Abstract
Ovarian carcinoma is most frequently detected when disease has already disseminated intra-abdominally, resulting in a 5-year survival rate of less than 20% owing to complications of metastasis. Peritoneal ascites is often present, establishing a unique microenvironmental niche comprised of tumor and inflammatory cells, along with a wide range of bioactive soluble factors, several of which stimulate the EGF-receptor (EGFR). Elevated EGFR is associated with less favorable disease outcome in ovarian cancer, related in part to EGFR activation of signaling cascades that lead to enhanced matrix metalloproteinase expression and/or function. The available data suggest that modulating the expression or activity of the EGFR and/or matrix metalloproteinases offers opportunity for targeted intervention in patients with metastatic disease.
Keywords: EGF-receptor, invasion, metastasis, MMP-9, MT1-MMP/MMP-14, ovarian carcinoma
Epithelial ovarian carcinoma (EOC) is the leading cause of death from gynecologic malignancy, resulting in 15,520 deaths in the USA in 2008 [201]. When disease is confined to the ovary, 5-year survival is greater than 90%. Unfortunately, the majority of women with EOC are diagnosed with already disseminated intra-abdominal disease and have a low 5-year survival rate of less than 20%. Thus, while early detection is a pressing clinical problem, analysis of factors that promote metastatic dissemination can ultimately improve the survival of women with ovarian cancer.
Ovarian tumor microenvironment
A dualistic model for ovarian tumorigenesis has been proposed that is characterized by specific genetic alterations and unique molecular signatures [1–3]. In this classification system, low-grade carcinomas are often confined to the ovary, arise from a recognized precursor lesion, and have an indolent clinical course, whereas high-grade tumors are more clinically aggressive, presenting with metastases, and are associated with poor clinical outcome. In addition to analysis of genetic alterations, further investigation of contributions of the unique ovarian carcinoma microenvironment to tumor progression and metastasis may provide novel insight into factors that regulate disease progression. Women with ovarian tumors often present with diffuse and multifocal intraperitoneal metastases [4]. Unlike most solid tumors, hematogenous metastasis of EOC is uncommon. Instead, dissemination occurs as a result of shedding of malignant cells as single cells and multicellular aggregates (MCAs) (Figure 1) [9–11]. These shed tumor cells may also block peritoneal lymphatics [12], contributing to the accumulation of peritoneal ascites, which can further facilitate metastatic implantation. Single cells and MCAs adhere to peritoneal mesothelium, where upon adhesive interactions and localized proteolysis enable anchoring of proliferative secondary lesions [9,10]. An exception is found in atypical proliferative serous tumors or micropapillary proliferative serous tumors (grouped as ‘borderline’ or ‘low malignant potential’), the prognosis of which requires evaluation of peritoneal implants that often accompany these tumors. While patients with ‘noninvasive implants’ have a 10-year survival of practically 100%, a 34% mortality within 7 years is observed for those patients with invasive implants [12].
Figure 1. Ovarian tumor microenvironment.
(A) Malignant transformation and proliferation of OSE cells forms the primary ovarian tumor. (B) Ovarian cancer metastasis occurs by direct extension, via shedding of malignant cells into the peritoneal cavity. Exfoliated cells exist as MCAs or individual cells. Malignant ascites is common and is believed to promote dissemination of tumor cells intraperitoneally. (C) Intraperitoneal adhesion and localized invasion anchors secondary lesions in the peritoneum and omentum, followed by proliferation to establish disseminated intraperitoneal metastases.
EMT: Epithelial–mesenchymal transition; MCA: Multicellular aggregates; OSE: Ovarian surface epithelium.
An unique microenvironmental niche is thereby established in the peritoneal cavity, comprised of tumor cells, inflammatory cells and a plethora of soluble factors secreted by – or in response to – tumor cells, including growth factors, inflammatory mediators, bioactive lipids and proteolytic enzymes [13–20]. In addition, products of matrix invasion or partially resolved fibrosis, as well as shed cell surface components such as CA125 (MUC16) and E-cadherin ectodomain [16], also accumulate in ascites. Direct contact is maintained, via ascites, among primary tumor, suspended ascitic tumor cells and peritoneally anchored metastatic cells, establishing a unique mechanism for microenvironmental regulation of all stages of tumor progression (Figure 1).
EGF-receptor activation in ovarian cancer
There are many bioactive compounds within the ovarian tumor microenvironment, including those that stimulate cell-surface receptors such as the EGF-receptor (EGFR). The EGFR is a single membrane-spanning receptor tyrosine kinase (Figure 2A) and a therapeutic target for several human tumors (reviewed in [7,8,21–26]). Ligand binding promotes EGFR homo- and heterodimerization with related ErbB family members such as ErbB2/HER-2, activation of the catalytic intracellular tyrosine kinase domain, and phosphorylation of specific tyrosine residues of the receptor cytoplasmic domain. This leads to assembly of signaling complexes and stimulation of numerous downstream signaling cascades associated with cell growth and survival, angiogenesis and tumor metastasis [7,8,21–26].
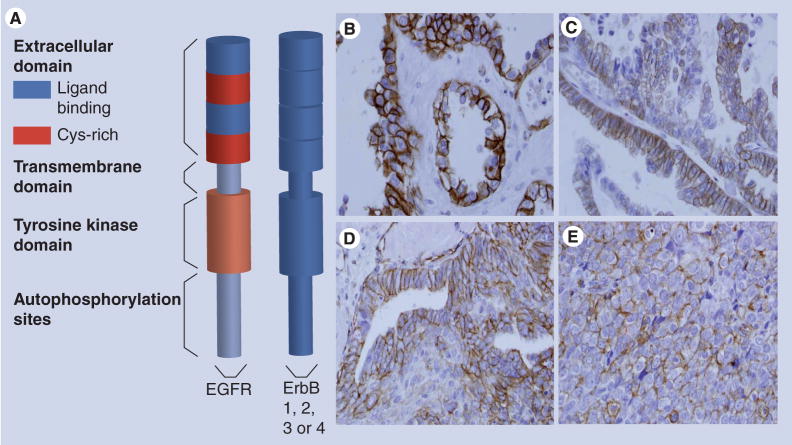
Figure 2. Activated EGF-receptor in ovarian tumors.
(A) Model of the EGFR. The extracellular N-terminal domain contains two subdomains that directly interact with ligand and two cysteine-rich subdomains. There is a single transmembrane domain that links the extracellular domain to the intracellular tyrosine kinase domain and the C-terminal tail that contains the autophosphorylation sites. The EGFR dimerizes with other ErbB receptors. (B–E) Immunohistochemical staining for activated (phospho-)-EGFR in ovarian tumors. Immunohistochemical analysis was performed retrospectively as described in [41], using antibodies to phospho-EGFR (1:400, Zymed®, CA, USA) according to standard procedures. EGFR activation (phospho-EGFR) was evident in 35% of the specimens analyzed (n = 146) and MMP-9 expression was statistically positively correlated with EGFR activation [41]. (B) Ovarian clear cell carcinoma; (C) mucinous ovarian carcinoma; (D) endometrioid ovarian carcinoma; (E) serous ovarian carcinoma.
EGFR: EGF-receptor.
Overall, elevated EGFR is associated with less favorable disease outcomes in a number of human tumors [5,7,8,24,26–29]. In ovarian cancer, estimates of the frequency of EGFR overexpression range from 10 to 70%, with EGFR expression reported on average in 48% of ovarian tumors (reviewed in [5]). There is no uniform agreement regarding the relationship between EGFR expression and various disease parameters, but despite differences in results from individual studies, there is evidence that dysregulation of EGFR expression or activity is a factor in ovarian cancer outcome (reviewed in [5,13]). When the EGFR level in tumors was compared with that of metaplastic ovarian surface epithelium and normal tubal epithelium as a reference control, EGFR overexpression was significantly correlated with aggressive disease characteristics [30]. In a multivariable analysis, EGFR expression status has been reported to be the most significant prognostic factor for disease-free and overall survival [31], and a meta-analysis revealed a relationship between EGFR and decreased survival [32].
There is accumulating evidence that activated (tyrosine phosphorylated, pEGFR), rather than total EGFR may be a more pertinent parameter for drawing relationships between EGFR and prognostics in human tumors [33–38]. There are a limited number of studies on pEGFR in ovarian tumors and, overall, little attention has been given to receptor activation status and disease parameters. In one study, 11.8% of ovarian tumors were positive for pEGFR, but no clinicopathological or survival differences were noted [39]. In another study, 24 heavily pretreated patients with epithelial ovarian cancer all had detectable EGFR and pEGFR (Y1148), suggesting that EGFR activation might be more evident in advanced disease [40]. We conducted an immunohistochemical analysis of pEGFR in microarrayed ovarian tumor tissues and found evidence for pEGFR in approximately a third of the samples (Figure 2B–D). EGFR activation (pEGFR) was positively correlated with matrix metalloproteinase (MMP)-9 expression, a protein associated with tumor invasion and metastasis [41]. Further analysis of a panel of paired primary tumors and peritoneal metastases (from the same patient) showed that approximately a third (35%) of metastases displayed elevated EGFR activation (pEGFR staining) relative to the paired primary tumor. MMP-9 expression was high in 100% of pEGFR-positive metastases [41]. Together, these in vivo data indicate that activated EGFR is present in ovarian tumor specimens. This is significant because EGFR activation stimulates numerous signaling cascades known to drive tumor proliferation and metastasis.
Although the exact mechanisms leading to EGFR activation in ovarian tumors are unknown at this time, three activators of the EGFR present in ascites (heparin-binding EGF [HB-EGF], endothelin-1 [ET-1] and lysophosphatidic acid [LPA]) have been studied in some detail. Although these three factors represent only a small subset of the total number of bioactive components in ascites, they illustrate the dynamic interplay between the ovarian tumor microenvironment and the potential for EGFR activation. There is accumulating evidence that the EGFR ligand HB-EGF is particularly important in ovarian cancer biology (reviewed in [42–44]). HB-EGF is elevated in advanced epithelial ovarian cancer tissues [45] and peritoneal fluid [46] when compared with ovarian cyst or normal controls. HB-EGF is present at higher levels than other EGFR ligands [45,46], and HB-EGF levels are significantly correlated with clinical outcome [45]. In addition to direct EGFR activation by ligands, transactivation can occur by stimulation of nonreceptor tyrosine kinases and/or G-protein coupled receptors (GPCRs) (reviewed in [5,24,44,47–50]). Activation of GPCRs by ligands such as ET-1, LPA and others can indirectly activate the EGFR through stimulation of the ADAM family of cell surface metalloproteinases, leading to cleavage of membrane-bound EGF family precursors such as HB-EGF [44,47–50]. An alternate mechanism for EGFR transactivation by GPCRs occurs by GPCR-dependent activation of nonreceptor tyrosine kinases such as c-Src [44,47–50]. These and other findings illustrate that pathophysiological levels of EGFR activators in ovarian peritoneal fluid and ascites regulate EGFR activity, providing a mechanism for modulation of metastasis-associated genes such as matrix metalloproteinases (MMPs).
EGFR regulation of MMP-9 & -14
MMPs are a family of zinc-dependent metallo-endopeptidases with activity directed against extracellular substrates, membrane-anchored receptors and soluble proteins and polypeptides (reviewed in [51,52]). MMP family proteinases share a common domain structure including a propeptide necessary for maintaining pro-enzyme latency, the catalytic domain containing the conserved zinc-binding consensus sequence HExxHxxGxxH, and a C-terminal hemopexin-like domain important for substrate recognition. MMP activity is linked to multiple physiologic and pathologic processes, including morphogenesis, angiogenesis, wound repair, arthritis and tumor invasion and metastasis.
Several MMPs, including MMP-1 (interstitial collagenase), -2 (gelatinase A), -7 (matrilysin), -9 (gelatinase B), -13 (collagenase-3) and -14 (membrane type 1 MMP, MT1-MMP) have been implicated in ovarian cancer pathology (reviewed in [51–53]). The soluble gelatinases MMP-2 and -9 and the membrane-anchored collagenase MT1-MMP (MMP-14) are the most extensively studied in ovarian cancer (Figure 3). High expression of these three MMPs has been detected in epithelial and stromal compartments of ovarian tumors [54]. MMP-2 and MMP-9 are present in ovarian tumor ascites [55,56]. High MMP-9 expression was associated with decreased overall survival [57,58] and high epithelial and stromal expression of MMP-2, MMP-9 and MT1-MMP were each significantly associated with shorter disease-specific survival [54]. Interestingly, expression of MMP-9, but not MMP-2, in short-term primary cultures of EOC cells derived from primary ovarian tumors, intraperitoneal metastases or ascites decreased with time in culture, suggesting that MMP-9 levels in tumor cells are regulated by the tumor microenvironment [55]. This observation is consistent with the positive correlation between EGFR activation and MMP-9 expression in ovarian tumor tissues [41] and the presence of EGFR activators in ascites fluids.
Figure 3. MMP domain structure.
(A) MT1-MMP (MMP-14) is a transmembrane protease comprised of a pro-peptide (processed intracellularly by furin in the secretory pathway; not shown) and a catalytic domain containing the Zn2+-binding consensus sequence HExxHxxGxxH. A flexible hinge region connects the catalytic domain to the hemopexin-like domain. The hemopexin-like domain may contain a dimerization interface and is important in substrate recognition. Following this region, a short stalk connects the transmembrane domain to the short cytoplasmic tail. In addition to degrading protein substrates such as interstitial (type I) collagen, MT1-MMP also catalyzes activation of pro-MMP-2. This reaction proceeds via an unusual mechanism requiring a trimeric complex among MT1-MMP, TIMP-2 and pro-MMP-2. Anchoring of pro-MMP-2 via trimeric complex formation enables cleavage of the pro-peptide domain of pro-MMP-2 by a second molecule of MT1-MMP, releasing soluble active MMP-2. (B) MMP-9 is initially secreted in zymogen form. Proteolytic processing of the pro-peptide region, catalyzed by numerous proteinases in the extracellular mileu, exposes the active Zn2+-binding catalytic domain. The active site of MMP-9 is also connected via a flexible hinge to a hemopexin-domain that imparts substrate specificity.
MMP: Matrix metalloproteinase; MT1: Membrane type 1; TIMP-2: Tissue inhibitor of metalloproteinase-2.
Transcriptional mechanisms
MMP gene regulation is largely due to transcriptional mechanisms, although post-transcriptional and epigenetic mechanisms also contribute to total MMP levels [59]. Regulatory sequences in the promoters of MMP genes are responsive to exogenous stimuli including growth factors, cytokines or bioactive lipids, such as those found in the ovarian tumor microenvironment. Signaling pathways implicated in MMP expression converge on numerous transcription regulatory sequences including AP-1, ETS, Sp1, β-catenin/TCF-4 and NF-κβ sites (reviewed in [59–61]). Increased transactivation through AP-1 or PEA3 occurs largely through mitogen-activated protein kinases (MAPKs) [59]. EGF activates the ERK, JNK and p38 MAPKs in EGF-responsive ovarian tumor cells [62], and EGFR stimulation of the ERK/MAPK pathway has been reported to activate several MMP genes, including MMP-1, MMP-3, MMP-7, MMP-9 and MT1-MMP [61]. EGF stimulation also promotes activation of AKT/PKB, a downstream target of PI3K [8,23,62].
EGF induces MMP-9 in ovarian tumor cells [43,63–65], and EGF regulates MT1-MMP expression in ovarian cell lines [65] and in other cell types [66,67]. Inhibition of the MAPK or PI3K signaling cascades interfered with EGF-stimulated pro-MMP-9 production and ovarian tumor cell in vitro invasion, indicating that multiple EGFR-regulated kinase pathways converge to regulate MMP-9 gene expression [62]. Interestingly, EGF does not induce MMP-9 in every ovarian tumor cell line expressing EGFR [63], suggesting that in some tumor cells signaling components downstream of the EGFR may be disrupted. Ultimately, cell- and disease-specific regulation of MMPs relies on integration of multiple signaling pathways and effective activation or recruitment of essential transcription factors.
A particular role for the ETS family transcription factor PEA-3 in EGF-dependent regulation of MMP-9 and MT1-MMP has been revealed [65]. PEA-3 is of interest because it is not detected in normal ovarian tissue, but is overexpressed in human ovarian tumors, and PEA-3 expression in ovarian tumors has been correlated with poor overall survival [68–71]. In one study, 92% of stage III and IV ovarian tumors (both primary lesions and metastases) expressed elevated PEA-3 [69], suggesting a relationship between PEA-3 and disease progression. In a variety of cell types, PEA-3 has been reported to regulate several MMPs that are implicated in cancer cell invasion, including MMP-1, MMP-7, MMP-9, MMP-13 and MT1-MMP [61,72–77]. Notably, there is significant overlap of MMPs regulated by EGF [62,63,65] and PEA3 [73], suggesting that PEA-3 may be a downstream mediator of EGFR activation.
We find that EGFR regulates PEA-3 nuclear localization and binding to endogenous MMP-9 and MT1-MMP promoters, and disruption of PEA-3 by short interfering RNA (siRNA) disrupts EGFR-stimulated MMP expression and cell invasion. Furthermore, ectopic expression of PEA-3 alone is sufficient to induce MMP-9 and MT1-MMP, and promote an invasive phenotype nearly equivalent to that detected in response to EGF [65]. Although EGFR regulates multiple ETS family transcription factors, there are distinctions in ETS protein activation and subsequent MMP targets in different model systems with selective regulation of MMP-9 and MT1-MMP by PEA-3 detected in ovarian tumor cells [65]. As tumor- specific changes in the transcription factor network (such as elevated PEA-3) may cooperate or synergize with EGFR signaling to regulate distinct cohorts of MMPs, further study of these potential inter-relationships in human tumors may reveal new avenues for therapeutic intervention.
EGFR regulation of MMP localization
Beyond transcriptional regulation of MMP gene expression, it is clear that localization of MMP activity to the cell surface contributes significantly to matrix remodeling events that occur during cellular migration and invasion. Although MMP-9 is a secreted protease, MMP-9 can also associate with the cell surface of diverse cell types ([78–84], reviewed in [85]). The association of pro-MMP-9 with the cell surface occurs by multiple mechanisms with distinct outcomes. Two proteins have been reported to mediate surface targeting (α2 [IV] and CD44), RECK inhibits MMP-9 and LRP directs the internalization of pro-MMP-9 [85]. In ovarian tumor cells, surface localization of pro-MMP-9 is stimulated by EGF and requires PI3K activity [62]. EGF promotes pro-MMP-9 binding to the cell surface through a mechanism that is independent of extracellular enzyme concentration. Interestingly, inhibition of PI3K activity abolishes EGF-induced cell surface association of pro-MMP-9, whereas inhibitors of MAPKs only partially block the response. These data suggest that EGF stimulation promotes the cell surface expression of an unidentified pro-MMP-9-binding component(s). As PI3K has been implicated in intracellular membrane and protein trafficking events, PI3K-mediated trafficking of pro-MMP-9 or its binding component to the cell surface may be a potential mechanism of gelatinase association.
While the function(s) of surface-localized MMP-9 in ovarian cancer remain unknown, surface localization may promote directed pericellular proteolysis. This is supported by data showing that MMP-9 is preferentially localized to the filopodial compartment in EGF-stimulated cells (Figure 4A & B), suggesting a contribution of MMP-9 to migratory and/or invasive activity. Furthermore, surface MMP-9 is associated with tumor cell migration and invasion in other model systems [80,81,83,84]. Thus, mechanisms that regulate MMP-9 expression and localization, such as EGFR activation, may play particularly important roles in the net MMP-9 activity present at the pericellular space.
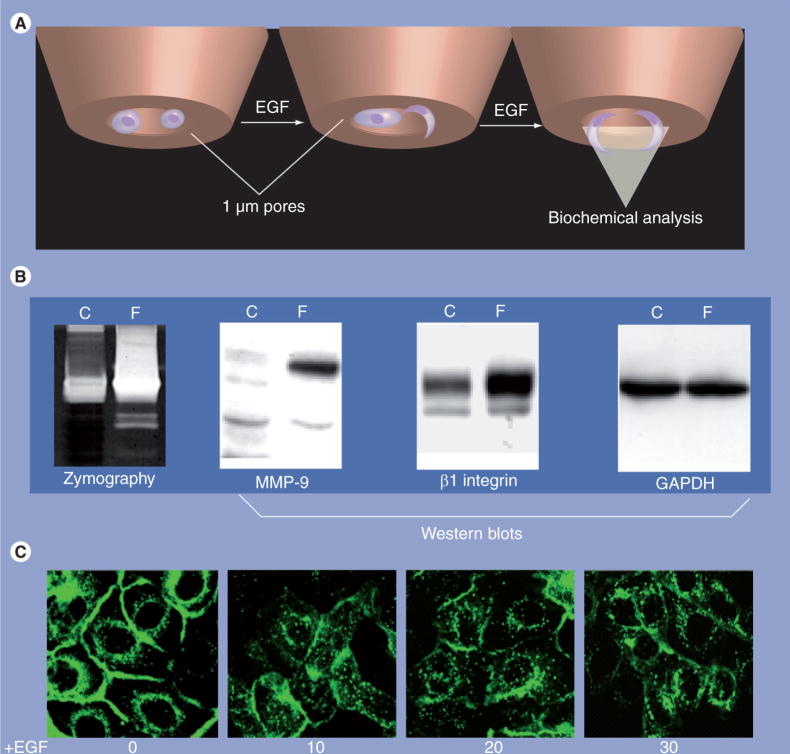
Figure 4. EGF-receptor activation regulates matrix metalloproteinase localization.
(A & B) Analysis of MMP-9 in filopodia of EGF-stimulated cells. OVCA429 cells (1 × 106) were serum-starved overnight and plated onto six-well plates containing 1.0 μm pore size membrane inserts. Each bottom chamber contained 2.5 ml of serum-free medium containing EGF. Following a 12-h incubation (empirically determined based on the lack of visible nuclei on the filter bottom), lysates were individually collected from the top chamber (cellular fraction) or the bottom chamber (filopodial fraction). Lysates were pooled from replicate chambers, normalized for protein concentration, and an equal amount (40 μg) of filopodial or total cellular protein was evaluated by gelatin zymography or western blotting, as indicated. Note that MMP-9 protein and activity are enriched in the filopodial pool following EGF treatment. β1 integrin is also concentrated in filopodia, while GAPDH is evenly distributed. (C) Cells expressing GFP-tagged MT1-MMP were seeded onto 35 mm Becton glass bottom dishes and serum-starved overnight prior to treatment with EGF (25 nM). Images were acquired at the indicated time points using a Nikon TE2000 inverted microscope (Nikon Inc., NY, USA) with a 60× oil immersion objective with numerical aperture 1.40 and analyzed using Metamorph Software (Molecular Devices, PA, USA). Note that EGF induces rapid internalization of MT1-MMP.
C: Cellular fraction; F: Filopodial fraction; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; GFP: Green fluorescent protein; MMP: Matrix metalloproteinase; MT1: Membrane type 1.
While there is an expansive body of literature outlining the central role of EGFR in the architecture of intracellular signaling networks, less in known about its ability to regulate transmembrane protein trafficking. EGFR signaling may modify the molecular landscape of the cell surface by regulating the trafficking, and consequently the function, of specific cell surface proteins, highlighting an underappreciated outcome of EGFR activation. Given the central role of MT1-MMP in cancer progression and metastasis, multiple mechanisms have evolved for transcriptional and post-translational regulation of MT1-MMP enzymatic activity. Using fluorescence resonance energy transfer imaging to visualize MT1-MMP activity in live cells, EGFR activation led to localization of active MT1-MMP at the leading edge of migrating cells [86].
Additional data support an EGF-dependent effect on MT1-MMP trafficking in ovarian cancer cells, as MT1-MMP is rapidly internalized from the cell surface following EGF stimulation (Figure 4C). In contrast to EGFR, which is endocytosed via clathrin-coated pits [87], internalized MT1-MMP is localized with caveosomal markers and is disrupted by pretreatment of cells with methyl-β-cyclodextrin, which disrupts caveolae [88]. The ability of EGFR activation to affect the trafficking of multiple transmembrane proteins that occupy caveolae (i.e., α2 integrin [87]; MT1-MMP, Figure 4C) suggests that EGF may regulate internalization of the caveolar compartment, and thereby alter the surface profiles of proteins occupying these domains. EGFR-dependent alterations in cell surface localization of MMP-9 and MT1-MMP may have significant consequences for tumor cell behavior and represent functions that are distinct from those of the stromally derived MMPs in ovarian cancer.
Actions of MMPs in ovarian cancer
Growth of ovarian tumors involves the formation of a provisional fibrin–fibronectin matrix around the tumor, which is subsequently replaced by a mature tumor stroma [89]. Fibrin degradation products, resulting from proteolysis of the provisional stroma, are elevated in the sera of women with advanced ovarian cancer [90,91]. In addition, polypeptides resulting from degradation of both fibrin and collagen, as well as procollagen peptides indicative of new collagen biosynthesis, are prevalent in carcinomatous ascites, as well as sera from women with ovarian cancer [92–95]. These data implicate aberrant proteolysis as a contributing factor to ovarian cancer pathobiology.
Proteolytic activity is important at several stages in intraperitoneal metastasis, including dissolution of cell–cell and cell–matrix junctions to enable shedding from the primary tumor surface, disruption of mesothelial cell junctions to facilitate access to the submesothelial matrix, intraperitoneal anchoring in the submesothelial interstitial collagen (types I and III) stroma, and for tumor angiogenesis [14,54,96–99]. MMPs have been implicated in all of these processes, and thus play a key role in ovarian cancer pathobiology (reviewed in [53]). Activity of these proteinases has been linked to key events in ovarian cancer metastasis, including detachment of primary tumor cells from the ovarian surface [100], shedding of cell–cell adhesion molecules such as the E-cadherin ectodomain [41,56], attachment to peritoneal mesothelium [14], invasion of submesothelial collagen [9–11,101–103] and removal of matrix barriers that constrain proliferation of secondary lesions [99–104]. As EGFR regulation of MMP-9 and MT1-MMP expression and/or activity has been demonstrated in ovarian cancer cells and tissues, the remaining discussion will focus on these two proteinases.
MMP-9 in ovarian cancer
Gelatinolytic soluble MMPs (MMP-2 and MMP-9) are readily detectable in ascites fluid obtained from women with ovarian cancer [41,55,105], suggesting prevalence in the ovarian tumor microenvironment. Although the cellular source of soluble MMPs in ascites cannot be determined, analysis of a panel of established ovarian cancer cell lines revealed expression of MMP-9 that was not detected in normal ovarian epithelium [106]. Further examination of MMP expression by short-term primary cultures of ovarian cancer cells showed presence of both latent and activated MMP-2 (indicative of constitutive MT1-MMP activity), while MMP-9 was detectable only in very early passage cells (passage 1–3), suggesting regulation of expression by a factor(s) in the in vivo microenvironment [55]. In human ovarian tumors, MMP-9 immunoreactivity is detectable in both the epithelial and stromal compartments [54,56,107], with epithelial MMP-9 immunoreactivity increased in malignant relative to borderline ovarian tumors [107]. Ovarian tumor-associated MMP-9 activity has been detected by gelatin zymography [58,108]. Three distinct studies have localized MMP-9 mRNA expression to the epithelial compartment of human ovarian tumors using in situ hybridization analysis [108–110]. High MMP-9 levels in both primary tumors and ascites correlate with more malignant tumors, disease recurrence and poor survival in univariate and multivariate analyses [57,107,110,111].
Additional in vitro and in vivo studies have provided evidence that MMP-9 activity may contribute to ovarian tumor progression. Using immunohistochemical analysis, expression of MMP-9 was shown to be elevated in preneoplastic lesions relative to normal ovarian tissue [112]. Expression was associated with loss of basement membrane and morphological transformation, suggesting a role for MMP-9 in early events in ovarian tumorigenesis. MMP-9 induces the release of biologically active VEGF from ovarian cancer cells [113]. A reciprocal relationship between VEGF and MMP-9 has been demonstrated, as VEGF-expressing tumor xenografts stimulate MMP-9 expression [114]. Further in vivo studies have demonstrated that both tumor and host MMP-9 contribute to tumor angiogenesis and progressive growth [109]. In addition to promoting angiogenesis, MMP-9 has also been shown to localize to the ovarian cancer cell surface and contribute to invasive activity, as function-blocking anti-MMP-9 antibodies block in vitro invasion [62,63].
A recently described role for MMP-9 in ovarian tumor progression involves processing of the extracellular domain (ectodomain) of the cell–cell adhesion molecule E-cadherin. Interestingly, analysis of MMP-9 and E-cadherin levels in ovarian tumors show an increase in the MMP:E-cadherin ratio with increasing disease stage and a significantly higher death rate in women whose tumors showed a high MMP:E-cadherin ratio [115]. Both activation of the EGFR and engagement of collagen binding integrins have been shown to downregulate E-cadherin levels in ovarian cancer cells [41,56]. A common mechanism for E-cadherin downregulation downstream of integrin signaling or EGFR activation is via ectodomain shedding as a consequence of MMP-9 induction. Specific blocking of MMP-9 activity using an anticatalytic antibody or siRNA abolishes E-cadherin ectodomain shedding [41,56]. Furthermore, addition of exogenous activated MMP-9 to ovarian cancer cells at a concentration representing the average level found in human ovarian cancer ascites (90 ng/ml [41]) decreases junctional E-cadherin staining. The effect of MMP-9 on E-cadherin junctional integrity was confirmed by analysis of MMP-9 transfected ovarian cancer cells that exhibit loss of the epithelial phenotype and have no detectable E-cadherin mRNA [41]. This is supported by analysis of human ovarian tumors, showing that tumors with high MMP-9 expression exhibit low or absent E-cadherin staining [56]. The shed full-length E-cadherin ectodomain (sEcad) accumulates to high concentrations (12 μg/ml) in ascites from women with ovarian cancer, and is significantly elevated relative to ascites from women with benign gynecologic conditions [56]. In contrast to other tumors wherein shed E-cadherin ectodomain is released into the circulation, the unique microenvironment of ovarian tumors enables the primary tumor to maintain direct contact with sEcad-rich ascites, providing a physiologically relevant model to address sEcad function. Studies show that the sEcad ectodomain disrupts preformed junctions and increases cell dispersion. Together these data support a novel mechanism whereby MMP-9 may promote intraperitoneal metastatic dissemination by catalyzing E-cadherin ectodomain shedding, and sEcad-induced cellular disaggregation of cellular aggregates [41,56].
MT1-MMP in ovarian cancer
MT1-MMP (MMP-14) is a transmembrane collagenolytic MMP that is essential to matrix remodeling during physiological processes [116,117] and key to acquisition of a metastatic phenotype in a variety of tumor cells, including lung, colon, breast, cervical and ovarian carcinomas [118–121]. Ovarian carcinomas express abundant MT1-MMP [65,96,97,99,104,110,122] and, consequently, high MT1-MMP levels correlate with poor survival of women with ovarian cancer [110]. Using both in situ hybridization and immunohistochemical analysis, MT1-MMP expression has been detected in both the stromal and epithelial compartments of ovarian tumors, and strong epithelial MT1-MMP is a significant predictor of poor disease-specific survival in univariate and multivariate analyses [54,104,111]. Similar results were obtained by analysis of human ovarian tumors grown in SCID mice, demonstrating enhanced human MT1-MMP in high-grade tumors with a minor contribution from the murine ovarian stroma [123]. In addition to functioning as a membrane-anchored collagenase, MT1-MMP also acts as a key physiologic activator of proMMP-2 (reviewed in [121]). Constitutive pro-MMP-2 activation has been observed in primary cultures of ovarian cancer cells, indicative of active cellular MT1-MMP [124]. This is supported by a clinical study showing that levels of active MMP-2 are significantly higher in stage III/IV ovarian cancers relative to stage I/II tumors, and that active MMP-2 levels correlate with disease progression [125].
Numerous studies have demonstrated that acquisition of MT1-MMP expression alone promotes cell migration [126,127], invasion of 3D collagen [101,102,104,121,128,129] and growth within a matrix-constrained 3D microenvironment [100,129] – key cellular processes that drive ovarian pathology. Metastasizing ovarian cancer cells often encounter a collagen-rich environment, as the submesothelial matrix is comprised primarily of interstitial collagens (types I and III), and ovarian tumors induce a fibro-proliferative response characterized by increased synthesis of collagen in the peritoneal cavity [94,130,131]. Indeed, even the noninvasive peritoneal implants found in 30% of women with atypical proliferative serous tumors (‘low malignant potential’ or ‘borderline’) often have desmoplastic features with significant fibrosis [12]. Ovarian cancer cells adhere preferentially to interstitial collagens via cellular integrins, activating a src-dependent signaling pathway that leads to upregulation of MT1-MMP expression [104]. MT1-MMP activity is likely key for peritoneal anchoring of invasive metastatic lesions, as inhibition of this proteinase blocks penetration of 3D collagen gels [101,102,123,132]. Successful growth of invasive implants to seed intraperitoneal metastasis also requires proliferation within the confines of the interstitial collagen-rich submesothelial matrix [133–136], and the ability of ovarian cancer cells to survive long-term and proliferate in 3D collagen gels is enhanced by MT1-MMP expression [15]. This observation is supported by data showing that expression of MT1-MMP in paired peritoneal metastases is greater than or equal to that of the primary tumor in more than 80% of cases [15].
In addition to its fairly well-described role in matrix invasion and expansive 3D growth, recent data support a novel role for MT1-MMP in ovarian cancer metastasis. An early event in the initial dissemination of ovarian cancer involves exfoliation of cells from the primary ovarian tumor into the peritoneal cavity as single cells or multicellular aggregates (MCAs) that range in size from 30 to 200 μm, survive anoikis, and form a free-floating population of highly malignant cells [9,10,13,137–139]. MCAs express higher levels of MT1-MMP relative to two-dimensional cultured ovarian cancer cells, and acquisition of MT1-MMP expression promotes cellular detachment and MCA formation [100]. Together these data suggest MT1-MMP activity is necessary to promote metastasis of primary ovarian tumor cells via enhanced tumor cell shedding as MCAs, as well as for subsequent intraperitoneal anchoring of malignant MCAs and expansive growth in the submesothelial matrix.
Conclusion & future perspective: opportunities for targeted intervention
As discussed in this review, the unique microenvironmental niche of ovarian cancer is rich in activators of the EGFR; EGFR activation stimulates signaling cascades that lead to increased expression and altered localization of certain MMPs such as MMP-9 and MT1-MMP, and MMPs contribute to ovarian tumor pathobiology. It then follows that modulating the expression or activity of the EGFR and/or MMPs offers an opportunity for targeted intervention in patients with metastatic disease (Figure 1). Metastatic processes have been proposed to offer new therapeutic targets based on blocking metastatic dissemination or disruption of reciprocal interactions between tumor cells and the microenvironment [140]. Interventions directed to the EGFR or MMPs fit these criteria (Figure 1). Based on the known consequences of EGFR activation in ovarian tumor cells and pre-clinical studies of EGFR antagonists, inhibition is predicted to confer benefit due to modulation of cell proliferation and survival, chemoresistance, epithelial–mesenchymal transition, migration and invasion (Figure 1) [13,25]. Similarly, selective targeting of specific MMPs may reduce tumor cell shedding, decrease proliferation and angiogenesis through inhibition of receptor ligand shedding, alter cell:cell and cell:matrix adhesive properties, inhibit epithelial–mesenchymal transition by reducing E-cadherin cleavage and decrease tumor cell invasion (Figure 1) [13,51,141–143].
Despite the recognized potential of these targets, the current status of MMP inhibitors and EGFR antagonists in cancer treatment is mixed. Preclinical trials of the broad-spectrum MMP inhibitor batimastat in mice harboring ovarian cancer xenografts led to solidification of the tumor, resolution of ascitic disease and increased survival [144]. In Phase I/II trials of a related compound, marimastat, ovarian cancer patients were monitored for a change in the rate of rise of the serum tumor marker CA-125 to assess drug effect. Although a promising decrease in the CA-125 detection rate was observed, a definitive correlation with drug efficacy can only be determined through Phase III trials [145–147]. Overall, clinical trials of broad-spectrum MMP inhibitors failed to provide a significant survival advantage in a variety of advanced stage cancers and were accompanied by significant side effects [142,143]. Several reasons have been offered to account for these clinical failures. One issue was a lack of sufficient information on the beneficial roles of specific MMPs in normal physiology, thereby contributing to the observed toxicities of broad-spectrum inhibitors [142]. New research suggests that MMPs have activities throughout cancer progression, so clinical trials focused solely on advanced disease may not have provided optimal benefit [51,142,143]. Furthermore, although MMPs play important roles in metastatic disease [51,148], MMPs also display protective effects in certain stages of tumor progression [142,143]. Many researchers now postulate that more selective targeting of specific MMPs based on validation of their substrates, coupled with a better understanding of the functions and contributions of individual MMPs to distinct stages of cancer progression, may result in more favorable outcomes with the next generations of MMP inhibitors.
Several EGFR-targeted therapeutics, including monoclonal antibodies (cetuximab and panitumumab), and small-molecule tyrosine kinase inhibitors (TKIs) (gefitinib, erlotinib and lapatinib) have been approved by the US FDA for cancer therapy. Additional agents are in development and being evaluated in clinical trials [25,26,149]. EGFR antagonists (TKI or monoclonal antibody) provide clear clinical benefit in certain cancers, but clinical trial results in ovarian tumor patients have been disappointing. The outcomes of ovarian cancer trials are summarized in a recent review [150], but briefly, results from Phase II trials of EGFR-targeted therapeutics suggest that their activity as single agents is modest in unselected women with advanced or recurrent ovarian cancer. In general, antitumor activity against platinum- and taxane-resistant ovarian cancers may be best characterized as cytostatic rather than cytotoxic [150].
As with MMP inhibitors, there are many likely reasons for these findings. First, EGFR inhibitors are effective in only a subset of patients for the best-studied tumors. Approximately 10–15% of patients with non-small-cell lung cancer have tumors that express activating EGFR mutations, and these patients are often highly responsive to treatment with erlotinib or gefitinib [25,26,149]. Interestingly, in one study of gefitinib in patients with recurrent or persistent epithelial ovarian cancer, the only objective response occurred in a patient with an EGFR mutation similar to those detected in non-small-cell lung cancer [151]. Another factor predictive of positive response is increased EGFR gene copy number [26,149]. Although estimates of increased EGFR protein expression in ovarian cancer exceed 50% on average [5], recent studies of EGFR gene copy number are significantly lower, ranging from 10–22% of ovarian cancer cases [30,152,153], suggesting that anti-EGFR therapies may be effective in a more select number of ovarian cancer cases. As with non-small-cell lung cancer, it will be important to validate predictive markers of patient response and identify ovarian cancer patients with tumors most likely to respond to EGFR antagonists [25,150,154]. This type of preselection is standard in breast cancer, for example, where the estrogen receptor status of a tumor plays a major role in the therapeutic decision-making strategy.
A second concern is that the doses of EGFR-based therapeutics may not optimally block EGFR signaling. In one study of gefitinib-treated patients with recurrent epithelial ovarian cancer, analysis of pre- and post-treatment tumor biopsies revealed that although a decrease in EGFR and pEGFR was evident in somewhat more than 50% of patients, detectable levels of pEGFR were present in all tumor biopsies regardless of treatment [40]. Especially under circumstances of elevated EGFR, partial inhibition may result in sufficient residual receptor activity to stimulate key signal transduction pathways associated with tumor growth, survival and metastasis. The development of imaging technologies for activated forms of the EGFR may help to facilitate patient selection and monitoring of patient response to EGFR signaling inhibitors, leading to more successful treatment choices [155]. Thirdly, in advanced cancers, other mutations that bypass or compensate for the EGFR may be present and have a negative impact on the success of EGFR-targeted therapies [26,145,150].
Despite the current limitations acknowledged previously, as we increase our understanding of the EGFR and/or MMPs in ovarian cancer, additional opportunities for targeted therapy will likely emerge. The presence of EGFR activators in the ovarian tumor microenvironment predicts that dual targeting of EGFR and EGFR activators such as HB-EGF or GPCRs would lead to more complete inhibition of EGFR signaling compared with monoclonal antibodies or TKIs alone. Targeting HB-EGF in preclinical in vivo models decreases peritoneal dissemination of ovarian tumor cell xenografts [42,156], suggesting that combinations of HB-EGF inhibitors and EGFR antagonists may improve outcomes. Similarly, ET-1 binding to its receptor transactivates the EGFR, and coadministration of an endothelin A receptor antagonist enhanced the antitumor effects of gefitinib in an ovarian tumor xenograft model [157]. Combined targeting of EGFR and specific MMPs also represent plausible approaches. The ADAM family of metalloproteinases is responsible for the ectodomain shedding of the ligands that are involved in direct (HB-EGF) and trans-EGFR activation, supporting the potential of these proteinases as novel antitumor targets [141]. EGFR activation promotes angiogenesis, and MMP-9 is a critical proangiogenic molecule [51,142], suggesting that dual inhibition of EGFR and select MMPs could be beneficial in advanced disease. Indeed, therapies targeted to EGFR and angiogenesis based on combining two agents highly selective against VEGF and EGFR, respectively, or a single dual targeted agent are under active investigation in many cancers [158]. The selection of optimal therapeutic combinations based on sound research holds potential to target metastatic disease and ultimately improve the efficacy of current treatment protocols [140]. Identification and validation of effective targets for management of metastatic disease would extend the future options for women with ovarian cancer [140,159].
Executive summary
Ovarian tumor microenvironment
▪ The peritoneal cavity provides a unique microenvironmental niche that facilitates direct contact between primary tumor, suspended ascitic tumor cells and anchored metastastic cells via carcinomatous ascites.
EGFR activation in ovarian cancer
▪ EGF-receptor (EGFR) is expressed in 48% of ovarian tumors; activated EGFR is present in 10–35% of ovarian tumors.
▪ Multiple EGFR ligands are present in ascites.
▪ In addition to ligand activation, EGFR transactivation is stimulated by ascites components.
EGFR regulation of MMP-9 & -14
▪ EGFR activation regulates matrix metalloproteinase (MMP) expression through transcriptional activation.
▪ The ETS family transcription factor PEA-3 is implicated in EGF-dependent MMP regulation, and PEA-3 expression is correlated with disease progression.
▪ Nontranscriptional mechanisms of MMP regulation occur via modulation of pericellular localization of transmembrane (MT1-MMP) or membrane-associated (MMP-9) proteinases.
Actions of MMPs in ovarian cancer
▪ MMPs participate in degradation of the provisional tumor stroma, dissolution of cell–cell and cell–matrix junctions to promote shedding of cells from primary tumor, intraperitoneal anchoring of metastatic cells and tumor angiogenesis.
▪ MMP-9 releases biologically active components, including VEGF and E-cadherin ectodomain, into ascites.
▪ MT1-MMP participates in initial shedding, intraperitoneal anchoring and regulation of expansive growth in the submesothelial matrix.
Opportunities for targeted intervention
▪ EGFR activation leads to increased expression and altered localization of MMPs, and MMPs contribute to ovarian tumor pathobiology.
▪ Modulation of the expression or activity of EGFR and/or MMPs offers opportunities for targeted intervention in metastatic disease processes.
▪ Inhibition of EGFR and/or MMP activity may impact cell shedding, cell proliferation and survival, chemoresistance, epithelial–mesenchymal transition, migration/invasion and angiogenesis.
▪ More selective MMP inhibitors may promote more favorable outcomes.
▪ Patient preselection, to identify women with activated EGFR and/or high MMP levels, may enhance therapeutic efficacy.
▪ Currently used doses of EGFR-based therapeutics may be insufficient to block EGFR signaling.
▪ Optimal therapeutic combinations hold potential to target metastatic disease.
Acknowledgments
Financial & competing interests disclosure
This work was supported by National Institutes of Health Research Grants CA90492 (LGH), CA86984 (MSS), CA86984-S1 (NMM) and CA109545 (MSS & LGH). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Contributor Information
Laurie G Hudson, Department of Pharmaceutical Sciences, College of Pharmacy, University of New Mexico, Albuquerque, NM, USA.
Natalie M Moss, Department of Cell & Molecular Biology, Northwestern University, Chicago, IL, USA.
M Sharon Stack, University of Missouri School of Medicine, Department of Pathology & Anatomical Sciences, 1 Hospital Drive, M214C Medical Sciences Building, Columbia, MO 65212, USA, Tel.: +1 573 884 7301, Fax: +1 573 884 8104, stackm@missouri.edu.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Shedden KA, Kshisager MP, Schwartz DR, et al. Histologic type, organ or origin and wnt pathway status: effect on gene expression in ovarian and uterine carcinomas. Clin Can Res. 2005;11:2123–2131. doi: 10.1158/1078-0432.CCR-04-2061. [DOI] [PubMed] [Google Scholar]
- 2.Shih IM, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Path. 2004;164:1511–1518. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz DR, Kardia SLR, Shedden KA, et al. Gene expression in ovarian cancer reflects both morphology and biological behavior, distinguishing clear cell from other poor-prognosis ovarian carcinomas. Can Res. 2002;62:4722–4729. [PubMed] [Google Scholar]
- 4.Scully RE, Young RH, Clement PB. Atlas of Tumor Pathology (Third Series, Fascicle 23) Armed Forces Institute of Pathology; Washington, DC, USA: 1998. Tumors of the ovary, maldeveloped gonads, fallopian tube and broad ligament. [Google Scholar]
- 5▪▪.Lafky JM, Wilken JA, Baron AT, Maihle NJ. Clinical implications of the ErbB/epidermal growth factor (EGF) receptor family and its ligands in ovarian cancer. Biochim Biophys Acta. 2008;1785(2):232–265. doi: 10.1016/j.bbcan.2008.01.001. This article is an exceptionally thorough review of the literature on the expression of ErbB receptors and ligands and clinical correlates in ovarian cancer. [DOI] [PubMed] [Google Scholar]
- 6.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5(5):341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 7.Mendelsohn J, Baselga J. Epidermal growth factor receptor targeting in cancer. Semin Oncol. 2006;33(4):369–385. doi: 10.1053/j.seminoncol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Normanno N, De Luca A, Bianco C, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366(1):2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Burleson KM, Hansen LK, Skubitz APN. Ovarian carcinoma spheroids disaggregate on type I collagen and invade live human mesothelial cell monolayers. Clin Exp Metastasis. 2004;21:685–697. doi: 10.1007/s10585-004-5768-5. [DOI] [PubMed] [Google Scholar]
- 10.Burleson KM, Casey RC, Skubitz KM, et al. Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecol Oncol. 2004;93:170–181. doi: 10.1016/j.ygyno.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 11.Burleson KM, Boente MP, Pambuccian SE, et al. Disaggreation and invasion of ovarian carcinoma ascites spheroids. J Translational Med. 2006;4:1–16. doi: 10.1186/1479-5876-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seidman JD, Russell P, Kurman RJ. Surface epithelial tumors of the ovary. In: Kurman RJ, editor. Blaustein’s Pathology of the Female Genital Tract. Springer Press; Germany: 2002. pp. 791–820. [Google Scholar]
- 13.Hudson LG, Zeineldin R, Silberberg M, Stack MS. Activated epidermal growth factor receptor in ovarian cancer. Cancer Treat Res. 2009 doi: 10.1007/978-0-387-98094-2_10. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14▪▪.Kenny HA, Krausz T, Yamada SD, et al. Use of a novel 3D culture model to elucidate the role of mesothelial cells, fibroblasts and extracellular matrices on adhesion and invasion of ovarian cancer cells to the omentum . Int J Cancer. 2007;121:1463–1472. doi: 10.1002/ijc.22874. Authors describe an elegant 3D organotypic model system to mimic omental metastasis of ovarian cancer. This model has efficacy for mechanistic evaluation of early events in intraperitoneal dissemination. [DOI] [PubMed] [Google Scholar]
- 15.Barbolina MV, Moss NM, Westfall SD, et al. Microenvironmental regulation of ovarian cancer metastasis. Cancer Treat Res. 2009 doi: 10.1007/978-0-387-98094-2_15. In press. [DOI] [PubMed] [Google Scholar]
- 16.Offner FA, Obrist P, Stadlmann S, et al. IL6 secretion by human peritoneal mesothelial and ovarian cancer cells. Cytokine. 1995;7:542–547. doi: 10.1006/cyto.1995.0073. [DOI] [PubMed] [Google Scholar]
- 17.Mustea A, Pirvulescu C, Konsgen D, et al. Decreased IL1 RA concentration in ascites is associated with a significant improvement in overall survival in ovarian cancer. Cytokine. 2008;42:77–84. doi: 10.1016/j.cyto.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Freedman RS, Deavers M, Liu J, et al. Peritoneal inflammation – a microenvironment for epithelial ovarian cancer. J Trans Med. 2004;2:23–33. doi: 10.1186/1479-5876-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gortzak-Uzan L, Ignatchenko A, Evangelou AI, et al. A proteome resource of ovarian cancer ascites: integrated proteomic and bioinformatics analyses to identify putative biomarkers. J Proteome Res. 2008;7:339–351. doi: 10.1021/pr0703223. [DOI] [PubMed] [Google Scholar]
- 20.Singh AP, Senapati S, Ponnusamy MP, et al. Clinical potential of mucins in diagnosis, prognosis and therapy of ovarian cancer. Lancet Oncol. 2008;9:1076–1085. doi: 10.1016/S1470-2045(08)70277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bazley LA, Gullick WJ. The epidermal growth factor receptor family. Endocr Relat Cancer. 2005;12(Suppl 1):S17–S27. doi: 10.1677/erc.1.01032. [DOI] [PubMed] [Google Scholar]
- 22.Linggi B, Carpenter G. ErbB receptors: new insights on mechanisms and biology. Trends Cell Biol. 2006;16(12):649–656. doi: 10.1016/j.tcb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Citri A, Yarden Y. EGF–ERBB signaling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7(7):505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 24.Wieduwilt MJ, Moasser MM. The epidermal growth factor receptor family: biology driving targeted therapeutics. Cell Mol Life Sci. 2008;65(10):1566–1584. doi: 10.1007/s00018-008-7440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harari PM, Allen GW, Bonner JA. Biology of interactions: antiepidermal growth factor receptor agents. J Clin Oncol. 2007;25(26):4057–4065. doi: 10.1200/JCO.2007.11.8984. [DOI] [PubMed] [Google Scholar]
- 26▪.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358(11):1160–1174. doi: 10.1056/NEJMra0707704. Addresses the mechanisms and clinical efficacy of EGF-receptor-targeted therapeutics in human cancers. [DOI] [PubMed] [Google Scholar]
- 27▪.Amit I, Wides R, Yarden Y. Evolvable signaling networks of receptor tyrosine kinases: relevance of robustness to malignancy and to cancer therapy. Mol Syst Biol. 2007;3:151. doi: 10.1038/msb4100195. Authors present a review of receptor tyrosine kinase signaling networks that provides a context for understanding both the potential and the challenge of targeting signaling proteins for cancer therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zandi R, Larsen AB, Andersen P, et al. Mechanisms for oncogenic activation of the epidermal growth factor receptor. Cell Signal. 2007;19(10):2013–2023. doi: 10.1016/j.cellsig.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 29.Riese D, Gallo R, Settleman J. Mutational activation of ErbB family receptor tyrosine kinases: insights into mechanisms of signal transduction and tumorigenesis. Bioessays. 2007;29(6):558–565. doi: 10.1002/bies.20582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lassus H, Sihto H, Leminen A, et al. Gene amplification, mutation, and protein expression of EGFR and mutations of ERBB2 in serous ovarian carcinoma . J Mol Med. 2006;84(8):671–681. doi: 10.1007/s00109-006-0054-4. [DOI] [PubMed] [Google Scholar]
- 31.Psyrri A, Kassar M, Yu Z, et al. Effect of epidermal growth factor receptor expression level on survival in patients with epithelial ovarian cancer. Clin Cancer Res. 2005;11(24 Pt 1):8637–8643. doi: 10.1158/1078-0432.CCR-05-1436. [DOI] [PubMed] [Google Scholar]
- 32.Crijns APG, Boezen HM, Schouten JP, et al. Prognostic factors in ovarian cancer: current evidence and future prospects The ECCO 12 Educational Book. Eur J Cancer. 2003;1(Suppl):127–145. [Google Scholar]
- 33.Tsakiridis T, Cutz JC, Singh G, et al. Association of phosphorylated epidermal growth factor receptor with survival in patients with locally advanced non-small cell lung cancer treated with radiotherapy. J Thorac Oncol. 2008;3(7):716–722. doi: 10.1097/JTO.0b013e31817c6094. [DOI] [PubMed] [Google Scholar]
- 34.Sonnweber B, Dlaska M, Skvortsov S, et al. High predictive value of epidermal growth factor receptor phosphorylation but not of EGFRvIII mutation in resected stage I non-small cell lung cancer (NSCLC) J Clin Pathol. 2006;59(3):255–259. doi: 10.1136/jcp.2005.027615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanematsu T, Yano S, Uehara H, et al. Phosphorylation, but not overexpression, of epidermal growth factor receptor is associated with poor prognosis of non-small cell lung cancer patients. Oncol Res. 2003;13(5):289–298. doi: 10.3727/096504003108748348. [DOI] [PubMed] [Google Scholar]
- 36.Papouchado B, Erickson LA, Rohlinger AL, et al. Epidermal growth factor receptor and activated epidermal growth factor receptor expression in gastrointestinal carcinoids and pancreatic endocrine carcinomas. Mod Pathol. 2005;18(10):1329–1335. doi: 10.1038/modpathol.3800427. [DOI] [PubMed] [Google Scholar]
- 37.Kong A, Leboucher P, Leek R, et al. Prognostic value of an activation state marker for epidermal growth factor receptor in tissue microarrays of head and neck cancer. Cancer Res. 2006;66(5):2834–2843. doi: 10.1158/0008-5472.CAN-05-2994. [DOI] [PubMed] [Google Scholar]
- 38.Guo L, Abraham J, Flynn DC, Castranova V, Shi X, Qian Y. Individualized survival and treatment response predictions for breast cancers using phospho-EGFR, phospho-ER, phospho-HER2/neu, phospho-IGF-IR/In, phospho-MAPK, and phospho-p70S6K proteins. Int J Biol Markers. 2007;22(1):1–11. doi: 10.1177/172460080702200101. [DOI] [PubMed] [Google Scholar]
- 39.De Graef P, Crijns AP, Ten Hoor KA, et al. The ErbB signaling pathway: protein expression and prognostic value in epithelial ovarian cancer. Br J Cancer. 2008;99:341–349. doi: 10.1038/sj.bjc.6604471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Posadas EM, Liel MS, Kwitkowski V, et al. A Phase II and pharmacodynamic study of gefitinib in patients with refractory or recurrent epithelial ovarian cancer. Cancer. 2007;109(7):1323–1330. doi: 10.1002/cncr.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cowden Dahl KD, Symowicz J, Ning Y, et al. Matrix metalloproteinase 9 is a mediator of epidermal growth factor-dependent e-cadherin loss in ovarian carcinoma cells. Cancer Res. 2008;68:4606–4613. doi: 10.1158/0008-5472.CAN-07-5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyamoto S, Yagi H, Yotsumoto F, et al. New approach to cancer therapy: heparin binding-epidermal growth factor-like growth factor as a novel targeting molecule. Anticancer Res. 2007;27(6A):3713–3721. [PubMed] [Google Scholar]
- 43.Miyamoto S, Yagi H, Yotsumoto F, et al. Heparin-binding epidermal growth factor-like growth factor as a new target molecule for cancer therapy. Adv Exp Med Biol. 2008;622:281–295. doi: 10.1007/978-0-387-68969-2_23. [DOI] [PubMed] [Google Scholar]
- 44.Braun AH, Coffey RJ. Lysophosphatidic acid, a disintegrin and metalloprotease-17 and heparin-binding epidermal growth factor-like growth factor in ovarian cancer: the first word, not the last. Clin Cancer Res. 2005;11(13):4639–4643. doi: 10.1158/1078-0432.CCR-05-0973. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka Y, Miyamoto S, Suzuki SO, et al. Clinical significance of heparin-binding epidermal growth factor-like growth factor and a disintegrin and metalloprotease 17 expression in human ovarian cancer. Clin Cancer Res. 2005;11(13):4783–4792. doi: 10.1158/1078-0432.CCR-04-1426. [DOI] [PubMed] [Google Scholar]
- 46.Miyamoto S, Hirata M, Yamazaki A, et al. Heparin-binding EGF-like growth factor is a promising target for ovarian cancer therapy. Cancer Res. 2004;64(16):5720–5727. doi: 10.1158/0008-5472.CAN-04-0811. [DOI] [PubMed] [Google Scholar]
- 47.Prenzel N, Zwick E, Daub H, et al. EGF receptor transactivation by G-protein-coupled receptors requires etalloproteinase cleavage of proHB-EGF. Nature. 1999;402(6764):884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 48.Hsieh M, Conti M. G-protein-coupled receptor signaling and the EGF network in endocrine systems. Trends Endocrinol Metab. 2005;16(7):320–326. doi: 10.1016/j.tem.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Fischer OM, Hart S, Ullrich A. Dissecting the epidermal growth factor receptor signal transactivation pathway. Methods Mol Biol. 2006;327:85–97. doi: 10.1385/1-59745-012-X:85. [DOI] [PubMed] [Google Scholar]
- 50.Bhola NE, Grandis JR. Crosstalk between G-protein-coupled receptors and epidermal growth factor receptor in cancer. Front Biosci. 2008;13:1857–1865. doi: 10.2741/2805. [DOI] [PubMed] [Google Scholar]
- 51.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25(1):9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 52.Itoh Y, Nagase H. Matrix metalloproteinases in cancer. Essays Biochem. 2002;38:21–36. doi: 10.1042/bse0380021. [DOI] [PubMed] [Google Scholar]
- 53.Ghosh S, Wu Y, Stack MS. Ovarian cancer-associated proteinases. Cancer Treat Res. 2002;107:331–354. doi: 10.1007/978-1-4757-3587-1_16. [DOI] [PubMed] [Google Scholar]
- 54▪.Kamat AA, Fletcher M, Gruman LM, et al. The clinical relevance of stromal matrix metalloproteinase expression in ovarian cancer. Clin Cancer Res. 2006;12(6):1707–1714. doi: 10.1158/1078-0432.CCR-05-2338. Evaluates matrix metalloproteinase (MMP) expression in both tumor and stromal tissues, concluding that proteinases from both sources are important predictors of clinical outcome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fishman DA, Bafetti LM, Banionis S, Kearns AS, Chilukuri K, Stack MS. Production of extracellular matrix-degrading proteinases by primary cultures of human epithelial ovarian carcinoma cells. Cancer. 1997;80(8):1457–1463. doi: 10.1002/(sici)1097-0142(19971015)80:8<1457::aid-cncr13>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 56.Symowicz J, Adley BP, Gleason KJ, et al. Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res. 2007;67:2030–2039. doi: 10.1158/0008-5472.CAN-06-2808. [DOI] [PubMed] [Google Scholar]
- 57.Demeter A, Sziller I, Csapo Z, et al. Molecular prognostic markers in recurrent and in non-recurrent epithelial ovarian cancer. Anticancer Res. 2005;25(4):2885–2889. [PubMed] [Google Scholar]
- 58.Lengyel E, Schmalfeldt B, Konik E, et al. Expression of latent matrix metalloproteinase 9 (MMP-9) predicts survival in advanced ovarian cancer. Gynecol Oncol. 2001;82(2):291–298. doi: 10.1006/gyno.2001.6243. [DOI] [PubMed] [Google Scholar]
- 59.Yan C, Boyd DD. Regulation of matrix metalloproteinase gene expression. J Cell Physiol. 2007;211(1):19–26. doi: 10.1002/jcp.20948. [DOI] [PubMed] [Google Scholar]
- 60.Mancini A, Di Battista JA. Transcriptional regulation of matrix metalloprotease gene expression in health and disease. Front Biosci. 2006;11:423–446. doi: 10.2741/1809. [DOI] [PubMed] [Google Scholar]
- 61.Vincenti MP, Brinckerhoff CE. Signal transduction and cell-type specific regulation of matrix metalloproteinase gene expression: can MMPs be good for you? J Cell Physiol. 2007;213(2):355–364. doi: 10.1002/jcp.21208. [DOI] [PubMed] [Google Scholar]
- 62.Ellerbroek SM, Halbleib JM, Benavidez M, et al. Phosphatidylinositol 3-kinase activity in epidermal growth factor-stimulated matrix metalloproteinase-9 production and cell surface association. Cancer Res. 2001;61(5):1855–1861. [PubMed] [Google Scholar]
- 63.Ellerbroek SM, Hudson LG, Stack MS. Proteinase requirements of epidermal growth factor-induced ovarian cancer cell invasion. Int J Can. 1998;78(3):331–337. doi: 10.1002/(SICI)1097-0215(19981029)78:3<331::AID-IJC13>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 64.Zhou HY, Pon YL, Wong AS. Synergistic effects of epidermal growth factor and hepatocyte growth factor on human ovarian cancer cell invasion and migration: role of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase. Endocrinology. 2007;148(11):5195–5208. doi: 10.1210/en.2007-0361. [DOI] [PubMed] [Google Scholar]
- 65.Cowden Dahl KD, Zeineldin R, Hudson LG. PEA3 is necessary for optimal epidermal growth factor receptor-stimulated matrix metalloproteinase expression and invasion of ovarian tumor cells. Mol Cancer Res. 2007;5:413–421. doi: 10.1158/1541-7786.MCR-07-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kheradmand F, Rishi K, Werb Z. Signaling through the EGF receptor controls lung morphogenesis in part by regulating MT1-MMP-mediated activation of gelatinase A/MMP2. J Cell Sci. 2002;115:839–848. doi: 10.1242/jcs.115.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Meter TE, Broaddus WC, Rooprai HK, Pilkington GJ, Fillmore HL. Induction of membrane-type-1 matrix metalloproteinase by epidermal growth factor-mediated signaling in gliomas. J Neurooncol. 2004;6:188–199. doi: 10.1215/S1152851703000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chotteau-Lelievre A, Desbiens X, Pelczar H, Defossez PA, de Launoit Y. Differential expression patterns of the PEA3 group transcription factors through murine embryonic development. Oncogene. 1997;15:937–952. doi: 10.1038/sj.onc.1201261. [DOI] [PubMed] [Google Scholar]
- 69.Davidson B, Goldberg I, Gotlieb WH, Kopolovic J, Ben-Baruch G, Reich R. PEA3 is the second Ets family transcription factor involved in tumor progression in ovarian carcinoma. Clin Cancer Res. 2003;9:1412–1419. [PubMed] [Google Scholar]
- 70.Hibbs K, Skubitz KM, Pambuccian SE, et al. Differential gene expression in ovarian carcinoma: identification of potential biomarkers. Am J Pathol. 2004;165:397–414. doi: 10.1016/S0002-9440(10)63306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koo TB, Song H, Moon I, et al. Differential expression of the PEA3 subfamily of ETS transcription factors in the mouse ovary and peri-implantation uterus. Reproduction. 2005;129:651–657. doi: 10.1530/rep.1.00656. [DOI] [PubMed] [Google Scholar]
- 72.Lynch CC, Crawford HC, Matrisian LM, McDonnell S. Epidermal growth factor upregulates matrix metalloproteinase-7 expression through activation of PEA3 transcription factors. Int J Oncol. 2004;24:1565–1572. [PubMed] [Google Scholar]
- 73.de Launoit Y, Baert JL, Chotteau-Lelievre A, et al. The Ets transcription factors of the PEA3 group: Transcriptional regulators in metastasis. Biochim Biophys Acta. 2006;1766:79–87. doi: 10.1016/j.bbcan.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 74.Gum R, Lengyel E, Juarez J, et al. Stimulation of 92-kDa gelatinase B promoter activity by ras is mitogen- activated protein kinase kinase 1-independent and requires multiple transcription factor binding sites including closely spaced PEA3/ets and AP-1 sequences. J Biol Chem. 1996;271:10672–10680. doi: 10.1074/jbc.271.18.10672. [DOI] [PubMed] [Google Scholar]
- 75.Habelhah H, Okada F, Kobayashi M, et al. Increased E1AF expression in mouse fibrosarcoma promotes metastasis through induction of MT1-MMP expression. Oncogene. 1999;18:1771–1776. doi: 10.1038/sj.onc.1202465. [DOI] [PubMed] [Google Scholar]
- 76.Pendas AM, Balbin M, Llano E, Jimenez MG, Lopez-Otin C. Structural analysis and promoter characterization of the human collagenase-3 gene (MMP13) Genomics. 1997;40:222–233. doi: 10.1006/geno.1996.4554. [DOI] [PubMed] [Google Scholar]
- 77.Tardif G, Reboul P, Dupuis M, et al. Transforming growth factor-α induced collagenase-3 production in human osteoarthritic chondrocytes is triggered by Smad proteins: cooperation between activator protein-1 and PEA-3 binding sites. J Rheumatol. 2001;28:1631–1639. [PubMed] [Google Scholar]
- 78.Olson MW, Toth M, Gervasi DC, Sado Y, Ninomiya Y, Fridman R. High affinity binding of latent matrix metalloproteinase-9 to the a2(IV) chain of collagen IV. J Biol Chem. 1998;273:10672–10681. doi: 10.1074/jbc.273.17.10672. [DOI] [PubMed] [Google Scholar]
- 79.Bourguignon LY, Gunja-Smith Z, Iida N, et al. CD44v(3,8–10) is involved in cytoskeleton-mediated tumor cell migration and matrix metalloproteinase (MMP-9) association in metastatic breast cancer cells. J Cell Physiol. 1998;176:206–215. doi: 10.1002/(SICI)1097-4652(199807)176:1<206::AID-JCP22>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 80.Yu Q, Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD440-mediated tumor invasion. Genes Dev. 1999;13:35–48. doi: 10.1101/gad.13.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14(2):163–176. [PMC free article] [PubMed] [Google Scholar]
- 82.Toth M, Gervasi CD, Fridman R. Phorbol ester induced cell surface association of matrix metalloproteinase 9 in human MCF10A breast epithelial cells. Cancer Res. 1997;57:3159–3167. [PubMed] [Google Scholar]
- 83.Koyama Y, Naruo H, Yoshitomi Y, et al. Matrix metalloproteinase-9 associated with heparan sulphate chains of GPI-anchored cell surface proteoglycans mediates motility of murine colon adenocarcinoma cells. J Biochem. 2008;143(5):581–592. doi: 10.1093/jb/mvn006. [DOI] [PubMed] [Google Scholar]
- 84.Peng ST, Su CH, Kuo CC, Shaw CF, Wang HS. CD44 crosslinking-mediated matrix metalloproteinase-9 relocation in breast tumor cells leads to enhanced metastasis. Int J Oncol. 2007;31(5):1119–1126. [PubMed] [Google Scholar]
- 85.Fridman R, Toth M, Chvyrkova I, Meroueh SO, Mobashery S. Cell surface association of matrix metalloproteinase-9 (gelatinase B) Cancer Metastasis Rev. 2003;22(2–3):153–166. doi: 10.1023/a:1023091214123. [DOI] [PubMed] [Google Scholar]
- 86.Ouyang M, Lu S, Li XY, et al. Visualization of polarized membrane type 1 matrix metalloproteinase activity in live cells by fluorescence resonance energy transfer imaging. J Biol Chem. 2008;283:17740–17748. doi: 10.1074/jbc.M709872200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ning Y, Buranda T, Hudson LG. Activated epidermal growth factor receptor induces integrin α2 internalization via caveolae/raft-dependent endocytic pathway. J Biol Chem. 2007;282(9):6380–6387. doi: 10.1074/jbc.M610915200. [DOI] [PubMed] [Google Scholar]
- 88.Moss NM, Liu Y, Johnson JJ, et al. Epidermal growth factor receptor-mediated membrane type 1 matrix metalloproteinase endocytosis regulates the transition between invasive versus expansive growth of ovarian carcinoma cells in three-dimensional collagen. Mol Can Res. 2009 doi: 10.1158/1541-7786.MCR-08-0571. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilhelm O, Hafter R, Coppenrath E, et al. Fibrin-fibronectin compunds in human ovarian tumor ascites and their possible relation to the tumor stroma. Cancer Res. 1988;48:3507–3514. [PubMed] [Google Scholar]
- 90.Hafter R, Klaubert W, Gollwitzer R, von Hugo G, Graeff H. Crosslinked fibrin derivatives and fibronectin in ascetic fluid from patients with ovarian cancer compared with ascetic fluid in liver cirrhosis. Thromb Res. 1984;35:53–64. doi: 10.1016/0049-3848(84)90312-8. [DOI] [PubMed] [Google Scholar]
- 91.Crickard K, Niedbala MJ, Crickard U, et al. Characterization of human ovarian and endometrial carcinoma cell lines established on extracellular matrix. Gynecol Oncol. 1989;32:163–173. doi: 10.1016/s0090-8258(89)80028-9. [DOI] [PubMed] [Google Scholar]
- 92.Cracchiolo BM, Hanauske-Abel HM, Schwartz PE, Chambers JT, Holland B, Chambers SK. Procollagen-derived biomarkers in malignant ascites of ovarian cancer. Gynecol Oncol. 2002;87:24–33. doi: 10.1006/gyno.2002.6798. [DOI] [PubMed] [Google Scholar]
- 93.Santala M, Risteli J, Risteli L, et al. Synthesis and breakdown of fibrillar collagens: concomitant phenomena in ovarian cancer. Br J Cancer. 1998;77:1825–1831. doi: 10.1038/bjc.1998.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu GG, Risteli J, Puistola U, et al. Progressive ovarian carcinoma induces synthesis of type I and type III procollagens in the tumor tissue and peritoneal cavity. Cancer Res. 1993;53:5028–5032. [PubMed] [Google Scholar]
- 95.Abdel Aziz MT, Abdel Aziz Wassef M, Kamel M, et al. Clinical evaluation of serum aminoterminal propeptide of type III procollagen as tumor marker in gynecologic malignancies. Tumori. 1993;79:219–223. doi: 10.1177/030089169307900313. [DOI] [PubMed] [Google Scholar]
- 96.Sood AK, Seftor EA, Fletcher MS, et al. Molecular determinants of ovarian cancer plasticity. Am J Pathol. 2001;158(4):1279–1288. doi: 10.1016/S0002-9440(10)64079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sood AK, Fletcher MS, Coffin JE, et al. Functional role of matrix metalloproteinases in ovarian tumor cell plasticity. Am J Obstet Gynecol. 2004;190(4):899–909. doi: 10.1016/j.ajog.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 98.Kenny HA, Kaur S, Coussens LM, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest. 2008;118:1367–1379. doi: 10.1172/JCI33775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Adley BP, Gleason KJ, Yang XJ, Stack MS. Expression of membrane type 1 matrix metalloproteinase (MMP-14) in epithelial ovarian cancer: high level expression in clear cell carcinoma. Gynecol Oncol. 2009;112(2):319–324. doi: 10.1016/j.ygyno.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moss NM, Barbolina MV, Liu Y, et al. Ovarian carcinoma cell detachment and multi-cellular aggregate formation are regulated by membrane type 1 matrix metalloproteinase: a potential role in intra-peritoneal metastatic dissemination. Cancer Res. 2009 doi: 10.1158/0008-5472.CAN-08-4151. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ellerbroek SM, Wu YI, Overall CM, et al. Functional interplay between type I collagen and cell surface matrix metalloproteinase activity. J Biol Chem. 2001;276:24833–24842. doi: 10.1074/jbc.M005631200. [DOI] [PubMed] [Google Scholar]
- 102.Ellerbroek SM, Fishman DA, Kearns AS, et al. Ovarian carcinoma regulation of matrix metalloproteinase-2 and membrane type 1 matrix metalloproteinase through β1 integrin. Cancer Res. 1999;59:1635–1641. [PubMed] [Google Scholar]
- 103.Kenny HA, Dogan S, Zillhardt M, et al. Organotypic models of metastasis: a 3 dimensional culture mimicking the human peritoneum and omentum for the study of the early steps of ovarian cancer metastasis. Cancer Treat Res. 2009 doi: 10.1007/978-0-387-98094-2_16. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barbolina MV, Adley BP, Ariztia EV, et al. Microenvironmental regulation of membrane type 1 matrix metalloproteinase activity in ovarian carcinoma cells via collagen-induced EGR1 expression. J Biol Chem. 2007;282:4924–4931. doi: 10.1074/jbc.M608428200. [DOI] [PubMed] [Google Scholar]
- 105.Young TN, Rodriguez GC, Rinehart AR, Bast RC, Jr, Pizzo SV, Stack MS. Characterization of gelatinases linked to matrix invasion in ovarian adenocarcinoma. Gynecol Oncol. 1996;62(1):89–99. doi: 10.1006/gyno.1996.0195. [DOI] [PubMed] [Google Scholar]
- 106.Moser TL, Pizzo SV, Bafetti LM, et al. Evidence for preferential adhesion of ovarian epithelial carcinoma cells to type I collagen mediated by the α2β1 integrin. Int J Cancer. 1996;67:695–701. doi: 10.1002/(SICI)1097-0215(19960904)67:5<695::AID-IJC18>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 107.Ozalp S, Tanir HM, Yalcin OT, Kabukcuoglu S, Oner U, Uray M. Prognostic value of matrix metalloproteinase-9 (gelatinase-B) expression in epithelial ovarian tumors. Eur J Gynaecol Oncol. 2003;24(5):417–420. [PubMed] [Google Scholar]
- 108.Naylor MS, Stamp GW, Davies BD, Balkwill FR. Expression and activity of MMPs and their regulators in ovarian cancer. Int J Cancer. 1994;58:50–56. doi: 10.1002/ijc.2910580110. [DOI] [PubMed] [Google Scholar]
- 109.Huang S, Van Arsdall M, Tedjarati S, et al. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J Natl Cancer Inst. 2002;94(15):1134–1142. doi: 10.1093/jnci/94.15.1134. [DOI] [PubMed] [Google Scholar]
- 110.Davidson B, Goldberg I, Gotlieb WH, et al. High levels of MMP-2, MMP-9, MT1-MMP and TIMP-2 mRNA correlate with poor survival in ovarian carcinoma. Clin Exp Metastasis. 1999;17:799–808. doi: 10.1023/a:1006723011835. [DOI] [PubMed] [Google Scholar]
- 111.Davidson B, Goldberg I, Gotlieb WH, et al. The prognostic value of metalloproteinases and angiogenic factors in ovarian carcinoma. Mol Cell Endocrinol. 2002;187:39–45. doi: 10.1016/s0303-7207(01)00709-2. [DOI] [PubMed] [Google Scholar]
- 112.Cai KQ, Yang WL, Capo-Chichi CD, et al. Prominent expression of metalloproteinases in early stages of ovarian tumorigenesis. Mol Carcinogen. 2007;46:130–143. doi: 10.1002/mc.20273. [DOI] [PubMed] [Google Scholar]
- 113.Belotti D, Paganoni P, Manenti L, et al. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res. 2003;63:5224–5229. [PubMed] [Google Scholar]
- 114.Belotti D, Calcagno C, Garofalo A, et al. Vascular endothelial growth factor stimulates organ-specific host matrix metalloproteinase-9 expression and ovarian cancer invasion. Mol Can Res. 2008;6:525–534. doi: 10.1158/1541-7786.MCR-07-0366. [DOI] [PubMed] [Google Scholar]
- 115.Herrera CA, Xu L, Bucana CD, et al. Expression of metastasis-related genes in humanepithelial ovarian tumors. Int J Oncol. 2002;20:5–13. [PubMed] [Google Scholar]
- 116.Holmbeck K, Bianco P, Caterina J, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99(1):81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 117.Zhou Z, Apte SS, Soininen R, et al. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci USA. 2000;97(8):4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sabeh F, Ota I, Holmbeck K, et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167(4):769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Seiki M, Yana I. Roles of pericellular proteolysis by membrane type-1 matrix metalloproteinase in cancer invasion and angiogenesis. Cancer Sci. 2003;94(7):569–574. doi: 10.1111/j.1349-7006.2003.tb01484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhai Y, Hotary KB, Nan B, et al. Expression of membrane type 1 matrix metalloproteinase is associated with cervical carcinoma progression and invasion. Cancer Res. 2005;65:6543–6550. doi: 10.1158/0008-5472.CAN-05-0231. [DOI] [PubMed] [Google Scholar]
- 121.Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol. 2006;206(1):1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 122.Afzal S, Lalani EN, Poulsom R, et al. MT1-MMP and MMP-2 mRNA expression in human ovarian tumors: possible implications for the role of desmoplastic fibroblasts. Hum Pathol. 1998;29(2):155–165. doi: 10.1016/s0046-8177(98)90226-x. [DOI] [PubMed] [Google Scholar]
- 123.Drew AF, Blick TJ, Lafleur MA, et al. Correlation of tumor-and stromal-derived MT1-MMP expression with progression of human ovarian tumors in SCID mice. Gynecol Oncol. 2004;95:437–448. doi: 10.1016/j.ygyno.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 124.Fishman DA, Kearns AM, Chilikuri K, et al. metastatic dissemination of human ovarian epithelial carcinoma is promoted by α2β1 integrin-mediated interaction with type I collagen. Invasion Metastasis. 1998;18:15–26. doi: 10.1159/000024495. [DOI] [PubMed] [Google Scholar]
- 125.Wu X, Li H, Kang L, Li L, Wang W, Shan B. Activated matrix metalloproteinase-2 – a potential marker of prognosis for epithelial ovarian cancer. Gynecol Oncol. 2002;84:126–134. doi: 10.1006/gyno.2001.6477. [DOI] [PubMed] [Google Scholar]
- 126.Kajita M, Itoh Y, Chiba T, et al. Membrane-type I matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Bio. 2001;153:893–904. doi: 10.1083/jcb.153.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gingras D, Bousquet-Gagnon N, Langlois S, Lachambre MP, Annabi B, Béliveau R. Activation of the extracellular signal-regulated protein kinase (ERK) cascade by membrane-type-1 matrix metalloproteinase (MT1-MMP) FEBS Lett. 2001;507(2):231–236. doi: 10.1016/s0014-5793(01)02985-4. [DOI] [PubMed] [Google Scholar]
- 128.Hotary K, Allen E, Punturieri A, Yana I, Weiss SJ. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J Cell Biol. 2000;149(6):1309–1323. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129▪▪.Hotary KB, Allen ED, Brooks PC, et al. Membrane type 1 matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. Introduces the important concept that matrix proteolysis is not simply a tool to facilitate invasion, but also removes physical constraints on cell shape to enable cytoskeletal rearrangements necessary for proliferation within a mechanically confining microenvironment. [DOI] [PubMed] [Google Scholar]
- 130.Harvey W, Amlot P. Colalgen production by human mesothelial cells in vitro. J Pathol. 1983;139:337–347. doi: 10.1002/path.1711390309. [DOI] [PubMed] [Google Scholar]
- 131.Stylianou E, Jenner LA, Davies M, et al. Isolation, culture and characterization of human peritoneal mesothelial cells. Kidney Int. 1990;37:1563–1570. doi: 10.1038/ki.1990.150. [DOI] [PubMed] [Google Scholar]
- 132.Sodek KL, Ringuette MJ, Brown TJ. MT1-MMP is the critical determinant of matrix degradation and invasion by ovarian cancer cells. Br J Cancer. 2007;97:358–367. doi: 10.1038/sj.bjc.6603863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Niedbala MJ, Crickard K, Bernacki RJ. Interactions of human ovarian tumor cells with human mesothelial cells grown on extracellular matrix An in vitro model system for studying tumor cell adhesion and invasion. Exp Cell Res. 1985;160(2):499–513. doi: 10.1016/0014-4827(85)90197-1. [DOI] [PubMed] [Google Scholar]
- 134.Niedbala MJ, Crickard K, Bernacki RJ. In vitro degradation of extracellular matrix by human ovarian carcinoma cells. Clin Exp Mets. 1987;5:181–197. doi: 10.1007/BF00058063. [DOI] [PubMed] [Google Scholar]
- 135.Sawada K, Mitra AK, Radjabi AR, et al. Loss of E-cadherin promotes ovarian cancer metastasis via α5 integrin, which is a therapeutic target. Cancer Res. 2008;68:2329–2339. doi: 10.1158/0008-5472.CAN-07-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cannistra SA, Kansas GS, Niloff J, et al. Binding of ovarian cancer cells to peritoneal mesothelium in vitro is partly mediated by CD44H. Can Res. 1993;53:3830–3838. [PubMed] [Google Scholar]
- 137.Casey RC, Burleson KM, Skubitz KM, et al. β1-integrins regulate the formation and adhesion of ovarian carcinoma multicellular spheroids. Am J Pathol. 2001;159(6):2071–2080. doi: 10.1016/s0002-9440(10)63058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Frankel A, Rosen K, Filmus J, Kerbel RS. Induction of anoikis and suppression of human ovarian tumor growth in vivo by down-regulation of Bcl-X(L) Cancer Res. 2001;61(12):4837–4841. [PubMed] [Google Scholar]
- 139.Kassis J, Lkominek J, Kohn EC. Tumor microenvironment: what can effusions teach us? Diagnostic Cytopathology. 2004;33:316–319. doi: 10.1002/dc.20280. [DOI] [PubMed] [Google Scholar]
- 140.Steeg PS. Theodorescu D/l Metastasis. a therapeutic target for cancer. Nat Clin Pract Oncol. 2008;5(4):206–219. doi: 10.1038/ncponc1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Higashiyama S, Iwabuki H, Morimoto C, Hieda M, Inoue H, Matsushita N. Membrane-anchored growth factors, the epidermal growth factor family: beyond receptor ligands. Cancer Sci. 2008;99(2):214–220. doi: 10.1111/j.1349-7006.2007.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Martin MD, Matrisian LM. The other side of MMPs: protective roles in tumor progression. Cancer Metastasis Rev. 2007;26(3–4):717–724. doi: 10.1007/s10555-007-9089-4. [DOI] [PubMed] [Google Scholar]
- 143.Fingleton B. MMPs as therapeutic targets – still a viable option? Semin Cell Dev Biol. 2008;19(1):61–68. doi: 10.1016/j.semcdb.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Davies B, Brown PD, East N, Crimmin MJ, Balkwill FR. A synthetic matrix metalloproteinase inhibitor decreases tumor burden and prolongs survival of mice bearing human ovarian carcinoma xenografts. Cancer Res. 1993;53:2087–2091. [PubMed] [Google Scholar]
- 145.Nemunaitis J, Poole C, Primrose J, et al. Combined analysis of studies of the effects of the matrix metalloproteinase inhibitor marimastat on serum tumor markers in advanced cancer: selection of a biologically active and tolerable dose for longer-term studies. Clin Cancer Res. 1998;4:1101–1109. [PubMed] [Google Scholar]
- 146.Malfetano J, Teng N, Barter J. Marimastat in patients with advanced cancer of the ovary: a dose-finding study. Proc Am Soc Clin Oncol. 1997;16:373a. [Google Scholar]
- 147.Poole C, Adams M, Barley V. A dose-finding study of marimastat, an oral matrix metalloproteinase inhibitor in patients with advanced ovarian cancer. Ann Oncol. 1996;7:68. [Google Scholar]
- 148▪▪.Overall CM, Kleifeld O. Tumour microenvironment – opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;(3):227–239. doi: 10.1038/nrc1821. Provides a cogent discussion of considerations to be taken into account for validation of MMPs as targets in therapeutic drug development. [DOI] [PubMed] [Google Scholar]
- 149.Bareschino MA, Schettino C, Troiani T, Martinelli E, Morgillo F, Ciardiello F. Erlotinib in cancer treatment. Ann Oncol. 2007;18(Suppl 6):vi35–vi41. doi: 10.1093/annonc/mdm222. [DOI] [PubMed] [Google Scholar]
- 150.Palayekar MJ, Herzog TJ. The emerging role of epidermal growth factor receptor inhibitors in ovarian cancer. Int J Gynecol Cancer. 2008;18(5):879–890. doi: 10.1111/j.1525-1438.2007.01144.x. [DOI] [PubMed] [Google Scholar]
- 151.Schilder RJ, Sill MW, Chen X, et al. Phase II study of gefitinib in patients with relapsed or persistent ovarian or primary peritoneal carcinoma and evaluation of epidermal growth factor receptor mutations and immunohistochemical expression: a Gynecologic Oncology Group study. Clin Cancer Res. 2005;11(15):5539–5548. doi: 10.1158/1078-0432.CCR-05-0462. [DOI] [PubMed] [Google Scholar]
- 152.Dimova I, Zaharieva B, Raitcheva S, Dimitrov R, Doganov N, Toncheva D. Tissue microarray analysis of EGFR and erbB2 copy number changes in ovarian tumors. Int J Gynecol Cancer. 2006;16(1):145–151. doi: 10.1111/j.1525-1438.2006.00286.x. [DOI] [PubMed] [Google Scholar]
- 153.Stadlmann S, Gueth U, Reiser U, et al. Epithelial growth factor receptor status in primary and recurrent ovarian cancer. Mod Pathol. 2006;19(4):607–610. doi: 10.1038/modpathol.3800575. [DOI] [PubMed] [Google Scholar]
- 154.Baselga J, Rosen N. Determinants of RASistance to anti-epidermal growth factor receptor agents. J Clin Oncol. 2008;26(10):1582–1584. doi: 10.1200/JCO.2007.15.3700. [DOI] [PubMed] [Google Scholar]
- 155.Gelovani JG. Molecular imaging of epidermal growth factor receptor expression-activity at the kinase level in tumors with positron emission tomography. Cancer Metastasis Rev. 2008;27(4):645–653. doi: 10.1007/s10555-008-9156-5. [DOI] [PubMed] [Google Scholar]
- 156.Yagi H, Yotsumoto F, Miyamoto S. Heparin-binding epidermal growth factor-like growth factor promotes transcoelomic metastasis in ovarian cancer through epithelial-mesenchymal transition. Mol Cancer Ther. 2008;7(10):3441–3451. doi: 10.1158/1535-7163.MCT-08-0417. [DOI] [PubMed] [Google Scholar]
- 157.Rosanò L, Di Castro V, Spinella F, et al. Combined targeting of endothelin A receptor and epidermal growth factor receptor in ovarian cancer shows enhanced antitumor activity. Cancer Res. 2007;67(13):6351–6359. doi: 10.1158/0008-5472.CAN-07-0883. [DOI] [PubMed] [Google Scholar]
- 158.Tortora G, Ciardiello F, Gasparini G. Combined targeting of EGFR-dependent and VEGF-dependent pathways: rationale, preclinical studies and clinical applications. Nat Clin Pract Oncol. 2008;5(9):521–530. doi: 10.1038/ncponc1161. [DOI] [PubMed] [Google Scholar]
- 159.Markman M. The promise and perils of ‘targeted therapy’ of advanced ovarian cancer. Oncology. 2008;74(1–2):1–6. doi: 10.1159/000138349. [DOI] [PubMed] [Google Scholar]
Website
- 201.National Cancer Institute. Surveillance Epidemiology and End Results (SEER) stat fact sheet . http://seer.cancer.gov/statfacts/html/ovary.html.