Abstract
The endometrial lining of the human uterus contains a population of phenotypically distinct (CD56bright, CD16dim), tissue-specific, natural killer (uNK) cells that play a key role in establishment of a successful pregnancy. An increase in the number of endometrial uNK cells occurs when the conceptus implants and there is a further rise during the early stages of placentation. Here we describe studies that have identified chorionic gonadotrophin (hCG), a glycoprotein synthesised by the pre-implantation conceptus, as a novel regulator of uNK cell proliferation. The impact of hCG on uNK cells was mediated via the mannose receptor (MR, CD206) rather than by the classical hCG/LH receptor that was not expressed. MR and hCG were co-localised on the surface of uNK cells and proliferation did not occur if cells were incubated with deglycosylated-hCG or intact hCG in the presence of excess D-Mannose. These novel observations provide new insight into the endocrine-immune dialogue that exists between the conceptus and immune cells within the receptive endometrium and have implications for the role of uNK cell-trophoblast interactions and pregnancy outcome.
Keywords: uterine natural killer cells, proliferation, hCG, mannose receptor
Introduction
The endometrium contains a population of CD56brightCD16dim endometrial natural killer cells (uNKs) that are distinct from circulating peripheral CD56dimCD16bright NK cells, reviewed in (1). The number of CD56bright cells increases at the onset of decidualisation during the secretory phase of the menstrual cycle (2, 3) with a further increase as a result of successful implantation. As a result uNK cells become the predominant lymphocyte subtype in the decidua during the first trimester of pregnancy (4).
Uterine NK cells play a key role in establishment, maintenance and regulation of early pregnancy (3) and a number of studies have suggested that they are aberrantly regulated in unexplained recurrent pregnancy loss (RPL) and in fetal growth restriction (5). For example, three studies have reported that the endometrium of women with RPL had increased numbers and/or activity of peripheral type NK cells (reviewed in (5)). In addition, although there may not be a difference in the total number of uNK cells in women with RPL the phenotype of the uNK cells may be altered and there is evidence that endometrium from women with RPL contain less CD56bright CD16dim uNK cells as compared to endometrium of fertile control patients (6). Reduced NK cell populations are also reported in pregnancies complicated by fetal growth restriction (FGR) with or without accompanying pre-eclampsia (7).
At present the lineage, origin and mechanism underlying the postovulatory and early pregnancy expansion of uNK cells remains to be established. Although variation in uNK cell number across the menstrual cycle suggests ovarian steroid regulation (5, 8) neither progesterone receptor (PR) nor oestrogen receptor alpha (ERα) have been detected in human uNK cells (9, 10). In addition, the rise in cell numbers that occurs at the time of implantation remains unexplained and although recruitment of migratory precursor cells may occur (1) the potential that local regulators may drive expansion within the tissue has prompted us to investigate what other factors might be involved in stimulating uNK cell proliferation.
Human chorionic gonadotrophin (hCG) is one of the earliest proteins secreted by the fetal trophoblast and is thus a candidate for regulation of paracrine embryo-endometrial dialogue. hCG is a heterodimeric glycoprotein that has an identical alpha subunit to that of luteinizing hormone (LH) originating from the pituitary gland, and a unique beta subunit. Both proteins can bind to the LH receptor, a transmembrane G-protein coupled receptor that is expressed on ovarian cells (11) and macrophages from term placenta and late secretory phase non-pregnant endometrium (12, 13). Mature hCG and LH are both modified by N-linked carbohydrate side chains that are important for the stability and assembly of the proteins (14).
We hypothesised that hCG secreted by the conceptus may directly mediate expansion of uNK cells during early stages of placentation. We therefore examined whether uNK cells express either the LH/hCG receptor or the mannose receptor (MR, CD206), a cell surface lectin that binds glycoproteins with N-linked carbohydrate side chains (15).
We report novel experimental data demonstrating that hCG can induce a signficant increase in uNK cell number. We propose that these findings necessitate a paradigm shift of the mechanisms that regulate this critical cell population during establishment of a fully functional placenta.
Materials and Methods
Human uterine tissue collection
Endometrial tissue specimens were obtained from non-pregnant women of reproductive age undergoing surgery for non-malignant gynaecological conditions (n = 9). Local ethical committee approval was granted and written, informed, patient consent was obtained prior to tissue collection. Histology, as assessed according to (16), was consistent with the patient’s reported last menstrual period and circulating steroid levels at time of tissue collection (17). Decidual tissue was obtained from women (n = 12; gestational age 7 -9 weeks) who had undergone first trimester surgical termination of pregnancy. All these women had an ultrasound scan to confirm a viable intrauterine pregnancy and gestational age. Decidua parietalis tissue was selected by macroscopic inspection.
Preparation of uNK cell population
NK cells were isolated from endometrial or decidual samples as previously described (18) and CD56bright uNKs were further purified by positive selection using CD56 antibody-coated magnetic Microbeads and the MACS® system (Miltenyi Biotec Ltd, Surrey, UK). Purified cells were seeded in 6-well plates at a concentration of 2.5 × 106 cells/ml and cultured in RPMI 1640 medium (Sigma) supplemented with 10 % FCS (Mycoplex), penicillin (50 μg/ml; Sigma), streptomycin (50 μg/ml; Sigma) and gentamycin (5 μg/ml; Sigma). Cells were incubated with hCG (10 ng/ml-10000 ng/ml) (Organon) in the presence or absence of D-Mannose (1 mg/ml) (Sigma) for 24 h at 37°C in 5 % (v/v) CO2 or with hCG that had been deglycosylated using PNGase F (Sigma) according to standard methods.
Cell proliferation assay
Cellular proliferation was measured by a non-radioactive cell proliferation assay system (MTT assay; Roche, Mannheim, Germany).
Semi Quantitative RT-PCR
RNA was isolated using the Qiagen RNeasy Mini Kit (Qiagen) and cDNA prepared using random hexamer primers (PE Biosystems, Warrington, UK). Target cDNAs, MR (Forward: CTACCCCTGCTCCTGGTTTTT, Reverse: TGAAACACTCATAATCTGAGATTC, 203 bp) and LH/hCG receptor (Forward: ATGAAGCAGCGGTTCTCG, Reverse: TTGACAGGGAGGTAGGCAAG, 203 bp), were amplifed using BioMix (BioMix Red, Bioline Ltd, London, UK) and BIOTAQ DNA Polymerase (Bioline Ltd). Amplification of GAPDH (Forward: CTGCACCACCAACTGCTTAGC, Reverse: ATGCCAGTGAGCTTCCGTTC, 205 bp) was used as an internal control for integrity of the RNA samples. 30 cycles of amplification were performed with an initial denaturing temperature of 95°C for 5 min for 1 cycle followed by 30 cycles of: denaturation at 95°C for 30 sec, annealing for 30 sec and extension at 72°C for 1 min 30 sec. Final extension at 72°C was carried out for 10 min. Amplified products were visualised on agarose gels. A sample of RNA extracted from human corpus luteum was included as a positive control for LHR.
Immunocytochemistry
uNK cells were prepared for immunostaining by Shandon Cytospin® (Thermo Electron Corporation, Cheshire, UK), briefly 1 × 105 cells were fixed in methanol for 10 min at room temperature and incubated with mouse monoclonal anti-human CD56 (Zymed) overnight at 4°C and then with peroxidase goat anti-mouse antibody (Dako UK Ltd) for 30 min followed by Tyramide Cy3 for 10 min at room temperature. Co-localisation of hCG and MR was explored by incubating the cells at 4°C overnight with rabbit polyclonal anti-human hCG (Abcam), then biotinylated goat anti-rabbit antibody (Dako UK Ltd) for 30 min followed by streptavidin 546 (Molecular Probes) for 60 min each at room temperature. After washing cells were incubated with mouse monoclonal anti-human MR (Abcam) followed by biotinylated goat anti-mouse antibody (Dako UK Ltd) for 30 min and streptavidin 488 (Molecular Probes) for 60 min each at room temperature. Negative controls were incubated with secondary antibodies alone and all nuclei were counterstained with DAPI. Fluorescent images were obtained on a LSM 510 confocal microscope (Carl Zeiss).
Flow cytometry
FACS analysis of was carried out on cells stained with phycoerythrin-conjugated mouse anti-human MR-specific (CD206) and fluorescein isothiocyanate-conjugated mouse anti-human CD56-specific antibody (BD Pharmingen).
Mass Spectrometry
Mass spectrometry was performed on an Applied Biosysetms Voyager DE-STR MALDI-TOF instrument using a sinapinic acid matrix to analyse hCG protein.
Statistical Analysis
Prior to statistical analysis data were tested for, and shown to exhibit, Gaussian distribution by applying the Shapiro-Wilk normality test to the data. Where appropriate, values were presented as mean ± S.E.M. Comparison of the different parameters for the various treatment groups was determined by repeated measures analysis of variance (ANOVA). Significant differences were assigned using Kruskal-Wallis post hoc test. The criterion for significance for all tests was set at p < 0.05. Specific software was used to assist in the data analysis (GraphPad Prism v4.0b for Macintosh, GraphPad Software, San Diego, USA).
Results
Isolation and characterisation of uNK cells from non-pregnant late secretory phase human endometrium and first trimester human decidua
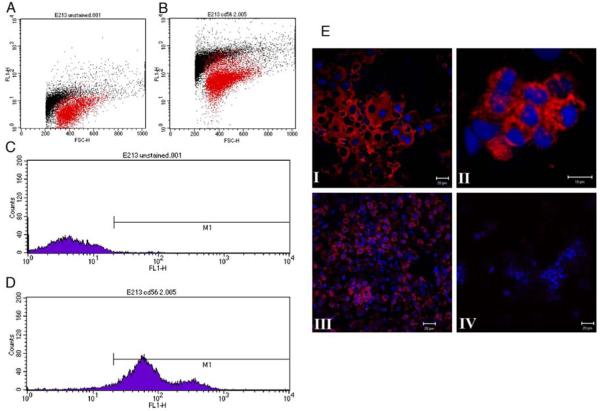
FACS analysis demonstrated that the purity of the purified uNK cells from non-pregnant late secretory phase human endometrium was ~95% (Fig. 1 D). Immunocytochemistry confirmed that these isolated cells expressed CD56 on their surface (Fig. 1 E). Decidual NK cells isolated using the same procedures were typically >90 % pure (Supplementary Fig S1).
Figure 1. Isolation and characterisation of uNK cells from non-pregnant late secretory phase human endometrium.
FACS analysis showing of cells incubated with the isotype-matched negative control (A) or CD56 antibody (B): y axis is forward scatter and x axis is the FITC value. In panels C and D the region represented by M1 indicates the number of cells that are positive for FITC. This is ~ 1% in isotype control (C) and ~ 95 % after incubation with anti-CD56 (D). Panel E (I-IV) shows uNK cells immunostained for CD56 (red) and ToPro (nuclear counterstain, blue), Panel I purified uNK cells stained with mouse anti-human CD56, Panel II, is a high power view of the same cells. Panel III, formalin-fixed section of 1st trimester decidua, (positive control), Panel IV negative control. Scale bar = 20μm.
Uterine NK cells express the mannose receptor (MR, CD206) but not the LH/hCG receptor
RNA was extracted from highly purified populations of NK cells obtained from both non-pregnant endometrium and first trimester decidua and RTPCR was performed using primers specific for LHR and MR. MR mRNA was detected in all samples of purified NK cells (Fig. 2A) but these samples did not contain measurable LHR mRNA even though this was readily detected in an extract from human corpus luteum (Fig. 2 B). Expression of MR protein on the surface of CD56 positive uNK cells was demonstrated using FACS (Fig. 2 C-H) and immunofluorescence (Fig. 3) with a specific anti-CD206 antibody. Although MR is a cell surface receptor it is internalised following binding to carbohydrates and thus the cells were permeabilised prior to immunostaining; ~40% of the CD56 positive uNK cells (Fig 2F) population were confirmed as positive for MR (CD206), using FACS (Fig 2 G, upper left quadrant). In parallel analyses ~35 % of uNK cells stained positive for both CD206 and CD56 (Fig 2H upper right quadrant). When immunostained cells were visualised using a confocal microscope MR was clearly localised to the purified uNK cells (Fig. 3 middle panel). Notably the antibody staining was not uniform but appeared clustered (Fig. 3 I, II, V) and the intensity of the immunopositive staining varied between cells (Fig. 3 II, V, VIII, XI).
Figure 2. Expression of receptors in uNK cells.

Panel A. Expression of MR mRNA (203bp, upper panel) was detected in isolated uNK cells from 1st trimester decidua and late-secretory phase endometrium, GAPDH (205bp,lower) was detected in all samples. Panel B. Expression of LH/hCG receptor (203 bp, upper panel) was not detected in isolated uNK cells from 1st trimester decidua or late-secretory phase endometrium but as expected was present in a sample of CL, GAPDH (205bp lower) was detectred in all samples. Lane M represents the DNA hyperladder. Lanes 1 to 4 show uNK cells from 1st trimester decidua, lanes 5 to 7 shows uNK cells from non-pregnant late secretory phase endometrium, lane 8 shows a first trimester decidual sample and lane 9 is a sample of corpus luteum (positive control for LHR). Panels C to H: FACS analysis of uNK cell surface antigen expression in all cases axis y is the PE [phycoerythrin] value and axis x is the FITC [fluorescein isothiocyanate] value; C are unstained cells (D), isotype-matched negative controls for FITC+PE, late-secretory endometrial MACS®–isolated uNK cell sample negative controls FITC (E) and stained for CD56 (F) or MR (G). Panel H shows analysis of cells co-stained for CD56 and MR, note cells positive for both markers in upper right quadrant. One representative data set is shown of nine experiments performed.
Figure 3. Localization of hCG and MR on uNK cells.
Staining of purified uNK cells with antibodies directed against MR (green) and/or hCG (red). Yellow staining shows areas of co localisation. Blue shows nuclear counterstain, To Pro. The left hand column shows both MR and hCG staining superimposed, the middle column-MR alone (green channel) and the right hand column hCG alone (red channel). Panels I to III: cells were untreated. Panels IV to VI: cells were incubated with hCG alone (10,000 ng/ml). Panels VII to IX: cells were incubated with hCG plus D-mannose (1mg/ml). Panels X-XII: cells were incubated with deglycosylated hCG alone (10,000 ng/ml).
Binding of hCG to uNK cells is dependent upon the presence of N-linked carbohydrate side chains containing terminal mannose residues
After intact hCG was added to incubation medium it could be detected on the surface of the majority of purified uNK cells (Fig 3 VI) and notably co-localisation with antigenic sites positive for MR was also demonstrated (Fig 3 IV, yellow staining) although there was not a complete/uniform overlap in immunoreactivity. As we expected binding of hCG to be dependent upon the presence of N-linked carbohydrate side chains containing terminal mannose residues we also performed immunostaining with intact hCG in the presence of excess D-mannose (Fig. 3 VII-IX) or with deglycosylated hCG (Fig. 3X-XII). In both cases, although MR immunoexpression was still detectable (green Fig 3 VIII, XI), no hCG was detected bound to the cell surface.
Human chorionic gonadotrophin stimulates uterine NK cell proliferation (Figure 4)
Figure 4. MTT proliferation analysis of uNK cell proliferation and mass spectrometry (MS) analysis demonstrating effective deglycosylation of hCG.
Panel A: Treatment with hCG alone induced proliferation of uNK cells in a dose-dependent manner, with maximal proliferation observed with 10,000 ng/ml hCG treatment (p<0.001) (green bars). Treatment of uNK cells with hCG plus D-mannose (1 mg/ml) did not induce proliferation and this was also the case when cells were incubated with deglycosylated hCG (10,000 ng/ml). a = p<0.001, 2-way ANOVA between groups. * = p<0.05, *** = p<0.001, 1-way ANOVA within group. n = 5 secretory phase endometrial uNK cell samples. Graph B shows MS analysis of glycosylated hCG, intact protein is ~36 kDa (arrow), α subunit is 13 kDa. Graph C shows MS analysis of deglycosylated hCG with the size of protein reduced to ~22 kDa (*), α subunit is 11.5 kDa. Graph D depicts the MS analysis of the deglycosylation enzyme, PNGase alone.
To investigate whether there was a link between expression of the MR in uNK cells, hCG binding and uNK cell number, we evaluated uNK cell proliferation in cells incubated with a range of concentrations of hCG alone or in the presence of excess D-mannose (1 mg/ml) for 24h (Fig. 4A). A significant dose-dependent increase in proliferation of uNK cells was observed at 1000 ng/ml hCG (Fig. 4A p<0.05) and 10000 ng/ml hCG (Fig 4A p<0.001). However no proliferation was induced at any concentration of hCG in the presence of excess D-mannose (1 mg/ml) (Fig 4A).
In order to test whether the protein backbone of hCG was able to alter uNK cell number the protein was deglycosylated with PNGase (Fig. 4 B-D) and incubated with uNK cells at identical concentrations to that used for the intact, glycosylated protein. Under these conditions no uNK cell proliferation was detected (Fig. 4A).
We were also unable to demonstrate any increase in numbers of uNK cells when cells were incubated with either LH (10000 ng/ml) or FSH (0.1 IU) for 24 h (data not shown).
Discussion
Natural killer cells represent the largest leukocyte population in the placenta of early pregnancy and although the increase in the number of uNK cells coincides with implantation and early stages of placentation the mechanism remains unknown. Chorionic gonadotrophin (hCG) is one of the earliest proteins secreted by the fetal trophoblast. In the present study we have described experimental results that have identified chorionic gonadotrophin (hCG), one of the earliest proteins secreted by the pre-implantation conceptus, as a novel regulator of uNK cell proliferation. The impact of hCG on uNK cells was shown to be mediated via the mannose receptor (MR, CD206) rather than by the classical hCG/LH receptor that was not expressed. MR and hCG were co-localised on the surface of uNK cells and proliferation did not occur if cells were incubated with deglycosylated-hCG or intact hCG in the presence of excess D-Mannose.
Our results suggest that cell surface lectins, that have the ability to recognise carbohydrate moieties in glycosylated proteins, may play a role in hCG-mediated uNK cell proliferation. The MR (CD206) contains eight tandemly arranged C-type lectin-like domains that mediate binding to mannose, fucose, or N-acetylglucosamine in a Ca2+-dependent manner (19). Binding of hCG to peripheral blood monocytes has previously been attributed to C-type lectins although the expression of MR was not investigated (20). MR mRNA, but not LHR mRNA, was detected in all samples of purified CD56brightNK cells and expression of MR protein was demonstrated on the surface of purified uNK cells using both FACS and immunofluorescence. Although the MR is a cell surface receptor it is internalised following binding to carbohydrates and this was the reason for permeabilising the cells prior to immunostaining. Variations in percentages between FACS and immunocytochemistry may be attributable to either the primary antibody masking antigenic sites or preventing binding of the other antibody to the cells, and may be influenced by shuttling of the MR. so that the pattern of staining observed is a reflection of the rapid recycling of the MR between the cell surface and intracellular compartments (15).
In order to establish whether hCG could bind directly to uNK cells CD56Bright NK cells were isolated from late secretory (non-pregnant) endometrium using positive selection with CD56 antibody-coated magnetic microbeads and magnetic assisted cell separation. We reasoned that as these cells had not been exposed to proteins from the conceptus any putative binding sites would not be blocked by endogenous protein(s). Mature hCG and LH are modified by N-linked carbohydrate side chains that are important for the stability and assembly of the proteins (14). We further demonstrated that binding of hCG to uNK cells was dependent upon the presence of N-linked carbohydrate side chains containing terminal mannose residues.
A significant dose-dependent increase in proliferation of uNK cells was observed in response to incubation with hCG whereas no proliferation was induced at any concentration of hCG in the presence of excess D-mannose. Furthermore no uNK cell proliferation was detected when the protein backbone of hCG was deglycosylated with PNGase and incubated with uNK cells at identical concentrations to that used for the intact, glycosylated protein. These observations indicated that not only is hCG-mediated binding to uNK cell carbohydrate specific but cell proliferation was also carbohydrate dependent.
We were unable to induce uNK cell proliferation with the other closely related glycoprotein hormones, LH and FSH. LH and FSH share a common α subunit with hCG, but their carbohydrate side chains are not identical (21). Despite a high degree of homology between the LH and CG beta subunits they are reported to differ in their effect on the N-linked oligosaccharide processing of the alpha subunit (22). Furthermore, a previous study has reported that a hyper-glycosylated form of hCG is formed early in pregnancy and is then rapidly replaced by less glycosylated isoforms, suggesting that the hCG glycoforms also change dynamically as pregnancy progresses (23).
These results are of particular interest when considered in parallel with the fluctuation in NK cell number during pregnancy; the dNK population is maximal in the first trimester of pregnancy but declines in the second trimester. These observations provide an explanation for the results of our study showing uNK proliferation was only stimulated by glycosylated hCG and not by LH and provide a mechanism by which the embryo signals its presence to local maternal immune systems facilitating trophoblast implantation and successful establishment of pregnancy. During the normal cycle a peak of LH occurs at the time of ovulation however a significant increase in numbers of uNK cells occurs several days later at the time of decidualisation and this is consistent with our results showing LHR was not expressed on uNK cells.
The daily rate of increase in hCG is lower in failing pregnancies and circulating hCG concentrations are predictive of viable or non-viable (miscarriage and ectopic gestation) pregnancy outcome (24). The endometrium of women with RPL exhibits a greater percentage of CD16Bright, CD56dim uNK cells and a smaller percentage of the CD16dim, CD56Bright uNK cell population, as compared to endometrium of fertile control patients (25, 26). As CD16Bright cells demonstrate greater cytolytic activity than CD16dim NK cells an increased number of CD16Bright cells in the decidua may be a factor in early miscarriage (5). Reduced NK cell populations are also reported in pregnancies complicated by fetal growth restriction (FGR) with or without accompanying pre-eclampsia (7). In this context reduced NK cell numbers in the decidua basalis of women with fetal growth restriction supports a crucial role for uterine NK cell-trophoblast interactions in adequate placental development (7). Indeed, it is likely that uNK cells play an important role at the earliest stage of pregnancy, both in the remodelling of the spiral arteries (27) and the decidualisation of the endometrium. Adequate decidualisation and vascular remodelling are essential for the establishment of a successful pregnancy.
CD56Bright NK cells are also considered to be distinct clinico-pathological entities in malignant conditions and are typically referred to as NK cell lymphoma (28). Clinically, most cases of NK cell lymphoma affect the nasal cavity or other parts of the upper aerodigestive tract (28). Other sites such as the skin, gastrointestinal tract and testis, have also been documented and are usually referred to as extra nasal NK cell lymphoma (28). hCG is an established tumor-associated antigen that is over-expressed in a variety of common cancers (29) and serum hCG levels have proved to be a useful diagnostic tool both for monitoring tumor burden and for evaluating the effectiveness of therapeutic intervention (30). For example, elevated hCG has been demonstrated to be an independent predictor of an unfavorable disease outcome and has been associated with a more aggressive disease course in renal, colorectal, bladder, and pancreatic cancers (31, 32). It has been proposed mechanistically that hCG may act at several different levels to facilitate cancer progression, as a transforming growth factor, an immunosuppressive agent, an inducer of metastasis, and/or as an angiogenic factor (29). To date, no data are available on hCG levels in patients with NK cell lymphomas however our new findings suggest that therapies blocking hCG production or action could provide a novel therapy for this type of cancer or others where the MR is expressed.
In summary, we have shown for the first time that the MR is expressed on CD56brightNK cells isolated from both non-pregnant endometrium and first trimester decidua. Furthermore we report that uterine NK cells can bind hCG but do not express the classical LH/hCG receptor. MR and hCG were co-localised to the surface of uNK cells and incubation with intact glycosylated hCG induced nNK cell proliferation that was blocked if cells were incubated with hCG in the presence of excess D-Mannose. We propose that the proliferation of uNK cells in the early stages of pregnancy is regulated by hCG produced by the trophoblast of the conceptus and that hCG-carbohydrate-side chain recognition by the MR is fundamental in propagating non-canonical hCG signaling. Therefore, carbohydrate recognition, a highly conserved process, may orchestrate the dynamic and complex milieu of the uterine environment in which an immune-endocrine network involving hCG and uNK cells exists, and could be the focus of future therapeutic interventions. These data have implications for management of miscarriage and the development of conditions such as fetal growth restriction that have a life-long impact on health and wellbeing.
Acknowledgements
The authors are very grateful for the support and dedication of Catherine Murray and Sharon McPherson in consenting patients and collection of tissue samples. We are also grateful to Dr Andrew Cronshaw for assistance with analysis of hCG using mass spectroscopy.
Funding: NK was the recipient of an MRC PhD studentship to the Human Reproductive Sciences Unit. RK and PTKS were funded by the core grant to MRC Reproductive Sciences Unit (U1276.00.002.00005.01). Support to HODC from MRC Programme Grant G0500047.
References
- 1.Kalkunte S, Chichester CO, Gotsch F, Sentman CL, Romero R, Sharma S. Evolution of non-cytotoxic uterine natural killer cells. Am J Reprod Immunol. 2008;59:425–432. doi: 10.1111/j.1600-0897.2008.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King A. Uterine leukocytes and decidualization. Human Reproduction Update. 2000;6:28–36. doi: 10.1093/humupd/6.1.28. [DOI] [PubMed] [Google Scholar]
- 3.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 4.Bulmer JN, Morrison L, Longfellow M, Ritson A, Pace D. Granulated lymphocytes in human endometrium: histochemical and immunohistochemical studies. Hum Reprod. 1991;6:791–798. doi: 10.1093/oxfordjournals.humrep.a137430. [DOI] [PubMed] [Google Scholar]
- 5.Dosiou C, Giudice LC. Natural killer cells in pregnancy and recurrent pregnancy loss: endocrine and immunologic perspectives. Endocr Rev. 2005;26:44–62. doi: 10.1210/er.2003-0021. [DOI] [PubMed] [Google Scholar]
- 6.Lachapelle MH, Miron P, Hemmings R, Roy DC. Endometrial T, B, and NK cells in patients with recurrent spontaneous abortion. Altered profile and pregnancy outcome. J Immunol. 1996;156:4027–4034. [PubMed] [Google Scholar]
- 7.Eide IP, Rolfseng T, Isaksen CV, Mecsei R, Roald B, Lydersen S, Salvesen KA, Harsem NK, Austgulen R. Serious foetal growth restriction is associated with reduced proportions of natural killer cells in decidua basalis. Virchows Arch. 2006;448:269–276. doi: 10.1007/s00428-005-0107-z. [DOI] [PubMed] [Google Scholar]
- 8.King A, Burrows T, Verma S, Hiby S, Loke YW. Human uterine lymphocytes. Hum Reprod Update. 1998;4:480–485. doi: 10.1093/humupd/4.5.480. [DOI] [PubMed] [Google Scholar]
- 9.King A, Gardner L, Loke YW. Evaluation of oestrogen and progesterone receptor expression in uterine mucosal lymphocytes. Hum Reprod. 1996;11:1079–1082. doi: 10.1093/oxfordjournals.humrep.a019300. [DOI] [PubMed] [Google Scholar]
- 10.Henderson TA, Saunders PT, Moffett-King A, Groome NP, Critchley HO. Steroid receptor expression in uterine natural killer cells. J Clin Endocrinol Metab. 2003;88:440–449. doi: 10.1210/jc.2002-021174. [DOI] [PubMed] [Google Scholar]
- 11.Acevedo HF. Human chorionic gonadotropin (hCG), the hormone of life and death: a review. J Exp Ther Oncol. 2002;2:133–145. doi: 10.1046/j.1359-4117.2002.01031.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang YM, Rao Ch V, Lei ZM. Macrophages in human reproductive tissues contain luteinizing hormone/chorionic gonadotropin receptors. Am J Reprod Immunol. 2003;49:93–100. doi: 10.1034/j.1600-0897.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- 13.Licht P, Fluhr H, Neuwinger J, Wallwiener D, Wildt L. Is human chorionic gonadotropin directly involved in the regulation of human implantation? Mol Cell Endocrinol. 2007;269:85–92. doi: 10.1016/j.mce.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Matzuk MM, Boime I. The role of the asparagine-linked oligosaccharides of the alpha subunit in the secretion and assembly of human chorionic gonadotrophin. J Cell Biol. 1988;106:1049–1059. doi: 10.1083/jcb.106.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.East L, Isacke CM. The mannose receptor family. Biochim Biophys Acta. 2002;1572:364–386. doi: 10.1016/s0304-4165(02)00319-7. [DOI] [PubMed] [Google Scholar]
- 16.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertility and Sterility. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 17.Critchley HO, Henderson TA, Kelly RW, Scobie GS, Evans LR, Groome NP, Saunders PT. Wild-type estrogen receptor (ERbeta1) and the splice variant (ERbetacx/beta2) are both expressed within the human endometrium throughout the normal menstrual cycle. J Clin Endocrinol Metab. 2002;87:5265–5273. doi: 10.1210/jc.2002-020502. [DOI] [PubMed] [Google Scholar]
- 18.Trundley A, Gardner L, Northfield J, Chang C, Moffett A. Methods for isolation of cells from the human fetal-maternal interface. Methods Mol Med. 2006;122:109–122. doi: 10.1385/1-59259-989-3:109. [DOI] [PubMed] [Google Scholar]
- 19.Taylor ME. Structure and function of the macrophage mannose receptor. Results Probl Cell Differ. 2001;33:105–121. doi: 10.1007/978-3-540-46410-5_6. [DOI] [PubMed] [Google Scholar]
- 20.Kosaka K, Fujiwara H, Tatsumi K, Yoshioka S, Sato Y, Egawa H, Higuchi T, Nakayama T, Ueda M, Maeda M, Fujii S. Human chorionic gonadotropin (HCG) activates monocytes to produce interleukin-8 via a different pathway from luteinizing hormone/HCG receptor system. J Clin Endocrinol Metab. 2002;87:5199–5208. doi: 10.1210/jc.2002-020341. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson B, Rosen SW, Weintraub BD, Zopf DA. Differences in the carbohydrate moieties of the common alpha-subunits of human chorionic gonadotropin, luteinizing hormone, follicle-stimulating hormone, and thyrotropin: preliminary structural inferences from direct methylation analysis. Endocrinology. 1986;119:2737–2743. doi: 10.1210/endo-119-6-2737. [DOI] [PubMed] [Google Scholar]
- 22.Corless CL, Matzuk MM, Ramabhadran TV, Krichevsky A, Boime I. Gonadotropin beta subunits determine the rate of assembly and the oligosaccharide processing of hormone dimer in transfected cells. J Cell Biol. 1987;104:1173–1181. doi: 10.1083/jcb.104.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovalevskaya G, Kakuma T, Schlatterer J, O’Connor JF. Hyperglycosylated HCG expression in pregnancy: cellular origin and clinical applications. Mol Cell Endocrinol. 2007;260-262:237–243. doi: 10.1016/j.mce.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Sugantha SE, Webster S, Sundar E, Lenton EA. Predictive value of plasma human chorionic gonadotrophin following assisted conception treatment. Hum Reprod. 2000;15:469–473. doi: 10.1093/humrep/15.2.469. [DOI] [PubMed] [Google Scholar]
- 25.Laird SM, Tuckerman EM, Cork BA, Linjawi S, Blakemore AI, Li TC. A review of immune cells and molecules in women with recurrent miscarriage. Hum Reprod Update. 2003;9:163–174. doi: 10.1093/humupd/dmg013. [DOI] [PubMed] [Google Scholar]
- 26.Tuckerman E, Laird SM, Prakash A, Li TC. Prognostic value of the measurement of uterine natural killer cells in the endometrium of women with recurrent miscarriage. Hum Reprod. 2007;22:2208–2213. doi: 10.1093/humrep/dem141. [DOI] [PubMed] [Google Scholar]
- 27.Bulmer JN, Lash GE. Human uterine natural killer cells: a reappraisal. Mol Immunol. 2005;42:511–521. doi: 10.1016/j.molimm.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 28.Siu LL, Chan JK, Kwong YL. Natural killer cell malignancies: clinicopathologic and molecular features. Histol Histopathol. 2002;17:539–554. doi: 10.14670/HH-17.539. [DOI] [PubMed] [Google Scholar]
- 29.Triozzi PL, Stevens VC. Human chorionic gonadotropin as a target for cancer vaccines. Oncol Rep. 1999;6:7–17. [PubMed] [Google Scholar]
- 30.Braunstein GD. Placental proteins as tumor markers. Immunol Ser. 1990;53:673–701. [PubMed] [Google Scholar]
- 31.Syrigos KN, Fyssas I, Konstandoulakis MM, Harrington KJ, Papadopoulos S, Milingos N, Peveretos P, Golematis BC. Beta human chorionic gonadotropin concentrations in serum of patients with pancreatic adenocarcinoma. Gut. 1998;42:88–91. doi: 10.1136/gut.42.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundin M, Nordling S, Lundin J, Alfthan H, Stenman UH, Haglund C. Tissue expression of human chorionic gonadotropin beta predicts outcome in colorectal cancer: a comparison with serum expression. Int J Cancer. 2001;95:18–22. doi: 10.1002/1097-0215(20010120)95:1<18::aid-ijc1003>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]