Abstract

Methods that introduce posttranslational modifications in a general, mild, and non-sequence-specific manner using biologically produced peptides have great utility for investigation of the functions of these modifications. In this study, the substrate promiscuity of a lantibiotic synthetase was exploited for the preparation of phosphopeptides, glycopeptides, and peptides containing analogs of methylated or acetylated lysine residues. Peptides attached to the C-terminus of the leader peptide of the lacticin 481 precursor peptide were phosphorylated on serine residues in a wide variety of sequence contexts by the R399M and T405A mutants of lacticin 481 synthetase (LctM). Serine residues located as many as 30 amino acids C-terminal to the leader peptide were phosphorylated. Wild-type LctM was shown to dehydrate these peptides to generate dehydroalanine-containing products that can be conveniently modified with external nucleophiles including thiosaccharides, 2-(dimethylamino)ethanethiol, and N-acetyl cysteamine, resulting in mimics of O-linked glycopeptides and acetylated and methylated lysines.
Posttranslational modifications (PTMs) greatly increase the functional and structural space accessible to genome-encoded proteins and are a key mechanism to control dynamic cellular processes, mostly via phosphorylation, glycosylation, and histone modification (1). Synthetic peptides carrying defined modifications have played important roles in a large number of studies, from use as substrates in enzyme assays to identification of protein−protein interactions. In addition, both global and selective methods have been developed for modification of preformed synthetic peptides (2−7). More recently, synthetic peptides have also found use in the construction of full length proteins carrying PTMs using orthogonal ligation techniques (8−11). Despite the great utility of these synthetic functionalized peptides, their synthesis has limitations for routine use in nonspecialist laboratories. Methods that introduce posttranslational modifications in a general, mild, and non-sequence-specific manner using biologically produced peptides are complementary and in some cases conceptually preferable as they can take advantage of rapid and inexpensive access to libraries and methods to screen them. Unfortunately, most enzymes that introduce posttranslational modifications are highly selective. Here we describe a facile enzymatic method to produce peptides containing phosphorylated serine residues by taking advantage of the substrate promiscuity of lacticin 481 synthetase (LctM). In addition, the methodology is shown to be amenable for the preparation of glycopeptides and peptides containing analogs of acetylated and methylated lysines found in histones.
LctM converts a linear ribosomally synthesized peptide (LctA) into a polycyclic structure characteristic for the class of lantibiotic antimicrobial peptides (12). The enzyme dehydrates serine and threonine residues in the C-terminal part of its substrate (the structural peptide) to the corresponding dehydroalanine (Dha) and dehydrobutyrine (Dhb) residues, respectively (1, panel a) (13). The polycyclic structures are formed by subsequent reaction of cysteine thiols with the dehydro amino acids in a Michael-type addition. The N-terminal leader peptide is not modified in this process but is important for efficient modification of the structural peptide (14). Previous studies have demonstrated high substrate promiscuity for LctM, which can dehydrate a range of nonlantibiotic peptides attached to the leader peptide (15−17). The mechanism of dehydration involves phosphorylation of the serine/threonine targeted for dehydration and subsequent elimination of the phosphate group (1, panel a) (18,19).
Figure 1.
LctM-catalyzed dehydration and phosphorylation. a) Posttranslational modification of the LctA peptide by the enzyme LctM to generate dehydroamino acids. Dehydration involves a two-step sequence of phosphorylation and elimination. b) Design of the substrate peptides to investigate the scope of peptide phosphorylation. The recognition sequence for Factor Xa protease is indicated in blue. All peptides have a His6-tag at their N-terminus originating from the pET15b plasmid with the following sequence: GSSHHHHHHSSGLVPRGSH.
A comprehensive site-directed mutagenesis study of LctM identified two residues that when mutated did not affect the phosphorylation step but compromised the elimination step (19). Hence, these mutant enzymes, LctM-R399M and LctM-T405A, were envisioned to be potentially useful as kinases that could phosphorylate peptides of interest attached to the C-terminus of the leader peptide of LctA. The current study demonstrates the potential of this methodology.
Results and Discussion
To explore the substrate specificity for phosphorylation, a library of plasmids was generated with oligonucleotides encoding nonapeptides with random sequences inserted in frame behind an lctA gene fragment encoding the leader peptide. All nonapeptides I were designed to contain a serine at position 5 (1, panel b). In order to provide the possibility to render the leader peptide traceless, a Factor Xa protease cleavage site was engineered N-terminal to the random peptide sequence. After transformation of Escherichia coli cells with the vector library, colonies were picked, and their plasmids were sequenced. On the basis of the diversity within their sequences, a subset was chosen that contained a variety of different residues flanking the serine (hydrophobic, hydrophilic, cationic, anionic, and cyclic). These peptides were heterologously expressed in E. coli and purified by nickel affinity chromatography (1).
Table 1. Results of incubation of LctM-T405A with peptide sequences attached to the leader peptide of LctA as depicted in Figure 1, panel b.
| Entry | Sequence | No. of phosphorylations | Reaction time | Time for full dehydration by wt LctM |
|---|---|---|---|---|
| 1 | RWVRSALLI | 1 | 30 min | <30 min |
| 2 | RLIKTFAYV | 1 | 1 h | <30 min |
| 3 | RLIKSFAYV | 1 | 30 min | <30 min |
| 4 | GHAGSAPPA | 1 | 3 h | |
| 5 | RLLRSDLVP | 1 | 2 h | |
| 6 | RPDESLNRL | 1 | 2 h | <30 min |
| 7 | LAYPSLRLL | 1 | 30 min | <30 min |
| 8 | ILILSVPVA | 1 | 30 min | <30 min |
| 9 | FAFYSCPVH | 1 | 30 min | |
| 10 | LCLCSALLC | 1 | 30 min | |
| 11 | CYCVSQGPA | 1 | 30 min | <30 min |
| 12 | LRRASVA | 1 | 1 h | <30 min |
| 13 | LASPLVNSHV | 2 | 4 h | |
| 14 | HPSPARPSDA | 1 | 6 h | |
| 15 | PLSLPALSRA | 2 | 1 h | <30 min |
| 16 | ARSLGAQSVL | 2 | 4 h | <30 min |
| 17 | RLIKSFAYVRLLRSLDVP | 2 | 1 h | <30 min |
| 18 | RLIKSFAYVHAIKSLRNRGRQLSKIPA | 3 | 4 h | |
| 19 | RLIKSFAYVRLLRSLDVPVARISHNKA | 3 | 4 h |
Incubation of the purified peptides with LctM-T405A or LctM-R399M, MgCl2, and ATP resulted in clean conversion to phosphorylated peptides as determined by MALDI-TOF mass spectrometric analysis. As a representative example, 2, panel a depicts the LctM-T405A catalyzed phosphorylation of a peptide containing RWVRSALLI behind the Factor Xa cleavage site (entry 1, 1; for all other entries spectral data is provided in Supporting Information). The T405A mutant enzyme displayed activity higher than that of LctM-R399M and was used for further study. For peptides that were good substrates, phosphorylation on a 2−4 nmol scale was complete within 1 h as determined by HPLC (see Supporting Information) resulting in yields in the 80% range after purification. For comparison, a subset of these peptides was tested for dehydration by wild-type (wt) LctM, resulting in complete dehydration within 30 min (1). As expected on the basis of the known activity of lantibiotic synthetases (12,15,20−22), LctM-T405A also phosphorylates threonine residues as shown in entry 2, 1. Similarly, incubation of peptide II (1, panel b) with LctM-T405A resulted in a monophosphorylated peptide in 30 min with the phosphate located on the threonine residue. The peptide in entry 2 was also phosphorylated on a larger scale (2.5 mg) resulting in full conversion as determined by HPLC. After HPLC purification, 1.2 mg of isolated phosphorylated peptide was obtained.
Figure 2.
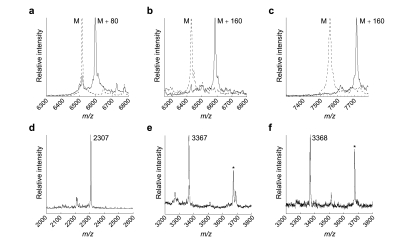
Representative examples of LctM-T405A catalyzed phosphorylation of peptides attached to the LctA leader sequence. MALDI-TOF mass spectra of the substrates are shown in dashed lines and assay products in solid lines. All entries refer to 1: a) entry 1; b) entry 15; c) entry 17. Panels d−f show the products after treatment with Factor Xa: d) entry 17; e) entry 18; f) entry 19. Factor Xa also cleaves after Arg16 in the His6-tag resulting in the LctA leader peptide with a GSH tripeptide at its N-terminus. This peptide is indicated with an asterisk in panels e and f.
The remarkable promiscuity of LctM-T405A is illustrated by the wide variety of residues flanking serine that are tolerated, which include hydrophobic, hydrophilic, acidic, basic, and aromatic residues (1). Flanking Gly residues have been reported to reduce serine/threonine dehydration activity by LctM (15), and the same was observed for phosphorylation by LctM-T405A in this study. Phosphorylation was also sluggish for serine residues flanked by two proline, two negatively charged residues, or combinations thereof (data not shown); one of these residues flanking a serine did not deactivate phosphorylation (e.g., entries 4−6, 1). A single flanking proline residue also did not prevent phosphorylation activity (entries 7, 13, and 14); this is an important observation given that a serine/threonine-proline motif is phosphorylated in many regulatory pathways by cyclin-dependent kinases (23) and mitogen-activated protein (MAP) kinases (24). The utility of the method is illustrated in entry 12, in which a previously reported potent inhibitor of human tripeptidyl peptidase II (25) was readily prepared.
Next, a series of decapeptides III (1, panel b) containing two serine residues located at positions 3 and 8 was investigated (e.g., entries 13−16, 1). LctM-T405A phosphorylated these peptides twice (2, panel b), simultaneously demonstrating the ability to generate bisphosphorylated peptides and that phosphorylation is not dependent on the position of the serine. This methodology is complementary to solid phase peptide synthesis of phosphopeptides using monobenzyl protected Fmoc-pSer (26,27) and may be particularly useful for longer peptides that are more difficult to prepare synthetically. To test the applicability toward longer peptides, the length of the sequences appended to the LctA leader was increased to 18 and 27 residues (entries 17−19, 1). These peptides contain two or three serine residues at positions 5 and 14 or 5, 14, and 23. LctM-T405A efficiently phosphorylated these peptides twice (for the peptide in entry 17, 2, panel c) and three times (entries 18 and 19), demonstrating that longer peptides are amenable to this methodology. In the latter two peptides, phosphorylation occurs on a serine located 30 residues C-terminal to the end of the leader peptide. Similarly, in vivo studies on lantibiotic dehydratases have shown that these enzymes can dehydrate serine/threonine residues as far as 42 residues C-terminal from the leader peptide in non-natural designed peptides (22). After phosphorylation by LctM-T405A, the leader peptide could be conveniently cleaved with Factor Xa, as shown for representative examples in 2, panels d−f. The convergent nature and mild conditions of the methodology as well as its use with bacterially expressed peptides bodes well for its potential application toward the preparation of peptide libraries, possibly in combination with phage display technology.
In addition to its utility to prepare phosphopeptides in a leader-peptide-dependent manner, the use of LctMfor the preparation of other peptide conjugates was investigated. Ribosomally synthesized peptides do not contain reactive electrophilic groups. Therefore, the ability to introduce dehydroalanines in a mild and efficient manner into a range of peptides with wild-type LctM provides opportunities to derivatize these reactive handles chemoselectively. We (28−30) and others (31,32) have shown that dehydroalanines can be used to decorate peptides and proteins with prenyl groups and thiosaccharides as well as with thialysine in various methylation states. In these previous studies, Dha was introduced using chemical methods. We show here that these structures can be readily introduced using enzymatic methods. As anticipated, treatment of the peptides listed in 1 with wild-type LctM, ATP, and Mg2+ resulted in the efficient conversion of serine and threonine residues to the corresponding Dha and Dhb. As shown in 2, subsequent addition of 1-thio glucose nucleophile to the Dha-containing peptides provided thia-analogs of O-linked glycopeptides. We have shown previously that this methodology can also be used with more elaborate synthetic thiosaccharides including tumor-associated antigens (30). Michael-type additions with N-acetyl cysteamine or 2-(dimethylamino)ethane thiol resulted in thia-analogs of methylated or acetylated lysines. Such methylated thialysines have been shown to be fully functional in histone H3 (33). The high conversion ob-served in these Michael-type additions is illustrated for some representative examples in 3 (for other examples see Supporting Information). An inherent potential disadvantage of the use of Michael-type additions for conjugation is the possible generation of stereoisomers at the α-carbon of the former dehydro amino acids. Whether this possibility presents a true limitation requires further investigation. Certainly, in many cases non-native linkages produced from various ligation methods have not prevented their successful use. Importantly, for the only case examined thus far, Michael ligation with N-acetyl cysteamine to generate an acetylated thiaLys in histone H3 resulted in a protein product that was a substrate for HDAC3 (32). Whether the ligation was stereoselective in the context of the H3 protein or whether HDAC3 has deacetylation activity for H3 containing d-N-acetyl thiaLys was not investigated.
Table 2. Enzymatic installation of dehydroamino acids and subsequent derivatization to S-linked glycopeptides, Nϵ-dimethyllysine, and Nϵ-acetyllysine analogs.
 |
Figure 3.
Representative examples of the transformation of dehydropeptides with various nucleophiles. MALDI-MS data for a) dehydropeptide substrate, b) product containing Nϵ-dimethylthialysine (entry 1, 2), c) product containing Nϵ-acetylthialysine (entry 2, 2), and d) product containing β-1-thioglucose (entry 3, 2).
In summary, we present methodology that allows rapid, mild, and convergent access to peptides containing serine phosphorylations, methylations and acetylations of lysine analogs, and S-linked glycosylations. The methodology is complementary to solid-phase peptide synthesis (SPPS) and may be particularly suited for laboratories that are not experts in SPPS. Genes encoding for the precursor peptides can be conveniently introduced into plasmids that already contain the oligonucleotide sequence encoding the LctA leader peptide, which can be rendered traceless after cleavage with a commercial protease.
Methods
Cloning, Expression, and Purification of Peptides
All materials and molecular biology methods used to generate the expression constructs for the substrate peptides are described in Supporting Information. Conditions used for expression of the peptides in E. coli and subsequent purification by nickel affinity chromatography followed by HPLC are also described in Supporting Information as is the protocol to purify LctM and LctM-T405A.
Phosphorylation of Serine-Containing Peptides by LctM-T405A on a 2−4 nmol Scale
HPLC-purified peptides (20−40 μM) were reacted with LctM-T405A or LctM-R399 M (2 μM final concentration) at 25 °C in 100 μL of buffer containing 50 mM Tris·HCl, pH 7.5, 1 mM ATP, and 10 mM MgCl2. Reactions were stopped at the times indicated in 1 by adding 5% TFA to give a final concentration of 0.5%. Phosphorylated peptides were purified by HPLC as described in Supporting Information. For MS analysis before purification, assay products were desalted using C18 ZipTips (Millipore) and eluted with 4 μL of α-cyano-4-hydroxy cinnamic acid. From this solution, 3 μL was applied to the MALDI target and analyzed for phosphorylation by MALDI-TOF MS.
Phosphorylation on a 0.38 μmol Scale
The peptide in entry 2 of 1 (2.5 mg) was divided over three eppendorf tubes, and to each tube was added 1 mL of 50 mM MOPS (pH 7.5) containing 10 mM MgCl2, 5 mM ATP, and 5 mM TCEP providing a peptide concentration of ∼120 μM. To two of the tubes was added His6-LctM-T405A (2 μM final concentration), and to the third tube was added His6-LctM-R399 M (final concentration 2 μM). The three tubes were incubated at 25 °C for 12 h and analyzed by HPLC, which showed that all substrate had been consumed for each reaction. The phosphopeptides were purified by analytical RP-HPLC with a Vydac C4 column (0.46 cm × 25 cm) using a linear gradient of 20% to 100% solvent B over 35 min (solvent A = 0.1% TFA in H2O; solvent B = 80% MeCN, 20% H2O, 0.086% TFA). The fractions containing phosphopeptide as determined by MALDI-TOF mass spectrometry were combined and lyophilized, giving 1.2 mg of a white powder (48%).
General Conjugation Protocol
Peptides (20−40 μM) were incubated under identical conditions as described above for phosphorylation except that His6LctM was added instead of a mutant enzyme. After 30 min of incubation, the products were purified by HPLC (Supporting Information), and to purified dehydrated peptide (1 μL, ∼1 μg) were added 5 μL of 50 mM phosphate buffer at pH 8.0 and thiol (either dissolved in the same buffer or added neat, in the case of N-acetyl cysteamine). The reactions were incubated for 1−2 h at 25 °C and then purified by Zip-tip for MALDI-TOF MS analysis. As shown in Supplementary Figure S3, an excess of just 5 equiv of thiol resulted in excellent conversion.
Acknowledgments
This work was supported by the National Institutes of Health (GM58822 to W.A.V.) and a Ruth L. Kirschstein National Research Service Award (T32 GM008276 to M.R.L.). We thank Scott Silverman (UIUC) for helpful discussions.
Supporting Information Available
This material is available free of charge via the Internet at http://pubs.acs.org.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Walsh C. T. (2005) Post-Translational Modification of Proteins: Expanding Nature’s Inventory, Roberts & Company, Greenwood Village, CO. [Google Scholar]
- Perich J. W.; Johns R. B. (1988) Di-t-butyl N,N-diethylphosphoramidite and N-dibenzylphosphoramidite. Highly reactive reagents for the phosphite triester phosphorylation of Ser-containing peptides. Tetrahedron Lett. 29, 2369. [Google Scholar]
- Perich J. W. (1997) Synthesis of phosphopeptides using modern chemical approaches. Methods Enzymol. 289, 245–266. [DOI] [PubMed] [Google Scholar]
- Lemieux G. A.; Bertozzi C. R. (1998) Chemoselective ligation reactions with proteins, oligosaccharides and cells. Trends Biotechnol. 16, 506–513. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Chan T. R.; Hilgraf R.; Fokin V. V.; Sharpless K. B.; Finn M. G. (2003) Bioconjugation by copper(I)-catalyzed azide-alkyne [3 + 2] cycloaddition. J. Am. Chem. Soc. 125, 3192–3193. [DOI] [PubMed] [Google Scholar]
- Kolb H. C.; Finn M. G.; Sharpless K. B. (2001) Click chemistry: diverse chemical function from a few good reactions. Angew. Chem., Int. Ed. 40, 2004–2021. [DOI] [PubMed] [Google Scholar]
- Kimmerlin T.; Seebach D. (2005) '100 years of peptide synthesis': ligation methods for peptide and protein synthesis with applications to β-peptide assemblies. J. Pept. Res. 65, 229–260. [DOI] [PubMed] [Google Scholar]
- Muir T. W.; Sondhi D.; Cole P. A. (1998) Expressed protein ligation: a general method for protein engineering. Proc. Natl. Acad. Sci. U.S.A. 95, 6705–6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellois J. P.; Muir T. W. (2006) Semisynthetic proteins in mechanistic studies: using chemistry to go where nature can’t. Curr. Opin. Chem. Biol. 10, 487–491. [DOI] [PubMed] [Google Scholar]
- Sun L.; Ghosh I.; Barshevsky T.; Kochinyan S.; Xu M. Q. (2007) Design, preparation and use of ligated phosphoproteins: a novel approach to study protein phosphatases by dot blot array, ELISA and Western blot assays. Methods 42, 220–226. [DOI] [PubMed] [Google Scholar]
- Schwarzer D.; Cole P. A. (2005) Protein semisynthesis and expressed protein ligation: chasing a protein’s tail. Curr. Opin. Chem. Biol. 9, 561–569. [DOI] [PubMed] [Google Scholar]
- Chatterjee C.; Paul M.; Xie L.; van der Donk W. A. (2005) Biosynthesis and mode of action of lantibiotics. Chem. Rev. 105, 633–684. [DOI] [PubMed] [Google Scholar]
- Xie L.; Miller L. M.; Chatterjee C.; Averin O.; Kelleher N. L.; van der Donk W. A. (2004) Lacticin 481: in vitro reconstitution of lantibiotic synthetase activity. Science 303, 679–681. [DOI] [PubMed] [Google Scholar]
- Levengood M. R.; Patton G. C.; van der Donk W. A. (2007) The leader peptide is not required for post-translational modification by lacticin 481 synthetase. J. Am. Chem. Soc. 129, 10314–10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee C.; Patton G. C.; Cooper L.; Paul M.; van der Donk W. A. (2006) Engineering dehydro amino acids and thioethers into peptides using lacticin 481 synthetase. Chem. Biol. 13, 1109–1117. [DOI] [PubMed] [Google Scholar]
- Levengood M. R.; van der Donk W. A. (2008) Use of lantibiotic synthetases for the preparation of bioactive constrained peptides. Bioorg. Med. Chem. Lett. 18, 3025–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton G. C.; Paul M.; Cooper L. E.; Chatterjee C.; van der Donk W. A. (2008) The Importance of the leader sequence for directing lanthionine formation in lacticin 481. Biochemistry 47, 7342–7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee C.; Miller L. M.; Leung Y. L.; Xie L.; Yi M.; Kelleher N. L.; van der Donk W. A. (2005) Lacticin 481 synthetase phosphorylates its substrate during lantibiotic production. J. Am. Chem. Soc. 127, 15332–15333. [DOI] [PubMed] [Google Scholar]
- You Y. O.; van der Donk W. A. (2007) Mechanistic investigations of the dehydration reaction of lacticin 481 synthetase using site-directed mutagenesis. Biochemistry 46, 5991–6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L.; van der Donk W. A. (2004) Post-translational modifications during lantibiotic biosynthesis. Curr. Opin. Chem. Biol. 8, 498–507. [DOI] [PubMed] [Google Scholar]
- Rink R.; Kuipers A.; de Boef E.; Leenhouts K. J.; Driessen A. J.; Moll G. N.; Kuipers O. P. (2005) Lantibiotic structures as guidelines for the design of peptides that can be modified by lantibiotic enzymes. Biochemistry 44, 8873–8882. [DOI] [PubMed] [Google Scholar]
- Rink R.; Wierenga J.; Kuipers A.; Kluskens L. D.; Driessen A. J. M.; Kuipers O. P.; Moll G. N. (2007) Production of dehydroamino acid-containing peptides by Lactococcus lactis. Appl. Environ. Microbiol. 73, 1792–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. W.; Adams P. D. (2001) Cyclin-dependent kinases. Chem. Rev. 101, 2511–2526. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Gibson T. B.; Robinson F.; Silvestro L.; Pearson G.; Xu B.; Wright A.; Vanderbilt C.; Cobb M. H. (2001) MAP kinases. Chem. Rev. 101, 2449–2476. [DOI] [PubMed] [Google Scholar]
- Tomkinson B.; Grehn L.; Fransson B.; Zetterquist O. (1994) Use of a dehydroalanine containing peptide as an efficient inhibitor of tripeptidyl peptidase II. Arch. Biochem. Biophys. 313, 276–279. [DOI] [PubMed] [Google Scholar]
- Wakamiya T.; Saruta K.; Yasuoka J.; Kusumoto S. (1994) An efficient procedure for solid-phase synthesis of phosphopeptides by the Fmoc strategy. Chem. Lett. 1099–1102. [Google Scholar]
- Vorherr T.; Bannwarth W. (1995) Phospho-serine and phospho-threonine building blocks for the synthesis of phosphorylated peptides by the Fmoc solid phase strategy. Bioorg. Med. Chem. Lett. 5, 2661–2664. [Google Scholar]
- Zhu Y.; Gieselman M.; Zhou H.; Averin O.; van der Donk W. A. (2003) Biomimetic studies on the mechanism of stereoselective lanthionine formation. Org. Biomol. Chem. 1, 3304–3315. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; van der Donk W. A. (2001) Convergent synthesis of peptide conjugates using dehydroalanines for chemoselective ligations. Org. Lett. 3, 1189–1192. [DOI] [PubMed] [Google Scholar]
- Galonić D. P.; van der Donk W. A.; Gin D. Y. (2003) Oligosaccharide-peptide ligation of glycosyl thiolates with dehydropeptides. Synthesis of S-linked mucin glycopeptide conjugates. Chem.−Eur. J. 24, 5997–6006. [DOI] [PubMed] [Google Scholar]
- Bernardes G. J.; Chalker J. M.; Errey J. C.; Davis B. G. (2008) Facile conversion of cysteine and alkyl cysteines to dehydroalanine on protein surfaces: versatile and switchable access to functionalized proteins. J. Am. Chem. Soc. 130, 5052–5053. [DOI] [PubMed] [Google Scholar]
- Guo J.; Wang J.; Lee J. S.; Schultz P. G. (2008) Site-specific incorporation of methyl- and acetyl-lysine analogues into recombinant proteins. Angew. Chem., Int. Ed. 47, 6399–6401. [DOI] [PubMed] [Google Scholar]
- Simon M. D.; Chu F.; Racki L. R.; de la Cruz C. C.; Burlingame A. L.; Panning B.; Narlikar G. J.; Shokat K. M. (2007) The site-specific installation of methyl-lysine analogs into recombinant histones. Cell 128, 1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.