Abstract
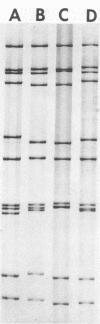
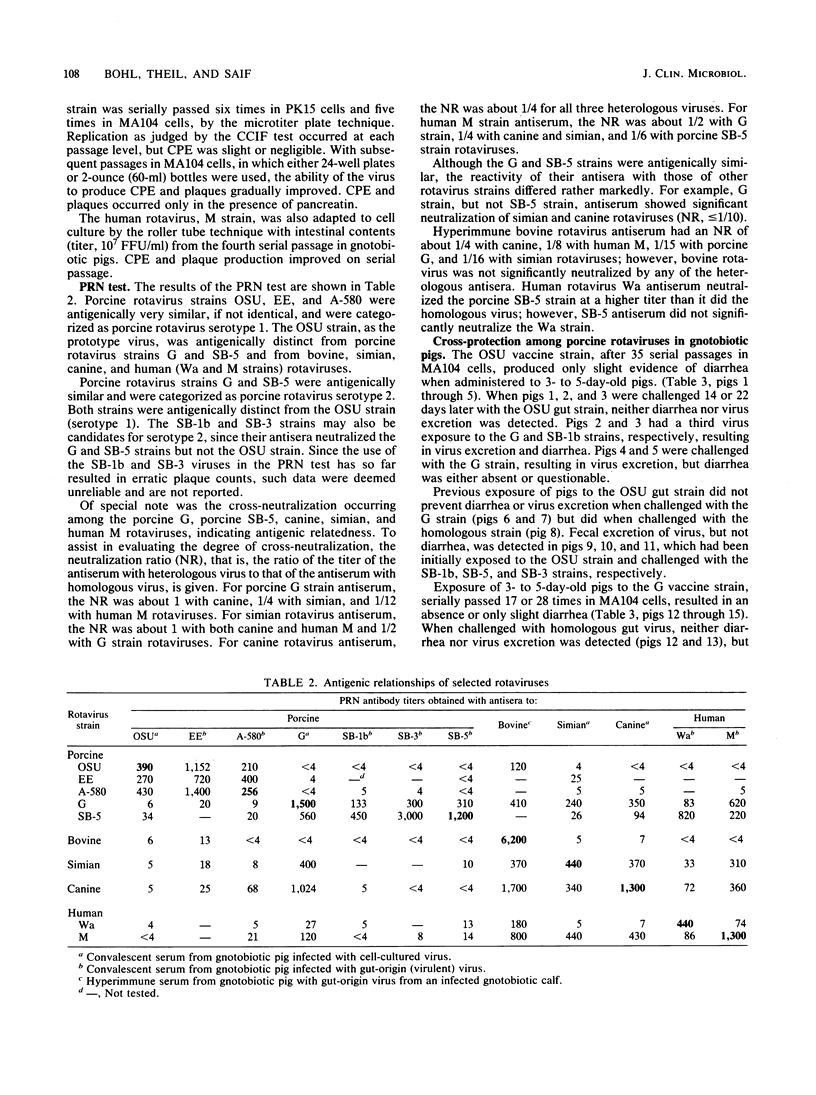
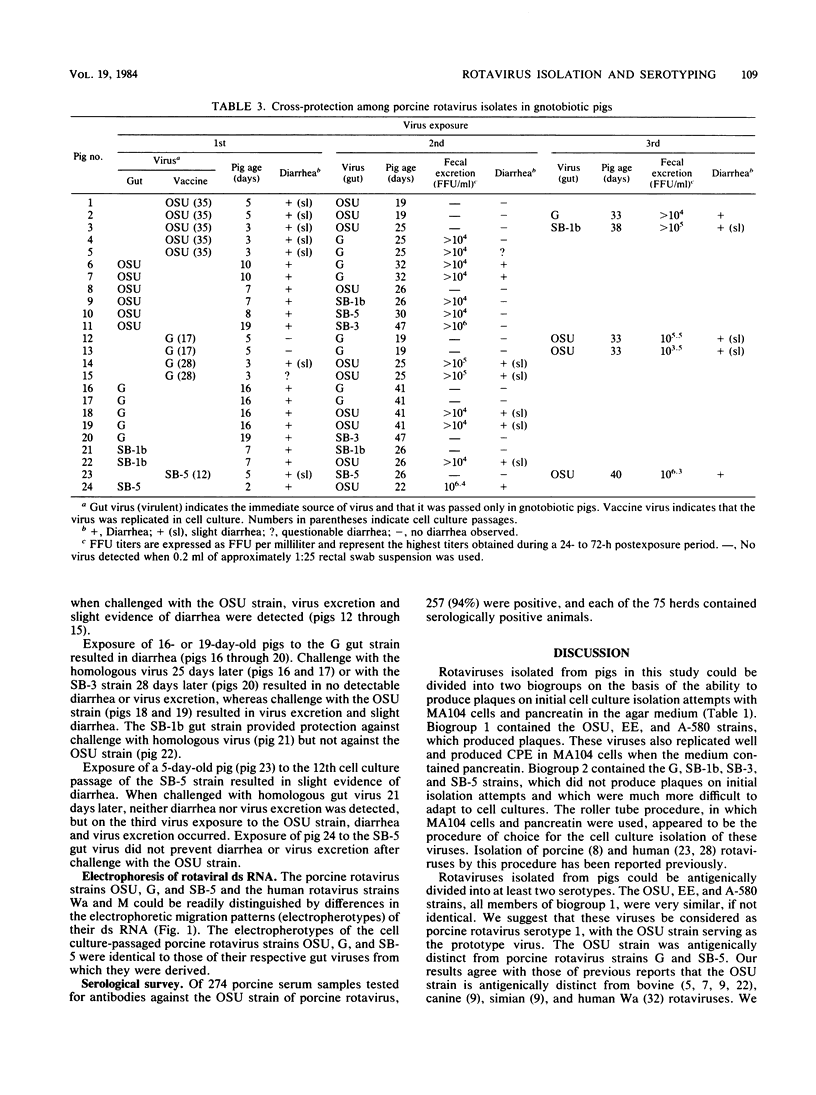
Seven rotavirus strains were isolated in cell cultures from the intestinal contents of piglets with diarrhea. MA104 cells with pancreatin in the cell culture medium was the host system of choice for virus isolation and replication. A cell culture immunofluorescence test in which MA104 cells were used in microtiter plates was very effective for detecting and assaying rotaviruses. A plaque reduction neutralization test, cross-protection studies in gnotobiotic pigs, and electrophoresis of rotaviral double-stranded RNA were used for comparing viruses. Three strains produced plaques on initial isolation attempts, replicated well in cell cultures, and were antigenically very similar. We suggest that these three strains be considered porcine rotavirus serotype 1, with The Ohio State University (OSU) strain serving as the prototype. The OSU strain was distinct from bovine, simian, canine, and human (Wa and M) rotaviruses by plaque reduction neutralization. Four strains did not produce plaques on initial isolation attempts, were difficult to adapt to cell cultures, and were related to each other but were distinct from the serotype 1 strains. We suggest that the Gottfried (G) strain be tentatively considered as a prototype for porcine rotavirus serotype 2. The G strain was antigenically closely related to canine and simian rotaviruses and less so to human M rotavirus (human rotavirus serotype 3). Canine, simian, and human M rotaviruses were closely related. All seven porcine rotavirus strains caused diarrhea in gnotobiotic pigs. Cell-cultured vaccines of the OSU and G strains caused only mild or no diarrhea in gnotobiotic pigs, and protection occurred when such pigs were challenged with homologous, bur not heterologous, virulent viruses. A survey indicated that 94% of 274 porcine serum samples and 100% of 75 herds were serologically positive to the porcine OSU rotavirus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohl E. H., Kohler E. M., Saif L. J., Cross R. F., Agnes A. G., Theil K. W. Rotavirus as a cause of diarrhea in pigs. J Am Vet Med Assoc. 1978 Feb 15;172(4):458–463. [PubMed] [Google Scholar]
- Bohl E. H. Rotaviral diarrhea in pigs: brief review. J Am Vet Med Assoc. 1979 Mar 15;174(6):613–615. [PubMed] [Google Scholar]
- Bohl E. H., Saif L. J., Theil K. W., Agnes A. G., Cross R. F. Porcine pararotavirus: detection, differentiation from rotavirus, and pathogenesis in gnotobiotic pigs. J Clin Microbiol. 1982 Feb;15(2):312–319. doi: 10.1128/jcm.15.2.312-319.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthier G., Vautherot J. F., Vannier P. Mise en évidence sérologique d'infections à rotavirus au sein de l'élevage porcin francais (région de Bretagne). Ann Rech Vet. 1979;10(1):65–69. [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y. Identification of rotaviruses of different origins by the plaque-reduction test. Am J Vet Res. 1980 Jan;41(1):151–152. [PubMed] [Google Scholar]
- Fukusho A., Shimizu Y., Ito Y. Isolation of cytopathic porcine rotavirus in cell roller culture in the presence of trypsin. Arch Virol. 1981;69(1):49–60. doi: 10.1007/BF01315265. [DOI] [PubMed] [Google Scholar]
- Gaul S. K., Simpson T. F., Woode G. N., Fulton R. W. Antigenic relationships among some animal rotaviruses: virus neutralization in vitro and cross-protection in piglets. J Clin Microbiol. 1982 Sep;16(3):495–503. doi: 10.1128/jcm.16.3.495-503.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H., McAuliffe V., Valdesuso J., Wyatt R., Flores J., Kalica A., Hoshino Y., Singh N. Serological analysis of the subgroup protein of rotavirus, using monoclonal antibodies. Infect Immun. 1983 Jan;39(1):91–99. doi: 10.1128/iai.39.1.91-99.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A. J., Inglis N. F., Ojeh C. K., Snodgrass D. R., Menzies J. D. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J Clin Microbiol. 1982 Sep;16(3):473–477. doi: 10.1128/jcm.16.3.473-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Kalica A. R., Flores J., Kapikian A. Z. Serological comparison of canine rotavirus with various simian and human rotaviruses by plaque reduction neutralization and hemagglutination inhibition tests. Infect Immun. 1983 Jul;41(1):169–173. doi: 10.1128/iai.41.1.169-173.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Scott F. W., Appel M. J. Isolation and characterization of a canine rotavirus. Arch Virol. 1982;72(1-2):113–125. doi: 10.1007/BF01314456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian A. Z., Cline W. L., Greenberg H. B., Wyatt R. G., Kalica A. R., Banks C. E., James H. D., Jr, Flores J., Chanock R. M. Antigenic characterization of human and animal rotaviruses by immune adherence hemagglutination assay (IAHA): evidence for distinctness of IAHA and neutralization antigens. Infect Immun. 1981 Aug;33(2):415–425. doi: 10.1128/iai.33.2.415-425.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lecce J. G., King M. W., Dorsey W. E. Rearing regimen producing piglet diarrhea (rotavirus) and its relevance to acute infantile diarrhea. Science. 1978 Feb 17;199(4330):776–778. doi: 10.1126/science.203032. [DOI] [PubMed] [Google Scholar]
- Lecce J. G., King M. W., Mock R. Reovirus-like agent associated with fatal diarrhea in neonatal pigs. Infect Immun. 1976 Sep;14(3):816–825. doi: 10.1128/iai.14.3.816-825.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebus C. A., Kono M., Underdahl N. R., Twiehaus M. J. Cell culture propagation of neonatal calf diarrhea (scours) virus. Can Vet J. 1971 Mar;12(3):69–72. [PMC free article] [PubMed] [Google Scholar]
- Sato K., Inaba Y., Miura Y., Tokuhisa S., Matumoto M. Antigenic relationships between rotaviruses from different species as studied by neutralization and immunofluorescence. Arch Virol. 1982;73(1):45–50. doi: 10.1007/BF01341726. [DOI] [PubMed] [Google Scholar]
- Sato K., Inaba Y., Shinozaki T., Fujii R., Matumoto M. Isolation of human rotavirus in cell cultures: brief report. Arch Virol. 1981;69(2):155–160. doi: 10.1007/BF01315159. [DOI] [PubMed] [Google Scholar]
- Stuker G., Oshiro L. S., Schmidt N. J. Antigenic comparisons of two new rotaviruses from rhesus monkeys. J Clin Microbiol. 1980 Feb;11(2):202–203. doi: 10.1128/jcm.11.2.202-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil K. W., Bohl E. H., Agnes A. G. Cell culture propagation of porcine rotavirus (reovirus-like agent). Am J Vet Res. 1977 Nov;38(11):1765–1768. [PubMed] [Google Scholar]
- Theil K. W., Bohl E. H. Porcine rotaviral infection of cell culture: effects of certain enzymes. Am J Vet Res. 1980 Jan;41(1):140–143. [PubMed] [Google Scholar]
- Theil K. W., McCloskey C. M., Saif L. J., Redman D. R., Bohl E. H., Hancock D. D., Kohler E. M., Moorhead P. D. Rapid, simple method of preparing rotaviral double-stranded ribonucleic acid for analysis by polyacrylamide gel electrophoresis. J Clin Microbiol. 1981 Sep;14(3):273–280. doi: 10.1128/jcm.14.3.273-280.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urasawa S., Urasawa T., Taniguchi K. Three human rotavirus serotypes demonstrated by plaque neutralization of isolated strains. Infect Immun. 1982 Nov;38(2):781–784. doi: 10.1128/iai.38.2.781-784.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G. N., Bridger J., Hall G. A., Jones J. M., Jackson G. The isolation of reovirus-like agents (rota-viruses) from acute gastroenteritis of piglets. J Med Microbiol. 1976 May;9(2):203–209. doi: 10.1099/00222615-9-2-203. [DOI] [PubMed] [Google Scholar]
- Wyatt R. G., Greenberg H. B., James W. D., Pittman A. L., Kalica A. R., Flores J., Chanock R. M., Kapikian A. Z. Definition of human rotavirus serotypes by plaque reduction assay. Infect Immun. 1982 Jul;37(1):110–115. doi: 10.1128/iai.37.1.110-115.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., James W. D., Bohl E. H., Theil K. W., Saif L. J., Kalica A. R., Greenberg H. B., Kapikian A. Z., Chanock R. M. Human rotavirus type 2: cultivation in vitro. Science. 1980 Jan 11;207(4427):189–191. doi: 10.1126/science.6243190. [DOI] [PubMed] [Google Scholar]