exposure to single drug in the three cancer cell lines evaluated here (Fig. 2B–D). The CI trends for both drugs in these cell lines were similar to the CI for endothelial cells. The CI was below 0.9 when higher concentrations of TW-37 (i.e. 0.5 µM) were used in combination with cisplatin in OSCC3 and UM-SCC-74A cells, indicating synergism between drugs (Fig. 2B–D). In contrast, the CI was between 0.9 and 1.1 at the IC50 concentration of TW-37, which demonstrates additive effects of the drug combination. The CI was >1.1 with most conditions using the lowest concentration of TW-37, which demonstrates lack of additive or synergistic effect of the combination.
Treatment sequence has a significant impact on the cytotoxicity of TW-37 and cisplatin in vitro
Next, we investigated the impact of treatment sequence on the effect of the combination cisplatin and TW-37. We either initiated treatment simultaneously with both drugs, or pre-treated with one drug for 24 hours and then completed the experimental period with both drugs together. The concentration of drugs was fixed at the 72-hour IC50 for both endothelial cells and head and neck cancer cells. The highest effect of combination treatment was observed when we started treatment with both drugs at the same time (Fig. 3A–D). Notably, pre-treatment with either drug essentially eliminated the benefit of combination therapy, when compared with single drug therapy (Fig. 3A–D). Together, these data demonstrate the critical impact of treatment sequence on the cytotoxic effect of these drugs in vitro.
Figure 3.
Effect of treatment sequence on the cytotoxicity of TW-37 and cisplatin. A–D, Cells were treated either with single drug alone or both drugs simultaneously for 72 hours or 96 hours. Alternatively, cells were pre-treated for 24 hours with either cisplatin or TW-37. This pre-treatment was followed by a 48- or 72-hour treatment with both drugs together. Asterisk (*) depicts significant difference (P<0.05) of the experimental condition against all the other conditions in the study. Each panel is representative of at least three independent experiments, performed in triplicate wells per condition.
Combination treatment enhances apoptotic level of endothelial cells and cancer cells
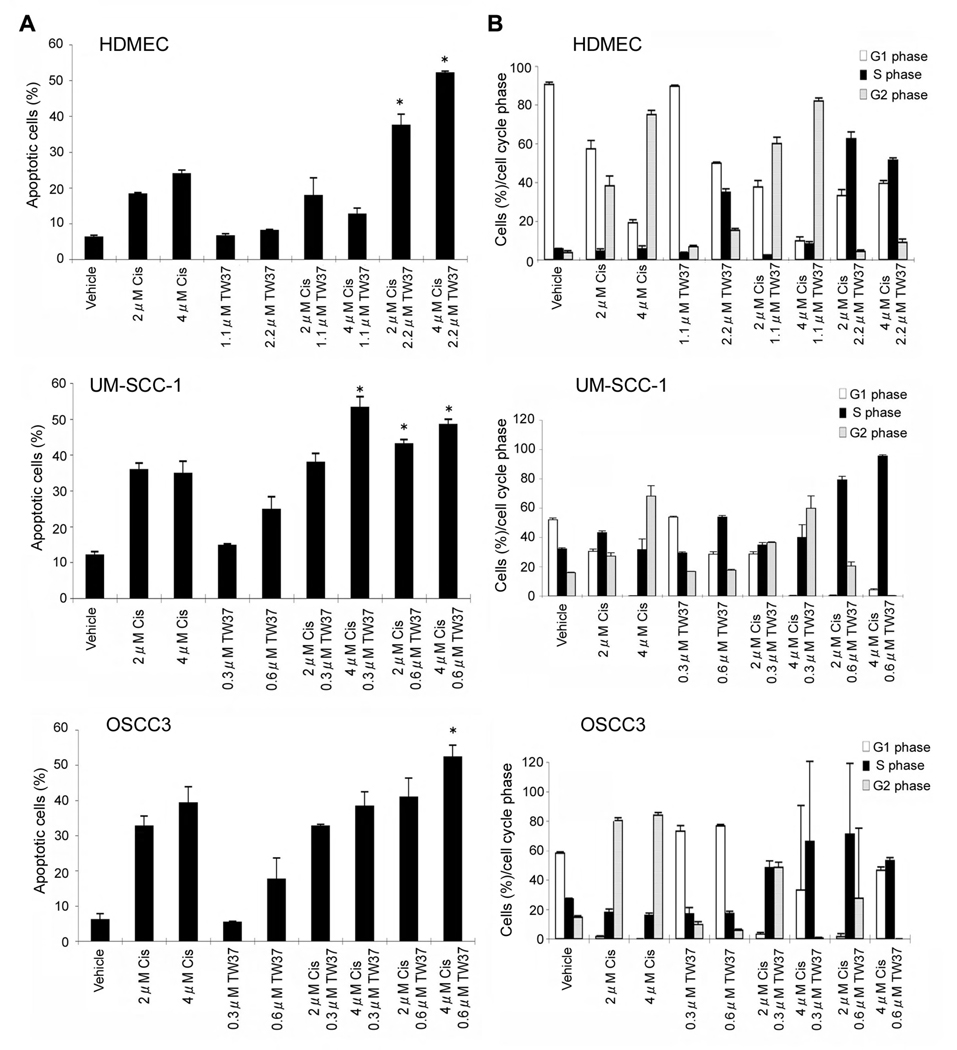
SRB assays are useful for the initial screen of cytotoxic effects of drugs, but they do not allow for the discrimination between the effects of drugs on cell survival versus effects on cell cycle. Therefore, we performed flow cytometric studies with propidium iodide to determine the effects of the drugs in the sub-G0/G1 fraction (i.e. apoptotic cells), as well as in the distribution of cells in different phases of cell cycle. We did not observe an increase in the percentage of apoptotic endothelial cells when 1.1 µM TW-37 was given by itself or in combination treatments (Fig. 4A). However, a significant increase in the proportion of apoptotic endothelial cells was observed when 2.2 µM TW-37 (i.e. 2× IC50) was used in combination with cisplatin (Fig. 4A), as compared to single drug treatment (cisplatin or TW-37). In contrast, 0.6 µM TW-37 was sufficient to induce a significant increase in the percentage of apoptotic head and neck tumor cells (Fig. 4A). In general, combination of 0.6 µM TW-37 with cisplatin was sufficient to mediate higher apoptotic indexes as compared to single drug treatment with either drug (Fig. 4A).
Figure 4.
Effect of cisplatin and TW-37 on apoptosis and cell cycle of endothelial or head and neck tumor cells. A, Cells were exposed to cisplatin and/or TW-37. After 72 hours, cells were stained with propidium iodide and subjected to flow cytometry for the analysis of the proportion of apoptotic cells (sub-G0/G1). Asterisk (*) depicts that the combination regimen is significantly higher than any of the single drug (cisplatin or TW-37) regimen by itself (P<0.05). B, Cells were treated as in panel A, stained with propidium iodide, and the percentage of cells in each cell cycle phase was determined by flow cytometry. Representative of at least three independent experiments performed in triplicate wells per condition.
While the effects of cisplatin in the cell cycle are very well known, i.e. it mediates G2 cell cycle arrest (33), the effects of a small molecule inhibitor of Bcl-2 are unclear. As expected, cisplatin treatment resulted in dose-dependent increase in the proportion of HDMEC and cancer cells in the G2 phase of cell cycle (Fig. 4B; Suppl. Fig. 2). In contrast, treatment of HDMEC or UM-SCC-1 with 2.2 µM TW-37 alone was associated with an increase in the proportion of cells in the S phase of cell cycle (Fig. 4B; Suppl. Fig. 2). Interestingly, when cisplatin was combined with lower concentrations of TW-37, it resulted in an increase in the proportion of endothelial cells in the G2 phase (Fig. 4B; Suppl. Fig. 2). This is consistent with a dominant effect of cisplatin on cell cycle. However, when cisplatin was combined with higher TW-37 concentrations, the combination resulted in a marked increase in endothelial cells and tumor cell in the S phase of cell cycle (Fig. 4B; Suppl. Fig. 2). Since TW-37 alone or in combination with cisplatin caused markedly lower cell numbers (Fig. 1 and Fig. 2), these data demonstrate that TW-37 is causing an S phase cell cycle arrest in endothelial and head and neck tumor cells. Notably, it is well known that phosphorylation of Chk1 triggers a signaling cascade that results in proteolysis of CDC25A, which in turn inhibits the replication machinery causing S-phase cell cycle arrest (34). Here, we observed that TW-37-induced “S” phase cell cycle arrest correlates with increase in Chk1 phosphorylation and a decrease in Cyclin D1 and CDK4 expression in endothelial cells (Suppl. Fig. 3).
Combination with TW-37 potentiates the anti-tumor effect of cisplatin
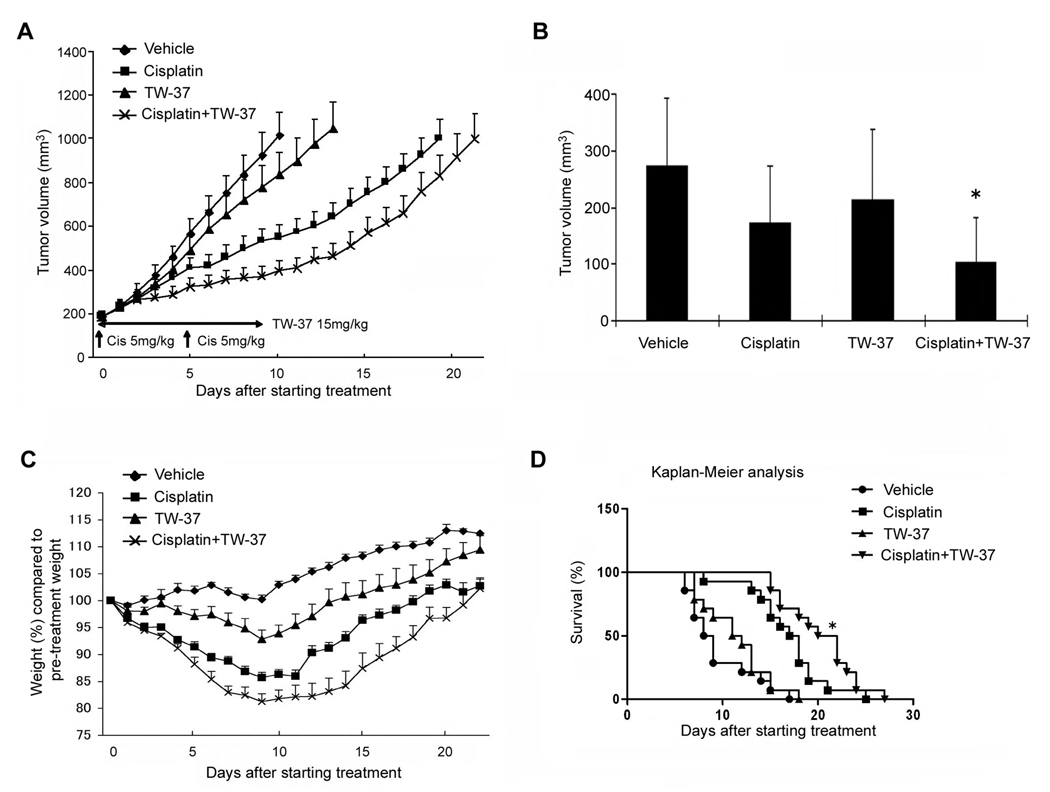
We have previously demonstrated that xenografted human tumors vascularized with human functional microvessels can be engineered in SCID mice (30, 31). Using this approach, we investigated the effect of cisplatin and TW-37 on tumor progression and tumor angiogenesis. We implanted HDMEC along with human oral squamous cell carcinoma (OSCC-3) in SCID mice, and observed the development of tumors (Fig. 5A). At the end of the treatment period (i.e. 10 days), the average volume of non-treated group mice increased by 5.7-fold (Fig. 5A). Four days after the beginning of the treatment, combination of TW-37 and cisplatin already showed an inhibition of tumor growth as compared to the other conditions (Fig. 5B). A significant loss of weight was observed in the groups treated with cisplatin or both drugs in combination (Fig. 5C). The degree of weight loss was more pronounced in the drug combination group than in the cisplatin monotherapy group, and mice treated with both drugs required more time to return to the original weight after completion of treatment (Fig. 5C). Minimal weight loss was observed with the regimen of TW-37 alone. Mice treated with TW-37 recovered weight quickly and surpassed baseline weight 9–10 days after completion of treatment (Fig. 5C). A Kaplan-Meier analysis was performed using as the criteria for failure a 4-fold increase in tumor volume as compared to pre-treatment tumor volume (Fig. 5D). The average times to failure were 8.5 days (control group), 17.5 days (cisplatin), 11.5 days (TW-37), and 21 days (combination treatment) (Fig. 5D). The combination treatment extended the time to failure of tumor significantly as compared with single treatment with either TW-37 or with cisplatin (Fig. 5D).
Figure 5.
Effect of cisplatin and/or TW-37 on time to tumor failure. Each SCID mouse was implanted with two scaffolds seeded with 1.0 × 105 OSCC-3 and 9.0 × 105 HDMEC. Eighteen days after implantation, mice were randomly assigned to four groups (n=14 tumors per experimental group) as follows: 5 mg/kg cisplatin on day 0 and day 5 via intraperitoneal injection; 15 mg/kg TW-37 daily for 10 days via intraperitoneal injection; combination of the regimens above for cisplatin and TW-37 for 10 days; or vehicle injected controls. A, tumor progression curve, starting on day 0 and finishing when the average tumor volume reached 1,000 mm3. Data represent mean values (±SE). B, Tumor volume four days after the beginning of the treatment. Asterisk (*) depicts significant difference (P<0.05) of one experimental condition against all the other conditions. C, Mouse weight during treatment. Data were normalized against initial mice weight. D, Kaplan-Meier end point analysis using a four-fold increase of tumor volume as compared to pre-treatment volume was used as the criterion for tumor failure. In the graph, survival depicts the percentage of mice bearing tumors that did not reach the four-fold increase in tumor volume. Asterisk (*) depicts significant difference (P<0.05) against all the other experimental conditions, using the Gehan-Breslow-Wilcoxon test.
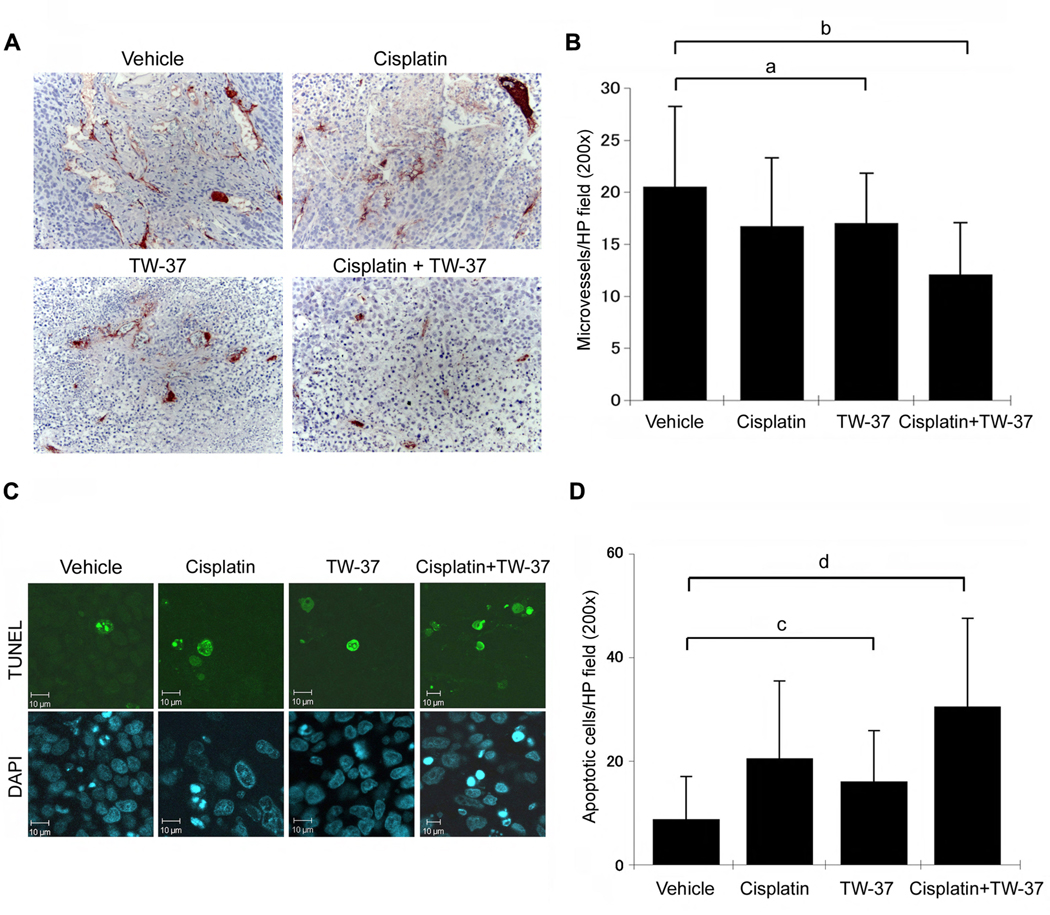
We have previously shown that TW-37 decreases the angiogenic potential of human endothelial cells in vitro, and mediates a significant decrease in microvessel density in vivo (21). To evaluate the effect of the drugs on xenografted head and neck tumor angiogenesis, we shortened the duration of treatment to 5 days and euthanized the mice the day after completion of treatment. Here, we observed a modest (but statistically significant) decrease in tumor microvessel density when TW-37 alone is compared to vehicle treated controls (Fig. 6A). Combination treatment further inhibited tumor microvessel density, and a significant decrease of blood vessel numbers was observed when compared with the single drug therapies evaluated here (Fig. 6B).
Figure 6.
Effect of cisplatin and/or TW-37 on tumor angiogenesis and apoptosis. Each SCID mouse was implanted with two scaffolds seeded with 1.0 × 105 OSCC-3 and 9.0 × 105 HDMEC. Eighteen days after implantation, mice were randomly assigned to four groups (n=14 tumors per experimental group) as follows: 5 mg/kg cisplatin on day 0 and day 5 via intraperitoneal injection; 15 mg/kg TW-37 daily for 5 days via intraperitoneal injection; combination of the regimens above for cisplatin and TW-37 for 5 days; or vehicle injected controls. Mice were euthanized on day 6, i.e. one day after the end of treatment. A,B, Tissue sections were stained for Factor VIII (red color) and counterstained with hematoxylin. Factor VIII positive vessels were counted under light microscopy at 200× magnification. C,D, Tissue sections were stained with in situ TUNEL and with DAPI. Images were prepared at 200× magnification and TUNEL positive cells were counted using the Image J software. Statistical significance (P<0.05) is depicted by lowercase letters, as follows: (a) blood vessel density is significantly lower in cisplatin or TW-37 treated tumors than in control tumors; (b) blood vessel density is significantly lower in combination treatment than in any other condition. (c) number of apoptotic cells is significantly higher in cisplatin or TW-37 treated tumors than in control tumors; (d) number of apoptotic cells is significantly higher in combination treatment than in any other condition.
We next analyzed the effect of combination treatment on apoptosis in the xenografted tumors by in situ TUNEL. TUNEL positive cells were only counted in regions of intact tumor, in such a way that the central core necrosis typically observed in xenografts did not interfere with quantification of apoptotic cells. Tumor samples from animals treated with TW-37 or cisplatin alone showed higher TUNEL-positive cells compared to untreated group (Fig. 6D). Notably, combination of the drugs caused approximately 3.5-fold increase in the number of apoptotic cells as compared to untreated controls, and 1.5∼2-fold increase when compared with single drug therapy (Fig. 6D).
Discussion
Organ preservation approaches incorporating chemotherapy minimize the need for radical surgery, and tend to improve the quality of life of patients with head and neck cancer (35). However, current drugs have shown relatively modest improvements in the survival of patients with head and neck cancer. Bcl-2 is upregulated in poorly differentiated head and neck carcinomas, and its expression correlates with positive nodal status (36, 37). A closely related member of the Bcl-2 family, Bcl-xl, is upregulated in laryngeal cancer and is associated with poor response to chemotherapy and radiation (17). We have shown that Bcl-2 gene expression is approximately 60,000 fold higher in the endothelial cells lining tumor blood vessels, as compared to the endothelial cells of adjacent normal oral mucosa in patients with head and neck tumors (38). Notably, Bcl-2 downregulation in tumor associated endothelial cells by gene silencing is sufficient to inhibit the growth of xenografted head and neck tumors (38). Therefore, Bcl-2 appears to be a compelling target for treatment of patients with head and neck cancer.
TW-37 has an anti-tumor effect on lymphoma and pancreatic tumor models (27, 28). We hypothesize that the anti-tumor action of TW-37 is due to a combination of a pro-apoptotic effect on the tumor cells, as well as a specific anti-angiogenic effect. This hypothesis is based on the following observations made by our research group: A) Bcl-2 initiates a pro-angiogenic signaling pathway that is mediated by NF-kB transcriptional activity and result in upregulated expression of the pro-angiogenic chemokines CXCL1 and CXCL8 in endothelial cells (39). B) Sub apoptotic concentrations of TW-37 inhibited the angiogenic potential of endothelial cells in vitro (21). And C) Sub-apoptotic concentrations of both the BH3 mimetics (-)-gossypol (BL193) and TW-37 inhibit the expression of the pro-angiogenic chemokines CXCL1 and CXCL8 in endothelial cells (21, 39). Notably, we have recently demonstrated that Bcl-2 functions as the orchestrator of a crosstalk between neovascular endothelial cells and tumor cells, which has a direct effect on head and neck tumor progression (38). Indeed, inhibition of Bcl-2 function in endothelial cells by gene silencing was sufficient to inhibit tumor cell proliferation in co-cultures in vitro, as well as to slow down tumor progression in vivo (38). These observations provided the rationale for the current investigation where we designed a detailed study of the effect of TW-37 alone or in combination with cisplatin in both, endothelial cells and head and neck tumor cells.
The use of multiple drugs with different mechanism or modes of action may increase the efficacy of the therapeutic effect, minimizing or slowing down the development of drug resistance, and providing selective synergism against target versus host (40). We chose cisplatin for combination studies with TW-37 because this drug is widely used in the treatment of head and neck cancer (5, 41), and because it has clearly a different mechanism of action. Cisplatin causes DNA damage by making platinum-DNA adducts, which leads to cell cycle arrest, inhibition of transcription, and initiation of the apoptotic cascade (33). Cisplatin’s effects are expected to be primarily in highly proliferative cells, such as tumor cells (4). The putative functions of TW-37 in a combined therapy with cisplatin are: A) TW-37 may sensitize the tumor cells to cisplatin by blocking the function of a critical pro-survival pathway. B) TW-37 will have an anti-angiogenic effect by inducing apoptosis of endothelial cells, and by inhibiting the secretion of pro-angiogenic chemokines by resistant endothelial cells. C) TW-37 will block endothelial cell-initiated crosstalk with tumor cells that lead to enhanced tumor progression. Here, we used cisplatin at maximum tolerated dose (MTD) for the mice in this study, as shown by a decrease in approximately 15% in weight by the end of treatment. In contrast, we used a sub-optimal dose of TW-37 for the in vivo studies, i.e. 15 mg/kg TW-37 daily. The MTD for this drug was determined to be 40 mg/kg daily (27). Nevertheless, combination of TW-37 and cisplatin at MTD was significantly more efficient in slowing down tumor progression when compared with single drug treatment with cisplatin. Likewise, combination treatment resulted in a significant decrease in tumor microvessel density and increase in the tumor apoptotic index when compared to treatment with cisplatin alone. Together, these results suggest that TW-37 may sensitize xenografted head and neck tumors to cisplatin.
We observed enhanced cytotoxic effects of the two drugs in endothelial cells when cells were exposed to higher concentrations of TW-37. In parallel experiments, we observed that the efficacy of the treatment with TW-37 or cisplatin presented an inverse relation with cell density, i.e. more cells correlated with lower efficacy of the drugs (data not shown). These results suggest that combination treatment might have a predominant effect in the highly proliferative endothelial cells of tumor neovessels, while sparing the more mature endothelial cells of physiological vessels. Indeed, here we observed that while there was a significant decrease in tumor microvessel density in mice treated with TW-37 and cisplatin, these animals did not show signs of overt toxicity.
Prior to the in vivo experiments, we performed a detailed study of the effect treatment sequence in the overall response to combination of TW-37 and cisplatin. Others have demonstrated that treatment schedule may have a profound effect on the anti-tumor effect of drugs. For example, pretreatment with paclitaxel before co-administration of paclitaxel and A-385358 (inhibitor of Bcl-xl) potentiated the activity of combination treatment (42). Here, we observed that pretreatment with TW-37 or with cisplatin abrogated the beneficial effect of combination treatment. Indeed, there was no observable advantage of the combination therapy when pretreatment with one of the drugs was performed, as compared to the use of a single drug. These results were somewhat unexpected. However, the trends observed here were highly reproducible in four independent experiments. We are currently performing experiments to evaluate in more depth possible mechanistic explanations for these results. Nevertheless, these results guided our decision to start both drugs at the same time in our in vivo studies.
Interestingly, TW-37 in the low to mid nano-molar range markedly reduced head and neck tumor cell density in vitro without an equivalent increase in cell apoptosis. This apparent conundrum was resolved, in part, when we performed cell cycle analysis. TW-37 treatment is accompanied by a marked accumulation of cells in the S phase of cell cycle. This was distinctively different than the effect of cisplatin, which resulted in the accumulation of cells in the G2 phase, as expected. Indeed, combination treatment showed a preponderant effect of TW-37 over cisplatin in tumor cells, since accumulation of cells in the S phase was observed in most experimental conditions involving both drugs. Others have shown that inhibition of the STAT3 signaling pathway lead to S phase cell cycle arrest in human hepatocellular carcinoma cells (43). We have shown that Bcl-2 induces STAT3 transcriptional activity (38). Therefore, we hypothesize that the therapeutic blockade of Bcl-2 function with TW-37 leads to an S phase cell cycle arrest by inhibiting STAT3 transcriptional activity. These results suggest a novel function for Bcl-2 in the regulation of cell cycle, and explain the marked reduction in cell numbers observed here with sub-apoptotic concentrations of TW-37.
This study demonstrated that TW-37, a small molecule inhibitor of Bcl-2, is a potent inhibitor of endothelial cell and head and neck tumor cell growth in vitro. In vivo, single therapy with daily administration of 15 mg/kg TW-37 showed modest anti-tumor effects. These results were somewhat expected, considering that the dosage used here was significantly below the MTD for single agent TW-37 that was determined to be 120 mg/kg given in three divided daily dosages of 40 mg/kg per injection i.v. (27). Notably, combination of TW-37 and cisplatin suppressed xenografted head and neck tumor angiogenesis and tumor progression. The small molecule inhibitors of Bcl-2 are emerging as a new class of molecularly targeted drugs that have both, a direct anti-tumor cell cytotoxic effect, as well as an anti-angiogenic effect (44). The evidence presented here suggests that therapeutic inhibition of Bcl-2 with a small molecule inhibitor such as TW-37 might benefit patients with head and neck squamous cell carcinomas.
Supplementary Material
Acknowledgments
The authors thank Chris Strayhorn for his help with the histology, and Chris Edwards for his help with the confocal microscopy. This work was supported by grant P50-CA97248 (University of Michigan Head & Neck SPORE) (TNT, SW, JEN) and grant U19-CA113317 (SW) from the NIH/NCI; and grants R01-DE14601, R01-DE15948, R01-DE16586, and R01-DE19279 from the NIH/NIDCR (JEN).
References
- 1.Kolker JL, Ismail AI, Sohn W, Ramaswami N. Trends in the incidence, mortality, and survival rates of oral and pharyngeal cancer in a high-risk area in Michigan, USA. Community Dent Oral Epidemiol. 2007;35:489–499. doi: 10.1111/j.1600-0528.2007.00371.x. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamieson ER, Lippard SJ. Structure, recognition, and processing of cisplatin-DNA adducts. Chem Rev. 1999;99:2467–2498. doi: 10.1021/cr980421n. [DOI] [PubMed] [Google Scholar]
- 5.Forastiere AA. Chemotherapy in the treatment of locally advanced head and neck cancer. J Surg Oncol. 2008;97:701–707. doi: 10.1002/jso.21012. [DOI] [PubMed] [Google Scholar]
- 6.Pignon JP, Bourhis J, Domenge C, Designe L. Chemotherapy added to locoregional treatment for head and neck squamous cell carcinoma: Three meta-analysis of updated individual data. Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- 7.El-Sayed S, Nelson N. Adjuvant and adjunctive chemotherapy in the management of squamous cell carcinoma of the head and neck region: A meta-analysis of prospective and randomized trials. J Clin Oncol. 1996;14:838–847. doi: 10.1200/JCO.1996.14.3.838. [DOI] [PubMed] [Google Scholar]
- 8.Browman GP. Evidence-based recommendations against neoadjuvant chemotherapy for routine management of patients with squamous cell head and neck cancer. Cancer Invest. 1994;12:662–671. doi: 10.3109/07357909409023052. [DOI] [PubMed] [Google Scholar]
- 9.The Department of Veterans Affairs Laryngeal Cancer Study Group. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324:1685–1690. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 10.Domenge C, Hill C, Lefebvre JL, et al. Randomized trial of neoadjuvant chemotherapy in oropharyngeal carcinoma. French Groupe d’Etude des Tumeurs de la Tete et du Cou(GETTEC) Br J Cancer. 2000;83:1594–1598. doi: 10.1054/bjoc.2000.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haddad R, Tishler RB, Norris CM, et al. Docetaxel, cisplatin, 5-fluorouracil(TPF)-based Induction chemotherapy for head and neck cancer and the case for sequential, combined-modality treatment. Oncologist. 2003;8:35–44. doi: 10.1634/theoncologist.8-1-35. [DOI] [PubMed] [Google Scholar]
- 12.Browman GP, Hodson DI, Mackenzie RJ, Bestic N, Zuraw l. Cancer Care Ontario Practice Guideline Initiative Head and Neck Cancer Disease Site Group. Choosing a concomitant chemotherapy and radiotherapy regimen for squamous cell head and neck cancer: A systematic review of the published literature with subgroup analysis. Head Neck. 2001;23:579–589. doi: 10.1002/hed.1081. [DOI] [PubMed] [Google Scholar]
- 13.Tsao AS, Garden AS, Kies MS, et al. Phase I/II Study of docetaxel, cisplatin, and concomitant boost radiation for locally advanced squamous cell cancer of the head and neck. J Clin Oncol. 2006;24:4163–4169. doi: 10.1200/JCO.2006.05.7851. [DOI] [PubMed] [Google Scholar]
- 14.Posner MR, Hershock DM, Blajman CR, et al. TAX 324 Study Group. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 15.Vermorken JB, Remenar E, van Herpen C, et al. EORTC 24971/TAX 323 Study Group. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–1704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 16.Reuter CW, Morgan MA, Eckardt A. Targeting EGF-receptor-signalling in squamous cell carcinomas of the head and neck. Br J Cancer. 2007;96:408–416. doi: 10.1038/sj.bjc.6603566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trask DK, Wolf GT, Bradford CR, et al. Expression of Bcl-2 family proteins in advanced laryngeal squamous cell carcinoma: correlation with response to chemotherapy and organ preservation. Laryngoscope. 2002;112:638–644. doi: 10.1097/00005537-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 19.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 20.Letai A, Sorcinelli MD, Beard C, Korsmeyer SJ. Antiapoptotic BCL-2 is required for maintenance of a model leukemia. Cancer Cell. 2004;6:241–249. doi: 10.1016/j.ccr.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Zeitlin BD, Joo E, Dong Z, et al. Antiangiogenic effect of TW37, a small-molecule inhibitor of Bcl-2. Cancer Res. 2006;66:8698–8707. doi: 10.1158/0008-5472.CAN-05-3691. [DOI] [PubMed] [Google Scholar]
- 22.Kirkin V, Joos S, Zornig M. The role of Bcl-2 family members in tumorigenesis. Biochim Biophys Acta. 2004;1644:229–249. doi: 10.1016/j.bbamcr.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Bauer JA, Trask DK, Kumar B, et al. Reversal of cisplatin resistance with a BH3 mimetic, (-)-gossypol, in head and neck cancer cells: role of wild-type p53 and Bcl-xL. Mol Cancer Ther. 2005;4:1096–1104. doi: 10.1158/1535-7163.MCT-05-0081. [DOI] [PubMed] [Google Scholar]
- 24.Bauer JA, Kumar B, Cordell KG, et al. Targeting apoptosis to overcome cisplatin resistance: a translational study in head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;69:S106–S108. doi: 10.1016/j.ijrobp.2007.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolter KG, Wang SJ, Henson BS, et al. (-)-Gossypol Inhibits growth and promotes apoptosis of human head and neck squamous cell carcinoma in vivo. Neoplasia. 2006;8:163–172. doi: 10.1593/neo.05691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang G, Nikolovska-Coleska Z, Yang CY, et al. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. J Med Chem. 2006;49:6139–6142. doi: 10.1021/jm060460o. [DOI] [PubMed] [Google Scholar]
- 27.Mohammad RM, Goustin AS, Aboukameel A, et al. Preclinical studies of TW-37, a new nonpeptidic small-molecule inhibitor of Bcl-2, in diffuse large cell lymphoma xenograft model reveal drug action on both Bcl-2 and Mcl-1. Clin Cancer Res. 2007;13:2226–2235. doi: 10.1158/1078-0432.CCR-06-1574. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Song W, Aboukameel A, et al. TW-37, a small-molecule inhibitor of Bcl-2, inhibits cell growth and invasion in pancreatic cancer. Int J Cancer. 2008;123:958–966. doi: 10.1002/ijc.23610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Nör JE, Hu Y, Song W, Spencer DM, Núñez G. Ablation of microvessels in vivo upon dimerization of iCaspase-9. Gene Ther. 2002;9:444–451. doi: 10.1038/sj.gt.3301671. [DOI] [PubMed] [Google Scholar]
- 30.Nör JE, Peters MC, Christensen JB, et al. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Invest. 2001;81:453–463. doi: 10.1038/labinvest.3780253. [DOI] [PubMed] [Google Scholar]
- 31.Nör JE, Christensen J, Liu J, et al. Up-Regulation of Bcl-2 in microvascular endothelial cells enhances intratumoral angiogenesis and accelerates tumor growth. Cancer Res. 2001;61:2183–2188. [PubMed] [Google Scholar]
- 32.Nör JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154:375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 34.Bartek J, Lukas C, Lukas J. Checking on DNA damage in S phase. Nat Rev Mol Cell Biol. 2004;5:792–804. doi: 10.1038/nrm1493. [DOI] [PubMed] [Google Scholar]
- 35.Fung K, Lyden TH, Lee J, et al. Voice and swallowing outcomes of an organ-preservation trial for advanced laryngeal cancer. Int J Radiat Oncol Biol Phys. 2005;63:1395–1399. doi: 10.1016/j.ijrobp.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Jordan RC, Catzavelos GC, Barrett AW, Speight PM. Differential expression of bcl-2 and bax in squamous cell carcinomas of the oral cavity. Eur J Cancer B Oral Oncol. 1996;32:394–400. doi: 10.1016/s0964-1955(96)00033-4. [DOI] [PubMed] [Google Scholar]
- 37.Teni T, Pawar S, Sanghvi V, Saranath D. Expression of bcl-2 and bax in chewing tobacco-induced oral cancers and oral lesions from India. Pathol Oncol Res. 2002;8:109–114. doi: 10.1007/BF03033719. [DOI] [PubMed] [Google Scholar]
- 38.Kaneko T, Zhang Z, Mantellini MG, et al. Bcl-2 orchestrates a cross-talk between endothelial and tumor cells that promotes tumor growth. Cancer Res. 2007;67:9685–9693. doi: 10.1158/0008-5472.CAN-07-1497. [DOI] [PubMed] [Google Scholar]
- 39.Karl E, Warner K, Zeitlin B, et al. Bcl-2 acts in a proangiogenic signaling pathway through nuclear factor-B and CXC chemokines. Cancer Res. 2005;65:5063–5069. doi: 10.1158/0008-5472.CAN-05-0140. [DOI] [PubMed] [Google Scholar]
- 40.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacological Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 41.Cohen EE, Lingen MW, Vokes EE. The expanding role of systemic therapy in head and neck cancer. J Clin Oncol. 2004;22:1743–1752. doi: 10.1200/JCO.2004.06.147. [DOI] [PubMed] [Google Scholar]
- 42.Shoemaker AR, Oleksijew A, Bauch J, et al. A small-molecule inhibitor of Bcl-XL potentiates the activity of cytotoxic drugs In vitro and In vivo. Cancer Res. 2006;66:8731–8739. doi: 10.1158/0008-5472.CAN-06-0367. [DOI] [PubMed] [Google Scholar]
- 43.Fuke H, Shiraki K, Sugimoto K, et al. Jak inhibitor induces S phase cell-cycle arrest and augments TRAIL-induced apoptosis in human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2007;363:738–744. doi: 10.1016/j.bbrc.2007.09.049. [DOI] [PubMed] [Google Scholar]
- 44.Zeitlin BD, Zeitlin IJ, Nör JE. An expanding circle of inhibition: small molecule inhibitors of Bcl-2 as anti-cancer cell and anti-angiogenic agents. J Clin Oncol. 2008;26:4180–4188. doi: 10.1200/JCO.2007.15.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.