Abstract
There have been rapid advances in the development and applications of semiconductor quantum dots (QDs) represented by CdSe/ZnS. However, a serious limitation of these QDs is the necessary use of toxic heavy metals. It is reported here that small carbon nanoparticles doped with inorganic salts serve as a highly promising new platform for brightly photoluminescent dots. The photoluminescent carbon dots with the carbon core doped with ZnO (CZnO-Dots) or ZnS (CZnS-Dots) in aqueous solutions are competitive to the commercially available CdSe/ZnS QDs in luminescence brightness.
Introduction
There have been rapid advances in the development and applications of semiconductor quantum dots (QDs), especially for the more fluorescent core—shell dots based on CdSe nanocrystals with a wide-bandgap semiconductor shell.1-3 Despite their demonstrated performance and widely discussed potentials, however, a major limitation is their necessary use of heavy metals such as cadmium.3a,4 In the continuing search for benign (nontoxic) alternatives,4-10 Sun and co-workers found and reported that nanosized pure carbon particles may be surface-passivated by organic molecules (dubbed “carbon dots”) to exhibit bright photoluminescence in the visible with either one- or two-photon excitation.8,9 Carbon dots compare favorably with the semiconductor QDs in many properties (carbon being a nontoxic element, no-blinking, etc.),8,9 but their brightness (emission quantum yields up to 15–20%) is still lower than that of the best-performing CdSe/ZnS core—shell dots. In this work, we found that carbon nanoparticles may be doped with inorganic salts such as ZnO or ZnS before their surface passivation by organic molecules to achieve much higher photoluminescence quantum yields. These new dots with a doped carbon core (Figure 1) are performance-wise competitive to the commercially available CdSe/ZnS dots, especially in aqueous solutions (where shortcomings of the CdSe/ZnS dots are known in the literature3b,11). The results suggest that small salt-doped carbon nanoparticles represent a new platform for quantum dotlike optical nanomaterials.
Figure 1.
Left: cartoon illustration on carbon dots with a doped carbon core (from an experimental HR-TEM image with ZnS lattice fringes circled). Right: aqueous solutions of CZnS-Dots and CZnO-Dots (450 nm excitation for both) compared with a commercial toluene solution of CdSe/ZnS dots (matching optical density at excitation), all photographed through a 475 nm cutoff filter.
Results and Discussion
Experimentally, carbon nanoparticles from laser ablation were processed in terms of the nitric acid treatment, dialysis, and then centrifugation to retain the supernatant, in which the suspended nanoparticles were generally less than 10 nm in size according to electron microscopy analyses. The doping of the carbon nanoparticles with ZnO or ZnS was achieved in an aqueous suspension of the nanoparticles with Zn(CH3COO)2 through hydrolysis with NaOH or precipitation with Na2S, respectively. For the former, the sample was thermally annealed to convert Zn(OH)2 to ZnO. No thermal annealing step was necessary for ZnS-doped carbon nanoparticles.
A sample (200 mg) containing either ZnO- or ZnS-doped carbon nanoparticles was dispersed in an aqueous solution of sodium dodecyl sulfate (1 wt %, 120 mL) via sonication for 30 min. Upon filtration, the filter cake was washed repeatedly with water, dried, and then mixed thoroughly with the diamine-polyethylene glycol H2NCH2(CH2CH2O)35CH2CH2CH2NH2 (PEG1500N, 1.9 g). The mixture was heated to 110 °C and stirred for 72 h under nitrogen protection. It was then cooled to room temperature and dispersed in water, followed by centrifuging at 25 000g to retain the supernatant. The reaction conditions were the same as those used previously in the functionalization of carbon nanotubes,12 where the PEG1500N amino groups and the carboxylic acid moieties on the oxidized carbon surface (still naked areas on ZnO- or ZnS-doped carbon nanoparticles) form zwitterion pairs.12,13 Additionally, there may also be strong PEG1500N adsorption on the particle surface, as also observed in the functionalized carbon nanotubes.
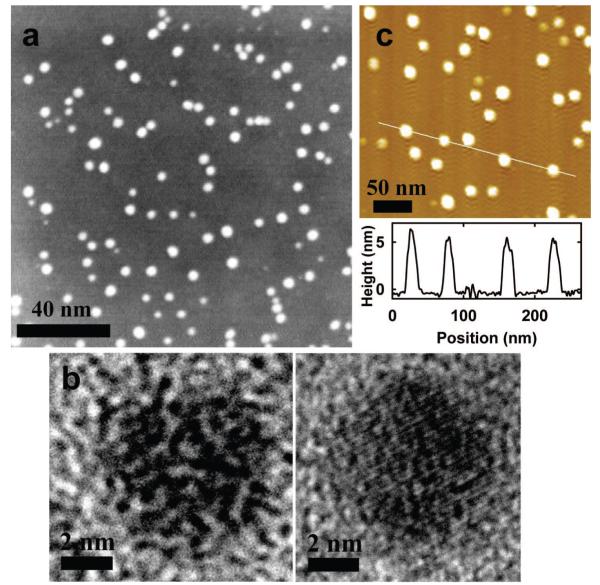
The carbon dots with ZnO- or ZnS-doped carbon cores (“CZnO-Dots” or “CZnS-Dots”, respectively) were characterized by using microscopy techniques. Shown in Figure 2 are the TEM images of CZnS-Dots, which suggest typical dot sizes around 4–5 nm. At a higher imaging resolution, the doping of a carbon particle with ZnS could be visualized (Figure 2 and also Figure 1). The results from energy dispersive X-ray (EDX) analyses of CZnS-Dots on a silicon grid confirmed the presence of C, Zn, and S. Also shown in Figure 2 are AFM images of CZnO-Dots on a mica substrate.
Figure 2.
(a) TEM (Z-contrast) images of CZnS-Dots. (b) High-resolution TEM images of individual carbon dots without doping (left) and with ZnS-doping (right, showing lattice fringes). (c) AFM topography images of CZnO-Dots on a mica substrate (and the height profile along the line).
Thermogravimetric analysis (TGA) measurements of the CZnO-Dots and CZnS-Dots samples were performed at 10 °C/min, first to 600 °C in nitrogen to remove the surface functional groups and then to 800 °C in air to oxidize the carbon core into carbon dioxide (purged out of the system). According to the TGA results, the core C:ZnO and C:ZnS ratios in CZnO-Dots and CZnS-Dots were approximately 3:1 and 2:1, respectively, in terms of weight (corresponding to 20:1 and 13:1, respectively, in molar ratios).
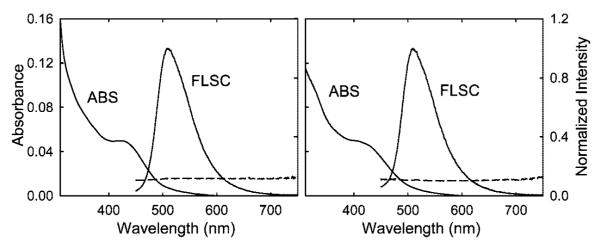
The absorption and luminescence emission spectra of CZnO-Dots and CZnS-Dots are rather similar (Figure 3). For both samples, the absorption spectra feature a shoulder in the blue region, where the absorptivities are on the order of 100 [(mole of core carbon atoms)/L]−1cm−1. The excitation into the absorption shoulder results in strong bluish green luminescence emissions (Figure 3 and also Figure 1). The observed emission quantum yields (440 nm excitation, quinine sulfate as the fluorescence standard) for CZnS-Dots in aqueous solution are consistently higher than 50% (varying somewhat from batch to batch, up to 15%, in about a dozen of repeated sample preparations). This is competitive to the performance of commercially available organic-based CdSe/ZnS core—shell dots (NN-LABS, LLC, Figure 1).
Figure 3.
Absorption (ABS) and luminescence emission (FLSC, 440 nm excitation, normalized against the peak intensity) spectra of CZnS-Dots (left) and CZnO-Dots (right) in aqueous solutions. As also shown for comparison, the carbon nanoparticles doped with ZnS or ZnO but without PEGs were not emissive (dashed lines, ×10 and offset by 0.1 for easier viewing).
The currently available CZnO-Dots in aqueous solution are slightly less luminescent than CZnS-Dots, with observed quantum yields around 45% (also varying somewhat from batch to batch, up to 15%, in repeated sample preparations).
Mechanistically, the photoluminescence in carbon dots has been attributed to passivated defects on the carbon particle surface acting as excitation energy traps, for which the covalently attached organic molecules serve as the passivation agents.8,9 While the role of ZnO or ZnS doping in the substantial enhancement of photoluminescence performance is not clear (no precedent to follow), we propose that the dopant may provide secondary yet more effective surface passivation in combination with the organic passivation agents. Results from repeated control experiments suggested that the functionalization of ZnO- or ZnS-doped carbon nanoparticles by the organic (PEG1500N) molecules is necessary for the observed very strong photoluminescence, as compared in Figure 3.
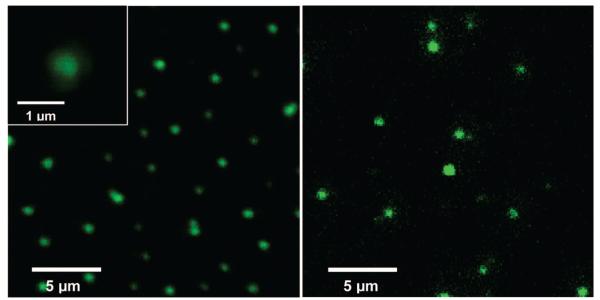
The CZnO-Dots and CZnS-Dots are both strongly luminescent under multiphoton excitation conditions, a property that they share with the original carbon dots.9 The two-photon excitation at 800 nm with a femtosecond pulsed laser (Spectra Physics Tsunami Ti-Sapphire) resulted in bright luminescence emissions in the visible region, which were generally similar to those observed with one-photon excitation at 458 nm (argon ion laser). For example, shown in Figure 4 are luminescence images of CZnS-Dots obtained on a confocal microscope (Leica DMIRE2 with TCS SP2 SE scanning system) with one- and two-photon excitations. Even with infinite dilution of the solution used in the preparation of the specimen, the resulting luminescence emissions from presumably individual dots could still be readily detected (Figure 4). These results suggest great potentials of these dots in one- and two-photon luminescence imaging applications.
Figure 4.
One- (left, 458 nm excitation) and two-photon (right, 800 nm excitation) luminescence images of the CZnS-Dots and that for the specimen from an infinitely diluted solution (left inset).
In summary, small carbon nanoparticles doped with inorganic salts apparently serve as a highly promising new platform in the development of quantum dotlike optical nanomaterials for imaging and other applications. The CZnO-Dots and CZnS-Dots in aqueous solutions are competitive to the commercially available organic-based CdSe/ZnS QDs in luminescence brightness. Beyond the blue—green regions, carbon dots with doped carbon cores for other colors are being pursued.
Experimental Section
Materials
Zinc acetate dihydrate and sodium sulfide were purchased from Alfa, sodium hydroxide from Aldrich, and the poly(ethylene glycol) diamine (∼35 repeating units for a molecular weight of 1500) from Fluka. N,N-Dimethylformamide and sodium dodecyl sulfate were supplied by Acros and VWR, respectively. Millipore Durapore membrane filters (0.22 μm, GV membrane) were obtained from Fisher Scientific, dialysis membrane tubing from Spectrum Laboratories, and carbon- and silicon-coated copper grids from Electron Microscopy Sciences. Water was deionized and purified by being passed through a Labconco WaterPros water purification system.
For the ZnS doping, the laser ablation-produced carbon nanoparticle sample8,9 (1 g) was refluxed in an aqueous nitric acid solution (2.6 M) for 12 h, neutralized via dialysis (membrane molecular weight cutoff ∼ 1000) against a large volume of fresh water, and then centrifuged at 1000g for 5 min. The supernatant was retained and evaporated to remove water. The recovered carbon nanoparticles (600 mg) were dispersed in DMF (200 mL) with the aid of ultrasonication (VWR model 250D) for 30 min. To the suspension was added zinc acetate dihydrate (680 mg, 3.1 mmol) under vigorous stirring, followed by slow dropwise addition of an aqueous Na2S solution (0.62 M, 5 mL) at room temperature. The mixture was centrifuged at 3000g, and the precipitate was retained and repeatedly washed with distilled water to obtain the ZnS-doped carbon nanoparticles (881 mg).
In the doping with ZnO, the same initial treatments of carbon nanoparticles were applied to obtain their dispersion in DMF (600 mg/200 mL). To the dispersion was added zinc acetate dihydrate (680 mg, 3.1 mmol) under vigorous stirring, followed by slow dropwise addition of an aqueous NaOH solution (1.25 M, 5 mL) at room temperature. The mixture was centrifuged at 3000g to discard the supernatant. The precipitate was repeatedly washed with water, evaporated to remove water, and then dried at 60 °C in a vacuum oven. The sample was annealed at 200 °C for 2h to obtain the ZnO-doped carbon nanoparticles (830 mg).
Measurements
Baxter Megafuge (model 2630) and Beckman-Coulter ultracentrifuge (Optima L90K with a type 90 Ti fixed-angle rotor) were used for low- and high-speed centrifugations, respectively. Thermogravimetric analysis (TGA) was performed on a TA Instruments Q500 TGA (up to 800 °C with air or nitrogen gas).
Transmission electron microscopy (TEM) imaging was carried out on a Hitachi HD-2000 S-TEM system and a Hitachi H-9500 TEM system. The same S-TEM system was used for the in situ energy dispersive X-ray spectroscopy (EDX) analysis. Atomic force microscopy (AFM) images were obtained in the acoustic AC mode on a Molecular Imaging PicoPlus system equipped with a multipurpose scanner and a NanoWorld Pointprobe NCH sensor. The height profile analysis was assisted by using the SPIP software distributed by Image Metrology.
UV/vis absorption spectra were recorded on a Shimadzu UV2101-PC spectrophotometer. Photoluminescence spectra were measured on a Spex Fluorolog-2 emission spectrometer equipped with a 450 W xenon source and a detector consisting of a Hamamatsu R928P photomultiplier tube operated at 950 V. A Leica laser scanning confocal fluorescence microscope (DMIRE2, with Leica TCS SP2 SE scanning system) was used for optical imaging and spectral measurements. The microscope was equipped with an argon ion laser (JDS Uniphase) and a femtosecond pulsed (∼100 fs at 80 MHz) Ti:Sapphire laser (Spectra-Physics Tsunami witha5W Millennia pump). An oil immersion objective lens (Leica X63/1.40) was used in both one- and two-photon imaging experiments. For the two-photon measurements, an external nondescanned detector (NDD) was used for higher signals.
Supplementary Material
Acknowledgment
Financial support from the NIH, the American Chemical Society Petroleum Research Fund, and the DoD Breast Cancer Research Program is gratefully acknowledged. The instrumentations used in this work were acquired with funding from the NSF.
Footnotes
Supporting Information Available: Figure S1. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- (1) (a).Colvin VL, Schlamp MC, Alivisatos AP. Nature. 1994;370:354–357. [Google Scholar]; (b) Alivisatos AP. Science. 1996;271:933–937. [Google Scholar]
- (2) (a).Hines MA, Guyot Sionnest, P. J. Phys. Chem. 1996;100:468–471. [Google Scholar]; (b) Peng XG, Schlamp MC, Kadavanich AV, Alivisatos AP. J. Am. Chem. Soc. 1997;119:7019–7029. [Google Scholar]; (c) Alivisatos AP. Nat. Biotechnol. 2004;22:47–52. doi: 10.1038/nbt927. [DOI] [PubMed] [Google Scholar]
- (3) (a).Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Medintz IL, Uyeda HT, Goldman ER, Mattoussi H. Nat. Mater. 2005;4:435–446. doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- (4) (a).Derfus AM, Chan WCW, Bhatia SN. Nano Lett. 2004;4:11–18. doi: 10.1021/nl0347334.Gao XH, Cui YY, Levenson RM, Chung LWK, Nie SM. Nat. Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994.Kirchner C, Liedl T, Kudera S, Pellegrino T, Javier AM, Gaub HE, Stolzle S, Fertig N, Parak WJ. Nano Lett. 2005;5:331–338. doi: 10.1021/nl047996m.Lovric J, Cho SJ, Winnik FM, Maysinger D. Chem. Biol. 2005;12:1227–1234. doi: 10.1016/j.chembiol.2005.09.008.It should be noted that toxicity is a complicated topic, and the reference here is only for the widely acknowledged fact that cadmium is a toxic heavy metal, irrespective of the extensive effort on minimizing or eliminating the effect in vivo. Carbon as an element is not intrinsically toxic.
- (5) (a).Wilson WL, Szajowski PF, Brus LE. Science. 1993;262:1242–1244. doi: 10.1126/science.262.5137.1242. [DOI] [PubMed] [Google Scholar]; (b) Li ZF, Ruckenstein E. Nano Lett. 2004;4:1463–1467. [Google Scholar]
- (6).Bharali DJ, Lucey DW, Jayakumar H, Pudavar HE, Prasad PN. J. Am. Chem. Soc. 2005;127:11364–11371. doi: 10.1021/ja051455x. [DOI] [PubMed] [Google Scholar]
- (7).Yu S-J, Kang M-W, Chang H-C, Chen K-M, Yu Y-C. J. Am. Chem. Soc. 2005;127:17604–17605. doi: 10.1021/ja0567081. [DOI] [PubMed] [Google Scholar]
- (8).Sun Y-P, Zhou B, Lin Y, Wang WJ, Fernando KAS, Pathak P, Meziani MJ, Harruff BA, Wang X, Wang H, Luo PJG, Yang H, Kose ME, Chen B, Veca LM, Xie SY. J. Am. Chem. Soc. 2006;128:7756–7757. doi: 10.1021/ja062677d. [DOI] [PubMed] [Google Scholar]
- (9).Cao L, Wang X, Meziani MJ, Lu FS, Wang HF, Luo PJG, Lin Y, Harruff BA, Veca LM, Murray D, Xie S-Y, Sun Y-P. J. Am. Chem. Soc. 2007;129:11318–11319. doi: 10.1021/ja073527l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10) (a).Zhou JG, Booker C, Li RY, Zhou XT, Sham TK, Sun XL, Ding ZF. J. Am. Chem. Soc. 2007;129:744–745. doi: 10.1021/ja0669070. [DOI] [PubMed] [Google Scholar]; (b) Liu HP, Ye T, Mao CD. Angew. Chem., Int. Ed. 2007;46:6473–6475. doi: 10.1002/anie.200701271. [DOI] [PubMed] [Google Scholar]; (c) Bourlinos AB, Stassinopoulos A, Anglos D, Zboril R, Karakassides M, Giannelis EP. Small. 2008;4:455–458. doi: 10.1002/smll.200700578. [DOI] [PubMed] [Google Scholar]; (d) Bourlinos AB, Stassinopoulos A, Anglos D, Zboril R, Georgakilas V, Giannelis EP. Chem. Mater. 2008;20:4539–4541. [Google Scholar]
- (11).Bakalova R, Zhelev Z, Aoki I, Kanno I. Nat. Photo. 2007;1:487–489. [Google Scholar]
- (12).Huang WJ, Fernando KAS, Allard LF, Sun Y-P. Nano Lett. 2003;3:565–568. [Google Scholar]
- (13).Chen J, Rao AM, Lyuksyutov S, Itkis ME, Hamon MA, Hu H, Cohn RW, Eklund PC, Colbert DT, Smalley RE, Haddon RC. J. Phys. Chem. B. 2001;105:2525–2528. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.