Abstract
The complications of amebic liver abscess (ALA) are underappreciated in developed countries, and are often misdiagnosed. We report a 16 month-old with ALA, initially misdiagnosed with pneumonia, who became critically ill with peritoneal, pleural and pericardial extension, and gastric perforation. In addition to highlighting the complications of ALA, this case demonstrates the value of PCR testing as a diagnostic and molecular tool.
Keywords: Entamoeba histolytica, invasive amebiasis, amebic liver abscess, thoracic amebiasis, complicated amebiasis
INTRODUCTION
Amebiasis is responsible for approximately 100,000 deaths per year worldwide (1). In some tropical countries, the antibody prevalence rate exceeds 50%; in the United States, the seroprevalence is estimated at 4%. The majority of those who are infected (90%) remain relatively asymptomatic. The other 10% manifest the disease in intestinal or extraintestinal forms, both of which can be life-threatening. Because the extraintestinal forms of this disease are relatively uncommon, particularly in children in developed nations, cases are prone to misdiagnosis. This is the first reported case of ALA with complications of peritonitis, pleural and pericardial involvement, and gastric rupture, and serves as an important reminder of these clinical complications. A discussion of management and molecular tools for diagnosis is also presented.
CASE REPORT
A 16 month-old male presented to the Emergency Department after having difficulty breathing and perioral cyanosis at home. His illness began three weeks prior, with afebrile diarrhea; several family members were also ill. Though the patient's diarrhea resolved, he continued to have decreased activity and preferred crawling to walking. Four days prior to his visit to the ER, he developed fever and on the day of admission his parents noticed sudden labored breathing and blueness around his lips. Past medical and social histories were contributory only for the patient being the child of Mexican immigrants. The child himself had no recent travel and no unusual exposures, and was fully vaccinated. Evaluation in the Emergency Department was significant for ill appearance, temperature 40.5° C, heart rate 180 beats/minute, respirations 62/minute, oxygen saturation 96%, decreased breath sounds at the left lung base, and tenderness and guarding of his upper abdomen. Laboratory studies were significant for a white cell count of 12.0 × 109/L, with 58% segmented neutrophils, 13% band forms, 15% lymphocytes, 6% monocytes, and 2% eosinophils. The hematocrit was 37.5%, platelet count 484 × 103/uL, erythrocyte sedimentation rate 33 mm/hr and C-reactive protein 57.4 mg/dL. Respiratory acidosis was present, with an arterial pH of 7.33, and normal liver function tests. Chest radiography revealed a left basilar opacity with pleural effusion (Fig 1). He was admitted with a diagnosis of lobar pneumonia and started on cefuroxime.
Fig. 1.
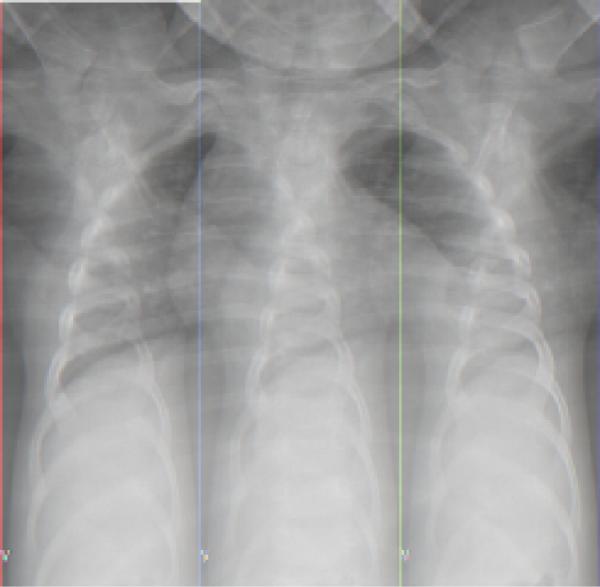
Chest radiograph upon admission demonstrating left lower lobe infiltrate and effusion
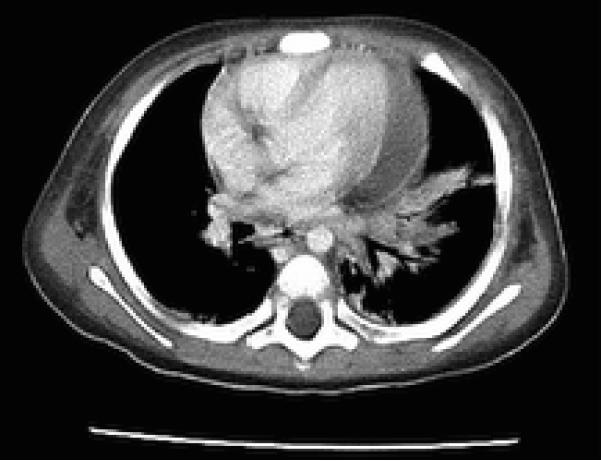
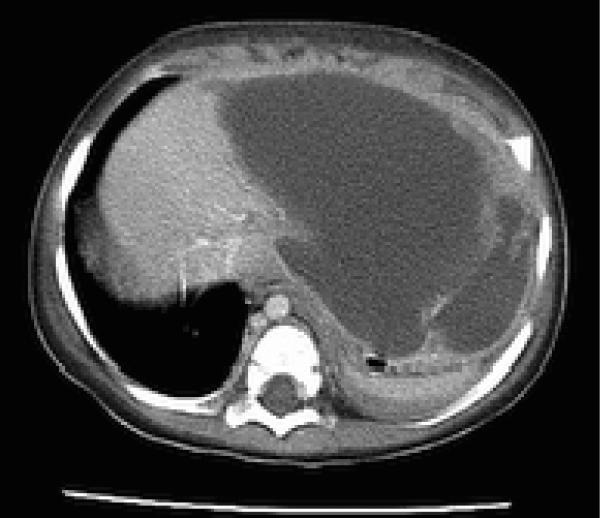
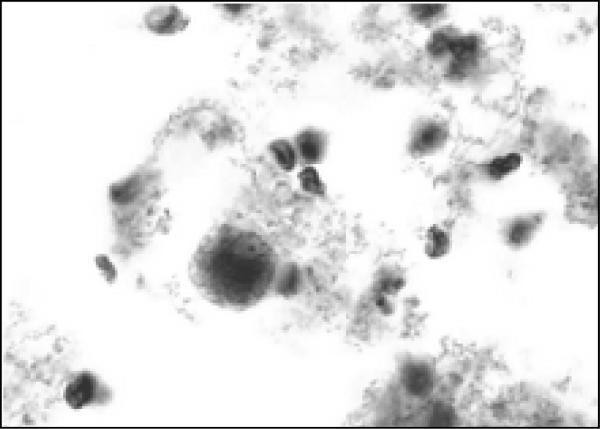
Over the next 48 hours, his clinical condition did not improve. A CT of the chest and abdomen confirmed consolidation at the left lung base with a left pleural effusion. In addition, a moderate pericardial effusion was observed and the left hemidiaphragm was elevated by a 12 cm cystic fluid collection that extended along the lesser curve of the stomach (Fig 2 and 3). Though the liver was compressed by this mass, there was no evidence of intrahepatic disease. The origin of the mass was unclear, and it was initially thought to be a gastric teratoma or duplication cyst; therefore surgical consultation was obtained. Intraoperatively, a hole through the left anterior lobe of the liver was noted, associated with a left-sided, perforated, subdiaphragmatic abscess filled with brown, foul-smelling fluid. There was no evidence of bowel or gastric perforation. The liver surface appeared normal. More than 300 mLs of purulent fluid was drained, and postoperatively the patient was started on vancomycin, piperacillin/tazobactam and metronidazole and transferred to the PICU. Examination of the drained fluid was significant for the absence of white cells and a negative Gram stain, and thus complicated amebic liver abscess (ALA) was suspected. On microscopic examination, trophozoites were observed in abscess fluid (Fig 4).
Fig. 2.
Chest CT demonstrating pericardial fluid
Fig. 3.
Abdominal CT demonstrating abdominal mass
Fig. 4.
Trophozoite in abdominal abscess fluid
Post-operatively, he deteriorated clinically, requiring intensified ventilatory and hemodynamic support. Pericardial and bilateral pneumonic effusions were drained, without therapeutic result; characteristics of the drained fluids are shown in Table 1. Nine days after the first surgery, additional debridement and exploration of his abdomen revealed a gastric rupture along the lesser curve, which was repaired. Thereafter, his condition improved. He received a 4-week course of metronidazole and, when he could tolerate oral medication, a 7-day course of the intraluminal amebicide paramomycin. He also received a 2-week course of bacterial coverage for the possibility of superinfection/contamination. His acute and convalescent E. histolytica enzyme-linked immunosorbent assays (ELISAs) (IVD Research, Inc) were positive at 1:64, and at 1:256, respectively.
Table 1.
Fluid analysis of specimens obtained from various sites in patient with complicated ALA
| SOURCE | GROSS DESCRIPTION | CYTOLOGY/MICROBIOLOGY | CHEMISTRIES | PCR |
|---|---|---|---|---|
| Stool | Normal | ND | ND | + |
| Abdominal abscess | Purulent Foul smelling |
No WBCs Trophozoites (Fig. 4) |
ND | + |
| Left pleural fluid | Purulent | ND | Protein 4mg/dL LDH 47,191 IU/L |
+ |
| Right pleural fluid | Straw colored | ND | - | |
| Pericardial fluid | Straw colored | WBCs 400 53% PMNs RBCs 32,800 |
Protein 2082 IU/L | + |
ND= not done, WBC= white blood cells, PMN= polymorphonuclear cells, RBC= red blood cells, LDH= lactate dehydrogenase. All cultures and Gram stains were negative for bacteria, and all samples except abdominal abscess fluid were negative for cysts and trophozoites.
Because of the potential value of molecular diagnostics for the evaluation of complicated ALA in a low prevalence setting, available fluids were sent for polymerase chain reaction (PCR) using species-specific primers. The amplification technique is described elsewhere (2, 3), and distinguishes E. histolytica from E. dispar (4). Specimens from stool, liver abscess, and left pleural and pericardial spaces were positive for E. histolytica. The right pleural fluid was negative, as expected. Of interest, the genotype of the stool isolate differed from the genotype of the invasive isolate; this may be a result of co-infection where only one of the co-infecting strains invades extraintestinally, or may be a result of a genotype switch that occurs with invasion (3).
DISCUSSION
Rupture of an ALA is relatively rare in children, leading to difficulty with diagnosis and management of the disease. The case reported here was complicated by initial misdiagnosis, peritonitis, pleural and pericardial disease, and gastric rupture. Though each of these complications are reported in the literature, this is the first case report in which all occurred in the same patient. Though PCR for E. histolytica DNA was not essential for diagnosis or management in this case, its use is of interest for its novel use on extraintestinal fluids.
Because of low suspicion for the disease and clinical mimicry, 50% of patients with complicated ALA are misdiagnosed (5). In developed countries where ALA is not common, elements of the history are either not sought or are not helpful; 50-75% of patients with ALA do not have a history of diarrhea (6). Though the literature suggests that the disease is associated with squalor, our experience, as reflected in this case, suggests that the typical patient in the US is a child of immigrants from an endemic area and not necessarily living in poverty. The clinical mimicry is due to involvement of organs besides the liver and referred pain (7,8), leading a clinician to alternative diagnoses. Though it is well known that inflammation in the chest can cause significant abdominal symptoms (i.e. pneumonia misdiagnosed as appendicitis), our patient demonstrates the opposite phenomenon: an abdominal process initially diagnosed as pneumonia. Indeed, chest complications of ALA are often confused with pulmonary tuberculosis, tuberculous pleurisy, bacterial pneumonia or tumor (5). However, the triad of pericardial effusion, pleural effusion and intraabdominal abscess is most commonly reported with complicated ALA.
Complications of ALA develop from rupture of the abscess into the abdomen or chest through transdiaphragmatic spread, though hematogenous spread is also possible (Table 2). Rupture is most common with left-sided ALA, though infection in that lobe is statistically less likely due to hepatic venous drainage patterns (7). It is estimated that 22% of ALAs rupture, though in our clinical experience, rupture is rare (5). The reported overall mortality of ruptured ALA ranges from 20-75% (1, 5, 9) and is highest with acute peritonitis and pericarditis. Peritonitis occurs in 2-7% of ALA, and manifests as either acute peritonitis from sudden abscess perforation, subacute localized peritonitis, or chronic peritonitis. Acute peritonitis is the most common and is associated with severe systemic illness (9, 10). Though intestinal perforation occurs with amebic peritonitis, gastric rupture, as in our patient, is rarely reported (5).
Table 2.
Complications of invasive E. histolytica
| ASSOCIATED WITH INTESTINAL AMEBIASIS | ASSOCIATED WITH ALA | ASSOCIATED WITH HEMATOLOGIC SPREAD |
|---|---|---|
| Intestinal perforation/peritonitis | Peritonitis | Cerebral abscess/meningitis |
| Appendicitis | Pleuropulmonary/pneumothorax | Pulmonary abscess |
| Hemorrhage | Pericarditis/pneumopericardium/tamponade | Urogenital tract infection |
| Ameboma | Mediastinitis/pneumomediastinum | Splenic abscess |
| Intussuception | Bacterial superinfection | Systemic inflammatory response syndrome |
| Amebic stricture | Obstructive jaundice | |
| Toxic megacolon | Inferior vena cava obstruction | |
| Cutaneous fistulization, perianal and perigenital | Cutaneous fistulization, chest and abdominal wall | |
| Internal fistulization | Internal fistulization | |
| Ulcerative postdysenteric colitis | Hemobilia/thoracobilia | |
| Venous and arterial thrombosis | Venous and arterial thrombosis | |
| Systemic inflammatory response syndrome | Systemic inflammatory response syndrome |
Pleuropulmonary amebiasis is the most common complication of ALA and has a clinical presentation similar to bacterial pneumonia. Distinguishing features include sudden onset respiratory distress and marked abdominal tenderness. The exact incidence is unknown, but clinical or radiographic abnormalities occur in approximately 50% of ALA cases; a significant percentage are likely secondary to hemidiaphragm elevation and atelectasis rather than extension (7). Pericardial disease occurs in 2% of ALA, with the potential for life-threatening tamponade (11-13). Intrathoracic effusions are either “anchovy paste”, as seen in liver abscesses, or serous appearing (12). When anchovy paste is seen, direct rupture from the liver is usually apparent. When serous, it is unclear if the effusion is infected, or a non-infectious inflammatory reaction (8, 14). In our case, the pericardial and pleural fluids macroscopically appeared serous, and chemical, cytologic and culture results were inconclusive for active infection. However, molecular diagnostics demonstrated the presence of E. histolytica DNA.
Management of complications of ALA, with its high mortality, is a source of constant worry for the clinician (1) (7, 8, 10, 13-17). Though reports describing series of cases exist, exact management strategies have not been extensively explored. Leaving clinicians in non-endemic areas without experience or literature for guidance. While aspiration of ALA is only indicated in select circumstances (left side of liver, abscess greater than 5cm, failure to respond to therapy after 5-7 days) (7), drainage of ruptured ALA, whether into the peritoneal, pleural or pericardial space, is generally recommended. E. histolytica is particularly cytotoxic, and destruction of gut, liver and lung parenchyma, as well as fluid accumulation to the point of cardiac tamponade, can ensue. Bacterial superinfection is an additional risk, leading to additional morbidity and mortality. Longer-term, post-inflammatory complications are possible, such as abdominal adhesions and fibrinous pericarditis. However, it is worth noting that there are reports of managing chest complications with liver abscess drainage alone (17) or, in the case of pericardial disease, drainage only when there is tamponade (13); comfort with this approach should be dictated by the clinical scenario. In our patient, the deteriorating clinical course and the possibility of superinfection led to drainage of the pericardial and pleural spaces, though significant improvement did not occur. His condition ultimately improved following discovery and repair of his gastric rupture and further intra-abdominal debridement.
Though laboratory testing aids in the diagnosis of ALA, early diagnosis still largely relies on clinical suspicion. Demonstration of E. histolytica in stool is not reliable in the diagnosis of extraintestinal amebiasis; cysts or trophozoites are detected in only 15-33% of the stools of those with ALA. Trophozoites are also not commonly seen in abscess fluids (13). E. histolytica abscesses lack the presence of leukocytes on microscopy despite the appearance of “pus”, due to cytolytic activity of the ameba. In addition, they usually are not malodorous unless superinfected with bacteria (7) (18). Eosinophilia is not a feature of amebiasis, and in general liver function tests are not elevated, except the alkaline phosphatase. Specific laboratory tests include antibody detection, antigen detection and DNA detection, the precise details of which are reviewed elsewhere (1, 2, 19). Serum antibody detection is hampered by the inability to distinguish past from current infection when only IgG is measured, a relatively lengthy turn-around time, and the influence of specimen timing and methodology on sensitivity (2). However, the sensitivity of paired acute and convalescent serology is over 90% (20). Antigen detection in stool has a sensitivity and specificity of over 95% for colitis, but for extra-intestinal disease, the test on stool is usually negative (21). Given that denaturation occurs with specimen fixation and processing, adequate specimens for antigen detection may not be available at the time the diagnosis is entertained (2). PCR for E. histolytica DNA in stool and other sources is currently limited to research, but holds hope as a sensitive and specific future diagnostic tool (22). In addition, this technique may give insight into the pathogenesis and epidemiology of amebiasis (3, 4, 21, 23-26).
CONCLUSION
This unusual case of ruptured ALA with peritonitis, gastric rupture and extension to the left pleura and pericardium serves as a reminder regarding the complications of this disease and highlights the use of molecular techniques that will aid in diagnosis and research.
SUPPORT AND ACKNOWLEDGEMENTS
The authors have indicated they have no financial relationships relevant to the article to disclose. We are indebted to the microbiology laboratory at The Children's Hospital in Denver for their exceptional microscopy skills and special handling of specimens. This work was supported in part by NIH grant AI043596 to WAP.
ABBREVIATIONS
- CT
computerized tomography
- PCR
polymerase chain reaction
- ALA
Amebic Liver Abscess
- ELISA
Enzyme-Linked Immunosorbance Assay
REFERENCES
* = of special interest
** = of outstanding interest
- 1.Walsh JA. Problems in recognition and diagnosis of amebiasis: estimation of the global magnitude of morbidity and mortality. Rev Infect Dis. 1986;8:228–38. doi: 10.1093/clinids/8.2.228. [DOI] [PubMed] [Google Scholar]
- 2.Tanyuksel M, Petri WA., Jr Laboratory diagnosis of amebiasis. Clin Microbiol Rev. 2003;16:713–29. doi: 10.1128/CMR.16.4.713-729.2003. ** [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali IK, Solaymani-Mohammadi S, Akhter J, Roy S, Gorrini C, Calderaro A, et al. Tissue Invasion by Entamoeba histolytica: Evidence of Genetic Selection and/or DNA Reorganization Events in Organ Tropism. PLoS Negl Trop Dis. 2008;2:e219. doi: 10.1371/journal.pntd.0000219. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali IK, Zaki M, Clark CG. Use of PCR amplification of tRNA gene-linked short tandem repeats for genotyping Entamoeba histolytica. J Clin Microbiol. 2005;43:5842–7. doi: 10.1128/JCM.43.12.5842-5847.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng XY, Wu JX. Perforated amebic liver abscess: clinical analysis of 110 cases. South Med J. 1994;87:985–90. doi: 10.1097/00007611-199410000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Peres LC, Saggioro FP, Dias LB, Jr, Alves VA, Brasil RA, Luiz VE, et al. Infectious diseases in paediatric pathology: experience from a developing country. Pathology. 2008;40:161–75. doi: 10.1080/00313020701816357. ** [DOI] [PubMed] [Google Scholar]
- 7.Hughes MA, Petri WA., Jr Amebic liver abscess. Infect Dis Clin North Am. 2000;14:565–82. viii. doi: 10.1016/s0891-5520(05)70121-5. [DOI] [PubMed] [Google Scholar]
- 8.Salles JM, Moraes LA, Salles MC. Hepatic amebiasis. Braz J Infect Dis. 2003;7:96–110. doi: 10.1590/s1413-86702003000200002. [DOI] [PubMed] [Google Scholar]
- 9.Archampong EQ. Peritonitis from amoebic liver abscess. Br J Surg. 1972;59:179–81. doi: 10.1002/bjs.1800590306. [DOI] [PubMed] [Google Scholar]
- 10.Sarda AK, Bal S, Sharma AK, Kapur MM. Intraperitoneal rupture of amoebic liver abscess. Br J Surg. 1989;76:202–3. doi: 10.1002/bjs.1800760231. [DOI] [PubMed] [Google Scholar]
- 11.Ganesan TK, Kandaswamy S. Amebic pericarditis. Chest. 1975;67:112–3. doi: 10.1378/chest.67.1.112. [DOI] [PubMed] [Google Scholar]
- 12.Ibarra-Perez C. Thoracic complications of amebic abscess of the liver: report of 501 cases. Chest. 1981;79:672–7. doi: 10.1378/chest.79.6.672. [DOI] [PubMed] [Google Scholar]
- 13.Shamsuzzaman SM, Hashiguchi Y. Thoracic amebiasis. Clin Chest Med. 2002;23:479–92. doi: 10.1016/s0272-5231(01)00008-9. [DOI] [PubMed] [Google Scholar]
- 14.Adeyemo AO, Aderounmu A. Intrathoracic complications of amoebic liver abscess. J R Soc Med. 1984;77:17–21. doi: 10.1177/014107688407700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyche KD, Jensen WA. Pleuropulmonary amebiasis. Semin Respir Infect. 1997;12:106–12. [PubMed] [Google Scholar]
- 16.Lyche KD, Jensen WA, Kirsch CM, Yenokida GG, Maltz GS, Knauer CM. Pleuropulmonary manifestations of hepatic amebiasis. West J Med. 1990;153:275–8. [PMC free article] [PubMed] [Google Scholar]
- 17.Takhtani D, Kalagara S, Trehan MS, Chawla Y, Suri S. Intrapericardial rupture of amebic liver abscess managed with percutaneous drainage of liver abscess alone. Am J Gastroenterol. 1996;91:1460–2. [PubMed] [Google Scholar]
- 18.Huston CD, Boettner DR, Miller-Sims V, Petri WA., Jr Apoptotic killing and phagocytosis of host cells by the parasite Entamoeba histolytica. Infect Immun. 2003;71:964–72. doi: 10.1128/IAI.71.2.964-972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruckner DA. Amebiasis. Clin Microbiol Rev. 1992;5:356–69. doi: 10.1128/cmr.5.4.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haque R, Huston CD, Hughes M, Houpt E, Petri WA., Jr Amebiasis. N Engl J Med. 2003;348:1565–73. doi: 10.1056/NEJMra022710. ** [DOI] [PubMed] [Google Scholar]
- 21.Stark D, van Hal S, Fotedar R, Butcher A, Marriott D, Ellis J, et al. Comparison of stool antigen detection kits to PCR for diagnosis of amebiasis. J Clin Microbiol. 2008;46:1678–81. doi: 10.1128/JCM.02261-07. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solaymani-Mohammadi S, Lam MM, Zunt JR, Petri WA., Jr Entamoeba histolytica encephalitis diagnosed by PCR of cerebrospinal fluid. Trans R Soc Trop Med Hyg. 2007;101:311–3. doi: 10.1016/j.trstmh.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Ali IK, Mondal U, Roy S, Haque R, Petri WA, Jr, Clark CG. Evidence for a link between parasite genotype and outcome of infection with Entamoeba histolytica. J Clin Microbiol. 2007;45:285–9. doi: 10.1128/JCM.01335-06. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solaymani-Mohammadi S, Rezaian M, Babaei Z, Rajabpour A, Meamar AR, Pourbabai AA, et al. Comparison of a stool antigen detection kit and PCR for diagnosis of Entamoeba histolytica and Entamoeba dispar infections in asymptomatic cyst passers in Iran. J Clin Microbiol. 2006;44:2258–61. doi: 10.1128/JCM.00530-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul J, Srivastava S, Bhattacharya S. Molecular methods for diagnosis of Entamoeba histolytica in a clinical setting: an overview. Exp Parasitol. 2007;116:35–43. doi: 10.1016/j.exppara.2006.11.005. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali IK, Clark CG, Petri WA., Jr Molecular epidemiology of amebiasis. Infect Genet Evol. 2008 May 14; doi: 10.1016/j.meegid.2008.05.004. * [DOI] [PMC free article] [PubMed] [Google Scholar]