Abstract
In nonhuman primate social groups, biological differences related to social status have proven useful in investigating mechanisms of sensitivity to various disease states. Physiological and neurobiological differences between dominant and subordinate monkeys have been interpreted in the context of chronic social stress. The present experiments were designed to investigate the relationships between basal cortisol and testosterone concentrations and the establishment and maintenance of the social hierarchy in male cynomolgus monkeys. Cortisol concentrations were measured at baseline and following suppression with dexamethasone (DEX) and subsequent administration of ACTH while monkeys were individually housed (n=20) and after 3 months of social housing (n=4/group), by which time dominance hierarchies had stabilised. Cortisol was also measured during the initial three days of social housing. Neither pre-social housing hormone concentrations nor HPA axis sensitivity predicted eventual social rank. During initial social housing, cortisol concentrations were significantly higher in monkeys that eventually became subordinate; this effect dissipated within three days. During the 12 weeks of social housing, aggressive and submissive behaviours were observed consistently, forming the basis for assignment of social ranks. At this time, basal testosterone and cortisol concentrations were significantly higher in dominant monkeys and, following dexamethasone suppression, cortisol release in response to a challenge injection of ACTH was significantly greater in subordinates. These results indicate that basal cortisol and testosterone concentrations and HPA axis function are state variables that differentially reflect position in the dominance hierarchy, rather than trait variables that predict future social status.
Keywords: cortisol, dexamethasone suppression, dominance hierarchy, testosterone, nonhuman primates
Introduction
Nonhuman primate social groups have proven useful for studying social status-related differences in disease vulnerability and resistance, with such differences linked to predictable variation in physiological, neurobiological and behavioural characteristics. For example, socially subordinate monkeys are more susceptible to immune, cardiovascular and reproductive dysfunction compared to dominant monkeys (1–5). Moreover, subordinate monkeys are more sensitive to the abuse-related effects of cocaine (6, 7) and can serve as a model of major depressive disorder (8, 9). The influence of social rank on health in monkeys parallels the direct relationship between control over resources and life expectancy in humans (10, 11) and the inverse relationship between socioeconomic status and susceptibility to disease (12, 13). These similarities have encouraged research into the physiological correlates and consequences of assuming a particular social rank.
The linear dominance hierarchies that characterise many nonhuman primate social groups are established in large part by dyadic agonistic interactions and maintained with aggressive, submissive and affiliative behaviours (14). Dominant monkeys (i.e., those above the median in social rank) typically have greater control over resources and maintain their status through physical aggression and/or intimidation; subordinate monkeys often experience a shortage of resources, fewer coping strategies and reproductive impairment (4). Thus, one explanation put forth to explain physiological and neurobiological differences between dominant and subordinate monkeys involves a relatively greater amount of stress experienced by subordinate monkeys (15). Subsequent studies have revealed that the extent to which different monkeys in such hierarchies experience stress, sometimes conceptualised as allostatic load (e.g., 16), varies widely according to the particulars of the social structure, including the stability of hierarchies, the availability of social support to subordinates and style of dominance, i.e., whether dominance is maintained through physical aggression or non-physical intimidation (e.g.,5,17,18).
Hypothalamic-pituitary-adrenal (HPA) axis function is routinely used as a measure of stress sensitivity in macaques. Exposure to stressors in the environment result in increases in release of CRH from the paraventricular nucleus of the hypothalamus, which acts to release ACTH from the anterior pituitary into the circulation. ACTH acts in the adrenal cortex to synthesise and release cortisol which mediates physiological responses to stress throughout the body. When evaluating HPA axis function in monkeys, three commonly assessed variables are circulating concentrations of cortisol, sensitivity to suppression by the glucocorticoid receptor agonist dexamethasone (DEX), a glucocorticoid receptor agonist which acts in the pituitary to suppress ACTH release via negative feedback and, following DEX suppression, adrenal responsiveness to a challenge injection of an amount of ACTH designed to be reflect stimulation due to exposure to a stressor. Although a review of the vast literature describing the relationship between glucocorticoids and social rank is beyond the scope of this paper, a considerable number of studies have been conducted in Old World monkeys. The hypothesis that differences in HPA axis function among socially housed monkeys are due to chronic stress experienced by subordinates is supported by the findings that subordinate monkeys have heavier adrenal glands and greater cortisol secretion in response to stressors than dominant monkeys (8, 19–22; but see 23). However, the relationship between social rank and basal cortisol concentrations in Old World monkeys is less clear, with studies reporting higher circulating cortisol in dominant monkeys (24), subordinate monkeys (8, 21, 25–30) or a lack of a relationship between cortisol concentration and social rank (20, 31–36). The relationship between social status and cortisol concentration is extremely sensitive to setting and procedural details such that it may not be possible to generalise from captive to free-ranging animals (cf. ref. 16). Moreover, the influence of sex on this relationship is unclear. Nonetheless, in light of the experimental control afforded by studying monkeys in captivity, a better understanding of the relationship between glucocorticoid function and social rank in the laboratory setting would be valuable.
The present studies extended our examination of the relationship between cortisol and social rank in male cynomolgus monkeys with the objective of determining the extent to which commonly used measures of HPA axis function could predict future social status or were reflective of achieved social status. In these experiments, we expanded our earlier investigations (34) by performing a more extensive characterisation of basal cortisol concentrations and by assessing changes in cortisol during the initial week of social housing. Based on previous studies in our laboratory and others (33, 34, 37–39), we hypothesised that basal cortisol concentrations, extent of suppression by DEX and sensitivity to ACTH administration would not predict future social rank. We hypothesised further that following social group formation and stabilisation, subordinate monkeys would display higher basal cortisol concentrations and a greater sensitivity to a pharmacological challenge in comparison to dominant monkeys, as a result of chronic exposure to social stress. In addition, we anticipated that testosterone concentrations would not predict social rank, but would be higher in dominant vs. subordinate monkeys once ranks were established, consistent with previous reports in Old World monkeys (e.g., 40–42).
Materials and Methods
Subjects
Twenty adult male cynomolgus monkeys (Macaca fascicularis) were used. Another monkey was added to the study to replace an injured monkey (see below). Monkeys were housed in cages (Allentown Caging, Allentown, New Jersey) divided into four equal quadrants by removable partitions. Each quadrant measured 0.71 × 0.84 × 0.84 m and allowed visual, auditory and limited tactile interactions. When partitions were removed, four monkeys occupied the entire cage (0.71 × 1.73 × 1.83 m). Monkeys were weighed weekly and fed enough food daily (LabDiet Monkey Chow and fresh fruit) to maintain body weights at approximately 90%–95% of free-feeding weights. Each quadrant was equipped with a spout from which water was available ad libitum. Nine subjects (aged 10–15 years) had previously self-administered cocaine and had previously been socially housed (see 6). However, these monkeys had not self-administered cocaine for at least 4 months and had been housed individually for 4–6 months at the onset of the present experiments. Twelve additional subjects (aged 5–8 years) were cocaine-naïve and had not been socially housed. Results of statistical analysis using one-way analyses of variance (ANOVA) indicated that no dependent variables differed significantly according to social housing or cocaine self-administration history (not shown). Each monkey was fitted with a collar (Primate Products, Redwood City, CA) and trained over several weeks to sit calmly in a standard primate restraint chair (Primate Products) using a specially designed stainless steel pole that attached to the collar.
Thirteen of the 21 subjects had previously been surgically prepared under sterile surgical conditions with an indwelling venous catheter in a jugular or femoral vein which was connected to a subcutaneous vascular access port (VAP; Access Technologies, Skokie, IL) as described previously (6). All manipulations were performed in accordance with the 2003 National Research Council Guidelines for the Care and Use of Mammals in Neuroscience and Behavioural Research and were approved by the Animal Care and Use Committee of Wake Forest University.
Introduction of social housing conditions
Cage partitions remained in place during the individual-housing phase of the study, although monkeys were allowed visual, auditory and limited tactile contact with each future pen-mate. One pen consisted of 4 previously subordinate animals and one pen consisted of 3 previously intermediate-ranked monkeys plus 1 monkey who had not been previously socially housed. The remaining 12 monkeys were randomly assigned to three pens by weight, with the three heaviest monkeys designated to three separate pens, the next three heaviest randomly placed into those pens, and so on. The average weight of all subjects on the first day of social housing was 5.4 kg (range = 4.3–6.2 kg). There were no significant differences in average body weight across pens or eventual social ranks at any time during the present studies. Initial evaluations of basal cortisol and HPA axis function were performed while animals were housed individually with partitions between quadrants in place (see Fig. 1 for sequence of experimental events).
Figure 1.
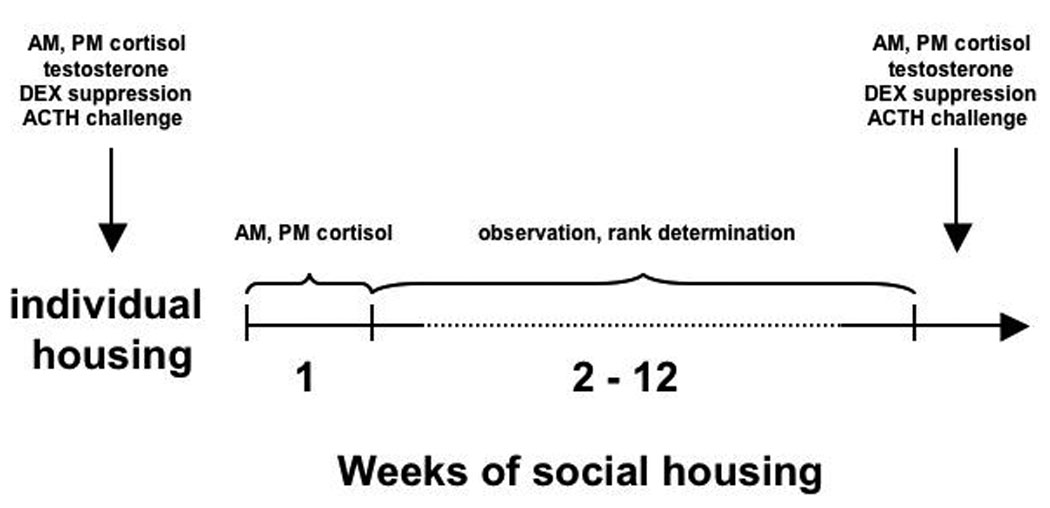
Timeline of experimental events.
At approximately 9:00AM on the first day of social housing, all animals in the pen received a low ketamine dose (1.0–3.0 mg/kg based on knowledge of the extent of tolerance to sedative effects of ketamine). Next, partitions were removed. For the next 5 days, partitions were replaced at approximately 4:15PM and monkeys remained separated overnight. This precaution was taken to prevent injury occurring when laboratory personnel were not present. It is important to note that, when monkeys were separated overnight, all interactions other than physical (i.e., visual, olfactory and auditory) were maintained, as monkeys remained in close proximity. After approximately one week, monkeys were socially housed overnight and separated each weekday morning, during which time monkeys were involved in operant behavioural sessions (data not shown) and fed. On weekends, pens were separated for one hour for feeding. Thus, time spent socially housed was approximately 15 hours on weekdays and 23 hours on weekends. In rare cases of an injury that required veterinary care or other routine veterinary procedures, the entire pen was individually housed overnight (or until the injury healed).
Deviations from this protocol occurred for two pens. During the first week of social housing, monkeys in the pen consisting of previously subordinate monkeys initially displayed a high frequency of aggressive behaviours. Specifically, three monkeys frequently cornered and bit the fourth, who made no attempt to escape. To facilitate establishment of the hierarchy, these monkeys were transferred to the Wake Forest University Primate Centre (WFUPC) where they were housed for 2.5 months in a larger enclosure (1.5 × 2.4 × 3 m), designed to provide more room for escape. The #4-ranked monkey in this pen was removed two weeks after being moved to the WFUPC due to injury. He was replaced with a monkey who had also been subordinate in a previous social group and he became #4-ranked in the new social group. A second pen of monkeys was housed in a similar enclosure at the WFUPC for three weeks at the start of social housing. None of the subsequently acquired measurements differed between the pens that underwent this manipulation and those that did not.
Determination of ranks
From week 2 through week 12 of social housing, two observers separately conducted a total of 26 observation sessions per pen. Each 15-min session began immediately after partitions were removed in the afternoon. Aggressive, submissive and affiliative behaviours were recorded according to an ethogram described previously (43, see ref. 34, Table I) utilising Noldus Observer software (Noldus Information Technology; Wageningen, The Netherlands). In these focal group sessions, both initiators and recipients of behaviours were recorded. The monkey in each pen aggressing towards all other monkeys and submitting to none was ranked #1 (most dominant). The monkey aggressing at everyone except the #1-ranked monkey and submitting only to the #1-ranked monkey was ranked #2, etc. The monkey designated #4 displayed a low frequency of aggressive behaviours and submitted to all other monkeys in the pen. Thus, a transitive, linear hierarchy was established in each pen.
Table 1.
Testosterone concentrations (ng/ml) before and after social housing according to eventual social rank.
| individually housed | socially housed | ||
|---|---|---|---|
| Eventual Rank | mean (S.E.M.) | mean (S.E.M.) | |
| 1 | 10.0 (3.1) | 14.0 (4.7) |  |
| 2 | 8.8 (2.3) | 10.5 (3.1) | |
| 3 | 6.5 (1.6) | 11.1 (4.7) | |
| 4 | 9.7 (4.1) | 5.3 (1.5) | |
p< 0.05.
Blood sampling: basal cortisol and testosterone measures
While monkeys (n=20) were seated in a primate chair, blood samples were collected at 7:00AM and 4:30PM at three stages of the present study: while monkeys were individually housed during 5 days of the week prior to initial social housing, during the first three days of social housing and during 5 days of the 12th week of social housing. Samples were not collected during the first week of social housing for the one pen that was moved to the WFUPC during the first week (see above). Subjects from one pen were each placed into a primate chair 15 minutes prior to sampling. Each monkey’s VAP site was cleaned with betadine and 95% alcohol. Catheters were first flushed with 0.5 ml 0.9% NaCl to evacuate old contents. Next, 0.5 ml of blood was extracted and discarded to minimise dilution from the saline flush. An additional 2–3 ml was then extracted and immediately transferred to a chilled K2-EDTA lined Vacutainer blood collection tube (Becton Dickinson, Franklin Lakes, NJ). After collection, 2 ml of heparinised saline (100 U/ml) was flushed through the catheter to prevent clotting. For the 7 subjects without indwelling catheters, samples were obtained through percutaneous sticks of the femoral vein while the monkey was in the primate chair. For each pen, all samples were acquired within 15 minutes of monkeys being placed in chairs. Samples were kept on ice until centrifuged under refrigeration at 4°C at 3,000 rpm for 15 min. Plasma was separated and stored at −30°C until assayed by the Biomarkers Core Lab, Yerkes National Primate Research Centre, Emory University using a commercially prepared kit (Diagnostic Systems Laboratories, Webster, TX). The inter-assay variance of the cortisol assay was 4.50% at 4.2 µg/dl and 8.74% at 19.7 µg/dl. Testosterone was assayed from the 7:00AM blood samples. The inter-assay variance of the testosterone assay was 5.95% at 0.7 µg/dl and 4.14% at 5.7 µg/dl. The intra-assay variances for cortisol and testosterone assays were 4.9% (n=8) and 6.3% (n=6), respectively.
Blood sampling: ACTH challenge
Experiments were performed on 20 subjects approximately 1 week prior to social housing and again approximately 19 weeks after social housing was initiated (range = 13–23 weeks). All four members of a pen underwent the experiment simultaneously. Samples collected at 11:00AM one week prior to the ACTH challenge served as a baseline measure. DEX (0.5 mg/kg, i.m.) was administered between 6:30 and 7:30 AM. Four hours later, each subject was placed into a primate chair and a blood sample was obtained to assess the extent of DAX suppression of cortisol concentrations. Approximately 15 min later, synthetic ACTH (Cortrosyn: 10 ng/kg, i.v.) was administered through the VAP or, in the 7 subjects without a VAP, through the saphenous vein via an external catheter using a 24 gauge angiocath (Insyte Autoguard, Becton Dickinson, NJ), followed by 2 ml 0.9% NaCl. Doses of DEX and cortrosyn were selected based on previous studies in cynomolgus monkeys (e.g., 8). Blood samples were collected 15 and 30 min after ACTH administration. DEX and Cortrosyn were purchased from the North Carolina Baptist Hospital pharmacy.
Data analysis
Comparisons were made using two-way, repeated-measures ANOVA with Fisher’s LSD post-hoc tests. Cortisol values (ng/ml) were used to assess whether social rank was predicted by AM or PM hormone concentrations while monkeys were individually housed (eventual rank and time of day were factors in the ANOVA) and whether cortisol concentrations differed according to social rank after 3 months of social housing (rank and time of day were factors). A one-way ANOVA assessed whether basal testosterone concentrations (ng/ml) varied across eventual rank in individually housed monkeys, or current rank for blood samples taken after social housing. The percent change from AM or PM baseline was used to assess rank-related differences in cortisol concentrations during initial social housing (eventual rank and time/day were factors). To assess whether the cortisol response to ACTH administration differed across eventual (when individually housed) or current (when socially housed) rank, percent change from DEX-suppressed baseline was analysed (rank and time after ACTH were factors). In addition, this approach was used to determine whether AM or PM cortisol concentrations or the cortisol response to ACTH changed from individual to social housing conditions. Finally, extent of DEX suppression was compared using a one-way ANOVA and post-hoc Fisher’s LSD test. Because we anticipated that moderate effects in intermediate monkeys could obscure detection of differences in the ANOVA, a decision was made a priori to conduct a post-hoc t-test between data from #1- and #4-ranked monkeys in the event that ANOVA generated a non-significant F value. In all cases, statistical significance was accepted at the 95% level of confidence (p<0.05). There were no qualitative or statistically significant differences (assessed using t-tests) in any measures depending on whether monkeys were experienced in cocaine self-administration or experimentally naïve at the start of the experiment, so data from all monkeys were used in all analyses.
Results
Basal cortisol and testosterone concentrations when individually housed
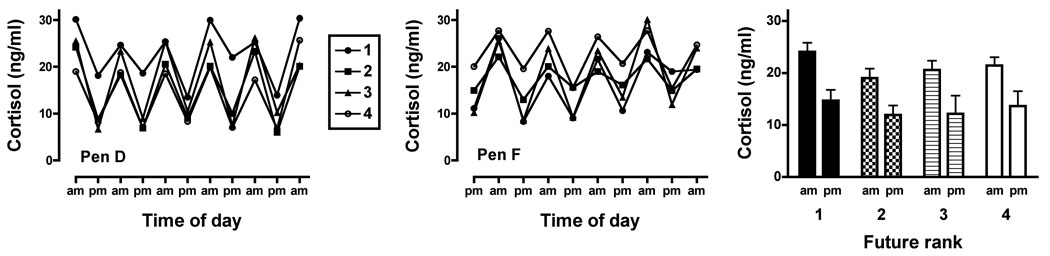
In blood samples obtained from individually housed monkeys, diurnal fluctuations in basal cortisol concentrations were observed in all subjects. Representative data from two pens of monkeys are shown in the left and centre panels of Fig. 2; mean (+SEM) cortisol concentrations from all groups are depicted in the right panel of Fig. 2. There was a significant main effect of time (F1,16=41.31, p<0.0001), but not eventual social rank, with no significant interaction. Post-hoc analysis indicated that average morning cortisol concentrations were significantly higher than average evening concentrations for all ranks (p<0.01 except eventual #2-ranked, p<0.05). There were no significant differences in AM or PM cortisol concentrations observed across ranks. Regarding testosterone concentrations, ANOVA revealed no significant differences as a function of eventual social rank when monkeys were individually housed (Table 1). In no case during this study were cortisol or testosterone concentrations correlated with age.
Figure 2.
Diurnal fluctuations in basal cortisol in individually housed monkeys. Left and centre panels depict data from individual monkeys in representative pens prior to social housing. For each rank, mean morning and evening cortisol concentrations were significantly different (all p<0.01 except future rank #2, p<0.05). Right panel shows mean (+S.E.M.) for 5 monkeys of each eventual rank.
Initial social housing
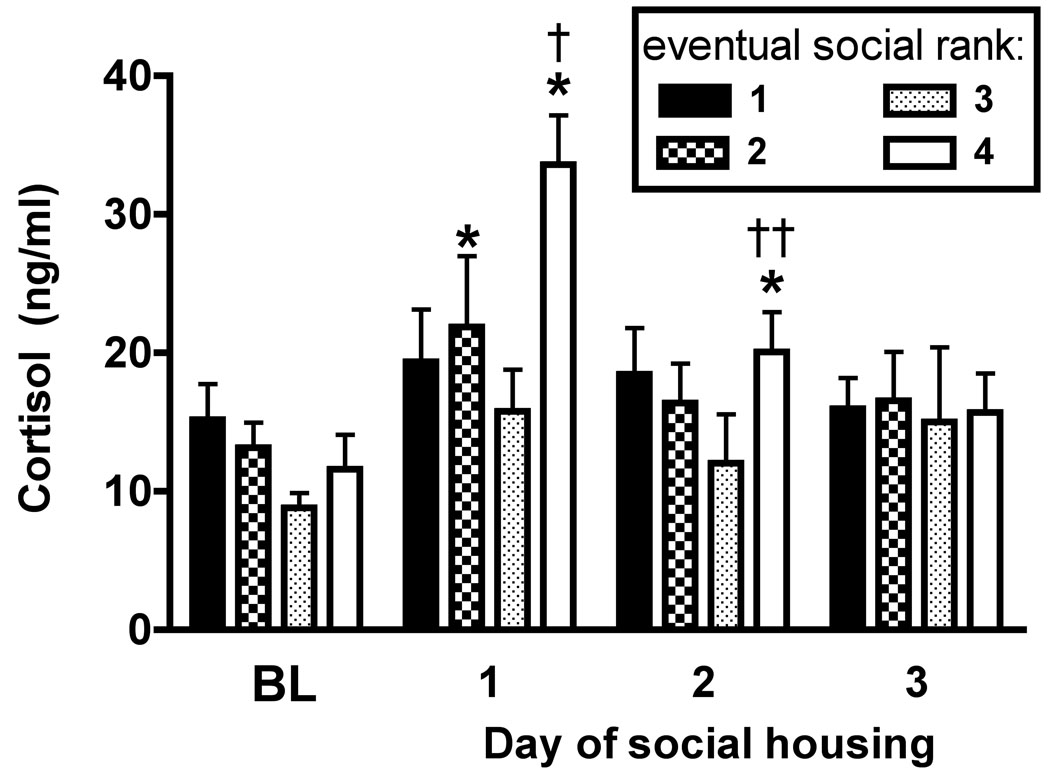
Cortisol concentrations measured in the evening immediately after monkeys’ initial experiences with social housing were elevated compared to monkeys’ normal PM baseline (Fig. 3). There was a significant main effect of day (F3,36=12.04; p<0.0001) but not rank, and a significant interaction between rank and time (F9,36=2.29; p<0.05). Post-hoc analysis indicated that, on the first evening after social housing, cortisol concentrations were higher than average PM concentrations for #2-ranked (p<0.05) and #4-ranked monkeys (p<0.01). Moreover, after the first day of social interaction, #4-ranked monkeys had significantly higher average cortisol concentrations than all other ranks (p<0.01). Evening concentrations after the second day of social housing were elevated significantly only in #4-ranked monkeys (p<0.05). PM concentrations on Day 2 were also significantly different between #3- and #4-ranked monkeys (p<0.05). No significant differences were observed after the third day of social housing. Morning cortisol concentrations did not differ from pre-social housing AM baselines (data not shown), a result that was expected because monkeys were individually housed overnight during the first week of social housing.
Figure 3.
PM cortisol concentrations at baseline and after each of the first three days of social housing. Points represent mean (+S.E.M., n=4) across social ranks. *, p<0.05 compared to baseline for the same rank; †, p<0.01 compared to #1-, #2-, and #3-ranked monkeys on Day 1; ††, p<0.05 compared to #3-ranked monkeys on Day 2.
Behavioural observations
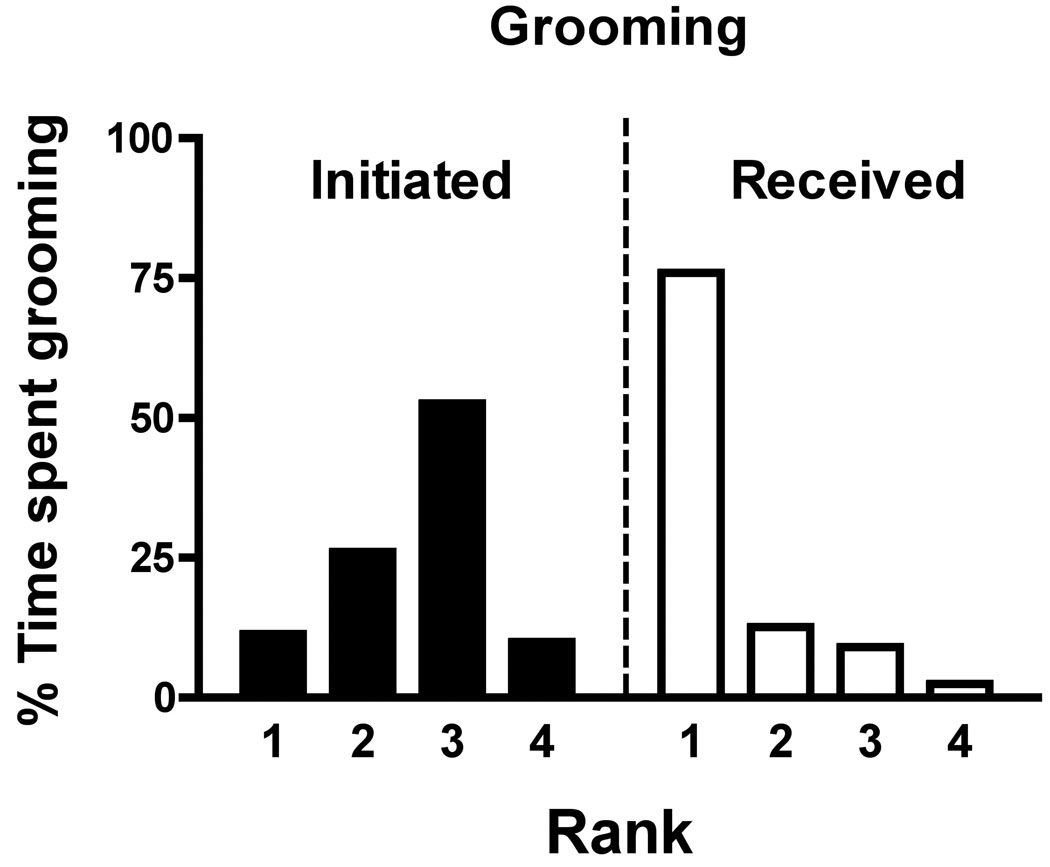
Considering that the rate of aggressive and submissive behaviours did not change across three months of sampling (data not shown), data were collapsed across months. Table 2 depicts the total aggressive and submissive behaviours across ranks, indicating the initiator and recipient. Similar rates of initiating aggression were observed in #1-, #2-, and #3-ranked monkeys, whereas rates of aggressive behaviour were much lower in #4-ranked monkeys. Receiving aggressive behaviour and initiating submissive behaviour correlated positively with rank and with each other while receiving submissive behaviour correlated negatively with rank. That is, monkeys tended to receive aggression from and submit towards higher ranking monkeys, and receive submission from lower ranking monkeys. Because ranks were assigned based on these data, these relationships are not surprising. They are presented here to demonstrate the extent of differences in frequency of the behavioural measures across ranks. Regarding affiliative behaviours, over 75% of total grooming was received by #1-ranked monkeys whereas less than 3% of the total grooming was directed to #4-ranked monkeys (Fig. 4). Moreover, very little grooming was initiated by #4-ranked monkeys. No significant correlations were observed between body weight or age and eventual social rank or aggressive, submissive or grooming behaviours (data not shown).
Table 2.
Sum of aggressive and submissive behaviours across the first three months of social housing according to the rank of the initiator and recipient
| Aggressive behaviours | ||||||
|---|---|---|---|---|---|---|
| Recipient | ||||||
| Rank | 1 | 2 | 3 | 4 | Total (% Initiated) |
|
| 1 | ----- | 60 | 56 | 196 | 312 (30.1) | |
| 2 | 14 | ----- | 166 | 135 | 315 (30.4) | |
| 3 | 4 | 12 | ----- | 376 | 392 (37.8) | |
| 4 | 5 | 7 | 6 | ----- | 18 (1.7) | |
| Total (% Received) |
23 | 79 | 228 | 707 | ||
| (2.2) | (7.6) | (22.0) | (68.2) | |||
| Submissive behaviours | ||||||
| Recipient | ||||||
| Rank | 1 | 2 | 3 | 4 | Total (% Initiated) |
|
| 1 | ----- | 4 | 0 | 1 | 5 (0.2) | |
| 2 | 203 | ----- | 6 | 0 | 209 (9.0) | |
| 3 | 495 | 177 | ----- | 7 | 679(29.3) | |
| 4 | 546 | 296 | 58 | ----- | 1426 (61.5) | |
| Total (% Received) |
1244 | 477 | 590 | 8 | ||
| (53.6) | (20.6) | (25.4) | (0.34) | |||
Figure 4.
Percent of total observation time spent grooming according to social rank and divided according to grooming initiated and received. Bars indicate the mean of 5 monkeys.
Basal cortisol and testosterone concentrations when socially housed
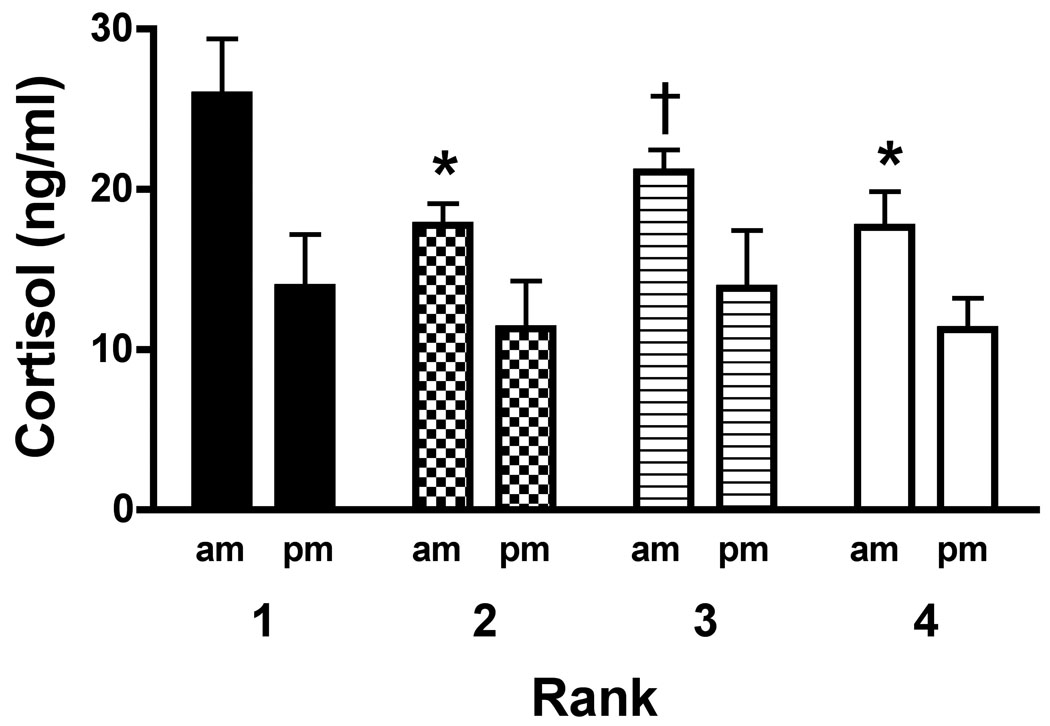
Morning and evening cortisol concentrations were again measured after at least 12 weeks of social housing by which time ranks had stabilised (Fig. 5). There was a significant main effect of time (F1,16=57.90; p<0.0001) but not rank, and no significant interaction. Post-hoc analysis indicated that morning cortisol concentrations in #1-ranked monkeys were significantly higher than those observed in #2-, #3- and #4-ranked monkeys (p<0.01 except #3-ranked, p<0.05). Within each rank, average morning cortisol concentrations were significantly higher than evening concentrations (p<0.01). When a comparison was made between morning values obtained during individual versus social housing, a main effect of rank was observed (F3,16=3.34; p<0.05) with no main effect of time and no interaction. Importantly, post-hoc testing revealed no significant differences in morning cortisol concentrations between individual vs. social housing within any social rank. A similar analysis of evening cortisol concentrations revealed no significant differences. Regarding testosterone concentrations, ANOVA revealed no main effect of rank when monkeys of all four ranks were considered, but a post-hoc t-test indicated that the average morning testosterone concentration in dominant monkeys was significantly higher than that of subordinates (t8=2.57, p<0.05; Table 1).
Figure 5.
Average morning and evening cortisol concentrations in socially housed monkeys. Each bar represents mean (+S.E.M.) for 4 (#4-ranked) or 5 (#1-, #2- and #3-ranked) monkeys. For each rank, mean morning and evening cortisol concentrations were significantly different (p<0.01). *, p<0.01 compared to #1-ranked monkeys’ AM cortisol concentration; †, p<0.05 compared to #1-ranked monkeys’ AM cortisol concentration.
Cortisol response to ACTH after dexamethasone suppression
When monkeys were individually housed, administration of DEX resulted in a decrease in circulating cortisol concentrations in all monkeys that was comparable across eventual social ranks (Fig. 6). A similar extent of suppression by DEX was observed when monkeys were socially housed. When monkeys were individually housed, administration of ACTH resulted in increased cortisol concentrations at 15 min in all monkeys, with concentrations approaching basal values at 30 min (Fig. 6). There was a significant main effect of time (F1,16=21.52; p<0.001), but not rank, and no significant interaction. Post-hoc testing indicated that 15 min after ACTH injection, #1 and #2 ranked monkeys differed significantly from #3-ranked monkeys (p<0.05). Cortisol concentrations at 15 min were significantly higher than at 30 min for monkeys ranked #1 (p<0.01), #2 and #4 (p<0.05). No significant rank-related differences were observed at 30 min. Thus, administration of DEX and subsequently, ACTH affected all monkeys similarly with the exception of #3-ranked monkeys which were somewhat less sensitive to ACTH.
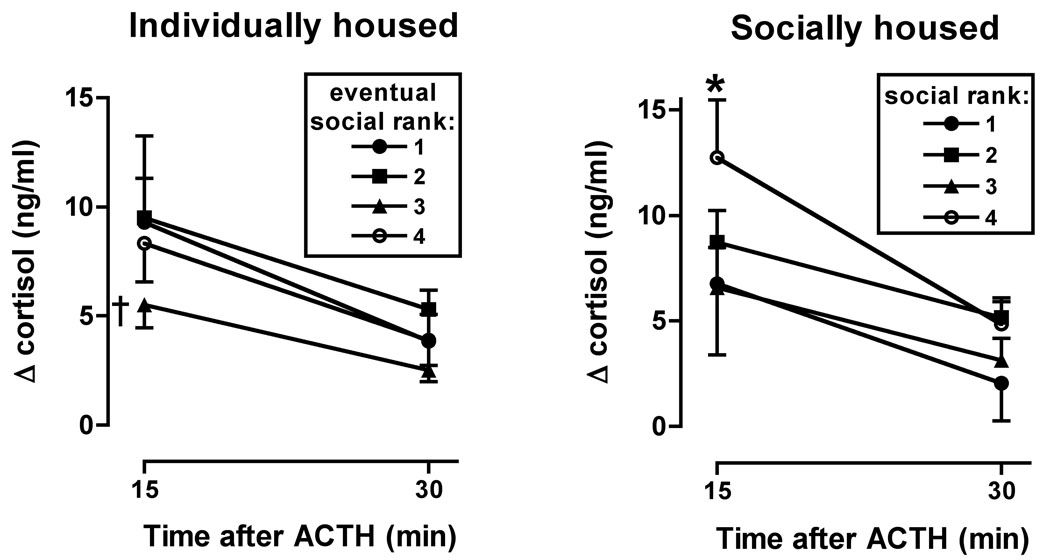
Figure 6.
Time course of effects of ACTH administration on cortisol concentrations determined when monkeys were individually (left) and socially housed. Data are expressed as mean (±S.E.M.) change in cortisol concentrations (ng/ml) from baseline. †, #3-ranked monkeys different from #1-and #2-ranked groups, p<0.05; *, #4-ranked monkeys different from #1-, #2- and #3-ranked groups, p<0.01.
When the ACTH challenge was repeated after 3 months of social housing, #4-ranked monkeys displayed greater sensitivity to ACTH than monkeys of other ranks (Fig. 6). There was a significant main effect of time (F1,16=26.67; p<0.0001) but not of rank, nor was there a significant interaction between time and rank. Post-hoc analyses revealed significant differences at the 15-min time point between #4 ranked monkeys and each of the other three groups (p<0.01), and no significant differences at the 30-min point. When data from 15 min or 30 min after ACTH were examined as a function of housing condition, there was no significant main effects or interactions. However, at both time points, post-ACTH cortisol concentrations were higher during the social housing condition for #4-ranked monkeys than when those monkeys were individually housed. For #1-, #2- and #3-ranked monkeys, no differences were observed at either time point when individual and socially housed effects were compared.
Discussion
Physiological and neurobiological differences have been described in monkeys as a function of social rank. The present studies extended our previous investigations of the relationship between HPA axis function and social rank in male cynomolgus monkeys (34) to more thoroughly characterise cortisol fluctuations and the relationship of cortisol concentrations and adrenal sensitivity to social rank. The results indicated that these measures did not predict eventual social rank. Once socially housed, subordinate monkeys performed the majority of submissive behaviours, received the majority of aggressive behaviours and rarely participated in grooming, reinforcing the conceptualisation of social subordination as characterised by chronic social stress. Although basal cortisol concentrations were, on average, higher in dominant monkeys, adrenal responsiveness to a pharmacological challenge was higher in subordinate monkeys.
Measures of HPA axis function were initially collected while monkeys were individually housed to determine whether these variables could predict eventual social rank. Similar diurnal fluctuations of plasma cortisol concentrations were observed in all subjects. Moreover, similar reductions in circulating cortisol were observed after DEX administration. On average, 15 min after ACTH administration, cortisol concentrations were elevated to approximately 150–200% of DEX-suppressed concentrations in all monkeys, similar to previous results (33, 34, 37–39). Basal, DEX-suppressed and ACTH-induced cortisol concentrations while monkeys were individually housed were unrelated to future social rank. Thus, differences in HPA axis function among socially housed monkeys appear to result from exposure to environmental variables associated with establishment of the dominance hierarchy (i.e., state variables), and do not pre-exist and determine social rank (i.e., trait variables). Consistent with previous studies in Old World monkeys (34, 40–42), testosterone concentrations also did not predict future social rank.
During the initial three days of social housing, monkeys were group-housed for approximately 7 hours per day. After the first day, evening cortisol was elevated in all monkeys relative to concentrations observed at that time of day when individually housed, as noted previously (e.g., 18, 38, 39, 44–46). In the present studies, the extent of elevation of cortisol was inversely related to future social rank. Specifically, monkeys who would eventually occupy the lowest position in the dominance hierarchy had significantly higher concentrations of cortisol than future dominant monkeys. These results are inconsistent with previous reports cited above, in which cortisol increased similarly across ranks, but support the view that the animals that would become subordinate experienced a greater degree of stress on the first day of social housing. The significant difference between #1- and #4-ranked monkeys was also observed after the second day of social housing. This effect was not simply carried over from the previous day, since cortisol concentrations in all monkeys returned to normal morning concentrations after monkeys were individually housed overnight. Because morning cortisol concentrations are high, and evening concentrations remained high after social housing in subordinate monkeys, these monkeys experienced a persistently high concentration of plasma cortisol for more than 48 hours after introduction of social housing. Such persistent activation of the HPA axis has been shown to exert a deleterious effect on resistance to a variety of disease states (for reviews see 5, 47). Although the frequency of aggressive and submissive behaviours were consistent throughout the first 12 weeks of social housing, the ability of several hours of social housing to elevate cortisol in subordinates dissipated by Day 3. Thus, in subordinates, the HPA axis response appeared to habituate rapidly to social stress experienced in the social housing condition. Such a rapid dissipation in cortisol response despite the continued presence of stressors has been observed previously in rhesus monkeys (48).
After 12 weeks of social housing, average morning cortisol concentrations were slightly but significantly higher in dominant monkeys compared to subordinates. The biological significance of this change is unclear considering that, despite this significant difference between ranks, basal cortisol concentrations did not significantly change from individually housed values for any rank. Clearly, however, these data are inconsistent with studies reporting higher basal circulating concentrations of cortisol in subordinate monkeys (8, 21, 25–30, 44, 49). In contrast to this discrepancy, the observations that DEX reduced basal cortisol similarly across ranks and that subordinate monkeys were significantly more sensitive to administration of a biologically relevant amount of ACTH were consistent with previously published studies in cynomolgus monkeys (8, 19, 21), and may be explained by the finding that adrenal glands were larger and heavier in subordinate monkeys (19, 22). Thus, although social stress-induced elevations in evening cortisol dissipated in #4-ranked monkeys within three days, subordinate monkeys remained more sensitive to activation of the HPA axis by ACTH administration. Although it is possible that assessments of HPA reactivity in the DEX-suppressed state do not accurately reflect the response in the normal state, an alternative explanation is that greater sensitivity to an acute pharmacological stressor was maintained in subordinates despite habituation of basal cortisol concentrations. As seen with cortisol, dominant monkeys had higher circulating concentrations of testosterone than subordinates, in agreement with previous studies in macaques (40–42) and other nonhuman primate species (27, 50, 51). One notable exception is our previous study (34), in which no rank-related difference in testosterone was observed. This discrepancy is likely due to variability in the time of sample collection. In the present study, samples were collected in all animals around 7:00AM, whereas in the Morgan et al. study, samples were collected in conjunction with concurrent brain imaging studies that occurred throughout the day. In addition, it should be noted that the studies involving DEX suppression and ACTH administration reflect only one component of HPA axis, the sensitivity of the adrenal gland to ACTH, and that adaptations could occur elsewhere in the axis that could compensate for adrenal hyper-responsiveness. A more complete understanding of social rank-related differences in HPA axis function will be gained through studies involving CRH administration.
Over the past 40 years, the relationship between HPA axis function and social rank has been examined in numerous primate species under diverse experimental settings with varying approaches and strikingly equivocal results. It is thus not surprising that there is inconsistency among results, particularly in relation to basal cortisol concentrations. However, stimulation of the HPA axis under controlled conditions appears to unmask a more consistent pattern in which subordinate monkeys exhibit increased HPA reactivity relative to dominants. In this regard, the current data from male cynomolgus monkeys are in agreement with studies that have reported higher circulating cortisol concentrations in dominant monkeys compared to subordinates (24, 39, 52–55) and with data indicating that the HPA axis of subordinates is more sensitive to acute stressors (8, 19–21). There are several factors that may contribute to the apparent discrepancies with other studies that have reported different relationships between cortisol concentrations and social rank.
Importantly, the social rank of the monkey that experiences the greatest amount of social stress can vary according characteristics of the social group including sex, social structure and stability of the hierarchy (for reviews see 3, 5, 16, 17, 47, 56). Dominant monkeys may experience relatively greater stress during periods of instability when dominance is being contested, and in settings in which dominance is maintained through physical aggression. Conversely, characteristics of social groups in which subordinates experience a higher amount of stress include hierarchy stability, an abundance of resources, reliance of dominant monkeys on non-physical intimidation, inability of subordinates to avoid dominants and a lack of social support as a means for coping with stress. Although dominant monkeys had higher basal cortisol concentrations, the social organisation of monkeys in the present experiments appears to share features of the latter type of hierarchy in which subordinates experience more stress. For example, cortisol was measured when the hierarchy appeared stable, competition for resources was minimal because monkeys were separated during feeding and had no access to females, the enclosure was small enough that subordinates had difficulty avoiding dominants, subordinates had few outlets for coping (as evidenced by low rates of grooming) and rates of aggression and submission remained as high at 12 weeks as they were during initial weeks of social housing. It should be noted, however, that judging stability of a hierarchy is not straightforward; instability may persist despite consistency in social ranks. Allowing more time to pass before measuring cortisol may have produced data more similar to those of Sapolsky (25) or others who have reported no difference in cortisol across ranks in stable social groups (33, 35). Additionally, it should be noted that the living space was relatively small compared to other studies of nonhuman primate social behaviour. The increased density of monkeys may have functioned in this study to elevate stress longer than has been observed in other studies.
An alternative interpretation of the present results which may reconcile the apparent difference between basal cortisol concentrations (higher in dominants) and greater reactivity to ACTH (in subordinates) involves adaptation in subordinate monkeys. In several studies, rank-related differences in basal cortisol concentrations only shortly after group formation, in some cases documenting dissipation of those differences (18, 38, 45, 46). In the present studies, cortisol concentrations were greatly elevated after the first day of social housing in monkeys that would become subordinate despite the fact that dominant monkeys had higher average cortisol concentrations 12 weeks later. Adaptation to the cortisol-elevating effect of social interaction took place within three days, suggesting that a reduction in circulating cortisol may have occurred as an adaptation to chronic stimulation of the HPA axis. Despite this adaptation of basal cortisol concentrations, adrenal responsiveness to a pharmacological challenge was significantly greater in subordinates. Whether subordinates are also more sensitive to more relevant social stressors would be hypothesised, but remains to be explored.
Acknowledgements
The authors are grateful to Michelle Icenhower, Chris Corcoran, Susan Nader and Tonya Calhoun for technical assistance, and to Dr. Jay R. Kaplan for helpful comments on an earlier version of this manuscript. This research was supported by NIDA grant DA 10584.
References
- 1.Cameron JL. Stress and behaviorally induced reproductive dysfunction in primates. Semin Reprod Endocrinol. 1997;15:37–45. doi: 10.1055/s-2008-1067966. [DOI] [PubMed] [Google Scholar]
- 2.Cohen S, Line S, Manuck SB, Rabin BS, Heise ER, Kaplan JR. Chronic stress, social status, and susceptibility to upper respiratory infections in nonhuman primates. Psychosom Med. 1997;59:213–221. doi: 10.1097/00006842-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan JR, Manuck SB. Status, stress, and atherosclerosis: the role of environment and individual behavior. Ann NY Acad Sci. 1998;896:145–161. doi: 10.1111/j.1749-6632.1999.tb08112.x. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan JR, Manuck SB. Ovarian dysfunction, stress, and disease: a primate continuum. ILAR J. 2004;45:89–115. doi: 10.1093/ilar.45.2.89. [DOI] [PubMed] [Google Scholar]
- 5.Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 6.Czoty PW, McCabe C, Nader MA. Assessment of the relative reinforcing strength of cocaine in socially housed monkeys using a choice procedure. J Pharmacol Exp Ther. 2005;312:96–102. doi: 10.1124/jpet.104.073411. [DOI] [PubMed] [Google Scholar]
- 7.Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- 8.Shively CA, Laber-Liard K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry. 1997;41:871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- 9.Shively CA, Register TC, Friedman DP, Morgan TM, Thompson J, Lanier T. Social stress-associated depression in adult female cynomolgus monkeys. Biol Psychology. 2005;69:67–84. doi: 10.1016/j.biopsycho.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Amick BC, 3rd, McDonough P, Chang H, Rogers WH, Pieper CF, Duncan G. Psychological and physical exposures in the United States labor market from 1968 to 1992. Psychosom Med. 2002;64:370–381. doi: 10.1097/00006842-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Wohlfarth T, van den Brink W. Social class and substance use disorder: the value of social class as distinct from socioeconomic status. Soc Sci Med. 1998;47:51–58. doi: 10.1016/s0277-9536(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 12.Adler NE, Matthews K. Health physiology: why do some people get sick and some stay well? Ann Rev Psychol. 1993;45:229–259. doi: 10.1146/annurev.ps.45.020194.001305. [DOI] [PubMed] [Google Scholar]
- 13.Krantz DS, McCeney MK. Effects of psychological and social factors on organic disease: a critical assessment of research on coronary heart disease. Ann Rev Psychol. 2002;53:341–369. doi: 10.1146/annurev.psych.53.100901.135208. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM. Social status, environment, and atherosclerosis in cynomolgus monkeys. Atherosclerosis. 1982;2:359–368. doi: 10.1161/01.atv.2.5.359. [DOI] [PubMed] [Google Scholar]
- 15.Henry JP, Stephens PM. Stress, Health and the Social Environment; A Sociobiologic Approach to Medicine. New York: Sprenger-Verlag; 1977. [Google Scholar]
- 16.Goymann W, Wingfield JC. Allostatic load, social status and stress hormones: the costs of social status matter. Animal Behaviour. 2004;67:591–602. [Google Scholar]
- 17.Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Jr, Sapolsky RM. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- 18.Clarke AS, Czekala NM, Lindburg DG. Behavioral and adrenocortical responses of male cynomolgus monkeys and lion-tailed macaques to social stimulation and group formation. Primates. 1995;36:41–56. [Google Scholar]
- 19.Kaplan JR, Adams MR, Koritnik DR, Rose JC, Manuck SB. Adrenal responsiveness and social status in intact and ovariectomized Macaca fascicularis. Am J Primatol. 1986;11:181–193. doi: 10.1002/ajp.1350110209. [DOI] [PubMed] [Google Scholar]
- 20.Sassenrath EN. Increased adrenal responsiveness related to social stress in rhesus monkeys. Horm Behav. 1970;1:283–298. [Google Scholar]
- 21.Shively CA. Social subordination stress, behavior and central monoaminergic function in female cynomolgus monkeys. Biol Psychiatry. 1998;44:882–891. doi: 10.1016/s0006-3223(97)00437-x. [DOI] [PubMed] [Google Scholar]
- 22.Shively CA, Kaplan JR. Effects of social factors on adrenal weight and related physiology of Macaca fascicularis. Physiol Behav. 1984;33:777–782. doi: 10.1016/0031-9384(84)90047-7. [DOI] [PubMed] [Google Scholar]
- 23.Hayama S. Correlation between adrenal weight and dominance rank in caged crab-eating monkeys (Macaca fascicularis) Primates. 1966;7:22–26. [Google Scholar]
- 24.Kimura K, Shimizu K, Hayashi M, Ishikawa T, Ago Y. Pituitary-adrenocortical responses to the first dyadic encounters in male rhesus monkeys: Effect of dominance relationship. Am J Primatol. 2000;50:247–256. doi: 10.1002/(SICI)1098-2345(200004)50:4<247::AID-AJP2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 25.Sapolsky RM. The endocrine stress-response and social status in the wild baboon. Horm Behav. 1982;16:279–292. doi: 10.1016/0018-506x(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 26.Sapolsky RM. Endocrine aspects of social instability in the olive baboon (Papio anubis) Am J Primatol. 1983;5:365–379. doi: 10.1002/ajp.1350050406. [DOI] [PubMed] [Google Scholar]
- 27.Sapolsky RM. Hypercortisolism among socially subordinate wild baboons originates at the CNS level. Arch Gen Psychiatry. 1989;46:1047–1051. doi: 10.1001/archpsyc.1989.01810110089012. [DOI] [PubMed] [Google Scholar]
- 28.Sapolsky RM. Cortisol concentrations and the social significance of rank instability among wild baboons. Psychoneuroendocrinology. 1992;17:701–709. doi: 10.1016/0306-4530(92)90029-7. [DOI] [PubMed] [Google Scholar]
- 29.Yodyingyuad U, de la Riva C, Abbott DH, Herbert J, Keverne EB. Relationship between dominance hierarchy, cerebrospinal fluid levels of amine transmitter metabolites (5-hydroxyindole acetic acid and homovanillic acid) and plasma cortisol in monkeys. Neuroscience. 1985;16:851–858. doi: 10.1016/0306-4522(85)90099-5. [DOI] [PubMed] [Google Scholar]
- 30.Sapolsky RM, Alberts SC, Altmann J. Hypercortisolism associated with social subordinance or social isolation among wild baboons. Arch Gen Psychiatry. 1997;54:1137–1143. doi: 10.1001/archpsyc.1997.01830240097014. [DOI] [PubMed] [Google Scholar]
- 31.Bercovitch FB, Clarke S. Dominance rank, cortisol concentrations, and reproductive maturation in male rhesus macaques. Physiol Behav. 1995;58:215–221. doi: 10.1016/0031-9384(95)00055-n. [DOI] [PubMed] [Google Scholar]
- 32.Chamove AS, Bowman RE. Rank, rhesus social behavior, and stress. Folia Primatol. 1976;26:57–66. doi: 10.1159/000155730. [DOI] [PubMed] [Google Scholar]
- 33.McGuire MT, Brammer GL, Raleigh MJ. Resting cortisol levels and the emergence of dominant status among male vervet monkeys. Horm Behav. 1986;20:106–117. doi: 10.1016/0018-506x(86)90033-4. [DOI] [PubMed] [Google Scholar]
- 34.Morgan D, Grant KA, Prioleau OA, Nader SH, Kaplan JR, Nader MA. Predictors of social status in cynomolgus monkeys (Macaca fascicularis) after group formation. Am J Primatol. 2000;52:115–131. doi: 10.1002/1098-2345(200011)52:3<115::AID-AJP1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 35.Stavisky RC, Adams MR, Watson SL, Kaplan JR. Dominance, cortisol and behavior in small groups of female cynomolgus monkeys (Macaca fascicularis) Horm Behav. 2001;39:232–238. doi: 10.1006/hbeh.2001.1650. [DOI] [PubMed] [Google Scholar]
- 36.Yodyingyuad U, Eberhart JA, Keverne EB. Effects of rank and novel females on behaviour and hormones in male talapoin monkeys. Physiol Behav. 1982;28:995–1005. doi: 10.1016/0031-9384(82)90166-4. [DOI] [PubMed] [Google Scholar]
- 37.Eberhart JA, Yodyingyuad U, Keverne EB. Subordination in male talapoin monkeys lowers sexual behavior in the absence of dominants. Physiol Behav. 1985;35:673–677. doi: 10.1016/0031-9384(85)90395-6. [DOI] [PubMed] [Google Scholar]
- 38.Goo GP, Sassenrath EN. Persistent adrenocortical activation in female rhesus monkeys after new breeding groups formation. J Med Primatol. 1980;9:325–334. doi: 10.1159/000460162. [DOI] [PubMed] [Google Scholar]
- 39.Mendoza SP, Coe CL, Lowe EL, Levine S. The physiological response to group formation in adult male squirrel monkeys. Psychoneuroendocrinology. 1979;3:221–229. doi: 10.1016/0306-4530(78)90012-4. [DOI] [PubMed] [Google Scholar]
- 40.Berstein IS, Gordon CP, Rose RM. The interaction of hormones, behavior, and social context in nonhuman primates. In: Savre BB, editor. Hormones and Aggressive Behavior. New York: Plenum; 1983. pp. 535–561. [Google Scholar]
- 41.Clarke AS, et al. J Med Primatol. 1986;15:419. [PubMed] [Google Scholar]
- 42.Rose RM, Berstein IS, Gordon TP. Consequences of social conflict on plasma testosterone levels in rhesus monkeys. Psychosom Med. 1975;37:50–61. doi: 10.1097/00006842-197501000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Kaplan JR, Heise ER, Manuck SB, Shively CA, Cohen S, Rabin BS, Kasprowicz AL. The relationship of agonistic and affiliative behavior patterns to cellular immune function among cynomolgus monkeys (Macaca fascicularis) living in unstable social groups. Am J Primato. 1991;25:157–173. doi: 10.1002/ajp.1350250303. [DOI] [PubMed] [Google Scholar]
- 44.Coe CL, Franklin D, Smith ER, Levine S. Hormonal responses accompanying fear and agitation in the squirrel monkey. Physiol Behav. 1982;29:1051–1057. doi: 10.1016/0031-9384(82)90297-9. [DOI] [PubMed] [Google Scholar]
- 45.Golub MS, Sassenrath EN, Goo GP. Plasma cortisol levels and dominance in peer groups of rhesus monkey weanlings. Horm Behav. 1979;12:50–59. doi: 10.1016/0018-506x(79)90026-6. [DOI] [PubMed] [Google Scholar]
- 46.Gust DA, Gordon TP, Wilson ME, Ahmed-Ansari A, Brodie AR, McClure HM. Formation of a new social group of unfamiliar female rhesus monkeys affects the immune and pituitary adrenocortical systems. Brain Behav Immun. 1991;5:296–307. doi: 10.1016/0889-1591(91)90024-5. [DOI] [PubMed] [Google Scholar]
- 47.Sapolsky RM. Endocrinology of the stress response. In: Becker J, Breedlove S, Crews D, McCarthy M, editors. Behavioral Endocrinology. 2nd Ed. Cambridge, MA: MIT Press; 2002. pp. 409–450. [Google Scholar]
- 48.Xiao E, Xia-Zhang L, Ferin M. Inadequate luteal function is the initial clinical cyclic defect in a 12-day stress model than includes a psychogenic component in the rhesus monkey. J Clin Endocrinol Metab. 2002;87:2232–2237. doi: 10.1210/jcem.87.5.8500. [DOI] [PubMed] [Google Scholar]
- 49.Manogue KR, Leshner AI, Candland DK. Dominance status and adrenocortical reactivity to stress in squirrel monkeys. Primates. 1975;16:457–463. [Google Scholar]
- 50.Winslow JT, Miczek KA. Androgen dependency of alcohol effects on aggressive behavior: a seasonal rhythm in high-ranking squirrel monkeys. Psychopharmacology. 1988;95:92–98. doi: 10.1007/BF00212774. [DOI] [PubMed] [Google Scholar]
- 51.Beehner JC, Phillips-Conroy JE, Whitten PL. Female testosterone, dominance rank, and aggression in an Ethiopian population of hybrid baboons. Am J Primatol. 2005;67:101–119. doi: 10.1002/ajp.20172. [DOI] [PubMed] [Google Scholar]
- 52.Batty KA, Herbert J, Keverne EB, Velluci SV. Differences in blood levels of androgens in female talapoin monkeys related to their social status. Neuroendocrinology. 1986;44:347–354. [Google Scholar]
- 53.Coe CL, Mendoza SP, Levine S. Social status constrains the stress response in the squirrel monkey. Physiol Behav. 1979;23:633–638. doi: 10.1016/0031-9384(79)90151-3. [DOI] [PubMed] [Google Scholar]
- 54.Gonzalez CA, Coe CL, Levine S. Cortisol responses under different housing conditions in female squirrel monkeys. Psychoneuroendocrinology. 1982;7:209–216. doi: 10.1016/0306-4530(82)90014-2. [DOI] [PubMed] [Google Scholar]
- 55.Leshner AI, Candland DK. Endocrine effects of grouping and dominance rank in squirrel monkeys. Physiol Behav. 1972;8:441–445. doi: 10.1016/0031-9384(72)90326-5. [DOI] [PubMed] [Google Scholar]
- 56.Creel S. Social dominance and stress hormones. Trends Ecology Evolution. 2001;16:491–497. [Google Scholar]