Abstract
Integrin-mediated adhesion of circulating neutrophils to endothelium during inflammation involves multiple adhesion molecules on both neutrophils and endothelium. Most studies of neutrophil adhesion have focused on adhesion to ICAM-1 (mediated by β2 integrins), but interaction with the endothelial ligand vascular cell adhesion molecule 1 (VCAM-1) may also play a role in neutrophil adhesion to activated endothelium. In this study we demonstrate significant adhesion between neutrophils and VCAM-1 mediated by β1 integrins, principally via α4β1 (VLA-4). We characterize the dynamics of adhesion in terms of rate constants for a two-step bond formation process, the first involving juxtaposition of active molecules with substrate and the second involving bond formation. The results indicate that the first step is rate limiting for VLA-4-VCAM-1 interactions. Changing divalent cation composition affects these coefficients, implicating molecular conformational changes as a key step in the process.
Introduction
Leukocyte trafficking is regulated by several families of cell adhesion molecules that function concomitantly or sequentially to mediate leukocyte adhesion to endothelium (1). A primary focus in studies of leukocyte adhesion has been the family of β2-integrins, an integrin subtype that is unique to leukocytes (2), and its principal endothelial ligand ICAM-1 (3). A major role for this adhesive pairing has been well established by a number of investigators (3–6). The role that members of the β1-integrin family play in leukocyte adhesion has also received attention. The principle endothelial counter-receptor for β1-integrins is vascular cell adhesion molecule-1 (VCAM-1, CD106), a cytokine-inducible adhesion member of immunoglobulin superfamily found on endothelium (7,8). Interactions between VLA-4 (α4β1) and VCAM-1 have been found to play a significant role in adhesion of lymphocytes (9,10), and monocytes (11), but a role for these proteins in mediating neutrophil adhesion in humans has been less clear. Early reports indicated that VLA-4 interactions with VCAM-1 were important in mediating adhesion of eosinophils and basophils but not neutrophils to cultured endothelium (12). But more recent reports have documented the presence of several members of the β1-integrin family on neutrophils including VLA-4 (α4β1), VLA-5 (α5 β1) and VLA-9 (α9β1)) (13–15). Reinhardt and colleagues (16) first demonstrated that significant upregulation of VLA-4 presentation on neutrophils occurs in response to inflammatory stimuli, and that VLA-4 can mediate neutrophil adhesion to TNF-α stimulated endothelium. They further showed that even in the absence of exogenous stimuli, significant numbers of neutrophils will adhere to immobilized VCAM-1 under flow conditions, although at much lower levels than other leukocytes (17). Thus, there is evidence that VLA-4−VCAM-1 interactions can mediate neutrophil adhesion to endothelium.
Most integrins exist in multiple affinity states, and therefore, an important step for integrin-adhesion to occur is the activation of the molecules to their high affinity conformation (18–21). In the circulation, this activation occurs as a result of inside-out signaling that is initiated by natural stimuli (22–24). It is also possible to activate integrins artificially by agents such as activating mAb (25,26) or changing divalent cation concentrations (27). The response to divalent ions differs among different integrins. Manganese (Mn2+) has been shown to activate a wide range of integrins, including all members of the β2-integrin family and many members of the β1-family (26–29). In contrast, magnesium (Mg2+) in the presence of calcium chelator has relatively little effect on the affinity of the β2-integrin Mac-1 (30,31) but causes activation of the other principle β2-integrin LFA-1 (28,30,32,33). The affinities of β1 integrins are also modulated by divalent cations to varying degrees (27). As in the case of the β2-integrins, Mn2+ had more substantial effects than Mg2+ in inducing an activation epitope detectable by monoclonal antibody, but, significantly, the presence of Mg2+ and chelation of calcium facilitated ligand binding via α5 β1 (27). In contrast, VLA-4 is thought to be unique among leukocyte integrins in that it is active in the presence of calcium (27) and can initiate the adhesion of leukocytes under physiological conditions without further activation (26). The ability to modulate integrin affinity by changing extracellular ion composition provides an opportunity to explore the particular importance of integrin activation for adhesion in the absence of general activation of the cell.
Neutrophil recruitment during the inflammatory response is a complex process involving multiple molecular interactions. Although most of the principal adhesion molecules involved in the process have been identified, much remains to be learned about the relative roles of different adhesion molecules in this complex dynamic process. The single cell micromechanical approach used in the current study enables us to explore the distinctive behavior of different integrin-receptor pairs and to measure the time dependence of the formation and strengthening of cellular adhesions. In a previous report, we have characterized the dynamics of adhesion between neutrophils and the endothelial ligand ICAM-1, and have obtained effective kinetic rate constants for LFA-1–ICAM-1 interactions (30). In the course of that study, we observed a surprisingly high level of adhesion between neutrophils and VCAM-1 in the presence of Mg2+ and EGTA. Moreover, we observed that the behavior of neutrophils binding to VCAM-1 did not follow the simple kinetic model that had served as an adequate framework for interpreting binding interactions to ICAM-1. In the companion article in this issue (34) we develop an alternative kinetic scheme that accounts for this behavior. In this article we characterize the dynamics of neutrophil adhesion to VCAM-1 in terms of this new framework, demonstrate significant contributions from the β1-integrins in mediating this adhesion, and explore the effects of changing divalent ion composition in the suspending medium on the kinetics of cell adhesion.
Materials and Methods
Cell preparation
Forpipette studies neutrophils were obtained from healthy donors by diluting a drop of peripheral blood in 4% fetal bovine serum (HyClone, Logan, UT) in BSS (balanced saline solution: 5 mM KCl, 140 mM NaCl, 11.1 mM Glucose made with low endotoxin water obtained from Invitrogen, Grand Island, NY) containing 10 mM N-[2-Hydroxyethyl] piperazine-N'-[2-ethanesulfonic acid] (HEPES, Sigma, Saint Louis, MO), pH 7.4, 290 mOsm. Divalent cation composition was adjusted by including either 3.0 mM Mn2+, 5.0 mM Mg2+ plus 0.5 mM EGTA, or 1.5 mM Ca2+. The suspension was placed in a small chamber on the microscope stage and two micropipettes were used to manipulate a single cell into contact with the ligand-coated bead for controlled durations. Neutrophils were selected based on their multi-lobular nuclei. The selection process was validated by transferring selected cells by pipette to a slide and verifying that the cells were neutrophils by histological staining (>85% of cells selected were neutrophils).
For flow cytometry measurements, neutrophils were first isolated from whole blood. Venous blood drawn from healthy donors was placed over a layer of Polymorphs (Accurate Chemical & Scientific Corporation, Westbury, NY). After centrifugation at 450g for 45 min, the band of polymorphonuclear cells was visible. Neutrophils were harvested by pipette, then washed in 4% fetal bovine serum (HyClone) in HANK's balanced salt solution (BioWhittaker, Walkersville, MD), containing 10 mM HEPES, without Ca2+ and Mg2+, and brought to the final concentration: 5 × 106 cells/mL. Then cells were treated at 4°C with saturating concentration of monoclonal anti-human fluorescein isothiocyanate conjugated antibodies: 0.015 mg/mL of anti-β1 integrin (CD29) B-D15 (BioSource International, Camarilo, CA), 0.02 mg/mL of anti-α4 integrin (CD49d) P1H4 (Chemicon International, Temecula, CA), 0.005 mg/mL of anti-α5 integrin (CD49e) SAM-1 (Chemicon International), 0.025 mg/mL of anti-α9β1 integrin, Y9A2 (Chemicon International). Isotype controls were obtained from the corresponding manufacturer: IgG1 (Beckman Coulter, Miami, FL), IgG2a (R&D Systems, Minneapolis, MN) and IgG2b (Chemicon International). Fluorescence detection was performed on an Epics Elite flow cytometer using forward and side scatter to gate on granulocytes. To correlate fluorescence intensity with the number of bound antibodies on the cells, the fluorescence signal was calibrated using Quantum Simply Cellular Beads (Flow Cytometry Standards Corp., Fishers, IN). A suspension of Simply Cellular Beads, containing five different populations with known numbers of antibody binding sites, was labeled to saturation with the same antibodies used to label the neutrophils. The fluorescence intensity was converted to the number of binding sites using software provided by the manufacturer.
In some pipette experiments, adhesion was inhibited by introducing blocking antibodies into the suspending medium in the pipette chamber. These experiments were performed at 0.1 mg/mL of anti-β1 integrin, 4B4 (Beckman Coulter) and at 0.01 mg/mL of anti-β2 (CD18) integrin, IB4 (Ancell, Bayport, MN), as well as at above mentioned concentrations of P1H4, SAM-1 and Y9A2.
Coating beads
The beads (tosylactivated paramagnetic M-450 Dynabeads (Dynal, Lake Success, NY)) were coated with soluble recombinant forms of human VCAM-1 (R&D Systems), as described previously (30). Briefly, 107 beads were incubated with ligand (3.3–8.3 μg/mL) at room temperature overnight. Then unreacted tosyl groups were blocked by incubation with 0.25 M ethanolamine, washed and stored in 0.1% bovine serum albumin (Calbiochem, La Jolla, CA) in phosphate buffered saline (Invitrogen) at 4°C.
The density of VCAM-1 on ligand-coated beads was measured by flow cytometry. The beads were pre-incubated at 4°C overnight with FITC-conjugated antibody against human VCAM-1 (R&D Systems). To assess the value for the nonspecific binding untreated beads were incubated with the same FITC-conjugated antibody against human VCAM-1. To correlate fluorescence intensity with the number of bound antibodies on the beads, the fluorescence signal was calibrated using Quantum Simply Cellular Beads, as described in the previous section.
Micropipette technique
The experiments were performed on the stage of an Olympus IX70 inverted microscope (Spectra Services Inc., Rochester, NY), using Hamamatsu RS-170 CCD (Microvideo Instruments, Avon, MA) as described previously (30). Two micropipettes were positioned in a dual entry chamber mounted on the microscope stage: one to hold the bead, another to manipulate the cell. (Fig. 1) The bead and the neutrophil were held in contact for a user-specified length of time, then separated. The experiments were recorded on videotape and analyzed subsequently using Scion Image software to determine the fraction of contacts resulting in adhesion and to measure the contact area. An adhesion event was scored when there was any visible deformation of the cell surface as the bead and cell were separated. The adhesion probability was calculated as the total number of adhesive events divided by total number of touches. All experiments were performed either at room temperature (22°C) or at 37°C as indicated.
Figure 1.
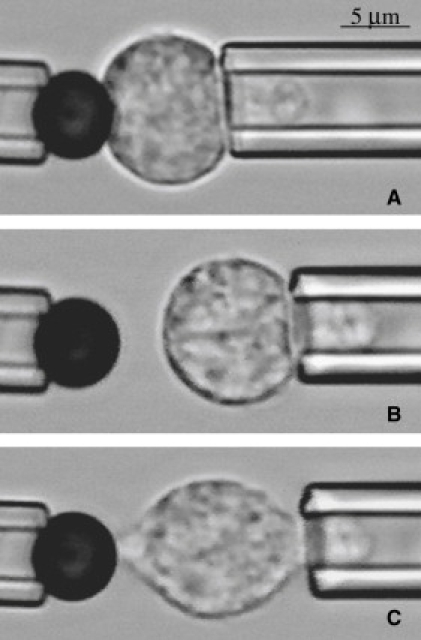
Interaction between the bead and the cell during the experiment: (A) initial contact; (B) no adhesion; (C) adhesive event. The adhesive event here resulted in the formation of the membrane strand between the cell and the bead. This occurred after some, but not all the adhesive contacts. Original magnification 200× for all panels. (Bar = 5 μm.)
Calculations
Adhesion probability is related to the formation of intermolecular bonds in the contact zone. Assuming that the formation of bonds is uncorrelated and therefore follows Poisson statistics, the expected bond number, 〈n〉, is related to the adhesion probability through the relationship (35):
| (1) |
The formation of adhesive bonds in the contact zone has been shown in other systems to follow first order kinetics. The expected bond number 〈n〉 is expected to depend on a number of parameters, including the contact time, t, and the contact area, Ac. In the companion article (34) we develop a new kinetic framework involving a two step process leading to the formation of VLA-4-VCAM-1 bonds. The first step involves the formation of a reaction zone, which is defined as the presence of an active form of the adhesion molecule in close apposition with the substrate. The second step involves the binding reaction itself. As shown in the companion article (34), for VCAM-1 substrate concentrations >200 sites/μm2, the expressions for the expected bond number as a function of time takes the form
| (2) |
where τRZ is a characteristic time for reaction zone formation, TRZ is the total concentration of possible reaction zones on the surface, and KDRZ is a dissociation constant for reaction zone formation. These coefficients are related to the kinetic parameters of the model (34): τRZ = 1/(k+ + k−BI), KDRZ = k−BI/k+ and . In comparing the multiple data sets, it is important to account for the fact that adhesion probability depends on the size of the contact area. The contact area between the bead and the cell was determined from the size of the contact zone measured from video recordings of the experiments (30). To avoid biasing the data because of the differences in contact area among different data sets, the measured adhesion probability (Padh) was corrected to the average contact area (A0 = 7.5 μm2) over the whole set of experiments performed in this study. This correction was made by first converting the adhesion probability to 〈n〉meas (Eq. 1) then adjusting 〈n〉 meas to a standard value 〈n〉 by
| (3) |
where Ameas is the mean contact area of the data set. Then 〈n〉 was converted back to the normalized adhesion probability Po related to the expected number of bonds via Eq. 1.
Results
Flow cytometry measurements revealed that the mean density of VCAM-1 molecules was 210 sites/μm2, 250 sites/μm2, 370 sites/μm2, 650 sites/μm2, 860 sites/μm2, 970 sites/μm2 and 990 sites/μm2 for the different bead preparations used in this study. Interestingly, when the surface concentration of VCAM-1 exceeded 200 sites/μm2, the adhesion probability was found to be independent of the ligand density (34), indicating that this concentration was saturating for the bond formation step. Surface concentrations of the different types of β1 integrins on human neutrophils (CD49d, CD49e, VLA-9) were also measured. Ten thousand cells from each of five different donors were tested (Fig. 2). The distribution of fluorescence labeling indicates single cell populations with a mean of ∼8,000 β1 subunits per cell, as well as significant numbers of α4, α5, and α9 subunits: 31%, 24% and 31% of the total respectively (Table 1). These results are in general agreement with previous findings (13–15). We also performed measurements to determine whether the expression levels of the β1 integrins were stable under the conditions of our experiments. Isolated neutrophils were incubated for 15, 60 or 120 min either in 3 mM Mn2+ at 21°C, or in 5 mM Mg2+ plus EGTA or 1.5 mM Ca2+ at 37°C. No significant changes in expression levels were observed (data not shown).
Figure 2.
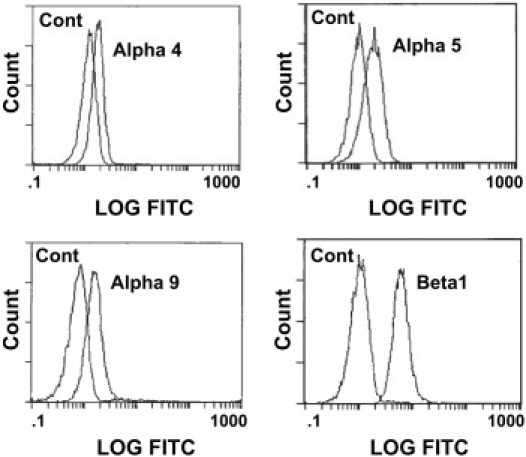
Immunofluorescent analysis of VCAM-1 receptor molecules expressed on human neutrophils. Cells were labeled for (top left) α4 (using anti-CD49d antibody P1H4); (top right) α5 (using anti-CD49e antibody SAM-1); (bottom left) α9 (using anti-α9β1 antibody Y9A2); (bottom right) β1 (using anti-CD29 antibody B-D15) and their corresponding isotype controls.
Table 1.
The antibody binding capacity for integrins, expressed on neutrophils
| Antibody | Targeted integrin | Antibody binding capacity |
|---|---|---|
| B-D15 | β1 (CD29) | 8000 |
| P1H4 | α4 (CD49d) | 2500 |
| SAM-1 | α5 (CD49e) | 2100 |
| Y9A2 | α9β1 (VLA-9) | 2500 |
In our initial experiments to determine the extent to which these integrins might mediate neutrophil adhesion to VCAM-1, adhesion measurements were performed on 15 bead-cell pairs from each of seven different donors using VCAM-coated beads in the presence of Mg2+ plus EGTA or Ca2+. Cells were perturbed as little as possible. A drop of whole blood was dispersed in buffer and cells were selected individually for testing based on their observed multi-lobular nuclear structure. In these initial experiments, each cell was placed in contact with a bead for 60 seconds at 37°C and then separated. The probability of adhesion to VCAM-1 coated beads was significantly higher in the presence of Mg2+ plus EGTA (53%) than in Ca2+ (23%), confirming expectations that replacement of calcium with magnesium should increase integrin-mediated adhesion. In the presence of Mg2+ plus EGTA at 37°C the adhesion probability for 2 s contacts (46%) was not statistically different from that measured for 60 s contacts (53%), but at room temperature, the adhesion probability increased significantly over this time frame: Padh = 59% for 60 s contacts versus 33% for 2 s (Fig. 3).
Figure 3.
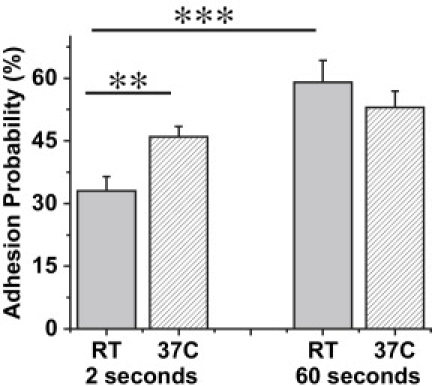
Time and temperature dependent adhesion probability for VCAM-1 coated bead - cell interactions. The experiments were performed in the presence of 5 mM Mg2+ plus EGTA at room temperature. For 2 s contacts each bar represents data from 10 cell-bead pairs from each of three donors (total of 30 cell-bead pairs). Each cell - bead pair was contacted 25 times. For 60 s contacts, n = 105 from seven donors. The surface concentration of VCAM was ∼650 sites per μm2. Error bars indicate standard error. The stars denote a statistically significant difference in adhesion probability (∗∗p < 0.01 and ∗∗∗p < 0.001, Student's t-test).
The finding that the adhesion process in 5 mM Mg2+ saturated at probabilities <100% at high concentrations of VCAM-1 (650 sites/μm2) and long contact times led to the conclusion that this process cannot be described with a simple bimolecular kinetic model (57). In the companion article (34), an alternative kinetic scheme is proposed in which the formation of adhesive contacts is limited by the formation of reaction zones at the interface of the cell and the substrate. The governing equation for this process at high VCAM-1 concentrations is given in Eq 2. The kinetics of adhesive contact formation at room temperature is thus characterized in terms of the coefficients τRZ and KDRZ. To determine these coefficients, adhesion probability was measured for 1, 2, 5, 10, 20 and 60 second contacts between neutrophils and VCAM-1 coated beads. Parallel experiments in the presence of the β1 blocking antibody 4B4 served as control. Adhesion probability was converted to the number of bonds formed per unit contact area according to Eq. 1. In the presence of a blocking antibody, the apparent number of bonds formed increased more or less linearly with time (Fig. 4 A). Adhesion in the absence of a blocking antibody appeared to consist of the sum of two contributions: specific interactions (those not blockable by 4B4) with an exponential dependence on time plus a linearly increasing nonspecific component. The nonspecific component was determined by linear regression to the data obtained in the presence of 4B4, and those coefficients were used to assess the kinetic rates for specific neutrophil/VCAM-1 interactions. Background adhesion was similar in the Mn2+, Mg2+ or Ca2+buffers. The increase in adhesion with time also showed similar behavior but different magnitudes of adhesion in the presence of 5 mM Mg2+ plus EGTA (Fig. 4 A), 3 mM Mn2+ (Fig. 4 B) or in the presence of 1.5 mM Ca2+ (Fig. 4 C). The kinetic constants were obtained from least squares regression to Eq. 2 accounting for background adhesion. For VCAM-1/neutrophil interactions in the presence of 5.0 mM Mg2+: τRZ = 14.8 ± 4.09 s, KDRZ = 53.7 ± 3.67, and 〈n〉o = 0.19, in the presence of 3.0 mM Mn2+: τRZ = 3.21 ± 2.894, KDRZ = 23.25 ± 4.53, and 〈n〉o = 0.29, and in the presence of 1.5 mM Ca2+, τRZ = 2.33 ± 1.15 s, KDRZ = 160 ± 23 and 〈n〉o ≈ 0. This corresponds to values for the kinetic coefficients: in Mn2+, k+ = 0.013 s−1, k−BI = 0.30 s−1, and k− = 1.65 s−1, Mg2+, k+ = 1.2×10−3 s−1, k−BI = 0.066 s−1, and k− = 0.25 s−1, and in Ca2+, k+ = 2.7×10−3 s−1, k−BI = 0.43 s−1, and k− is too large for us to measure.
Figure 4.
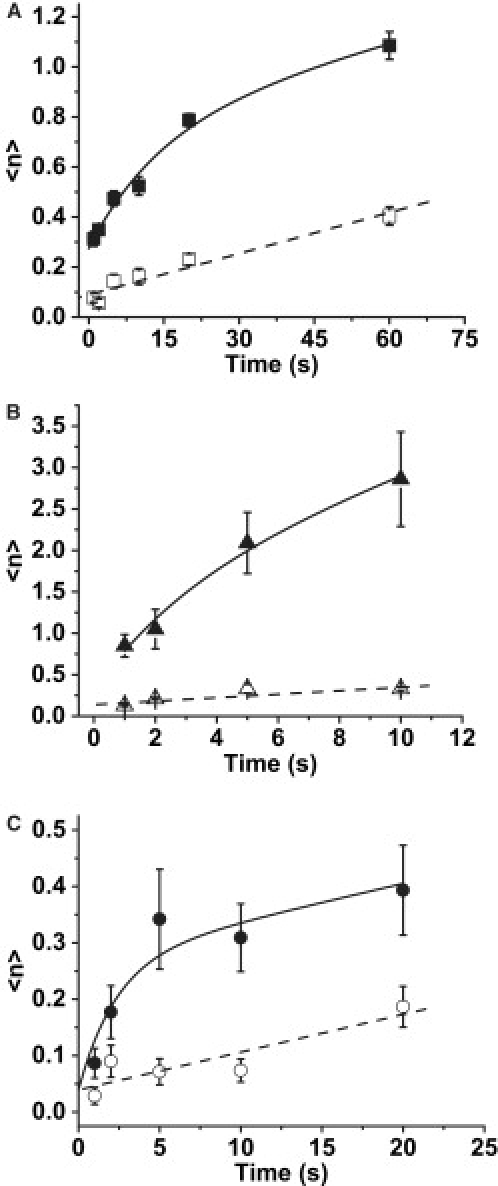
Dependence of adhesion probability on contact time. The experiments were performed at room temperature as repeated tests of cell-bead touches for different contact durations. The total adhesion probability was converted to the expected number of bonds 〈n〉 (see Eq. 1) and plotted as 〈n〉 versus time. Error bars indicate standard error. (A) The experiments were performed in the presence of 5 mM Mg2+ plus EGTA for 1, 2, 5, 10, 20 and 60 seconds duration. The model used to fit the upper data was Eq 2 plus a linear portion reflecting background adhesion: 0.0055x + 0.089. From nonlinear least squares regression, 〈n〉o = 0.19 ± 0.035, τRZ = 14.8 ± 4.09 s, and KDRZ = 53.7 ± 3.67. (B) The experiments were performed in 3 mM Mn for 1, .2, .5, 10, and 20 seconds duration. Model used to fit the data was Eq 2 with a linear portion reflecting background: 0.137 + 0.021x. The fitted parameters were 〈n〉o = 0.29 ± 0.418, τRZ = 3.21 ± 2.894 s, and KDRZ = 23.25 ± 4.533. (C) The experiments were performed in the presence of 1.5 mM Ca2+ for 1, 2, 5, 10 and 20 seconds duration. Model used to fit the data was Eq 2 with a linear portion reflecting background: 0.039 + 0.0067x. The fitted parameters were 〈n〉o = 0.0 (fixed), τRZ = 2.33 ± 1.15 s, and KDRZ = 160 ± 23.
To evaluate which ligands on the neutrophil mediate adhesion to VCAM-1, additional experiments with blocking antibodies were performed. The addition of anti-β1 blocking antibody 4B4 caused a 70% reduction in binding to VCAM-1 in every buffer tested in the study (Fig. 5 A). The presence of β2 blocking antibody had no significant effect on the adhesion probability (Fig. 5 B). The significant reduction caused by β1 blocking antibody is shown for comparison. Thus, the specific binding of neutrophils to VCAM-1 is attributable to members of the β1 integrin family. Additional blocking experiments were performed to determine which β1-integrins on the neutrophil surface mediate the adhesion to VCAM-1. Cells were tested for 20 second contacts at room temperature. In 5 mM Mg2+, the presence of Y9A2 antibody (anti-α9) or SAM-1 (anti-α5) had little effect on adhesion probability, but P1H4 (anti-α4) as well as the combination of SAM-1 and P1H4 gave a reduction in adhesion that was significant and statistically indistinguishable from the baseline levels (presence of 4B4 mAb) (Fig. 5 C). In 1.5 mM Ca2+, only VLA-4 is expected to exhibit appreciable affinity for VCAM-1 (27,36). Consistent with this, the α4-blocking antibody P1H4 reduced neutrophil adhesion in Ca2+ to baseline levels (Fig. 5 D). Similar results were obtained for two-second contacts, but the observed differences were less distinct because of the lower adhesion probabilities.
Figure 5.
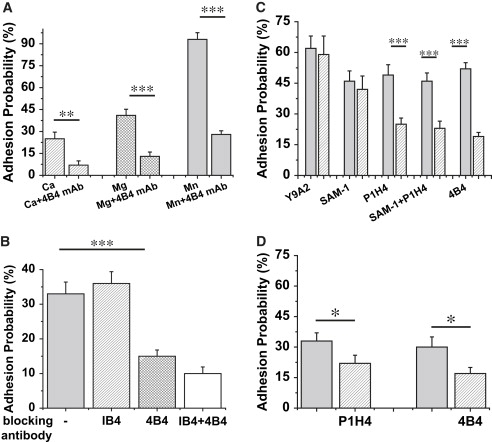
Effect of blocking antibodies on adhesion to VCAM-coated beads. (A) Every bar represents the adhesion probability to VCAM-1 coated beads for 10 second contacts. The amount of VCAM is 650 sites/μm2. Addition of anti-β1 blocking antibody 4B4 caused the 70% reduction in adhesion probability in all buffers tested in the study: 1.5 mM Ca2+, 5 mM Mg2+, 3 mM Mn2+. (B) Each bar represents 30 cell - VCAM-bead pairs interacting for 2 seconds (25 contacts for each pair) in the presence of 5.0 mM Mg2+ plus EGTA at room temperature. The amount of VCAM is 650 sites/μm2. The β2 blocking antibody IB4 had no effect on the adhesion probability, but the β1 blocking antibody 4B4 reduced the adhesion probability to 15%. The slight additional degrease in Padh when 4B4 and IB4 were used in combination was not statistically significant. (C and D) Experiments were performed as repeated tests of 20 contacts for each cell-bead pair. Contact time was 20 seconds. Each bar represents data from 8 to 36 cells total. Gray bars represent control condition for the sparsed bars (condition labeled on the x axis). (C) In the presence of 5 mM Mg2+ plus EGTA; (D) In the presence of 1.5 mM Ca2+. Error bars represent standard error. The asterisk denotes a statistically significant difference in adhesion probability (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; Student's t-test).
Discussion
Mediators of adhesion to VCAM-1
Our results indicate that neutrophil binding to VCAM-1 under the conditions of our experiments is primarily mediated by VLA-4. Other candidates for binding to VCAM-1 that are present on neutrophils include VLA-5 and VLA-9 (α5β1 and α9β1), but blocking antibodies against these forms had negligible effect on adhesion. For the case of α9β1, this is consistent with solution studies that showed a much lower affinity of α9β1 than α4β1 for VCAM-1 (37). The β2 integrin family members αXβ2 (CD11c/CD18) and αDβ2 have also been detected on neutrophils (38,39). The latter, αDβ2, can be rapidly upregulated in response to stimulus (40), and both are known to bind preferentially to VCAM-1(40–43). However, the lack of inhibition of adhesion by the β2 blocking antibody IB4 indicates that most, if not all, adhesion of neutrophils to VCAM-1 is mediated through β1-integrins, and that β2-integrins play a minor, if any, role in mediating the adhesive contacts for resting cells to VCAM-1 coated surfaces. A possible explanation for this is that the other integrins are confined to regions of the surface that do not make physical contact with the substrate, or that these forms are simply not present in a high affinity conformation under the conditions of our study.
Divalent ions and integrin activation
The use of Mn2+ and Mg2+, rather than other more physiological activators of neutrophils, to induce the high affinity conformation of integrins enabled us to study the dynamics of integrin-VCAM-1 interactions in the absence of whole cell activation. Prior studies in our laboratory have shown that Mg2+ has no effect on L-selectin or β2 integrin expression on the cell surface (44), an important sign that the neutrophils are not activated even though integrins assume the high affinity conformation. (Upregulation of the β2 integrin Mac-1 and downregulation of the surface expression of L-selectin are early markers of cell activation (45–47).) Our observation that Mg2+ and Ca2+ caused increases in cell adhesion over controls is consistent with prior studies of cell adhesion to VCAM-1 coated substrates that demonstrated that VLA-4 can mediate adhesion in the presence of either of these metal ions (48). Other reports confirm this and also show that VLA-4 affinity is higher in Mg2+ than in Ca2+, and highest in the presence of Mn2+ (49). The ability of VLA-4 to bind ligand effectively in the presence of Ca2+ is unusual among integrins, most of which assume a low affinity conformation in the presence of Ca2+. That we observe neutrophil adhesion to VCAM-1 in the presence of Ca2+ supports our conclusion that VLA-4 is the principal mediator of cell adhesion in this study.
Meaning of the coefficients
The probability that a cell will adhere to a surface depends directly on the rate at which bonds between the molecules on the opposing surfaces can form. When molecules are confined to the surfaces of cells, formation of adhesive bonds reflects contributions from multiple mechanisms operating on molecular and microscopic scales. In addition to the intrinsic reactivity of the molecules themselves (which may itself vary with conditions), adhesion depends on several extrinsic factors, such as the size of the contact area, the lateral distribution and mobility of the molecules on the cell membrane, and the topography of both the cell and substrate surfaces. A significant aspect of our study is the finding that the probability of adhesion is independent of the concentration of VCAM-1 on the bead surface over the range of surface concentrations >200 sites/μm2. This observation led to the development of a novel kinetic model that explicitly recognizes the need to have an active molecule in close contact with the substrate if bond formation is to proceed (34). We refer to these localized sites as reaction zones. In that model, the kinetics of binding involves five kinetic coefficients: k+ and k−, governing the formation of a reaction zone; kf and kr, representing the kinetics of transition between bonded and unbonded reaction zones; and kBI−, representing the transition of a bond to an inactive zone.
Of the five parameters, the ones associated with reaction zone formation, k+, k−, and, to some extent, kBI−, reflect most of the influence of extrinsic influences on bond formation, including surface topography and the nonuniform distribution of molecules on the cell surface. Although it has never been critically tested, these factors are not expected to be influenced by different divalent ion compositions in the extracellular space. In this regard, our finding that k+, k−, and kBI− are all affected by divalent ion composition may seem surprising. But reaction zone formation also requires that molecules in regions of close contact be in a high affinity state and capable of forming a bond in the interface. Evidence that divalent ions affect molecular conformation and affinity comes from the work of Chigaev et al (49), who used fluorescence energy transfer to reveal changes in molecular folding in concentrations of different divalent cations. Thus, the results from this report indicate that changes in molecular conformation between low and high affinity forms are a key mechanism underlying the kinetics of reaction zone formation.
Insights from solution kinetics
It is instructive to review the effects of divalent ions on the kinetics of VLA-4–VCAM-1 interactions in the solution. Measurements of kinetic constants for VLA-4–VCAM-1 interactions in Mn2+ gave values for Kd, kr and kf of 30 nM, 0.03 s−1, and ∼10.0 × 105 M−1 s−1, respectively (49). The value of Kd increased upon the addition of Ca2+ as a result of increasing off rate, e.g. at 10 mM Ca2+, Kd = 500 nM, and kr = 1.2 s−1. Using a small peptide to mimic VCAM-1 binding to VLA-4, Chen et al. (50) also found that off rates in the presence of calcium were substantially higher than for Mg2+ or Mn2+. This trend is also evident in our own findings that the reverse rate for molecular activation is very large in the presence of Ca2+. In contrast, solution studies show very little dependence of forward rates on divalent ion composition. This led Chen and colleagues to speculate that in their studies of small molecule binding from solution, it was the binding reaction (rather than conformational change) that was facilitated by the presence of Mg2+ or Mn2+. This view is contradicted by studies using activation-sensitive antibodies that show substantial increases in binding in the presence of activating cations (27,51). It is also contradicted by our own results that k+, reflecting the forward rate for molecular activation, is an order of magnitude larger in the presence of Mn2+, indicating that Mn2+ facilitates the conformational change leading to high affinity.
Is VLA-4 relevant for neutrophil adhesion in human health?
Conventional wisdom that VLA-4 should play a relatively minor role in neutrophil adhesion in the human vasculature seems well justified based on the relative numbers of β1 and β2 integrins on human neutrophils (e.g., 2500 copies of VLA-4 vs. 25,000 copies of LFA-1 (αLβ2)). Although there is strong evidence for the importance of VLA-4–VCAM-1 interactions in mice (52,53), extrapolation of observations in mice to the human system are of questionable value in this case because, unlike their human counterparts, mouse neutrophils express VLA-4 at high levels (54,55). In addition to lower expression of VLA-4 in human systems, the expression of VCAM-1 on human endothelial cells appears to be lower than expression of ICAM-1, for example, although there is evidence that VCAM-1 expression can be significant. Human umbilical vein endothelial cells (HUVEC) increase their expression of VCAM-1 roughly 10-fold after treatment with inflammatory agents such as TNFα, although the levels remain substantially lower (30-fold) than those for ICAM-1 in the same system (56). Hentzen et al. (57) report ICAM-1 densities in the range of 9000 sites/μm2 for HUVEC, suggesting VCAM-1 levels should be on the order of 300 sites/μm2, a number that is intriguingly close to the density at which neutrophil interaction becomes independent of VCAM-1 density in our study. Thus, although direct measurements of VCAM-1 expression in human vasculature are not available, it seems probable that the VLA-4 ligand VCAM-1 is expressed at substantial levels in the human system.
Even though the expression levels of VLA-4 are substantially lower than the β2 integrins in human neutrophils, the observations presented here indicate that their ability to form bonds is comparable. For example, for the specific case of a 2s contact with a surface having a ligand density of 200 sites/μm2 the expected bond number for fully activated VLA-4 (in Mn2+) binding to VCAM-1 is ∼0.13/μm2, whereas the expected bond number for LFA-1 mediated binding to ICAM-1 under comparable conditions is ∼0.04/μm2. (Compare Fig. 4 B herein with Fig. 6 in reference (30). Thus, even though the surface density of VLA-4 on neutrophils is ten times smaller than LFA-1, its ability to form bonds under comparable conditions appears to be threefold higher! Furthermore, VLA-4 is known to mediate adhesion of lymphocytes to HUVECs (17), yet expression levels of VLA-4 on lymphocytes is only about fourfold higher than on neutrophils, ∼10,000 copies per cell (50). Based on these comparisons, a significant role for VLA-4–VCAM-1 interactions appears likely for human neutrophils.
Conclusions
Measurements of adhesion frequency between neutrophils and bead surfaces coated with VCAM-1 reveal substantial β1-integrin mediated adhesion, principally via α4β1 (VLA-4). Although adhesion is observed in the presence of Ca2+, it is enhanced in the presence of Mg2+, and substantially enhanced in the presence of Mn2+, consistent with known characteristics of VLA-4. The system behaves in a manner consistent with a kinetic scheme in which the formation of a reaction zone, i.e., a region of membrane in close contact with a substrate containing at least one adhesion molecule in the high affinity state, is, in this case, a key rate-limiting step. The small number of VLA-4 molecules on the neutrophil surface and their different affinity states in different cationic environments has enabled us to distinguish and characterize this distinct step. The dependence of reaction zone formation on the divalent cations present in the solution and the likelihood that different divalent ions may alter the distribution of VLA-4 between high and low affinity forms suggests that the kinetics of reaction zone formation involves fluctuation of VLA-4 molecules between low and high affinity states. These measurements represent the first, to our knowledge, quantitative characterization of adhesion dynamics between neutrophils and VCAM-1, and reveal a more complex kinetic behavior than previously supposed.
Acknowledgments
The authors thank Richard Bauserman and Donna Brooks for technical support and Christopher Spillmann for scientific discussions.
This work was supported by the National Institute of Health, NHLBI, Program project grant No. PO1-HL18208 and by supplemental funds from the University of Rochester School of Medicine and Dentistry.
References
- 1.Butcher E.C., Picker L.J. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 2.Harris E.S., McIntyre T.M., Prescott S.M., Zimmerman G.A. The leukocyte integrins. J. Biol. Chem. 2000;275:23409–23412. doi: 10.1074/jbc.R000004200. [DOI] [PubMed] [Google Scholar]
- 3.Springer T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence M.B., Smith C.W., Eskin S.G., McIntire L.V. Effect of Venous Shear Stress on CD18-Mediated Neutrophil Adhesion to Cultured Endothelium. Blood. 1990;75:227–237. [PubMed] [Google Scholar]
- 5.Lum A.F., Green C.E., Lee G.R., Staunton D.E., Simon S.I. Dynamic regulation of LFA-1 activation and neutrophil arrest on intercellular adhesion molecule 1 (ICAM-1) in shear flow. J. Biol. Chem. 2002;277:20660–20670. doi: 10.1074/jbc.M202223200. [DOI] [PubMed] [Google Scholar]
- 6.Jones D.A., Smith C.W., McIntire L.V. Leucocyte adhesion under flow conditions: principles important in tissue engineering. Biomaterials. 1996;17:337–347. doi: 10.1016/0142-9612(96)85572-4. [DOI] [PubMed] [Google Scholar]
- 7.Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59:1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- 8.Rice G.E., Bevilacqua M.P. An inducible endothelial cell surface glycoprotein mediates melanoma adhesion. Science. 1989;246:1303–1306. doi: 10.1126/science.2588007. [DOI] [PubMed] [Google Scholar]
- 9.Alon R., Kassner P.D., Carr M.W., Finger E.B., Hemler M.E. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J. Cell Biol. 1995;128:1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berlin C., Bargatze R.F., Campbell J.J., von Andrian U.H., Szabo M.C. α4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 11.Luscinskas F.W., Kansas G.S., Ding H., Pizcueta P., Schleiffenbaum B.E. Monocyte rolling, arrest and spreading on IL-4-activated vascular endothelium under flow is mediated via sequential action of L-selectin, β1-integrins, and β2-integrins. J. Cell Biol. 1994;125:1417–1427. doi: 10.1083/jcb.125.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bochner B.S., Luscinskas F.W., Gimbrone M.A., Newman W., Sterbinsky S.A. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J. Exp. Med. 1991;173:1553–1557. doi: 10.1084/jem.173.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shang T., Yednock T., Issekutz A.C. α9β1 integrin is expressed on human neutrophils and contributes to neutrophil migration through human lung and synovial fibroblast barriers. J. Leukoc. Biol. 1999;66:809–816. doi: 10.1002/jlb.66.5.809. [DOI] [PubMed] [Google Scholar]
- 14.van den Berg J.M., Mul F.P., Schippers E., Weening J.J., Roos D. β1 integrin activation on human neutrophils promotes β2 integrin-mediated adhesion to fibronectin. Eur. J. Immunol. 2001;31:276–284. doi: 10.1002/1521-4141(200101)31:1<276::AID-IMMU276>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 15.Taooka Y., Chen J., Yednock T., Sheppard D. The integrin α9β1 mediates adhesion to activated endothelial cells and transendothelial neutrophil migration through interaction with vascular cell adhesion molecule-1. J. Cell Biol. 1999;145:413–420. doi: 10.1083/jcb.145.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinhardt P.H., Elliott J.F., Kubes P. Neutrophils can adhere via α4β1-integrin under flow conditions. Blood. 1997;89:3837–3846. [PubMed] [Google Scholar]
- 17.Reinhardt P.H., Kubes P. Differential leukocyte recruitment from whole blood via endothelial adhesion molecules under shear conditions. Blood. 1998;92:4691–4699. [PubMed] [Google Scholar]
- 18.Bazzoni G., Hemler M.E. Are changes in integrin affinity and conformation overemphasized? Trends Biochem. Sci. 1998;23:30–34. doi: 10.1016/s0968-0004(97)01141-9. [DOI] [PubMed] [Google Scholar]
- 19.Lollo B.A., Chan K.W., Hanson E.M., Moy V.T., Brian A.A. Direct evidence for two affinity states for lymphocyte function-associated antigen 1 on activated T cells. J. Biol. Chem. 1993;268:21693–21700. [erratum appears in J. Biol. Chem. 1994 Apr 1;269(13):10184] [PubMed] [Google Scholar]
- 20.Woodside D.G., Liu S., Ginsberg M.H. Integrin Activation. Thromb. Haemost. 2001;86:316–323. [PubMed] [Google Scholar]
- 21.Shimaoka M., Takagi J., Springer T.A. Conformational regulation of integrin structure and function. Annu. Rev. Biophys. Biomol. Struct. 2002;31:485–516. doi: 10.1146/annurev.biophys.31.101101.140922. [DOI] [PubMed] [Google Scholar]
- 22.Takagi J., Petre B., Walz T., Springer T. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 2002;110:599–611. doi: 10.1016/s0092-8674(02)00935-2. [DOI] [PubMed] [Google Scholar]
- 23.Kim M., Carman C.V., Springer T.A. Bidirectional Transmembrane Signaling by Cytoplasmic Domain Separation in Integrins. Science. 2003;301:1720–1725. doi: 10.1126/science.1084174. [DOI] [PubMed] [Google Scholar]
- 24.Hogg N., Harvey J., Cabanas C., Landis R.C. Control of leukocyte integrin activation. Am. Rev. Respir. Dis. 1993;148:S55–S59. doi: 10.1164/ajrccm/148.6_Pt_2.S55. [DOI] [PubMed] [Google Scholar]
- 25.Kovach N.L., Carlos T.M., Yee E., Harlan J.M. A monoclonal antibody to β 1 integrin (CD29) stimulates VLA-dependent adherence of leukocytes to human umbilical vein endothelial cells and matrix components. J. Cell Biol. 1992;116:499–509. doi: 10.1083/jcb.116.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yednock T.A., Cannon C., Vandevert C., Goldbach E.G., Shaw G. α 4 β 1 integrin-dependent cell adhesion is regulated by a low affinity receptor pool that is conformationally responsive to ligand. J. Biol. Chem. 1995;270:28740–28750. doi: 10.1074/jbc.270.48.28740. [DOI] [PubMed] [Google Scholar]
- 27.Bazzoni G., Ma L., Blue M.L., Hemler M.E. Divlent cations and ligands induce conformational changes that are highly divergent among β1 integrins. J. Biol. Chem. 1998;273:6670–6678. doi: 10.1074/jbc.273.12.6670. [DOI] [PubMed] [Google Scholar]
- 28.Dransfield I., Cabanas C., Craig A., Hogg N. Divalent cation regulation of the function of the leukocyte integrin LFA-1. J. Cell Biol. 1992;116:219–226. doi: 10.1083/jcb.116.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu C., Shimaoka M., Zang Q., Takagi J., Springer T.A. Locking in alternate conformations of the integrin αLβ2 I domain with disulfide bonds reveals functional relationships among integrin domains. Proc. Natl. Acad. Sci. USA. 2001;98:2393–2398. doi: 10.1073/pnas.041618598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lomakina E.B., Waugh R.E. Micromechanical Tests of Adhesion Dynamics between Neutrophils and Immobilized ICAM-1. Biophys. J. 2004;86:1223–1233. doi: 10.1016/S0006-3495(04)74196-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diamond M.S., Springer T.A. A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J. Cell Biol. 1993;120:545–556. doi: 10.1083/jcb.120.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leitinger B., Hogg N. Effects of I domain deletion on the function of the β2 integrin lymphocyte function-associated antigen-1. Mol. Biol. Cell. 2000;11:677–690. doi: 10.1091/mbc.11.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Labadia M.E., Jeanfavre D.D., Caviness G.O., Morelock M.M. Molecular regulation of the interaction between leukocyte function-associated antigen-1 and soluble ICAM-1 by divalent metal cations. J. Immunol. 1998;161:836–842. [PubMed] [Google Scholar]
- 34.Lomakina E.B., Waugh R.W. Active site formation, not bond kinetics, limits adhesion rate between human neutrophils and immobilized vascular cell adhesion molecule 1. Biophsy. J. 2009;96:268–275. doi: 10.1016/j.bpj.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chesla S.E., Selvaraj P., Zhu C. Measuring two-dimentional receptor-ligand binding kinetics by micropipette. Biophys. J. 1998;75:1553–1572. doi: 10.1016/S0006-3495(98)74074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masumoto A., Hemler M.E. Mutation of putative divalent cation sites in the α 4 subunit of the integrin VLA-4: distinct effects on adhesion to CS1/fibronectin, VCAM-1, and invasin. J. Cell Biol. 1993;123:245–253. doi: 10.1083/jcb.123.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pepinsky R.B., Mumford R.A., Chen L.L., Leone D., Amo S.E. Comparative assessment of the ligand and metal ion binding properties of integrins α9β1 and α4β1. Biochemistry. 2002;41:7125–7141. doi: 10.1021/bi020024d. [DOI] [PubMed] [Google Scholar]
- 38.Arnaout M.A. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990;75:1037–1050. [PubMed] [Google Scholar]
- 39.Grayson M.H., Van der Vieren M., Gallatin W.M., Hoffman P.A., Bootman M.D. Expression of novel β2 integrin (alfaDβ2) on human leukocytes and mast cells. J. Allergy Clin. Immunol. 1997;99:S386. [Google Scholar]
- 40.Grayson M.H., Van der Vieren M., Sterbinsky S.A., Michael Gallatin W., Hoffman P.A. αdβ2 integrin is expressed on human eosinophils and functions as an alternative ligand for vascular cell adhesion molecule 1 (VCAM-1) J. Exp. Med. 1998;188:2187–2191. doi: 10.1084/jem.188.11.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grayson M.H., Van der Vieren M., Sterbinsky S.A., Gallantin W.M., Hoffman P. αdβ2 integrin is a ligand for vascular cell adhesion molecule-1. Int. Arch. Allergy Immunol. 1999;118:263–264. doi: 10.1159/000024094. [DOI] [PubMed] [Google Scholar]
- 42.Van der Vieren M., Crowe D.T., Hoekstra D., Vazeux R., Hoffman P.A. The leukocyte integrin α D β 2 binds VCAM-1: evidence for a binding interface between I domain and VCAM-1. J. Immunol. 1999;163:1984–1990. [PubMed] [Google Scholar]
- 43.Sadhu C., Ting H.J., Lipsky B., Hensley K., Garcia-Martinez L.F. CD11c/CD18: novel ligands and a role in delayed-type hypersensitivity. J. Leukoc. Biol. 2007;81:1395–1403. doi: 10.1189/jlb.1106680. [DOI] [PubMed] [Google Scholar]
- 44.Spillmann C., Osorio D., Waugh R.E. Integrin activation by divalent ions affects neutrophil homotypic adhesion. Ann. Biomed. Eng. 2002;30:1002–1011. doi: 10.1114/1.1511241. [DOI] [PubMed] [Google Scholar]
- 45.Anderson S.I., Hotchin N.A., Nash G.B. Role of the cytoskeleton in rapid activation of CD11b/CD18 function and its subsequent downregulation in neutrophils. J. Cell Sci. 2000;113:2737–2745. doi: 10.1242/jcs.113.15.2737. [DOI] [PubMed] [Google Scholar]
- 46.Erlandsen S.L., Hasslen S.R., Nelson R.D. Detection and spatial distribution of the β 2 integrin (Mac-1) and L-selectin (LECAM-1) adherence receptors on human neutrophils by high-resolution field emission SEM. J. Histochem. Cytochem. 1993;41:327–333. doi: 10.1177/41.3.7679125. [DOI] [PubMed] [Google Scholar]
- 47.Taylor A.D., Neelamegham S., Hellums J.D., Smith C.W., Simon S.I. Molecular dynamics of the transition from L-selectin- to β 2-integrin-dependent neutrophil adhesion under defined hydrodynamic shear. Biophys. J. 1996;71:3488–3500. doi: 10.1016/S0006-3495(96)79544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Day E.S., Osborn L., Whitty A. Effect of divalent cations on the affinity and selectivity of α4 integrins towards the integrin ligands vascular cell adhesion molecule-1 and mucosal addressin cell adhesion molecule-1: Ca2+ activation of integrin α4β1 confers a distinct ligand specificity. Cell Commun. Adhes. 2002;9:205–219. doi: 10.1080/15419060216014. [DOI] [PubMed] [Google Scholar]
- 49.Chigaev A., Zwartz G., Graves S.W., Dwyer D.C., Tsuji H. α4β1 integrin affinity changes govern cell adhesion. J. Biol. Chem. 2003;278:38174–38182. doi: 10.1074/jbc.M210472200. [DOI] [PubMed] [Google Scholar]
- 50.Chen L.L., Whitty A., Lobb R.R., Adams S.P., Pepinsky R.B. Multiple activation states of integrin α4β1 detected through their different affinities for a small molecule ligand. J. Biol. Chem. 1999;274:13167–13175. doi: 10.1074/jbc.274.19.13167. [DOI] [PubMed] [Google Scholar]
- 51.Takamatsu Y., Simmons P.J., Levesque J.P. Dual control by divalent cations and mitogenic cytokines of α 4 β 1 and α 5 β 1 integrin avidity expressed by human hemopoietic cells. Cell Adhes. Commun. 1998;5:349–366. doi: 10.3109/15419069809010781. [DOI] [PubMed] [Google Scholar]
- 52.Tasaka S., Richer S.E., Mizgerd J.P., Doerschuk C.M. Very late antigen-4 in CD18-independent neutrophil emigration during acute bacterial pneumonia in mice. Am. J. Respir. Crit. Care Med. 2002;166:53–60. doi: 10.1164/rccm.2105034. [DOI] [PubMed] [Google Scholar]
- 53.Bowden R.A., Ding Z.M., Donnachie E.M., Petersen T.K., Michael L.H. Role of α4 integrin and VCAM-1 in CD18-independent neutrophil migration across mouse cardiac endothelium. Circ. Res. 2002;90:562–569. doi: 10.1161/01.res.0000013835.53611.97. [DOI] [PubMed] [Google Scholar]
- 54.Ridger V.C., Wagner B.E., Wallace W.A., Hellewell P.G. Differential effects of CD18, CD29, and CD49 integrin subunit inhibition on neutrophil migration in pulmonary inflammation. J. Immunol. 2001;166:3484–3490. doi: 10.4049/jimmunol.166.5.3484. [DOI] [PubMed] [Google Scholar]
- 55.Henderson R.B., Lim L.H., Tessier P.A., Gavins F.N., Mathies M. The use of lymphocyte function-associated antigen (LFA)-1-deficient mice to determine the role of LFA-1, Mac-1, and alpha4 integrin in the inflammatory response of neutrophils. J. Exp. Med. 2001;194:219–226. doi: 10.1084/jem.194.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fiehn C., Paleolog E.M., Feldmann M. Selective enhancement of endothelial cell VCAM-1 expression by interleukin-10 in the presence of activated leucocytes. Immunology. 1997;91:565–571. doi: 10.1046/j.1365-2567.1997.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hentzen E.R., Neelamegham S., Kansas G.S., Benanti J.A., McIntire L.V. Sequential binding of CD11a/CD18 and CD11b/CD18 defines neutrophil capture and stable adhesion to intercellular adhesion molecule-1. Blood. 2000;95:911–920. [PubMed] [Google Scholar]


