Abstract
Itch, an unpleasant sensation associated with the desire to scratch, is symptomatic of dermatologic and systemic disorders that often resist antihistamine treatment. Histamine-independent itch mediators include serotonin (5-HT) and agonists of the protease-activated receptor-2 (PAR-2). We used behavior, Fos immunohistochemistry, and electrophysiology to investigate if these mediators activate spinal dorsal horn neurons in a manner consistent with itch. Intradermal (id) injection of the PAR-2 agonist SLIGRL-NH2 in the rostral back evoked bouts of directed hindlimb scratches over 20–30 min. Hindpaw injection of SLIGRL-NH2 produced Fos staining in superficial dorsal horn which was then targeted for single-unit recording. Small id microinjections of SLIGRL-NH2 or 5-HT identified responsive single units in the superficial dorsal horn of mice anesthetized with pentobarbital. Thirty-eight units characterized as wide dynamic range, nociceptive specific, or mechanically insensitive exhibited significantly increased firing after id SLIGRL-NH2 for 9 min, to partial (25%) tachyphylaxis with repeated injection. A majority additionally responded to 5-HT (70%), mustard oil (79%), and capsaicin (71%). Seven units isolated with the 5-HT search stimulus exhibited significant and prolonged responses to 5-HT with tachyphylaxis to repeated injections. The majority also responded to SLIGRL-NH2, mustard oil, and capsaicin. The prolonged responses of superficial dorsal horn neurons to SLIGRL-NH2 and 5-HT suggest a role in signaling itch. However, their responsiveness to algogens is inconsistent with itch specificity. Alternatively, such neurons may signal itch, whereas noxious stimulus levels recruit these and a larger population of pruritogen-insensitive cells to signal pain which masks or occludes the itch signal.
Introduction
Chronic itch is a common clinical problem associated with a variety of dermatologic conditions such as atopic dermatitis, as well as kidney and liver disease (Ikoma et al., 2006). Itch is often poorly controlled by antihistamines (Twycross et al., 2003), and there is a pressing need for improved treatments of chronic itch. The protease-activated receptor-2 (PAR-2) has been implicated in pain and inflammation (Cottrell et al., 2003; Dai et al., 2004), as well as itch (Steinhoff et al., 2003). PAR-2 agonists elicit dose-related scratching in mice (Shimada et al., 2006; Ui et al., 2006; Iodi Carstens et al., 2008) consistent with a role for PAR-2 in peripheral itch transduction. PAR-2 may represent a nonhistaminergic mechanism of itch, offering a novel target for development of antipruritic treatments. The bean plant, cowhage, has pods with spicules containing a protease, mucunain, that induces itch without flare when applied to human skin (Johanek et al., 2007) and excites C-polymodal nociceptors (Namer et al., 2008). Mucunain was recently shown to act at PAR-2 and PAR-4 (Reddy et al., 2008). In contrast, histamine, the prototypical itch mediator in human skin, induces a local flare and itch sensation via activation of mechanically insensitive C-fibers (Schmelz et al., 1997), which in turn contact histamine-responsive spinothalamic tract (STT) neurons in the superficial spinal dorsal horn (Andrew and Craig, 2001) that transmit itch signals to higher centers. Furthermore, itch evoked by histamine and cowhage may activate separate subpopulations of primary afferent C-fibers (Namer et al., 2008) and STT neurons (Davidson et al., 2007). Since most behavioral studies of itch mechanisms use mice, we investigated if a PAR-2 agonist activates neurons in superficial laminae of the murine spinal cord dorsal horn in a manner consistent with a role in signaling itch.
Serotonin (5-hydroxytryptamine, 5-HT) is another inflammatory mediator that elicits dose-dependent scratching behavior in rats (Thomsen et al., 2001; Jinks and Carstens, 2002; Nojima and Carstens, 2003a,b; Nojima et al., 2003) and mice (Yamaguchi et al., 1999; Cuellar et al., 2003). Intraplantar injection of 5-HT elicits dose-related and naltrexone-sensitive biting and licking directed to the injection site but not limb scratching (Hagiwara et al., 1999). This suggests that itch in distal extremities is associated with biting, gnawing, or licking, which may substitute for scratching to relieve itch and justifies investigating itch mechanisms in lumbar neurons receiving hindpaw afferents. 5-HT excites rat superficial dorsal horn neurons over a prolonged time course matching scratching behavior, although most units also responded to algogenic stimuli (Jinks and Carstens, 2002). We presently also investigated if 5-HT excites mouse superficial dorsal horn neurons in a manner consistent with itch. We used a chemical search strategy (Jinks and Carstens, 2000, 2002) to increase the likelihood of identifying neurons responsive to the PAR-2 agonist or 5-HT. We also tested their chemical selectivity by determining if they additionally responded to mustard oil and capsaicin, which elicit sensations of burning pain rather than itch. An abstract of parts of this study has appeared previously (Carstens et al., 2008).
Materials and Methods
All experiments were conducted using adult male ICR mice (Harlan) (25–52 g) under a protocol approved by the University of California, Davis Animal Care and Use Committee.
Fos expression.
Mice were anesthetized with sodium pentobarbital (60 mg/kg, i.p.). A unilateral intraplantar microinjection of SLIGRL-NH2 (50 μg in 5 μl of isotonic saline; Bachem) or vehicle (isotonic saline) was made, and the animal was perfused transcardially 2 h later with PBS followed by 4% paraformaldehyde as described previously (Merrill et al., 2006). The lumbosacral spinal cord was removed and postfixed for 8 h, cryoprotected in a 30% sucrose solution, and cut in 50 μm sections. Every third section was washed and blocked in goat serum (3%) and then incubated in primary c-Fos antibody (1:50,000; Arnel Products) for 2 d. Sections were then treated with a secondary biotinylated (goat-anti-rabbit) antibody followed by an avidin–biotin–peroxidase complex reaction enhanced with biotinyl tyramide/H2O2. Immunoreactivity was visualized using a nickel-enhanced diaminobenzidine reaction. Sections were mounted on slides, coverslipped, and examined by a blinded investigator under the light microscope (Nikon E-200). Counts of cells in the superficial dorsal horn expressing Fos-like immunoreactivity (FLI) were averaged for five sections per animal and between-group comparisons made by ANOVA.
Scratching behavior.
Animals were habituated to the Plexiglas recording arena in three daily 1 h sessions. They then received an intradermal (id) injection of SLIGRL-NH2 (50 μg/5 μl in isotonic saline) or vehicle (isotonic saline) in the rostral back and immediately placed in the arena and videotaped for 45 min, as described in a previous study (Cuellar et al., 2003). Tests using the same mouse were conducted at least 1 week apart. Bouts of hindlimb scratching directed toward the injection site were counted in 5 min intervals. Spontaneous scratching was similarly counted in the same animals (no treatment). We used number of scratch bouts, rather than other parameters such as within-bout scratching frequency, because only counts of scratch bouts were significantly correlated with the concentration of the pruritic stimulus (Nojima and Carstens, 2003b). In contrast, the within-bout frequency of individual scratches (∼12 Hz in mice), and duration of scratch bouts (∼1.5–3 s), remained constant across various stimulus conditions and did not correlate significantly with stimulus intensity (Nojima and Carstens, 2003b).
Electrophysiology.
Anesthesia was induced with sodium pentobarbital (60 mg/kg, i.p.) and maintained by constant infusion of pentobarbital through a jugular cannula at a rate sufficient to maintain areflexia. The lumbosacral spinal cord was exposed by laminectomy and the animal fixed in a stereotaxic frame as described previously (Cuellar et al., 2004). A tungsten microelectrode (FHC) was driven into the spinal cord by hydraulic microdrive (David Kopf Instruments) to record extracellular single-unit activity which was amplified, digitized (Powerlab; AD Instruments), and displayed on-line using Chart 5 software (AD Instruments).
A chemical search strategy (Jinks and Carstens, 2000, 2002) was used to isolate units in the superficial dorsal horn. A 30-gauge needle connected to PE 10 tubing filled with either SLIGRL-NH2 (50 μg/μl in saline) or 5-HT (14 mm/μl in saline) was inserted id in the plantar skin. A small volume (∼0.25 μl) was microinjected, and the spinal recording electrode was advanced until a unit exhibiting ongoing firing was isolated. If no unit was isolated, the procedure was repeated at least 10 min later at a different site on the plantar surface, or on the opposite side. When an active unit was isolated, we then waited until firing decreased to zero or a steady low level over a period of at least 10 min, based on the assumptions that (1) the unit had a low level of activity before the search stimulus, (2) ongoing activity was attributable to injection of the search stimulus, and (3) stimulus-evoked activity would eventually decline to close to the presearch level. SLIGRL-NH2 or 5-HT (1 μl) was then reinjected. The 1 μl injection volume was chosen consistent with our previous studies (Carstens, 1997; Jinks and Carstens, 2000, 2002), allowing repeated injections at the same site on the assumption that the small volume is rapidly cleared from the id injection site. Units exhibiting increased firing (>30% above preinjection baseline) were studied further. In most cases, the same chemical (i.e., either SLIGRL-NH2 or 5-HT) was reinjected twice more at 10 min interstimulus intervals to test for tachyphylaxis. Then, the injection needle was removed and replaced with another one for microinjection of a different chemical. In most (n = 54) experiments, units were searched with SLIGRL-NH2 and tested usually with three (but occasionally 2, 4, or 5) successive injections, followed by three successive microinjections of 5-HT. In some experiments (n = 17), 5-HT was used as a search stimulus, and the same stimulus sequence was followed in reverse order.
After completion of testing with SLIGRL-NH2 and 5-HT, unit receptive fields were mapped using mechanical (touch-pressure) stimuli. Since we used a chemical search strategy, it was not possible to assess mechanical or thermal sensitivity before the pruritogen search stimulus was injected. This approach also avoided any potential effects of the physical stimuli on pruritogen-evoked neuronal activity. Units were classified as wide dynamic range (WDR) if they responded to heat and responded at higher firing rate to pinch than light touch. They were classified as nociceptive specific (NS) if they responded to pinch (and often also noxious heat) but not light touch. Two units were mechanically insensitive. An array of additional mechanical, thermal, and chemical stimuli was then delivered. Mechanical stimuli included light brushing with a cotton wisp, pinch with forceps, and graded mechanical indentation with a series of calibrated von Frey filaments (usually 4, 12, and 76 g). Thermal stimuli included brief (10 s) increases in temperature up to 44–54°C, or cooling down to 0°C, from an adapting temperature of 34°C, delivered by a computer-controlled Peltier thermode (Physitemp NTE-2A). Chemical stimuli included isotonic saline (1 μl id; vehicle control), mustard oil (75% in mineral oil, delivered topically in a 2 μl volume), capsaicin (3.3 mm/1 μl), and in a few experiments, histamine (1%/1 μl). Mechanical and thermal stimuli were always tested before other chemical stimuli; mustard oil was usually applied before capsaicin, but in a few experiments, the order was reversed.
Unit action potentials were counted using Chart software. Unit activity was usually quantified as number of action potentials per minute and displayed in peristimulus-time histogram (PSTH) format with 1 s bins. Group responses at 1 min intervals after a given stimulus were compared with activity 1 min before the stimulus by repeated-measures ANOVA (PSS 9.0), with p < 0.05 set as significant.
At the conclusion of recording, an electrolytic lesion was made. The spinal cord was fixed in 10% buffered formalin, and 50 μm sections were cut and mounted on slides for microscopic verification of the lesion site.
Results
Spinal Fos expression
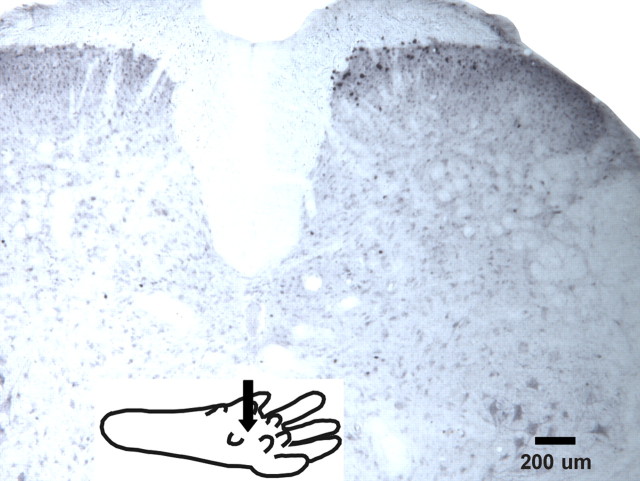
We initially used Fos expression to identify locations of lumbar neurons activated by intraplantar microinjection of the PAR-2 agonist SLIGRL-NH2. Figure 1 shows a typical example of FLI located superficially in laminae I–II. No FLI was observed in the contralateral dorsal horn. Counts of FLI in the ipsilateral superficial dorsal horn after SLIGRL-NH2 were significantly greater compared with vehicle (saline)-treated controls [PAR-2 agonist: mean, 15.28 per section ±2.25 (SEM); saline: 1.1 per section ±0.97, respectively; p < 0.05], the low number of which was similar to that observed in our previous study (Merrill et al., 2006).
Figure 1.
FLI in lumbar superficial dorsal horn after intraplantar microinjection of PAR-2 agonist SLIGRL-NH2. Photomicrograph of lumbar section showing FLI (black nuclei) after intraplantar microinjection of PAR-2 agonist SLIGRL-NH2 (50 μg in 5 μl). Arrow in inset shows injection site on ipsilateral hindpaw.
Scratching behavior
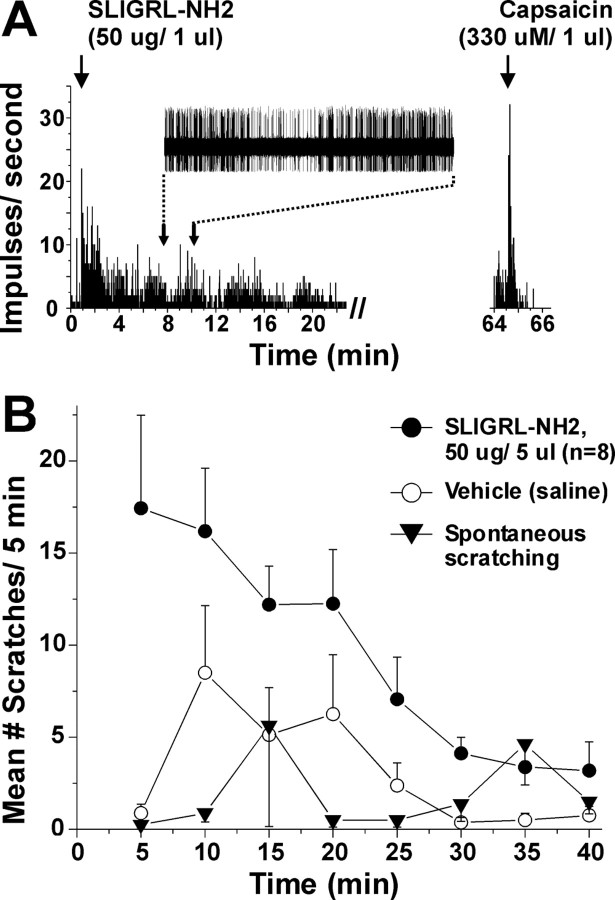
We and others (Shimada et al., 2006; Ui et al., 2006; Iodi Carstens et al., 2008) have shown that the PAR-2 agonist SLIGRL-NH2 elicits dose-related scratching. Figure 2 shows comparable time courses of neuronal (Fig. 2A) and behavioral (Fig. 2B) responses to id injection of SLIGRL-NH2 over a 20 min period. The superficial dorsal horn unit shown in Figure 2A additionally responded to capsaicin (right, PSTH). Figure 2B shows the time course of scratch bouts elicited by id injection of SLIGRL-NH2 (50 μg) into the nape of the neck, along with vehicle-evoked and spontaneous scratching. The total number of SLIGRL-NH2-evoked scratch bouts was significantly greater compared with vehicle or untreated (spontaneous scratching) conditions (p < 0.01; p < 0.05, respectively).
Figure 2.
Excitation of superficial lumbar dorsal horn unit by SLIGRL-NH2 over a time course consistent with scratching. A, PSTH (1 s bins) of superficial lumbar dorsal horn unit's response to intraplantar id microinjection of SLIGRL-NH2 (arrow). PSTH (right) shows same unit's response to id capsaicin. Inset shows train of action potentials. Time axis is aligned with graph of scratching shown in B. Note that unit firing increased after injection and persisted for 20 min. B, Graph plots mean number of scratch bouts versus time. ●: Mice received id microinjection of SLIGRL-NH2 into the nape of the neck at time 0. Bouts of hindlimb scratching directed toward the injection site were counted at 5 min intervals. Scratching peaked at 5 min after injection and persisted for 25 min. Error bars indicate SEM; n = 8. ○: Vehicle (isotonic saline; tested separately in same mice). ▾: Spontaneous scratching in same group of mice. There was a significant difference in mean number of scratch bouts over time between SLIGRL-NH2-evoked and spontaneous scratching (p < 0.05, ANOVA).
Electrophysiology: PAR-2 agonist search
Unit characterization
We then targeted the superficial dorsal horn using a chemical search strategy to identify units responsive to SLIGRL-NH2. Thirty-eight of fifty-four (70%) units responded to a second id injection of SLIGRL-NH2 given more than 10 min later. Of these, 68% were classified as WDR type, 27% as NS, and 5% were mechanically insensitive (Table 1). The majority of units additionally responded to noxious heating, cooling, and other irritant chemical stimuli (Table 1). It should be cautioned, however, that mechanical and thermal responses might have been affected by previous application of the PAR-2 agonist, which was unavoidable using our search strategy. A typical example of a unit responsive to all irritant chemical stimuli is shown in Figure 3. Most unit recording sites were in the superficial dorsal horn based on micrometer depth (mean, 189.1 μm ±24.8 SEM), and histologically identified recordings sites were mainly in lamina I (Fig. 4B, inset).
Table 1.
Incidence of activation of SLIGRL-NH2-responsive units by other stimuli tested
| Brush | Pinch | Heat | Cold | 5-HT | Mustard oil | Capsaicin |
|---|---|---|---|---|---|---|
| 68% (26 of 38) | 95% (36 of 38)a | 78% (28 of 36) | 53% (19 of 36) | 70% (14 of 20) | 79% (23 of 29) | 71% (17 of 24) |
Sixty-eight percent were classified as WDR and 27% as NS. Heat stimulus was 44–54°C over 30 s, cold was 36–0°C over 30 s, by Peltier thermode. Mustard oil (70%) applied topically, 5-HT and capsaicin injected intradermally. Responsive units exhibited >30% increase in spikes per minute after stimulus versus prestimulus baseline.
aTwo units unresponsive to pinch were unresponsive to brush with cotton = mechano-insensitive.
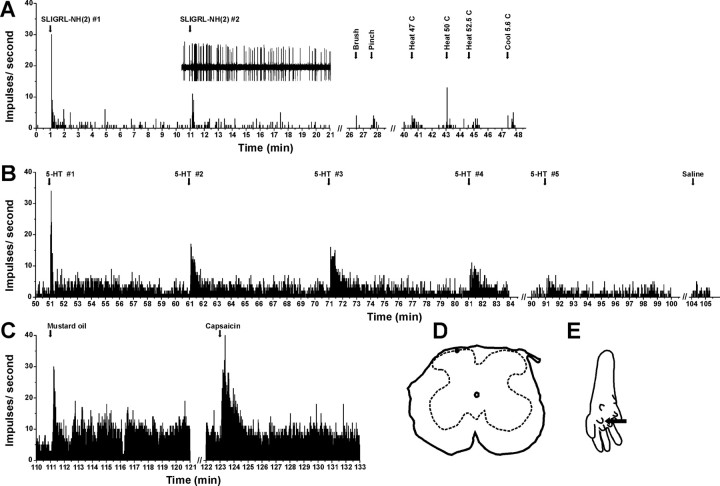
Figure 3.
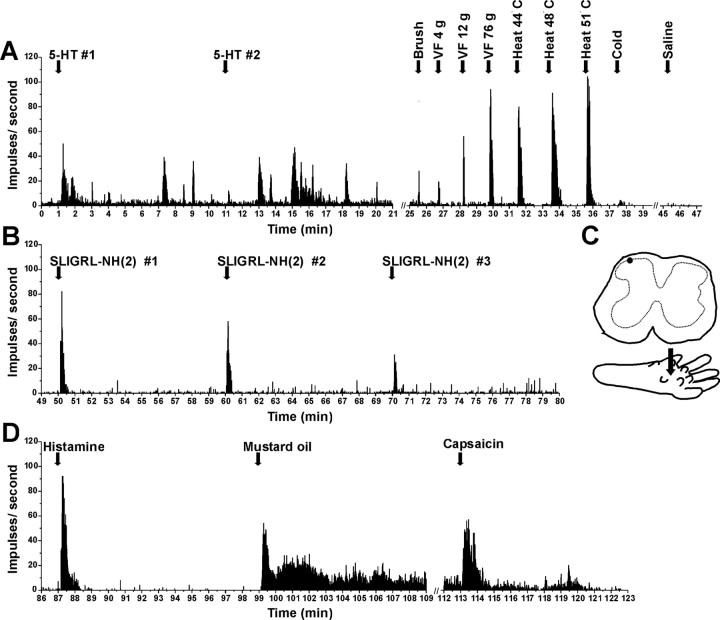
Example of unit identified by SLIGRL-NH2 search stimulus. A, PSTH shows successive responses to id SLIGRL-NH2 (arrows), followed by mechanical and thermal stimuli. Inset, Raw action potentials. B, Continued recording from unit in A shows responses to successive id 5-HT and lack of response to vehicle (saline). C, Responses to mustard oil, capsaicin. D, Recording site in lamina I. E, Arrow shows injection site.
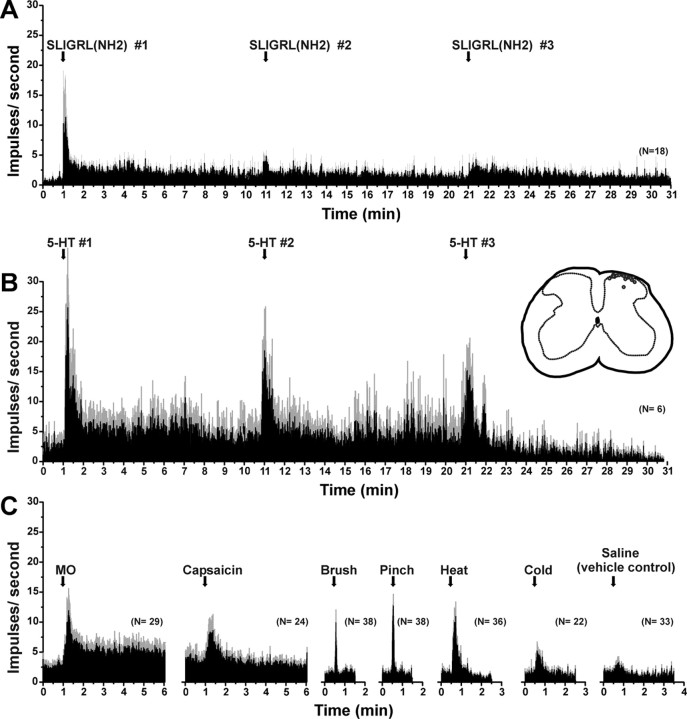
Figure 4.
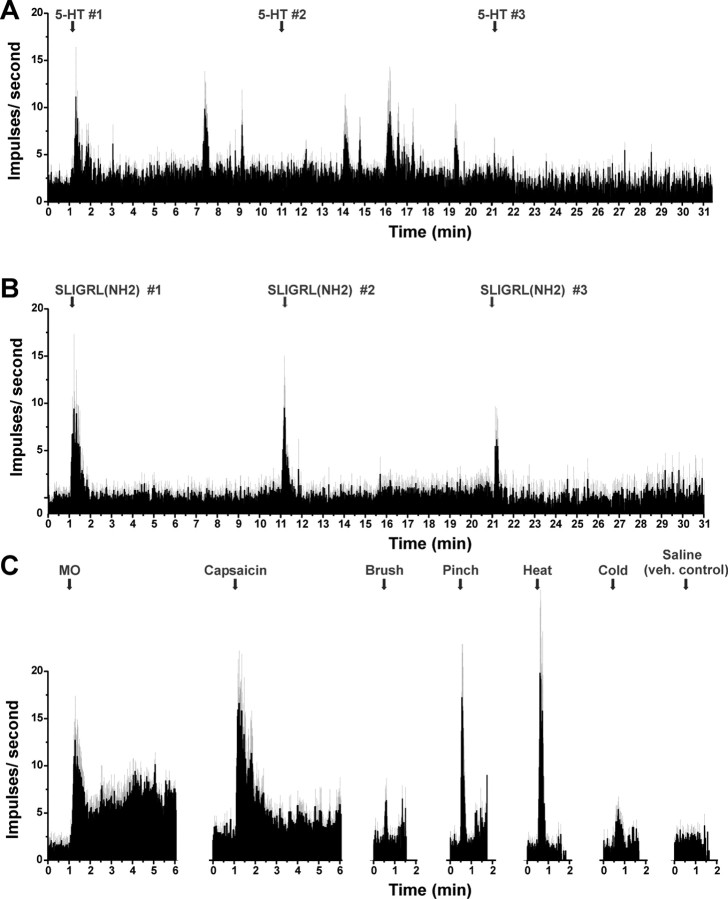
Mean responses to pruritic and algesic stimuli for superficial dorsal horn units isolated using a PAR-2 agonist search strategy. A, PSTH of averaged response of 20 units to three successive id injections of SLIGRL-NH2 (50 μg/1 μl) at 10 min intervals. Error bars (gray) indicate SEM. B, Mean responses of six SLIGRL-sensitive units to three successive injections of 5-HT. Inset to right, Histologically recovered recording sites (○) compiled on midlumbar section. C, PSTHs of averaged responses of SLIGRL-NH2-responsive units to mustard oil (MO), capsaicin, mechanical and thermal stimuli, and id saline (vehicle control).
SLIGRL-NH2
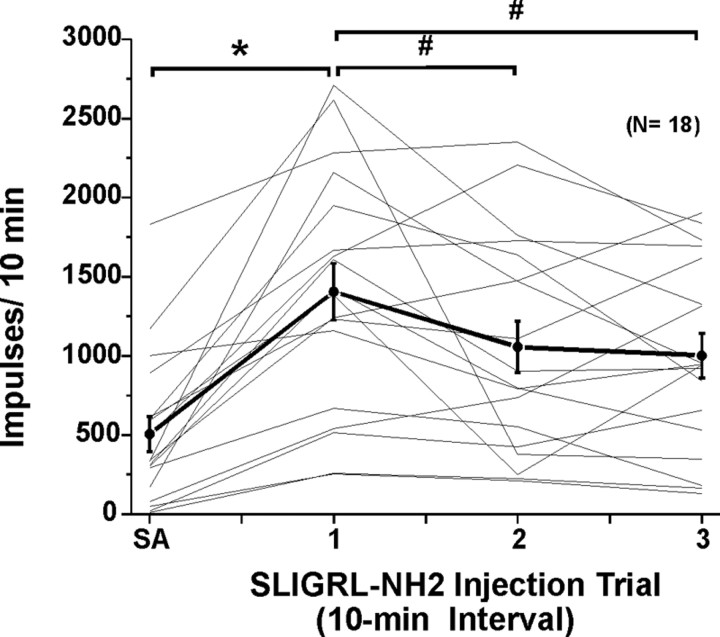
Responses to SLIGRL-NH2 usually consisted of an initial high-frequency component adapting to a steady-state level that sometimes persisted for 10 min or more (Figs. 2A, 3A). Figure 4A shows averaged responses of 18 units to three repeated injections of SLIGRL-NH2. After the first injection, the mean firing rate increased significantly relative to preinjection baseline (F(1,17) = 58.8, p < 0.001) out to 9 min after injection (Fig. 4A). After the second injection of SLIGRL-NH2, mean firing was significantly (p < 0.001) elevated for 5 min (i.e., to minute 16 in Fig. 4A) before returning to baseline. After the third injection, mean firing was also significantly (p < 0.001) elevated for 4 min (i.e., to minute 25 in Fig. 4A). It is also apparent from Figure 4A that the second and third responses to SLIGRL-NH2 were lower compared with the first, indicating tachyphylaxis. This is confirmed in Figure 5, which plots individual (thin lines) and mean (thick line) unit responses to three successive injections of SLIGRL-NH2 at 10 min intervals. Most (but not all) units exhibited a lower response to the second versus first injection, and on average, the second response was significantly lower (by 24.8%) than the first (p < 0.05, paired t test) but did not differ significantly from the third response (Fig. 5).
Figure 5.
Responses of lumbar superficial dorsal horn units to repeated id microinjections of SLIGRL-NH2. Thin lines plot each individual unit's SA (recorded for 1 min × 10) and response to three repeated id microinjections of SLIGRL-NH2 at 10 min intervals. Thick black line connecting dots with error bars (SEM) shows mean of 18 units tested. *Mean response to first injection of SLIGRL-NH2 significantly different from SA (p < 0.05, ANOVA).#Second and third responses to SLIGRL-NH2 significantly different from first (p < 0.05); i.e., tachyphylaxis. Third mean response not significantly different from second; second and third responses significantly different from SA.
5-HT
Seventy percent of the PAR-2 agonist-responsive units tested also responded to id 5-HT (Table 1). Figure 3B shows an individual unit's responses to five repeated injections of 5-HT at 10 min intervals. Responses were characterized by an initial high-frequency discharge followed by a prolonged period of elevated firing, with gradual tachyphylaxis to repeated injections. Figure 4B plots mean responses of six units to repeated 5-HT. After each 5-HT injection, the mean firing per minute was significantly (p < 0.5) elevated for the first minute. Successive responses to 5-HT decreased, indicating tachyphylaxis. When averaged over the 10 min postinjection period, the second 5-HT response was significantly lower than the first (p < 0.001), and the third was significantly lower than the second (p < 0.001).
Other stimuli
The majority of units additionally responded to the other mechanical, thermal, and chemical stimuli tested (Table 1). Figure 4C shows mean responses of SLIGRL-NH2-responsive units to additional stimuli. Both mustard oil and capsaicin elicited significant increases in firing (p < 0.01 and p < 0.05, respectively) during the first minute after injection compared with preinjection baseline. Similarly, brush and pinch stimuli both significantly increase firing averaged over 10 s, and heating and cooling significantly increased firing over 30 s, whereas saline did not significantly affect the firing rate.
5-HT search
Seventeen units were isolated using an id 5-HT search stimulus, and seven (41%) responded to subsequent injection of 5-HT (WDR, six; NS, one). The mean recording depth was 172.8 μm ±36.4 (SEM), and histologically recovered sites were in lamina I (Fig. 4B, inset).
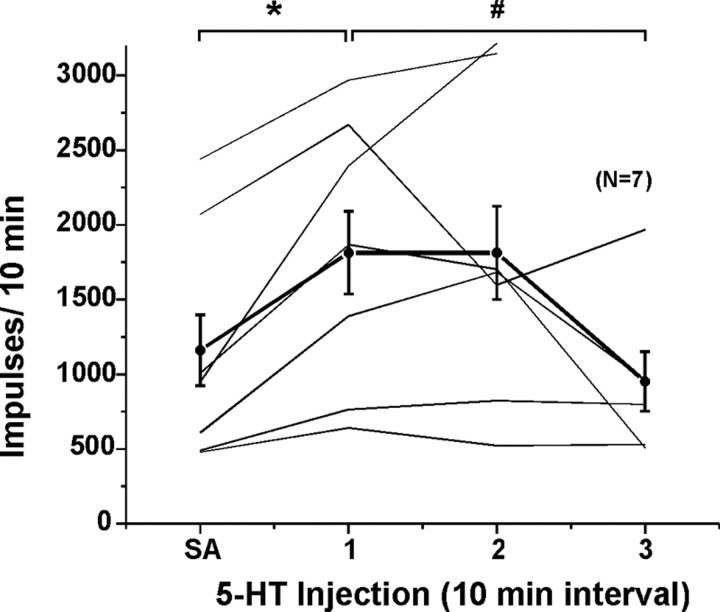
Figure 6A shows a WDR unit that responded to the initial 5-HT with a prolonged and variable increase in firing. It responded weakly to a second injection of 5-HT followed by variable prolonged activity. This WDR unit located in lamina I (Fig. 6C) responded with phasic, decrementing responses to repeated injections of SLIGRL-NH2 (Fig. 6B) and additionally responded to histamine, mustard oil, and capsaicin (Fig. 6D). Figure 7A shows averaged responses of seven units to three successive applications of 5-HT. After the first injection of 5-HT, mean firing per minute was significantly greater for 9 min compared with the pre-5-HT baseline (p < 0.01 for all time points). After the second 5-HT injection, mean firing during the first min (Fig. 7A, min 11–12) differed significantly (p < 0.05) from baseline activity preceding the initial 5-HT injection (minute 0–1) but not from activity during the immediately preceding minute (minute 10–11). This indicates that the increase in firing elicited by the first 5-HT injection had not completely returned to baseline before the second 5-HT injection was made. There was no significant increase in mean firing above baseline at any time after the third 5-HT injection. Figure 8 plots individual (thin lines) and mean (thick line) responses to 5-HT. Mean activity after the first and second 5-HT injections did not differ significantly from each other, with both significantly greater (p < 0.05) than baseline [spontaneous activity (SA)]. Mean activity after the third 5-HT injection was significantly lower (p < 0.05) compared with the first and second 5-HT injections and did not differ significantly from baseline (SA).
Figure 6.
Individual example of responses of lamina I neuron isolated by 5-HT search stimulus (format as in Fig. 3). A, Responses to 5-HT (14 mm/1 μl), mechanical and thermal stimuli, and lack of response to saline. B, Successive responses to SLIGRL-NH2 (same unit as in A). C, Lamina I recording site (top) and intraplantar microinjection site (bottom). D, Responses to id histamine (1%/1 μl), topical mustard oil, and id capsaicin.
Figure 7.
Responses to pruritic and algesic stimuli for superficial dorsal horn units isolated using a 5-HT search strategy (format as in Fig. 4). A, Averaged response of seven units to three successive id injections of 5-HT at 10 min intervals. B, Mean responses of units in A to three successive injections of SLIGRL-NH2. C, Responses of units in A and B to mustard oil, capsaicin, mechanical and thermal stimuli, and id saline [vehicle (veh) control].
Figure 8.
Responses of lumbar superficial dorsal horn units to repeated id microinjections of 5-HT. Format as in Figure 6. *Mean response to first injection of 5-HT significantly different from SA (p < 0.05, ANOVA). #First response to 5-HT significantly different from third (p < 0.05); i.e., tachyphylaxis. Third mean response not significantly different from SA.
Most (80%) of the 5-HT-responsive units also responded to subsequent SLIGRL-NH2 (Fig. 6B). Figure 7B shows mean responses to three successive injections of SLIGRL-NH2. Mean firing per minute significantly increased for 2 min after the first and second injections (p < 0.01) and increased for 1 min after the third injection (p < 0.01), after which it decreased (p < 0.05). When averaged over the 10 min postinjection period, mean responses to the first and second injections of SLIGRL-NH2 were not significantly different, whereas the third response was significantly lower compared with the second (p < 0.001).
Averaged responses to the additional stimuli tested are shown in Figure 7C. All 5-HT-responsive units tested also responded to mustard oil, capsaicin, and noxious heat, and 80% responded to cooling. Evoked responses averaged over 60 s (mustard oil and capsaicin), 10 s (brush and pinch), or 30 s (heat and cool) were all significantly greater (p < 0.05) compared with prestimulus baseline. Saline injection did not have any significant effect.
Discussion
The present study identified populations of WDR and NS neurons in the superficial dorsal horn that exhibited prolonged responses to the PAR-2 agonist SLIGRL-NH2, as well as 5-HT, suggesting a possible role in the transmission of itch. However, the vast majority of these units additionally responded to noxious stimuli including mustard oil and/or capsaicin that are generally associated with burning pain rather than itch. These data are discussed in terms of the potential role of such neurons to signal itch versus pain.
PAR-2
PAR-2 has been implicated in pain and inflammation (Cottrell et al., 2003; Dai et al., 2004), as well as itch (Steinhoff et al., 2003). PAR-2 agonists elicit dose-related scratching in mice (Shimada et al., 2006; Ui et al., 2006; Iodi Carstens et al., 2008), consistent with a role in itch rather than pain, since scratching would tend to enhance pain and thus be avoided. The present data confirm recent studies showing that the PAR-2 agonist SLIGRL-NH2 induces Fos expression in murine superficial dorsal horn neurons (Dai et al., 2004; Nakano et al., 2008) and extend them by showing electrophysiologically that a subpopulation of superficial neurons is excited by this agonist. Neuronal responses continued for 9 min on average and often longer. This contrasts with the shorter duration of capsaicin-evoked responses. However, mustard oil also elicited prolonged neuronal firing (Figs. 3C, 4C, 6D, 7C), mitigating against the argument that itch is signaled by longer-duration (and possibly lower frequency) neuronal firing, as opposed to a shorter-duration (and possibly higher frequency)-firing pattern for pain.
Cowhage, a bean plant whose pods have spicules containing the protease mucunain, induces nonhistaminergic itch in human skin (Johanek et al., 2007). Mucunain was recently shown to act at PAR-2 and -4 (Reddy et al., 2008), and we speculate that cowhage excites peripheral sensory fibers expressing PAR-2 to, in turn, excite superficial dorsal horn neurons that signal itch from cowhage. Recent human microneurography studies indicate the existence of separate populations of histamine- and cowhage-sensitive primary afferent C-fibers. Histamine excites mechanically insensitive C-afferents having widespread receptive fields over a time course matching concomitant itch sensation (Schmelz et al., 1997; Namer et al., 2008), whereas cowhage excites mechanically sensitive C-fiber polymodal nociceptors (Namer et al., 2008). Interestingly, most of the cowhage- and histamine-sensitive fibers were also excited by capsaicin, consistent with our data on spinal neurons. Subpopulations of identified primate STT neurons responded to either histamine or cowhage but not both (Davidson et al., 2007), consistent with the human C-fiber data and nearly all histamine- or cowhage-sensitive STT neurons additionally responded to capsaicin. In monkey, many C-polymodal nociceptors were excited by both histamine and cowhage, whereas relatively few were additionally activated by capsaicin (Johanek et al., 2008). We are currently investigating if histamine and the PAR-2 agonist excite separate populations of mouse superficial dorsal horn neurons. In any event, the bulk of data suggests the existence of separate neural mechanisms and pathways for itch elicited by histamine versus cowhage. Further investigation of these mechanisms is crucial to the rational development of strategies to interfere with itch transmission, particularly for the various types of clinical itch that are resistant to antihistamines.
Responses of monkey C-polymodal nociceptors (Johanek et al., 2008) and STT neurons (Davidson et al., 2007) to repeated application of cowhage did not exhibit significant tachyphylaxis. There was also no significant tachyphylaxis to scratching elicited by repeated injections of SLIGRL-NH2 at a 40 min interval (Iodi Carstens et al., 2008), in partial contrast to the present data showing a significant 25% decrease in the mean response of superficial neurons to a second application of SLIGRL-NH2 (Figs. 4A, 5). If such neurons are involved in itch-related scratching, the 25% change in mean firing may be insufficient to be expressed behaviorally.
5-HT
A minority (41%) of units isolated by the 5-HT search stimulus responded to a second injection of 5-HT. Lack of neuronal response may be attributed to absence of input from 5-HT-sensitive afferents and/or tachyphylaxis. Indeed, neuronal responses exhibited significant tachyphylaxis to repeated 5-HT (Figs. 4B, 7A, 8), and we observed significant tachyphylaxis for behavioral scratching to repeated injection of 5-HT (our unpublished observations). Furthermore, the 5-HT search stimulus may have elicited prolonged activity, since baseline firing was greater for units searched with 5-HT compared with SLIGRL-NH2 (compare SA in Figs. 8, 5). Despite these limitations, several units responded to subsequent 5-HT, indicating that tachyphylaxis was incomplete. In addition, there was no significant cross-tachyphylaxis between 5-HT and SLIGRL-NH2, since a high percentage of neurons isolated by each agent responded to the other one (Table 1, Fig. 7). By the same reasoning, neither 5-HT nor SLIGRL-NH2 exhibited significant cross-tachyphylaxis to mustard oil or capsaicin.
Identification of itch-signaling neurons
A prerequisite for an itch-signaling neuron would be that it responds to the itch mediator over a time course matching that of itch sensation or related scratching behavior. Presently, superficial neurons exhibited significantly increased firing for 9–10 min after id injections of the PAR-2 agonist or 5-HT. However, scratching behavior persisted for 20–30 min after id injection of the PAR-2 agonist (Fig. 2B) or 5-HT (Cuellar et al., 2003). There are several explanations for the briefer duration of neuronal responses versus scratching. (1) Tachyphylaxis of neuronal responses caused by the search stimulus would reduce subsequent responses to the same stimulus. (2) Anesthesia in electrophysiology experiments may have blunted neuronal response duration. (3) Use of a smaller injection volume in electrophysiology versus behavioral experiments. (4) Different injection sites in behavioral (nape of neck) versus electrophysiological (hindpaw) studies. However, hindpaw injection of 5-HT evoked itch-related biting over a 20–30 min period (Hagiwara et al., 1999), similar to that of PAR-2 agonist-evoked scratching observed presently. Conceivably, the spinal neuronal response may initiate itch and scratching, which are subsequently maintained by supraspinal mechanisms, even after spinal neuronal activity wanes. Thus, the present data are consistent with the possibility that pruritigen-responsive superficial dorsal horn neurons contribute to itch and the initiation of scratching.
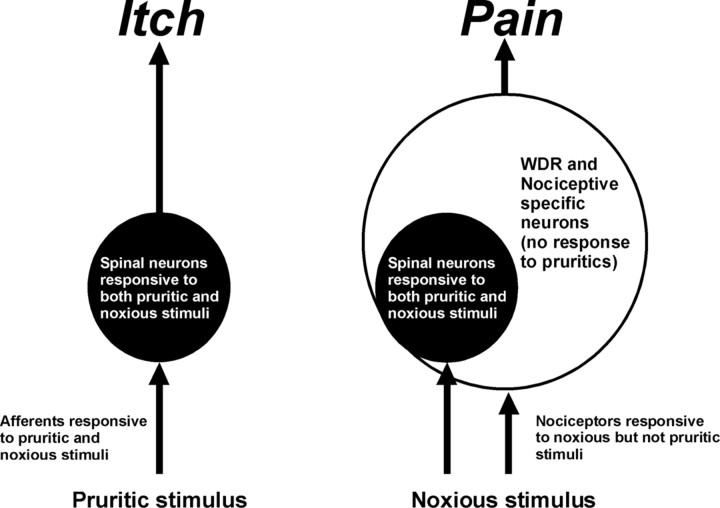
The present search strategy was purposefully biased toward isolating chemosensitive units, allowing the identification of superficial dorsal horn neurons responsive to the PAR-2 agonist and 5-HT. This strategy should reveal neurons selectively responsive to the agent, if they exist. However, we found no neurons that responded exclusively to the PAR-2 agonist or 5-HT. Instead, the vast majority of units additionally responded to mustard oil and/or capsaicin, thus exhibiting broad tuning for algesic and pruritic mediators. This is consistent with our previous study of rat superficial dorsal horn units that responded to 5-HT over a time course consistent with behavioral scratching (Jinks and Carstens, 2002). These units additionally responded to algogens mustard oil and capsaicin (Jinks and Carstens, 2002). Similarly, ∼50% of identified primate STT WDR and high-threshold neurons responded to id histamine but nearly all additionally responded to capsaicin (Simone et al., 2004). A more recent study from the same group found that subpopulations of superficial and deep dorsal horn primate STT neurons responded to histamine or cowhage but not both (Davidson et al., 2007a). In the study of cat lamina I STT neurons that responded to cutaneous application of histamine, two of four units tested also responded to mustard oil (Andrew and Craig, 2001). Thus, regardless of whether units are identified using an unbiased antidromic stimulation approach or a heavily biased chemical search strategy, the available data suggest that neurons selectively responsive to one chemical agent are rare and that the majority of spinal neurons responds nonselectively to multiple pruritic and algesic agents. These findings argue against the dominance of an itch-selective pathway and suggest instead that itch may be signaled (at least partly) by chemically nonselective neurons. A population-coding mechanism consistent with the neurophysiologic data is illustrated in Figure 9. In this scenario, itch is signaled by the activation of subpopulations of spinal WDR and NS neurons responsive to both pruritogens and algogens. Noxious stimulation additionally recruits larger populations of pruritogen-insensitive WDR and NS neurons that signal pain while simultaneously masking or occluding itch sensation.
Figure 9.
Population coding of itch versus pain. Pruritogens excite primary afferent fibers, which in turn excite WDR, NS, and mechano-insensitive neurons in the superficial dorsal horn that transmit itch (left). Noxious stimulation excites nociceptors to recruit a larger population of pruritogen-insensitive spinal WDR and NS neurons that signal pain and simultaneously mask or occlude itch (right).
Pruritogen-evoked responses are also expected to be inhibited by scratching of the receptive field but not by administration of the analgesic morphine which can induce or enhance itch (Carstens, 1997). Consistent with this, scratching inhibited the ongoing activity of monkey STT neurons after id histamine and cowhage (Davidson et al., 2007b). Moreover, systemic morphine did not reduce 5-HT-evoked scratching behavior or spinal Fos expression in rats, although it significantly reduced capsaicin-evoked spinal Fos expression (Nojima et al., 2003). Systemic morphine (1 mg/kg) did not affect, whereas the μ-opioid antagonist naltrexone significantly attenuated, SLIGRL-NH2-evoked scratching behavior in mice (Iodi Carstens et al., 2008). These observations further support a role for PAR-2 agonist- and 5-HT-responsive neurons in the central transmission of itch, and it will be of future interest to determine if such neuronal responses are modulated by opioid agonists and antagonists in a manner consistent with itch.
Footnotes
This work was supported by National Institutes of Health Grant DE013685 and AR057194.
References
- Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci. 2001;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- Carstens E. Responses of rat spinal dorsal horn neurons to intracutaneous microinjection of histamine, capsaicin, and other irritants. J Neurophysiol. 1997;77:2499–2514. doi: 10.1152/jn.1997.77.5.2499. [DOI] [PubMed] [Google Scholar]
- Carstens E, Merrill AW, Akiyama T, Iodi Carstens M. Intradermal PAR-2 agonist excites superficial dorsal horn neurons in the mouse: potential role in itch. Soc Neurosci Abstr. 2008;34 doi: 10.1523/JNEUROSCI.6103-08.2009. 771.10/MM1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell GS, Amadesi S, Schmidlin F, Bunnett N. Protease-activated receptor 2: activation, signalling and function. Biochem Soc Trans. 2003;31:1191–1197. doi: 10.1042/bst0311191. [DOI] [PubMed] [Google Scholar]
- Cuellar JM, Jinks SL, Simons CT, Carstens E. Deletion of the preprotachykinin A gene in mice does not reduce scratching behavior elicited by intradermal serotonin. Neurosci Lett. 2003;339:72–76. doi: 10.1016/s0304-3940(02)01458-1. [DOI] [PubMed] [Google Scholar]
- Cuellar JM, Antognini JF, Carstens E. An in vivo method for recording single unit activity in lumbar spinal cord in mice anesthetized with a volatile anesthetic. Brain Res Brain Res Protoc. 2004;13:126–134. doi: 10.1016/j.brainresprot.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Dai Y, Moriyama T, Higashi T, Togashi K, Kobayashi K, Yamanaka H, Tominaga M, Noguchi K. Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J Neurosci. 2004;24:4293–4299. doi: 10.1523/JNEUROSCI.0454-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ., Jr The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007a;27:10007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ., Jr Responses and modulation of monkey spinothalamic tract neurons to itch-producing and itch-inhibiting stimuli. Acta Derm Venereol. 2007b;87:475. [Google Scholar]
- Hagiwara K, Nojima H, Kuraishi Y. Serotonin-induced biting of the hind paw is itch-related response in mice. Pain Res. 1999;14:53–59. [Google Scholar]
- Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- Iodi Carstens M, Akiyama T, Merrill AW, Zanotto KL, Carstens E. Scratching behavior and Fos expression in superficial dorsal horn elicited by protease-activated receptor agonists and other itch mediators in mice. Soc Neurosci Abstr. 2008;34 doi: 10.1124/jpet.109.152256. 771.11/MM2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks SL, Carstens E. Superficial dorsal horn neurons identified by intracutaneous histamine: chemonociceptive responses and modulation by morphine. J Neurophysiol. 2000;84:616–627. doi: 10.1152/jn.2000.84.2.616. [DOI] [PubMed] [Google Scholar]
- Jinks SL, Carstens E. Responses of superficial dorsal horn neurons to intradermal serotonin and other irritants: comparison with scratching behavior. J Neurophysiol. 2002;87:1280–1289. doi: 10.1152/jn.00431.2001. [DOI] [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, LaMotte RH, Ringkamp M. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci. 2007;27:7490–7497. doi: 10.1523/JNEUROSCI.1249-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, Hartke T, LaMotte RH, Ringkamp M. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci. 2008;28:7659–7669. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill AW, Barter LS, Rudolph U, Eger EI, 2nd, Antognini JF, Carstens MI, Carstens E. Propofol's effects on nociceptive behavior and spinal c-fos expression after intraplantar formalin injection in mice with a mutation in the gamma-aminobutyric acid-type(A) receptor beta3 subunit. Anesth Analg. 2006;103:478–483. doi: 10.1213/01.ane.0000223847.50233.1b. [DOI] [PubMed] [Google Scholar]
- Nakano T, Andoh T, Lee JB, Kuraishi Y. Different dorsal horn neurons responding to histamine and allergic itch stimuli. Neuroreport. 2008;19:723–726. doi: 10.1097/WNR.0b013e3282fdf6c5. [DOI] [PubMed] [Google Scholar]
- Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100:2062–2069. doi: 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima H, Carstens E. 5-Hydroxytryptamine (5-HT)2 receptor involvement in acute 5-HT-evoked scratching but not in allergic pruritus induced by dinitrofluorobenzene in rats. J Pharmacol Exp Ther. 2003a;306:245–252. doi: 10.1124/jpet.103.049239. [DOI] [PubMed] [Google Scholar]
- Nojima H, Carstens E. Quantitative assessment of directed hind limb scratching behavior as a rodent itch model. J Neurosci Methods. 2003b;126:137–143. doi: 10.1016/s0165-0270(03)00074-8. [DOI] [PubMed] [Google Scholar]
- Nojima H, Simons CT, Cuellar JM, Carstens MI, Moore JA, Carstens E. Opioid modulation of scratching and spinal c-fos expression evoked by intradermal serotonin. J Neurosci. 2003;23:10784–10790. doi: 10.1523/JNEUROSCI.23-34-10784.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci. 2008;28:4331–4335. doi: 10.1523/JNEUROSCI.0716-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada SG, Shimada KA, Collins JG. Scratching behavior in mice induced by the proteinase-activated receptor-2 agonist, SLIGRL-NH2. Eur J Pharmacol. 2006;530:281–283. doi: 10.1016/j.ejphar.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Simone DA, Zhang X, Li J, Zhang JM, Honda CN, LaMotte RH, Giesler GJ., Jr Comparison of responses of primate spinothalamic tract neurons to pruritic and algogenic stimuli. J Neurophysiol. 2004;91:213–222. doi: 10.1152/jn.00527.2003. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, Luger TA, Schmelz M. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003;23:6176–6180. doi: 10.1523/JNEUROSCI.23-15-06176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen JS, Petersen MB, Benfeldt E, Jensen SB, Serup J. Scratch induction in the rat by intradermal serotonin: a model for pruritus. Acta Derm Venereol. 2001;81:250–254. doi: 10.1080/00015550152572868. [DOI] [PubMed] [Google Scholar]
- Twycross R, Greaves MW, Handwerker H, Jones EA, Libretto SE, Szepietowski JC, Zylicz Z. Itch: scratching more than the surface. QJM. 2003;96:7–26. doi: 10.1093/qjmed/hcg002. [DOI] [PubMed] [Google Scholar]
- Ui H, Andoh T, Lee JB, Nojima H, Kuraishi Y. Potent pruritogenic action of tryptase mediated by PAR-2 receptor and its involvement in anti-pruritic effect of nafamostat mesilate in mice. Eur J Pharmacol. 2006;530:172–178. doi: 10.1016/j.ejphar.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Nagasawa T, Satoh M, Kuraishi Y. Itch-associated response induced by intradermal serotonin through 5-HT2 receptors in mice. Neurosci Res. 1999;35:77–83. doi: 10.1016/s0168-0102(99)00070-x. [DOI] [PubMed] [Google Scholar]