Abstract
Background
Severe malaria is characterized by microvascular obstruction, endothelial dysfunction, and reduced levels of L-arginine and nitric oxide (NO). L-Arginine infusion improves endothelial function in moderately severe malaria. Neither the longitudinal course of endothelial dysfunction nor factors associated with recovery have been characterized in severe malaria.
Methods
Endothelial function was measured longitudinally in adults with severe malaria (n = 49) or moderately severe malaria (n = 48) in Indonesia, using reactive hyperemia peripheral arterial tonometry (RH-PAT). In a mixed-effects model, changes in RH-PAT index values in patients with severe malaria were related to changes in parasitemia, lactate, acidosis, and plasma L-arginine concentrations.
Results
Among patients with severe malaria, the proportion with endothelial dysfunction fell from 94% (46/49 patients) to 14% (6/42 patients) before discharge or death (P <.001). In severe malaria, the median time to normal endothelial function was 49 h (interquartile range, 20–70 h) after the start of antimalarial therapy. The mean increase in L-arginine concentrations in patients with severe malaria was 11 μmol/L/24 h (95% confidence interval [CI], 9–13 μmol/L/24 h), from a baseline of 49 μmol/L (95% CI, 37–45 μmol/L). Improvement of endothelial function in patients with severe malaria correlated with increasing levels of L-arginine (r = 0.56; P =.008) and decreasing levels of lactate (r = −0.44; P =.001).
Conclusions
Recovery of endothelial function in severe malaria is associated with recovery from hypoargininemia and lactic acidosis. Agents that can improve endothelial NO production and endothelial function, such as L-arginine, may have potential as adjunctive therapy early during the course of severe malaria.
The endothelium and microcirculation are thought to play key roles in the pathogenesis of severe Plasmodium falciparum infection [1, 2]. Autopsy studies of malaria have demonstrated widespread endothelial activation [3] and sequestration of parasitized erythrocytes [4, 5]. These processes are believed to result in microcirculatory obstruction, decreased oxygen delivery, tissue hypoxia, metabolic acidosis, hyperlactatemia, and organ damage.
Endothelial function is defined as the vasodilatory capacity of blood vessels in response to shear stress or pharmacological stimuli and is primarily dependent on endothelial cell production of nitric oxide (NO) [6]. Endothelial dysfunction has been described in a variety of illnesses, including cardiovascular [6] and sickle cell [7] disease. In these diseases, the common pathogenic mechanism is a reduction in vascular bioavailability of NO that results in impaired vasodilatory ability and endothelial activation with increased expression of endothelial receptors, such as intercellular adhesion molecule (ICAM)–1 [6].
Severe falciparum malaria in children and adults is associated with impaired systemic and pulmonary NO production [8, 9], reduced expression of type 2 (inducible) NO synthase (NOS) in mononuclear cells [8] and decreased plasma concentrations of L-arginine [9, 10], the substrate for NOS. We have recently shown marked impairment of endothelial function in adults with severe malaria, compared with that in adults with moderately severe malaria and in healthy control subjects [9]. Endothelial dysfunction in malaria was associated with increases in blood lactate concentrations, plasma ICAM-1 concentrations, and measures of intravascular hemolysis, with increased levels of plasma arginase and cell-free hemoglobin potentially increasing L-arginine metabolism and NO quenching, respectively [9]. Taken together, these findings suggest that reduced vascular NO bioavailability may contribute to the pathogenesis of severe malaria by impairing endothelial function and vascular perfusion, in addition to increasing endothelial activation and sequestration of parasitized red blood cells.
Extracellular L-arginine concentrations affect intracellular NO production by endothelial NOS [11]. The estimates for the half-saturating concentration (Km) of extracellular L-arginine for intracellular NO production (73–150 μmol/L) [12, 13] approximate the range of normal plasma L-arginine concentrations but are higher than concentrations observed in human malaria. The findings of hypoargininemia in severe malaria and a dose-dependent improvement in endothelial function after L-arginine infusion in moderately severe malaria [9] suggest that plasma L-arginine concentrations may also affect endothelial NO production in severe malaria. However, to date no studies have examined the effects of adjunctive L-arginine on endothelial function in severe malaria.
It is unclear whether preexisting impairment of endothelial function and hypoargininemia make patients more susceptible to severe malaria or whether these processes are a consequence of severe disease. The natural history of endothelial function and its relationship with plasma L-arginine concentrations during the course of human malarial infection have not been reported but have implications for the required duration of potential adjunctive therapies to improve endothelial function. We hypothesized that endothelial function would return to normal after successful treatment of severe malaria and that the recovery would be associated with an increase in plasma L-arginine concentrations. We therefore undertook a prospective longitudinal study measuring endothelial function and L-arginine concentrations in adult patients with severe and moderately severe malaria in Papua, Indonesia, and investigating the clinical and biochemical factors influencing their course.
METHODS
Study design and site
A prospective longitudinal study was conducted at Mitra Masyarakat Hospital, Timika, Papua, Indonesia, in an area with documented unstable transmission of malaria [14].
Patients and sampling
The study enrolled patients ≥18 years old who were hospitalized with P. falciparum malaria and were included in a cross-sectional study of endothelial function described elsewhere [9]. Moderately severe malaria was defined as fever or history of fever in the past 48 h along with >1000 asexual P. falciparum parasites/μL that required inpatient parenteral therapy because of an inability to tolerate oral therapy but that exhibited no World Health Organization (WHO) warning signs or criteria for severe malaria [15, 16]. Severe falciparum malaria was defined as the presence of P. falciparum parasitemia and at least 1 modified WHO criterion for severity [17]. Individuals with moderately severe malaria were treated with intravenous quinine, in accordance with national guidelines. Of the patients with severe malaria, 20 were initially randomized to either intravenous quinine or artesunate as part of a multicenter clinical trial [18]. After a national policy change, all patients with severe malaria subsequently received intravenous artesunate. Both groups also received doxycycline or clindamycin. All decisions for provision of supportive care, including antibiotic and fluid administration, were made by the treating physicians, who were independent of the study.
On enrollment, a standardized history was obtained from either the patient or attendant relatives, after which a clinical examination was performed, venous blood was collected, and endothelial function was measured. Each of these procedures was repeated daily until death, discharge from the hospital, or, for those patients still hospitalized after day 4, until the reactive hyperemia peripheral arterial tonometry (RH-PAT) index was above an a priori–defined cutoff indicating endothelial dysfunction (1.67) [19] on 2 consecutive days.
Parasitological and biochemical observations
Parasite counts were determined from microscopy of Giemsa-stained thick and thin blood films. Hemoglobin concentration, routine biochemistry values, acid-base parameters (including base deficit [20]), and lactate were determined with a portable biochemical analyzer (i-STAT). Venous blood collected in lithium heparin was centrifuged within 30 min of collection, and plasma was stored at −70°C. Amino acids were extracted from 50 μL of plasma after the addition of 50 μL of internal standard (norleucine) and 200 μL of cold ethanol. Deproteinized plasma was derivitized with AccQFluor reagent (Waters), and amino acids were measured by high-performance liquid chromatography (Shimadzu), using a method modified from van Wandelen and Cohen [21] and Strydom and Cohen [22]. Plasma concentrations of the endothelial activation markers, soluble ICAM-1 and E-selectin, were assayed by ELISA (R&D Systems). To quantify total parasite biomass, plasma histidine-rich protein 2 was measured by ELISA, as described elsewhere [23]. Plasma haptoglobin and lactate dehydrogenase (LDH) were quantified by ELISA and a calorimetric assay, respectively (both Roche Diagnostics). Plasma arginase activity was measured using a radiometric assay, as described elsewhere; findings and reported in micromoles per milliliter per hour [7].
Endothelial function assessment
Endothelial function is defined by the ability of vessels to dilate in response to increased sheer stress or chemical agonists [6], a response that is inversely related to endothelial activation [6]. Endothelial function was measured noninvasively on the basis of the change in pulse wave amplitude of the digits, determined using peripheral arterial tonometry (EndoPAT; Itamar), in response to reactive hyperemia (yielding an RH-PAT index [6, 24]). Changes in the pulse wave amplitude reflect changes in digital blood flow, and a value of <1.67 represents an impaired response [9, 19]. Systemic effects on the pulse wave amplitude are accounted for by normalization to the control arm [24]. RH-PAT is at least 50% dependent on endothelial NO production [25] and correlates with the more labor-intensive flow-mediated dilation method [24] as well as with endothelial function in other vascular beds [26]. The internal validation and repeatability of RH-PAT in this population has been reported elsewhere [9].
Statistical methods
Proportions and paired proportions were compared using χ2 and McNemar χ2 tests, respectively. Univariate and multivariate-linear mixed-effects methods were used to model the longitudinal changes of the RH-PAT index and L-arginine concentrations during the course of hospitalization (Stata software; version 9.2; StataCorp). The model evaluated longitudinal correlations of RH-PAT index with potential covariates, including pulse rate, temperature, blood pressure, Glasgow Coma Score, base excess, lactate concentration, peripheral parasitemia, and L-arginine concentration. These models allow for incomplete follow-up data and a variable number of late measurements, including those in patients who may die early in the study or refuse further examination. The Bonferroni correction was used to adjust for the multiple comparisons. Baseline predictors influencing the longitudinal course of the RH-PAT index and plasma L-arginine concentrations were identified by linear regression analysis.
Ethics
Ethical approval was obtained from the health research ethics committees of the National Institute of Health Research and Development, Indonesia, and Menzies School of Health Research, Australia. Written informed consent was obtained from patients or relatives.
RESULTS
Patients
In total, 99 hospitalized patients were included in the study, 51 with severe and 48 with moderately severe malaria. Restlessness precluded measurement of baseline RH-PAT index in 2 patients with severe malaria, who were subsequently excluded from the analysis. Baseline clinical and laboratory characteristics of the remaining 97 patients are summarized in table 1.
Table 1.
Baseline characteristics of patients according to clinical status.
| Baseline characteristica | Moderately severe malaria (n = 48) | Severe malaria (n = 49) |
|---|---|---|
| Age, mean (range), years | 28 (18–56) | 29 (18–56) |
| Sex, male | 32 (67) | 36 (73) |
| Weight, mean (range), kg | 58 (43–77) | 57 (45–76) |
| Ethnicity, Papuan highlandera | 37 (77) | 27 (55) |
| Current smoker | 19 (40) | 22 (45) |
| Former smoker | 7 (15) | 8 (16) |
| Days of fever before presentation, median (IQR) | 3 (1–5) | 4 (1–7) |
| Systolic blood pressure, mean (range), mm Hgb | 114 (88–152) | 107 (60–154) |
| Hypertensive at enrollment | 1 (2) | 1 (2) |
| Pulse rate, mean (range), beats/minb | 86 (56–118) | 97 (61–138) |
| Respiratory rate, mean (range), breaths/minb | 25 (14–42) | 29 (16–60) |
| Temperature, mean (range), °Cb | 36.5 (34.1–39.8) | 37.1 (34.8–40.3) |
| Comaa | 0 (0) | 27 (55) |
| White blood cell count, mean (range), 103 cells/μLb | 5.9 (2.6–10.8) | 9.4 (3.2–17.3) |
| Hemoglobin concentration, mean (range), g/dLb | 12.8 (7.1–16.7) | 10.4 (6–16.3) |
| Plasma L-arginine concentration, mean (95% CI), μmol/L | 42 (37–45) | 50 (44–56) |
| Lactate concentration, mean (95% CI), mmol/L | 1.29 (1.1–1.5) | 2.94 (2.38–3.5) |
| HRP2 concentration, mean (range), loge ng/mLb | 5.78 (1.70–8.79) | 8.14 (2.5–10.98) |
| Plasma arginase activity, mean (95% CI), μmol/mL/hb | 0.21 (0.14–0.26) | 0.27 (0.22–0.31) |
| Parasite density, geometric mean (range), parasites/μLb | 13,297 (850–127,350) | 34,811 (125–725,340) |
| Lactate dehydrogenase concentration, mean (95% CI), IU/Lb | 660 (563–757) | 1661 (1439–1884) |
| RH-PAT index, mean (95% CI)b | 1.82 (1.7–2.02) | 1.41 (1.33–1.47) |
| Time from start of antimalarial therapy to physiological testing, mean (95% CI), hb | 4.5 (3–6) | 8 (7–9) |
NOTE. Data are no. (%) of patients, unless otherwise specified. CI, confidence interval; HRP2, histidine-rich protein 2; IQR, interquartile range; RH-PAT, reactive hyperemia peripheral arterial tonometry.
P <.01 (χ2 test comparing patients with severe vs. moderately severe malaria).
P <.01 (2-sided t test comparing patients with severe vs. moderately severe malaria).
Longitudinal course of endothelial function
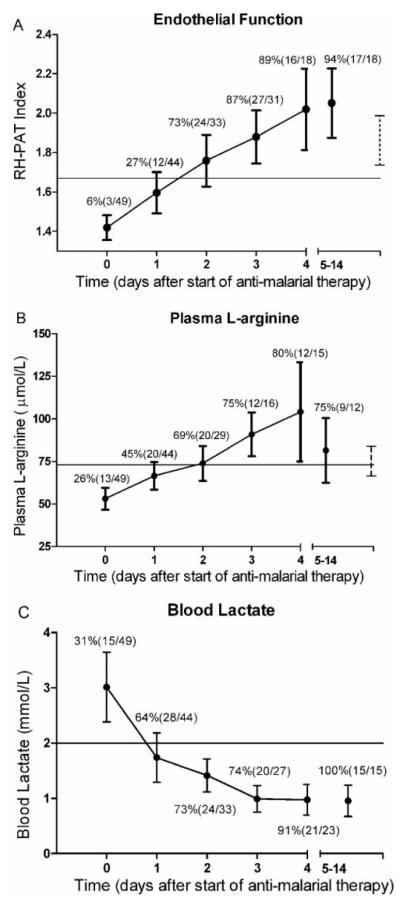
The mean RH-PAT index on enrollment in patients with severe malaria (1.41 [95% confidence interval {CI}, 1.33–1.47]) was significantly lower than that in patients with moderately severe malaria (1.82 [95% CI, 1.70–2.02]) (P <.0001) (table 1). The mean RH-PAT index increased after antimalarial treatment in the severe malaria group, although 3 patients (6%) had normal baseline endothelial function that did not change significantly during the course of their illness. Seven of the 8 patients who had severe malaria with a fatal outcome died before a repeated measurement could be obtained, 5 within 24 h of enrollment and the other 2 within 48 h. The mean baseline RH-PAT index in the patients with fatal severe malaria was not significantly different from that in survivors (P =.66). In the remaining 39 patients with severe malaria and impaired endothelial function on admission, there was a significant improvement in the RH-PAT index during the first 2 days of hospitalization. The mean admission RH-PAT index increased from 1.41 (95% CI, 1.33–1.47) to 1.75 (95% CI, 1.63–1.82) (P <.001) at 48 h (figure 1A), with a mean rate of improvement of 0.17/24 h (95% CI, 0.10–0.21/h). The improvement slowed thereafter, with an increase of 0.09/24 h (95% CI, 0.06–0.13/h) from 48 to 96 h. The proportion of patients with severe malaria with endothelial dysfunction (defined as an RH-PAT index <1.67 [9]) fell from 94% (46/49 patients) on admission to 14% (6/42 patients) before discharge or death (P <.001). Among the 39 patients with repeated RH-PAT index measurements, the median time to achieve normal endothelial function was 49 h (interquartile range [IQR], 20–70 h) after the start of intravenous antimalarial therapy.
Figure 1.
Longitudinal course of reactive hyperemia peripheral arterial tonometry (RH-PAT) indexes (A), plasma L-arginine concentrations (B), and blood lactate concentrations (C) in patients with severe malaria. Mean values (circles) and 95% confidence intervals (CIs) (bars) are displayed for each time point. The X-axis values show time from the start of antimalarial therapy (day 0, 0 –12 h; day 1, 13–36 h; day 2, 37– 60 h; day 3, 61– 84 h; day 4, 85–109 h; and days 5–14, >110 h). A, RH-PAT index. The horizontal line indicates the cutoff defining endothelial dysfunction (RH-PAT index, >1.67). Percentages and ratios indicate proportions of patients with an RH-PAT index >1.67 among all patients tested during that time interval. The dashed vertical line and bars indicate the mean and 95% CIs for a control adult population in Papua, Indonesia [9]. B, Plasma L-arginine concentration. The horizontal line indicates the lower estimate of the half-saturating concentration of extracellular L-arginine concentration for intracellular nitric oxide synthesis (73 μmol/L). Percentages and ratios indicate proportions of patients with plasma L-arginine concentrations >73 μmol/L among all patients tested during that time interval. The dashed line and bars indicate mean and 95% CI for a control adult population in Papua [9]. C, Blood lactate concentration. Percentages and ratios indicate proportions of patients with lactate concentrations <2 mmol/L; the horizontal line indicates the published normal upper limit of lactate concentrations.
In the group with moderately severe malaria, measurements were repeated ~24 h after the start of treatment in 40 of 48 patients, after which most patients were discharged. There were no deaths in this group. The moderately severe malaria group had a baseline mean RH-PAT index above the predefined criterion for endothelial dysfunction (RH-PAT index, <1.67), and there were no further increases during the course of hospitalization. However, in the 33% of patients with moderately severe malaria and a baseline RH-PAT index <1.67 (n = 18), there was an increase during the first 24 h (mean, 0.22/24 h [95% CI, 0.15–0.3]; P =.01).
Longitudinal course of plasma L-arginine concentrations
The patients with severe malaria had a progressive increase in plasma L-arginine concentrations until hospital separation (P <.001) (figure 1B); their L-arginine concentrations increased by 11 μmol/L/24 h (95% CI, 9–13 μmol/L/24 h), from a baseline of 49 μmol/L (95% CI, 43–55 μmol/L/24 h). It took a median of 43 h (IQR, 24–55 h) after the start of antimalarial therapy for plasma L-arginine concentrations to rise above the lower estimate of the Km of extracellular L-arginine for cellular NO production (73 μmol/L) (figure 1B). In the patients with moderately severe malaria, L-arginine concentrations increased by 7 μmol/L/24 h (95% CI, 5–8 μmol/L/24 h; P <.001).
Longitudinal correlates of endothelial function recovery in severe malaria
In a linear mixed-effects model in the severe malaria group, the improvement in RH-PAT index within 48 h was correlated with an increase in plasma L-arginine concentrations (r = 0.56; P =.008) and was inversely correlated with the fall in blood lactate concentrations (figure 1C) (r = −0.44; P =.001) and standard base deficit (r = − 0.31; P =.01). The association with L-arginine and lactate concentrations remained significant after controlling for confounding factors. There was no association between recovery of RH-PAT index and peripheral parasitemia, temperature, pulse rate, Glasgow Coma Score, or blood pressure.
Baseline predictors of recovery of endothelial function and plasma L-arginine concentrations in severe malaria
In multiple linear regression analysis, none of the admission variables predicted the longitudinal course of RH-PAT index during the first 48 h. These included sex, ethnicity, intravenous antimalarial drug used, smoking status, weight, age, baseline plasma L-arginine concentration, parasite biomass, plasma LDH, plasma arginase, lactate, and ICAM-1 concentrations. By means of the same methodology to predict the recovery of plasma L-arginine concentrations, body weight (P =.02) and baseline LDH concentrations (P =.008) were found to be associated with a more rapid increase in plasma L-arginine concentrations, whereas baseline plasma arginase activity was associated with slower recovery (P =.003). After adjustment for multiple testing, LDH and arginase concentrations remained significant.
DISCUSSION
Endothelial function is almost universally impaired in patients with severe malaria [9] but returns to normal in the majority of patients who survive severe malaria within ~48 h after the start of antimalarial therapy. In the present study, L-arginine concentrations in these patients also returned to normal in parallel with the improvement in endothelial function. These observations indicate that the endothelial dysfunction and hypoargininemia seen in severe malaria are reversible processes related to the disease process itself rather than preexisting risk factors for the development of severe disease.
Our previous L-arginine infusion studies in patients with moderately severe malaria showed recovery of endothelial dysfunction after exogenous L-arginine administration [9]. The longitudinal association between recovery of plasma L-arginine concentrations and improvement in endothelial function in severe malaria suggests that endothelial function may also depend on plasma L-arginine concentrations in severe disease. These data provide evidence that the hypoargininemia and impaired NO bioavailability found in both children [10] and adults [9] with severe malaria contribute to endothelial dysfunction and may contribute to disease severity.
The mechanisms leading to endothelial dysfunction are likely to be complex and multifactorial. First, endothelial NO production depends on the intracellular movement of plasma L-arginine by cationic amino acid transporter protein (CAT)–1 [27] with a Km of 100–150 μmol/L [11], which is within the estimated range of the Km of extracellular L-arginine for intracellular NO production (73–150 μmol/L) [12, 13]. In severe malaria, mean pretreatment L-arginine concentrations are considerably lower than this range and are likely to be rate limiting for optimal NO production. This notion is supported by the increase to L-arginine concentrations approaching the lower estimates of the Km during clinical recovery in parallel with the recovery of endothelial function.
Second, in the absence of sufficient L-arginine, endothelial cell NOS types 2 and 3 generate increased amounts of superoxide instead of NO [28]. The resulting NO quenching by superoxide is also likely to contribute to endothelial dysfunction [28]. Finally, impaired endothelial NO production may result from not only an absolute reduction in plasma L-arginine concentrations but also a decrease in the plasma concentration of L-arginine relative to endogenous inhibitors of NOS (e.g., asymmetric dimethylarginine) [29] or from amino acids that compete for intracellular transport by CAT-1 (e.g., symmetric dimethylarginine) [27].
Plasma concentrations of L-arginine, a semiessential amino acid, have been reported to be low in patients with systemic inflammatory response syndrome arising from different causes, including trauma [30] and sepsis [31, 32]. In human sepsis, this finding has been shown to result from increased catabolism and decreased production [32, 33]. In our malaria study, the baseline activity of plasma arginase was associated with a slower rise in plasma L-arginine concentrations after treatment, suggesting that arginase-mediated catabolism of L-arginine contributes to both baseline hypoargininemia and a slower recovery of L-arginine concentrations after the treatment of severe malaria.
In adults with sepsis, endothelial and microvascular dysfunction have been associated with increased lactic acidosis [34] and an increased risk of organ failure and mortality [35, 36]. These complications are thought to result from tissue dysoxia due to a decrease in functional capillary density with compromised oxygen delivery and impaired oxygen utilization [37]. In malaria, mechanical obstruction by cytoadherent parasites is believed to play the major role in microcirculatory compromise [4, 5, 38]. An animal model of malaria has demonstrated reduced functional capillary density [39], suggesting that the vasomotor impairment demonstrated in the present study may also contribute to microcirculatory compromise [38] in severe malaria. The endothelial dysfunction found in this study is a measure of endothelial activation in other vascular beds [6], which likely include the postcapillary venule sites of parasite sequestration. Another possible pathogenic mechanism arising from endothelial dysfunction in severe malaria includes further increases in endothelial adhesion receptor expression, parasite sequestration, microcirculatory obstruction, and compromise of tissue oxygenation. In malaria, increasing lactic acid concentrations and severity of metabolic acidosis are indicators of a poor prognosis [20]. In severe malaria, we found that the improvement in endothelial function was associated with a fall in blood lactate concentrations. This suggests that the recovery of endothelial dysfunction influences the recovery of microvascular perfusion, tissue oxygen delivery, and/or oxygen utilization in severe malaria.
Our study had some limitations. Because of the paucity of repeated examinations in patients who died, we cannot necessarily extrapolate our results to the most severely ill patients. The number of patients who returned after discharge was too small for meaningful analysis of measurements on or after day 5, although the mean values for endothelial function and plasma L-arginine concentrations were within the normal range at discharge.
The South East Asian Quinine Artesunate Malaria Trial demonstrated that case-fatality rates in severe malaria are reduced from 22% with use of quinine to 15% with use of artesunate, a more rapidly parasiticidal antimalarial drug [18]. Although this trial demonstrated an overall mortality benefit with artesunate, there was no difference in mortality between the 2 drugs during the first 48 h of therapy. Additional interventions will be required to reduce mortality rates to <15% for severe malaria, and they will need to target pathogenic mechanisms within this 48-h window. Our data showing that endothelial dysfunction has largely resolved within 48 h in most patients recovering from severe malaria supports the use of adjunctive therapies targeting endothelial dysfunction within this time frame.
Clinical trials of a limited number of adjunctive treatments have not yet demonstrated efficacy in severe malaria [40], but none have specifically targeted the endothelium or the arginine-NO pathway. Results from the present study and our previous safety and efficacy studies of L-arginine therapy for moderately severe malaria [9, 41] provide a rationale for clinical trials of adjunctive treatments, including L-arginine, to improve endothelial NO bioavailability and endothelial function in severe malaria. Potential benefits resulting from improved endothelial NO bioavailability and the reversal of endothelial dysfunction include reduced endothelial activation and superoxide generation, improved oxygen delivery, reduced parasite sequestration and microvascular obstruction, and improved organ perfusion. An animal model of severe malaria has recently shown that increasing NO bioavailability improves survival [42]. Agents with demonstrated clinical ability to improve endothelial function in such chronic diseases as diabetes mellitus and atherosclerosis, including statins, are also available [43], but these have not yet been evaluated in acute malaria.
In summary, recovery of endothelial function and clinically significant hypoargininemia occur in the majority of patients who survive severe malaria within ~48 h after the start of anti-malarial chemotherapy. In severe malaria, the improvement in endothelial function is associated with clinical recovery and an increase in plasma L-arginine concentrations, with the latter likely contributing to the recovery of endothelial function. Recovery of endothelial function is also related to a decrease in blood lactate, a biomarker of improved oxygen delivery and utilization. Agents such as L-arginine that improve endothelial NO production and endothelial function may have a role as adjunctive therapy early during the course of severe malaria.
Acknowledgments
We thank Ferryanto Chalfein, Kim Piera, Prayoga, Youwei Chen, Roesmini, Yoshi Elvi, Betsy Hill, Govert Waramori, and Sri Rahayu for technical and logistical assistance; Marlini Malisan and Margaretha Ferre for nursing assistance; Mitra Masyarakat Hospital staff for clinical support; Mauritz Okeseray, Erna Tresnaningsih, Jeanne Rini, Paul Harijanto, Julie Simpson, and Paulus Sugiarto for support; Joseph McDonnell for statistical advice; and David Sullivan for kindly providing the purified histidine-rich protein 2.
Financial support: National Health and Medical Research Council of Australia (International Collaborative Research Grant [ICRG] 283321 to the study and Practitioner Fellowship to N.M.A.), Wellcome Trust (ICRG GR071614MA to the study and Career Development Award to R.N.P.), Tudor Foundation; Veterans Affairs Research Service; National Institutes of Health (grants AI55982 and AI041764).
Footnotes
Potential conflicts of interest: N.M.A., D.L.G., and J.B.W. are named as inventors on a US patent for the use of L-arginine as treatment for severe malaria but have transferred all their rights to their respective institutional malaria research collaborations. This patent is issued for US rights only, and no rights are being sought in other countries. The authors have no other competing interests.
Presented in part: 56th annual meeting of the American Society of Tropical Medicine and Hygiene, Philadelphia, 4 – 8 November 2007.
References
- 1.Dondorp AM, Pongponratn E, White NJ. Reduced microcirculatory flow in severe falciparum malaria: pathophysiology and electron-microscopic pathology. Acta Trop. 2004;89:309–17. doi: 10.1016/j.actatropica.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–9. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 3.Turner GD, Morrison H, Jones M, et al. An immunohistochemical study of the pathology of fatal malaria: evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol. 1994;145:1057–69. [PMC free article] [PubMed] [Google Scholar]
- 4.MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA. Human cerebral malaria: a quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor TE, Fu WJ, Carr RA, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10:143–5. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- 6.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–95. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 7.Morris CR, Kato GJ, Poljakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anstey NM, Weinberg JB, Hassanali MY, et al. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med. 1996;184:557–67. doi: 10.1084/jem.184.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeo TW, Lampah DA, Gitawati R, et al. Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med. 2007;204:2693–704. doi: 10.1084/jem.20070819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopansri BK, Anstey NM, Weinberg JB, et al. Low plasma arginine concentrations in children with cerebral malaria and decreased nitric oxide production. Lancet. 2003;361:676–8. doi: 10.1016/S0140-6736(03)12564-0. [DOI] [PubMed] [Google Scholar]
- 11.Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iyengar R, Stuehr DJ, Marletta MA. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci USA. 1987;84:6369–73. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granger DL, Hibbs JB, Jr, Perfect JR, Durack DT. Metabolic fate of L-arginine in relation to microbiostatic capability of murine macrophages. J Clin Invest. 1990;85:264–73. doi: 10.1172/JCI114422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratcliff A, Siswantoro H, Kenangalem E, et al. Two fixed-dose artemisinin combinations for drug-resistant falciparum and vivax malaria in Papua, Indonesia: an open-label randomised comparison. Lancet. 2007;369:757–65. doi: 10.1016/S0140-6736(07)60160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–90. [PubMed] [Google Scholar]
- 16.Davis TM, Phuong HL, Ilett KF, et al. Pharmacokinetics and pharmaco-dynamics of intravenous artesunate in severe falciparum malaria. Anti-microb Agents Chemother. 2001;45:181–6. doi: 10.1128/AAC.45.1.181-186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tran TH, Day NP, Nguyen HP, et al. A controlled trial of artemether or quinine in Vietnamese adults with severe falciparum malaria. N Engl J Med. 1996;335:76–83. doi: 10.1056/NEJM199607113350202. [DOI] [PubMed] [Google Scholar]
- 18.The SEAQUAMAT Trial Group. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366:717–25. doi: 10.1016/S0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 19.Yinon D, Lowenstein L, Suraya S, et al. Pre-eclampsia is associated with sleep-disordered breathing and endothelial dysfunction. Eur Respir J. 2006;27:328–33. doi: 10.1183/09031936.06.00010905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day NP, Phu NH, Mai NT, et al. The pathophysiologic and prognostic significance of acidosis in severe adult malaria. Crit Care Med. 2000;28:1833–40. doi: 10.1097/00003246-200006000-00025. [DOI] [PubMed] [Google Scholar]
- 21.van Wandelen C, Cohen SA. Using quaternary high-performance liquid chromatography eluent systems for separating 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate-derivatized amino acid mixtures. J Chromatogr A. 1997;763:11–22. [Google Scholar]
- 22.Strydom DJ, Cohen SA. Comparison of amino acid analyses by phenyl-isothiocyanate and 6–aminoquinolyl-N-hydroxysuccinimidyl carbamate precolumn derivatization. Anal Biochem. 1994;222:19–28. doi: 10.1006/abio.1994.1448. [DOI] [PubMed] [Google Scholar]
- 23.Dondorp AM, Desakorn V, Pongtavornpinyo W, et al. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 2005;2:e204. doi: 10.1371/journal.pmed.0020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–74. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 25.Nohria A, Gerhard-Herman M, Creager MA, et al. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol. 2006;101:545–8. doi: 10.1152/japplphysiol.01285.2005. [DOI] [PubMed] [Google Scholar]
- 26.Bonetti PO, Pumper GM, Higano ST, et al. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–41. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 27.Zani BG, Bohlen HG. Transport of extracellular L-arginine via cationic amino acid transporter is required during in vivo endothelial nitric oxide production. Am J Physiol Heart Circ Physiol. 2005;289:H1381–90. doi: 10.1152/ajpheart.01231.2004. [DOI] [PubMed] [Google Scholar]
- 28.Stuehr D, Pou S, Rosen GM. Oxygen reduction by nitric-oxide synthases. J Biol Chem. 2001;276:14533–6. doi: 10.1074/jbc.R100011200. [DOI] [PubMed] [Google Scholar]
- 29.Cooke JP. ADMA: its role in vascular disease. Vasc Med. 2005;10(Suppl 1):S11–7. doi: 10.1177/1358836X0501000103. [DOI] [PubMed] [Google Scholar]
- 30.Loehe F, Bruns CJ, Nitsch SM, Angele MK. The role of L-arginine following trauma and blood loss. Curr Opin Clin Nutr Metab Care. 2007;10:80–7. doi: 10.1097/MCO.0b013e328011bb1b. [DOI] [PubMed] [Google Scholar]
- 31.Freund H, Atamian S, Holroyde J, Fischer JE. Plasma amino acids as predictors of the severity and outcome of sepsis. Ann Surg. 1979;190:571–6. doi: 10.1097/00000658-197911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villalpando S, Gopal J, Balasubramanyam A, Bandi VP, Guntupalli K, Jahoor F. In vivo arginine production and intravascular nitric oxide synthesis in hypotensive sepsis. Am J Clin Nutr. 2006;84:197–203. doi: 10.1093/ajcn/84.1.197. [DOI] [PubMed] [Google Scholar]
- 33.Argaman Z, Young VR, Noviski N, et al. Arginine and nitric oxide metabolism in critically ill septic pediatric patients. Crit Care Med. 2003;31:591–7. doi: 10.1097/01.CCM.0000050291.37714.74. [DOI] [PubMed] [Google Scholar]
- 34.De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166:98–104. doi: 10.1164/rccm.200109-016oc. [DOI] [PubMed] [Google Scholar]
- 35.Creteur J, Carollo T, Soldati G, et al. The prognostic value of muscle StO2 in septic patients. Intensive Care Med. 2007;33:1549–56. doi: 10.1007/s00134-007-0739-3. [DOI] [PubMed] [Google Scholar]
- 36.Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32:1825–31. doi: 10.1097/01.ccm.0000138558.16257.3f. [DOI] [PubMed] [Google Scholar]
- 37.Trzeciak S, Rivers EP. Clinical manifestations of disordered microcirculatory perfusion in severe sepsis. Crit Care. 2005;9(Suppl 4):S20–6. doi: 10.1186/cc3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dondorp AM, Ince C, Charunwatthana P, et al. Direct in vivo assessment of microcirculatory dysfunction in severe falciparum malaria. J Infect Dis. 2008;197:79–84. doi: 10.1086/523762. [DOI] [PubMed] [Google Scholar]
- 39.Martini J, Gramaglia I, Intaglietta M, van der Heyde HC. Impairment of functional capillary density but not oxygen delivery in the hamster window chamber during severe experimental malaria. Am J Pathol. 2007;170:505–17. doi: 10.2353/ajpath.2007.060433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohanty S, Patel DK, Pati SS, Mishra SK. Adjuvant therapy in cerebral malaria. Indian J Med Res. 2006;124:245–60. [PubMed] [Google Scholar]
- 41.Yeo TW, Lampah DA, Gitawati R, et al. Safety profile of L-arginine infusion in moderately severe falciparum malaria. PLoS One. 2008;3:e2347. doi: 10.1371/journal.pone.0002347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gramaglia I, Sobolewski P, Meays D, et al. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat Med. 2006;12:1417–22. doi: 10.1038/nm1499. [DOI] [PubMed] [Google Scholar]
- 43.Tousoulis D, Antoniades C, Koumallos N, et al. Novel therapies targeting vascular endothelium. Endothelium. 2006;13:411–21. doi: 10.1080/10623320601061714. [DOI] [PubMed] [Google Scholar]