Summary
The interplay between excitation and inhibition in the auditory cortex is crucial for the processing of acoustic stimuli. However, the precise role that inhibition plays in the distributed cortical encoding of natural vocalizations has not been well studied. We recorded single units (SU) and local field potentials (LFP) in the awake mouse auditory cortex while presenting pup isolation calls to animals that either do (mothers) or do not (virgins) recognize the sounds as behaviorally relevant. In both groups, we observed substantial call-evoked inhibition. However, in mothers this was earlier, longer, stronger, and more stereotyped compared to virgins. This difference was most apparent for recording sites tuned to tone frequencies lower than the pup calls’ high-ultrasonic frequency range. We hypothesize that this auditory cortical inhibitory plasticity improves pup call detection in a relatively specific manner by increasing the contrast between call-evoked responses arising from high-ultrasonic and lateral frequency neural populations.
Introduction
One of the fundamental tasks of the auditory system is to process species-specific communication sounds. In mammals, the auditory cortex is thought to be essential for this (Rauschecker, 1998; Scott and Johnsrude, 2003). Facilitatory combination-sensitivity (Fitzpatrick et al., 1993; Rauschecker et al., 1995; Razak and Fuzessery, 2008; Washington and Kanwal, 2008) and precisely-timed inhibitory input (Razak and Fuzessery, 2006) both help shape excitatory selectivity for calls at the single cortical neuron level. At the population level, anesthetized studies suggest distributed cortical excitation can help improve signal-to-noise for downstream processing (Liu and Schreiner, 2007; Medvedev and Kanwal, 2004; Wallace et al., 2005; Wang et al., 1995). However, this picture ignores a possible role for call-evoked inhibition at the population level – an issue that has been overlooked in most vocalization studies. We address that here in an awake mouse preparation using SU and LFP recordings to reveal the widespread presence, plasticity and potential coding function of a purely inhibited cortical response to species-specific communication calls.
We took advantage of a previously described ultrasonic communication system between mouse pups and adult females (Liu et al., 2006; Liu et al., 2003; Liu and Schreiner, 2007). Ultrasonic whistles emitted by mouse pups are recognized as behaviorally-relevant by mothers, but not by pup-naïve virgins (Ehret, 2005; Ehret et al., 1987). Multi-neuronal unit (MU) correlates for this behavioral distinction have been found as early as auditory cortex, but only in anesthetized animals (Liu et al., 2006; Liu and Schreiner, 2007). Here, we report on electrophysiological recordings in the auditory cortices of fully awake, head-restrained mice for the first time. We focused on how neural responses could contribute to the collective detection of a class of natural pup calls, by contrasting pooled responses to all calls between virgins and mothers. We found that communication sounds can generally excite as well as purely inhibit cortical spiking. Comparing animal groups, pooling the various forms of evoked excitation did not reveal a significant response difference during the calls. However, the timing and strength of call-evoked inhibition was systematically altered in mothers – particularly for the frequency band lateral to the ~60–80 kHz frequency of the pup whistles. We suggest this lateral band inhibitory plasticity as a mechanism to enhance the signal-to-noise in the neural population representation of a pup call, consequently improving the downstream detection of calls.
Results
In order to investigate cortical responses to communication sounds in conscious animals, we developed a head-restrained, awake electrophysiology preparation for mice (see Experimental Procedures). We targeted recording locations using a stereotaxically-laid grid of holes over auditory cortex. SUs and LFPs were first characterized by their traditional responses to tones (Fig. 1, bottom row). We classified SUs as tone-excited or -inhibited depending on whether spiking increased or only decreased following tone presentation, respectively (see Supplemental Information Fig. S2); some SUs were tone-nonresponsive or not isolated during tone stimulation (Table 1). A best frequency (BF) was selected for each tone-excited SU by finding the frequency eliciting the greatest spike rate in a window around its peristimulus time histogram (PSTH) peak. A BF for each LFP site was determined similarly based on the largest average negative deflection within the first 100 ms after tone onset. In our data set, mothers and virgins were mostly similar in their distributions of both SU and LFP BFs (see Supplemental Information and Fig. S3). Recording sites were then similarly characterized by their responsiveness to a pool of 18 different pup ultrasounds (Fig. S1) as pup call-excited (Fig. 1A and B, S2O and P), -inhibited only (Fig. 1C, S2M and N), or -nonresponsive (Fig. S2Q and R).
Figure 1. Typical SU and LFP recordings.
Three examples of different SU and LFP pairs co-recorded off the same electrode in response to pup calls and tones demonstrate the quality of our recordings.
(A–C top row) Waveforms of all spikes (left) and inter-spike interval distributions (right) for pup call stimulation. Note the absence of refractory spikes within 1ms.
(A–C middle row) Responses to pup calls. Trial-by-trial SU spike raster (upper) and 10 ms-binned PSTH (middle) for call-excited (A and B) and -inhibited SUs. The mean call-evoked LFP responses (bottom) at the same site are also shown. Stimuli presented during the interval denoted by the horizontal black bar.
(A–C bottom row) Tonal tuning curves for SU spike count (solid black line, left axis) and the amplitude of the negative LFP deflection (dotted black line, right axis. Correlation coefficients (CC) between the full frequency tuning curves for SU and LFP varied from −0.58 to 0.95 over our tone-excited population of SUs, suggesting these neural signals do not reflect the same processes.
| Mothers | Virgins | |
|---|---|---|
| Total Recorded SU’s | 47 | 39 |
| Pup Call-Responsive | 35/47 (74%) | 21/39 (54%) |
| No Tone Data | 9 | 3 |
| & Pup Call-Excited | 4 | 0 |
| & Pup Call-Inhibited | 3 | 0 |
| & Pup Call-Nonresponsive | 2 | 3 |
| Tone-Responsive | 32/38 | 35/36 |
| Tone-Excited | 18/32 | 26/35 |
| & Pup Call-Excited | 12/18 (66%) | 8/26 (31%) |
| & Pup Call-Inhibited | 1/18 | 6/26 |
| & Pup Call-Nonresponsive | 5/18 | 12/26 |
| Tone-Inhibited | 14/32 | 9/35 |
| & Pup Call-Excited | 2/14 | 2/9 |
| & Pup Call-Inhibited | 10/14 (71%) | 5/9 (56%) |
| & Pup Call-Nonresponsive | 2/14 | 2/9 |
| Tone-Nonresponsive | 6/38 | 1/36 |
| & Pup Call-Excited | 0 | 0 |
| & Pup Call-Inhibited | 3 | 0 |
| & Pup Call-Nonresponsive | 3 | 1 |
Plasticity in SU Responses
We found a larger proportion of SUs in mothers (35/47) compared to virgins (21/39) that were either excited or inhibited by pup calls (z-test, z=1.81, p<0.05, 1-tailed). In both animal groups though, about equal proportions of these responsive SUs showed either pure inhibition or some form of excitation (18 excited vs. 17 inhibited in mothers, z-test, z=0, p>0.05, 2-tailed; 10 vs. 11 in virgins, z-test, z=0, p>0.05, 2-tailed), indicating a previously under-reported prevalence of communication call-evoked cortical inhibition (Table 1).
Focusing first on excited responses, we found a significantly higher proportion of tone-excited SUs in mothers (12/18) compared to virgins (8/26) which were also pup-excited (z-test, z=2.04, p<0.05, 2-tailed). Furthermore, in previous anesthetized MU studies, nearly all excitation had occurred near sound onset, and mothers showed better temporal alignment across BF ranges than virgins (Liu and Schreiner, 2007). By contrast, we now found that latencies to the excitation onset, maximum or half-maximum (Fig. 2A1) varied over a wide range of times. This reflected the many different ways by which excitation occurred in the awake animal, such as transient onsets and delayed offsets. Therefore, the distributions of these excitation latencies and durations (Fig. 2A2) overlapped between the two animal groups. Meanwhile, the magnitude of the time-dependent, population-averaged excitatory spike rate across all calls was higher in mothers, although this was not significant (note the large error bars in Fig. 2A3). When we computed the strength of SU excitation by integrating the normalized spike rate over the stimulus period (Fig. 2A3 Inset), differences between mothers and virgins were not significant. Although we did not find significant changes in the latency, duration, and strength of the pooled cortical excitation in awake animals, we cannot exclude the possibility that our methods may have failed to uncover more subtle changes in excitation.
Figure 2. Plasticity in SU responses.
Pure SU inhibition, but not excitation, evoked by calls was systematically earlier, longer, and stronger in mothers compared to virgins.
(A1 and B1) Examples of raw (gray bars, 5 ms bins) and Gaussian-smoothed (black line) PSTH’s for a call-excited and -inhibited SU. The stars indicate the half-max or half-min values for calculating the excitation or inhibition latency and duration.
(A2 and B2 scatter plot) Call-excited (upward triangles) and -inhibited (downward triangles) latencies and durations for mothers (black in this and other figures) and virgins (gray in this and other figures). Stars refer to SUs depicted in A1 and B1. Excited Nmothers=16, Nvirgins=10. Inhibited Nmothers=17, Nvirgins=11.
(A2 and B2 bottom box plots) Group comparison of call-evoked excitatory and inhibitory latencies. For this and later figures, box plots show lines at the lower quartile, median and upper quartile, and whiskers extending out to extreme data points that are not outliers. The difference between mothers and virgins for call-excited SUs was not significant (Mann-Whitney, U=80, Nmothers=16, Nvirgins=10, p>0.05, 2-tailed), but was significant for call-inhibited SUs (t-test, t=2.9, df=26, p<0.01). For this and later figures, n.s./asterisk indicates a non-significant/significant comparison.
(A2 and B2 right box plots) Group comparison of call-evoked excitatory and inhibitory durations. Mothers and virgins were not significantly different for call-excited SUs (t-test, t=.54, df=24, p>0.05), but were for call-inhibited SUs (t-test, t=4.7, df=26, p<0.0001).
(A3 and B3) Population-averaged time course of spike rate normalized by the spontaneous rate, for call-excited and -inhibited SUs. In this and later figures, the gray rectangles marked by asterisks denote regions where significant differences (anovan, followed by protected multiple t-test comparisons, p<0.05) were found between traces, and the error bars represent standard errors. Significant differences occurred only for call-inhibited responses. Dotted lines represent the baseline spontaneous rate. Insets show the normalized rate derived by integrating the SU spike count over the stimulus period and dividing by the spontaneous level. Call-excited SUs were not different between mothers and virgins (Mann-Whitney, U=51, Nmothers=16, Nvirgins=10, p>0.05, 2-tailed), but call-inhibited SUs were significantly different (t-test, t=2.8, df=26, p<0.01, 2-tailed).
Turning to inhibited cells, the time course of responses was more stereotyped, in contrast to excited cells. The proportion of call-inhibited SUs in mothers compared to virgins was higher but not significantly so, whether all SUs (20/47 for mothers, 11/39 for virgins, z-test, z=1.2, p>0.05, 2-tailed) or only tone-inhibited SUs (10/14 for mothers, 5/9 for virgins, z-test, z=0.33, p>0.05, 2-tailed) were considered. More importantly, unlike excitation, the latency to the half-minimum point in each call-inhibited SU PSTH (Fig. 2B1) was significantly shorter, and the duration of inhibition was significantly longer, so that mothers and virgins occupied distinct regions in the latency-duration plane (Fig. 2B2). Comparing the magnitude of inhibition, we found that the inhibition was significantly deeper in mothers on a time point by time point basis (Fig. 2B3). Integrated over the duration of the stimulus, call-evoked SU inhibition resulted in a significantly lower normalized firing rate in mothers (Fig. 2B3, inset). Thus, in the awake mouse, pooled inhibition of cortical spiking by the class of pup calls was systematically earlier, longer and stronger in mothers compared to virgins.
What function might these changes have, and how specific are they for processing pup calls? Because our SU data alone did not permit us to fully address these questions, as explained below, we turned next to analyzing the LFP. This allowed us to both corroborate and extend our evidence for functionally-relevant inhibitory plasticity.
Plasticity in LFP Responses
What causes a neuron to be inhibited or excited depends on the nature of its inputs from other neurons. To monitor this, we used the LFP, which is sensitive to the slow currents generated at excitatory and inhibitory synapses (Lopes da Silva and Kamp, 1987), and to spiking afterpotentials from neurons across this network (Logothetis, 2003). While distant, synchronous currents contribute to this signal, a recent study suggests that local contributions within ~250 um are dominant (Katzner et al., 2009). In principle, such local currents could be different around SUs that are being inhibited versus excited, not least of all because of the absence of the SUs own spiking in the former case. Indeed, simultaneous in vivo intracellular and extracellular recordings have shown that a SUs membrane potential often mirrors the LFP, so that depolarizations (hyperpolarizations) co-occur with relative negativities (positivities) in the extracellular potential (Kaur et al., 2005; Poulet and Petersen, 2008).
Given this possibility, we separately examined LFPs depending on whether a co-recorded SU was excited or inhibited by calls, in case the local network supporting each response type changed in a systematic way that would be reflected in the LFP. For this limited purpose, we focused only on the wide-band LFP (up to 100 Hz), rather than consider specific spectral bands (e.g. theta-band) individually (Galindo-Leon and Liu, in preparation). We used standard time domain methods (see Experimental Procedures and Supplemental Information) to generate the Hilbert phase time-series for each trial’s LFP signal. This describes when various shape features in a signal – such as local minima (~π), maxima (~0 and 2π) and zero-crossings (~0.5π and 1.5π) – occur.
Using this analysis, we found that Hilbert phases corresponding to points near the valley of the LFP (just after π up to just before 1.25π) occurred significantly earlier in mothers (Fig. 3A1 and B1), but only at call-inhibited SU sites. If upward and downward fluctuations of the LFP signal represent periods of relative hyperpolarization and depolarization, respectively (Haslinger et al., 2006; Kaur et al., 2005), then this would indicate that the delay to when the extracellular potential around an inhibited SU begins hyperpolarizing must occur earlier in mothers (Fig. 3A2 and B2), consistent with the SU itself being inhibited earlier (Fig. 2B2). Hence, plasticity in the mean shape of the call-evoked LFP around inhibited cortical SUs corroborates our SU finding that average features of call-evoked cortical inhibition are significantly altered in mothers.
Figure 3. Plasticity in the shape of the call-evoked LFP.
The timing of when the call-evoked LFP reached specific phases was significantly different between mothers and virgins for sites around call-inhibited but not -excited SUs.
(A1 and B1 box plots) Group comparison of the times at which the Hilbert phase of a site’s average call-evoked LFP reached values of π and 1.16π for call-excited and -inhibited SU sites. These times were not different between mothers and virgins for call-excited sites (Mann-Whitney, Nmothers=16, Nvirgins=10, π: U=56, p>0.05, 1.16π: U=52, p>0.05, 2-tailed), but were for phases between π and 1.25π at call-inhibited sites (Mann-Whitney, Nmothers=14, Nvirgins=11, π: U=45, p>0.05, 1.16π: U=30, p<0.05, 2-tailed). These phases corresponded to the initial rise from the minimum of the LFP. Note: Sample sizes for LFPs may be less than or equal to that of SUs if more than one SU was isolated at a site.
(A2 and B2) Comparison of the population-averaged LFP for all call-excited or -inhibited sites. The location of π (circle) and 1.25π (square) Hilbert phase values are marked. Notice that the timing difference in the Hilbert phase seen in (B1) manifests as a sizeable shift in the timing of the valley between mothers and virgins.
(C) Variability in the LFP shape depicted as a time-dependent probability histogram of trial-by-trial Hilbert phases. Whiter (Darker) colors indicate higher (lower) probabilities for a specific phase at a specific time.
Besides these changes in mean LFP activity, we also discovered plasticity in the call-evoked variability of the local network activity around call-inhibited SUs. To characterize variability, we constructed at each recording site a time-dependent histogram of the Hilbert phase trajectories across the different trials of all the calls (Fig. 3C). Before stimulus onset, the instantaneous Hilbert phase was essentially random. However, shortly after the onset of the calls, the Hilbert phase began concentrating near 0.5π to π, corresponding to the descent of the LFP toward its valley. The Hilbert phase distribution then became very sharp, and eventually widened back to a uniform distribution. We quantified the trial-by-trial variability of this local network response by a phase precision measure indicating how well aligned the instantaneous Hilbert phases from different trials were: a value of 1 at a particular time implies that all trials had exactly the same phase, while randomly distributed phases would yield a value of 0. Examples of the LFP phase precision at call-excited and -inhibited sites are shown in the upper panels of Fig. 4A1 and B1, respectively. In general, call onset reliably reset the wide-band LFPs Hilbert phase and drove a rapid increase in the precision of the local network response at each site. Over time, this phase drifted as intrinsic, non-stimulus-locked fluctuations began dominating the signal again.
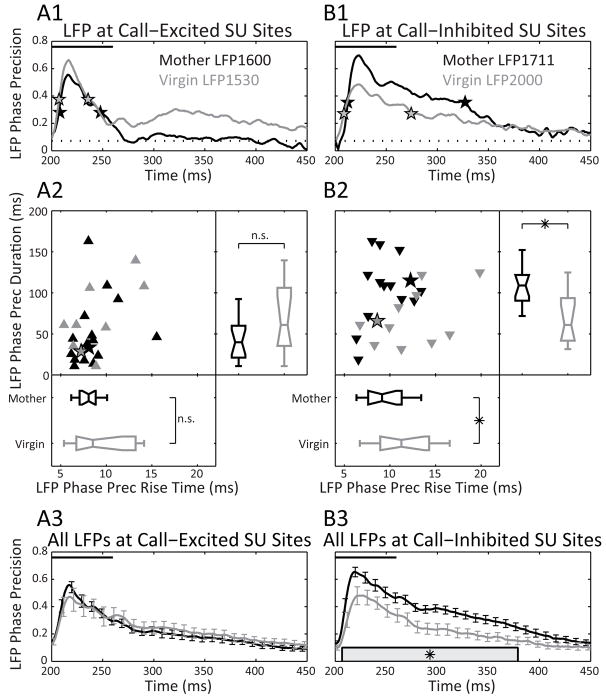
Figure 4. Plasticity in the LFP phase precision.
Mothers have an earlier, longer, and greater LFP phase precision at call-inhibited but not-excited SU sites.
(A1 and B1) Example LFP phase precision trajectories and their rise times and durations (stars) for both a call-excited and -inhibited site in a mother and virgin. Phase precision values lying above the dashed line are significant (see Experimental Procedures). (A2 and B2 scatter plot) Rise times and durations of the LFP phase precision at call-excited (upward triangles) and -inhibited SU sites (downward triangles) for mothers and virgins. Excited Nmothers=16, Nvirgins=10. Inhibited Nmothers=14, Nvirgins=11.
(A2 and B2 bottom box plots) Group comparison of call-evoked LFP phase precision rise times. Differences between mothers and virgins were not significant at call-excited SU sites (Mann-Whitney, U=70, Nmothers=16, Nvirgins=10, p>0.05, 2-tailed), but were significant at call-inhibited SU sites (t-test, t=2.0, df=23, p=0.05, 2-tailed).
(A2 and B2 right box plots) Group comparison of call-evoked LFP phase precision durations. Differences between mothers and virgins were not significant at call-excited SU sites (Mann-Whitney, U=54, Nmothers=16, Nvirgins=10, p>0.05, 2-tailed), but were significant at call-inhibited SU sites (t-test, t=2.3, df=23, p<0.05, 2-tailed).
(A3 and B3) Population-averaged phase precision trajectories. LFPs from call-excited SU sites did not show a significant difference between mothers and virgins. LFPs from call-inhibited sites in mothers had a significantly higher phase precision trajectory than virgins beginning near sound onset until more than 100 ms after sound offset.
In order to detect systematic differences in local network variability, we compared the rise times, durations, and the population-averaged phase precision time courses between animal groups. Mirroring the results found when comparing SU firing between mothers and virgins, the LFPs at call-excited SU sites (Fig. 4A2 and A3) were not different in any of these measures. In contrast, the LFP phase precision at call-inhibited sites in mothers rose significantly faster, and stayed higher for longer, even beyond the duration of the pup call stimulus (Fig. 4B2 and B3). Thus, the local network near call-inhibited SUs in mothers responded trial-by-trial with earlier and more stereotyped activity than in virgins.
Furthermore, the LFP phase precision at call-inhibited SU sites in mothers increased even beyond the level of precision at call-excited sites. Although there was no difference in the rise time for phase precision between call-excited and -inhibited sites in mothers, the precision became greater for inhibited sites after about 18 ms, and stayed higher until 190 ms after stimulus onset (comparison not illustrated). This result indicates that local network level changes between virgins and mothers consistently increased the precision of presumed synaptic and membrane currents associated with inhibiting cortical neurons. In fact, on a site-by-site basis, we found that the stronger the SU inhibition, as measured by a lower normalized spike rate averaged across all calls (integrated over the maximum call duration), the greater the peak LFP phase precision (corrcoef, cc=−0.55, df=26, p<0.005, 2-tailed). This did not occur for excitation (cc=0.33, df=24, p>0.05, 2-tailed). Thus, earlier, longer and deeper inhibition of SU spiking in mothers correlates with more rapid, sustained and higher precision in the LFP.
Plasticity Dominated by Laterally-Tuned Sites
The SU and LFP data both suggest significant changes in the nature of call-evoked inhibition in the mother’s auditory cortex. Is this plasticity globally distributed, or might changes in inhibition between virgins and mothers depend on the specificity of a recording site’s tuning to the frequencies in pup calls? We addressed this by separating our SUs and LFPs at call-excited and -inhibited sites depending on the LFP BF. Sites with LFP BF<50 kHz (lateral band) nevertheless responded to high-ultrasonic calls presented at moderate sound levels. These sites showed a significant difference in the degree of call-evoked SU inhibition between mothers and virgins (Fig. 5B1 Inset). In parallel, there were large differences in the strength of the phase precision for LFPs recorded around call-inhibited SUs (Fig. 5B1). Differences were not apparent for call-excited SUs (Fig. 5A1 and Inset).
Figure 5. Plasticity from call-inhibited sites tuned to lateral frequencies.
Significant differences in LFP phase precision mainly arose from call-inhibited sites with LFP BF<50 kHz. SUs from these same lateral band sites had greater call-evoked inhibition in mothers than in virgins.
(A1 and A2) Population-averaged phase precision trajectories for LFPs at call-excited SU sites, grouped by LFP BF. No significant differences in trajectories were found between mothers and virgins for either lateral (A1) or high-ultrasonic (A2) band sites. Similarly, differences in the normalized, integrated SU firing (see Insets of Fig. 2A3 and B3) did not reach significance for either the lateral (t-test, t=.98, df=10, p>0.05, 2-tailed) or the high-ultrasonic band (Mann-Whitney, U=16, Nmothers=9, Nvirgins=5, p>0.05, 2-tailed).
(B1 and B2) Population-averaged phase precision trajectories for LFPs at call-inhibited SU sites, grouped by LFP BF. The trajectory for mothers was significantly higher than virgins at lateral (B1) but not high-ultrasonic (B2) band sites. Similarly, differences in the normalized, integrated SU firing were significant for the lateral (t-test, t=2.3, df=13, p<0.05, 2-tailed) but not the high-ultrasonic band (t-test, t=1.7, df=11, p>0.05, 2-tailed; Nmothers=9, Nvirgins=4).
(A3 and B3) Group comparison of call-evoked LFP phase precision rise times for lateral (hatched) and high-ultrasonic (solid) bands. No differences between mothers and virgins were found at call-excited sites for both the lateral (Mann-Whitney, U=12, Nmothers=7, Nvirgins=5, p>0.05, 2-tailed) and high-ultrasonic bands (t-test, t=1.6, df=12, p>0.05, 2-tailed). However, there was a significant difference at call-inhibited sites in the lateral (t-test, t=2.3, df=12, p<0.05, 2-tailed), but not high-ultrasonic band (t-test, t=0.05, df=9, p>0.05, 2-tailed). When comparing across frequency ranges within the same group, there was no significant differences between the LFP phase precision rise times for mothers for excited (Mann-Whitney, U=12, NBF>50kHz=9, NBF<50kHz=7, p>0.05, 2-tailed) or inhibited (t-test, t=1.7, df=12, p>0.05, 2-tailed). However, the virgins showed differences between LFP sites with BF<50 kHz and BF>50 kHz for both excited (t-test, t=2.5, df=8, p<0.05, 2-tailed) and inhibited (t-test, t=3.0, df=9, p<0.05, 2-tailed) SUs.
In fact, the lateral BF range was mainly responsible for the population differences in normalized SU firing (Fig. 2A3 and B3, Insets) and LFP phase precision (Fig. 4A3 and B3). When we compared LFP sites tuned to high-ultrasonic frequencies (BF>50 kHz), the differences in SUs and LFPs at both call-excited and -inhibited SU sites (Fig. 5A2 and B2) were not significant. For the call-excited SU responses, the median SU normalized firing rate was higher in mothers, but not significantly so. Mirroring the SU results, the LFP phase precision at call-excited and -inhibited sites also did not show significant differences between mothers and virgins. Thus, using both SUs and LFPs, we conclude that there is a robust plasticity in call-evoked inhibition within auditory cortical regions tuned to frequencies lower than the high-ultrasonic frequencies where pup calls are found.
The LFP plasticity may partially reflect a change in the reliability of feed-forward inputs into the lateral band. This conclusion is based on how quickly the phase precision rises at the onset of vocalizations. The high-ultrasonic regions in both virgins and mothers presumably receive strong inputs from pup calls, and the rise times for both animal groups at both call-excited and -inhibited (Fig. 5A3 and B3, solid bars) sites were correspondingly short and not significantly different. On the other hand, pup calls do not normally drive very robust inputs to the lateral band (hatched bars), since the phase precision here took significantly longer to build up compared to the high-ultrasonic band, for both call-excited and -inhibited sites (Fig. 5A3 and B3, top asterisks). This changed in mothers, particularly for call-inhibited sites, so that the lateral band exhibited more robust evoked responses whose precision rose significantly faster than in virgins (bottom asterisk in Fig. 5B3). This produced a uniformly rapid rise in phase precision across both the high-ultrasound and lateral bands not only at call-inhibited but also -excited SU sites in mothers (top n.s. in Fig. 5B3 and A3), generating a more temporally “synchronized” onset of precise neural activity.
Lateral Band Plasticity Enhanced for Pup Call Frequencies
Both the SU and the LFP data suggest that the main changes in call-evoked inhibition occurred for neural sites tuned to lateral frequencies. We next asked whether these changes were in any way specific for pup calls, or whether they reflected a more generic difference in auditory processing in mothers. To address this, we turned to pure tonal stimuli, since tone frequency is one of the main parameters that defines these whistle-like pup calls (Liu et al., 2003). We looked to see whether the lateral band’s inhibitory plasticity was specific for stimulus frequencies in the high-ultrasonic range, and analyzed tone responses from lateral sites the same way we did for natural pup calls. However, because we had far fewer trials for our tone stimuli (~15/tone) compared to our pup call stimuli (~50/call × 18 calls), we had to pool responses for 5 adjacent, logarithmically-spaced tones. Even still, the reduced trials made our normalized SU firing rate estimates noisy. Moreover, because there is a floor in SU spiking, relative changes in the strength of SU inhibition are harder to quantify. Thus, since we found that SU inhibition is correlated with LFP phase precision, we relied on the latter for this analysis.
Comparing LFP phase precisions for high-ultrasonic tones between 60–80 kHz, we found a significant increase in mothers compared to virgins at lateral band call-inhibited SU sites (Fig. 6B1, top panel). This was a more than 50% improvement, computed by taking the (bootstrap) mean difference in the group-averaged phase precisions during the tone. This enhancement was relatively specific for the natural pup call frequency range: varying the center tone frequency outside of this range caused the improvement to drop from its peak (Fig. 6C). Some weak frequency generalization was nevertheless apparent. When tone frequencies between 30–40 kHz were examined, a group difference was still found (Fig. 6B1, lower panel), but it was smaller, and the time interval for significance was shorter. Thus, call-inhibited SU sites in mothers had a significant increase in tone-evoked phase precision for frequencies starting above ~30 kHz, with greatest enhancement spanning the natural pup call range.
Figure 6. Tone-evoked LFP phase precision for lateral band LFP sites.
Differences between mothers and virgins were particularly enhanced for tone frequencies falling in the natural pup call range.
(A1 and B1) Comparison of 60–80 kHz (upper panels) and 30–40 kHz (lower panels) tone-evoked LFP phase precision trajectories, averaged only across lateral band LFPs. The magnitude of phase precisions was higher for the latter frequencies since lateral band sites should be more responsive to them. No significant differences between mothers and virgins were found for call-excited sites for either tonal stimulus. However, mothers and virgins did differ significantly at call-inhibited sites for both tonal stimuli. Excited Nmothers=7, Nvirgins=5. Inhibited Nmothers=7, Nvirgins=6.
(A2 and B2) Group comparison of tone-evoked LFP phase precision rise times. Bars depict the mean and standard error of the rise times for each group. No significant differences between mothers and virgins were found for call-excited sites, regardless of the frequency of tonal stimulation (60–80 kHz Mann-Whitney, U=13, Nmothers=7, Nvirgins=5, p>0.05, 2-tailed; 30–40 kHz: Mann-Whitney, U=14, Nmothers=7, Nvirgins=5, p>0.05, 2-tailed). The same was true for call-inhibited sites (60–80 kHz: Mann-Whitney, U=14, Nmothers=7, Nvirgins=6, p>0.05, 2-tailed; 30–40 kHz: t-test, t=0.78, df=11, p>0.05, 2-tailed).
(C) Frequency-dependence of tone-evoked LFP phase precision enhancement in mothers compared to virgins for call-excited (blue) and -inhibited (red) sites. Each point pooled trials from 5 logarithmically-spaced tone frequencies centered on that point. Population-averaged phase precision trajectories were computed separately for call-excited and -inhibited SU sites from each animal group. The average difference (solid lines) between trajectories (mother – virgin) over the duration of the tone quantified the precision enhancement relative to virgins. Shaded bands represent 95% confidence intervals computed by bootstrapping across sites. We found a significant increase only for LFPs at call-inhibited SU sites, with the greatest differences for frequencies falling in the natural pup call range (dashed vertical lines). A smaller but still significant difference was also apparent for tone frequencies above ~30 kHz.
In contrast, at call-excited SU sites, the phase precision time course for 60–80 kHz tones was not different between virgins and mothers (Fig. 6A1, top panel). The percentage increase relative to virgins was also non-significant across all tone frequencies (0% line lies within the 95% confidence interval, blue band in Fig. 6C). Finally, neither call-excited nor -inhibited sites with LFP BF>50 kHz exhibited significant differences (data not shown).
Despite the similar increase in phase precision in the local network response to high-ultrasonic tones and to pup calls at call-inhibited SU sites, responses to these two stimuli were not entirely identical. In Fig. 5B3, call-inhibited SU sites in the lateral band had a significantly faster rise in call-evoked phase precision in mothers compared to virgins. However, a significant difference was absent for tones (Fig. 6A2 and B2). This is remarkable since there was actually more acoustic variability in the onset of the natural calls used (Fig. S1) than of the tones used (all 10 ms rise times), yet the local network supporting SU inhibition in mothers increased its precision more quickly (compared to virgins) for natural calls, but not for tones.
Discussion
Earlier studies of auditory cortical communication sound encoding have almost entirely focused on excitatory neural responses (Huetz et al., 2009; Liu et al., 2006; Liu and Schreiner, 2007; Medvedev and Kanwal, 2004; Recanzone, 2008; Syka et al., 2005; Wallace et al., 2005; Wang et al., 1995). Inhibition has previously been considered only in so far as it shapes an individual cortical neuron’s receptive field and excitatory responsiveness to calls (Narayan et al., 2005; Razak and Fuzessery, 2006). Our study of ultrasonic call processing in the awake mouse demonstrates an alternative role for inhibition in the distributed cortical representation of species-specific vocalizations. Its importance was revealed through a plasticity that yielded more robust inhibition to ultrasonic pup call frequencies by neural sites tuned to lateral frequencies. Our data suggests lateral band inhibition can enhance the cortical contrast in the population representation of a communication call. Here we relate this work to prior studies, interpret our plasticity data, and propose its function for improving call detection in background noise.
Relation to Prior Studies
This work took advantage of a known behavioral change in the recognition of a natural communication call to search for auditory cortical correlates of its behavioral relevance, a strategy previously applied in anesthetized mice (Liu et al., 2006; Liu and Schreiner, 2007). Here we investigated cortical coding in awake mice for the first time, and focused only on neural changes relevant for detection by pooling responses across calls. Some conclusions remained consistent. Both the current and earlier work suggest plasticity in feedforward activity because mothers showed a more “synchronized” response onset across the auditory cortex (compare the non-significant differences in rise times in mothers in Fig. 5A3 and B3 to the near simultaneous PSTH onsets in Fig. 1 of (Liu and Schreiner, 2007)). Moreover, the faster rise in LFP precision found here (Fig. 4B2) is consistent with our earlier finding of increased call detection information near the onset of MU spiking, since reduced stimulus-evoked variability generally enhances transmission of stimulus information.
Nevertheless, the current data are fundamentally different and could not have been predicted from the anesthetized work. Whereas earlier conclusions were based on changes in excitatory neural responses, here we found that half of our SUs that responded to calls did so in a purely inhibitory manner (Fig. 2). In fact, call-evoked pure inhibition has rarely been reported, perhaps for methodological reasons. MU recordings may obscure the inhibition of individual neurons by sounds. Ketamine anesthesia may disinhibit (Behrens et al., 2007; Bergman, 1999) or otherwise modulate (Syka et al., 2005) or synchronize (Greenberg et al., 2008) cortical excitation. Neuron search strategies may also differ. Most studies first characterize units by their excitatory tone response area and BF, yet many of our call-inhibited SUs did not have excitatory tonal responses (18 of 25, Table 1); a “best” frequency for all SUs was instead based on the surrounding populations’ response to tones (i.e. LFP BF). Even when inhibited SU responses to natural calls have been reported in an awake animal though (Recanzone, 2008), the fraction of neurons has been very small, and has varied according to the call (0–10%). Hence, a final possibility is that single frequency ultrasonic calls may be more likely to evoke pure inhibition than the broad-band calls used in other studies.
Robust Plasticity in Inhibition Rather Than Excitation
Our data suggests this inhibition may be functionally relevant for detecting pup calls since it systematically changed in its timing and strength, particularly in the lateral band, in a manner that correlated with the call’s behavioral significance. These results were observed both directly in the SU data (Fig. 2B, 5B Insets), and indirectly through associated changes in the surrounding local network (Fig. 3B, 4B, 5B). Although inhibitory plasticity for vocalization selectivity has been reported in developing bats (Razak and Fuzessery, 2007; Razak et al., 2008), we are not aware of prior studies demonstrating inhibitory plasticity in adult auditory cortex that would impact call detection. Whether the changes here arise from pup experience, hormonal changes associated with pregnancy or lactation, or attention remains to be investigated.
Despite the robust plasticity in evoked SU inhibition, our data did not demonstrate a significant change between virgins and mothers in pooled SU excitation (Fig. 2A) for either the high-ultrasonic or lateral frequency bands (Fig. 5A Insets), although there may be a trend towards greater strengths for high-ultrasonic SUs in mothers. In other words, enhancing the “average” excitation per “typical” SU may not be necessary to improve the detection of pup calls. Distributed excitation may serve a different role in communication processing, such as discriminating calls.
There are two qualifications to this. First, cortical neurons are not all identical, and we likely do not record equally from all types. In particular, our high-impedance tungsten electrodes may be less sensitive to spikes from smaller interneurons than to larger pyramidal cells (Gold et al., 2006; Towe and Harding, 1970). The latter is likely the “typical” SU that we detect, as they make up 70–80% of cortical neurons (DeFelipe and Farinas, 1992). Such a recording bias may explain why we did not see a systematic change in SU excitation timing arising from inhibitory interneurons that presumably generate the earlier inhibition we reported here. In support of this, when we used lower-impedance electrodes to record (under anesthesia) thresholded MU activity, which likely has a greater contribution from interneurons, we found an earlier onset of excitation only in the lateral band (Fig. 1 of (Liu and Schreiner, 2007)). That would coincide here with the stronger SU (e.g. pyramidal cell) inhibition and earlier rise in LFP phase precision (e.g. reliable interneuron depolarization) only at laterally-tuned sites (Fig. 5B).
Second, lack of change in “average” excitation does not preclude differential plasticity that depends on systematic variations within our pool of “typical” SUs. This may be especially relevant for high-ultrasound-tuned excitatory neurons. For example, in addition to one statistically-excluded outlier (see Experimental Procedures), two other SUs in mothers had noticeably higher normalized spike rates (affecting the PSTH in Fig. 2A3 and the box plot in the inset of Fig. 5A2). These may be part of a specialized neuronal subcategory that emerged in mothers. The high variability in types of excitation makes it difficult to distinguish these. Thus, we did not further sub-classify SUs based on their excitatory response time courses, since sample sizes would have been too small to make reliable conclusions. In support of possible subtle changes in excitation that elude our current methods, Fig. 2A2 does show potentially more offset (latency >40 ms) and sustained (duration >50 ms) SUs in mothers. Furthermore, individual excitatory receptive fields may also be changing, which could improve call discrimination. This could occur without affecting overall evoked excitation and call detection.
In parallel with these SU results, our LFP data did not show changes in the network activity associated with SU excitation, although it did for inhibition (Fig. 3, 4). Although this is a serendipitous finding for our plasticity analysis, it is not entirely clear why a coarse measure of neural activity such as the LFP would show different changes depending on the response of a co-recorded SU. A detailed study of the relation between the SU and LFP is needed, but beyond the current scope (Galindo-Leon and Liu, in preparation). Instead, we mention here two possible, non-exclusive scenarios. First, there may be spatial clustering in SU response types, as has been reported for vocalization responses in the anesthetized guinea pig (Wallace et al., 2005). Second, the dominant sources contributing to the LFP may arise from a spatially restricted region (Katzner et al., 2009), and these sources differ depending on whether the co-recorded SU is inhibited or excited. For example, inhibition of a pyramidal cell can come from fast perisomatic inhibition by nearby basket cells (Freund and Katona, 2007). Each basket cell initiates synchronous inhibitory postsynaptic potentials in many pyramidal cells within its localized region of innervation (Miles et al., 1996). Such currents may be less consistent or weaker around pyramidal cells that are excited by calls. If plasticity occurs primarily in the inhibitory network, the LFP around excited SUs might not then easily reveal this.
A difference between excitation and inhibition was also apparent in how the LFP phase precision correlates to the strength of SU spiking, irrespective of the plasticity between animal groups. Phase precision measures response variability across trials, which can arise here from either random neural noise for each call, or systematic variation for acoustically different calls (Fig. S1). If different calls elicit similar neural responses, then the latter component is minimized. This is the case for call-inhibited but less so for -excited SU sites. Most of our call-inhibited SUs were uniformly inhibited by most (Fig. 1C), if not all (Fig. S2M and N), the calls, whereas the response to different calls by call-excited SUs was typically more varied (Fig. 1B, S2O and P). Hence, our pooled phase precision at call-inhibited SU sites reflects neural noise more directly, and reveals an intriguing correlation with the strength of SU inhibition: LFP trajectories, which include both synaptic and spiking contributions, become less variable as the co-recorded SU’s spiking drops to zero. This relation justified interpreting increases in tone-evoked phase precision as indicative of enhanced inhibitory strength (Fig. 6). In further support of this, differences between virgins and mothers were similar even when we compared average call-specific (instead of call-pooled) phase precision trajectories (data not shown). This suggests that mean response differences across calls were not a major contribution to the LFP variability at call-inhibited sites. For call-excited SUs, systematic variations in the pattern of mean firing for individual calls could degrade a site’s pooled LFP phase precision, independent of the SU’s excitatory strength. Thus, these measures were uncorrelated at call-excited sites.
Hypothesized Role of Enhanced Inhibition in the Lateral Band
How might a more robust inhibitory response at the population level functionally improve communication sound detection? Accumulating evidence suggests the auditory cortex changes to more powerfully represent sounds that acquire behavioral meaning (Fritz et al., 2003; Weinberger, 2004). Inhibitory plasticity may help achieve this by enhancing the neural contrast in a sound’s distributed cortical code. A cartoon model based on our results illustrates how this might work (Fig. 7). An emitted pup call (upper right) excites the ultrasound region of the basilar membrane, and is transduced into a neural signal that feeds forward through subcortical stations to the auditory cortex, evoking a distributed response spanning both the high-ultrasonic (solid bars) as well as lateral (hatched bars) frequency bands. In each region, (presumed) pyramidal cell activity is divided into call-excited or -inhibited classes. Normalized evoked spike rates (relative to spontaneous activity) for a virgin (gray) or mother (black) are represented by bar heights. If pooled spike rates are not significantly different between groups, rates are depicted as equal for simplicity. Hence, only the call-inhibited sites in the mother’s lateral band are shown as significantly lower. Assuming simple one-to-one integration of call-excited and -inhibited activity in a frequency band-specific fashion, this would produce a downstream representation with a greater contrast in mothers between the frequency region that should represent the pup call (high-ultrasound) and lateral frequency areas.
Figure 7. Hypothesized model to enhance a pup call’s neural contrast.
CN: cochlear nucleus; SOC: superior olivary complex nuclei; IC: inferior colliculus; MGB: medial geniculate body. See text for details.
Why would enhancement of population-level contrast be advantageous for call processing? If calls were emitted in the presence of broadband background noise, call-evoked lateral band inhibition could help suppress this noise, helping the neural activity in the high-ultrasonic band to stand out more clearly. In fact, we actually found some generalization in mothers of the enhanced inhibition at laterally-tuned sites for lower frequencies as well (Fig. 6). Whether this inhibition can add to the call-evoked inhibition must be tested in future two-tone or masking experiments. Finally, such a coding scheme is reminiscent of attention-related gain changes of auditory cortical neurons during a tone-in-broadband noise detection task in ferrets (Atiani et al., 2009). That study observed stronger suppression of excitatory activity for neurons tuned to sites “far” from the target frequency. On the other hand, our main effect was stronger inhibition at these sites to the target sound (pup call) alone. In both scenarios, the hypothesized outcome would be enhanced neural contrast for the target.
Experimental Procedures
The Emory University Institutional Animal Care and Use Committee approved all procedures. Experiments were carried out on 8 virgin female, and 7 mother CBA/CaJ mice, all between 14 and 24 weeks old at the time of surgery. All mothers had their pups weaned within the 2 weeks prior to surgery. Animals were housed under a reversed light cycle (14 hours light/10 hours dark), and had access to food and water ad libitum.
Acoustic stimulation
Stimuli were generated using Tucker-Davis Technologies (TDT, Alachua, FL, USA) System 3 Gigabit hardware and software and presented through the Brainware application via modules programmed in the RPvdsEx environment. Noise bursts, frequency sweeps and tones were used as search sounds to locate auditory responses. Tuning curves were derived at ~60 dBSPL by playing a set of 40 tones, 60 ms long plus 10 ms cos2 onset and offset, with logarithmically-spaced frequencies ranging from 6.4 to 95 kHz were presented. Different frequencies were randomly selected every 600 ms and repeated 5 or 15 times.
Eighteen pup calls (Fig. S1) were drawn from a large library of natural ultrasonic CBA/CaJ vocalizations for playback (Liu et al., 2003). Sound snippets were high pass filtered in software (25 kHz corner, 8-order Butterworth filter, MATLAB), spectrally denoised (Liu et al., 2003), and then Hilbert transformed to extract the instantaneous frequency and amplitude envelope. These were used to resynthesize a clean version of each pup call, which were then multiplied by a 0.5 ms cos2 onset and offset function, and scaled to a target root-mean-square (RMS) amplitude corresponding to 65 dBSPL.
A maximum of fifty trials (600 ms long) of each pup call along with a blank stimulus were presented in random order, with sound onset usually beginning at 200 ms after trial onset. Occasionally, a single unit would drift sufficiently in amplitude that it could no longer be isolated, in which case the call stimuli were terminated with fewer trials. Recordings of adult CBA/CaJ calls were also played back, but were not analyzed in this work.
Extracellular recordings
To record neural activity in completely awake, restrained mice, we developed a protocol for relatively short (up to ~3 hours), but chronic (up to ~1 week) electrophysiology sessions. The details are presented in the Supplemental Information. All recording experiments were conducted during an animal’s dark phase. SUs (high-pass filtered at 300 Hz and low-pass filtered at 3 or 6 kHz) and LFPs (high-pass filtered at 2 Hz, low-pass filtered at 300 Hz, with a notch filter at 60 Hz) were recorded off single 4–6 M tungsten electrodes (FHC Inc, Bowdoin, ME). Recordings were performed between 300 and 700 μm. Both SU and LFP signals were sampled at a rate of 24414.0625 samples/s. Offline, the LFP was despiked, decimated (keep every 24th point), low pass filtered (MATLAB Parks-McClellan optimal equiripple FIR filter, transition band between 90 and 100Hz) forward and backward (filtfilt) to eliminate traces of action potentials without introducing phase delays. The initial despiking could be accomplished either (1) by subtracting the (dynamically-updated) “average” spike at each spike time, or (2) by simply deleting a [−0.5, 4] ms window around each spike and replacing it with a spline-interpolated signal; both yielded similar results, so (2) was used for simplicity. This procedure effectively attenuated the residual power leaking into the low frequency region from large-amplitude spikes.
The targeting of recording sites was guided by our intent to record high quality SUs. Details of this isolation are described in the Supplemental Information. We generally focused on recording at sites with SU responses to high frequency tones above ~20 kHz (either excitation or inhibition), since this range has shown more prominent responses to the ~64 kHz pup sounds (Liu and Schreiner, 2007). On a few occasions, initially-isolated SUs would be lost, leaving the SU and its corresponding LFP sites incompletely characterized by either the pup calls or tones. Alternatively, new SUs could appear after characterization had already begun. Thus, the sites with pup calls or tone responses did not always agree (see Table 1).
SU analysis
To assess offline whether a SU exhibited any kind of response to ultrasound vocalizations, we took the majority decision from independent classifications by 3 individuals of each SUs response to pup calls. The set of call-ordered rasters (e.g. as in Fig. S2, right column) and overall pup call PSTH was deemed excited if any consistent (over trials) increase in spiking beyond the spontaneous level was evident around the time of the calls for any of the calls; inhibited if only a consistent decrease in spiking was apparent; and nonresponsive if neither excitation nor pure inhibition was clearly discernible. If the PSTH showed both excitation and inhibition, the SU was classified as excitatory. Most classifications were unanimous across observers (57/86). Examples from each category are shown in Fig. S2. Because of occasionally complex response structure to different calls, we decided a manual majority rather than automated pattern recognition algorithm would provide a more robust classification for the purposes of this paper.
SU response latency was determined by finding the half-max or half-min of the smoothed spike rate (convolution of individual spikes with a Gaussian smoothing function, 5 ms standard deviation). The half-max (half-min) was determined based on the spike rates at stimulus onset and at the maximum (minimum). The response latency was the time relative to stimulus onset for the smoothed, pooled spiking response to reach the halfway point. The duration of SU inhibition was the time over which the smoothed spike rate stayed below the half-min value. Similarly, the duration of SU excitation was the time over which the smoothed spike rate stayed above the half-max value.
To determine if there were differences between mothers and virgins in the pooled spike rate for call-excited or -inhibited SUs, we normalized each smoothed, time-dependent spike rate function by the average spontaneous rate during the blank trial and then averaged the SUs together. We quantified the strength of SU excitation or inhibition by integrating the actual spike count over a period from 205 to 265 ms (accounting for the shortest neural delay to the auditory cortex, and the longest duration pup call, 60 ms). One high-ultrasound SU in a mother was removed from SU normalized rate analyses by Peirce’s criterion for statistically detecting outliers (4 standard deviations greater than the mean), although its inclusion would not change our results (Peirce, 1852).
Wide-band LFP analysis
The LFP is usually analyzed in spectral bands – such as theta (~4–10 Hz), beta (~10–35 Hz) and gamma (~35–90 Hz) – consistent with an oscillatory view of neural activity. We took a complementary approach by instead studying the wide-band (2–100 Hz) signal. Although our LFP was spectrally peaked around 4–10 Hz, this nevertheless better preserves the shape of transients, such as those induced by the acoustic stimulation (Shah et al., 2004). Our analysis applied the Hilbert transform to each LFP trace to generate its unique analytic signal in the complex domain (Boashash, 1992; Pikovsky et al., 2001). We focused on the Hilbert phase trajectory, where specific phases approximately correspond to specific shape features in the signal (Fig. S4). See Supplemental Information for further details and discussion about this mathematical transformation.
LFP phase precision is defined at each time point by the mean resultant length of the trial-by-trial wide-band Hilbert phases over the N trials: . This quantity has also been called phase concentration (Lakatos et al., 2005), and is algebraically related to the circular variance (Mardia and Jupp, 2000) or phase reliability (Montemurro et al., 2008).
Phase precision rise time was calculated as the delay from stimulus onset to the half-max, and the duration was the time it exceeded this level. For tone responses, the percentage increase in tone-evoked phase precision over the virgin was defined as:
The integration period was from 205 to 275 ms to account for the duration, including onset/offset ramps, of the tone. To determine whether there was a significant increase in the integrated phase precision, we performed a bootstrap. We sampled each distribution of sites for mothers and virgins separately with replacement 1000 times and found the 95% confidence interval. Differences were taken as significant when the confidence bound did not include 0.
Statistical tests
Statistical tests were carried out in MATLAB. When justified, we preferred parametric statistical tests. In testing the differences between two populations, we first performed a two-tailed Lilliefors goodness-of-fit test for normality and a two-tailed F-test for variance, using a p<0.05 level for significance. If one population was statistically different from a normal distribution or if the two populations had unequal variances, we used a Mann-Whitney U test to assess whether the medians of two data sets were the same. If both populations were not statistically different from a normal distribution and had equal variances, we used a two-tailed t-test for equal means. When comparing two time traces from different animal groups, we used an N-way analysis of variance followed by multcompare using Fisher’s least significant difference method for correcting for multiple pair-wise t-test comparisons at each time point. Correlations were tested using corrcoef at a p<0.05 significance level.
Significance of the phase precision was computed by the Rayleigh statistic (Fisher, 1993), and depended only on the number of trials. For pup calls and responses to tones, at a significance level of p ≤ 0.05, should be larger than 0.058 for N=900 trials, and 0.199 for N=75 trials respectively. We discarded the phase precision for a LFP site if it did not exceed this significance level during the stimulus period. Finally, a z-test for two proportions assuming equal variances was used to assess whether our SU classification percentages differed between mothers and virgins.
Supplementary Material
Acknowledgments
RCL conceived the experiments. EEG and RCL developed the awake recording preparation. EEG and FGL collected and analyzed the data. FGL and RCL generated the figures and wrote the paper with substantial input from EEG. The authors thank Brian Kocher and Yongkui Zhang for electrophysiology assistance; Tamara Ivanova for mouse husbandry; Dieter Jaeger, Christoph Schreiner, Garrett Stanley and Jason Miranda for comments on earlier versions, anonymous reviewers for their constructive comments, and the NIDCD (008343), the NSF CBN (IBN-9876754) and IGERT (DGE-0333411) for funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atiani S, Elhilali M, David SV, Fritz JB, Shamma SA. Task difficulty and performance induce diverse adaptive patterns in gain and shape of primary auditory cortical receptive fields. Neuron. 2009;61:467–480. doi: 10.1016/j.neuron.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Bergman SA. Ketamine: review of its pharmacology and its use in pediatric anesthesia. Anesth Prog. 1999;46:10–20. [PMC free article] [PubMed] [Google Scholar]
- Boashash B. Estimating and Interpreting The Instantaneous Frequency of a Signal - Part 1: Fundamentals. Proceedings of the IEEE. 1992;80:520–538. [Google Scholar]
- DeFelipe J, Farinas I. The pyramidal neuron of the cerebral cortex: morphological and chemical characteristics of the synaptic inputs. Prog Neurobiol. 1992;39:563–607. doi: 10.1016/0301-0082(92)90015-7. [DOI] [PubMed] [Google Scholar]
- Ehret G. Infant rodent ultrasounds -- a gate to the understanding of sound communication. Behav Genet. 2005;35:19–29. doi: 10.1007/s10519-004-0853-8. [DOI] [PubMed] [Google Scholar]
- Ehret G, Koch M, Haack B, Markl H. Sex and parental experience determine the onset of an instinctive behavior in mice. Naturwissenschaften. 1987;74:47. doi: 10.1007/BF00367047. [DOI] [PubMed] [Google Scholar]
- Fisher NI. Statistical analysis of circular data. Cambridge [England]; New York, NY, USA: Cambridge University Press; 1993. [Google Scholar]
- Fitzpatrick DC, Kanwal JS, Butman JA, Suga N. Combination-sensitive neurons in the primary auditory cortex of the mustached bat. J Neurosci. 1993;13:931–940. doi: 10.1523/JNEUROSCI.13-03-00931.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat Neurosci. 2003;6:1216–1223. doi: 10.1038/nn1141. [DOI] [PubMed] [Google Scholar]
- Gold C, Henze DA, Koch C, Buzsaki G. On the origin of the extracellular action potential waveform: A modeling study. J Neurophysiol. 2006;95:3113–3128. doi: 10.1152/jn.00979.2005. [DOI] [PubMed] [Google Scholar]
- Greenberg DS, Houweling AR, Kerr JN. Population imaging of ongoing neuronal activity in the visual cortex of awake rats. Nat Neurosci. 2008;11:749–751. doi: 10.1038/nn.2140. [DOI] [PubMed] [Google Scholar]
- Haslinger R, Ulbert I, Moore CI, Brown EN, Devor A. Analysis of LFP Phase Predicts Sensory Response of Barrel Cortex. J Neurophysiol. 2006;96:1658–1663. doi: 10.1152/jn.01288.2005. [DOI] [PubMed] [Google Scholar]
- Huetz C, Philibert B, Edeline JM. A spike-timing code for discriminating conspecific vocalizations in the thalamocortical system of anesthetized and awake guinea pigs. J Neurosci. 2009;29:334–350. doi: 10.1523/JNEUROSCI.3269-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzner S, Nauhaus I, Benucci A, Bonin V, Ringach DL, Carandini M. Local origin of field potentials in visual cortex. Neuron. 2009;61:35–41. doi: 10.1016/j.neuron.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Rose HJ, Lazar R, Liang K, Metherate R. Spectral integration in primary auditory cortex: Laminar processing of afferent input, in vivo and in vitro. Neuroscience. 2005;134:1033–1045. doi: 10.1016/j.neuroscience.2005.04.052. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE. An Oscillatory Hierarchy Controlling Neuronal Excitability and Stimulus Processing in the Auditory Cortex. J Neurophysiol. 2005;94:1904–1911. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- Liu RC, Linden JF, Schreiner CE. Improved cortical entrainment to infant communication calls in mothers compared with virgin mice. Eur J Neurosci. 2006;23:3087–3097. doi: 10.1111/j.1460-9568.2006.04840.x. [DOI] [PubMed] [Google Scholar]
- Liu RC, Miller KD, Merzenich MM, Schreiner CE. Acoustic variability and distinguishability among mouse ultrasound vocalizations. J Acoust Soc Am. 2003;114:3412–3422. doi: 10.1121/1.1623787. [DOI] [PubMed] [Google Scholar]
- Liu RC, Schreiner CE. Auditory Cortical Detection and Discrimination Correlates with Communicative Significance. PLoS Biol. 2007;5:e173. doi: 10.1371/journal.pbio.0050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci. 2003;23:3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes da Silva FH, Kamp A. Biophysical aspects of EEG and magnetoencephalogram generation. In: Lopes da Silva FH, Niedermeyer E, editors. Electroencephalography: basic principles, clinical applications, and related fields. Baltimore: Urban & Schwarzenberg; 1987. pp. 29–42. [Google Scholar]
- Mardia KV, Jupp PE. Directional Statistics. New York, NY: John Wiley & Sons; 2000. [Google Scholar]
- Medvedev AV, Kanwal JS. Local field potentials and spiking activity in the primary auditory cortex in response to social calls. J Neurophysiol. 2004;92:52–65. doi: 10.1152/jn.01253.2003. [DOI] [PubMed] [Google Scholar]
- Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Montemurro MA, Rasch MJ, Murayama Y, Logothetis NK, Panzeri S. Phase-of-firing coding of natural visual stimuli in primary visual cortex. Curr Biol. 2008;18:375–380. doi: 10.1016/j.cub.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Narayan R, Ergun A, Sen K. Delayed inhibition in cortical receptive fields and the discrimination of complex stimuli. J Neurophysiol. 2005;94:2970–2975. doi: 10.1152/jn.00144.2005. [DOI] [PubMed] [Google Scholar]
- Peirce B. Criterion for the rejection of doubtful observations. Astron J II. 1852;45:161–163. [Google Scholar]
- Pikovsky A, Rosenblum M, Kurths J. Synchronization: A universal concept in nonlinear sciences. New York, NY: Cambridge University Press; 2001. [Google Scholar]
- Poulet JF, Petersen CC. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454:881–885. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Cortical processing of complex sounds. Curr Opin Neurobiol. 1998;8:516–521. doi: 10.1016/s0959-4388(98)80040-8. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B, Hauser M. Processing of complex sounds in the macaque nonprimary auditory cortex. Science. 1995;268:111–114. doi: 10.1126/science.7701330. [DOI] [PubMed] [Google Scholar]
- Razak KA, Fuzessery ZM. Neural mechanisms underlying selectivity for the rate and direction of frequency-modulated sweeps in the auditory cortex of the pallid bat. J Neurophysiol. 2006;96:1303–1319. doi: 10.1152/jn.00020.2006. [DOI] [PubMed] [Google Scholar]
- Razak KA, Fuzessery ZM. Development of inhibitory mechanisms underlying selectivity for the rate and direction of frequency-modulated sweeps in the auditory cortex. J Neurosci. 2007;27:1769–1781. doi: 10.1523/JNEUROSCI.3851-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razak KA, Fuzessery ZM. Facilitatory mechanisms underlying selectivity for the direction and rate of frequency modulated sweeps in the auditory cortex. J Neurosci. 2008;28:9806–9816. doi: 10.1523/JNEUROSCI.1293-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razak KA, Richardson MD, Fuzessery ZM. Experience is required for the maintenance and refinement of FM sweep selectivity in the developing auditory cortex. Proc Natl Acad Sci U S A. 2008;105:4465–4470. doi: 10.1073/pnas.0709504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH. Representation of con-specific vocalizations in the core and belt areas of the auditory cortex in the alert macaque monkey. J Neurosci. 2008;28:13184–13193. doi: 10.1523/JNEUROSCI.3619-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK, Johnsrude IS. The neuroanatomical and functional organization of speech perception. Trends Neurosci. 2003;26:100–107. doi: 10.1016/S0166-2236(02)00037-1. [DOI] [PubMed] [Google Scholar]
- Shah AS, Bressler SL, Knuth KH, Ding M, Mehta AD, Ulbert I, Schroeder CE. Neural Dynamics and the Fundamental Mechanisms of Event-related Brain Potentials. Cereb Cortex. 2004;14:476–483. doi: 10.1093/cercor/bhh009. [DOI] [PubMed] [Google Scholar]
- Syka J, Suta D, Popelár J. Responses to species-specific vocalizations in the auditory cortex of awake and anesthetized guinea pigs. Hearing Research. 2005;206:177–184. doi: 10.1016/j.heares.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Towe AL, Harding GW. Extracellular microelectrode sampling bias. Exp Neurol. 1970;29:366–381. doi: 10.1016/0014-4886(70)90065-8. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Shackleton TM, Anderson LA, Palmer AR. Representation of the purr call in the guinea pig primary auditory cortex. Hear Res. 2005;204:115–126. doi: 10.1016/j.heares.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Wang X, Merzenich MM, Beitel R, Schreiner CE. Representation of a species-specific vocalization in the primary auditory cortex of the common marmoset: temporal and spectral characteristics. J Neurophysiol. 1995;74:2685–2706. doi: 10.1152/jn.1995.74.6.2685. [DOI] [PubMed] [Google Scholar]
- Washington SD, Kanwal JS. DSCF neurons within the primary auditory cortex of the mustached bat process frequency modulations present within social calls. J Neurophysiol. 2008;100:3285–3304. doi: 10.1152/jn.90442.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci. 2004;5:279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.