Abstract
Introduction
Quantitative fiber tracking with diffusion tensor imaging (DTI) provides a new approach for assessing deficits in the microstructural integrity of white matter circuits that may underlie cognitive deficits associated with conditions affecting white matter, including chronic alcoholism.
Methods
Alcoholic men and women (n=87) and healthy controls (n=88) performed the Digit Symbol (DS) test and underwent structural and diffusion tensor imaging. Measures of fractional anisotropy (FA) of fibers passing through genu and splenium were computed, as were size of genu and splenium fiber target regions of interest (ROI).
Results
Alcoholics scored lower than controls on the DS and had even greater deficits in genu than splenium fiber FA. In alcoholics, fiber FA of the genu selectively predicted DS scores after accounting for splenium FA. Neither fiber FA measure predicted incidental recall of the symbols used in the task. Size of genu and splenium ROI, although reduced in alcoholics, did not predict DS score or incidental recall.
Conclusions
Quantitative tractography of frontal fibers connecting left and right hemispheres selectively predicted performance by alcoholics on a coordinated psychomotor task and provide support for frontally based systems in Digit Symbol performance, both of which are compromised in recovering alcoholics.
Keywords: Fiber tractography, Diffusion tensor imaging, Corpus callosum, Alcoholism, Digit Symbol test, Genu, Splenium
Introduction
Demonstration of in vivo selective brain-behavior relationships, particularly in patients who have diffuse or partial rather than focal or complete brain lesions, is often elusive. Such a condition is alcoholism, in which the characteristic deficits in component processes of executive function, visuospatial function, and balance (Fein et al. 1990; Oscar-Berman 2000; Parsons et al. 1987; Sullivan et al. 2000; Tarter and Alterman 1984) are probably related to disruption of circuits that link fronto-cerebellar neural nodes (Sullivan 2003; Sullivan and Pfefferbaum 2005) and provide interhemispheric integration (Schulte et al. 2004) rather than to focal deficits in specific regions.
Diffusion tensor imaging (DTI) with quantitative tractography provides a powerful tool for assessing the integrity of specific white matter tracks and may be more useful than either quantitative measures of regional brain macrostructure or DTI measures of regional microstructural integrity for demonstrating selective brain-behavior relationships. DTI may be particularly sensitive in the identification of brain substrates of cognitive and motor tasks involving interhemispheric integration in alcoholism and other conditions characterized by partial lesions. A primary measure in DTI is fractional anisotropy (FA), which reflects the extent to which water molecules move in a common restricted orientation in white matter tracts, with higher values representing more consistency, and by implication greater integrity of its microstructural components, such as myelin. Quantitative tractography is a method that extends the use of FA, on an intervoxel basis, to visualize and quantify white matter connectivity, i.e., neural tracts, in the brain.
The corpus callosum, a complex band of white matter fibers linking homologous regions of left and right hemispheres (Gazzaniga 2005), is a key structure for integrating functions between the hemispheres. Interhemispheric connections have been demonstrated postmortem in humans (Aboitiz et al. 1992; Gross et al. 1977; Seltzer and Sherwin 1983) and in non-human primates (Pandya and Seltzer 1986). Recent in vivo studies using DTI and white matter FA orientational color maps and fiber tractography (Abe et al. 2004; Hofer and Frahm 2006; Huang et al. 2005; Park et al. 2007; Sullivan et al. 2006) are consistent with postmortem studies in suggesting that fibers of the genu, the anterior portion of the corpus callosum, connect left and right ventral prefrontal cortex and parts of the dorsal prefrontal cortex, whereas fibers of the splenium, the posterior portion of the corpus callosum, connect bilaterally distributed sites of the temporal, parietal, and occipital cortices (Pandya and Seltzer 1986). Thus, complete or partial lesions at the anterior extent of the corpus callosum could affect the execution of bilaterally distributed executive functions, whereas posterior callosal lesions could affect the transfer of visuospatial information between hemispheres.
Patients with alcoholism have compromised callosal morphology variously described in vivo and postmortem as “thinning” or reduced area or volume compared with age-matched controls (Cardenas et al. 2007; Estruch et al. 1997; Harper and Kril 1990; Hommer et al. 1996; Lee et al. 2005; Oishi et al. 1999; Pfefferbaum et al. 1996, 2006a). Recent studies quantifying the microstructural integrity of callosal white matter have found DTI to be more sensitive than conventional MRI in detecting callosal compromise in alcoholic women (Pfefferbaum and Sullivan 2002). Other DTI studies of patients with a history of chronic heavy alcohol use have noted a greater predilection for compromised integrity in frontal than posterior callosal regions (Pfefferbaum and Sullivan 2002; Pfefferbaum et al. 2000). Furthermore, a twin study showed that while genetic influences on regional morphology are similar from anterior to posterior extent, white matter integrity is more susceptible to environmental than genetic influences in the anterior portion than in the posterior portion (Pfefferbaum et al. 2001). DTI thus enhances the ability to differentiate regional sensitivity of the corpus callosum to the environmental toxin of heavy alcohol consumption.
Both macrostructural size and microstructural DTI measures of the anterior genu of the corpus callosum based on a region-of-interest approach have been associated with age or alcohol related impaired performance on several frontally mediated executive tasks, including Trail Making and Symbol Digit tests (Jokinen et al. 2007), episodic memory retrieval time (Bucur et al. 2007), and interhemispheric transfer performance (Schulte et al. 2005). These findings support the functional significance of the topographic organization of the corpus callosum. Few studies (Pfefferbaum et al. 2007; Sullivan et al. 2006), however, have used quantitative fiber tracking to test for associations and dissociations of selective brain-behavioral relations that reflect interhemispheric integration.
The Digit Symbol (DS) subtest of the WAIS-R (Wechsler 1981) has been widely used as a test of psychomotor performance and visual perception in neuropsychological research and clinical settings because of its sensitivity to brain insult from trauma, neuropsychiatric disease, and environmental toxins including alcohol (Lezak 1995). Patients with alcoholism are commonly impaired on the DS (Beatty et al. 2000; Davies et al. 2005; Harris et al. 2003; Hochla et al. 1982; Sullivan et al. 2002a; Sullivan et al. 2000), as are many patients with any brain dysfunction. Although DS was not designed as an interhemispheric transfer task, coordination of the executive, visuospatial, motor, and mnemonic processes is required to perform the DS test successfully (Glosser et al. 1977; Joy et al. 2000, 2003a, b; Kaplan et al. 1991) and likely invokes frontally-based systems and interhemispheric communication. In support of this possibility, we recently reported that executive abilities contributed over and above the contribution of visuospatial, motor, and mnemonic function to prediction of DS scores in patients with alcoholism (Sassoon et al. 2007).
In the present study, we analyzed the contribution of fiber tractography FA of fiber bundles passing through the genu and splenium into their left and right hemisphere cortical targets to performance on the standard DS test. For comparison, we also examined the contribution of these brain measures to performance on an incidental recall test of the symbols. This comparison task was used because it permits examination of a non-frontally based function using the same material as used for the target coordinated psychomotor test. Based on our earlier analysis of factors contributing to DS performance (Sassoon et al. 2007), we tested the hypothesis that integrity of fibers crossing at the genu would contribute more to DS performance than integrity of fibers crossing at the splenium. By contrast, we hypothesized that neither genu nor splenium fiber integrity would contribute selectively to incidental recall.
Material and methods
Participants
We combined data from alcoholic and control participants from two studies in our laboratory on the effects of chronic alcoholism on brain structure and cognitive and motor abilities. The first study involved only alcoholics and controls (46 alcoholics, 50 controls). The second study provided an additional 41 patients with alcoholism and 38 controls. Neuroimaging data from the first study (Pfefferbaum et al. 2006a, b; Schulte et al. 2005) and a sample combining subjects from both studies (Pfefferbaum et al. 2006c, 2007; Schulte et al. 2007) have appeared in other reports. Extensive clinical and demographic descriptions and analysis of the first (Rosenbloom et al. 2005) and second (Rosenbloom et al. 2007) cohorts are also provided elsewhere. A summary of the demographic characteristics of the 175 men and women in the current analysis appears in Table 1.
Table 1.
Demographic and clinical characteristics of study participants
| Descriptor | Control (n=88) |
Alcoholic (n=87) |
p Value | ||||
|---|---|---|---|---|---|---|---|
| Ratio | Mean | SD | Ratio | Mean | SD | ||
| Men/Women | 42/46 | 59/28 | 0.007 | ||||
| Ethnicitya (Caucasian/minority) | 54/34 | 64/23 | 0.09 | ||||
| Current or prior smoker | 25% | 73% | 0.0001 | ||||
| Age (years) | 44.5 | 9.9 | 46.6 | 9.0 | 0.15 | ||
| Socioeconomic statusb | 26.5 | 13.0 | 30.7 | 13.2 | 0.042 | ||
| Years of education | 15.8 | 2.3 | 14.2 | 2.3 | 0.0001 | ||
| Handedness (Crovitz)c | 24.4 | 13.7 | 26.2 | 15.1 | 0.38 | ||
| Body mass index | 25.8 | 4.7 | 26.1 | 4.4 | 0.71 | ||
| GAF rating (from SCID) | 81.6 | 8.9 | 55.6 | 11.5 | 0.0001 | ||
| BDI-II scored | 2.6 | 2.9 | 9.8 | 8.1 | 0.0001 | ||
| Lifetime alcohol (kg) | 42.0 | 60.6 | 854.4 | 770.6 | 0.0001 | ||
| Length sober (days) | – | – | 113.9 | 154.0 | |||
Caucasian non-Hispanic vs Hispanic plus other Minorities
Hollingshead 2-factor score, (higher score=lower status)
Twelve alcoholics and six controls were left handed (scored>50)
Fifteen alcoholics and no controls scored over 14 (mildly depressed)
Men and women with alcoholism were recruited by referral from several San Francisco Bay Area outpatient substance abuse treatment centers. Control subjects were recruited by referral from patient participants, by Internet posting, newspaper advertisements, flyers and word of mouth. Referrals and inquiries were followed up with a brief screening interview designed to identify subjects who would be ineligible for the study by virtue of a diagnosis of schizophrenia, bipolar disorder, neurological disease not related to alcohol use, or inability to undergo MRI. Those who met initial criteria were invited for a more detailed assessment at our laboratory (Cohort 1) or the AIDS Community Research Consortium (ACRC; Cohort 2), where a trained nurse informed them about the full scope of the study and obtained informed consent.
Clinical evaluation
All alcoholic patients and controls underwent a series of structured interviews designed to characterize alcohol history and other pertinent medical and psychiatric information. Admission to or exclusion from the study was based on the Structured Clinical Interview for DSM-IV (SCID) (First et al. 1998), administered by clinicians. Any prospective subject who met lifetime criteria for schizophrenia or bipolar disorder or for nonalcohol substance dependence or abuse within the prior 3 months was excluded, as were prospective controls that met DSM-IV criteria for any other Axis I disorder. All subjects classified as alcoholics met DSM-IV criteria for alcohol dependence. The SCID was also used to rate functioning using the Global Assessment of Functioning (GAF) scale (Endicott et al. 1976). All subjects were HIV negative either by self-reported medical history (Cohort 1) or by blood test (cohort 2). A history of alcohol consumption (Pfefferbaum et al. 1992; Skinner 1982; Skinner and Sheu 1982) yielded quantitative lifetime consumption of alcohol and time since last drink. Interviews and questionnaires assessed current depression symptoms using the Beck Depression Inventory—II (Beck et al. 1996); socioeconomic status (SES) using a two-factor scale based on education and occupation (Hollingshead and Redlich 1958); handedness (Crovitz and Zener 1962); history of smoking (current, past or never), and body mass index (height, cm/weight kg2), an index of nutritional status. Statistics of these and other demographic values are presented in Table 1.
MRI acquisition and processing
Magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) protocols were used to acquire brain data on a General Electric 1.5T system. Methods of acquisition and analysis were described in detail in our previous report (Pfefferbaum et al. 2007), and are summarized briefly here. MRI data were acquired in the coronal plane and included: (1) a dual-echo fast spin echo (FSE) sequence (47 contiguous, 4 mm thick slices; TR/TE1/TE2=7,500/14/98 ms; matrix=256 × 192); and (2) a SPoiled Gradient Recalled Echo (SPGR) sequence (94 2 mm thick slices; TR/TE=25/5 ms, flip angle=30°, matrix=256 × 192) to provide neuroanatomical context for the DTI data. DTI was also acquired in the coronal plane with the same slice location parameters as the dual-echo FSE, using a single shot spin-echo echo-planar imaging technique with a 24 cm field of view (47 contiguous, 4 mm thick slices, TR/TE=10,000/103 ms, matrix=128 × 128, in-plane resolution=1.875 mm2). Diffusion was measured along six noncollinear directions with alternating signs to minimize the need to account for cross terms between imaging and diffusion gradients. Six images were acquired and averaged for each gradient direction. The coronal MRI and DTI acquisitions produced either 2 or 4 mm thick slices and were prescribed for consistent slice locations so that each 4-mm slice encompassed a pair of 2 mm thick slices. To align the structural and DTI images, the dual-echo FSE images were passed through the FSL Brain Extraction Tool (BET; Smith 2002) to extract the brain and exclude dura, skull, scalp and other non-brain tissue. Eddy-current correction preceded alignment of DTI data with FSE data using a non-linear 3D warp (Woods et al. 1998).
On DTI images, the apparent diffusion coefficient was mapped, and the general diffusion tensor was then diagonalized, yielding eigenvalues λ1, λ2, λ3 as well as eigenvectors that define the predominant diffusion orientations. Based on these eigenvalues, fractional anisotropy (FA) was calculated on a voxel-by-voxel basis yielding new images for analysis after alignment with the anatomical images.
Identification of the corpus callosum and its sectors
Alignment enabled anatomical identification of the corpus callosum in a common space for each subject from FA images. The corpus callosum was identified on the midsagittal slice extracted from the FA data with a semi-automated edge identification procedure with high interrater reliability (r=0.99; Schulte et al. 2004; Sullivan et al. 2002b). Genu and splenium regions of interest (ROI) were defined geometrically by projecting radii at +15° (genu + rostrum) and +160° (splenium) from the midpoint of a plane connecting the anterior extreme of the genu and the posterior extreme of the splenium and computing the area of these regions.
Quantitative fiber tracking of the genu and splenium
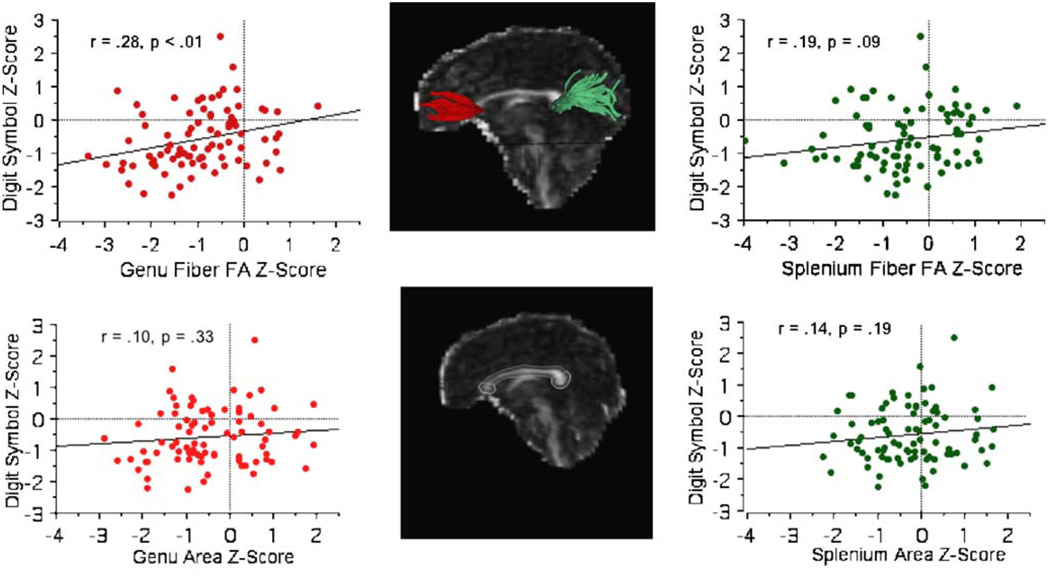
The genu and splenium ROIs served as targets for fiber tracking. The fiber sources were parallel planes perpendicular to the midsagittal plane and located 10 mm anterior to the genu target and 10 mm posterior to the splenium target on the aligned FA data. Targets and sources were then transformed back to each subject’s coordinates on the native unwarped DTI image and fiber tracking performed for each callosal region of interest separately, using software distributed by Gerig and colleagues (Gerig et al. 2005) based on the method of Mori and colleagues (Mori and van Zijl 2002; Xu et al. 2002; Xue et al. 1999). This process identified individual fibers for which the FA of each voxel was determined. Quantification yielded the number and mean length of fibers coursing through either genu or splenium and mean FA for each “fiber system.” After fiber detection the fiber locations were transformed back to common coordinates for display (Fig. 1).
Fig. 1.
Center column Fiber tracking from genu and splenium (upper panel) and area of genu and splenium (lower panel) for a 33-year-old alcoholic woman. Values for all alcoholics for genu fiber FA (upper left), genu target region of interest (ROI; lower left), splenium fiber FA (upper right), and splenium ROI (lower right) plotted against performance on the Digit Symbol test. All values are normalized against a control sample. Values above zero for each measure represent individuals whose behavior or brain values were better than would be expected for their age, education or head size. Values below zero for each measure represent individuals whose behavior or brain values were worse than would be expected for their age, education or head size. The simple regression line is also plotted
Behavioral testing
Digit Symbol (DS) subtest of the WAIS-R (Wechsler 1981)
Participants were presented with 93 randomly assigned digits from one to nine, each in a box with a blank box below and were required to fill in the blank box by pairing each digit with a symbol from a key displayed above the grid. The score was the number of boxes correctly completed in 90 s. As previously reported (Sassoon et al. 2007), subjects also completed the entire grid and following a 30 s delay, they were given a sheet containing the nine digits and instructed to fill in the matching symbol for each number from memory.
Statistical analysis
Group differences for brain and behavioral data were assessed with repeated measures analysis of variance (ANOVA) and t-tests. Relations between variables were tested with Pearson product-moment correlations (r). When the association between measures of either genu or splenium and a behavioral measure was significant, multiple regression analyses were used to examine the relative contribution of genu and splenium measures to predict these cognitive scores. For group comparisons α=.05.
To control for the normal effects of age on white matter fiber integrity (Sullivan et al. 2006), fiber FA measures were converted to age-corrected Z scores, using age effects detected in the larger control sample (n=120), as described in an earlier report (Pfefferbaum et al. 2007). Genu and splenium areas were converted to both age and brain size corrected Z scores, while DS and incidental recall scores were converted to age- and education-corrected Z scores based on the performance of the normal control sample.
Results
Group differences in number and length of fibers, fiber FA, and region size
Raw scores for number and mean length of fibers are summarized in Table 2. Significantly more fibers were identified in the splenium than the genu bundle, with alcoholics showing a trend for fewer fibers than controls. There was no group by region interaction. On average, fibers in the splenium bundle were longer than those in the genu bundle. Groups did not differ in fiber length, but alcoholics had longer fibers in the splenium whereas controls had longer fibers in the genu.
Table 2.
Mean, SD and SE for number and mean length of fibers in bundles identified at genu and splenium with results for repeated measures analysis of variance (ANOVA) for group (control and alcoholic) and region (genu and splenium)
| Genu |
Splenium |
Effect | F | p Value | |||
|---|---|---|---|---|---|---|---|
| Control | Alcohol | Control | Alcohol | ||||
| Number of fibers | |||||||
| Mean | 98.61 | 84.84 | 178.66 | 176.68 | Group | 3.61 | 0.0592 |
| SD | 28.22 | 33.10 | 43.48 | 38.54 | Region | 571.8 | 0.0001 |
| SE | 3.01 | 3.55 | 4.64 | 4.13 | Gp × region | 2.69 | 0.1025 |
| Mean fiber length (mm) | |||||||
| Mean | 6.938 | 6.731 | 8.76 | 8.858 | Group | 0.471 | 0.49 |
| SD | 0.713 | 0.638 | 0.626 | 0.371 | Region | 868.7 | 0.0001 |
| SE | 0.077 | 0.068 | 0.068 | 0.075 | Gp × region | 5.30 | 0.02 |
Both raw and age-corrected Z scores for fiber FA and age and intracranial volume corrected size of the target ROIs are summarized in Table 3. Statistics were performed on the Z scores. Fiber FA was significantly lower in the alcohol group than controls, and the deficit was disproportionately greater in the genu than the splenium. Genu and splenium size were both reduced in alcoholics relative to the controls, with greater deficits at the genu (−0.46 SD) than the splenium (−0.21 SD) but the group-by-region interaction was not significant. ANOVAs for group and sex for fiber FA or size of each region revealed no significant effects for sex or group-by-sex interactions.
Table 3.
Mean, SD and SE for raw and Z scores of fiber bundle FA and regional size with results for repeated measures analysis of variance (ANOVA) for group (control and alcoholic) and target region (genu and splenium)
| Genu |
Splenium |
Z Score effect | F | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control |
Alcohol |
Control |
Alcohol |
||||||||
| Raw Score | Z score | Raw Score | Z score | Raw Score | Z score | Raw Score | Z score | ||||
| Target ROI size (mm2) | |||||||||||
| Mean | 54.99 | 0.00 | 51.07 | −0.457 | 89.78 | 0.00 | 97.12 | −0.210 | Group | 6.22 | 0.0136 |
| SD | 8.31 | 0.994 | 9.23 | 1.128 | 12.31 | 0.994 | 13.55 | 1.083 | Region | 2.05 | 0.154 |
| SE | 0.886 | 0.106 | 0.99 | 0.121 | 1.31 | 0.106 | 1.45 | 0.116 | Gp × region | 2.08 | 0.151 |
| Fiber bundle FA | |||||||||||
| Mean | 0.46 | −0.022 | 0.43 | −0.938 | 0.56 | 0.008 | 0.54 | −0.526 | Group | 29.676 | 0.0001 |
| SD | 0.003 | 0.985 | 0.003 | 0.977 | 0.003 | 1.039 | 0.003 | 1.087 | Region | 7.831 | 0.0057 |
| SE | 0.004 | 0.105 | 0.004 | 0.105 | 0.003 | 0.111 | 0.003 | 0.117 | Gp × region | 5.868 | 0.0164 |
Group differences in DS and incidental recall performance
Alcoholics performed more poorly than controls on the DS test but did not show deficits in incidental recall of the symbols (see Table 4). ANOVAs for group and sex revealed that women as a whole performed better than men on the DS test (F(1,163)=24.39, p<0.0001), but there was no sex by diagnosis interaction.
Table 4.
Mean, SD and SE for raw and Z scores of Digit Symbol scaled score and incidental recall score and group differences for Z scores
| Control |
Alcohol |
Effect | F | p Value | |||
|---|---|---|---|---|---|---|---|
| Raw score | Z score | Raw score | Z score | ||||
| Digit Symbol test score | |||||||
| Mean | 9.6 | 0.000 | 7.8 | −0.568 | Group | 15.16 | 0.0001 |
| SD | 2.6 | 0.994 | 2.4 | 0.891 | |||
| SE | 0.3 | 0.11 | 0.3 | 0.097 | |||
| Incidental recall score | |||||||
| Mean | 6.8 | 0.000 | 6.4 | −0.18 | Group | 1.26 | 0.26 |
| SD | 1.9 | 0.994 | 2.1 | 1.06 | |||
| SE | 0.2 | 0.112 | 0.2 | 0.115 | |||
Other contributing factors to callosal fiber or DS measures
Greater lifetime alcohol consumption among the alcoholics modestly correlated with lower DS score (r=−0.297, p<0.006) but not with fiber FA of the genu or splenium. Length of sobriety before testing was not associated with DS, incidental recall, or fiber FA of genu or splenium. Alcoholic patients were significantly more likely (73%) to have ever been a cigarette smoker than controls (25%; see Table 1). However, among alcoholics, ANOVA for the factor of nicotine use (never, past, current) did not show a significant effect on fiber FA at either splenium (F(2,83)=0.61, p=0.54) or genu (F(2,83)=0.89, p=0.41). Smoking also had no effect on DS performance among the alcoholics (F(2,83)=0.42, p=0.65) and did not account for group differences in DS performance; ANOVA for diagnosis and nicotine use revealed no effect for nicotine use or a diagnosis-by-nicotine use interaction. Half of the alcoholic sample reported remote (median=3 years) prior abuse or dependence on other substances. However, former-users of other substances were not significantly different from never-users on DS performance or fiber FA at genu or splenium.
Fiber tractography and ROI size predictors of performance
Simple regressions analyzed genu and splenium measures separately as associates of performance. Where significant associations were found, both brain measures were entered into multiple regressions to see if one measure persisted as a predictor of behavior after accounting for the contribution of the other. This analysis does not necessarily imply causality or a longitudinal process.
Among the alcoholics, Pearson product-moment correlations showed that higher DS scores were significantly associated with higher fiber FA in the genu but not the splenium. A similar pattern of results was found for the absolute number of fibers in the genu and splenial bundles (Table 5 and Fig. 1). By contrast neither genu nor splenium size was associated with DS score in the control group. Simple and multiple regression results for fiber FA are presented in Table 5 and indicated that FA in both these fiber bundles together accounted for 8% of the variance in performance and the contribution of the genu persisted after accounting for that of the splenium. This genu effect was also seen in alcoholic men (n=59) and women (n=28) examined alone but was significant only in the larger sample of men.
Table 5.
Simple with follow-up multiple regressions for genu and splenium fiber bundle FA and fiber number as predictors of Digit Symbol (DS) and incidental recall tests
| Genu |
Splenium |
r 2 | F | p Value | |||
|---|---|---|---|---|---|---|---|
| Control | Alcohol | Control | Alcohol | ||||
| DS test | |||||||
| Fiber bundle FA | |||||||
| Simple regression r value | 0.14 | 0.29** | 0.01 | 0.19 | |||
| Multiple regression t value | 2.03* | 0.39 | 0.08 | 3.61 | 0.03 | ||
| Multiple regression beta coefficient | 0.255 | 0.049 | |||||
| Number of fibers | |||||||
| Simple regression r value | 0.06 | 0.29** | 0.18 | 0.01 | |||
| Multiple regression t value | 2.81** | −0.51 | 0.09 | 3.97 | 0.03 | ||
| Multiple regression beta coefficient | 0.30 | −0.06 | |||||
| Incidental recall test | |||||||
| Fiber bundle FA | |||||||
| Simple regression r value | 0.01 | 0.16 | 0.05 | 0.12 | |||
p<0.05
p<0.01
Neither fiber FA nor size of the genu or splenium ROIs was significantly associated with incidental recall of DS symbols in simple regressions in either alcoholics or controls.
Discussion
This study provides evidence for a selective relation between DS performance and fiber tract integrity of the genu of the corpus callosum—specifically, FA of fiber tracking targeted at the genu predicted coordinated psychomotor performance in chronic alcoholics after accounting for the contribution from FA of fiber bundles coursing through the splenium. This selective relationship demonstrates a predominantly frontal contribution to the DS test, a visually based psychomotor task, requiring speeded eye-hand coordinated actions. By contrast, although genu and splenium of the corpus callosum are reduced in size (based on the size of the target ROIs) in patients with alcoholism, neither region size was associated with performance on the DS test.
Higher FA represents more consistency in water molecule diffusion, and by implication greater integrity of local white matter. The resolution at which these DTI data were acquired, however, precludes direct axonal measurement. Nonetheless, the fibers identified by the tractography software as passing through the genu putatively link the left and right ventral prefrontal cortex and parts of the dorsal prefrontal cortex, whereas fibers passing through the splenium putatively link regions of left and right parietal, temporal and occipital cortices. Thus, a positive association of FA in the genu bundle, reflecting the microstructural integrity of those white matter fibers, with DS performance in alcoholics is consistent with behavioral evidence for a strong executive component to successful DS task performance (Sassoon et al. 2007). Even though FA in splenium fibers showed some association with DS task performance in alcoholics, consistent with the visual scanning and associative learning skills required for this task, it did not contribute independently. Although the DS certainly requires simple psychomotor skills—tracking and filling in the boxes—our analysis suggests that performance also benefits from higher order skills, such as directed attention. Performance on the incidental recall task was not impaired in the alcoholic patients and, as predicted, was not associated with either genu or splenium fiber bundle FA. The contrast between fiber tractography and regional size measures as predictors of behavior suggests the possibility that integrity and number of fibers reaching out into the cortical areas from the corpus callosum, rather than size of the section of corpus callosum from which they originate better predict performance.
A limitation of this study is that the DS task was the only behavioral task available across this entire subject sample with which to assess the functional ramifications of impaired white matter fiber integrity in genu and splenium of the corpus callosum. The two measures yielded by this task—number of boxes completed in 90 s and score on incidental recall of the symbols—were, nonetheless, successful in showing differential associations with genu and splenium.
As expected, and regardless of diagnosis, women as a group performed the DS test better than men (Snow and Weinstock 1990), but there was no evidence to suggest that group differences in performance were an artifact of the fact that there were a larger proportion of women in the control sample than in the alcoholic sample. Furthermore, among the alcoholics, similar patterns of association between DS performance and genu fiber FA were observed in both men and women, albeit with a stronger effect in the larger sample of men.
Functional ramifications of the topographic organization of the corpus callosum in humans have been studied by administering cognitive tests to patients with callosal lesions resulting from relatively discrete trauma (e.g., Peru et al. 2003) to more extensive surgically induced lesions (e.g., Funnell et al. 2000; Gazzaniga 2005). Such studies have illuminated complex patterns of interhemispheric communication as well as compensatory mechanisms that develop when the normal functional connections either fail to develop or are damaged. Recent brain imaging studies using DTI are revealing not only the integrity of the corpus callosum and its classically defined subsections, but are redefining those sections in terms of the source and target of white matter fiber tracts that course through them (Hofer and Frahm 2006). The application of quantitative tractography also permits the investigation of incomplete lesions in the corpus callosum, such as those associated with chronic alcohol use, and provides evidence that impaired integrity of white matter tracks coursing through the genu, the anterior portion of the corpus callosum, contributes to impaired performance on tasks such as the DS that rely on interhemispheric coordination of frontal lobe processes. As new analysis approaches permit greater specificity in identifying and quantifying the integrity of white matter circuits linking not only left and right hemispheres but also cortical-subcortical and anterior-posterior circuits they will allow further investigations of the neural underpinnings of a wide range of cognitive processing.
Acknowledgment
This study was supported by the National Institute on Alcohol Abuse and Alcoholism (AA10723, AA12388, AA05965).
Contributor Information
Margaret J. Rosenbloom, Neuroscience Program, SRI International, Menlo Park, CA, USA Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, 401 Quarry Road, Stanford, CA 94305-5723, USA.
Stephanie A. Sassoon, Neuroscience Program, SRI International, Menlo Park, CA, USA
Rosemary Fama, Neuroscience Program, SRI International, Menlo Park, CA, USA.
Edith V. Sullivan, Email: edie@stanford.edu, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, 401 Quarry Road, Stanford, CA 94305-5723, USA.
Adolf Pfefferbaum, Neuroscience Program, SRI International, Menlo Park, CA, USA; Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, 401 Quarry Road, Stanford, CA 94305-5723, USA.
References
- Abe O, Masutani Y, Aoki S, Yamasue H, Yamada H, Kasai K, et al. Topography of the human corpus callosum using diffusion tensor tractography. Journal of Computer Assisted Tomography. 2004;28:533–539. doi: 10.1097/00004728-200407000-00016. [DOI] [PubMed] [Google Scholar]
- Aboitiz F, Scheibel A, Fisher R, Zaidel E. Fiber composition of the human corpus callosum. Brain Research. 1992;598:143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- Beatty W, Tivis R, Stott H, Nixon S, Parsons O. Neuropsychological deficits in sober alcoholics: Influences of chronicity and recent alcohol consumption. Alcoholism: Clinical and Experimental Research. 2000;24:149–154. [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory—II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, et al. Age-related slowing of memory retrieval: Contributions of perceptual speed and cerebral white matter integrity. Neurobiology of Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.02.008. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. NeuroImage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crovitz HF, Zener KA. Group test for assessing hand and eye dominance. American Journal of Psychology. 1962;75:271–276. [PubMed] [Google Scholar]
- Davies SJ, Pandit SA, Feeney A, Stevenson BJ, Kerwin RW, Nutt DJ, et al. Is there cognitive impairment in clinically ‘healthy’ abstinent alcohol dependence? Alcohol and Alcoholism. 2005;40:498–503. doi: 10.1093/alcalc/agh203. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- Estruch R, Nicolas JM, Salamero M, Aragon C, Sacanella E, Fernandez-Sola J, et al. Atrophy of the corpus callosum in chronic alcoholism. Journal of the Neurological Sciences. 1997;146:145–151. doi: 10.1016/s0022-510x(96)00298-5. [DOI] [PubMed] [Google Scholar]
- Fein G, Bachman L, Fisher S, Davenport L. Cognitive impairments in abstinent alcoholics. Western Journal of Medicine. 1990;152:531–537. [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders (SCID) version 2.0. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1998. [Google Scholar]
- Funnell MG, Corballis PM, Gazzaniga MS. Insights into the functional specificity of the human corpus callosum. Brain. 2000;123(Pt 5):920–926. doi: 10.1093/brain/123.5.920. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. Forty-five years of split-brain research and still going strong. Nature Reviews Neuroscience. 2005;6:653–659. doi: 10.1038/nrn1723. [DOI] [PubMed] [Google Scholar]
- Gerig G, Corouge I, Vachet C, Krishnan KR, MacFall JR. Quantitative analysis of diffusion properties of white matter fiber tracts: A validation study; Paper presented at the 13th Proceedings of the International Society for Magnetic Resonance in Medicine; Miami, FL. 2005. [Google Scholar]
- Glosser G, Butters N, Kaplan E. Visuoperceptual processes in brain damaged patients on the digit symbol substitution test. International Journal of Neuroscience. 1977;7:59–66. doi: 10.3109/00207457709147202. [DOI] [PubMed] [Google Scholar]
- Gross CG, Bender DB, Mishkin M. Contributions of the corpus callosum and the anterior commissure to visual activation of inferior temporal neurons. Brain Research. 1977;131:227–239. doi: 10.1016/0006-8993(77)90517-0. [DOI] [PubMed] [Google Scholar]
- Harper CG, Kril JJ. Neuropathology of alcoholism. Alcohol and Alcoholism. 1990;25:207–216. doi: 10.1093/oxfordjournals.alcalc.a044994. [DOI] [PubMed] [Google Scholar]
- Harris CR, Albaugh B, Goldman D, Enoch MA. Neurocognitive impairment due to chronic alcohol consumption in an American Indian community. Journal of Studies on Alcohol. 2003;64:458–466. doi: 10.15288/jsa.2003.64.458. [DOI] [PubMed] [Google Scholar]
- Hochla NA, Fabian MS, Parsons OA. Brain-age quotients in recently detoxified alcoholic, recovered alcoholic and nonalcoholic women. Journal of Clinical Psychology. 1982;38:207–212. doi: 10.1002/1097-4679(198201)38:1<207::aid-jclp2270380135>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited—comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Hollingshead A, Redlich F. Social class and mental illness. New York: Wiley; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Rawlings R, Ragan P, Williams W, Rio D, et al. Decreased corpus callosum size among alcoholic women. Archives of Neurology. 1996;53:359–363. doi: 10.1001/archneur.1996.00550040099019. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhang J, Jiang H, Wakana S, Poetscher L, Miller MI, et al. DTI tractography based parcellation of white matter: Application to the mid-sagittal morphology of corpus callosum. NeuroImage. 2005;26:195–205. doi: 10.1016/j.neuroimage.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Jokinen H, Ryberg C, Kalska H, Ylikoski R, Rostrup E, Stegmann MB, et al. Corpus callosum atrophy is associated with mental slowing and executive deficits in subjects with age-related white matter hyperintensities: the LADIS Study. Journal of Neurology, Neurosurgery, and Psychiatry. 2007;78:491–496. doi: 10.1136/jnnp.2006.096792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy S, Fein D, Kaplan E. Decoding digit symbol: speed, memory, and visual scanning. Assessment. 2003a;10:56–65. doi: 10.1177/0095399702250335. [DOI] [PubMed] [Google Scholar]
- Joy S, Fein D, Kaplan E, Freedman M. Speed and memory in WAIS-R-III digit symbol performance among healthy older adults. Journal of the International Neuropsychological Society. 2000;6:770–780. doi: 10.1017/s1355617700677044. [DOI] [PubMed] [Google Scholar]
- Joy S, Kaplan E, Fein D. Digit symbol–incidental learning in the WAIS-III: Construct validity and clinical significance. Clinical Neuropsychology. 2003b;17:182–194. doi: 10.1076/clin.17.2.182.16495. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Fein D, Morris R, Delis DC. WAIS-R as a neuropsychological instrument. New York: The Psychological Corporation; 1991. [Google Scholar]
- Lee ST, Jung YM, Na DL, Park SH, Kim M. Corpus callosum atrophy in Wernicke's encephalopathy. Journal of Neuroimaging. 2005;15:367–372. doi: 10.1177/1051228405278352. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. 3rd ed. New York: Oxford University Press; 1995. [Google Scholar]
- Mori S, van Zijl PC. Fiber tracking: Principles and strategies—A technical review. NMR in Biomedicine. 2002;15:468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- Oishi M, Mochizuki Y, Shikata E. Corpus callosum atrophy and cerebral blood flow in chronic alcoholics. Journal of Neurological Sciences. 1999;162:51–55. doi: 10.1016/s0022-510x(98)00279-2. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M. Neuropsychological vulnerabilities in chronic alcoholism. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA’s neuroscience and behavioral research portfolio, NIAAA research monograph no. 34. Bethesda, MD: National Institutes of Health; 2000. pp. 437–472. [Google Scholar]
- Pandya DN, Seltzer B. The topography of commissural fibers. In: Lepore F, Ptito M, Jasper HH, editors. Two hemispheres—one brain: Functions of the corpus callosum. New York: Alan R. Liss, Inc.; 1986. pp. 47–74. [Google Scholar]
- Park H-J, Kim JJ, Lee S-K, Seok JH, Chun J, Kim DI, et al. Corpus callosal connection mapping using cortical gray matter parcellation and DT-MRI. Human Brain Mapping. 2007 doi: 10.1002/hbm.20314. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons OA, Butters N, Nathan PE, editors. Neuropsychology of alcoholism: Implications for diagnosis and treatment. New York: Guilford; 1987. [Google Scholar]
- Peru A, Beltramello A, Moro V, Sattibaldi L, Berlucchi G. Temporary and permanent signs of interhemispheric disconnection after traumatic brain injury. Neuropsychologia. 2003;41:634–643. doi: 10.1016/s0028-3932(02)00203-8. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and micro structural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiology of Aging. 2006a;27:994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biological Psychiatry. 2006b;59:364–372. doi: 10.1016/j.biopsych.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Desmond JE, Sullivan EV. Thinning of the corpus callosum in older alcoholic men: A magnetic resonance imaging study. Alcoholism: Clinical and Experimental Research. 1996;20:752–757. doi: 10.1111/j.1530-0277.1996.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, et al. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: A quantitative MRI study. Alcoholism: Clinical and Experimental Research. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV. Diffusion tensor imaging with quantitative fiber tracking in HIV infection and alcoholism comorbidity: Synergistic white matter damage. Brain. 2007;130:48–64. doi: 10.1093/brain/awl242. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Adalsteinsson E, Kemper CA, Deresinski S, et al. Contribution of alcoholism to brain dysmorphology in HIV infection: Effects on the ventricles and corpus callosum. NeuroImage. 2006c;33:239–251. doi: 10.1016/j.neuroimage.2006.05.052. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Microstructural but not macro structural disruption of white matter in women with chronic alcoholism. NeuroImage. 2002;15:708–718. doi: 10.1006/nimg.2001.1018. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Carmelli D. Genetic regulation of regional micro structure of the corpus callosum in late life. Neuroreport. 2001;12:1677–1681. doi: 10.1097/00001756-200106130-00032. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, Moseley M. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcoholism: Clinical and Experimental Research. 2000;24:1214–1221. [PubMed] [Google Scholar]
- Rosenbloom MJ, O’Reilly A, Sassoon SA, Sullivan EV, Pfefferbaum A. Persistent cognitive deficits in community-treated alcoholic men and women volunteering for research: Limited contribution from psychiatric comorbidity. Journal of Studies on Alcohol. 2005;66:254–265. doi: 10.15288/jsa.2005.66.254. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MJ, Sullivan EV, Sassoon SA, O’Reilly A, Fama R, Kemper CA, et al. Alcoholism, HIV infection and their comorbidity: Factors affecting self-rated health-related quality of life. Journal of Studies on Alcohol and Drugs. 2007;68:115–125. doi: 10.15288/jsad.2007.68.115. [DOI] [PubMed] [Google Scholar]
- Sassoon SA, Fama R, Rosenbloom MJ, O'Reilly A, Pfefferbaum A, Sullivan EV. Component cognitive and motor processes of the digit symbol test: Differential deficits in alcoholism, HIV infection and their comorbidity. Alcoholism: Clinical and Experimental Research. 2007;31:1315–1324. doi: 10.1111/j.1530-0277.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Pfefferbaum A, Sullivan EV. Callosal compromise differentially affects conflict processing and attentional allocation in alcoholism, HIV infection, and their comorbidity. Brain Imaging and Behavior. 2007 doi: 10.1007/s11682-007-9014-z. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Pfefferbaum A, Sullivan EV. Parallel interhemispheric processing in aging and alcoholism: Relation to corpus callosum size. Neuropsychologia. 2004;42:257–271. doi: 10.1016/s0028-3932(03)00155-6. [DOI] [PubMed] [Google Scholar]
- Schulte T, Sullivan EV, Müller-Oehring EM, Adalsteinsson E, Pfefferbaum A. Corpus callosal microstructural integrity influences interhemispheric processing: a diffusion tensor imaging study. Cerebral Cortex. 2005;15:1384–1392. doi: 10.1093/cercor/bhi020. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Sherwin I. A comparison of clinical features in early and late-onset primary degenerative dementia: One entity or two. Archives of Neurology. 1983;40:143–146. doi: 10.1001/archneur.1983.04050030037006. [DOI] [PubMed] [Google Scholar]
- Skinner HA. Development and validation of a lifetime alcohol consumption assessment procedure. Toronto, Canada: Addiction Research Foundation; 1982. [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices: The lifetime drinking history and the MAST. Journal of Studies on Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Smith S. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow WG, Weinstock J. Sex differences among nonbrain-damaged adults on the Wechsler Adult Intelligence Scales: A review of the literature. Journal of Clinical and Experimental Neuropsychology. 1990;12:873–886. doi: 10.1080/01688639008401028. [DOI] [PubMed] [Google Scholar]
- Sullivan EV. Compromised pontocerebellar and cerebellothalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcoholism: Clinical and Experimental Research. 2003;27:1409–1419. doi: 10.1097/01.ALC.0000085586.91726.46. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cerebral Cortex. 2006;16:1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, Pfefferbaum A. A profile of neuropsychological deficits in alcoholic women. Neuropsychology. 2002a;16:74–83. doi: 10.1037//0894-4105.16.1.74. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: A substrate of disruption and repair. Psychopharmacology. 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A, Adalsteinsson E, Swan GE, Carmelli D. Differential rates of regional change in callosal and ventricular size: A 4-year longitudinal MRI study of elderly men. Cerebral Cortex. 2002b;12:438–445. doi: 10.1093/cercor/12.4.438. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcoholism: Clinical and Experimental Research. 2000;24:611–621. [PubMed] [Google Scholar]
- Tarter RE, Alterman AI. Neuropsychological deficits in alcoholics: Etiological considerations. Journal of Studies on Alcohol. 1984;45:1–9. doi: 10.15288/jsa.1984.45.1. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—Revised. San Antonio, TX: The Psychological Corporation; 1981. [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Xu D, Mori S, Solaiyappan M, van Zijl PC, Davatzikos C. A framework for callosal fiber distribution analysis. NeuroImage. 2002;17:1131–1143. doi: 10.1006/nimg.2002.1285. [DOI] [PubMed] [Google Scholar]
- Xue R, van Zijl PC, Crain BL, Solaiyappan M, Mori S. In vivo three-dimensional reconstruction of rat brain axonal projections by diffusion tensor imaging. Magnetic Resonance in Medicine. 1999;42:1123–1127. doi: 10.1002/(sici)1522-2594(199912)42:6<1123::aid-mrm17>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]