Abstract
Formation of the Drosophila larval body wall muscles requires the specification, coordinated cellular behaviors and fusion of two cell types: Founder Cells (FCs) that control the identity of the individual muscle and Fusion Competent Myoblasts (FCMs) that provide mass. These two cell types come together to control the final size, shape and attachment of individual muscles. However, the spatial arrangement of these cells over time, the sequence of fusion events and the contribution of these cellular relationships to the fusion process have not been addressed. We analyzed the three dimensional arrangements of FCs and FCMs over the course of myoblast fusion and assayed whether these issues impact the process of myoblast fusion. We examined the timing of the fusion process by analyzing the fusion profile of individual muscles in wildtype and fusion mutants. We showed that there are two temporal phases of myoblast fusion in wildtype embryos. Limited fusion events occur during the first 3 hours of fusion, while the majority of fusion events occur in the remaining 2.5 hours. Altogether, our data have led us to propose a new model of myoblast fusion where the frequency of myoblast fusion events may be influenced by the spatial arrangements of FCs and FCMs.
Keywords: muscle, fusion, Drosophila, Founder Cell, Fusion Competent Myoblast
INTRODUCTION
Muscles are highly specialized, multinucleate cells formed by the fusion of mononucleate myoblasts. Multiple studies have demonstrated the conservation of cellular and molecular events required for fusion. Myoblasts in both Drosophila and vertebrates undergo the same cellular behaviors during fusion, including cell recognition, adhesion and membrane alignment. This conservation extends to the subcellular events observed by electron microscopy including the detection of electron dense plaques at the site of membrane breakdown (Abmayr and Kocherlakota, 2006; Beckett and Baylies, 2006; Doberstein et al., 1997; Horsley and Pavlath, 2004). However, myoblast fusion in Drosophila occurs over a relatively short period of hours, in contrast to vertebrate systems where fusion takes place over several days. Consequently, the larval body wall muscles of Drosophila have provided a relatively simple system in which to study muscle development and myoblast fusion in vivo.
Muscles in the Drosophila embryo arise from two populations of myoblasts that are specified in the developing somatic mesoderm. These are Founder Cells (FCs), which are thought to guide muscle formation, and Fusion Competent Myoblasts (FCMs) that are thought to play a more passive role (Baylies et al., 1998; Frasch, 1999). Within the somatic mesoderm of each hemisegment, FCs are specified at stereotypical positions. Each FC determines the position of a specific muscle with respect to the anterior-posterior (A–P) and dorsal-ventral (D–V) axes of the embryo. The FC then fuses to a stereotypical number of surrounding FCMs to give each muscle its characteristic size (Bate, 1990; Bate, 1993).
Several Drosophila mutants have been identified that block myoblast fusion (Abmayr and Kocherlakota, 2006; Beckett and Baylies, 2006). One class of fusion mutants appeared to block all myoblast fusion and includes myoblast city (mbc) (Erickson et al., 1997; Rushton et al., 1995). A second class of mutants were reported to show some fusion events and includes blown fuse (blow) (Doberstein et al., 1997; Schroter et al., 2004), rolling pebbles (rols, also know as antisocial) (Chen and Olson, 2001; Menon and Chia, 2001; Rau et al., 2001) and kette (Schroter et al., 2004). Based on these observations it has been proposed that there are two steps of myoblast fusion: each FC fuses 2–3 times to form a precursor cell in the first step of fusion and then all subsequent fusion events occur in the second step. Each step requires a specific subset of genes (Rau et al., 2001; Schroter et al., 2004).
Other studies focused on the subcellular events that occur between fusing myoblasts using transmission electron microscopy (TEM). This analysis has been incorporated into the two-step model to suggest that only the second step of fusion requires those subcellular behaviors (Schroter et al., 2004). However, there are aspects of this model that need further investigation. First, this model suggested that two distinct subcellular mechanisms responsible for myoblast fusion have arisen during evolution, but how this would have occurred is unclear. Second, a detailed and quantitative analysis, including TEM, has not been performed for all fusion mutants.
The description of myoblast cell behaviors and arrangements during fusion is largely limited to a seminal paper by Michael Bate in 1990. These studies showed that myoblast fusion begins at the onset of germband retraction at stage 12 (7.5 hours After Egg Laying [AEL]) and continues until stage 15 (13 hours AEL). As fusion begins, the mesoderm consists of a loosely organized sheet of cells in direct contact with the ectoderm and central nervous system (CNS), with a variable number of cells below. The initial 2–3 nuclei-containing myotubes are found in the outer layer of the mesoderm in direct contact with the ectoderm or CNS (Bate, 1990). However, the precise three-dimensional arrangements of FCs and FCMs, and how these spatial relationships change during the period of myoblast fusion, were not investigated. In addition, the timing of fusion in individual muscles has not been analyzed. The absence of this essential knowledge is currently hampering our understanding of both the fusion process and the contribution of individual genes to this process.
Using imaging techniques and markers that label both FCs/myotubes and FCMs, we have developed a new model of myoblast fusion. We have determined the spatial arrangements of these cell types and uncovered new cell behaviors throughout the course of myoblast fusion. By combining these approaches with FC identity markers we have constructed the first map showing the arrangement of FCs as fusion begins. Using this information, we have analyzed the timing of fusion events in individual muscles (the “fusion profile”). These data revealed that there are two temporal phases of fusion. We have then examined the fusion profile of individual muscles in fusion mutants. Based on these data we propose a new model of myoblast fusion where the frequency of fusion events is influenced by FC and FCM spatial arrangements. This work highlights the importance of understanding the spatial arrangements of cells that contribute to organ formation in Drosophila and, by extension, in vertebrate systems.
MATERIALS AND METHODS
Drosophila genetics
All stocks were grown on standard cornmeal medium at 25°C. Fly stocks used were: rp298-lacZ (Nose et al., 1998), twi-CD2 (Borkowski et al., 1995), kettej4–48 (Hummel et al., 2000), ants/rolsT627 (Chen and Olson, 2001), lonerT1032 (Chen et al., 2003), mbcC1 (Rushton et al., 1995), blow1 (Doberstein et al., 1997) and Rac1J11 Rac2Δ mtlΔ (Hakeda-Suzuki et al., 2002). Mutants were balanced using CyO P[w+ wgen11 lacZ] or TM3 Sb1 Dfd-lacZ and homozygotes were identified by absence of β-galactosidase staining.
Immunohistochemistry
Embryos were fixed in 4% paraformaldehyde/heptane for all immunohistochemistry. Antibodies were preabsorbed (PA) 1:10 against fixed wild type embryos where stated. Antibody dilutions used were: mouse anti-β-gal (1:2000; Promega), rabbit anti-β-gal (1:5000; Cappel), chicken anti-β-gal (1:1000; Abcam), rabbit anti-Lmd (1:250; PA; a gift from H. Nguyen), mouse anti-GFP (1:400; PA; Clonetech), mouse anti-Cyclin B (1:20; Developmental Studies Hybridoma Bank), rabbit anti-Eve (1:3000; PA; a gift from M. Frasch), guineau pig anti-Kr (1:500; PA; a gift from J. Reinitz), rabbit anti-Collier (1:150; PA; a gift of A. Vincent; used in combination with TSA-FITC system from PerkinElmer), guinea pig anti-Runt (1:2000; PA; a gift of J. Reinitz) and rabbit anti-Slouch (1:200; PA) (Cox and Baylies, 2005). Alexa488, Alexa555 and Alexa647 conjugated secondary antibodies were used (1:400; Molecular Probes). Alexa546 and Alexa647 conjugated phalloidin was used to visualize F-actin (1:100; Molecular Probes). Embryos were mounted in ProLong Gold antifade reagent (Molecular Probes).
Confocal Imaging and 3-D rendering
Fluorescent images were acquired on a Zeiss LSM 510 confocal scanning system mounted on an Axiovert 100M microscope with a 63× 1.2NA C-Apochromat water objective. For confocal microscopy, all pinholes were set to capture an optical slice of 1.0 µm with optical sections captured every 0.7–0.8 µm. 488nm, 543nm, and 633nm lasers were used to excite the fluorochromes. All fluorescent images were exported from Zeiss LSM software v.3.2. Volocity software was used for 3-D rendering of single mesodermal hemisegments. Images were processed using Adobe Photoshop 7.0 and movies were created from image sequences using Apple Quicktime.
Staging Embryos and Nuclei counting
For precise staging all embryos were counterstained using Alexa 546 conjugated phalloidin. In addition to embryonic morphology, the tracheal system was used as an internal control for staging (Manning and Krasnow, 1993). Nuclei were counted using 40× magnification on a Zeiss Axiophot microscope using standard fluorescence settings. For each muscle/mutant condition analyzed, 50 hemisegments (abdominal hemisegments 2–4) at stages 12–15 were counted (17 embryos total). Graphing of data and statistical analysis was performed with Microsoft Excel.
RESULTS
Spatial arrangements of FCs and FCMs during myoblast fusion
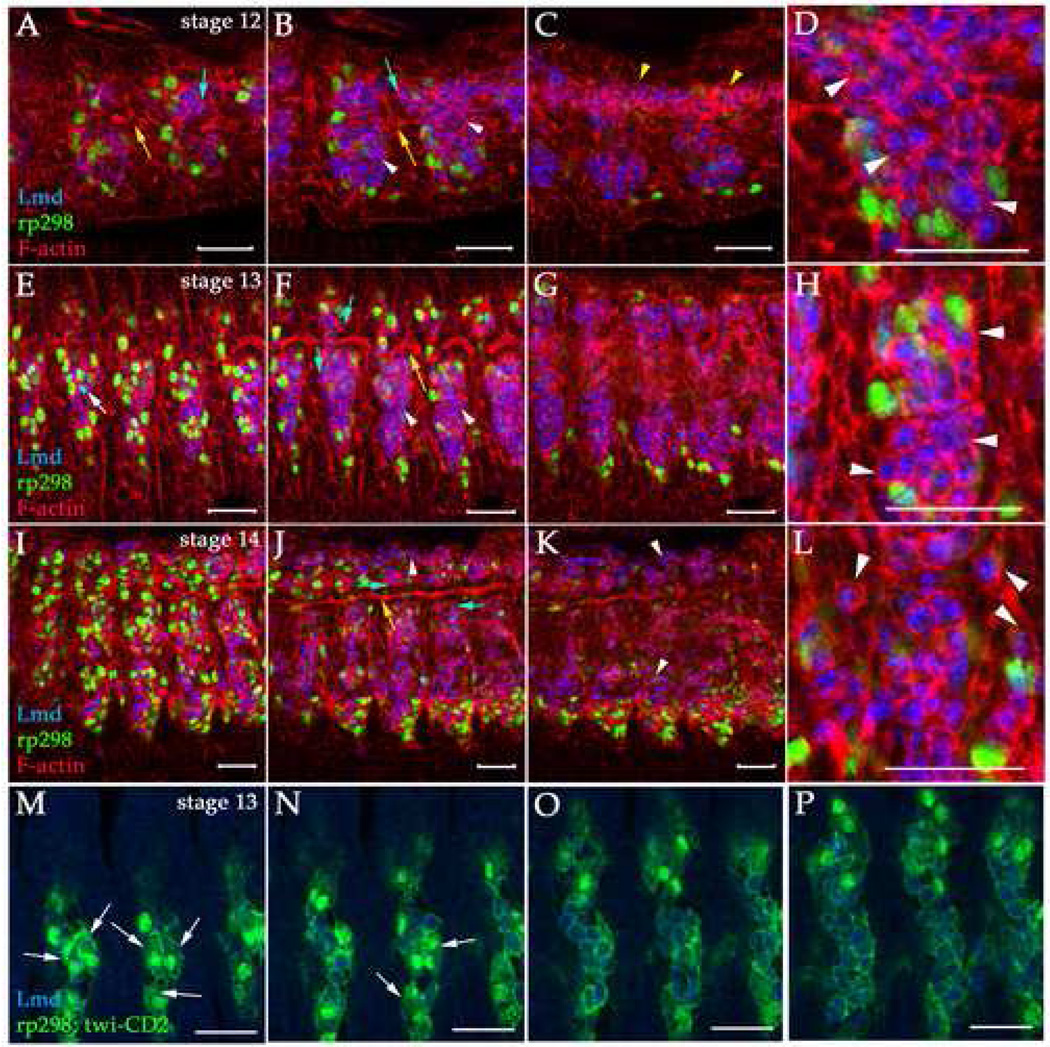
We used recent advances in imaging techniques and markers for different mesodermal cell types to examine the process of myoblast fusion and understand the three-dimensional arrangements of FCs and FCMs over the course of this process. We examined embryos expressing the rp298-lacZ transgene, which labels the nuclei of all muscle progenitors, FCs and myotubes (Nose et al., 1998), in combination with an antibody against Lameduck (Lmd) that labels the nuclei and cytoplasm of all FCMs (Duan et al., 2001). To mark out individual cells (rather than nuclei), we used phalloidin to label the F-actin at the cell cortex or twi-CD2 expressing embryos to label mesodermal cell membranes (Borkowski et al., 1995). In this way, all relevant cells are clearly identified. Using confocal microscopy, we imaged z-stacks through the somatic mesoderm during stages 12–15 (7.5–13 hours AEL). During this period, FC specification is completed, myoblast fusion occurs and the muscles seek out their attachment sites in the epidermis (Bate, 1990; Bate, 1993). As shown in Figure 1, the arrangement of somatic mesodermal cells changes dramatically over this period due to germband retraction and dorsal closure. Mesodermal hemisegments lengthened along the dorsal-ventral (D–V) axis and narrowed along the anterior-posterior (A–P) axis (compare Fig. 1A–C to Fig. 1I–K). The depth of the mesodermal hemisegment changed over this time as well. At stage 12, the ventral somatic mesoderm had a depth of 15–20 µm (3–4 cell diameters), but by stage 14 this had narrowed to 10–15 µm (2–3 cell diameters). Using this approach, we found that, in general, FCs/myotubes were in the most external cell layers in contact with the epidermis and CNS, while FCMs were more internal (Fig. 1).
Figure 1. FC and FCM arrangements during myoblast fusion.
Single optical slices of rp298-lacZ (A–L) or rp298-lacZ; twi-CD2 (M–P) stage 12 (A–D), 13 (E–F, M–P) and 14 (I–L) embryos labeled with anti-βgal to label FC/myotube nuclei (green), anti-Lmd to label FCMs (blue), phalloidin to label F-actin (red) and anti-CD2 to label mesodermal cell membranes (green) are shown. Panels on the left (A, E, I, M) are more external than those on the right (C, G, K, P). Panels D, H and L show close-ups of FCMs in panels B, F and J respectively. Dorsal is up and anterior is left and scale bars are 20 µm in all panels. The developing trachea (yellow arrows, A, B, F, H, K) were used for accurate staging of embryos (Manning and Krasnow, 1993). Mesodermal hemisegments narrow along the A–P axis and extend along the D–V axis during these stages due to germband retraction and dorsal closure (compare A–C with I–K). FCs/myotubes (green) are present in more external panels (A, E, I, M, N), but not more internal where the majority of FCMs (blue) are located (B, C, F, G, J, K, O, P). (A–H) FCs and FCMs are tightly packed together at stages 12 and 13 (white arrowheads, B–D, F–H, M–P). (I–L) However, during stage 14 the FCMs separate from one another and round up to become migratory (white arrowheads, J–L). FCMs in similar locations contact different cell types such as other FCMs, FCs/myotubes and epithelial cells (blue arrows, A, B, F, J). Fusion events can be visualized by colocalization of rp298-lacZ and Lmd (white arrow, E) and can be visualized using this approach from stage 13 onwards. (M–P) Myotubes containing two nuclei can be observed in external cell layers at late stage 13. No FCMs are observed in these cells layers at this stage. The visceral mesoderm is beneath the somatic mesoderm at stage 12 and expresses high levels of F-actin (yellow arrowheads, C).
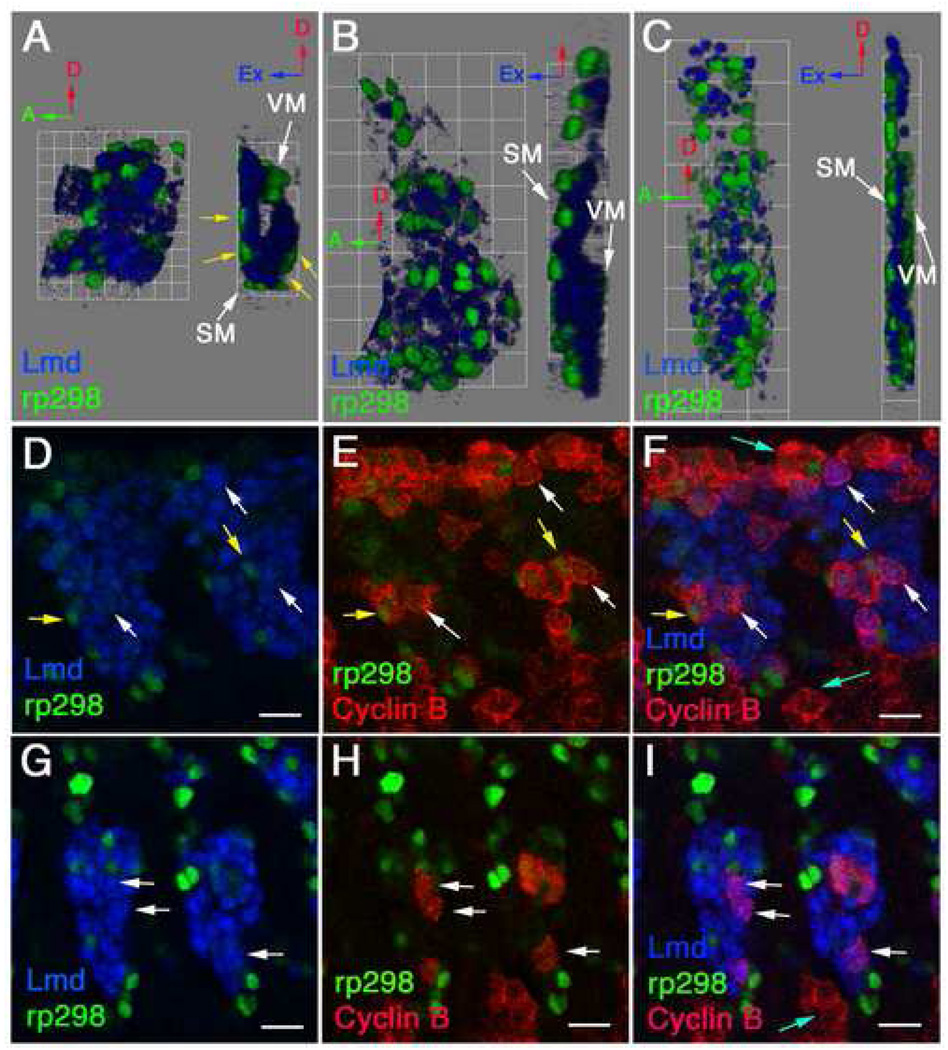
We next created three-dimensional renderings of single mesodermal hemisegments from stages 12–14 (Fig. 2A–C, Supplementary Movies 1–3). This allowed us to visualize the spatial arrangements of FCs and FCMs in three dimensions during myoblast fusion. We observed that the somatic mesoderm contains multiple layers at stage 12 with the most ventral somatic FCMs contacting the dorsal visceral FCs (marked by the expression of high levels of structural proteins such as F-actin, data not shown). The somatic FCs were located around the outside of the FCMs and therefore were concurrently the most external and internal cells in the somatic mesoderm at this stage (Fig. 2A, Supplementary Movie 1). This arrangement of FCs and FCMs had not previously been described. As germband retraction proceeds the mesodermal hemisegment lengthens along the D–V axis and the internal ventral FCs and FCMs move to directly underlay the epidermis. From these fixed samples, it appeared that the cells maintain their positions relative to one another. However, we cannot exclude the possibility of a limited amount of cell movement during this process. From stage 13 onwards, the FCs and developing myotubes were found externally with varying layers of FCMs internally depending on their position along the D–V axis (Fig. 2B–C, Supplementary Movies 2–3).
Figure 2. Three-dimensional analysis of FC and FCM arrangements demonstrates that FCMs are not a uniform cell population.
Stage 12 (A, D–F), 13 (B, G–I) and 14 (C) rp298-lacZ embryos were stained with antibodies against β-gal to label FC/myotube nuclei (green), Lmd to label FCMs (blue) and Cyclin B to label dividing cells (red). (A–C) Three-dimensional renderings of single mesodermal hemisegments at stage 12 (A, 1 grid unit = 5.7 µm), 13 (B, 1 grid unit = 10.9 µm) and 14 (C, 1 grid unit = 14.1 µm) are shown. Each panel shows an external view (left) and a side view rotated 90° clockwise (right). Red arrows point to dorsal, green arrows point to anterior and blue arrows point to external. SM stands for somatic mesoderm and VM stands for visceral mesoderm. The visceral mesoderm was identified based on position and expression of high levels of F-actin by phalloidin staining (Figure 1, data not shown). (A) At stage 12 the somatic mesoderm contains multiple layers and the FCs (green) are concurrently the most external and internal cells (yellow arrows, A), with the FCMs (blue) in between. The most ventral and interior FCMs contact the visceral FCs. (B) At stage 13, the internal FCs and FCMs have moved externally to underlay the overlaying epidermis (not labeled). The FCs (green) appear to rest on top of the FCMs (blue) at this stage and the cells are tightly packed together. (C) By stage 14, the number of rp298-lacZ expressing nuclei (green) have increased due to fusion. The FCMs (blue) have separated from one another. (D–I) Single confocal sections showing that a subset of FCMs undergo cell division during stages 12 and 13. At stage 12 (D–F) a small number of progenitor cells are still dividing to form FCs (yellow arrows, D–F). In addition a subset of FCMs are dividing in dorsal, lateral (white arrows, D–F) and ventral (data not shown) positions. By stage 13 no rp298-lacZ expressing cells are undergoing cell division, but a small number of FCMs are still dividing (white arrows, G–I). Proliferating non-mesodermal cells are located in close proximity to dividing FCMs (blue arrows, F, I).
FCMs are not a uniform cell population
Our spatial analysis of FC and FCM arrangements indicated that FCMs that arise in similar locations touched different cell types. For example, while some FCMs only touched other FCMs, some touched FCs or epithelial tissues such as the epidermis and trachea (Fig. 1). Our data also showed that during the initial stages of myoblast fusion (stages 12–13), the FCs and FCMs were tightly packed together (Fig. 1A–H). The tight cell contacts persisted until stage 14 when the internal FCMs separated from one another and rounded up, acquiring what appears to be a migratory morphology (Fig. 1I–L). During stage 13, bi-nucleate myotubes were observed in external cell layers and FCMs in those layers were no longer detected. Internal FCMs appeared to be still present in their original position (Fig. 1M–P), however, limited cell movements cannot be ruled out by this analysis. These data indicated that the external FCMs are responsible for initial fusion events. The internal FCMs, which do not directly contact FCs, presumably undergo cell migration and fuse later.
In addition, in contrast to the increase in rp298-expressing nuclei that we observed due to fusion (Fig 1–Fig 3), we did not observe the concomitant reduction in the number of FCMs that we expected at stage 14. While we hypothesized that this could be partly explained by the alterations in cell arrangements and interactions during this time, we tested whether the FCMs divided after their initial specification. Although it has been assumed that FCMs are all post-mitotic, analysis of markers for dividing cells, Cyclin B (G1-S phase, Fig. 2D–I) and phospho-Histone H3 (mitosis; Supplementary Fig. 1), revealed that a subset of FCMs undergo cell division during stages 12–13. FCM division is most frequent at early-mid stage 12 (Fig. 2D–F, 21% FCMs express Cyclin B in a given optical section, n=278 FCMs) and decreases during late stage 12 to stage 13 (Fig. 2G–I, 6% FCMs express Cyclin B in a given optical section, n=449 FCMs). No FCM divisions were observed from the end of stage 13 onwards. These dividing FCMs appeared in clusters in dorsal, lateral and ventral positions and occurred both externally and internally. They often contacted proliferating non-mesodermal cells (Fig. 2D–I). In addition, we observed division of rp298-expressing cells during stage 12 (Fig. 2D–F). This corresponds to the final FC progenitor cell divisions that occur at this stage (Bate, 1990; Bate, 1993).
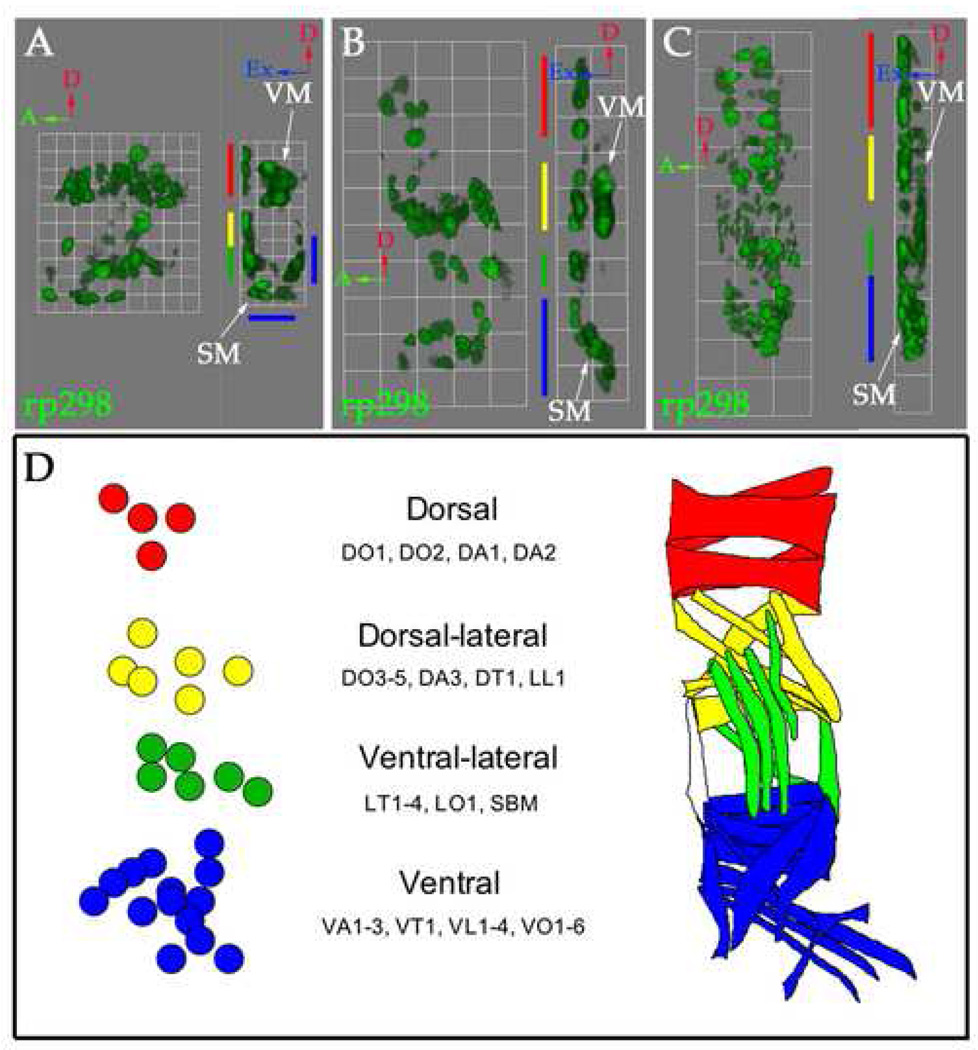
Figure 3. Three-dimensional analysis of FC arrangements shows organization into four groups.
Stage 12 (A), 13 (B) and 14 (C) rp298-lacZ embryos were stained with an antibody against βgal to label FC/myotube nuclei (green). (A–C) Three-dimensional renderings of single mesodermal hemisegments at stage 12 (A, 1 grid unit = 5.7 µm), 13 (B, 1 grid unit = 10.9 µm) and 14 (C, 1 grid unit = 14.1 µm) are shown. Each panel shows an external view (left) and a side view rotated 90° clockwise (right). Red arrows point to dorsal, green arrows point to anterior and blue arrows point to external. SM stands for somatic mesoderm and VM stands for visceral mesoderm. The visceral mesoderm was identified based on position and expression of high levels of F-actin by phalloidin staining (data not shown). During these stages, FCs are organized into four groups in the dorsal (red), dorsal-lateral (yellow), lateral (green) and ventral (blue) somatic mesoderm. At stage 12 (A) the most ventral FCs are located internally. After germband retraction these cells move externally (B–C). Visceral FCs can be clearly seen in the absence of FCMs (put in arrows). These data gathered at each stage have been confirmed with specific FC identity markers. (D) A map of FC arrangements, at stage 13 when 30 FCs can be counted, based on the position and known sibling relationships of the FCs. This map was made by drawing over a flattened version of panel 3B to mark the positions of these cells. A map labeling the identity of each muscle is shown in Supplemental Figure 1.
A three-dimensional map of FCs
An understanding of the fusion process requires knowledge of both the identity and the spatial arrangement of FCs in relation to each other and to the FCMs in the somatic mesoderm. As shown in Figure 1 and Figure 2, the positions of FCs changed during germband retraction as the internal ventral FCs moved to underlay the epidermis. However, throughout this process, the FCs were located in four groups that appear to be maintained throughout stages 12–14. These groups are shown in Figure 3. This was most apparent at stage 13, when approximately 30 rp298-lacZ positive FCs can be counted. Due to the lag time of p-galactosidase expression, this pattern reflects an earlier stage of development (stage 12) (Fig. 3B). There were consistently 4 FCs in the dorsal group, 6 FCs in the dorsal-lateral group, 6 FCs in the lateral group and 14 FCs in the ventral group, some external and some internal (Fig. 3A–C). While this grouping was reproducible, the exact positioning of FCs within a group often varied. However, the relative position of FCs to one another within a group formed a characteristic pattern. For example, in the dorsal group there was always a most dorsal and a most ventral FC with two FCs between, one more anterior to the other (Fig. 3B).
Based on the final position of the muscles we predicted the identity of the FCs within each group (Fig. 3D, Supplementary Fig. 2). We proposed that the dorsal group contains the DO1, DO2, DA1 and DA2 muscles. The dorsal-lateral group contains the DO3–5, DA3, DT1 and LL1 muscles. The lateral group contains the LT1–4, LO1 and SBM muscles and the ventral group contains the VT1, VA1–3, VL1–4 and VO1–6 muscles. Although the sibling relationships of all FCs are not currently known, the published data supported our grouping of the FCs (Supplementary Fig. 3) (Bourgouin et al., 1992; Carmena et al., 1995; Carmena et al., 2002; Crozatier and Vincent, 1999; Dohrmann et al., 1990; Halfon et al., 2000; Jagla et al., 1998; Knirr et al., 1999; Nose et al., 1998; Ruiz-Gomez et al., 1997).
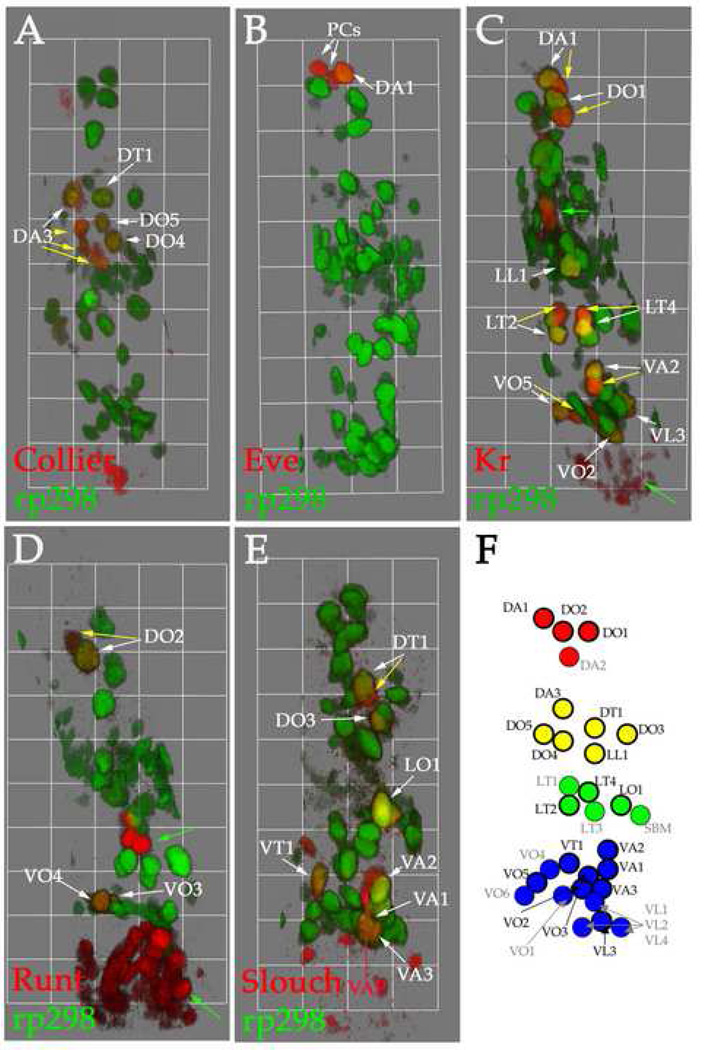
To confirm the identity of the FCs in each group and create a spatial map of FCs, we combined our three-dimensional imaging approach with FC identity markers (Fig. 4). For this analysis we used FC identity markers that are expressed during stage 13 and that, together, label the majority of FCs. These are Collier, Even-skipped (Eve), Krüppel (Kr), Runt and Slouch (Carmena et al., 1995; Carmena et al., 2002; Crozatier and Vincent, 1999; Dohrmann et al., 1990; Halfon et al., 2000; Ruiz-Gomez et al., 1997). Figure 4F shows the spatial map of FCs. FCs that have been confirmed using FC identity markers are outlined and labeled in black, while the others that have been identified based solely on position are labeled in grey. Double-labeling experiments with the identity markers verify these assignments (Crozatier and Vincent, 1999; Carmena et al., 2002; Halfon et al., 2000; Ruiz-Gomez et al., 1997; Knirr et al., 1999; data not shown). These data allowed us to account for all rp298-lacZ-expressing FCs based on their position.
Figure 4. Analysis of FC identity markers outlines a three-dimensional map of FCs at stage 13.
Stage 13 rp298-lacZ embryos were stained with antibodies against βgal (green, A–E), Collier (red, A), Eve (red, B), Kr (red, C), Runt (red, D) and Slouch (red, E). Three-dimensional renderings of single mesodermal hemisegments in each panel shows an external view. Dorsal is up in all panels and anterior is to the left. Each grid unit represents 10.6 µm (A, D), 11.2 µm (B), 11.3 µm (C) and 10.3 µm (E). Green arrows mark non-mesodermal expression of FC identity genes in the CNS or PNS. (A) Collier is expressed in the FCs for the DA3, DT1, DO4 and DO5 muscles. Fusion of the DA3 muscle has already begun at this stage (yellow arrows, A). (B) Eve is expressed in the FC for the DA1 muscle and two PCs. (C) Kr is expressed in the FCs for the DA1, DO1, LL1, LT2, LT4, VA2, VO2, VO5 and VL3 muscles. Fusion has begun in all Kr-positive muscles at this stage (yellow arrows, C). (D) Runt is expressed in the FCs for the DO2, VO3 and VO4 muscles. Fusion of the DO2 muscle has begun at this stage (yellow arrow, D). (E) Slouch is expressed in the FCs for the DT1, DO3, LO1, VT1 and VA1-3 muscles at this stage. Fusion has begun in the DT1 muscle at this stage (yellow arrow, E). The Slouch expressing ventral AP is also labeled (red arrow, E). (F) Map showing the identity and location of all FCs at stage 13. The dorsal (red), dorsal-lateral (yellow), lateral (green) and ventral (blue) groupings of the FCs are shown. FCs labeled in black are those confirmed using FC identity markers, while those labeled in grey show those identified based on position. A map showing the position and identity of all muscles in the final muscle pattern in shown in Supplemental Figure 1.
The fusion profile of individual muscles
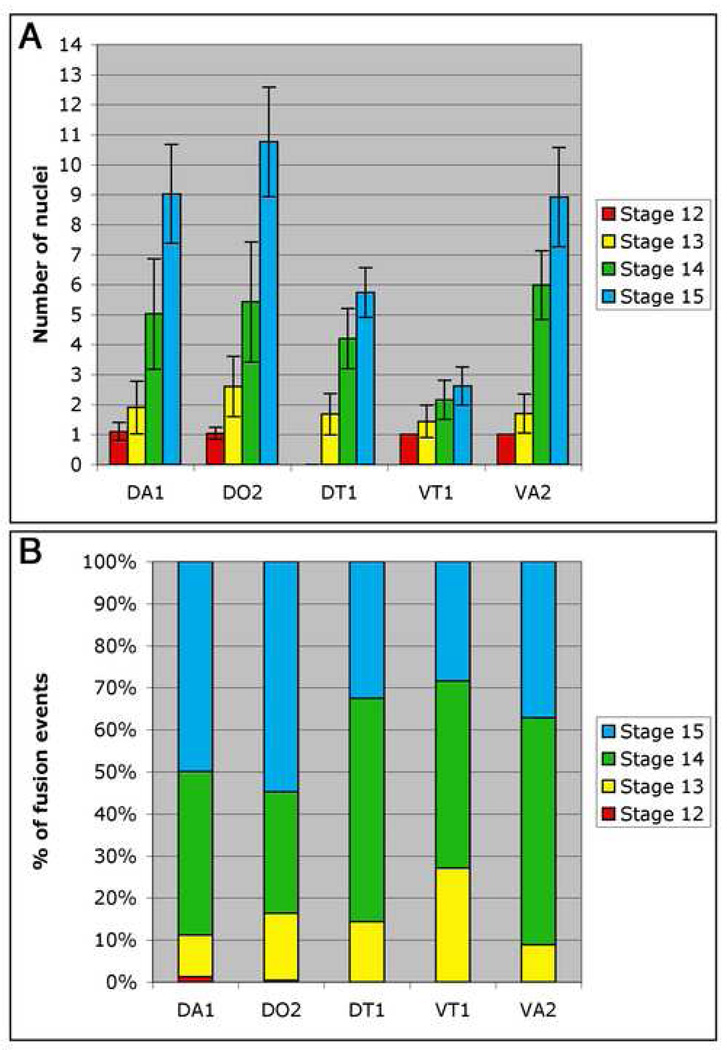
To examine the impact these behaviors could have on fusion, we next determined the timing of fusion in individual muscles over the course of the fusion process. To perform a detailed analysis of the fusion profile of individual muscles, we used FC identity markers to label individual FC/myotube nuclei. All embryos were additionally stained using phalloidin to assist in precise and consistent staging. The complete fusion profile of a subset of muscles (two dorsal DA1, DO2; one dorsal-lateral DT1 and two ventral VT1, VA2) was determined.
We began our analysis with the Eve-expressing DA1 muscle (Halfon et al., 2000; Carmena et al., 2002), as this marker and muscle have been used as an assay for the number of fusion events in previous studies (Menon et al., 2005; Schroter et al., 2004). We counted the number of Eve-expressing FC/myotube nuclei from stage 12 to 15 in 50 abdominal hemisegments (A2–4) for each stage. We focused on a specific set of hemisegments to control for differences in the number of fusion events occurring along the A–P axis (K.B. and M.K.B., unpublished observations) (Bate, 1990). Fusion of the DA1 FC began at stage 12 and by stage 15 an average of 9.02 ± 1.65 nuclei were observed per muscle (n= 50 muscles; Fig. 5A, Table 1, Supplementary Fig. 4A). We found that the relative proportion of fusion events that occurred at each stage were distinctly different. During the first half of myoblast fusion (stage 12–13, 7.5–10.5 hours AEL) only 11% of fusion occurred, while the remaining 89% of fusion occurred in the second half (stage 14–15, 10.5–13 hours AEL) (Fig. 5B, Supplementary Table 1). This indicated that there may be two temporal phases of fusion.
Figure 5. Wild type fusion profiles of individual muscles.
Wild type stage 12–15 embryos were stained with antibodies against Eve (DA1), Runt (DO2) or Slouch (DT1, VT1 and VA2) in combination with phalloidin to assist accurate staging. The number of nuclei for each muscle and stage were counted in 50 hemisegments (A2–4). (A) Bar graph showing the mean number of nuclei for each muscle at each stage. Error bars show one standard deviation from the mean. For each muscle, the majority of fusion occurs in stages 14–15. The stage 12 value for the DT1 muscle was not determined due to an inability to detect Slouch expression. (B) Histogram showing the percentage of fusion events that occur during each stage for each muscle during the course of fusion (7.5–13 hours AEL). The mean number of nuclei observed for each muscle at stage 15 is 100% and a single nucleus is 0%. The numbers used to plot this graph are shown in Supplemental Table 1. 9–27% of fusion occurs during stages 12–13 (7.5–10.5 hours AEL), while the remaining 73–91% of fusion occurs during stages 14–15 (10.5–13 hours AEL).
Table 1.
Wild type fusion profiles of individual muscles.
| Muscle | Stage 12 | Stage 13 | Stage 14 | Stage 15 |
|---|---|---|---|---|
| DA1 | 1.10 ± 0.30 (1–2) | 1.90 ± 0.89 (1–4) | 5.02 ± 1.85 (2–9) | 9.02 ± 1.65 (6–16) |
| DO2 | 1.04 ± 0.20 (1–2) | 2.60 ± 1.01 (1–5) | 5.42 ± 2.00 (3–9) | 10.76 ± 1.82 (8–17) |
| DT1 | n.d.* | 1.68 ± 0.68 (1–3) | 4.20 ± 1.01 (2–6) | 5.74 ± 0.83 (4–8) |
| VT1 | 1.00 ± 0.00 (1) | 1.44 ± 0.54 (1–3) | 2.16 ± 0.65 (1–4) | 2.62 ± 0.64 (2–4) |
| VA2 | 1.00 ± 0.00 (1) | 1.70 ± 0.65 (1–4) | 5.98 ± 1.51 (4–8) | 8.92 ± 1.54 (6–13) |
For each stage and muscle the average number of nuclei +/− standard deviation are shown (n=50). The range of nuclei numbers observed are shown in brackets.
Not determined due to inability to detect Slouch expression in the DT1 FC at this stage.
The fusion profile of other muscles was similar. The Runt positive DO2 muscle (Carmena et al., 2002) also began to fuse at stage 12 and by stage 15 contained an average of 10.76 ± 1.82 nuclei (n=50 muscles; Fig. 5A, Table 1, Supplementary Fig. 4B). Again we observed that 16% of fusion occurred during stages 12–13, while 84% occurred during stages 14–15 (Fig. 5B, Supplementary Table 1). Analysis of the Slouch-positive DT1, VT1 and VA2 muscles (Carmena et al., 1995; Dohrmann et al., 1990; Knirr et al., 1999) supported these results (14%, 27% and 9% of fusion during stage 12–13 respectively) (Fig. 5, Table 1, Supplementary Fig. 4C–E, Supplementary Table 1). Taken together, these data clearly demonstrated that there are two temporal phases of myoblast fusion in all of the muscles analyzed. For each muscle analyzed, 9–27% of fusion events occur in the first half of the fusion process, while the remaining 73–91% occur in the second half (Fig. 5B, Supplementary Table 1). This result was consistent for both larger (e.g. DA1, DO2, DT1 and VA2) and smaller (e.g. VT1) muscles. These data also indicated that the ventral muscles do not necessarily begin to fuse before the dorsal muscles as previously proposed (Bate, 1990; Bate, 1993). For example, the DA1 and DO2 muscles begin to fuse during stage 12, prior to the VA2 and VT1 muscles that begin to fuse during stage 13.
The fusion profile of fusion mutants
As stated in the Introduction a two-step model of myoblast fusion has been proposed based on the analysis of mutants that block the process at different stages. While the first class of mutants (e.g. mbc) have been reported to block all fusion (Erickson et al., 1997; Rushton et al., 1995), the second class of mutants (e.g. blow, kette, rols) appear to block fusion after the formation of a 2–3 nuclei precursor cell (Menon and Chia, 2001; Rau et al., 2001; Schroter et al., 2004). However a precise analysis of the degree of fusion block in many cases has not been performed. Our data indicated that there are two temporal phases of fusion (Fig. 5B, Supplementary Table 1). To test if the first phase of fusion corresponded to the first step of fusion in the two-step model, we analyzed the fusion profile of individual muscles in fusion mutants. If the two phases of fusion that we described corresponded to the two-step model, we would expect that in fusion mutants in which 2–3 fusion events occur, fusion would proceed normally until the end of stage 13 and then stop.
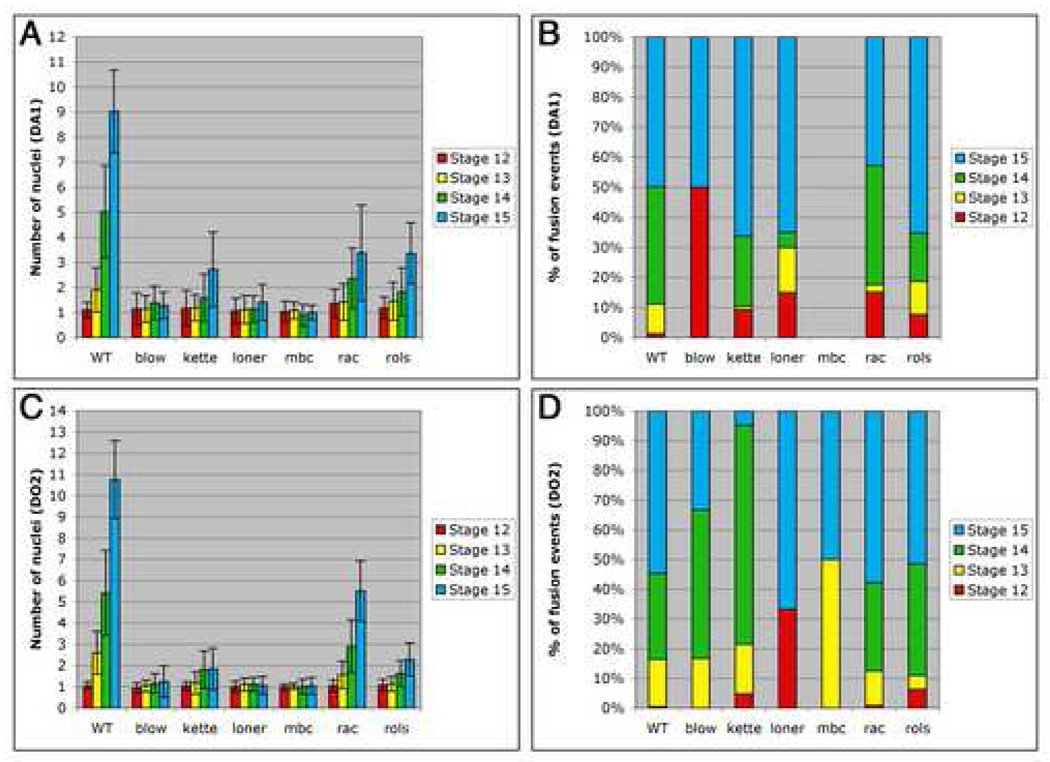
We again began by analyzing the fusion profile of the Eve-expressing DA1 muscle in fusion mutants (Fig. 6A–B, Table 2, Supplementary Table 2). We indeed observed two classes of fusion mutants. The first class of mutants showed almost no fusion events and included blow (1.28 ± 0.54 nuclei at stage 15, n=50 muscles), loner (1.40 ± 0.73 nuclei at stage 15, n=50) and mbc (1.00 ± 0.29 nuclei at stage 15, n=50). The second class of mutants showed 2–4 fusion events at stage 15 and included kette (2.72 ± 1.50 nuclei at stage 15, n=50 muscles), rac (3.38 ± 1.92 nuclei at stage 15, n=50) and rols (3.36 ± nuclei at stage 15, n=50) (Fig. 6A, Table 2). In contrast to previously published results (Schroter et al., 2004), blow mutant embryos, which had been described as showing 2–3 fusion events per muscle, showed almost no fusion in our hands. We propose that this discrepancy was due to the incomplete penetrance of the blow mutant phenotype. At stage 15, 70% of hemisegments showed no fusion (Table 2) and only 26% of hemisegments showed 1–2 fusion events (n=50). Because the average number of Eve-expressing nuclei per hemisegment in blow mutant embryos was 1.28 and hence comparable to that observed for mbc mutant embryos (Table 2), we classified blow mutants in the first class of fusion mutants. Likewise, we classified loner in the first class of fusion mutants, as we found 1.40 Eve-expressing nuclei per hemisegment in loner mutant embryos at stage 15 (Fig. 6A and Table 2). Previous work (Chen et al., 2003) had not precisely identified the extent of myoblast fusion defect in loner mutant embryos, yet it had been classified as blocking the first step of fusion (Schroter et al., 2004). Therefore the first class of fusion mutants included blow, loner and mbc, while the second class of fusion mutants included kette, rac, and rols.
Figure 6. Fusion profile of the DA1 and DO2 muscles for wild type and fusion mutant embryos shows two classes of fusion mutants.
Stage 12–15 wildtype, blow, loner, mbc, kette, rac and rols mutant embryos were stained with antibodies against Eve (DA1, A–B) or Runt (DO2, C–D) in combination with phalloidin (see text). The number of nuclei for each muscle and stage were counted in 50 hemisegments (A2–4). (A, C) Bar graphs showing the mean number of nuclei in the DA1 (A) and DO2 (C) muscles at each stage in each genotype. Error bars show one standard deviation from the mean. For each muscle two classes of fusion mutants were observed. The first class showed almost no fusion and included blow, loner and mbc. The second class showed limited fusion and included kette, rac and rols. The second class of fusion mutants showed fusion at all stages. (B, D) Histograms showing the percentage of fusion events that occur during each stage for each mutant during the course of fusion (7.5–13 hours AEL). The mean number of nuclei observed for each mutant at stage 15 is 100% and a single nucleus is 0%. The numbers used to plot these graphs are shown in Supplemental Table 2 and Supplemental Table 3. Fusion is observed at all stages of fusion independent of the number of fusion events that occur.
Table 2.
Fusion profile of the DA1 muscle for wild type and fusion mutant embryos.
| Genotype | Stage 12 | Stage 13 | Stage 14 | Stage 15 | % lacking fusion* |
|---|---|---|---|---|---|
| Wild type | 1.10 ± 0.30 (1–2) | 1.90 ± 0.89 (1–4) | 5.02 ± 1.85 (2–9) | 9.02 ± 1.65 (6–16) | 0% |
| blow | 1.14 ± 0.64 (0–3) | 1.14 ± 0.53 (0–3) | 1.38 ± 0.67 (1–4) | 1.28 ± 0.54 (0–3) | 70% |
| kette | 1.16 ± 0.71 (0–3) | 1.18 ± 0.52 (0–2) | 1.58 ± 0.95 (0–5) | 2.72 ± 1.50 (0–6) | 14% |
| loner | 1.06 ± 0.51 (0–3) | 1.12 ± 0.56 (0–2) | 1.14 ± 0.53 (0–3) | 1.40 ± 0.73 (0–3) | 68% |
| mbc | 1.04 ± 0.40 (0–2) | 1.08 ± 0.34 (0–2) | 0.88 ± 0.44 (0–2) | 1.00 ± 0.29 (0–2) | 92% |
| rac | 1.36 ± 0.56 (1–3) | 1.42 ± 0.73 (0–3) | 2.36 ± 1.21 (0–6) | 3.38 ± 1.92 (0–8) | 8% |
| rols | 1.18 ± 0.44 (0–2) | 1.44 ± 0.76 (0–4) | 1.82 ± 0.96 (0–4) | 3.36 ± 1.22 (0–6) | 2% |
For each stage and genotype the average number of nuclei +/− standard deviation are shown (n=50). The range of nuclei numbers observed are shown in brackets.
% of hemisegments lacking any fusion at stage 15 (n=50).
Our analysis showed that in mutants that showed limited fusion, those fusion events occurred over the entire period of muscle fusion. A size of 2–4 nuclei per myotube was only achieved by stage 15 in these mutants, in contrast to stage 13–14 in wild type embryos (Fig. 6A–B, Table 2, Supplementary Table 2). This contradicted the prediction of the two-step model, namely, that fusion should proceed normally in these mutants, forming a 2–3 nuclei precursor cell, then stop. Also, no fusion was observed in 14%, 8% and 2% of hemisegments in kette, rac and rols mutant embryos respectively (n=50, Table 2). This strongly suggested that these genes are required for fusion at all stages of the process. In addition, the rare fusion events observed in blow and loner mutant embryos occurred at all stages (Fig. 6A–B, Table 2, Supplementary Table 2). To confirm that this was not unique to the DA1 muscle, we performed the same analysis on the Runt-expressing DO2 muscle. Analysis of the DO2 muscle confirmed the results for the DA1 muscle (Fig. 6C–D, Table 3, Supplementary Table 3). blow, mbc and loner mutant embryos showed almost no fusion, while kette, rac and rols mutant embryos showed limited fusion events that occurred over the entire period of myoblast fusion. These data clearly showed that the fusion profile of the fusion mutants does not support the two-step model of fusion. We therefore concluded that the limited amount of fusion observed in the second class of fusion mutants was due to inefficient fusion.
Table 3.
Fusion profile of the DO2 muscle for wild type and fusion mutant embryos.
| Genotype | Stage 12 | Stage 13 | Stage 14 | Stage 15 | % lacking fusion* |
|---|---|---|---|---|---|
| Wild type | 1.04 ± 0.20 (1–2) | 2.60 ± 1.01 (1–5) | 5.42 ± 2.00 (3–9) | 10.76 ± 1.82 (8–17) | 0% |
| blow | 0.94 ± 0.24 (0–1) | 1.04 ± 0.28 (0–2) | 1.16 ± 0.47 (0–3) | 1.24 ± 0.74 (0–3) | 64% |
| kette | 1.04 ± 0.20 (1–2) | 1.18 ± 0.52 (0–3) | 1.80 ± 0.88 (0–4) | 1.84 ± 0.98 (0–4) | 32% |
| loner | 1.02 ± 0.25 (0–2) | 1.10 ± 0.30 (1–2) | 1.12 ± 0.33 (1–2) | 1.06 ± 0.42 (0–2) | 82% |
| mbc | 0.98 ± 0.14 (0–1) | 1.02 ± 0.14 (1–2) | 1.00 ± 0.35 (0–2) | 1.04 ± 0.40 (0–3) | 90% |
| rac | 1.04 ± 0.28 (0–2) | 1.56 ± 0.64 (0–3) | 2.90 ± 1.25 (0–5) | 5.50 ± 1.42 (3–9) | 0% |
| rols | 1.08 ± 0.27 (1–2) | 1.14 ± 0.35 (1–2) | 1.62 ± 0.60 (0–3) | 2.28 ± 0.78 (0–4) | 8% |
For each stage and genotype the average number of nuclei +/− standard deviation are shown (n=50). The range of nuclei numbers observed are shown in brackets.
% of hemisegments lacking any fusion at stage 15 (n=50).
DISCUSSION
Organogenesis relies on the coordination of multiple cell types in space and time. During Drosophila muscle formation, two distinct myoblast cell types, FCs and FCMs, must organize their behaviors in space and time to undergo cell-cell fusion and form individual muscles of a particular size, shape and orientation. The spatial relationships and behaviors amongst these cell types, the molecular underpinnings of these behaviors over time and the contributions of these to the fusion process were unknown. We have examined the spatial arrangements of the FCs and FCMs during myoblast fusion and morphogenesis. As a result, we constructed the first three-dimensional map of FCs and FCMs. In addition, we have identified novel FCM behaviors that occur during fusion, including cell divisions. The quantitative analysis of fusion profiles of individual muscles has forced us to reexamine the existing model of myoblast fusion, the two-step model. This model formed the framework for the incorporation of new fusion mutants and has been a paradigm for vertebrate myogenesis. Our data, however, suggested a new model of myoblast fusion whereby there are two temporal phases of fusion with the timing or frequency of individual fusion events changing dramatically over the course of the fusion process (Fig. 7). This work highlights the fact that detailed analysis of cell behaviors and cell spatial arrangements over time are essential to understand complex processes of organogenesis, which involve multiple cell types and cell behaviors as described here for Drosophila muscle formation and myoblast fusion.
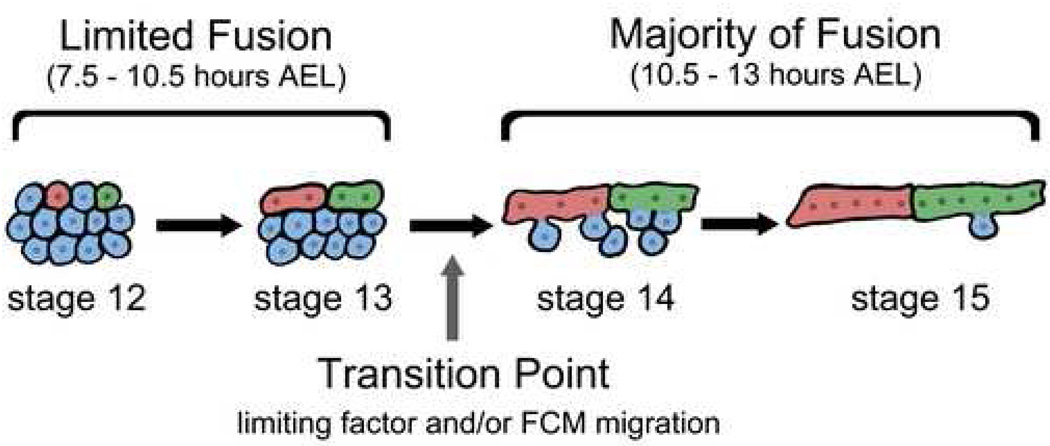
Figure 7. Model figure showing two temporal phases of myoblast fusion.
During stage 12–13 (7.5–10.5 hours AEL) FCs (red and green) are located externally to the FCMs (blue) and all cells are tightly packed together. There is limited fusion during this time. During stage 14–15 (10.5–13 hours AEL) the FCMs (blue) separate from one another, migrate externally and fuse to growing myotubes (red and green). The majority of fusion events occur during this time. We propose that these two temporal phases are due to differences in the frequency of individual fusion events and that expression of limiting factors during the first phase, and the initiation of FCM migration at the beginning of the second phase, are responsible for the transition between the two phases. This model combined with detailed analysis of fusion mutants predicts that subcellular behaviors such as prefusion complex and plaque formation occur at all stages of the fusion process.
Cell arrangements during myoblast fusion
The FCs/myotubes are generally the most external mesodermal cells from stage 12 onwards (Fig 2A–C, Fig 7). While this has been described and correlates with the fact that the muscles form directly underneath the epidermis (Bate, 1990; Doberstein et al., 1997), our work significantly extends these observations by showing the placement and organization of FCs with regard to each other and to the contributing FCMs. The use of phalloidin to label F-actin at the cell cortex allowed visualization of the spatial relationships between somatic mesodermal cells. We found that these cells are packed tightly together during stages 12–13 and appeared to form close cell-cell contacts (Fig 1A–H, Fig 7), although the nature of these contacts remains unknown. However, at the beginning of stage 14 we observed that the cells separated from one another and the FCMs round up and, we interpret, become migratory (Fig 1I–L, Fig 7). These data suggested that regulation of cell adhesion is very important during these stages of muscle development. The nature of these adhesions, the proteins responsible and the signal instructing cells to alter cell adhesion await identification. It is likely that the regulation of these cell contacts and migratory behavior play an essential role in determining the efficiency of the fusion process.
FCMs make an important contribution to muscle morphogenesis
Previous studies have shown that FCMs have their own developmental program (Artero et al., 2003; Duan et al., 2001; Estrada et al., 2006; Furlong et al., 2001; Ruiz-Gomez et al., 2002) and suggest that FCMs are not a uniform cell population. The differences in FCM identity are postulated based on the identification of genes expressed in subsets of FCMs (Artero et al., 2001; Estrada et al., 2006; Ruiz-Gomez et al., 2002). Our work has suggested that FCM’s position within the hemisegment has important consequences for their behaviors. FCMs form cell contacts with FCs, epithelial cells of the epidermis and trachea, as well as with each other (Fig. 1). It is likely that these diverse cell contacts contribute to the FCMs differential identity. For example, our data suggested that FCMs that contact an FC may be responsible for initial fusion events, while those that do not, become migratory and contribute to later fusion events (Fig 1, Fig 7). In addition, we observed that a subset of FCMs at stages 12–13 undergo cell division (Fig. 2D–I). These dividing cells appear clustered together in a segmentally repeated pattern indicating that they are responding to a localized mitogenic signal. It has been shown that both EGF and FGF are expressed in these regions at these times and therefore we propose these signaling proteins as possible candidates (Rutledge et al., 1992; Sutherland et al., 1996). We are now in a position to determine whether expression of genes in subsets of FCMs corresponds with the distinct cellular behaviors described above. These observations will give us a foothold to test the requirement of these behaviors for myoblast fusion and muscle morphogenesis.
Moving from a two-step to a two-phase model of myoblast fusion
The two-step model of myoblast fusion in the Drosophila embryo states that for each muscle, a 2–3 nuclei precursor cell is a critical intermediate step during the formation of a multinucleate myotube. This was based on the classification of fusion mutant into two classes: those showing no fusion and those showing 1–2 fusion events at the end of muscle development (Rau et al., 2001; Schroter et al., 2004). Our data examining the fusion profile of the fusion mutants in detail again showed two classes of mutants, but with important differences (Fig. 6, Table 2, 3, Supplemental Table 2, Supplemental Table 3). First, we have shown that in contrast to published data (Schroter et al., 2004), the amount of fusion observed in blow mutant embryos is directly comparable to that observed in loner and mbc mutant embryos. Second, we showed that in fusion mutants where limited fusion occurs (kette, rols and rac), these fusion events occurred throughout the period of fusion (stages 12–15). This is inconsistent with a model where after the formation of a 2–3 nuclei precursor cell, all subsequent fusion events stop in these mutants. In addition, the observation that in some hemisegments no fusion was detected in these mutant embryos (Table 2, Table 3) indicates that these gene products are required for all fusion events. Taken together, these data suggested to us that the fusion process occurs inefficiently in these mutant embryos. We propose this is due to remaining gene function in these mutants due to maternal loading or absence of a true null allele. It has been documented that Rac and Kette are maternally loaded (Luo et al., 1994; Schroter et al., 2006) and we observed low levels of Rols protein in rols mutant embryos (B. Richardson and M. K. B, unpublished observations). The similarity in the fusion profiles of kette, rols and rac mutant embryos is consistent with a similar cause in each.
The current two-step model indicates that the subcellular events described by EM are unique to the second step of fusion and therefore that two distinct mechanisms for myoblast fusion exist. This model is based on the conclusions that mbc is required for the first step of fusion, while blow and kette are required for the second step (Schroter et al., 2004). The detailed level of analysis presented here shows that there is almost no fusion in blow mutant embryos (Fig. 6, Table 2, Table 3). This indicates that mutants showing both almost no (mbc and blow) and limited (kette) fusion show defects at different stages of the fusion process as analyzed by EM. This suggests that the same subcellular mechanisms occur for all fusion events and underscores the difficulty of trying to link the genetic regulation of cellular and subcellular events in reiterative processes such as myoblast fusion.
While our data challenges the current two-step model of fusion and is inconsistent with two distinct mechanisms of myoblast fusion, it strongly suggested that fusion occurs in two temporal phases (Fig. 7). 9–27% of fusion occurs in the first 3 hours of fusion, while the remaining 73–91% occurs in the latter 2.5 hours (Fig. 5). This model is consistent with published data (Chen and Olson, 2001; Chen et al., 2003; Erickson et al., 1997; Kim et al., 2007; Massarwa et al., 2007; Menon and Chia, 2001; Menon et al., 2005; Rau et al., 2001; Rushton et al., 1995; Schroter et al., 2004). No known fusion mutants showed a block at the transition between these two phases (stage 13 to 14). However, genetic screens to identify fusion mutants have not yet been performed to saturation.
How can two temporal phases of fusion occur? We propose that this is due to either differences in the timing or frequency of individual fusion events. Live imaging studies indicate that the timing of individual fusion events does not vary between stage 13 and 14 (B. Richardson, K.B., S. Nowak and M.K.B., manuscript submitted). Therefore we favor the hypothesis that the frequency of fusion events changes over time. This is likely due to a limiting factor and/or FCM availability during the first phase of fusion. The limiting factor could be a recognition or adhesion protein such as Dumbfounded (Duf) or an unknown factor. Duf levels at the cell surface are known to be tightly controlled during fusion making it a good candidate (Menon et al., 2005). FCM availability may be explained by the correspondence between the time of transition between the two phases of fusion and the time at which FCMs separate from one another and become migratory. It is likely that during stage 12–13 FCs/myotubes can only fuse with the FCMs that they contact (consistent with our observation that the most external FCMs are depleted first). The subsequent, and majority of, fusion events are prevented until the FCMs are able to migrate externally to contact FCs/myotubes. We currently favor a combination of these two models and testing these models is a topic of active investigation.
Supplementary Material
Three-dimensional rendering of single mesodermal hemisegment of rp298-lacZ stage 14 embryo stained with antibodies against βgal (green) and Lmd (blue). One grid unit = 14.1 µm. Exterior is up and red arrow points to ventral.
Three-dimensional rendering of single mesodermal hemisegment of rp298-lacZ stage 12 embryo stained with antibodies against βgal (green) and Lmd (blue). One grid unit = 5.7 µm. Exterior is up and red arrow points to dorsal. Phalloidin was also used for precise staging and to identify individual cells. This information was removed for presentation.
Single optical slices of a stage 12 rp298-lacZ; twi-CD2 embryo labeled with anti-βgal to label FC/myotube nuclei (red), anti-CD2 to label mesodermal cell membranes (red) and anti-phospho-Histone H3 to label mitotic cells (green) are shown. Dorsal is up and anterior is left. Panel A is more external to panel B. (A-B) Several FCMs per hemisegment are dividing at this stage (white arrowheads).
The muscles are labeled according to their grouping and identity. Red = dorsal, yellow = dorsal-lateral, green = lateral, blue = ventral. Muscle identification as in Bate (1993) and (Crossley, 1978).
(A) In the dorsal group, 4 FCs (giving rise to muscles DO1, DO2, DA1, DA2), two pericardial cells (PCs) and two adult muscle progenitors (APs) are formed. Previous studies have shown that both of the PCs and the DO2 muscle arise from a common progenitor cell, while the DA1 and DA2 muscle are also siblings (Carmena et al., 2002). Our data suggested that the DO1 muscle and 2APs also share a common progenitor. (B) In the dorsal-lateral group, 6 FCs (giving rise to muscles DO3-5, DT1 and LL1) and 1 AP are formed. Previous studies showed that DA3 and DO5 share a common progenitor, as do DO3/4 and DT1 (Carmena et al., 1995; Crozatier and Vincent, 1999; Dohrmann et al., 1990). We proposed that the remaining DO3/4 and LL1 muscles and the AP arise from 1 or 2 dorsal-lateral progenitor cells. (C) 6 FCs (giving rise to muscles LT1-4, LO1 and SBM) and 2 APs form in the lateral group. The LT muscles arise from 2 common progenitor cells (Bourgouin et al., 1992; Ruiz-Gomez et al., 1997). Another single progenitor cell gives rise to the SBM and 2 APs (Jagla et al., 1998). Finally the lateral LO1 muscle and ventral VT1 muscles also shared a common progenitor. The two muscles arise in distinct locations due to the migration of the LO1 FC into the adjacent anterior hemisegment (Dohrmann et al., 1990). (D) The ventral group forms 14 FCs (giving rise to muscles VA1-3, VT1, VO1-6 and VA1-4) and a single AP. As described above, the VT1 FC shares a common progenitor with the lateral LO1 muscle (Dohrmann et al., 1990). It has also been shown that a single progenitor cell divides to form the FCs for VA1 and VA2, while another progenitor cell forms the FC for VA3 and an AP (Carmena et al., 1995; Dohrmann et al., 1990; Ruiz-Gomez et al., 1997). For the remaining 6 VO FCs and 4 VL FCs, we predicted that they are formed from 5 ventral progenitor cells. From this summary of what is currently known about the sibling relationships between FCs, we correlated the approximate 18 progenitor cells required to make the 30 FCs, 6 APs and 2 PCs in each hemisegment to the 18 premuscle clusters described by Carmena et al (1995).
Bar graphs showing the frequency of nuclei number in wild type DA1 (A), DO2 (B), DT1 (C), VT1 (D) and VA2 (E) muscles at stage 15 (n=50).
Three-dimensional rendering of single mesodermal hemisegment of rp298-lacZ stage 13 embryo stained with antibodies against βgal (green) and Lmd (blue). One grid unit = 10.9 µm. Exterior is up and red arrow points to dorsal. Phalloidin was also used for precise staging and to identify individual cells. This information was removed for presentation.
Acknowledgements
We thank K. V. Anderson, A. Martinez-Arias, J. T. Blankenship, B. Richardson and M. C. Wong for discussions and critical reading of the manuscript. We also thank G. Erskine for help with the model figure, M. Frasch, J. Reinitz, A. Vincent, E. Chen, D. Menon, W. Chia, H. Ngyugen, C. Klambt and the Developmental Hybridoma Bank for reagents. This work was supported by the Sloan Kettering Institute and NIH grants (GM 586989 and GM78318) to M.B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abmayr SM, Kocherlakota KS. Muscle Morphogenesis: The Process of Embryonic Myoblast Fusion. In: Sink H, editor. Muscle Development in Drosophila. New York: Springer Science + Business Media, Inc.; 2006. [Google Scholar]
- Artero R, Furlong EE, Beckett K, Scott MP, Baylies M. Notch and Ras signaling pathway effector genes expressed in fusion competent and founder cells during Drosophila myogenesis. Development. 2003;130:6257–6272. doi: 10.1242/dev.00843. [DOI] [PubMed] [Google Scholar]
- Artero RD, Castanon I, Baylies MK. The immunoglobulin-like protein Hibris functions as a dose-dependent regulator of myoblast fusion and is differentially controlled by Ras and Notch signaling. Development. 2001;128:4251–4264. doi: 10.1242/dev.128.21.4251. [DOI] [PubMed] [Google Scholar]
- Bate M. The embryonic development of larval muscles in Drosophila. Development. 1990;110:791–804. doi: 10.1242/dev.110.3.791. [DOI] [PubMed] [Google Scholar]
- Bate M. The mesoderm and its derivatives. In: MMA Bate A, editor. The Development of Drosophila melanogaster. New York: Cold Spring Harbor Laboratory Press; 1993. [Google Scholar]
- Baylies MK, Bate M, Ruiz Gomez M. Myogenesis: a view from Drosophila. Cell. 1998;93:921–927. doi: 10.1016/s0092-8674(00)81198-8. [DOI] [PubMed] [Google Scholar]
- Beckett K, Baylies MK. The development of the Drosophila larval body wall muscles. Int Rev Neurobiol. 2006;75:55–70. doi: 10.1016/S0074-7742(06)75003-6. [DOI] [PubMed] [Google Scholar]
- Borkowski OM, Brown NH, Bate M. Anterior-posterior subdivision and the diversification of the mesoderm in Drosophila. Development. 1995;121:4183–4193. doi: 10.1242/dev.121.12.4183. [DOI] [PubMed] [Google Scholar]
- Bourgouin C, Lundgren SE, Thomas JB. Apterous is a Drosophila LIM domain gene required for the development of a subset of embryonic muscles. Neuron. 1992;9:549–561. doi: 10.1016/0896-6273(92)90192-g. [DOI] [PubMed] [Google Scholar]
- Carmena A, Bate M, Jimenez F. Lethal of scute, a proneural gene, participates in the specification of muscle progenitors during Drosophila embryogenesis. Genes Dev. 1995;9:2373–2383. doi: 10.1101/gad.9.19.2373. [DOI] [PubMed] [Google Scholar]
- Carmena A, Buff E, Halfon M, Gisselbrecht S, Jimenez F, Baylies MK, Michelson AM. Reciprocal Regulatory Interactions between Notch and Ras Signaling Pathways in the Drosophila Embryonic Mesoderm. Dev Biol. 2002;15:226–242. doi: 10.1006/dbio.2002.0606. [DOI] [PubMed] [Google Scholar]
- Chen EH, Olson EN. Antisocial, an intracellular adaptor protein, is required for myoblast fusion in Drosophila. Dev Cell. 2001;1:705–715. doi: 10.1016/s1534-5807(01)00084-3. [DOI] [PubMed] [Google Scholar]
- Chen EH, Pryce BA, Tzeng JA, Gonzalez GA, Olson EN. Control of myoblast fusion by a guanine nucleotide exchange factor, loner, and its effector ARF6. Cell. 2003;114:751–762. doi: 10.1016/s0092-8674(03)00720-7. [DOI] [PubMed] [Google Scholar]
- Cox VT, Baylies MK. Specification of individual Slouch muscle progenitors in Drosophila requires sequential Wingless signaling. Development. 2005;132:713–724. doi: 10.1242/dev.01610. [DOI] [PubMed] [Google Scholar]
- Crossley AC. The morphology and development of the Drosophila muscular system. In: Ashburner M, Wright T, editors. The genetics and biology of Drosophila. Vol. 2b. New York: Academic Press; 1978. pp. 499–560. [Google Scholar]
- Crozatier M, Vincent A. Requirement for the Drosophila COE transcription factor Collier in formation of an embryonic muscle: transcriptional response to notch signalling. Development. 1999;126:1495–1504. doi: 10.1242/dev.126.7.1495. [DOI] [PubMed] [Google Scholar]
- Doberstein SK, Fetter RD, Mehta AY, Goodman CS. Genetic analysis of myoblast fusion: blown fuse is required for progression beyond the prefusion complex. J Cell Biol. 1997;136:1249–1261. doi: 10.1083/jcb.136.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrmann C, Azpiazu N, Frasch M. A new Drosophila homeo box gene is expressed in mesodermal precursor cells of distinct muscles during embryogenesis. Genes Dev. 1990;4:2098–2111. doi: 10.1101/gad.4.12a.2098. [DOI] [PubMed] [Google Scholar]
- Duan H, Skeath JB, Nguyen HT. Drosophila Lame duck, a novel member of the Gli superfamily, acts as a key regulator of myogenesis by controlling fusion-competent myoblast development. Development. 2001;128:4489–4500. doi: 10.1242/dev.128.22.4489. [DOI] [PubMed] [Google Scholar]
- Erickson MR, Galletta BJ, Abmayr SM. Drosophila myoblast city encodes a conserved protein that is essential for myoblast fusion, dorsal closure, and cytoskeletal organization. J Cell Biol. 1997;138:589–603. doi: 10.1083/jcb.138.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada B, Choe SE, Gisselbrecht SS, Michaud S, Raj L, Busser BW, Halfon MS, Church GM, Michelson AM. An Integrated Strategy for Analyzing the Unique Developmental Programs of Different Myoblast Subtypes. PLoS Genet. 2006;2:e16. doi: 10.1371/journal.pgen.0020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch M. Controls in patterning and diversification of somatic muscles during Drosophila embryogenesis. Curr Opin Genet Dev. 1999;9:522–529. doi: 10.1016/s0959-437x(99)00014-3. [DOI] [PubMed] [Google Scholar]
- Furlong EE, Andersen EC, Null B, White KP, Scott MP. Patterns of gene expression during Drosophila mesoderm development. Science. 2001;293:1629–1633. doi: 10.1126/science.1062660. [DOI] [PubMed] [Google Scholar]
- Hakeda-Suzuki S, Ng J, Tzu J, Dietzl G, Sun Y, Harms M, Nardine T, Luo L, Dickson BJ. Rac function and regulation during Drosophila development. Nature. 2002;416:438–442. doi: 10.1038/416438a. [DOI] [PubMed] [Google Scholar]
- Halfon MS, Carmena A, Gisselbrecht S, Sackerson CM, Jimenez F, Baylies MK, Michelson AM. Ras pathway specificity is determined by the integration of multiple signal-activated and tissue-restricted transcription factors. Cell. 2000;103:63–74. doi: 10.1016/s0092-8674(00)00105-7. [DOI] [PubMed] [Google Scholar]
- Horsley V, Pavlath GK. Forming a multinucleated cell: molecules that regulate myoblast fusion. Cells Tissues Organs. 2004;176:67–78. doi: 10.1159/000075028. [DOI] [PubMed] [Google Scholar]
- Hummel T, Leifker K, Klambt C. The Drosophila HEM-2/NAP1 homolog KETTE controls axonal pathfinding and cytoskeletal organization. Genes Dev. 2000;14:863–873. [PMC free article] [PubMed] [Google Scholar]
- Jagla T, Bellard F, Lutz Y, Dretzen G, Bellard M, Jagla K. Ladybird determines cell fate decisions during diversification of Drosophila somatic muscles. Development. 1998;125:3699–3708. doi: 10.1242/dev.125.18.3699. [DOI] [PubMed] [Google Scholar]
- Kim S, Shilagardi K, Zhang S, Hong SN, Sens KL, Bo J, Gonzalez GA, Chen EH. A critical function for the actin cytoskeleton in targeted exocytosis of prefusion vesicles during myoblast fusion. Dev Cell. 2007;12:571–586. doi: 10.1016/j.devcel.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Knirr S, Azpiazu N, Frasch M. The role of the NK-homeobox gene slouch (S59) in somatic muscle patterning. Development. 1999;126:4525–4535. doi: 10.1242/dev.126.20.4525. [DOI] [PubMed] [Google Scholar]
- Luo L, Liao YJ, Jan LY, Jan YN. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- Manning G, Krasnow MA. Development of the Drosophila Tracheal System. In: Bate M, Martinez Arias A, editors. The Development of Drosophila melanogaster. New York: Cold Spring Harbor Laboratory Press; 1993. [Google Scholar]
- Massarwa R, Carmon S, Shilo BZ, Schejter ED. WIP/WASp-based actin-polymerization machinery is essential for myoblast fusion in Drosophila. Dev Cell. 2007;12:557–569. doi: 10.1016/j.devcel.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Menon SD, Chia W. Drosophila rolling pebbles: a multidomain protein required for myoblast fusion that recruits D-Titin in response to the myoblast attractant Dumbfounded. Dev Cell. 2001;1:691–703. doi: 10.1016/s1534-5807(01)00075-2. [DOI] [PubMed] [Google Scholar]
- Menon SD, Osman Z, Chenchill K, Chia W. A positive feedback loop between Dumbfounded and Rolling pebbles leads to myotube enlargement in Drosophila. J Cell Biol. 2005;169:909–920. doi: 10.1083/jcb.200501126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A, Isshiki T, Takeichi M. Regional specification of muscle progenitors in Drosophila: the role of the msh homeobox gene. Development. 1998;125:215–223. doi: 10.1242/dev.125.2.215. [DOI] [PubMed] [Google Scholar]
- Rau A, Buttgereit D, Holz A, Fetter R, Doberstein SK, Paululat A, Staudt N, Skeath J, Michelson AM, Renkawitz-Pohl R. rolling pebbles (rols) is required in Drosophila muscle precursors for recruitment of myoblasts for fusion. Development. 2001;128:5061–5073. doi: 10.1242/dev.128.24.5061. [DOI] [PubMed] [Google Scholar]
- Ruiz-Gomez M, Coutts N, Suster ML, Landgraf M, Bate M. Myoblasts incompetent encodes a zinc finger transcription factor required to specify fusion-competent myoblasts in Drosophila. Development. 2002;129:133–141. doi: 10.1242/dev.129.1.133. [DOI] [PubMed] [Google Scholar]
- Ruiz-Gomez M, Romani S, Hartmann C, Jackle H, Bate M. Specific muscle identities are regulated by Kruppel during Drosophila embryogenesis. Development. 1997;124:3407–3414. doi: 10.1242/dev.124.17.3407. [DOI] [PubMed] [Google Scholar]
- Rushton E, Drysdale R, Abmayr SM, Michelson AM, Bate M. Mutations in a novel gene, myoblast city, provide evidence in support of the founder cell hypothesis for Drosophila muscle development. Development. 1995;121:1979–1988. doi: 10.1242/dev.121.7.1979. [DOI] [PubMed] [Google Scholar]
- Rutledge BJ, Zhang K, Bier E, Jan YN, Perrimon N. The Drosophila spitz gene encodes a putative EGF-like growth factor involved in dorsal-ventral axis formation and neurogenesis. Genes Dev. 1992;6:1503–1517. doi: 10.1101/gad.6.8.1503. [DOI] [PubMed] [Google Scholar]
- Schroter RH, Buttgereit D, Beck L, Holz A, Renkawitz-Pohl R. Blown fuse regulates stretching and outgrowth but not myoblast fusion of the circular visceral muscles in Drosophila. Differentiation. 2006;74:608–621. doi: 10.1111/j.1432-0436.2006.00080.x. [DOI] [PubMed] [Google Scholar]
- Schroter RH, Lier S, Holz A, Bogdan S, Klambt C, Beck L, Renkawitz-Pohl R. kette and blown fuse interact genetically during the second fusion step of myogenesis in Drosophila. Development. 2004;131:4501–4509. doi: 10.1242/dev.01309. [DOI] [PubMed] [Google Scholar]
- Sutherland D, Samakovlis C, Krasnow MA. branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell. 1996;87:1091–1101. doi: 10.1016/s0092-8674(00)81803-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Three-dimensional rendering of single mesodermal hemisegment of rp298-lacZ stage 14 embryo stained with antibodies against βgal (green) and Lmd (blue). One grid unit = 14.1 µm. Exterior is up and red arrow points to ventral.
Three-dimensional rendering of single mesodermal hemisegment of rp298-lacZ stage 12 embryo stained with antibodies against βgal (green) and Lmd (blue). One grid unit = 5.7 µm. Exterior is up and red arrow points to dorsal. Phalloidin was also used for precise staging and to identify individual cells. This information was removed for presentation.
Single optical slices of a stage 12 rp298-lacZ; twi-CD2 embryo labeled with anti-βgal to label FC/myotube nuclei (red), anti-CD2 to label mesodermal cell membranes (red) and anti-phospho-Histone H3 to label mitotic cells (green) are shown. Dorsal is up and anterior is left. Panel A is more external to panel B. (A-B) Several FCMs per hemisegment are dividing at this stage (white arrowheads).
The muscles are labeled according to their grouping and identity. Red = dorsal, yellow = dorsal-lateral, green = lateral, blue = ventral. Muscle identification as in Bate (1993) and (Crossley, 1978).
(A) In the dorsal group, 4 FCs (giving rise to muscles DO1, DO2, DA1, DA2), two pericardial cells (PCs) and two adult muscle progenitors (APs) are formed. Previous studies have shown that both of the PCs and the DO2 muscle arise from a common progenitor cell, while the DA1 and DA2 muscle are also siblings (Carmena et al., 2002). Our data suggested that the DO1 muscle and 2APs also share a common progenitor. (B) In the dorsal-lateral group, 6 FCs (giving rise to muscles DO3-5, DT1 and LL1) and 1 AP are formed. Previous studies showed that DA3 and DO5 share a common progenitor, as do DO3/4 and DT1 (Carmena et al., 1995; Crozatier and Vincent, 1999; Dohrmann et al., 1990). We proposed that the remaining DO3/4 and LL1 muscles and the AP arise from 1 or 2 dorsal-lateral progenitor cells. (C) 6 FCs (giving rise to muscles LT1-4, LO1 and SBM) and 2 APs form in the lateral group. The LT muscles arise from 2 common progenitor cells (Bourgouin et al., 1992; Ruiz-Gomez et al., 1997). Another single progenitor cell gives rise to the SBM and 2 APs (Jagla et al., 1998). Finally the lateral LO1 muscle and ventral VT1 muscles also shared a common progenitor. The two muscles arise in distinct locations due to the migration of the LO1 FC into the adjacent anterior hemisegment (Dohrmann et al., 1990). (D) The ventral group forms 14 FCs (giving rise to muscles VA1-3, VT1, VO1-6 and VA1-4) and a single AP. As described above, the VT1 FC shares a common progenitor with the lateral LO1 muscle (Dohrmann et al., 1990). It has also been shown that a single progenitor cell divides to form the FCs for VA1 and VA2, while another progenitor cell forms the FC for VA3 and an AP (Carmena et al., 1995; Dohrmann et al., 1990; Ruiz-Gomez et al., 1997). For the remaining 6 VO FCs and 4 VL FCs, we predicted that they are formed from 5 ventral progenitor cells. From this summary of what is currently known about the sibling relationships between FCs, we correlated the approximate 18 progenitor cells required to make the 30 FCs, 6 APs and 2 PCs in each hemisegment to the 18 premuscle clusters described by Carmena et al (1995).
Bar graphs showing the frequency of nuclei number in wild type DA1 (A), DO2 (B), DT1 (C), VT1 (D) and VA2 (E) muscles at stage 15 (n=50).
Three-dimensional rendering of single mesodermal hemisegment of rp298-lacZ stage 13 embryo stained with antibodies against βgal (green) and Lmd (blue). One grid unit = 10.9 µm. Exterior is up and red arrow points to dorsal. Phalloidin was also used for precise staging and to identify individual cells. This information was removed for presentation.