Abstract
Imitation ability has consistently been shown to be impaired in individuals with autism. A dysfunctional execution/observation matching system has been proposed to account for this impairment. The EEG mu rhythm is believed to reflect an underlying execution/observation matching system. This study investigated evidence of differential mu rhythm attenuation during the observation, execution, and imitation of movements and examined its relation to behaviorally assessed imitation abilities. Fourteen high-functioning adults with autism spectrum disorder (ASD) and 15 IQ- and age-matched typical adults participated. On the behavioral imitation task, adults with ASD demonstrated significantly poorer performance compared to typical adults in all domains of imitation ability. On the EEG task, both groups demonstrated significant attenuation of the mu rhythm when executing an action. However, when observing movement, the individuals with ASD showed significantly reduced attenuation of the mu wave. Behaviorally assessed imitation skills were correlated with degree of mu wave attenuation during observation of movement. These findings suggest that there is execution/observation matching system dysfunction in individuals with autism and that this matching system is related to degree of impairment in imitation abilities.
Keywords: autism, imitation, EEG, mu rhythm, mirror neurons
Introduction
Imitative deficits in individuals with autism have consistently been observed (Williams, Whiten & Singh, 2004). In fact, several researchers have suggested that imitation deficits are one of the core impairments of autism spectrum disorders (ASD; Dawson & Adams, 1984; Dawson & Lewy, 1989; Rogers & Pennington, 1991; Williams, Whiten, Suddendorf & Perrett, 2001). Imitation impairments in autism were first reported by DeMyer and colleagues (DeMeyer, Alpern, Barons, DeMyer, Churchill, Hingtgen, Bryson, Pontius & Kimberlin, 1972) and in the thirty years since this initial report, over twenty studies have examined varying aspects of imitation in ASD. Most of these studies have focused on imitation impairments in children with ASD. Children with autism under six years of age show impaired imitation skills compared to children with mental retardation, developmental delay, and communication disorders as well as compared to typically developing children (Sigman & Ungerer, 1984; Stone, Lemanek, Fishel, Fernandez & Altemeir, 1990; Charman, Swettenham, Baron-Cohen, Cox, Baird, & Drew, 1997, 1998; Stone, Ousley & Littleford, 1997; Dawson et al., 1998; Rogers, Hepburn, Stackhouse, & Wehner, 2003; Aldridge, Stone, Sweeney & Bower, 2000). School age children with autism also show imitation deficits (Hammes & Langdell, 1981; Ohta, 1987, Smith & Bryson, 1998; Green et al., 2002; Jones & Prior, 1985). Such impairments persist into adolescence and adulthood although there are fewer studies that have examined this age group (Rogers et al., 1996; Avikainen et al., 2003; Hobson & Lee, 1999). Hobson and Lee (1999) found that teens with autism were able to imitate goal directed actions but failed to imitate the style in which the examiner performed the task with greater frequency than the control group. Further, during the imitative acts, the subjects with autism made considerable reversal errors, errors in which the imitator replicates the gestures but in the perspective of how the gesture was observed. Similarly, Avikainen et al. (2003) examined imitation in adults through a task in which participants imitated the experimenter's actions using either the same hand or opposite hand. They found that while typical adults used the mirror image of the experimenter's actions to increase response speed and accuracy, the individuals with Asperger syndrome and high functioning autism failed to capitalize on the mirror image.
It has been proposed that a deficit in self other mapping is the most parsimonious explanation for the imitation impairments found in ASD (Williams et al., 2004). The self-other mapping hypothesis, which posits that a biological dysfunction of cross modal processes prevents the individual from forming and coordinating representations of the self and others, was earlier presented by Meltzoff and Gopnik (1993) and Rogers and Pennington (1991). Evidence from infant imitation work suggests that cross modal processes necessitate an execution/observation matching system (Meltzoff & Moore, 1997; Meltzoff & Decety, 2003). That is, in order for an infant to imitate faces, the infant must cross modally map an observed facial expression with his or her own motor execution of that expression. But the infant cannot see his or her face and so must have a supramodal internal representation that enables a match between the observed and executed expression.
Mu activity and the execution/observation system
The first neurophysiological evidence of an execution/observation matching system in humans comes from EEG work conducted in the 1950s which focused on the mu rhythm band oscillations. The EEG mu rhythm band falls between 8 and 13 Hz and is recorded from scalp electrodes over the sensorimotor cortex. Attenuation of resting EEG mu rhythm reflects desynchronization of the underlying cell assemblies, which suggests an increased load on those cells (Pfurtscheller, Neuper, Andrew & Edlinger, 1997). Attenuation of the resting EEG mu rhythm was observed in adult subjects in response to the execution of actions, both passive or reflex movements (Chatrian, Petersen, & Lazarete, 1959). Attenuation was also observed in adults who were watching others' movements (boxing) on film (Gastaut & Bert, 1954). More recently, the finding of mu wave attenuation when observing and executing actions has been replicated in both adults (Babiloni, Percio, Babiloni, Carducci, Cincotti, Moretti, & Rossini, 2003; Muthukumaraswamy, Johnson & McNair, 2004; Muthukumaraswamy & Johnson, 2004; Cochin, Barthelemy, Roux, Martineau, 1999; Cochin, Barthelemy, Lejeune, Roux, Martineau, 1998; Pfurtscheller et al, 1997; Babiloni, Carducci, Cincotti, Rossini, Neuper, Pfurtscheller, & Babiloni, 1999; Babiloni, Babiloni, Carducci, Cincotti, Cocozz, Del Pecrio, Moretti & Rossini, 2002; Arroyo, Lesser, Gordon, Uematsu, Jackson, Webber, 1993) and children (Stroganova, Orekhova & Posikero, 1999; Martineau & Cochin, 2003; Cochin, Barthelemy, Roux & Martineau, 2001; Lepage & Theoret, 2006). Mu wave attenuation during observation of action is strictly a central phenomenon. That is, movement in the body does not account for the mu wave attenuation when observing other's actions. In two studies of typical adults, Muthukumaraswamy and colleagues determined that subtle activity in the hand did not account for the reduced attenuation when observing human movement (Muthukumaraswamy, Johnson & McNair, 2004; Muthukumaraswamy & Johnson, 2004). Further, imagining movement is sufficient to attenuate the mu rhythm (Pfurtscheller, Brunner, Schlogl, & Lopes de Silva, 2006). In adults mu wave attenuation was found during the imagining of left and right hand motor movements. These consistent findings of attenuation of the mu wave during the observation or imagining of human movement, as well as during the execution of movement, suggest that the mu wave reflects an execution/observation matching system (Pineda, 2005).
In a study of 10 individuals with autism and typical individuals (6-47 years of age), Oberman and colleagues (2005) reported attenuation of the mu rhythm in the 8-13 Hz band during the execution and observation conditions for the typical group, whereas the autism group showed attenuation only during the execution condition. They concluded that the results were consistent with execution/observation system dysfunction in individuals with autism.
Although individuals with autism appear to fail to show attenuation of mu activation during observation and clearly show behavioral impairments in imitation, the relation between these to findings is unclear. This study aims to further explore the imitation deficits in a sample of high functioning adults with autism including the relation between behaviorally assessed imitative skills and the EEG index of the execution/observation matching system. It is hypothesized that adults with autism will demonstrate imitation impairments as well as reduced mu wave attenuation when observing biological movement. We further propose that degree of EEG mu wave attenuation will be correlated with level of imitative ability.
Methods
Participants
The original sample of participants included 17 adult males with an idiopathic autism spectrum disorder (ASD) and 16 neuropsychiatrically and medically healthy male adults, all with full scale IQ above 80. Groups were matched on IQ, chronological age, and handedness. Informed consent was obtained for all participants.
Participants with autism were diagnosed using the Autism Diagnostic Interview - Revised (ADI-R; Lord, Rutter, & LeCouteur, 1994), the Autism Diagnostic Observation Schedule - Generic (ADOS-G; Lord et al., 2000), and experienced clinician judgment using DSM-IV criteria (American Psychiatric Association, 2000). Intellectual ability was assessed in both groups using the Wechsler Adult Intelligence Scale-3rd Edition (WAIS-III; Wechsler, 1997).
Of the original sample of 17 males with ASD and 16 typical adults, three participants with ASD and one typical participant were excluded prior to analysis due to excessive movement artifacts that resulted in insufficient EEG data in one or more conditions. As a result, the final sample consisted of 14 males with ASD and 15 typical participants. Table 1 presents demographic information for both groups, including chronological age, IQ, ethnicity, and handedness. Groups did not significantly differ on any of these factors.
Table 1.
Participant descriptive characteristics
| Age (Years) | IQ: mean (S.D.) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | N | Ethnicity | Handedness | Min. | Max. | M (SD) | FSIQ | VIQ | PIQ |
| ASD | 14 | 13 White | 12 Right | 19.4 | 37.5 | 23.6 | 114.0 | 117 | 107.6 |
| 1 Other | 2 Left | (4.9) | (14.2) | (14.1) | (15.4) | ||||
| Typical | 15 | 13 White | 11 Right | 18.6 | 43.7 | 26.7 | 108.9 | 110.1 | 105.4 |
| 2 Other | 4 Left | (8.7) | (15.6) | (14.7) | (18.2) | ||||
The ASD group was comprised of 6 individuals with Autistic disorder, 5 individuals with Asperger Syndrome, and 3 individuals with pervasive developmental disorder – not otherwise specified.
Behavioral assessments
Mature Imitation Task
The behavioral imitation assessment battery used for this study was developed by Rogers and colleagues for use with high functioning individuals with autism (Rogers, Cook & Greiss-Hess, 2005). Participants were instructed to observe a videotaped model producing a variety of single and sequenced hand gestures, single and sequenced facial expressions, hand gestures requiring both hands, short meaningless hand movements, and actions on objects. The videotaped model was presented by computer monitor at a distance of 36 inches from the participant. Following the single trial of each gesture, expression, movement or action, the screen became blank and participants imitated the observed behavior. The sequences differed in length of presentation based on modeled action. For example, the shortest presentation was a single step hand gesture lasting 2.5 seconds while the longest modeled action (rubbing a lintbrush along one arm three times) lasted 20 seconds. Participants were provided with as much time as needed to attempt to imitate the model.
Participant responses were videotaped and later coded by a trained undergraduate who was blind to the diagnostic status of the participant. Imitations were coded following procedures outlined by Rogers and colleagues using the Mature Imitation Task Manual (Rogers et al., 2005). Gestures, expressions, movements, and actions on objects were coded on scales specific to each task. For example, hand gestures were coded on two scales: finger placement and orientation and were scored with one point for correct imitation and zero for failure while the meaningless movements were scored on four scales: start position, movement, posture change, and end position, with a total of two points earned on the start and end position scales and one point for the movement and posture change scales. Errors, such as groping and overshooting, were coded and tallied separately. Groping errors consisted of additional or incorrect movements that resulted in an improved response and included visual inspection of the gesture while overshooting errors were coded when the position of the body was in the correct area but the hand or arm extended beyond that demonstrated in the model. Style of action (harsh or gentle) on objects was also coded. The coder was initially trained to reliability, and inter-rater reliability was maintained between the coder and first author on 18% of the protocols. For rater discrepancies of more than one point for an item, consensus scores were determined and utilized. Inter-rater reliability was calculated using intraclass correlations with a mean correlation of .92 and range of .68 to .99 across the 7 scales as well as the error and style rating scales.
Summary scores were calculated. An overall total score was calculated by summing the scores for each imitation task. An overall error score was calculated by summing the errors across all imitation tasks. Both single and sequenced hand tasks were combined for a ‘hand total’ score and the same was done for the facial expression tasks (‘face total’). The single face and single hand tasks were combined to yield a ‘single total’ score and the same was done for the sequenced tasks (‘sequenced total’). A style total was calculated by summing the number of style points earned during the actions on objects task.
EEG assessment
EEG recording
Electrical brain activity was recorded using a dense-array EEG system (EGI Inc, Eugene, OR). EEG was recorded from a 128 channel Geodesic sensor net and impedances were below 50 kΩ. EEG signals were analog filtered (0.1 Hz high-pass, 100 Hz elliptical low-pass) amplified, and digitized at a 250 Hz. The participant sat approximately 75 cm from a video monitor that delivered the stimuli. The video stimuli were presented on the monitor at a size of 17.5 cm by 26.25 cm and subtended a visual angle of 13.3 by 19.8. All 128 channels were recorded continuously and stimulus onset and cessation and event markers were registered with the physiological record for off-line segmentation of the data. The vertex electrode was used as a reference, and the data were later re-referenced to an average reference. Data were post processed using software provided by Electrical Geodesics, Inc.
EEG imitation paradigm
Following the paradigm developed by Muthukumaraswamy & Johnson (2004) the experiment consisted of 4 conditions: resting, observe, execute, and imitate. All stimuli were presented on videotape to ensure standardization of administration of the hand movements. In the resting condition, the participant was asked to sit quietly with hands in lap. In the observe condition participants viewed an adult gripping a manipulandum (Muthukumaraswamy et al., 2004) with thumb and index finger as shown in Figure 1.
Figure 1.
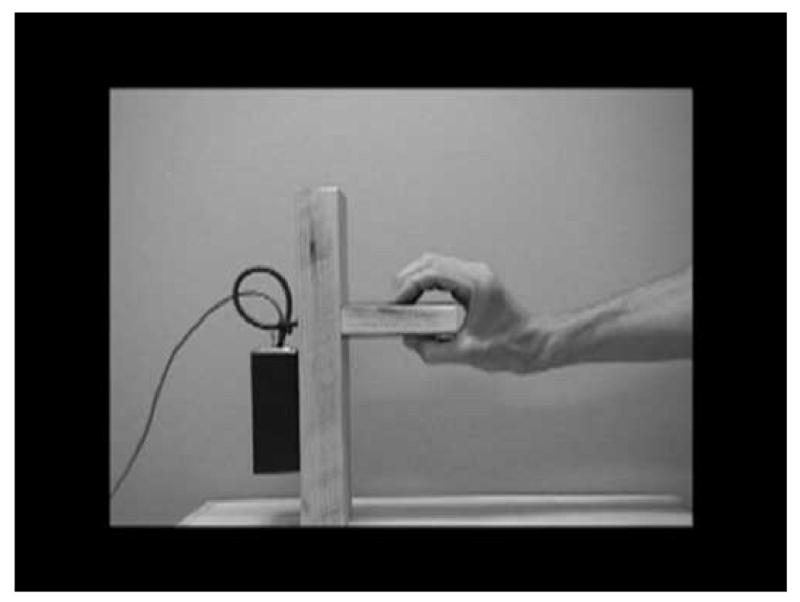
Hand grasping the manipulandum.
In the observe condition, participants sat with hands in lap and observed the action. In the execute condition, participants were verbally instructed to grip the manipulandum identical to that shown in the videotape in the same manner as shown in the videotape. The video monitor showed only the manipulandum at this time and no movement. The manipulandum was placed on the table 50 cm directly in front of the participant. In the imitate condition, the participant was instructed to imitate the experimenter gripping the manipulandum as shown on the videotape. Each trial lasted approximately three seconds and there was a 7 second inter trial interval between each trial. Twenty trials in each condition were presented with condition order randomized.
A synchronization pulse was sent to the EEG recording equipment at the moment the participant grasped the manipulandum. These pulses served as the event markers for the execute and imitate conditions. During the observe condition an event marker was sent to the EEG recording when the model on the videotaped hand grasped the manipulandum. These event markers, recorded during acquisition, were sent directly to the EEG record for use in EEG segmentation.
EEG analysis
Following procedures from Muthukumaraswamy et al., 2004, the EEG recording was segmented into units extending 2 seconds before and after the event markers. Segments with movement artifact were identified and removed through visual inspection as well as through NetStation's (Electrical Geodesic) automated artifact detection algorithm. Following the parameters of the automated algorithm, channels were identified as bad if fast average amplitude exceeded 200 μVs, if the differential average amplitude exceeded 100 μVs, or if the channel had zero variance. Channels were marked bad for all trials if they were marked bad in 20% of all trials and trials were discarded if they contained more than 10 bad channels. The mean trial rejection rate was 32.28% across both groups. There were no differences between groups in rate of trial rejection (Typical M = 32.27%; Autism M = 32.29%).
Fast Fourier Transforms (FFTs) were performed in Matlab (Natick, MA) on two second segments of clean EEG data. Power spectra were averaged across trials for each condition. As the mu rhythm, distinct from other EEG bands, has been proposed to reflect an execution/observation matching system, the study focused on mu rhythm activity only. The mu rhythm was defined as the frequency band ranging from 8-13 Hz that is topographically centered on the standard C3 and C4 positions (Pineda, 2005; Oberman et al., 2005; Muthukumaraswamy et al., 2004). As shown in figure 2, following Muthukumaraswamy et al (2004), a cluster of eight electrodes on each hemisphere surrounding the standard C3 and C4 positions were used for statistical analyses.
Figure 2.
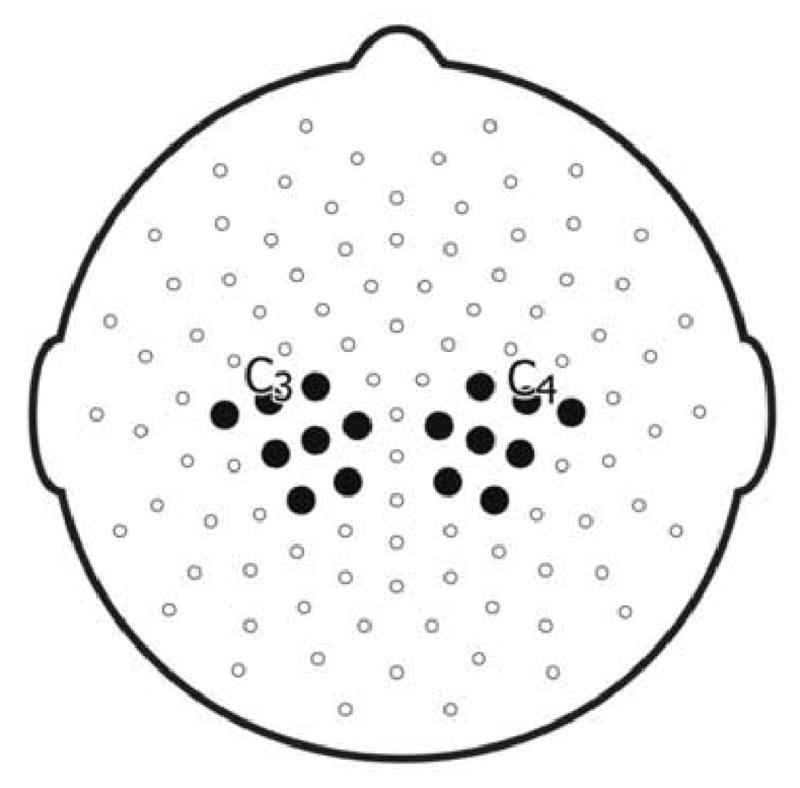
Cluster of eight electrodes surrounding C3 and C4 used for statistical analyses.
Two measures of mu attenuation were used in this study. The first measure was a ratio of the 8-13 Hz power during each of the execute, imitate, and observe conditions relative to 8-13 Hz power in the resting condition. This ratio was used to account for individual variability in overall EEG power in this frequency range. Due to the inherent non-normality of ratio data, a log transform was calculated for each ratio. The log transform results in a negative value representing attenuation, a value of zero indicating no attenuation, and a positive value representing mu rhythm augmentation. This measure was used to examine attenuation differences between groups and conditions.
The second measure of mu attenuation used was the difference between an individual's resting condition and the condition of interest (i.e. imitate or observe). For this measure of attenuation, greater values equal greater attenuation. Similar to the log ratio, this measure controls for individual differences in baseline EEG power. This measure of attenuation was used to examine relations between EEG and behaviorally assessed imitation skills
Results
Behavioral Imitation Skills
As shown in Table 2, on the Mature Imitation Task, the ASD group scored significantly worse than the typical adults. Typical adults were able to more accurately imitate the model's expressions and gestures and demonstrated fewer errors than the adults with autism.
Table 2.
Results for Mature Imitation Task
| Typical Group | Autism Group | |||
|---|---|---|---|---|
| Summary Scale | Mean (S.D.) | Mean (S.D.) | t-Test | Sig. p< |
| Total Score | 185.2 (13.1) | 155.1 (19.4) | 4.92 | .001 |
| Face Summary Score | 57.7 (4.3) | 46.1 (7.7) | 5.076 | .001 |
| Hand Summary Score | 45.9 (7.8) | 35.1 (12.4) | 2.832 | .01 |
| Single Task Summary | 28.8 (2.4) | 24.3 (3.5) | 4.047 | .001 |
| Sequenced Task Summary | 74.9 (8.8) | 56.9 (14.7) | 4.037 | .001 |
| Style Total | 9.7 (1.9) | 4.9 (2.9) | 5.299 | .001 |
| Number of Errors | 12.4 (5.2) | 19.9 (10.1) | 2.544 | .05 |
Mu Wave Attenuation
Figure 3 presents the log ratio of mu rhythm attenuation for each condition for both groups. Significant attenuation in mu from baseline was found for both groups for each condition. For the typical group: execute t(14) = −5.798, p < 0.001; imitate t(14) = −6.361, p < 0.001; observe t(14) = −6.211, p < 0.001. For the autism group: execute t(13) = −4.445, p < 0.001; imitate t(13) = −4.443, p < 0.001; observe t(13) = −2.747, p < 0.017. A 2 (group) by 3 (condition) ANOVA for repeated measures yielded a main effect of condition F(2,54) = 12.929, p <.001 but no main effect for group or interaction effects.
Figure 3.
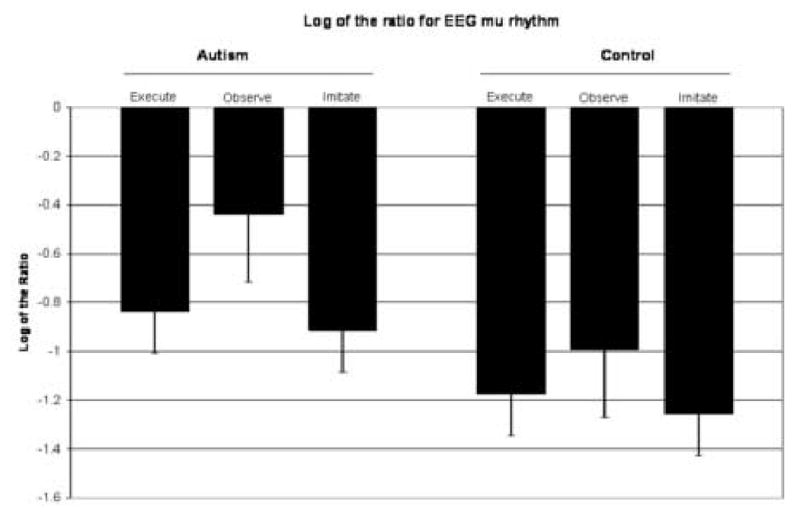
Mu power as a function of condition.
Analysis for the typical group of the log ratio showed no differences in degree of mu rhythm attenuation between the execute and observe conditions, t(14) = 1.803, p < ns. Similarly, there were no differences between degree of attenuation of the execute and imitate conditions, t(14) = .781, p < ns. However, for the typical group, greater attenuation was found for the imitate condition relative to the observe condition, t(14) = 2.275, p < 0.05.
Analyses for the autism group revealed significant differences in degree of attenuation between the execute, imitate, and observe conditions. The observe condition showed significantly less attenuation than the execute and imitate conditions, t(13) = 3.291, p < 0.01, t(13) = 3.653, p < 0.005, respectively. There were no differences between the imitate and execute conditions, t(13) = 1.106, p < ns.
Given that different patterns were found in EEG power attenuation between the execute and observe conditions for the autism group versus typical group, further analyses were conducted between groups using the log ratio. While the groups did not differ in mu power ratio in the execute condition (t(27) = 1.230, p = n.s.) or the imitate condition (t(27) = 1.201, p = n.s.), the groups did significantly differ in mu power ratio for the observe condition, with the autism group showing significantly lower mu wave power ratio as compared to the typical group (t(27) = 2.447, p < .05).
To assure that the effects of suppression were specific to the mu rhythm and not the result of other activity, such as the more powerful, posterior alpha activity, lead clusters were selected from other scalp regions. Six leads were selected from the frontal region (three around AF3 in the left hemisphere and three around AF4 in the right hemisphere) and six leads were selected from the occipital parietal region (three around O1 in the left and three around O2 in the right). Across the conditions no consistent pattern of suppression was observed in the frequency band under investigation at these other electrode clusters. This indicates that the observed suppression was specific to the central electrodes and not the result of other activity, such as alpha desynchronization.
Given the heterogeneity in individuals with diagnoses on the autism spectrum, power values were calculated for each participant with autism for each of the four experimental conditions and are presented in table 3.
Table 3.
Mu Wave Power Values for each autism participant (in μV2)
| Subject Number | Condition: mean (standard deviation) | |||
|---|---|---|---|---|
| Execute | Observe | Imitate | Resting | |
| 1 | .303940 (.125) | .685585 (.302) | .336325 (.132) | 1.08213 (.438) |
| 2 | .155272 (.064) | .143876 (.040) | .165551 (.058) | .407575 (.146) |
| 3 | .203481 (.081) | .127112 (.074) | .158918 (.050) | .214257 (.072) |
| 4 | .255224 (.070) | .302710 (.093) | .175809 (.092) | .783554 (.350) |
| 5 | .846268 (.478) | 2.36863 (1.72) | 1.22539 (.895) | 3.45046 (1.75) |
| 6 | .549620 (.222) | .880590 (.434) | .508607 (.221) | .853169 (.750) |
| 7 | .183697 (.031) | .248708 (.060) | .303731 (.103) | 1.62923 (.417) |
| 8 | .424599 (.205) | .666416 (.308) | .359777 (.110) | .546059 (.137) |
| 9 | .571214 (.372) | .842045 (.464) | .301757 (.149) | 2.12702 (.423) |
| 10 | .706779 (.365) | 2.00470 (.957) | .572928 (.167) | 3.09828 (.587) |
| 11 | .532764 (.175) | 1.01521 (.388) | .515492 (.190) | 3.65624 (1.32) |
| 12 | .123849 (.027) | .133719 (.056) | .106281 (.038) | .152444 (.086) |
| 13 | .211769 (.040) | .358013 (.134) | .203514 (.058) | .285538 (.138) |
| 14 | .175790 (.069) | .150766 (.091) | .226580 (.071) | .117707 (.058) |
Intercorrelations of EEG power between each electrode for both hemisphere clusters were calculated. Of the 168 comparisons across the three conditions of execute, observe, and imitate, 144 were significant at the .05 level (138 at the .01 level). This did not differ by condition.
The latency to grasp the manipulandum during the imitation condition was calculated for a random subset of the sample (11 participants from each group). This was calculated to check participants' attention to the imitation task. The average times from onset of trial to grasp for the autism and typical groups were 1.47 and 1.59 seconds, respectively. There were no differences between groups in latency to grasp the manipulandum, t(21)= .816, p = n.s.
Relation Between Imitation and Mu Attenuation
Correlational analyses revealed relations between mu wave attenuation and behavioral imitation skills. To capture attenuation of mu power, a difference score was calculated by subtracting the condition of interest (observe or imitate) from the resting condition for each individual.
Mu wave attenuation for the observe condition was correlated with the face imitation summary scale for both groups combined r (29) = .567, p<.001 and also for both groups when examined separately: typical group: r (15) = .551, p<.05; autism group: r (14) = .536, p<.05 (Figure 4). Mu wave attenuation for the observe condition was also correlated with the total imitation score (r (29) = .509, p<.01), hand summary score (r (29) = .388, p<.05), sequenced task summary score (r (29) = .522, p<.01), and single task summary score (r (29) = .401, p<.05).
Figure 4.
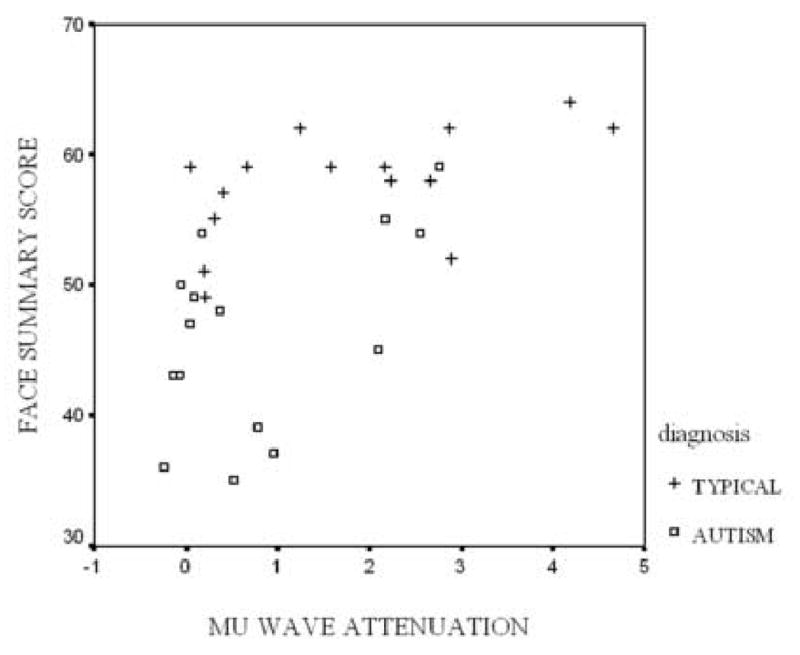
Relation between mu wave attenuation during observation and behaviorally assessed facial imitation task summary score. Attenuation is calculated as difference between power during observation and power during resting.
For mu wave attenuation in the imitate condition with both diagnostic groups combined, correlations were found with total score (r (29) = .450, p<.05), face summary score (r (29) = .433, p<.05), hand summary score (r (29) = .422, p<.05), and sequence summary score (r (29) = .507, p<.01). When these relations were examined for the typical group separately, only the correlation between imitation mu wave attenuation and the face summary scale remained, r (15) = .571, p<.05. No correlation was found between these two variables for the autism group alone. However for the autism group, correlations were found for the total sample between imitation mu attenuation and total score (r (14) = .574, p<.05), hand summary score (r (14) = .537, p<.05), and sequence summary score (r (14) = .625, p<.05).
Discussion
The primary goals of this study were to clarify the nature of the imitation deficits in autism and explore a possible neurophysiological correlate of these deficits. Both adults with ASD and typical adults showed significant mu wave attenuation during the execute condition as compared to the resting condition. However, during the observation condition, adults with autism showed reduced mu wave attenuation, as compared to the typical adults. To the extent to which mu wave attenuation is a sensitive index of the functioning of the execution/observation matching system, these results suggest that this neural system may be disrupted in autism. A class of neurons, called mirror neurons, first identified in monkeys in the prefrontal cortex, provides a possible neurological mechanism for an execution/observation matching system. Indirect evidence from imaging and psychophysiological studies suggests there is a mirror neuron system in humans. This system in humans consists of the posterior inferior frontal gyrus and inferior parietal cortex, and activates both when an action is observed and when it is executed (Rizzolatti & Craighero, 2004). While it is unclear what the relationship is between the mu rhythm and mirror neurons, it has been proposed that the mu rhythm might reflect “downstream modulation of motor neurons by cells in the premotor cortex, some of which are mirror neurons” (Pineda, 2005, p.57, Muthukumaraswamy, Johnson & McNair, 2004).
This study found support for continued impairments in imitation skills for adults with ASD who have intellectual abilities in the average range. While there have been numerous studies examining imitation skills in autism, the vast majority of these studies have been conducted with children. Two studies conducted with teens found continued evidence of imitation impairments (Rogers et al., 1996; Loveland et al., 1994) and one other study of adults with ASD found significantly more errors and no benefits in using a mirror image to enhance performance (Avikainen et al., 2003). The current study found continued impairments in single and multiple step acts that involved both hand and face imitation, imitative acts that required both hands, and actions that involved meaningless movements. The imitative deficits observed in childhood in previous studies clearly persist into adulthood.
Moreover, the degree of mu wave attenuation when observing an action was found to be correlated with behaviorally assessed imitation skills. Lower levels of mu attenuation were associated with poorer imitation skills, and this was most robust for facial imitation skills. This result is consistent with the hypothesis that functioning of execution/observation matching system is associated with level of imitation skill, and more specifically, that the imitation impairments found in individuals with autism might be related to dysfunction of the execution/observation matching system.
The more robust correlation with facial imitation as compared to hand or stylistic imitation may be due to differences in the subject of imitation. The imitation of facial expressions relies more heavily on cross modal communication than imitation of styles or hand gestures because there is no visual feedback. That is, when imitating a hand gesture, it is entirely possible for the imitator to look down at his or her hand to help with the imitation. This was, in fact, observed numerous times during the behavioral task. As such, facial imitation tasks may require more activation of the execution/observation matching system, which underlies cross modal communication, than imitation of hand gestures or action styles. This greater recruitment of the underlying execution/observation system may then translate into greater desynchronization of the underlying neural assemblies resulting in greater mu wave attenuation. A dysfunctional execution/observation system, therefore, could result in greater impairments in facial imitation than in other aspects of imitation.
While the typical adults demonstrated the same level of attenuation of the mu rhythm when both observing and executing an action, the autism group failed to show the same degree of attenuation when observing the action. However, the autism group did demonstrate attenuation when executing an action, suggesting intact sensorimotor systems underlying self initiated actions but not those underlying the observation of biologically relevant movement. A disruption in the execution/observation matching system could contribute to deficits in social behavior as this system allows for translation of others' observed actions into understanding through motor representation of that action. A disruption of the neural processes that allow for understanding others actions would then lead to difficulties in social learning, communication, and interaction.
Williams et al. (2001) first proposed that dysfunction of an execution/observation matching system, in the form of mirror neurons, underlies the imitation impairments in autism. Recently, several researchers have conducted studies relevant to this execution/observation matching system in individuals with autism (Theoret, Halligan, Kobayashi, Fregni, Tager-Flusberg, Pascual-Leone, 2005; Avikainen, Kulomaki & Hari, 1999; Williams, Waiter, Gilchrist, Perrett, Murray & Whiten, 2006; Oberman, Hubbard, McCleery, Dapretto, Davies, Pfeifer, Scott, Sigman, Bookheimer & Iacoboni, 2006; Hadjikhani, Joseph, Snyder & Tager-Flusberg, 2005). Differences between children and teens with autism and controls have been found in activation of the mirror neuron system in humans during functional MRI (Dapretto et al., 2006; Williams et al., 2006). Structural brain differences in mirror neuron regions have also been observed in adults and correlate with autism symptomology (Hadjikhani et al., 2006).
There are alternative explanations for our findings of reduced mu attenuation in individuals with autism during the observation of actions other than a faulty execution/observation matching system. First, it is possible that the autism group was performing some other mental task when watching the hand motion. It is also possible that the autism group was attending to different aspects of the action on the video screen. That is, their eye fixations could have focused on irrelevant parts of the screen, such as the manipulandum or a portion of the blank, gray background. If this were the case, during the imitation condition, the autism group might fail to imitate the action or show a significant delay when performing the imitation. First, attention was carefully monitored during the task and all subjects maintained fixation on the monitor during stimulus presentation. Second, average latency to grasp the manipulandum during the imitation condition was assessed and no differences were found between groups. None of the participants in the autism group failed to imitate the grasping of the manipulandum for any trials; none showed a significant delay when imitating the hand grasping the wooden block. Thus, it is unlikely that attention factors can account for these findings.
Another possibility is that disruption of the visual processing system or a disruption to other inputs system may disrupt the circuitry in the execution/observation matching system. While first order motion processing appears to be intact in individuals with autism (Dakin & Frith, 2005), there is evidence to suggest that there is a disruption in the processing of biological motion and specifically processing in the superior temporal sulcus (Pelphrey, Morris, & McCarthy, 2005; Boddaert, Chabane, Gervaise, Good, Bourgeouis, Plumet, Barthelemy, Mouren, Artiges, Samson et al., 2004; Waiter, Williams, Murray, Gilchrist, Perrett, & Whiten, 2004). Further research is necessary to clarify the role that the processing of biological motion plays in the execution/observation matching system, imitation, and the social deficits in autism. The most parsimonious explanation is that disruptions to multiple circuits acting in concert result in the pattern of social impairments seen in autism.
However, the execution/observation system cannot be entirely dysfunctional, as individuals with autism are able to imitate, albeit more poorly than individuals with typical development. Likewise, there is much variability in theory of mind abilities, empathy, and other social cognition deficits in individuals with autism. Therefore it could be degrees of dysfunction of this system, acting in concert with degrees of disruptions in other neural systems, such as the superior temporal sulcus, the limbic system, the cerebellum, and the ventromedial prefrontal cortex, that potentially lead to the range and variety of deficits observed in autism.
In summary, the execution/observation matching system is believed to serve as the underlying neural circuitry for imitation. This system allows the observer to translate observed actions into an understanding of the action through the activation of the observer's motor system. Disruptions in the circuitry that translates visual input into motor understanding can significantly impact social interaction. In light of the fact that deficits in social cognition are a core feature of autism, it is logical to examine this circuitry as one of the many sources of impairment in autism. This study provides further evidence of a disruption in the execution/observation system in autism and the relation between this circuitry and imitation skills.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldridge M, Stone K, Sweeney M, Bower T. Preverbal children with autism understand the intentions of others. Developmental Science. 2000;3:294–301. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: APA; 2000. Text Revision. [Google Scholar]
- Arroyo S, Lesser R, Gordon B, Uematsu S, Jackson D, Webber R. Functional significance of the mu rhythm of human cortex: an electrophysiologic study with subdural electrodes. Electroencephalography Clinical Neurophysiology. 1993;87:76–87. doi: 10.1016/0013-4694(93)90114-b. [DOI] [PubMed] [Google Scholar]
- Avikainen S, Kulomaki T, Hari R. Normal movement reading in Asperger subjects. Neuroreport. 1999;10:3467–70. doi: 10.1097/00001756-199911260-00001. [DOI] [PubMed] [Google Scholar]
- Avikainen S, Wohlschlager A, Liuhanen S, Hanninen R, Hari R. Impaired mirror-image imitation in Asperger and high-functioning autistic subjects. Current Biology. 2003;13:339–341. doi: 10.1016/s0960-9822(03)00087-3. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Babiloni F, Carducci F, Cincotti F, Cocozza G, Del Percio C, Moretti D, Rossini P. Human cortical electroencephalography (EEG) rhythms during the observation of simple aimless movements: a high-resolution EEG study. Neuroimage. 2002;17:559–572. [PubMed] [Google Scholar]
- Babiloni C, Del Percio C, Babiloni F, Carducci F, Cincotti F, Moretti D, Rossini P. Transient human cortical responses during the observation of simple finger movements: a high-resolution EEG study. Human Brain Mapping. 2003;20:148–57. doi: 10.1002/hbm.10135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C, Carducci F, Cincotti F, Rossini P, Neuper C, Pfurtscheller G, Babiloni F. Human movement-related potentials vs desynchronization of EEG alpha rhythm: a high-resolution EEG study. Neuroimage. 1999;10:658–65. doi: 10.1006/nimg.1999.0504. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Gervaise H, Good C, Bourgeois M, Plumet M, Barthelemy C, Mouren C, Artiges E, Samson Y, Brunelle S, Frackowiak R, Zilbovicius M. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 2004;23:364–369. doi: 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Charman T, Swettenham J, Baron-Cohen S, Cox A, Baird G, Drew A. Infants with autism: An investigation of empathy, pretend play, joint attention, and imitation. Developmental Psychology. 1997;33:781–789. doi: 10.1037//0012-1649.33.5.781. [DOI] [PubMed] [Google Scholar]
- Chatrian G, Petersen M, Lazarte J. The blocking of the rolandic wicket rhythm and some central changes related to movement. Electroencephalography Clinical Neurophysiology Supplement. 1959;11:497–510. doi: 10.1016/0013-4694(59)90048-3. [DOI] [PubMed] [Google Scholar]
- Cochin S, Barthelemy C, Lejeune B, Roux S, Martineau J. Perception of motion and qEEG activity in human adults. Electroencehalography and Clinical Neurophysiology. 1998;107:287–295. doi: 10.1016/s0013-4694(98)00071-6. [DOI] [PubMed] [Google Scholar]
- Cochin S, Barthelemy C, Roux S, Martineau J. Observation and execution of movement: Similarities demonstrated by quantified electroencephalography. European Journal of Neuroscience. 1999;11:1839–1842. doi: 10.1046/j.1460-9568.1999.00598.x. [DOI] [PubMed] [Google Scholar]
- Cochin S, Barthelemy S, Rous S, Martineau J. Electroencephalographic activity during perception of motion in childhood. European Journal of Neuroscience. 2001;13:1791–1796. doi: 10.1046/j.0953-816x.2001.01544.x. [DOI] [PubMed] [Google Scholar]
- Dakin S, Frith U. Vagaries of visual perception in autism. Neuron. 2005;48:597–507. doi: 10.1016/j.neuron.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies M, Pfeifer J, Scott A, Sigman M, Bookheimer S, Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Adams A. Imitation and social responsiveness in autistic children. Journal of Abnormal Child Psychology. 1984;12:209–226. doi: 10.1007/BF00910664. [DOI] [PubMed] [Google Scholar]
- Dawson G, Lewy A. Arousal, attention, and socioemotional impairments of individuals with autism. In: Dawson G, editor. Autism: Nature, diagnosis and treatment. New York: Guilford; 1989. pp. 49–74. [Google Scholar]
- Dawson G, Meltzoff A, Osterling J, Rinaldi J. Neuropsychological correlates of early symptoms of autism. Child Development. 1998;69:1276–1285. [PMC free article] [PubMed] [Google Scholar]
- DeMeyer M, Alpern G, Barons S, DeMyer W, Churchill D, Hingtgen J, Bryson C, Pontius W, Kimberlin C. Imitation in autistic, early schizophrenic, and non-psychotic subnormal children. Journal of Autism and Child Schizophrenia. 1972;2:264–287. doi: 10.1007/BF01537618. [DOI] [PubMed] [Google Scholar]
- Gastaut H, Bert J. EEG changes during cinematographic presentation; moving picture activation of the EEG. Electroencephalography Clinical Neurophysiology Supplement. 1954;6:433–44. doi: 10.1016/0013-4694(54)90058-9. [DOI] [PubMed] [Google Scholar]
- Green D, Baird G, Barnett A, Henderson L, Huber J, Henderson S. The severity and nature of motor impairment in Asperger's syndrome: a comparison with specific developmental disorder of motor function. Journal of Child Psychology and Psychiatry. 2002;43:655–68. doi: 10.1111/1469-7610.00054. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph R, Snyder J, Tager-Flusberg H. Anatomical Differences in the Mirror Neuron System and Social Cognition Network in Autism. Cerebral Cortex. 2005 Nov 23; doi: 10.1093/cercor/bhj069. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Hammes J, Langdell T. Precursors of symbol formation and childhood autism. Journal of Autism and Developmental Disorders. 1981;11:331–46. doi: 10.1007/BF01531515. [DOI] [PubMed] [Google Scholar]
- Hobson R, Lee A. Imitation and identification in autism. Journal of Child Psychology and Psychiatry. 1999;40:649–659. [PubMed] [Google Scholar]
- Jones V, Prior M. Motor imitation abilities and neurological signs in autistic children. Journal of Autism and Developmental Disorders. 1985;15:37–46. doi: 10.1007/BF01837897. [DOI] [PubMed] [Google Scholar]
- Lepage J, Theoret H. EEG evidence for the presence of an action observation-execution system in children. European Journal of Neuroscience. 2006;23:2505–2510. doi: 10.1111/j.1460-9568.2006.04769.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, LeCouteur A. Autism Diagnostic Interview – Revised: A revised versions of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook E, Leventhal B, DiLavore P, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–23. [PubMed] [Google Scholar]
- Loveland K, Tunali Kotoski B, Pearson D, Brelsford K, Ortegon J, Chen R. Imitation and expression of facial affect in autism. Development and Psychopathology. 1994;6:433–444. doi: 10.1017/s0954579497001351. [DOI] [PubMed] [Google Scholar]
- Meltzoff A, Decety J. What imitation tells us about social cognition: A rapprochement between developmental psychology and cognitive neuroscience. Philosophical transactions of the Royal Society of London, series B, biological sciences. 2003;358:491–500. doi: 10.1098/rstb.2002.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff A, Moore M. Explaining facial imitation: A theoretical model. Early Development & Parenting. 1997;6:179–192. doi: 10.1002/(SICI)1099-0917(199709/12)6:3/4<179::AID-EDP157>3.0.CO;2-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff A, Gopnik A. The role of imitation in understanding persons and developing theories of mind. In: Baron-Cohen S, Tager-Flusberg H, editors. Understanding other minds: Perspectives from autism. Oxford: Oxford University Press; 1993. [Google Scholar]
- Muthukumaraswamy S, Johnson B, McNair N. Mu rhythm modulation during observation of an object-directed grasp. Cognitive Brain Research. 2004;19:195–201. doi: 10.1016/j.cogbrainres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy S, Johnson B. Changes in rolandic mu rhythm during observation of a precision grip. Psychophysiology. 2004;41:152–156. doi: 10.1046/j.1469-8986.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- Oberman L, Hubbard E, McCleery J, Altschuler E, Ramachandran V, Pineda J. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cognitive Brain Research. 2005;24:190–198. doi: 10.1016/j.cogbrainres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Ohta M. Cognitive disorders of infantile autism: A study employing the WISC, spatial relationships, conceptualization, and gestural imitation. Journal of Autism and Developmental Disorders. 1987;17:45–62. doi: 10.1007/BF01487259. [DOI] [PubMed] [Google Scholar]
- Pelphrey J, Morris J, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128:1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Pineda J. The functional significance of mu rhythms: Translating “seeing” and “hearing” into “doing”. Brain Research Reviews. 2005;50:57–68. doi: 10.1016/j.brainresrev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Andrew C, Edlinger A. Foot and hand area mu rhythms. International Journal of Psychophysiology. 1997;26:121–135. doi: 10.1016/s0167-8760(97)00760-5. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rogers S, Pennington B. A theoretical approach to the deficits in infantile autism. Development and Psychopathology. 1991;3:137–162. [Google Scholar]
- Rogers S, Bennetto L, McEvoy R, Pennington B. Imitation and pantomime in high-functioning adolescents with autism spectrum disorders. Child Development. 1996;67:2060–2073. [PubMed] [Google Scholar]
- Rogers S, Hepburn S, Stackhouse T, Wehner E. Imitation performance in toddlers with autism and those with other developmental disorders. Journal of Child Psychology and Psychiatry. 2003;44:763–781. doi: 10.1111/1469-7610.00162. [DOI] [PubMed] [Google Scholar]
- Rogers S, Cook I, Greiss-Hess L. Unpublished coding manual. M.I.N.D Institute, University of California; Davis: 2005. Mature Imitation Task. [Google Scholar]
- Sigman M, Ungerer J. Attachment behaviors in autistic children. Journal of Autism and Developmental Disorders. 1984;14(3):231–244. doi: 10.1007/BF02409576. [DOI] [PubMed] [Google Scholar]
- Smith I, Bryson S. Gesture imitation in autism I: Nonsymbolic postures and sequences. Cognitive Neuropsychology. 1998;15:747–770. doi: 10.1080/026432998381087. [DOI] [PubMed] [Google Scholar]
- Stone W, Lemanek K, Fishel P, Fernandez M, Altemeier W. Play and imitation skills in the diagnosis of autism in young children. Pediatrics. 1990;64:1688–1705. [PubMed] [Google Scholar]
- Stone W, Ousley O, Littleford C. Motor imitation in young children with autism: What's the object? Journal of Abnormal Child Psychology. 1997;25:475–485. doi: 10.1023/a:1022685731726. [DOI] [PubMed] [Google Scholar]
- Stroganova T, Orekhova E, Posikera I. EEG alpha rhythm in infants. Clinical Neurophysiology. 1999;110:997–1012. doi: 10.1016/s1388-2457(98)00009-1. [DOI] [PubMed] [Google Scholar]
- Theoret H, Halligan E, Kobayashi M, Fregni F, Tager-Flusberg H, Pascual-Leone A. Impaired motor facilitation during action observation in individuals with autism spectrum disorder. Current Biology. 2005;15:R84–5. doi: 10.1016/j.cub.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Waiter G, Williams J, Murray A, Gilchrist A, Perrett D, Whiten A. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage. 2004;22:619–625. doi: 10.1016/j.neuroimage.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale-third edition (WAIS-III) San Antonio: TX Psych Corp; 1997. [Google Scholar]
- Williams J, Whiten A, Suddendorf T, Perrett D. Imitation, mirror neurons and autism. Neuroscience and Biobehavioral Reviews. 2001;25:287–295. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]
- Williams J, Whiten A, Singh T. A systematic review of action imitation in autistic spectrum disorder. Journal of Autism and Developmental Disorders. 2004;34(3):285–299. doi: 10.1023/b:jadd.0000029551.56735.3a. [DOI] [PubMed] [Google Scholar]
- Williams J, Waiter G, Gilchrist A, Perrett D, Murray A, Whiten A. Neural mechanisms of imitation and ‘mirror neuron’ functioning in autism spectrum disorder. Neuropsychologia. 2006;44:610–621. doi: 10.1016/j.neuropsychologia.2005.06.010. [DOI] [PubMed] [Google Scholar]


