Abstract
Purpose
Because of the prominence of central vision in primates, it has generally been assumed that signals from the fovea dominate refractive development. To test this assumption, the authors determined whether an intact fovea was essential for either normal emmetropization or the vision-induced myopic errors produced by form deprivation.
Methods
In 13 rhesus monkeys at 3 weeks of age, the fovea and most of the perifovea in one eye were ablated by laser photocoagulation. Five of these animals were subsequently allowed unrestricted vision. For the other eight monkeys with foveal ablations, a diffuser lens was secured in front of the treated eyes to produce form deprivation. Refractive development was assessed along the pupillary axis by retinoscopy, keratometry, and A-scan ultrasonography. Control data were obtained from 21 normal monkeys and three infants reared with plano lenses in front of both eyes.
Results
Foveal ablations had no apparent effect on emmetropization. Refractive errors for both eyes of the treated infants allowed unrestricted vision were within the control range throughout the observation period, and there were no systematic interocular differences in refractive error or axial length. In addition, foveal ablation did not prevent form deprivation myopia; six of the eight infants that experienced monocular form deprivation developed myopic axial anisometropias outside the control range.
Conclusions
Visual signals from the fovea are not essential for normal refractive development or the vision-induced alterations in ocular growth produced by form deprivation. Conversely, the peripheral retina, in isolation, can regulate emmetropizing responses and produce anomalous refractive errors in response to abnormal visual experience. These results indicate that peripheral vision should be considered when assessing the effects of visual experience on refractive development.
Emmetropization is an active process that uses visual feedback to regulate eye growth in a manner that normally eliminates the refractive errors common in neonates.1–3 The mechanisms responsible for emmetropization remain active well into early adult life and probably play an important role in maintaining the optimal refractive state and the proper interocular balance of refractive errors.4–7 However, it is also likely that visual experience acting through these mechanisms contributes to the development of refractive errors in many persons.8–11
Because resolution acuity in humans is highest at the fovea, is sensitive to optical defocus, and decreases rapidly with eccentricity, it has generally been assumed that visual signals processed in the fovea dominate the emmetropization process and presumably the genesis of common refractive errors in children.12 Although this fundamental assumption is logical, it is important to note that the vision-dependent mechanisms that regulate refractive development appear to have evolved from species without foveas (e.g., fish13) and to have been highly conserved across species.12,14 In this respect, the high degree of precision of the emmetropization process in tree shrews15 and chicks,16 which have relatively low visual acuities,17,18 demonstrates that high levels of acuity are not essential for emmetropization and suggests that visual signals from the fovea may not necessarily dominate emmetropization in primates. Moreover, observations in humans and animals suggest that peripheral vision can have a substantial influence on refractive development. For example, clinical observations suggest that peripheral retinal abnormalities11,19 and treatment strategies that selectively impair the periphery20,21 are frequently associated with foveal refractive errors. In addition, the pattern of refractive errors in the periphery has been implicated in the onset and progression of myopia at the fovea.22–24 However, the most direct evidence that peripheral vision can alter central refractive development comes from studies in which peripheral vision was selectively manipulated. For example, in the chick, altering the image in one hemiretina produces regional alterations in eye growth that demonstrate that the physiological processes mediating emmetropization integrate visual signals over restricted spatial areas and that visual experience in the periphery can alter local eye shape, potentially influencing the refractive error for central vision.25,26 Similarly, recent experiments in chicks (Morgan I, et al. IOVS 2006;47:ARVO E-Abstract 3328) and monkeys27 suggest that selective peripheral form deprivation or optical defocus can alter central refractive development. For example, rearing infant monkeys with diffuser lenses that have apertures centered on the pupils to provide unrestricted central vision but that eliminate form vision in the periphery produces axial myopia.27 Thus, it is feasible that peripheral retinal mechanisms participate in the visual regulation of eye growth.
Few attempts have been made to directly assess the relative role of the fovea in emmetropization or in the development of vision-induced refractive errors. The fact that selective peripheral form deprivation can produce axial myopia in infant monkeys, even in the presence of unrestricted central vision, suggests that foveal mechanisms do not dominate refractive development.27 Moreover, reduction experiments in which the fovea has been eliminated by laser photocoagulation indicate that central vision is not essential for emmetropizing responses. For example, ablating the fovea and perifovea in one eye each of infant monkeys with experimentally induced refractive errors does not alter the course of recovery in the treated eye when unrestricted vision is restored.27 However, by themselves, these experiments are not conclusive. Although recovery from induced refractive errors has been shown to be primarily mediated by visual feedback in chicks28 and tree shrews29 and although the rapid time course and the axial nature of the recovery from induced refractive errors in monkeys with intact retinas30 and those with foveal ablations27 are in agreement with this concept, it is possible that some aspects of this recovery were mediated by nonvisual homeostatic processes that were, for example, sensitive to the overall size of the eye.31 Therefore, the purpose of this investigation was to determine whether an intact fovea is essential for normal emmetropization, a process known to be regulated by visual feedback in primates,30,32,33 and whether an intact fovea is essential for the development of form-deprivation myopia, a refractive error produced by anomalous visual experience.34–36
Materials and Methods
Subjects
Data are presented for 43 infant rhesus monkeys (Macaca mulatta). The animals were obtained at 1 to 3 weeks of age and were housed in our primate nursery, maintained on a 12-hour light/12-hour dark lighting cycle.30 All rearing and experimental procedures were reviewed and approved by the University of Houston Institutional Animal Care and Use Committee and were in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
After the initial biometric measurements, which were performed when the monkeys were approximately 3 weeks of age, the monkeys were randomly assigned to either the control or the treatment group. The control group consisted of 21 infant monkeys reared with normal unrestricted vision and three monkeys reared wearing lightweight helmets that held zero-powered spectacle lenses in front of both eyes.30,37 Plano lens–reared monkeys wore the helmets continuously from 3 to 18–20 weeks of age and served as controls for our helmet-rearing procedures and the resultant restrictions in visual field. Refractive data for all the control animals have been reported.14,27,30,37
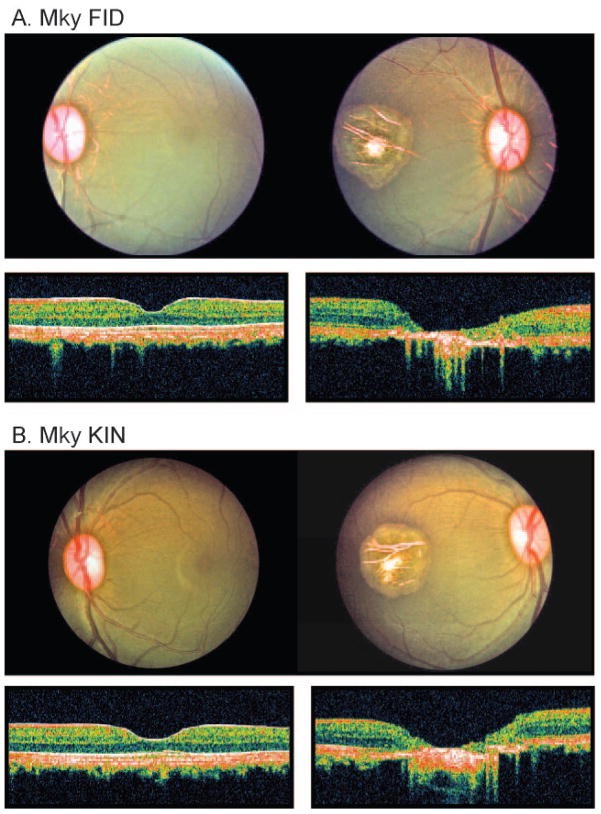
A reduction strategy was used to assess the relative role of the fovea in refractive development. Specifically, either an argon or a frequency-doubled YAG laser was used to photocoagulate the fovea of one eye in each of 13 experimental monkeys. Laser procedures were performed immediately after the initial biometric measurements, with the intent of eliminating all the fovea and part of the perifovea. Although at 3 weeks of age the fovea in an infant monkey eye is anatomically immature and the center of the fovea cannot be identified as readily by ophthalmoscopy as in adults, the distance between the fovea and the center of the optic disc is constant during postnatal development in monkeys.38 Therefore, the position of the fovea was inferred using the optic disc as a reference and the pattern of the central retinal vessels, which converge to a central avascular area that will be occupied by the future fovea. However, because of a degree of uncertainty concerning the position of the center of the fovea, the sizes of the ablations were approximately 20% larger than those in our previous studies of older monkeys. Diameters of the ablation zone were approximately twice the horizontal dimension of the optic disc and corresponded to approximately the central 10° to 12° of the retina. To make the foveal ablations, the monkeys were anesthetized (intramuscular injection [ketamine hydrochloride, 15–20 mg/kg; acepromazine maleate, 0.15–0.2 mg/kg] and topical instillation [1–2 drops of 0.5% tetracaine hydrochloride]), and the laser was delivered to the eye through a slit lamp or an indirect ophthalmoscope. The argon laser was operated in the blue-green mode and had a nominal spot size of 500 μm. Argon laser power was varied from 100 to 250 mW and was presented in 50-msec pulses. The frequency-doubled YAG laser was operated at 150 mW and was presented as 150-msec pulses. Foveal burns were overlapped to ensure complete photoablation of the fovea. Subsequently, ophthalmoscopy, optical coherence tomography (Stratus OCT; Carl Zeiss Meditec, Inc., Oberkochen, Germany), and fundus photography confirmed the sizes and positions of the lesions. As illustrated in the fundus photographs and the OCT retinal thickness scans for the treated and fellow eyes of two representative monkeys in Figure 1, the photoablations included all the foveas and substantial portions of the perifoveas in each animal. Sizes and positions of the ablations were similar in all 13 treated monkeys. OCT retinal thickness scans also showed that the photoablations were effective in destroying the neural retina in the treatment zones and that the effective treatment zones were substantially larger than the foveas.
Figure 1.
Fundus photographs and OCT retinal thickness scans for the treated (right) and fellow (left) eyes of two representative monkeys, (A) FID and (B) KIN. In each case, the lesions included the fovea and much of the perifoveas. OCT scans, which were obtained along the 1:00 to 7:00 o’clock meridians in both monkeys, show that the photoablations were effective in destroying the neural retina in the treatment zone and that the effective treatment zones were substantially larger than the foveas.
To examine the relative role of the fovea in normal emmetropization, five treated monkeys were allowed unrestricted vision after the laser procedures. For the other eight monkeys with monocular foveal ablations, form deprivation was imposed on the treated eyes immediately after the laser procedures. Monocular form deprivation was produced by fitting the monkeys with helmets that held a diffuser spectacle lens in front of the treated eye and a clear, zero-powered lens in front of the fellow eye. Diffuser lenses, each consisting of a zero-powered carrier lens covered with a commercially available occlusion foil (Bangerter Occlusion Foils; Fresnel Prism and Lens Co., Prairie, MN), were the strongest diffuser lenses used in our previous studies of form-deprivation myopia in infant monkeys.14,39 These diffuser lenses reduced the contrast sensitivity of normal adult humans by more than 1 log unit for grating spatial frequencies of 0.125 cyc/deg, with a resultant cutoff spatial frequency near 1 cyc/deg, and consistently produced axial myopia in infant monkeys with intact foveas. The lens-rearing period, which extended from approximately 3 weeks of age (22.8 ± 2.8 days) to 5 months of age (153 ± 17 days), encompassed the bulk of the rapid phase of emmetropization in rhesus monkeys. For comparison, data are also presented for six monkeys that had intact retinas and that were subjected to the same monocular form-deprivation regimen. Data for these form-deprived monkeys have been previously reported.14,39
Ocular Biometry
Details of our biometric measurements, which were performed every 2 to 4 weeks during the observation period, have been described elsewhere.30,40 Briefly, to perform the measurements, the animals were anesthetized (intramuscular injection [ketamine hydrochloride, 15–20 mg/kg; acepromazine maleate, 0.15–0.2 mg/kg] and topical instillation [1–2 drops of 0.5% tetracaine hydrochloride]) and cyclopleged (multiple drops of 1% tropicamide topically 20–30 minutes before retinoscopy). Refractive errors along the pupillary axis were determined independently by two experienced investigators using streak retinoscopy and hand-held trial lenses, averaged,41 and specified as spherical-equivalent, spectacle-plane refractive corrections. The 95% limits of agreement for our retinoscopy measures (spherical-equivalent refractive error) are ±0.6 D.42 Ocular axial dimensions were measured by A-scan ultrasonography implemented with either a 7-MHz (Image 2000; Mentor, Norwell, MA) or a 12-MHz transducer (OTI Scan 1000; OTI Ophthalmic Technologies, Inc., North York, ON, Canada). Intraocular distances were calculated from the average of 10 separate measurements using velocities of 1532 m/s, 1641 m/s, and 1532 m/s for the aqueous, lens, and vitreous, respectively. Corneal curvature was measured with a hand-held keratometer (Alcon Auto-Keratometer; Alcon Systems Inc., St. Louis, MO) or a videotopographer (EyeSys 2000; EyeSys Technologies Inc., Houston, TX). Both instruments provide repeatable and comparable measures of corneal curvature in infant monkeys.40
Statistical Analysis
Two-sample t-tests and Mann–Whitney U tests were used to compare the means and medians for treated and control monkeys. Paired t-tests were used to examine interocular differences in individual animals. Relationships between refractive error and vitreous chamber depth and corneal power were determined using linear regression. All analyses were executed using Minitab software (Release 12.21; Minitab Inc., State College, PA).
Results
At the first measurement session, before the laser and lens-rearing procedures, no systematic interocular differences were observed in refractive error or vitreous chamber depth in the control group or in either of the two laser-treated monkey groups (paired t-test; P = 0.20–0.99). Moreover, the eyes of the treated and control monkeys were similar; average refractive errors for the right eyes of the control and treated monkeys were moderately hyperopic (control [+3.78 ± 1.75 D] vs. treated [+4.18 ± 1.48 D]; two-sample t-test, P = 0.46), and the vitreous chamber depths for the control and treated monkeys were comparable (control [8.64 ± 0.31 mm] vs. treated [8.61 ± 0.13 mm]; two-sample t-test, P = 0.66).
Monocular Foveal Ablation
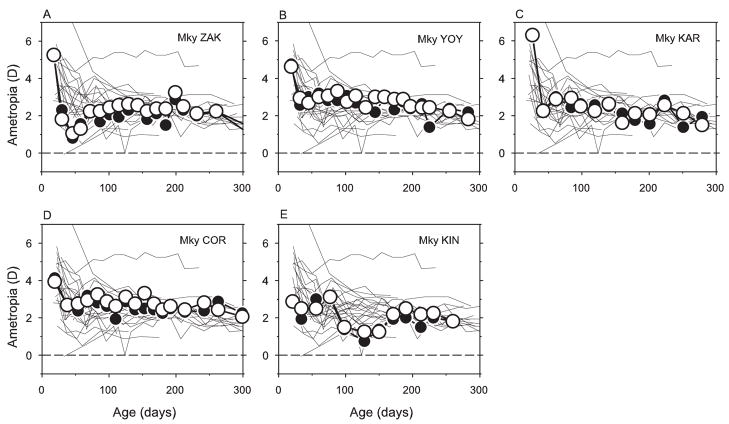
If visual signals from the fovea are essential for emmetropization or play a dominant role in normal refractive development, it would be expected that eliminating the fovea would alter the efficiency, time course, or possibly the target refractive error of the emmetropization process in the treated eyes relative to control eyes. In addition, because the vision-dependent mechanisms that regulate refractive development operate in a relatively autonomous fashion in each eye,30,43 monocular foveal ablations would be expected to result in differences in the course of emmetropization between the treated eye and the fellow eye of a given experimental monkey. Figure 2 illustrates the spherical-equivalent refractive errors plotted as a function of age for the right eyes of the control monkeys (thin lines) and the treated (filled symbols) and fellow eyes (open symbols) of the monkeys that had monocular foveal ablations. At the start of the observation period, four of the five treated monkeys exhibited hyperopic errors of +4.0 D or greater. All these animals exhibited the rapid reductions in hyperopia characteristic of normal emmetropization and thereafter maintained relatively moderate degrees of hyperopia in both eyes throughout the observation period. The fifth monkey with a monocular foveal ablation exhibited comparatively low degrees of hyperopia at the start of the treatment period and showed relatively small subsequent changes in refractive error. The key points are that there were no systematic differences in refractive development between treated and fellow eyes and refractive errors for both eyes of the treated monkeys were well within the range of refractive errors for control animals at all ages.
Figure 2.
Spherical-equivalent, spectacle-plane refractive corrections plotted as a function of age for individual control animals and the treated monkeys reared with monocular foveal ablations and unrestricted vision. Thin solid lines: right eyes of the control animals. Open and filled circles: control and laser-treated eyes of the experimental monkeys, respectively. Laser procedures were performed during the first measurement session at approximately 3 weeks of age.
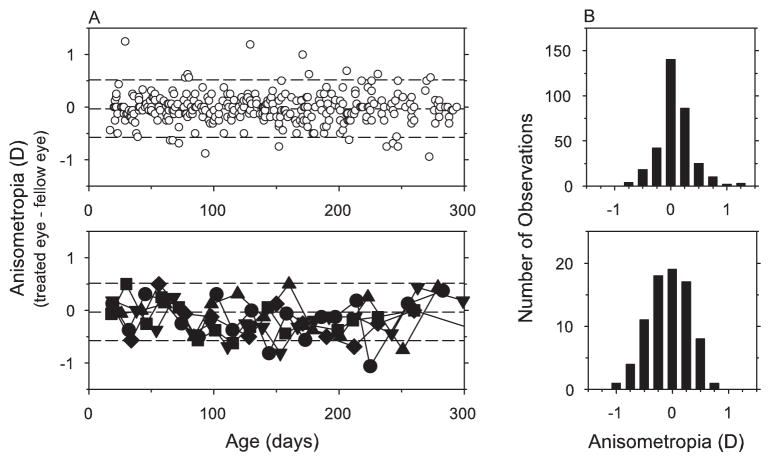
Interocular differences in refractive error are plotted as a function of age in Figure 3A for the five monkeys with monocular foveal ablations and all the control animals. As illustrated in the upper plot, both eyes of each control monkey grew in a coordinated manner so that clinically significant anisometropias were rare in the normal and plano-control monkeys. During the observation period, the largest anisometropia found in a control animal measured 1.25 D, which was observed at the initial measurement session for one animal. Anisometropias larger than 0.75 D were found on only 7 of 330 occasions in the control animals, and the average anisometropia was −0.03 ± 0.27 D (the right eye was considered the treated eye; paired t-test, P = 0.24). Although small anisometropias (e.g., 0.25–0.50 D) were relatively more common in the treated monkeys (compare the upper and lower frequency distributions in Fig. 3B), none of the animals with monocular foveal ablations exhibited anisometropia that fell outside the range of anisometropias found in the control animals. During the observation period, treated eyes were on average −0.15 ± 0.35 D less hyperopic than their fellow control eyes. Although these differences were not statistically significant (paired t-test, P = 0.23), the reduction in retinal thickness produced by our photoablation procedures could have resulted in small myopic shifts in retinoscopy measures. Regardless, there were no indications that the degree or direction of anisometropia in the treated animals changed in a systematic manner during the observation period.
Figure 3.
(A) Interocular differences in refractive error (right or treated eye – left or fellow eye) plotted as a function of age for control animals (upper plot, open circles) and the treated monkeys reared with monocular foveal ablations and unrestricted vision (lower plot, filled symbols). Dashed lines: mean ± 2 SD for control animals. (B) Frequency histograms of the anisometropic errors found during the observation period for the control (upper plot) and treated (lower plot) monkeys.
Foveal Ablation and Form Deprivation
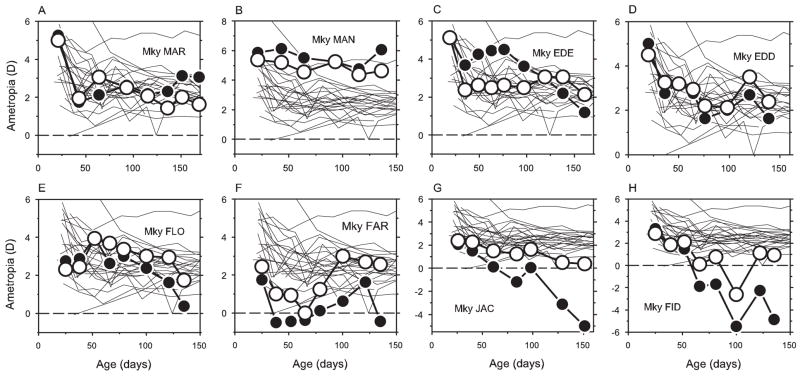
If signals from the fovea were essential for the development of vision-induced refractive anomalies, it would be expected that eliminating the fovea would prevent form deprivation from altering the course of emmetropization. Specifically, in this experiment, it was expected that refractive development would be similar in the treated and fellow eyes of the animals reared with monocular foveal ablation and form deprivation. Figure 4 compares the course of refractive development during the lens-rearing period between the right eyes of the control animals (thin lines) and the form-deprived (filled symbols) and nontreated fellow eyes (open symbols) of the monkeys with monocular foveal ablations.
Figure 4.
Spherical-equivalent, spectacle-plane refractive corrections plotted as a function of age for control animals and treated monkeys reared with monocular foveal ablations and form deprivation. Thin solid lines: right eyes of control animals. Open and filled circles: control and laser-treated eyes of the experimental monkeys. Laser procedures were performed during the first measurement session at approximately 3 weeks of age, immediately before the onset of form deprivation.
As a group, the treated monkeys exhibited substantial variability in refractive development. At the end of the diffuser-rearing period, the range of refractive errors for the treated eyes was obviously greater than that for the control animals (−5.00 to +6.06 D vs. +0.81 to +5.50 D), and the median refractive error for the treated eyes was significantly less hyperopic or more myopic (+0.78 D vs. +2.50 D; Mann–Whitney U test; P = 0.05). Most important, all the treated monkeys had obvious interocular differences in refractive error. By the end of the diffuser-rearing period, the treated eyes of six of the eight treated animals were more myopic or less hyperopic than their fellow eyes. Within this group, the onset and progression of form deprivation myopia was obvious in some animals (e.g., Fig. 4, lower row); however, the rate at which the relative myopia and the final degree of form-deprivation myopia emerged varied substantially among animals. Interestingly, in two treated animals, the form-deprived eyes showed relative hyperopic shifts in refractive error at the end of the rearing period (Figs. 4A, 4B). In addition, a third monkey (Fig. 4C, monkey EDE) showed an initial hyperopic shift in its treated eye, but, after approximately 50 days of form deprivation, the treated eye showed systematic reductions in the degree of hyperopia. By the end of the diffuser-rearing period, the treated eye had become more myopic than its fellow non-treated eye.
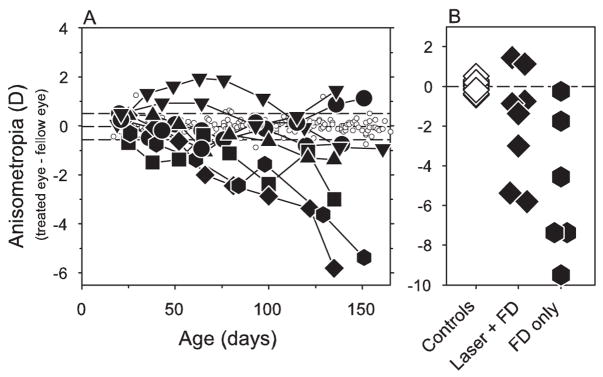
Figure 5 summarizes the effects of the treatment regimen on the interocular differences in refractive error. As illustrated in the left panel, which shows the direction and degree of anisometropia as a function of age for individual animals, all the treated animals had anisometropias that were outside the range for the control animals at some point during the rearing period. In particular, at the end of the treatment period, six of the eight treated monkeys exhibited myopic anisometropias that fell outside the control range, and the other two treated monkeys had hyperopic anisometropias that were larger than any anisometropia found in age-matched control animals. The average absolute degree of anisometropia for the treated animals at the end of the diffuser-rearing period was significantly larger than that for the age-matched control animals (2.52 ± 2.02 D vs. 0.19 ± 0.12 D; two-sample t-test, P = 0.01), and the median anisometropia for the treated animals was significantly more myopic (−1.16 D vs. −0.03 D; Mann–Whitney U test, P = 0.04). As expected, after approximately 75 days of age (7–8 weeks of lens wear), the deprived eyes of the treated animals were also significantly less hyperopic or more myopic than their fellow nontreated eyes (paired t-test, P = 0.003).
Figure 5.
(A) Interocular differences in refractive error (right or treated eye – left or fellow eye) plotted as a function of age for control animals (open circles) and treated monkeys reared with monocular foveal ablations and form deprivation (filled symbols). The first and last symbols for each animal represent the start and end of the period of form deprivation. Dashed lines: mean ± 2 SD for the control animals. (B) Degree of anisometropia at the end of the lens-rearing period for the monocularly form-deprived animals with monocular foveal ablations (filled diamonds), monocularly form-deprived animals with intact retinas (filled hexagons), and age-matched control animals (open diamonds).
Interocular differences in refractive error in the form-deprived animals with foveal ablations were comparable to those in form-deprived monkeys that had intact foveas (Fig. 5, filled hexagons). Ranges of anisometropias in these two groups of form-deprived monkeys were similar (laser + form deprivation [+1.44 to −5.81 D] vs form deprivation only [−0.25 to −9.5 D]). In addition, though an overall shift of the results occurred for the laser-treated monkeys in the hyperopic direction, no significant differences were observed in either the mean (−1.18 ± 2.76 D vs. −5.14 ± 3.60 D; two-sample t-test, P = 0.09) or the median (−1.16 D vs. −5.97 D; Mann–Whitney U test, P = 0.11) anisometropias between these two groups of form-deprived monkeys.
In addition to the direct effects of the form deprivation on the treated eyes, it was evident that the monocular treatment regimen had altered refractive development in the fellow non-treated eyes in some animals. For example, for three animals (Figs. 4F–H), the nontreated fellow eye exhibited relative myopic errors that fell outside the normal range at some point during the treatment period. In addition, the nontreated fellow eye of monkey MAN (Fig. 4B) showed no evidence of emmetropization and by the end of the diffuser rearing period had a refractive error of +4.63 D, which was >2 SD above the control mean. However, the mean (+2.05 ± 1.27 vs. +2.42 ± 0.94 D; two-sample t-test, P = 0.46) and median (+1.94 D vs. 2.50 D; two-sample t-test, P = 0.23) refractive errors for the nontreated fellow eyes were not significantly different from those for the control animals.
The observed interocular differences in refractive error in the form-deprived monkeys cannot be attributed to either laser-induced or vision-induced changes in corneal power. Although at the end of the lens-rearing period there was a trend for the form-deprived eyes of the laser-treated monkeys to have slightly steeper corneas than their fellow eyes (average inter-ocular difference, +0.33 D), these interocular differences were not significant (paired t-test, P = 0.09), and the absolute interocular differences in corneal power for the form-deprived monkeys with foveal lesions were not significantly larger than those for either the control animals (0.46 D vs. 0.25 D; two-sample t-test, P = 0.12) or the treated monkeys reared with unrestricted vision (0.46 D vs. 0.19, P = 0.07). Moreover, at the end of the diffuser-rearing period, the interocular differences in corneal power and refractive error were not significantly correlated (r2 = 0.04; P = 0.25).
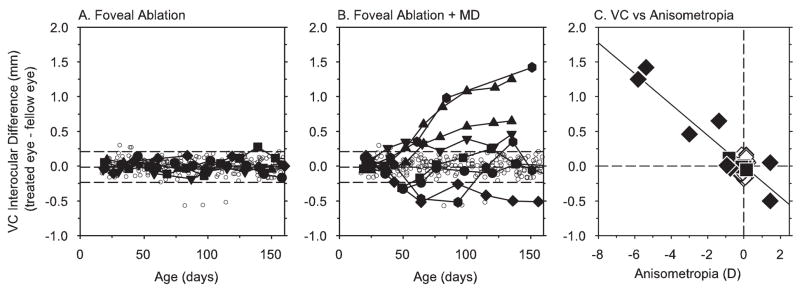
Anisometropias observed in the form-deprived animals with foveal ablations were primarily axial in nature. In Figures 6A and 6B, the interocular differences in vitreous chamber depth are plotted as a function of age for control animals (open circles), the treated monkeys that were reared with unrestricted visual experience (Fig. 6A, solid symbols), and the monocularly form-deprived monkeys that had foveal ablations (Fig. 6B, solid symbols). Monocular foveal ablation by itself had no apparent effect on vitreous chamber depth. No systematic interocular differences were observed in vitreous chamber depth in the treated monkeys allowed unrestricted vision (paired t-test, P = 0.71), and, with the exception of one observation, their interocular differences in vitreous chamber depth were always within 2 SD of the control mean. On the other hand, many of the monkeys that had foveal ablations and experienced monocular form deprivation exhibited obvious interocular differences in vitreous chamber depth. Beginning after approximately 1 month of form deprivation, the treated eyes of four monkeys (FLO, FAR, JAC, FID) showed consistently longer vitreous chambers than their fellow eyes, and the interocular differences for these four animals were well outside the control range for the remainder of the diffuser-rearing period. On the other hand, the vitreous chamber in the treated eye of one of the monkeys that developed a relative hyperopia in the treated eye (monkey MAR, filled diamonds) was consistently shorter than that for its fellow eye for most of the lens-rearing period.
Figure 6.
Interocular differences in vitreous chamber depth (right or treated eye – fellow eye) plotted as a function of age for control animals (open symbols), treated monkeys that experienced unrestricted vision (A, filled symbols), and monocularly form-deprived monkeys with foveal lesions (B, filled symbols). Dashed lines: mean ± 2 SD for control animals. (C) Interocular differences in vitreous chamber depth plotted as a function of the interocular differences in refractive error (right or treated eye – fellow eye) for control animals (open diamonds), treated monkeys reared with unrestricted vision (filled squares), and monocularly form-deprived animals with monocular foveal ablations (filled diamonds). Data were obtained at end of the diffuser rearing period for the monocularly form-deprived monkeys and at equivalent ages for the control animals and the treated monkeys reared with unrestricted vision. Solid line: best-fitting regression line (r2 = 0.81).
The observed changes in refractive error and vitreous chamber depth were well correlated. Figure 6C shows the interocular differences in vitreous chamber depth obtained at the end of the diffuser-rearing period plotted as a function of the interocular differences in refractive error for individual form-deprived animals (filled diamonds). Data obtained at equivalent ages are also shown for the control animals (open diamonds) and the laser-treated monkeys reared with unrestricted vision (filled squares). At the end of the lens-rearing period, five of the form-deprived animals with foveal ablations exhibited interocular differences in vitreous chamber depth that fell outside the range of interocular differences for control animals and monkeys with monocular foveal ablations that experienced unrestricted vision. Average interocular differences in vitreous chamber depth for form-deprived monkeys with foveal ablations (0.55 ± 0.55 mm) were significantly greater than for age-matched control animals (0.10 ± 0.05 mm; two-sample t-test, P = 0.05) or monkeys reared with monocular foveal ablations (0.04 ± 0.04 mm; two-sample t-test, P = 0.03). Regression analysis that included data from all the animal groups showed that the interocular differences in vitreous chamber depth were significantly correlated with the degree of anisometropia (P < 0.001) and that the interocular differences in vitreous chamber depth accounted for 81% of the variance in the anisometropias.
Discussion
Relative to refractive development in normal and nontreated eyes, the course of emmetropization was not altered by foveal ablation in the treated eyes of the infant monkeys allowed unrestricted vision. Interpretation of these results is dependent on the degree to which emmetropization is independent in the two eyes. Based on the fact that monocular manipulations can have interocular effects, it has been argued that the mechanisms that regulate refractive development in the two eyes are yoked.39,43–51 In fact, the nondeprived eyes of our monkeys subjected to monocular form deprivation showed evidence of interocular effects. The mechanisms that mediate these interocular effects are not well understood. In birds, it has been speculated that interocular effects are mediated by humoral factors of central and ocular origin (Li T, et al. IOVS 1998;39: ARVO Abstract 3287)46,52 and neural coupling between the mechanisms that regulate refractive growth in the two eyes.49–52 Therefore, it could be argued that refractive development in the treated eyes of our monkeys with foveal ablations was guided by signals from the nontreated fellow eyes. However, it is unclear whether similar pathways exist in primates because some of the manipulations that reveal interocular interactions in birds (e.g., continuous light exposure53) do not have any obvious effects on emmetropization in primates,54,55 and anatomic differences between birds and primates suggest that the neural pathways likely to be involved in any interocular neural interactions are less prominent in primates. For example, primates have 1000 times fewer centrifugal fibers that project to the retina than have birds.56 Moreover, the interocular effects in monkeys may have an optical basis. As a result of consensual accommodation and accommodation–convergence interactions in primates, we have previously argued that many interocular effects in monkeys could be caused by changes in the time-averaged clarity of the retinal image in the fellow eye as a result of manipulations of the contralateral eye.14 In other words, the presence of interocular effects does not necessarily imply that the mechanisms that regulate refractive development are directly yoked in the two eyes.
Conversely, strong evidence indicates that refractive development proceeds primarily in an independent manner in the two eyes. For example, in response to optically induced anisometropia, infants of many species exhibit differential interocular growth and develop axial anisometropias that compensate for the optically imposed errors.1–3,57 In Figure 4, the differential interocular growth produced by monocular form deprivation is another example of the relative independence of refractive development in the two eyes. However, in species with highly developed binocular vision, the nature of visual experience in the two eyes is highly correlated. In particular, because accommodation is yoked in the two eyes of primates and because primates typically bifoveally fixate objects in space, the retinal images in the two eyes are normally similar. Consequently, refractive development, even if it is regulated by independent mechanisms in the two eyes, normally proceeds similarly in each eye.
Assuming that the vision-dependent mechanisms that regulate emmetropization are largely independent in the two eyes of infant monkeys, results in our animals that had monocular foveal ablations and that experienced unrestricted vision indicated that visual signals from the fovea are not essential for emmetropization. Instead the mechanisms that mediate emmetropization can respond appropriately to optical defocus associated with refractive error in the absence of visual signals from the fovea. These results are in agreement with our previous finding that foveal ablation did not alter the ability of infant monkeys to recover from experimentally induced refractive errors,27 a process mediated by optical defocus.28,29 Results from both studies also imply that visual signals from the periphery, in isolation, can be used to determine the direction of axial growth required to eliminate refractive error and to determine when ocular growth has eliminated that refractive error (i.e., when emmetropia is achieved). Moreover, because the treated and fellow eyes of these laser-treated monkeys experienced comparable retinal images during development (given that accommodation is consensual in monkeys and that the animals were orthotropic and had little or no anisometropia), it can be argued that the emmetropization process driven by the periphery alone can apparently operate as effectively and efficiently as that driven by an intact eye. In other words, in an intact eye, the overall contribution of the visual signals from the fovea to emmetropization is probably small under ordinary circumstances (ignoring the role of central vision in directing accommodation).
Clinical observations in humans also suggest that the periphery can have a significant impact on emmetropization. For example, children with retinal diseases show a larger than normal range of refractive errors and have larger average refractive errors. It is likely that these refractive errors come about because the disease processes have interfered with the mechanisms responsible for emmetropization. In this respect, children who have conditions or diseases that primarily affect the peripheral retina usually exhibit larger refractive errors than children with eye diseases that primarily affect central vision.11 The pattern of peripheral refractive errors may also influence the course of refractive development in humans. For example, young adults who are undergoing pilot training and show compound hyperopic astigmatism in the periphery are more likely to exhibit myopic shifts in central refractive error than those who exhibit myopic peripheral refractive errors.22 Similarly, children with prolate posterior segments and relative hyperopia in the periphery are more likely to develop myopia than children with oblate eyes and relative myopia in the periphery.24,58 Based on our findings in monkeys, it is reasonable to speculate that the hyperopic defocus produced by peripheral refractive errors in these children24,25 and adults,22 which would be constant over time, dominated refractive development and led to central axial myopia.
The second major finding of this study was that laser ablation of the fovea did not prevent form deprivation from altering refractive development in infant monkeys. In many respects, the refractive changes produced by form deprivation in the monkeys with foveal ablations were similar to those produced by form deprivation in intact eyes. In intact eyes and in eyes with foveal ablations, vision-induced myopia is axial in nature and caused primarily by an increase in vitreous chamber depth.34,35,39 In intact eyes and eyes with foveal ablation, substantial intersubject variability occurred in the time course and degree of myopic anisometropia produced by monocular deprivation. In both cases, form deprivation produced hyperopia in the treated eyes in a small number of animals, which in some cases was transient and eventually resulted in myopia; in other cases, the treated eye remained relatively more hyperopic than the nontreated fellow eye throughout the treatment period.14,35,36,39 Interocular effects, which are manifest as alterations in the course of emmetropization of the fellow eye, can also be seen in monocularly form-deprived monkeys with intact eyes and when the fovea in the treated eye has been ablated.14,44 Thus, in response to form deprivation, the periphery can produce the same kinds of refractive error changes that are produced in monkeys with intact eyes.
It appears that the degree of form-deprivation myopia produced in the laser-treated eyes was smaller than that produced by the same diffuser regimen in intact eyes. Although this difference was not statistically significant, it is possible that these differences represented a true quantitative difference reflecting a reduction in overall growth signals that resulted from eliminating the central retina. Given the inherently high intersubject variability in the degree of myopia produced by form deprivation in monkeys, it will take substantially larger sample sizes than those available to definitively address this issue. However, it seems reasonable to suppose that in an intact eye the signals from the fovea normally contribute to the magnitude of the eye’s response to form deprivation and that laser foveal ablation reduces the magnitude of this signal.
The pattern of results in our laser-treated, form-deprived monkeys complements our previous observation that diffuser lenses with central apertures, which produce form deprivation in the periphery but allow unrestricted foveal vision, also result in axial myopia.27 The diffuser lenses used in these previous studies were designed to produce selective peripheral form deprivation; however, the optical consequence of the treatment lenses depended on the animals’ viewing behaviors. In this respect, the animals were motivated to fixate through the apertures, and observations throughout the treatment period showed that the infants rapidly adapted to the lenses and consistently fixated through the apertures, resulting in potentially clear central vision and form deprivation in the periphery. However, it is likely that the images presented to the foveas of these monkeys were at times degraded. Given the nonlinear temporal integration properties of the emmetropization process (Vingrys AJ, et al. IOVS 1991;32:ARVO Abstract 1203),14,59,60 it is unlikely that these brief periods of reduced central vision could have significantly contributed to the phenomenon of form-deprivation myopia. Results from the form-deprived monkeys with foveal ablations confirm our previous conclusion that selective peripheral form deprivation can produce myopia.27 Together, the results from our two studies indicate that in the absence of a visual signal from the fovea, or when conflicting visual signals exist between the fovea and the periphery, the effects of peripheral vision can dominate central refractive development.
Results of this study add to the growing body of data that indicate central/axial refractive development is influenced by image quality across the retina. Unfortunately, we know little about the spatial integration properties of the emmetropization process in primates. In particular, it will be important to learn how the signals from different parts of the eye are weighted to determine central axial elongation rates and the refractive status at the fovea and how visual experience, particularly regional variations in image quality, influence ocular shape and peripheral refractive error pattern. With respect to the development of optical treatment strategies to slow or prevent myopic progression, the results of the study reinforce the idea that peripheral vision must be taken into account to optimize any beneficial treatment effects.
Acknowledgments
The authors thank Ronald Harwerth and Joe Wheat for providing OCT retinal thickness scans for the treated monkeys.
Supported by National Eye Institute Grants RO1 EY03611 and P30 EY70551; the Vision Cooperative Research Centre, Sydney, Australia; and the Greeman-Petty Professorship, UH Foundation.
Footnotes
Disclosure: E.L. Smith III, P; R. Ramamirtham, None; Y. Qiao-Grider, None; L.-F. Hung, None; J. Huang, None; C. Kee, None; D. Coats, None; E. Paysse, None
References
- 1.Smith EL., III . Environmentally induced refractive errors in animals. In: Rosenfield M, Gilmartin B, editors. Myopia and Nearwork. Oxford, UK: Butterworth-Heinemann; 1998. pp. 57–90. [Google Scholar]
- 2.Norton TT. Animal models of myopia: learning how vision controls the size of the eye. Inst Lab Anim Res J. 1999;40:59–77. doi: 10.1093/ilar.40.2.59. [DOI] [PubMed] [Google Scholar]
- 3.Wildsoet CF. Active emmetropization—evidence for its existence and ramifications for clinical practice. Ophthalmic Physiol Opt. 1997;17:279–290. [PubMed] [Google Scholar]
- 4.Zhong X, Ge J, Nie H, Smith EL., III Compensation for experimentally induced hyperopic anisometropia in adolescent monkeys. Invest Ophthalmol Vis Sci. 2004;45:3373–3379. doi: 10.1167/iovs.04-0226. [DOI] [PubMed] [Google Scholar]
- 5.Troilo D, Nickla DL, Wildsoet CF. Form deprivation myopia in mature common marmosets (Callithrix jaccbus) Invest Ophthalmol Vis Sci. 2000;41:2043–2049. [PubMed] [Google Scholar]
- 6.Smith EL, III, Bradley DV, Fernandes A, Boothe RG. Form deprivation myopia in adolescent monkeys. Optom Vis Sci. 1999;76:428–432. doi: 10.1097/00006324-199906000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Papastergiou GI, Schmid GF, Laties AM, Pendrak K, Lin T, Stone RA. Induction of axial elongation and myopic refractive shift in one year old chickens. Vision Res. 1998;38:1883–1888. doi: 10.1016/s0042-6989(97)00347-7. [DOI] [PubMed] [Google Scholar]
- 8.Rabin J, Van Sluyters RC, Malach R. Emmetropization: a vision-dependent phenomenon. Invest Ophthalmol Vis Sci. 1981;20:561–564. [PubMed] [Google Scholar]
- 9.Robb RM. Refractive errors associated with hemangiomas of the eyelids and orbit in infancy. Am J Ophthalmol. 1977;83:52–58. doi: 10.1016/0002-9394(77)90191-x. [DOI] [PubMed] [Google Scholar]
- 10.O’Leary DJ, Millodot M. Eyelid closure causes myopia in humans. Experientia. 1979;35:1478–1479. doi: 10.1007/BF01962795. [DOI] [PubMed] [Google Scholar]
- 11.Nathan J, Kiely PM, Crewther SG, Crewther DP. Disease-associated image degradation and spherical refractive errors in children. Am J Optom Physiol Opt. 1985;62:680–688. doi: 10.1097/00006324-198510000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Stone RA, Flitcroft DI. Ocular shape and myopia. Ann Acad Med Singapore. 2004;33:7–15. [PubMed] [Google Scholar]
- 13.Shen W, Wijayan M, Sivak JG. Inducing form-deprivation myopia in fish. Invest Ophthalmol Vis Sci. 2005;46:1797–1803. doi: 10.1167/iovs.04-1318. [DOI] [PubMed] [Google Scholar]
- 14.Smith EL, III, Hung L-F, Kee C-s, Qiao Y. Effects of brief periods of unrestricted vision on the development of form-deprivation myopia in monkeys. Invest Ophthalmol Vis Sci. 2002;43:291–299. [PubMed] [Google Scholar]
- 15.Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew. Vision Res. 1992;32:833–842. doi: 10.1016/0042-6989(92)90026-f. [DOI] [PubMed] [Google Scholar]
- 16.Wallman J, Adams JI, Trachtman JN. The eyes of young chickens grow toward emmetropia. Invest Ophthalmol Vis Sci. 1981;20:557–561. [PubMed] [Google Scholar]
- 17.Petry HM, Fox R, Casagrande VA. Spatial contrast sensitivity of the tree shrew. Vision Res. 1984;24:1037–1042. doi: 10.1016/0042-6989(84)90080-4. [DOI] [PubMed] [Google Scholar]
- 18.Schmid KL, Wildsoet CF. Assessment of visual acuity and contrast sensitivity in the chick using an optokinetic nystagmus paradigm. Vision Res. 1998;38:2629–2634. doi: 10.1016/s0042-6989(97)00446-x. [DOI] [PubMed] [Google Scholar]
- 19.Sieving PA, Fishman GA. Refractive errors of retinitis pigmentosa patients. Br J Ophthalmol. 1978;62:163–167. doi: 10.1136/bjo.62.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knight-Nanan DM, O’Keefe M. Refractive outcome in eyes with retinopathy of prematurity treated with cryotherapy or diode laser: 3-year follow-up. Br J Ophthalmol. 1996;80:998–1001. doi: 10.1136/bjo.80.11.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connolly BP, Ng EY, McNamara JA, Regillo CD, Vander JF, Tasman W. A comparison of laser photocoagulation with cryotherapy for threshold retinopathy of prematurity at 10 years, part 2: refractive outcome. Ophthalmology. 2002;109:936–941. doi: 10.1016/s0161-6420(01)01015-6. [DOI] [PubMed] [Google Scholar]
- 22.Hoogerheide J, Rempt F, Hoogenboom WP. Acquired myopia in young pilots. Ophthalmologica. 1971;163:209–215. doi: 10.1159/000306646. [DOI] [PubMed] [Google Scholar]
- 23.Mutti DO, Sholtz RI, Friedman NE, Zadnik K. Peripheral refraction and ocular shape in children. Invest Ophthalmol Vis Sci. 2000;41:1022–1030. [PubMed] [Google Scholar]
- 24.Schmid G. Retinal steepness vs. myopic shift in children (Abstract) Optom Vis Sci. 2004;12S:23. doi: 10.1097/OPX.0b013e3182152646. [DOI] [PubMed] [Google Scholar]
- 25.Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek L. Local retinal regions control local eye growth and myopia. Science. 1987;237:73–77. doi: 10.1126/science.3603011. [DOI] [PubMed] [Google Scholar]
- 26.Diether S, Schaeffel F. Local changes in eye growth induced by imposed local refractive error despite active accommodation. Vision Res. 1997;37:659–668. doi: 10.1016/s0042-6989(96)00224-6. [DOI] [PubMed] [Google Scholar]
- 27.Smith EL, III, Kee C-s, Ramamirtham R, Qiao-Grider Y, Hung L-F. Peripheral vision can influence eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2005;46:3965–3972. doi: 10.1167/iovs.05-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wildsoet CF, Schmid KL. Optical correction of form deprivation myopia inhibits refractive recovery in chick eyes with intact or sectioned optic nerves. Vision Res. 2000;40:3273–3282. doi: 10.1016/s0042-6989(00)00138-3. [DOI] [PubMed] [Google Scholar]
- 29.Norton TT, Amedo AO, Siegwart JR., Jr Darkness causes myopia in visually inexperienced tree shrews. Invest Ophthalmol Vis Sci. 2006;47:4700–4707. doi: 10.1167/iovs.05-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith EL, III, Hung L-F. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39:1415–1435. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- 31.Troilo D, Wallman J. The regulation of eye growth and refractive state: an experimental study of emmetropization. Vision Res. 1991;31:1237–1250. doi: 10.1016/0042-6989(91)90048-a. [DOI] [PubMed] [Google Scholar]
- 32.Guyton DL, Greene PR, Scholz RT. Dark-rearing interference with emmetropization in the rhesus monkey. Invest Ophthalmol Vis Sci. 1989;30:761–764. [PubMed] [Google Scholar]
- 33.Whatham A, Judge S. Compensatory changes in eye growth and refraction induced by daily wear of soft contact lenses in young marmosets. Vision Res. 2001;41:267–273. doi: 10.1016/s0042-6989(00)00250-9. [DOI] [PubMed] [Google Scholar]
- 34.Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266:66–68. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- 35.Smith EL, III, Harwerth RS, Crawford MLJ, von Noorden GK. Observations on the effects of form deprivation on the refractive status of the monkey. Invest Ophthalmol Vis Sci. 1987;28:1236–1245. [PubMed] [Google Scholar]
- 36.Tigges M, Tigges J, Fernandes A, Eggers HM, Gammon JA. Postnatal axial eye elongation in normal and visually deprived rhesus monkeys. Invest Ophthalmol Vis Sci. 1990;31:1035–1046. [PubMed] [Google Scholar]
- 37.Hung L-F, Crawford MLJ, Smith EL., III Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat Med. 1995;1:761–765. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- 38.Springer AD, Hendrickson AE. Development of the primate area of high acuity, 2: quantitative morphological changes associated with retinal and pars plana growth. Vis Neurosci. 2004;21:775–790. doi: 10.1017/S0952523804215115. [DOI] [PubMed] [Google Scholar]
- 39.Smith EL, III, Hung L-F. Form-deprivation myopia in monkeys is a graded phenomenon. Vision Res. 2000;40:371–381. doi: 10.1016/s0042-6989(99)00184-4. [DOI] [PubMed] [Google Scholar]
- 40.Kee C-s, Hung L-F, Qiao Y, Habib A, Smith EL., III Prevalence of astigmatism in infant monkeys. Vision Res. 2002;42:1349–1359. doi: 10.1016/s0042-6989(02)00060-3. [DOI] [PubMed] [Google Scholar]
- 41.Harris WF. Algebra of sphero-cylinders and refractive errors, and their means, variance, and standard deviation. Am J Optom Physiol Opt. 1988;65:794–902. doi: 10.1097/00006324-198810000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Kee C-s, Hung L-F, Qiao-Grider Y, Roorda A, Smith EL., III Effects of optically imposed astigmatism on emmetropization in infant monkeys. Invest Ophthalmol Vis Sci. 2004;45:1647–1659. doi: 10.1167/iovs.03-0841. [DOI] [PubMed] [Google Scholar]
- 43.Raviola E, Wiesel TN. An animal model of myopia. N Engl J Med. 1985;312:1609–1615. doi: 10.1056/NEJM198506203122505. [DOI] [PubMed] [Google Scholar]
- 44.Bradley DV, Fernandes A, Boothe RG. The refractive development of untreated eyes of rhesus monkeys varies according to the treatment received by their fellow eyes. Vision Res. 1999;39:1749–1757. doi: 10.1016/s0042-6989(98)00177-1. [DOI] [PubMed] [Google Scholar]
- 45.Sivak JG, Barrie DL, Weerheim JA. Bilateral experimental myopia in chicks. Optom Vis Sci. 1989;66:854–858. doi: 10.1097/00006324-198912000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995;35:1175–1194. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- 47.Schmid KL, Wildsoet CF. Effects on the compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Res. 1996;36:1023–1036. doi: 10.1016/0042-6989(95)00191-3. [DOI] [PubMed] [Google Scholar]
- 48.Siegwart JTJ, Norton TT. The time course of changes in mRNA levels in tree shrew sclera during induced myopia and recovery. Invest Ophthalmol Vis Sci. 2002;43:2067–2075. [PMC free article] [PubMed] [Google Scholar]
- 49.Diether S, Schaeffel F, Lambrou GN, Fritsch C, Trendelenburg A-U. Effects of intravitreally and intraperitoneally injected atropine on two types of experimental myopia in chicken. Exp Eye Res. 2007;84:266–274. doi: 10.1016/j.exer.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 50.Simon P, Feldkaimper M, Bitzer M, Ohngemach S, Schaeffel F. Early transcription changes of retinal and choroidal TGFβ-2, RALDH-2, and ZENK following imposed positive and negative defocus in chickens. Mol Vis. 2004;10:588–597. [PubMed] [Google Scholar]
- 51.Bitzer M, Schaeffel F. Defocus-induced changes in ZENK expression in the chicken retina. Invest Ophthalmol Vis Sci. 2002;43:246–252. [PubMed] [Google Scholar]
- 52.Li T, Howland HC. Role of the pineal gland in ocular development of the chick in normal and constant light conditions. Invest Ophthalmol Vis Sci. 2006;47:5132–5136. doi: 10.1167/iovs.05-0671. [DOI] [PubMed] [Google Scholar]
- 53.Li T, Troilo D, Glasser A, Howland H. Constant light produces severe corneal flattening and hyperopia in chickens. Vision Res. 1995;35:1203–1209. doi: 10.1016/0042-6989(94)00231-a. [DOI] [PubMed] [Google Scholar]
- 54.Smith EL, III, Bradley DV, Fernandes A, Hung L-F, Boothe RG. Continuous ambient lighting and eye growth in primates. Invest Ophthalmol Vis Sci. 2001;42:1146–1152. [PubMed] [Google Scholar]
- 55.Smith EL, III, Hung L-F, Kee C-s, Qiao-Grider Y, Ramamirtham R. Continuous ambient lighting and lens compensation in infant monkeys. Optom Vis Sci. 2003;80:374–382. doi: 10.1097/00006324-200305000-00012. [DOI] [PubMed] [Google Scholar]
- 56.Repérant J, Ward R, Miceli D, et al. The centrifugal visual system of vertebrates: a comparative analysis of its functional anatomical organization. Brain Res Rev. 2006;52:1–57. doi: 10.1016/j.brainresrev.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 57.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Mutti DO, Zadnik K, Hayes JR, Mitchell GL, Jones LA, Moeschberger ML. Axial length and ocular shape before and after the onset of myopia (Abstract) Optom Vis Sci. 2004;12S:24. [Google Scholar]
- 59.Winawer J, Wallman J. Temporal constraints on lens compensation in chicks. Vision Res. 2002;42:2651–2668. doi: 10.1016/s0042-6989(02)00300-0. [DOI] [PubMed] [Google Scholar]
- 60.Shaikh AW, Siegwart JT, Norton TT. Effect of interrupted lens wear on compensation for a minus lens in tree shrews. Optom Vis Sci. 1999;76:308–315. doi: 10.1097/00006324-199905000-00019. [DOI] [PubMed] [Google Scholar]