Abstract
Lymphocytes migrate from the blood into tissue by binding to and migrating across endothelial cells. One of the endothelial cell adhesion molecules that mediate lymphocyte binding is VCAM-1. We have reported that binding to VCAM-1 activates endothelial cell NADPH oxidase for the generation of reactive oxygen species (ROS). The ROS oxidize and stimulate an increase in protein kinase C (PKC)α activity. Furthermore, these signals are required for VCAM-1-dependent lymphocyte migration. In this report, we identify a role for protein tyrosine phosphatase 1B (PTP1B) in the VCAM-1 signaling pathway. In primary cultures of endothelial cells and endothelial cell lines, Ab cross-linking of VCAM-1 stimulated an increase in serine phosphorylation of PTP1B, the active form of PTP1B. Ab cross-linking of VCAM-1 also increased activity of PTP1B. This activation of PTP1B was downstream of NADPH oxidase and PKCα in the VCAM-1 signaling pathway as determined with pharmacological inhibitors and antisense approaches. In addition, during VCAM-1 signaling, ROS did not oxidize endothelial cell PTP1B. Instead PTP1B was activated by serine phosphorylation. Importantly, inhibition of PTP1B activity blocked VCAM-1-dependent lymphocyte migration across endothelial cells. In summary, VCAM-1 activates endothelial cell NADPH oxidase to generate ROS, resulting in oxidative activation of PKCα and then serine phosphorylation of PTP1B. This PTP1B activity is necessary for VCAM-1-dependent trans-endothelial lymphocyte migration. These data show, for the first time, a function for PTP1B in VCAM-1-dependent lymphocyte migration.
Leukocyte migration from the blood into tissues is vital for immune surveillance and inflammation (1–5). Recent studies on endothelial cell adhesion molecules indicate that lymphocyte binding triggers active endothelial cell signals that regulate leukocyte migration (6–8). One of the endothelial cell adhesion molecules is VCAM-1 (9, 10). Binding to VCAM-1 is required for eosinophil infiltration into the lung in experimental OVA-induced asthma (1) and T cell infiltration across the blood-brain barrier in experimental allergic encephalomyelitis (2). VCAM-1 also functions in combination with other adhesion molecules during chronic inflammation and tumor metastasis. Moreover, the VCAM-1 null mice are embryonic lethal (11). Therefore, understanding VCAM-1 signaling may have important implications for disease intervention.
We have reported signals activated by VCAM-1 that are required for VCAM-1-dependent leukocyte migration (7, 9). VCAM-1 stimulates endothelial cell NADPH oxidase-catalyzed production of nontoxic levels of reactive oxygen species (ROS)4 (1 μM H2O2) (9, 12). In contrast, binding to VCAM-1 does not activate other ROS-generating enzymes in endothelial cells such as NO synthase (9). The 1 μM H2O2 stimulates endothelial cells by oxidation and consequently activation of both endothelial cell-associated matrix metalloproteinases (MMPs) (13) and endothelial cell protein kinase C (PKC)α (14). The VCAM-1-stimulated ROS generation, MMP activity, and PKCα activity are required for VCAM-1-dependent leukocyte migration (9, 13, 14).
We have also reported that VCAM-1 signals in endothelial cells cause actin structural changes in the endothelium (9). These endothelial cell actin changes are in response to endothelial cell NADPH oxidase-dependent generation of low levels of H2O2 (9). H2O2 can inhibit activity of Ser/Thr phosphatases or tyrosine phosphatases through reversible oxidation of their catalytic site (15–17). The modulation of phosphatase activity is important because it regulates cell shape. It has been shown in smooth muscle cells, in HUVECs, and in human microvascular endothelial cells (HMEC)-1 that inhibition of Ser/Thr phosphatases causes cell rounding, cytoskeletal disorganization, and cellular detachment (18, 19). The protein tyrosine phosphatase PTP1B also modulates the actin cytoskeleton in fibroblasts (20, 21). Whether VCAM-1 signaling through ROS modulates phosphatase activity has not been reported.
In addition to ROS modulation of protein tyrosine phosphatase (PTP) activity, PTP activity is regulated by phosphorylation. When HeLa cells are treated with okadaic acid, a Ser/Thr phosphatase inhibitor, and stimulated with cAMP, there is an increase in activity of PTP1B, an intracellular PTP (22). Phosphorylation of PTP1B by PKC also increases PTP activity (23). Therefore, PTPs are modulated by ROS and PKC, and we have reported that ROS and PKC are intermediates in the VCAM-1 signaling pathway (9, 13, 14). It is not known whether VCAM-1 activates PTPs through PKCα or inhibits PTP activity by ROS-mediated oxidation of PTPs.
To examine whether VCAM-1 signaling modulates PTP activity, we used primary cultures of cytokine-activated human lung microvascular endothelial (HMEC-L) cells as well as two independently derived endothelial cell lines. The endothelial cell lines, mHEVa and mHEVc, constitutively express VCAM-1 and do not express other ligands for lymphocyte migration such as ICAM-1, PECAM-1, and E-selectin as determined by immunofluorescence labeling (24) or cDNA microarray analysis (data not shown). Furthermore, adhesion and migration across the endothelial cells are dependent on binding to VCAM-1 and the constitutive expression of MCP-1 by the endothelial cells (9, 24, 25). Abs for other adhesion molecules other than VCAM-1 or its ligand α4β1 integrin do not block lymphocyte migration across the endothelial cells (12). Therefore, the endothelial cell lines provide a model to examine signals during VCAM-1-dependent lymphocyte migration, while avoiding complexity from signals induced by lymphocyte binding to other adhesion molecules on endothelial cells (9, 12, 26, 27). This is important so that signaling intermediates can be inhibited in the endothelial cells and then the functional consequence on VCAM-1-dependent migration examined. Using the HMEC-L cells and endothelial cell lines, we demonstrate here that VCAM-1 signaling through ROS and PKCα activates PTP1B. Moreover, this increase in PTP1B activity is required for VCAM-1-dependent lymphocyte migration.
Materials and Methods
Animals
Male 6–8 wk old BALB/c mice (Harlan Industries) were the source of splenic lymphocytes.
Cells
The endothelial cell lines mHEVa and mHEVc were derived from BALB/c mouse axillary and cervical lymph nodes, respectively, and cultured as previously described (26). The mHEVa and mHEVc cells have been independently spontaneously immortalized but are not transformed (26). The mHEV cells constitutively express VCAM-1 (26). HMEC-L cells (Clonetics) were grown in endothelial growth medium (Clonetics) plus 5% FCS and were used at passage 3–4. The HMEC-L cells do not express VCAM-1 until stimulated by cytokines such as TNF-α.
Single cell suspensions of spleen cells were prepared from freshly isolated BALB/c mouse spleens as previously described (9). RBC were lysed by hypotonic shock (26).
Reagents
Apocynin was obtained from Acros Organics. CinnGEL (catalog no. PR-114), CinnGEL-2ME (catalog no. PR-115), Gö-6976 (catalog no. EI-269), Rö-32–0432 (catalog no. EI-284), and rabbit anti-PKCα (catalog no. SA-144) were obtained from Biomol. Rat anti-mouse VCAM-1 (clone MV-CAM.A), mouse anti-human VCAM-1 (clone 51-10C9), rat IgG (isotype Ab, clone R35-95), and FITC-conjugated goat anti-rabbit Ig (mouse adsorbed) (catalog no. 554020) were purchased from BD Pharmingen. Zenon Alexa Fluor 568-labeled (catalog no. Z25106; Molecular Probes) rat anti-mouse CD45 (clone I3/2.3), goat anti-mouse IgG1 (catalog no. 1070-01), and goat anti-rat IgG (catalog no. 3050-01) were obtained from Southern Biotech. Rabbit anti-phospho-PKCα Thr (638) (catalog no. 9375) was purchased from Cell Signaling Technology. Rabbit anti-phosphoserine (catalog no. 61–8100) was from Zymed Laboratory. Mouse anti-β-actin (catalog no. ab6276) was from Abcam. HRP-conjugated donkey anti-rabbit Ab was from Amersham Biosciences. HRP-conjugated goat anti-mouse IgG was from Bio-Rad. Dominant-negative PKCα in the plasmid pCMV was a gift from A. Descoteaux (University of Québec, Montréal, Canada). This inactive transdominant mutant PKCα has the lysine in the ATP-binding domain replaced (28). Iodoacetamidofluorescein (catalog no. I9271) and anti-FITC (catalog no. F5636) were obtained from Sigma-Aldrich. Lipo-fectAMINE 2000 transfection reagent (catalog no. 11668-019) was purchased from Invitrogen Life Technologies.
Ab-coated beads
For anti-mouse VCAM-1-coated beads, streptavidin-coated 9.9 μm diameter beads (80 μl) (Bangs Laboratories) were labeled with 24 μg of biotin-conjugated goat anti-rat Ig in 375 μl of PBS with gentle rocking for 1 h at 4°C and then washed three times (9). These beads were incubated with 16 μg of rat anti-mouse VCAM-1 or a rat isotype control Ab in 375 μl of PBS with gentle rocking for 1 h at 4°C and then washed. For experiments with HMEC-L cells, the streptavidin-conjugated beads were coated with 12 μg of biotin-conjugated goat anti-mouse IgG1 (12062D; BD Pharmingen) and 8 μg of mouse anti-human VCAM-1 (clone 51-10C9; BD Pharmingen) or a control Ab (mouse anti-human PECAM-1, clone C-20; Santa Cruz Biotechnology).
Gene chip microarray
mRNA from one endpoint of nonstimulated mHEVa and mHEVc cells were collected using Copy Kit (Invitrogen Life Technologies) according to the manufacturer’s instructions. Qiagen columns were used to prepare the RNA. High quality of the RNA was determined by the Affymetrix Gene-Chip Microarray core facility at Cincinnati Childrens’ Hospital Medical Research Foundation (Cincinnati, OH) using the Agilent 2100 Bioanalyzer and the criteria of distinct 28S and 18S rRNA peaks and a 28S/18S ratio of 1.6–2.0. The RNA passed these criteria for use in the microarray. mRNA expression was analyzed using Affymetrix GeneChip technology with the MOE430A and MOE430B mouse gene chips (core facility, Cincinnati Children’s Hospital Medical Research Foundation). The microarray screen indicated expression by a present call (P) for p < 0.05 or an absent call (A) for p > 0.05 within the Affymetrix microarray. The microarray P and A results were also consistent with our previous reports on protein expression or lack of protein expression of multiple receptors and signaling molecules including NADPH oxidase, VCAM-1, MHC class I, ICAM-1, PECAM-1, scavenger receptors, chemokines, Rac-1, protein kinases, metalloproteinases, etc. (9, 13, 14, 24–27). Modulation of gene expression was not examined for these studies because there is not enough time for changes in gene expression during the 15 min for VCAM-1-dependent lymphocyte migration. In summary, the microarray was a screen for gene expression that was followed up with protein expression analysis of the gene of interest.
Total tyrosine phosphatase activity
mHEV cells were grown in 24-well plates and stimulated with 50 μl anti-ICAM-1 Ab coated beads. The cells were dissociated with cold PBS containing 0.3% EDTA and washed. Cells were lysed in 50 μl of degased/deoxygenated lysis buffer containing 0.2% Triton X-100 and incubated on ice for 20 min. Insoluble material was removed by centrifugation for 10 min at 10,000 × g. Endogenous phosphate was then reduced by filtering the lysate through a G25 Microspin column (Amersham). Cell lysates were added to 50ul degased/deoxygenated assay buffer (25 mM HEPES (pH 7.8), 5 mM EDTA) containing 10 mM L-o-phosphotyrosine (Sigma-Aldrich) in the absence or presence of 1 mM sodium vanadate or a 1/100 dilution of a phosphatase inhibitor mixture (sodium orthovanadate, sodium molybdate, sodium tartrate, and imidazole, pH 10) (Sigma-Aldrich). The reaction was allowed to proceed for 20 min at 37°C and then stopped by adding 150 μl 25% TCA on ice for 15 min. Protein precipitation was facilitated by adding 50 μl of 10 mg/ml BSA and lysates were centrifuged for 10 min at 12,000 × g. The supernatant was then incubated for 3 h at room temperature with incubation buffer (2 volumes H2O, 1 volume 6N sulfuric acid, 1 volume 2.5% ammonium molybdenate, and 1 volume 10% ascorbic acid). Free phosphate was measured by spectrophotometry at 750 nm. KH2PO4 was used to generate a standard curve. The data are presented as U/mg protein where units are the OD 750 nm reading and protein was determined by the Bio-Rad protein assay.
[32P]poly(glu:tyr) substrate
Poly(glu:tyr) (Sigma-Aldrich) was labeled with 32P using crude membrane extracts from A431 cell (American Type Culture Collection) lysates expressing high levels of the EGF receptor. Crude lysates were made by scraping two T75 flasks into 4 ml PBS. The cells were centrifuged and lysed in 100 μl PTP lysis buffer (25 mM sodium acetate, 1% Triton X-100, 150 mM NaCl, 10% glycerol, 5000U catalase, 500U SOD, 10 mg/ml leupeptin, 1 mM PMSF, and 10 μg/ml aprotinin). Briefly, 1 mg of poly(glu:tyr) was incubated in 0.5 ml of 150 mM NaCl, 2 mM MnCl2, 0.02% Triton X-100, 5% glycerol, 0.1 mM sodium orthovanadate, 0.2 mM ATP, 50 mM HEPES (pH 7.4), EGF (200 nM), 0.25 mCi [32P]-γATP (PerkinElmer), and 50 μl of crude A431 cell membranes were added to the reaction buffer. The reaction was incubated with shaking at room temperature for 4 h. The membranes were removed by centrifugation for 1 min. at 10,000 × g. The supernatant was collected and the poly(glu:tyr) was precipitated by adding an equal volume of 20% trichloracetic acid for 30 min at 4°C. The poly(glu:tyr) was recovered by centrifugation at 10,000 × g for 10 min. The pellet was dissolved in 100 μl of 2 M Tris-base, passed over a G50 Microspin column (Amersham) equilibrated with 50 mM imidazole, pH 7.2. Incorporation of 32P was measured by scintillation counting. The [32P]poly(glu:tyr) substrate was used for the PTP in-gel activity assay.
PTP in-gel activity assay (30)
mHEV cells were stimulated as described in Fig. 1, washed with PBS, and lysed in degassed/deoxygenated PTP lysis buffer (25 mM sodium acetate, 1% Triton X-100, 150 mM NaCl, 10% glycerol, 5000U catalase, 500U SOD, 10 mg/ml leupeptin, 1 mM PMSF, and 10 μg/ml aprotinin). Lysates were centrifuged for 10 min at 10,000 × g at 4°C. Cell lysates (2.5 μg protein/lane) in reducing sample buffer were separated by SDS-PAGE. Polyacrylamide-SDS gels (10%) were run according to Laemmli (31) except the separating gels contained 0.13% bisacrylamide. [32P]poly(glu:tyr) was incorporated into the gel by adding 105 cpm/ml into the acrylamide mixture before polymerization. After electrophoresis, all incubations were done at room temperature in 100 ml of the following buffers. The gels were first incubated in 50 mM Tris-Cl (pH 8.0) and 20% isopropanol for 1.5 h or overnight to remove SDS. The gels were then washed twice in 50 mM Tris-Cl (pH 8.0) and 0.3% 2-ME for 30 min. They were incubated in the same buffer plus 6 M guanidine-HCl and 1 mM EDTA for 1.5 h, incubated three times for 1h in renaturation buffer (1 mM EDTA, 50 mM Tris-Cl (pH 8.0), 0.3% 2-ME, and 0.04% Tween 40) and then, incubated overnight in renaturation buffer plus 4 mM DTT during which the PTPs dephosphorylate the [32P]poly(glu:tyr). The gels were stained with Coomassie brilliant blue, destained with 40% methanol and 10% acetic acid, dried, and analyzed by autoradiography.
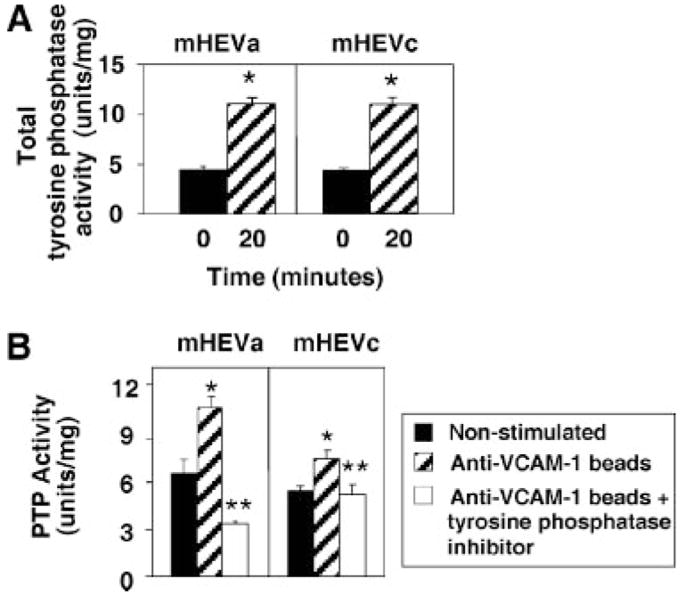
FIGURE 1.
Total tyrosine phosphatase activity in endothelial cells is increased by VCAM-1 binding. mHEV cell lines were stimulated with anti-VCAM-1 Ab-coated beads for 20 min in the absence (A) or presence (B) of the phosphatase inhibitor mixture (1/100) (Sigma-Aldrich). Tyrosine phosphatase activity is the phosphatase inhibitor-blocked free phosphate measured by the colorimetric PTP assay. Background in nonstimulated cells is likely the result of free phosphate in the cells during cell metabolism as the assay measures free phosphate. Units per milligram were calculated as the OD units for free phosphate per milligram of cell protein. Data presented are the mean ± SD from two experiments. *, p < 0.05 compared with nonstimulated mHEV cells.
Transfection
mHEVa were grown to 90% confluence in 12 well plates or on slides. The cells were transfected with 1 μg of dominant negative PKCα or the vector pCMV per well. Each of these transfections were performed in the presence of 2 μl/well LipofectAMINE 2000 Transfection Reagent (Invitrogen Life Technologies) according to instructions in culture medium without gentamicin, penicillin, or streptomycin. After 3.5 h, the medium was removed and replaced with medium containing antibiotics (9) for 24 h.
Immunoprecipitation (32)
mHEV cells in 12 well plates were pretreated with the PTP1B inhibitor 10 μM CinnGel (Biomol) or the vehicle control DMSO (0.1%) for 30 min and then stimulated in duplicates with anti-VCAM-1 Ab-coated beads or 1 μM H2O2 for the time indicated. The cells were washed with PBS and lysed with 100 μl/well radioimmunoprecipitation buffer (1% Triton X-100, 0.5% sodium deoxycholate, 0.2% SDS, 150 mM NaCl, 10 mM, HEPES (pH 7.3), 2 mM EDTA, 10 mg/ml leupeptin, 10 mg/ml aprotinin, 100 mg/ml iodoacetamide, and 1 mM PMSF). Duplicate samples were combined, and lysates were incubated on ice for 20 min. Lysates were homogenized by passing 20 times through a 22-gauge needle and then centrifuged for 10 min at 15,000 × g at 4°C. Total protein concentration was determined by a protein assay kit (Bio-Rad), and protein concentration was adjusted to 1 mg/ml. Equal volumes of lysate were then precleared with 50 μl protein G beads with rabbit serum for 1 h at 4°C with gentle rocking. Lysates were incubated with 50 μl protein G beads and rabbit anti-mouse PTP1B (2.5 μg/ml) overnight at 4°C with gentle rocking. The beads were washed three times with radioimmunoprecipitation buffer and one time each with 0.5 M NaCl followed by 10 mM Tris, pH 8. Protein was eluted from the beads by heating for 5 min at 100°C in SDS reducing sample buffer.
Western blotting
Cell lysates or immunoprecipitates in SDS sample buffer were analyzed by SDS-PAGE (7.5%) and transferred to PVDF membranes according to manufacturer’s instructions (Bio-Rad) (300 mA for 3 h). The membranes were blocked in 1% BSA in TBST or for 1 h at room temperature. Primary Abs rabbit anti-mouse PTP1B (1:1000), rabbit anti-phospho PKCα/β Thr (638) (2 μg/ml), or mouse anti-phosphoserine (2 μg/ml) were incubated with the membranes in TBST plus 1% BSA overnight and then washed three times for 5 min in TBST. HRP-conjugated secondary Abs (1:1000) were then incubated with the membrane for 1 h at room temperature and washed three times for 5 min in TBST. Protein expression was detected by ECL (Amersham) and autoradiography.
Cell association and migration assays with laminar flow
The parallel plate flow chamber was used to examine migration under conditions of laminar flow. Endothelial cells were grown to confluence on slides and then the slide was placed in a parallel plate flow chamber (33). In vivo, in the absence of inflammation, the rapid fluid dynamics of the blood result in blood cells located midstream of the vascular flow (34). However, during inflammation, there is a change of fluid dynamics (34–36). With inflammation, vascular permeability increases yielding fluid flow from the blood into the tissues which contributes to contact of blood cells with the endothelium (“margination”) (34, 36). There is also cell contact as the blood cells leave the capillaries and enter the postcapillary venules (35). Therefore, spleen cells (3 × 106) were added to the flow chamber (3.5 cm2) at 2 dynes/cm2. Spleen cells were added at concentrations below saturation of monolayer cell binding or migration (12, 24). To initiate spleen cell contact with the endothelial cells in vitro, the spleen cells were allowed to settle in the chamber as monitored by microscopy and then 2 dynes/cm2 was applied for the 15 min laminar flow assay. We observed by microscopy that during the assay under laminar flow, the spleen cells in contact with the endothelial cells either roll, roll and detach, or roll, firmly attach and migrate. For this assay, the coculture was exposed to laminar flow at 2 dynes/cm2 at 37°C for 3 min to examine spleen cell association with the endothelial cells or for 15 min to examine spleen cell migration. After 3 or 15 min at 2 dynes/cm2, the cells were washed at 2 dynes/cm2 with PBS/0.2 mM CaCl2/0.1 mM MgCl2 because cations are required for cell adhesion. The slide was removed from the flow chamber and the cells were fixed with 3% paraformaldehyde for 1 h. To quantify migrated spleen cells at 15 min, phase contrast microscopy was used to count migrated cells that are phase dark (37). It has been reported that the transendothelial migration of an individual leukocyte, after it has rolled to a site of migration, occurs in 2 min (26). However, the transendothelial migration of the leukocytes is asynchronous. Under laminar flow, spleen cell migration is detected at 15 min. The number of spleen cells that were associated but not migrated (phase light cells) at 15 min is low since in 15 min, the majority of non-migrating cells roll off the monolayer of endothelial cells as determined by microscopy (data not shown). Therefore, the number of spleen cells associated with the endothelial cells at 3 min of laminar flow are those cells that mediated cell-cell contact.
Statistics
Data were analyzed by a one-way ANOVA followed by Tukey’s multiple comparisons test (SigmaStat).
Results
VCAM-1 signaling increases total tyrosine phosphatase activity
We have reported that VCAM-1 signaling is activated by lymphocyte binding to VCAM-1 or Ab cross-linking of VCAM-1 (9, 27). VCAM-1 is constitutively expressed by the endothelial cell lines mHEVa and mHEVc (26). Therefore, to determine whether tyrosine phosphatases are modulated by VCAM-1 signaling, mHEV cells were stimulated with anti-VCAM-1 Ab-coated beads for 0 or 20 min. The cell monolayers were dissociated by PBS/EDTA, washed, and lysed. Total tyrosine phosphatase activity was examined using a colorimetric assay for free phosphate with an o-phosphotyrosine substrate. The background free phosphate is likely due to release of phosphate during cell metabolism as the assay measures free phosphate. Anti-VCAM-1-coated beads stimulated an increase in total free phosphate at 20 min (Fig. 1A). To verify specificity of this increase as tyrosine phosphatase activity, endothelial cells were pretreated for 30 min with a tyrosine phosphatase inhibitor mixture (Fig. 1B). The tyrosine phosphatase inhibitor blocked the anti-VCAM-1-coated bead-stimulated PTP activity (Fig. 1B) indicating that the increase in free phosphate in this assay detected tyrosine phosphatase activity. In summary, anti-VCAM-1 stimulated an increase in activity of total endothelial cell tyrosine phosphatases.
Identification of a VCAM-1-stimulated 50kDa PTP as PTP1B
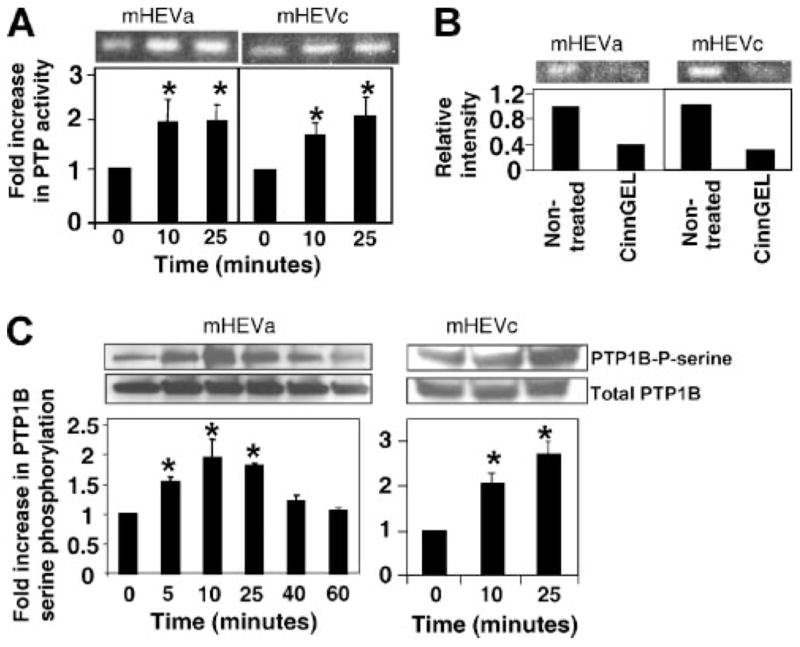
There are over 100 known phosphatase genes (38). Therefore, an in-gel assay was used to determine the m.w.(s) of the tyrosine phosphatase(s) activated by VCAM-1 signaling. The mHEV cells were stimulated with anti-VCAM-1 Ab-coated beads for 0, 10, and 25 min. The cell monolayers were washed with PBS, scraped into tyrosine phosphatase lysis buffer, and analyzed in an in-gel PTP activity assay. The in-gel assay detected four bands with low tyrosine phosphatase activity in nonstimulated mHEV cells (data not shown) and a 50 kDa band of tyrosine phosphatase activity that was increased by anti-VCAM-1 (Fig. 2). This 50 kDa tyrosine phosphatase activity was induced within 10 min (Fig. 2A). To further limit the number of potential tyrosine phosphatases, the expression of PTPs by the mHEV cell lines was examined using cDNA from the mHEVa and mHEVc cells and gene chip microarray analysis. There was differential expression of some PTPs by the mHEVa and mHEVc cell lines (Table I). This aided in focusing the studies to identify 50 kDa PTPs that were expressed by both cell lines because binding to VCAM-1 activated the 50 kDa in both cell lines. The mHEVa and mHEVc cells expressed one phosphatase at 50 kDa, PTP1B (Table I). Total PTP1B protein expression was verified by western blot analysis (Fig. 2C). It was then determined whether the 50 kDA PTP activity in the in-gel assay was blocked by an inhibitor of PTP1B, CinnGEL. Although the active sites of PTPs are conserved, CinnGEL binds to the active site of PTP1B and it has a chemical arm that binds to a neighboring cleft only found in PTP1B’s three dimensional structure (39). It has been reported that CinnGEL blocks activity of PTP1B but not other tyrosine phosphatases (39). The lysates were separated on a SDS-PAGE gel impregnated with the 32P-poly(glu:tyr) substrate and the in-gel activity examined in the presence or absence of 10 μM CinnGEL. CinnGEL blocked the activity of the 50 kDa band in both cell lines (Fig. 2B).
FIGURE 2.
VCAM-1 increases activity of a 50 kDa PTP. A, mHEVa and mHEVc cells were incubated with anti-VCAM-1 Ab-coated beads for 0, 10, and 25 min. The cells were lysed, and equal cell protein was analyzed by the PTP in-gel activity assay followed by autoradiography. Shown is the 50 kDa band. Data are from five experiments. B, mHEVa and mHEVc cells were lysed and equal protein was separated by SDS-PAGE. The gel was divided and incubated in the presence or absence of 10 μM CinnGel in the activation buffer plus 4 mM DTT for the PTP in-gel assay. C, mHEV cells were stimulated with anti-VCAM-1-coated beads. Cells were washed with PBS and lysed. PTP1B was immunoprecipitated, separated by SDS-PAGE, and analyzed by Western blot using anti-phosphoserine Ab. The membrane was stripped and probed with anti-total PTP1B Ab to determine protein loading. Densitometry analysis was performed using Image J software from the National Institutes of Health. Data presented are the mean ± SD from three to five experiments. *, p < 0.05 compared with mHEV cells at 0 min.
Table I.
PTPs expressed by the mHEVa and mHEVc cells. mRNA was isolated from the mHEV cells using Copy Kit (Invitrogen Life Technologies) and analyzed by GeneChip microarray analysis. Numbers in parentheses are the molecular weights of the mouse protein
| mHEVa | mHEVc | |
|---|---|---|
| Receptor PTP | RPTP A (93) | RPTP A |
| RPTP E (75) | RPTP E | |
| RPTP F (212) | RPTP F | |
| RPTP G (161) | RPTP G | |
| RPTP J (137) | RPTP K | |
| RPTP K (164) | RPTP M | |
| RPTP N (95) | RPTP S | |
| RPTP S (168) | ||
| RPTP T (163) | ||
| RPTP V (187) | ||
| Cytosolic PTP | PTPN1 (PTP1B) (50) | PTPN1 (PTP1B) |
| PTPN2 (45) | PTPN2 | |
| PTPN9 (68) | PTPN9 | |
| PTPN11 (67) | PTPN12 | |
| PTPN12 (87) | PTPN13 | |
| PTPN13 (217) | PTPN14 | |
| PTPN14 (135) | PTPN21 | |
| PTPN20 (49) | ||
| PTPN21 (133) | ||
| DSP | DSP 1 (40 | DSP 1 |
| DSP 2 (35) | DSP 6 | |
| DSP 6 (42) | DSP 9 | |
| DSP 9 (42) | DSP 10 | |
| DSP 10 (53) | DSP 12 | |
| DSP 12 (37) | DSP 14 | |
| DSP 14 (22) | ||
| Other | FYVE zinc finger PTP (133) | FYVE zinc finger PTP |
| PTP IVa1,2,3 (20) | PTP IVa1,2,3 | |
| PTPLB (28) | PTPLB | |
| PTEN | PTEN |
DSP, dual specificity phosphatase.
PTP1B activity is increased by serine phosphorylation (22). To examine whether VCAM-1 stimulates serine phosphorylation of PTP1B, mHEV cells were stimulated with anti-VCAM-1-coated beads for 0 to 60 min. The cells were washed, lysed, and equal protein equivalents were immunoprecipitated with an anti-PTP1B Ab. Immunoprecipitates were subjected to SDS-PAGE gel electrophoresis followed by western blot with anti-phosphoserine Abs. Protein loading was verified by stripping the membrane and probing for total PTP1B. Anti-VCAM-1-coated beads stimulated an increase in PTP1B serine phosphorylation in the endothelial cells (Fig. 2C). With equal cell equivalents loaded per lane, there was no change in total PTP1B (Fig. 2C). PTP1B was activated rapidly (10 min) in both the in-gel activity assay (Fig. 2A) and the Western blot for active phospho-PTP1B (Fig. 2C). This activation of PTP1B was transient (Fig. 2C). Anti-CD98-coated control beads, which bind to the endothelial cells (data not shown), did not activate phosphorylation of PTP1B as in Figs. 4 and 6. In summary, VCAM-1-induced-signals stimulate PTP1B activity by increasing serine phosphorylation of PTP1B.
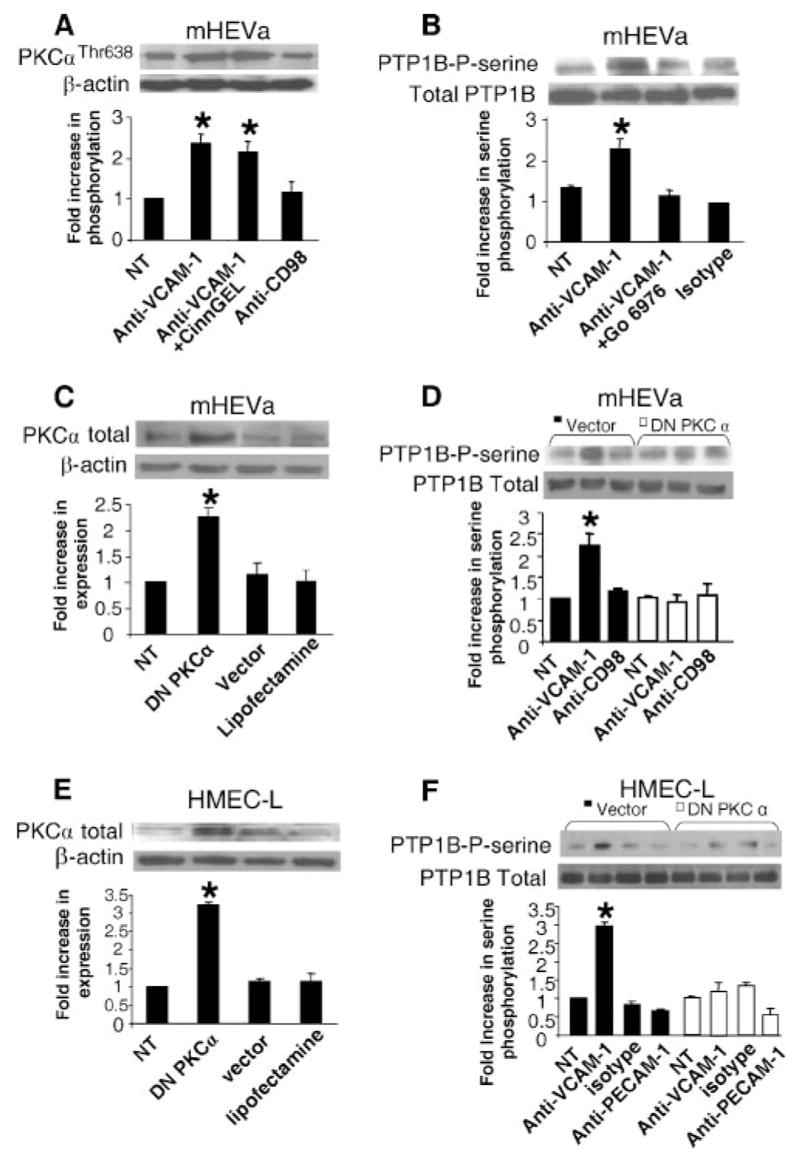
FIGURE 4.

VCAM-1 signaling through ROS activates PTPs by a mechanism independent of oxidation of the active site. A, mHEVa cells were pretreated with the NADPH oxidase inhibitor apocynin or the solvent control DMSO. The cells were stimulated with anti-VCAM-1-coated beads or the binding control (anti-CD98-coated beads). The cells were washed with PBS and lysed. PTP1B was immunoprecipitated, separated by SDS-PAGE, and analyzed by Western blot using anti-phosphoserine Ab. The membrane was stripped and probed with anti-PTP1B Ab. B, mHEVa cells were stimulated for 7 min with H2O2. C, Purified PTP1B was incubated for 5 min with H2O2. D, mHEVa cells were stimulated for 0, 10, and 25 min with anti-VCAM-1 Ab-coated beads. The cells were washed and scraped into PTP lysis buffer ± IAA. B–D, PTP activity was examined by the PTP1B in-gel assay followed by autoradiography. A–C, Data presented are the mean ± SD from three experiments. *, p < 0.05 compared with nonstimulated cells.
FIGURE 6.
PTP1B functions downstream of PKCα during VCAM-1 signaling. Nontreated mHEVa cells were pretreated with 10 μM CinnGEL-2ME (A) or 2.3 nM Go-6976 for 30 min (B). The cells were washed five times and then were stimulated with anti-VCAM-1-coated beads or control Ab-coated beads for 10 min. The cells were washed with PBS and scraped into SDS-PAGE loading buffer. Cell lysates were separated by SDS-PAGE followed by Western blot. A, Phosphorylated PKCα was detected with a rabbit-anti-mouse phospho-Thr (638)-PKCα Ab (1/1000) and an HRP-conjugated donkey anti-rabbit Ab (1/4000) followed by detection with ECL. The membrane was stripped and equal protein loading was verified using a mouse anti-β-actin Ab (1/5000). B, PTP1B was immunoprecipitated and Western blots were performed with anti-phosphoserine and then with anti-total PTP1B Abs. C–F, mHEVa cells or HMEC-L cells were LipofectAMINE transfected with DN PKCα. C and E, Western blots for total PKCα demonstrate increases in PKCα expression after transfection. D and F, The cells were stimulated with anti-VCAM-1-coated beads or control Ab-coated beads (isotype Ab, anti-CD98, or anti-PECAM-1) for 10 min and washed with PBS. PTP1B was immunoprecipitated. The immunoprecipitates were separated by SDS-PAGE followed by Western blot using a rabbit anti-phosphoserine and HRP-conjugated donkey anti-rabbit Ab followed by detection with ECL. The membrane was stripped and equal protein loading was verified using an anti-total PTP1B Ab. The inhibitors and DN PKCα did not affect cell viability (data not shown). Data presented are the mean ± SD from two experiments. *, p < 0.05 compared with non-treated mHEV cells.
Inhibition of PTP1B blocks lymphocyte migration
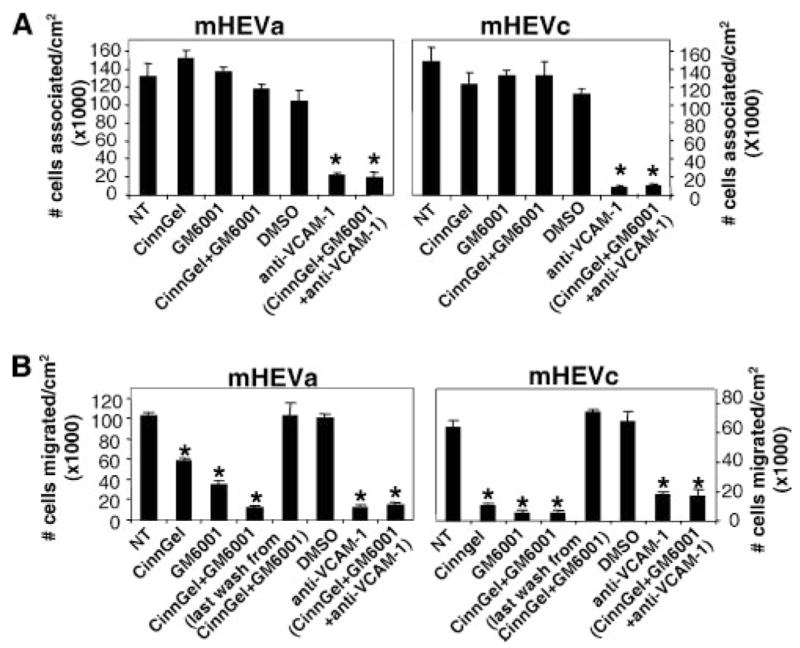
The VCAM-1 signaling cascade includes activation of NADPH oxidase and then subsequent oxidation of PKCα and membrane-bound MMPs (9, 13, 14, 27). These signals are required for VCAM-1-dependent lymphocyte migration (9, 13, 14). Therefore, it was determined whether VCAM-1 activation of endothelial cell PTP1B activity was involved in VCAM-1-dependent lymphocyte migration laminar flow conditions. Furthermore, to determine whether intracellular PTP1B functions in concert with extracellular endothelial-associated MMPs, mHEV cells were pretreated with 10 μM CinnGEL-2ME, a membrane permeable inhibitor of PTP1B, and/or with the MMP inhibitor GM6001 (10 μM) for 60 min and washed five times to remove excess inhibitor. As we have reported that lymphocyte adhesion to VCAM-1 is required for migration across the mHEV cells (9), we first determined whether the inhibitor pretreatment affected lymphocyte adhesion under static or laminar flow conditions (Fig. 3A). There was no effect of Cinn-GEL-2ME and/or GM6001 on lymphocyte association with endothelial cells under static or laminar flow (Fig. 3A). Furthermore, the inhibitors did not alter the VCAM-1 dependence of the spleen cell association with the endothelial cells (Fig. 3A). Next, we examined whether CinnGEL-2ME and/or GM6001 blocked spleen cell migration. Pretreatment with CinnGEL-2ME blocked lymphocyte migration across the endothelial cells under laminar flow conditions for 15 min (Fig. 3B). The last wash from inhibitor-pretreated endothelial cells was added to nontreated cells and this did not affect lymphocyte migration (Fig. 3B), indicating that the cells were sufficiently washed to remove excess inhibitor. In addition, for the mHEVa cells, the combination of CinnGEL with GM6001 enhanced inhibition of migration at 15 min under laminar flow as compared with cell pretreated with either the CinnGEL or the GM6001 alone (Fig. 3B). In summary, endothelial cell PTP1B functions in VCAM-1-dependent lymphocyte migration.
FIGURE 3.
Inhibition of PTP1B blocks lymphocyte migration but not adhesion under laminar flow conditions. Confluent monolayers of mHEVa and mHEVc cells on slides were treated for 60 min with 10 μM CinnGEL-2-ME, 10 μM GM6001 or the solvent control, 0.1% DMSO. The culture was washed five times to remove excess inhibitor. Spleen cells (3 × 106) were added on top of the mHEV monolayer (3.5 cm2). The spleen cells were allowed to briefly settle to mediate cell contact with the endothelial cell monolayer as determined by microscopy and then exposed to 2 dyne/cm2 laminar flow for 3 min for cell association (A) or 15 min for migration analysis (B). Cells were washed and fixed in 3% paraformaldehyde for 1 h. Migrated spleen cells are phase dark by phase contrast microscopy (37), whereas nonmigrated spleen cells are phase light (37). The spleen cells that migrate are >88% lymphocytes (12). CinnGel-2-ME and GM6001 had no affect on cell viability, as determined by trypan blue exclusion (data not shown). Data in each panel are from two to three experiments. *, p < 0.05 compared with nontreated or DMSO controls.
The VCAM-1 signaling intermediate H2O2 stimulates PTP1B activity
VCAM-1 signaling stimulates production of only 1–2 μM H2O2 (12), whereas it has been shown that high levels of H2O2 (>50 μM) inhibit PTP activity by oxidation of a critical cysteine residue in the active site (16). Therefore, it was determined whether phosphorylation of PTP1B occurred downstream of VCAM-1 activation of endothelial cell NADPH oxidase. For the rest of the studies, we focus on the mHEVa cell line and then on cultures of HMEC-L cells since, for both the mHEVa and mHEVc cell lines, there was activation of PTP1B as well as PTP1B dependence for migration. To examine mechanisms for activation of PTP1B, confluent monolayers of mHEVa cells were treated with apocynin to inhibit NADPH oxidase activity, or treated with the solvent control 0.1% DMSO for 30 min. We previously reported that apocynin blocks NADPH oxidase activity during VCAM-1 signaling without affecting cell viability or adhesion of lymphocytes to endothelial cells (9). The mHEVa cells were stimulated for 10 min with anti-VCAM-1-coated beads or control anti-CD98-coated beads. Anti-VCAM-1 increased endothelial cell PTP1B serine phosphorylation and this anti-VCAM-1 stimulation of PTP1B was blocked by apocynin (Fig. 4A). The anti-CD98 control Ab did not stimulate PTP1B phosphorylation and the DMSO solvent did not affect anti-VCAM-1-induced PTP1B phosphorylation (Fig. 4A). Background PTP1B phosphorylation was not affected by apocynin (Fig. 4A). In summary, VCAM-1 activates PTP1B in mHEVa cells. In addition, this PTP1B activation is downstream of VCAM-1-induced endothelial cell NADPH oxidase activity.
VCAM-1 induces exogenous production of H2O2 as we have reported that exogenous addition of catalase blocks VCAM-1 signals and VCAM-1-dependent lymphocyte migration (9, 13, 14). Furthermore, we have reported that addition of 1 μM H2O2 mimics VCAM-1 activation of endothelial cell-associated MMPs (13) and it has been reported that H2O2 diffuses through cell membranes at 100 μm/sec (40). Therefore, it was determined whether exogenous H2O2 activated endothelial cell PTP1B. To examine a dose response of cellular PTP1B to H2O2, the mHEVa cells were stimulated with 0, 0.3, 1, 10, and 50 μM H2O2. The cells were washed, lysed, and PTP activity examined using the in-gel PTP assay. Low levels of H2O2 (0.3, and 1 μM) activated PTP1B whereas high levels of H2O2 (50 μM) inhibited PTP1B (Fig. 4B). It has been reported that oxidation of the active site of PTP1B inhibits its activity (16, 17, 30). However, because PTP1B contains nonactive site cysteines that can be modified by allosteric inhibitors (41), we examined whether low concentrations of H2O2 can directly activate purified PTP1B by oxidizing PTP1B at a site distinct from the active site cysteine. Therefore, purified PTP1B was incubated for 5 min with 0, 0.3, 1, 10 and 50 μM H2O2. However, all concentrations of H2O2 inhibited the activity of purified PTP1B (Fig. 4C).
To further determine whether cellular PTP1B was oxidized during VCAM-1 signaling, the endothelial cells were stimulated with anti-VCAM-1 Ab-coated beads for 0, 10, or 25 min. The mHEVa cells were washed with PBS and scraped into PTP lysis buffer with or without iodoacetic acid (IAA) (10 mM). IAA binds to cysteines in the active site of nonoxidized PTPs and irreversibly inhibit their activity in the in-gel assay (30). In contrast, if a PTP is oxidized, IAA will not bind, DTT in the assay buffer will reverse the oxidation, and the PTP will be active in the in-gel assay and a clear band will appear in the gel (30). Anti-VCAM-1-coated beads stimulated the activation of PTP1B; however, this activity was blocked by IAA (Fig. 4D). Therefore, PTP1B was not oxidized at the active site by VCAM-1 signaling. Together, these data suggest that after stimulation of VCAM-1, the 1 μM H2O2 generated by endothelial cells, modifies signals upstream of endothelial cell PTP1B and that PTP1B in the cell is protected from H2O2-mediated oxidation. In summary, VCAM-1 signaling activated PTP1B.
VCAM-1 signaling activates PTP1B in primary cultures of HMEC-L cells
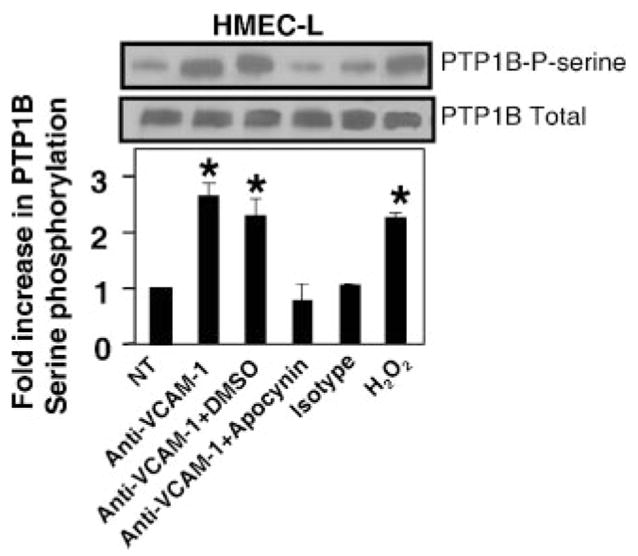
It was determined whether VCAM-1 stimulates PTP1B in primary cultures (passage 3–4) of human lung microvascular endothelial cells (HMEC-L cells) and it was determined whether phosphorylation of PTP1B occurred downstream of VCAM-1 activation of endothelial cell NADPH oxidase. HMEC-L cells do not constitutively express VCAM-1. Therefore, confluent monolayers of HMEC-L cells were induced to express VCAM-1 by treatment with TNF-α for 24 h. The cells were washed and then treated with apocynin to inhibit NADPH oxidase activity, or treated with the solvent control 0.1% DMSO for 30 min. The HMEC-L cells were stimulated for 10 min with anti-VCAM-1 or an isotype-matched Ab complexed with a secondary Ab. Alternatively, the endothelial cells were stimulated with 1 μM H2O2 to mimic the anti-VCAM-1 stimulation. The HMEC-L cells expressed VCAM-1 as determined by immunolabeling and flow cytometry (data not shown). Anti-VCAM-1 and H2O2 increased HMEC-L PTP1B serine phosphorylation and this anti-VCAM-1 stimulation of PTP1B was blocked by apocynin (Fig. 5). The isotype control Ab did not stimulate PTP1B phosphorylation and the DMSO solvent did not affect anti-VCAM-1-induced PTP1B phosphorylation (Fig. 5). In summary, VCAM-1 activates PTP1B in mHEV cell lines and in HMEC-L cells. In addition, this PTP1B activation is downstream of VCAM-1-induced NADPH oxidase activity.
FIGURE 5.
In HMEC-L cells, VCAM-1 signaling through NADPH oxidase increases serine phosphorylation of PTP1B. HMEC-L cells were treated with 1 ng/ml TNF-α for 24 h to induce VCAM-1 expression. The confluent HMEC-L cells were washed and then pretreated with 4 mM apocynin or the solvent control 0.1% DMSO for 20 min. There was no effect of apocynin on cell viability (data not shown). The HMEC-L cells were stimulated with 27 μg/ml mouse anti-human VCAM-1 Ab or an isotype-matched Ab plus 15 μg/ml goat anti-mouse IgG Ab for 10 min. HMEC-L cells were also stimulated with 1 μM H2O2 for 10 min. Cells were washed with PBS and lysed. PTP1B was immunoprecipitated, separated by SDS-PAGE, and analyzed by Western blot using anti-phosphoserine Ab. The membrane was stripped and probed with anti-PTP1B Ab to determine protein loading. Densitometry analysis was performed using Image J software from the National Institutes of Health. Data presented are the mean ± SD of two experiments. *, p < 0.05 compared with nontreated cells.
PTP1B functions downstream of PKCα in the VCAM-1 signal transduction pathway
We have reported that VCAM-1 signaling via NADPH oxidase or 1 μM H2O2 results in oxidation and consequently, transient activation of PKCα at 10–15 min (14). This occurs without altering total PKCα expression (14). Therefore, it was determined whether PTP1B functions upstream or downstream of PKCα during VCAM-1 signaling. mHEVa cells were pretreated with the PTP1B inhibitor CinnGEL (10 μM) for 30 min and then stimulated with anti-VCAM-1 for 15 min. mHEVa cells were scraped into SDS-PAGE loading buffer and lysates were analyzed by SDS-PAGE followed by western blot using an Ab that recognizes an active form of PKCα, phospho-Thr (638) PKCα. Equal protein loading was verified by stripping the membrane and probing for mouse anti-β-actin. Anti-VCAM-1 activated PKCα, but this activation was not blocked by the PTP1B inhibitor CinnGEL (Fig. 6A). The control anti-CD98 did not activate PKCα (Fig. 6A). These data suggests that PTP1B activity is not upstream of PKCα. Therefore, we determined whether PTP1B activation was downstream of PKCα during VCAM-1 signaling using a PKCα inhibitor and dominant negative PKCα. The mHEVa cells were pretreated for 30 min with the PKCα inhibitor Gö-6976 (2.3 nM) and washed five times. Gö-6976 inhibits PKCα and PKCβI with IC50 values in the nanomolar range, whereas up to 3 μM Gö-6976 has no effect on the activity of PKC-δ, -ε, and -ζ (42, 43). Gö-6976 blocked anti-VCAM-1-stimulated serine phosphorylation of PTP1B (Fig. 6B). The control Ab did not stimulate phosphorylation of PTP1B (Fig. 6B). Another inhibitor of PKCα, Rö-32–0432 (100 nM), also blocked anti-VCAM-1 stimulated phosphorylation of PTP1B in mHEV cells (data not shown).
It was then determined whether transient transfection of mHEVa cells or primary cultures of HMEC-L cells (passage 3–4) with a dominant-negative (DN) PKCα blocks anti-VCAM-1 activation of PTP1B. mHEVa cells or cytokine-treated HMEC-L cells were LipofectAMINE-transfected with 1 μg DN PKCα plasmid or vector pCMV for 3.5 h, washed and cultured for 24 h. These transfected cells were examined for an increase in total PKCα expression by western blot as well as examined for VCAM-1-stimulated serine phosphorylation of PTP1B. The transfection did not affect mHEVa cell expression of VCAM-1 as determined by immunolabeling and flow cytometry (data not shown). As expected, transfection with the DN PKCα increased PKCα expression in the endothelial cells (Fig. 6, C and E), indicating successful transfection. Importantly, transfection with the DN PKCα inhibited anti-VCAM-1 stimulation of PTP1B serine phosphorylation as compared with the vector control (Fig. 6, D and F). In summary, the data are consistent with the following signaling cascade: VCAM-1 activates endothelial cell NADPH oxidase to generate ROS; these ROS directly oxidize PKCα and transiently increase PKCα activity; this PKCα activity then transiently increases serine phosphorylation of PTP1B in endothelial cells; these signals function in VCAM-1-mediated lymphocyte migration.
Discussion
We have previously demonstrated that lymphocyte migration across mHEV cells and lymphocyte stimulation of VCAM-1 signaling are blocked by anti-VCAM-1 Abs (9, 24), by inhibition of endothelial cell NADPH oxidase activity with apocynin, DPI, or antisense to the gp91 phox subunit of NADPH oxidase (9, 13, 27), by scavenging ROS with exogenous catalase or superoxide dismutase (9), or by inhibition of endothelial cell PKCα with dominant negative PKCα or pharmacological inhibitors (14). Thus, endothelial cells have an active role in promotion of VCAM-1-dependent lymphocyte migration by an endothelial signal transduction cascade involving NADPH oxidase, ROS, and PKCα. In this study, we demonstrate that endothelial cell PTP1B activity participates in this VCAM-1-dependent signal transduction. PTP1B functioned downstream of VCAM-1-activated NADPH oxidase as inhibition of NADPH oxidase blocked anti-VCAM-1 activation of PTP1B. This was supported by the data showing that the exogenous addition of 1 mM H2O2, which corresponds to the levels of H2O2 produced after VCAM-1 stimulation (9, 12, 26), mimicked VCAM-1-induced PTP1B activity and serine phosphorylation of PTP1B. This serine phosphorylation of PTP1B required PKCα activity. Furthermore, VCAM-1 activation of PTP1B was required for VCAM-1-dependent lymphocyte migration. This is the first report of VCAM-1 signaling through PTP1B and we identify a mechanism for this activation.
It has been reported that PTP1B can be phosphorylated on Ser (378) by PKC (23) and this serine phosphorylation of PTP1B increases PTP1B activity (22). Furthermore, we previously reported that PKCα is activated by direct oxidation and it functions downstream of VCAM-1-stimulated endothelial cell NADPH oxidase activity (14). In the present report, VCAM-1-stimulated PKCα activity results in an increase in PTP1B activity as inhibition of PKCα blocks VCAM-1 activation of serine phosphorylation of PTP1B. This role of PKCα in activation of PTP1B does not preclude an increase in PTP1B phosphorylation, due in part, from an inhibition of ser/thr phosphatase activity induced by oxidation of the cysteine in the catalytic domain of ser/thr phosphatases. Whether ser/thr phosphatases are modulated during VCAM-1 signaling is in studies in progress.
In contrast to the ROS-dependent indirect activation of the tyrosine phosphatase PTP1B in endothelial cells reported here, tyrosine phosphatases can be directly inhibited by ROS through oxidation of the cysteine in their catalytic domain (16, 17). Consistent with this, we showed that purified PTP1B is inhibited by exogenous addition of 1 μM H2O2, the level generated by VCAM-1 signaling (9, 12, 26). However, VCAM-1 signaling via ROS increased PTP1B activity in endothelial cells. Furthermore, we demonstrated that cellular PTP1B was not directly oxidized by ROS during VCAM-1 signaling in endothelial cells. Instead, PTP1B was activated by PKCα-dependent serine phosphorylation of PTP1B. Therefore, during VCAM-1 signaling, there is restricted access of the low concentration of H2O2 to PTP1B by cellular compartmentalization. It has been reported that activation of PTP1B induces changes in localization of PTP1B in the cell (44). Alternatively, PTP1B may be insensitive to oxidation because it has been shown that PTPs are differentially oxidized in cells depending on the microenvironment of the catalytic cysteine (45). Therefore, the cellular microenvironment may influence the reactivity of PTPs within cells.
In vivo, in addition to VCAM-1, multiple endothelial cell adhesion molecules can mediate leukocyte infiltration into tissues, albeit through different signaling pathways (7, 46). However, during VCAM-1-dependent inflammation, anti-VCAM-1 Abs block infiltration of eosinophils into the lung in experimental asthma and block T cell infiltration into the brain in experimental allergic encephalomyelitis (1, 2). Abs against the ligand for VCAM-1, α-4 integrin, have been administered as therapy for patients with multiple sclerosis or inflammatory bowel disease (47–50). We have reported that in vivo treatment of mice with the ROS scavenger bilirubin blocks VCAM-1-dependent leukocyte migration but not the migration of leukocytes that use multiple endothelial cell adhesion molecules (46). These reports further emphasize that different adhesion molecules have different signaling pathways. In addition, the in vivo studies are consistent with in the vitro studies identifying different signaling pathways for adhesion molecules (7, 46). Therefore, in our studies on VCAM-1 signaling, VCAM-1 on HMEC-L cells, which express multiple adhesion molecules, is specifically triggered by anti-VCAM-1 Abs rather than by lymphocyte binding as lymphocyte binding to multiple adhesion molecules on TNF-α-activated HMEC-L cells trigger a complex set of intracellular signals. For the same reasons, the migration of spleen cells across TNF-α-activated HMEC-L cells was not examined to address VCAM-1-mediated signals as the HMEC-L cells express multiple receptors that support leukocyte migration through different signaling pathways (7, 46). Nevertheless, our studies performed with HMEC-L cells demonstrate that VCAM-1 on micro-vascular endothelial cells activates endothelial cell PTP1B through VCAM-1-triggered endothelial cell NADPH oxidase and PKCα. The identification of mechanisms for VCAM-1 signaling is important for proposing intervention of VCAM-1-dependent processes in vivo such as VCAM-1-dependent lung eosinophilia, VCAM-1-dependent T cell migration into the brain in multiple sclerosis, or VCAM-1-dependent T cell migration into the bowel in inflammatory bowel disease (1, 2, 47–50).
Signals mediated by endothelial cell adhesion molecules must be localized as endothelial cells retract only at the site of contact with a leukocyte. Therefore, we discuss here a model for localization of VCAM-1-induced extracellular production of H2O2. Cell membrane NADPH oxidases generate extracellular superoxide which rapidly dismutates to H2O2. Therefore, the question arises as to how extracellular H2O2, which would either be washed away by the blood or rapidly diffuse through cell membranes at 100 μm/second (40), could result in stimulation of localized endothelial function to induce endothelial cell shape changes at the site of leukocyte contact and allow VCAM-1-dependent leukocyte passage? This report and previous studies using exogenous addition of catalase that scavenges H2O2 to block VCAM-1 signals (9, 13), suggests that VCAM-1 engagement results in extracellular H2O2. Specifically, catalase blocks VCAM-1-dependent lymphocyte migration (9) and VCAM-1 activation of endothelial cell-associated MMPs (13). Furthermore, washing by blood flow as modeled by fluid flow at 2 dynes/cm2 does not alter VCAM-1-dependent endothelial cell ROS oxidation and activation of endothelial cell-associated matrix metalloproteinases (13). Thus, a working model for VCAM-1 signals is that 1) extracellular ROS produced by localized endothelial cell membrane NADPH oxidase oxidize localized proteins such as membrane-associated MMPs and 2) these ROS diffuse into the cell at 100 μm/second and act on localized intracellular PKCα, thereby affecting localized endothelial cell functions. This PKCα then activates PTP1B. To localize the ROS signal in the cell, the modest concentration of H2O2 (1 μM) (12, 27) likely oxidizes localized targets but, as the H2O2 diffuses, it would be too dilute at distant sites for a functional effect or is scavenged intracellulary at distant sites. In addition, extracellular ROS that is washed away by the blood flow would also be diluted and therefore have little effect at distant sites. The detection of localized VCAM-1-dependent changes in endothelial cell junctions is recently under investigation in our lab and beyond the scope of this manuscript.
In summary, VCAM-1-dependent lymphocyte migration is mediated by an increase in NADPH oxidase-dependent and PKCα-dependent phosphorylation of PTP1B, increasing PTP1B activity. Thus, the current model for VCAM-1 signaling is the following: binding to VCAM-1 stimulates calcium fluxes and Rac-1; this activates NADPH oxidase for the generation of extracellular ROS; H2O2 rapidly diffuses through membranes at 100 μm/sec; the 1 μM H2O2 transiently oxidizes localized PKCα; PKCα mediates phosphorylation and activation of PTP1B; these signals are required for VCAM-1-dependent lymphocyte migration. This is the first report demonstrating a mechanism for VCAM-1 activation of PTP1B for lymphocyte migration.
Acknowledgments
This manuscript is dedicated in memory of Norman Cook who constructed the parallel plate flow chamber.
Footnotes
This study was supported by National Institutes of Health Grant RO1 HL69428 (to J.M.C.-M.).
Abbreviations used in this paper: ROS, reactive oxygen species; MMP, matrix metalloproteinase; PKC, protein kinase C; HMEC, human microvascular endothelial cell; PTP, protein tyrosine phosphatase; DN, dominant-negative; IAA, iodoacetic acid.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Chin JE, Hatfield CA, Winterrowd GE, Brashler JR, Vonderfecht SL, Fidler SF, Griffin RL, Kolbasa KP, Krzesicki RF, Sly LM, et al. Airway recruitment of leukocytes in mice is dependent on α4-integrins and vascular cell adhesion molecule-1. Am J Physiol. 1997;272:L219–L229. doi: 10.1152/ajplung.1997.272.2.L219. [DOI] [PubMed] [Google Scholar]
- 2.Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA., Jr Surface expression of α4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tummala PE, Chen XL, Sundell CL, Laursen JB, Hammes CP, Alexander RW, Harrison DG, Medford RM. Angiotensin II induces vascular cell adhesion molecule-1 expression in rat vasculature: a potential link between the renin-angiotensin system and atherosclerosis. Circulation. 1999;100:1223–1229. doi: 10.1161/01.cir.100.11.1223. [DOI] [PubMed] [Google Scholar]
- 4.Wright PS, Cooper JR, Kropp KE, Busch SJ. Induction of vascular cell adhesion molecule-1 expression by IL-4 in human aortic smooth muscle cells is not associated with increased nuclear NF-κB levels. J Cell Physiol. 1999;180:381–389. doi: 10.1002/(SICI)1097-4652(199909)180:3<381::AID-JCP9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.Balogh P, Aydar Y, Tew JG, Szakal AK. Appearance and phenotype of murine follicular dendritic cells expressing VCAM-1. Anat Rec. 2002;268:160–168. doi: 10.1002/ar.10148. [DOI] [PubMed] [Google Scholar]
- 6.Lorenzon P, Vecile E, Nardon E, Ferrero E, Harlan JM, Tedesco F, Dobrina A. Endothelial cell E- and P-selectin and vascular cell adhesion molecule-1 function as signaling receptors. J Cell Biol. 1998;142:1381–1391. doi: 10.1083/jcb.142.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook-Mills JM, Deem TL. Active endothelial cell function during inflammation. J Leukocyte Biol. 2005;77:487–495. doi: 10.1189/jlb.0904554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etienne-Manneville S, Manneville JB, Adamson P, Wilbourn B, Greenwood J, Couraud PO. ICAM-1-coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. J Immunol. 2000;165:3375–3383. doi: 10.4049/jimmunol.165.6.3375. [DOI] [PubMed] [Google Scholar]
- 9.Matheny HE, Deem TL, Cook-Mills JM. Lymphocyte migration through monolayers of endothelial cell lines involves VCAM-1 signaling via endothelial cell NADPH oxidase. J Immunol. 2000;164:6550–6559. doi: 10.4049/jimmunol.164.12.6550. [DOI] [PubMed] [Google Scholar]
- 10.van Wetering S, van den Berk N, van Buul JD, Mul FPJ, Lommerse I, Mous R, ten Klooster J-P, Zwaginga J-J, Hordijk PL. VCAM-1-mediated Rac signaling controls endothelial cell-cell contacts and leukocyte transmigration. Am J Physiol. 2003;285:C343–C352. doi: 10.1152/ajpcell.00048.2003. [DOI] [PubMed] [Google Scholar]
- 11.Gurtner GC, Davis V, Li H, McCoy MJ, Sharpe A, Cybulsky MI. Targeted disruption of the murine VCAM1 gene: essential role of VCAM-1 in chorioallantoic fusion and placentation. Genes Dev. 1995;9:1–14. doi: 10.1101/gad.9.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Tudor KSRS, Hess KL, Cook-Mills JM. Cytokines modulate endothelial cell intracellular signal transduction required for VCAM-1-dependent lymphocyte transendothelial migration. Cytokine. 2001;15:196–211. doi: 10.1006/cyto.2001.0922. [DOI] [PubMed] [Google Scholar]
- 13.Deem TL, Cook-Mills JM. Vascular cell adhesion molecule-1 (VCAM-1) activation of endothelial cell matrix metalloproteinases: role of reactive oxygen species. Blood. 2004;104:2385–2393. doi: 10.1182/blood-2004-02-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdala-Valencia H, Cook-Mills JM. Vascular cell adhesion molecule (VCAM-1) signals activate endothelial cell protein kinase Cα via oxidation. J Immunol. 2006;177:6379–6387. doi: 10.4049/jimmunol.177.9.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 16.Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med. 2002;166:S4–S8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 17.Sommer D, Coleman S, Swanson SA, Stemmer PM. Differential susceptibilities of serine/threonine phosphatases to oxidative and nitrosative stress. Arch Biochem Biophys. 2002;404:271–278. doi: 10.1016/s0003-9861(02)00242-4. [DOI] [PubMed] [Google Scholar]
- 18.Gabel S, Benefield J, Meisinger J, Petruzzelli GJ, Young M. Protein phosphatases 1 and 2A maintain endothelial cells in a resting state, limiting the motility that is needed for the morphogenic process of angiogenesis. Otolaryngol Head Neck Surg. 1999;121:463–468. doi: 10.1016/S0194-5998(99)70238-X. [DOI] [PubMed] [Google Scholar]
- 19.Hosoya N, Mitsui M, Yazama F, Ishihara H, Ozaki H, Karaki H, Hartshorne DJ, Mohri H. Changes in the cytoskeletal structure of cultured smooth muscle cells induced by calyculin-A. J Cell Sci. 1993;105:883–890. doi: 10.1242/jcs.105.4.883. [DOI] [PubMed] [Google Scholar]
- 20.Dadke S, Chernoff J. Protein-tyrosine phosphatase 1B mediates the effects of insulin on the actin cytoskeleton in immortalized fibroblasts. J Biol Chem. 2003;278:40607–40611. doi: 10.1074/jbc.M306772200. [DOI] [PubMed] [Google Scholar]
- 21.Liu F, Sells MA, Chernoff J. Protein tyrosine phosphatase 1B negatively regulates integrin signaling. Curr Biol. 1998;8:173–176. doi: 10.1016/s0960-9822(98)70066-1. [DOI] [PubMed] [Google Scholar]
- 22.Brautigan DL, Pinault FM. Serine phosphorylation of protein tyrosine phosphatase (PTP1B) in HeLa cells in response to analogues of cAMP or diacylglycerol plus okadaic acid. Mol Cell Biochem. 1993;127–128:121–129. doi: 10.1007/BF01076763. [DOI] [PubMed] [Google Scholar]
- 23.Flint AJ, Gebbink MF, Franza BR, Jr, Hill DE, Tonks NK. Multi-site phosphorylation of the protein tyrosine phosphatase, PTP1B: identification of cell cycle regulated and phorbol ester stimulated sites of phosphorylation. EMBO J. 1993;12:1937–1946. doi: 10.1002/j.1460-2075.1993.tb05843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tudor KS, Deem TL, Cook-Mills JM. Novel α4-integrin ligands on an endothelial cell line. Biochem Cell Biol. 2000;78:99–113. [PubMed] [Google Scholar]
- 25.Qureshi MH, Cook-Mills J, Doherty DE, Garvy BA. TNF-α-dependent ICAM-1- and VCAM-1-mediated inflammatory responses are delayed in neonatal mice infected with Pneumocystis carinii. J Immunol. 2003;171:4700–4707. doi: 10.4049/jimmunol.171.9.4700. [DOI] [PubMed] [Google Scholar]
- 26.Cook-Mills JM, Gallagher JS, Feldbush TL. Isolation and characterization of high endothelial cell lines derived from mouse lymph nodes. In Vitro Cell Dev Biol Anim. 1996;32:167–177. doi: 10.1007/BF02723682. [DOI] [PubMed] [Google Scholar]
- 27.Cook-Mills JM, Johnson JD, Deem TL, Ochi A, Wang L, Zheng Y. Calcium mobilization and Rac1 activation are required for VCAM-1 (vascular cell adhesion molecule-1) stimulation of NADPH oxidase activity. Biochem J. 2004;378:539–547. doi: 10.1042/BJ20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.St-Denis A, Chano F, Tremblay P, St-Pierre Y, Descoteaux A. Protein kinase C-α modulates lipopolysaccharide-induced functions in a murine macrophage cell line. J Biol Chem. 1998;273:32787–32792. doi: 10.1074/jbc.273.49.32787. [DOI] [PubMed] [Google Scholar]
- 29.Fialkow L, Chan CK, Downey GP. Inhibition of CD45 during neutrophil activation. J Immunol. 1997;158:5409–5417. [PubMed] [Google Scholar]
- 30.Burridge K, Nelson A. An in-gel assay for protein tyrosine phosphatase activity: detection of widespread distribution in cells and tissues. Anal Biochem. 1995;232:56–64. doi: 10.1006/abio.1995.9961. [DOI] [PubMed] [Google Scholar]
- 31.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 32.Chen YH, Lu Q, Goodenough DA, Jeansonne B. Nonreceptor tyrosine kinase c-Yes interacts with occludin during tight junction formation in canine kidney epithelial cells. Mol Biol Cell. 2002;13:1227–1237. doi: 10.1091/mbc.01-08-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawrence MB, McIntire LV, Eskin SG. Effect of flow on polymorphonuclear leukocyte/endothelial cell adhesion. Blood. 1987;70:1284–1290. [PubMed] [Google Scholar]
- 34.Nobis U, Pries AR, Cokelet GR, Gaehtgens P. Radial distribution of white cells during blood flow in small tubes. Microvasc Res. 1985;29:295–304. doi: 10.1016/0026-2862(85)90020-2. [DOI] [PubMed] [Google Scholar]
- 35.Smith ML, Long DS, Damiano ER, Ley K. Near-wall micro-PIV reveals a hydrodynamically relevant endothelial surface layer in venules in vivo. Biophys J. 2003;85:637–645. doi: 10.1016/s0006-3495(03)74507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipowsky HH. Microvascular rheology and hemodynamics. Microcirculation. 2005;12:5–15. doi: 10.1080/10739680590894966. [DOI] [PubMed] [Google Scholar]
- 37.Ager A, Mistry S. Interaction between lymphocytes and cultured high endothelial cells: an in vitro model of lymphocyte migration across high endothelial venule endothelium. Eur J Immunol. 1988;18:1265–1274. doi: 10.1002/eji.1830180818. [DOI] [PubMed] [Google Scholar]
- 38.Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 39.Moran EJ, Sarshar S, Cargill JF, Shahbaz MM, Lio A, Mjalli AMM, Arstrong RW. Radio frequency tag encoded combinatorial library method for the discovery of tripeptide-subsituted cinnamic acid inhibitors of the protein tyrosine phosphatase PTP1B. J Am Chem Soc. 1995;117:10787–10788. [Google Scholar]
- 40.Mathai JC, Sitaramam V. Stretch sensitivity of transmembrane mobility of hydrogen peroxide through voids in the bilayer: role of cardiolipin. J Biol Chem. 1994;269:17784–17793. [PubMed] [Google Scholar]
- 41.Hansen SK, Cancilla MT, Shiau TP, Kung J, Chen T, Erlanson DA. Allosteric inhibition of PTP1B activity by selective modification of a non-active site cysteine residue. Biochemistry. 2005;44:7704–7712. doi: 10.1021/bi047417s. [DOI] [PubMed] [Google Scholar]
- 42.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 43.Ma R, Kudlacek PE, Sansom SC. Protein kinase Cα participates in activation of store-operated Ca2+ channels in human glomerular mesangial cells. Am J Physiol. 2002;283:C1390–C1398. doi: 10.1152/ajpcell.00141.2002. [DOI] [PubMed] [Google Scholar]
- 44.Ezumi Y, Takayama H, Okuma M. Differential regulation of protein-tyrosine phosphatases by integrin α IIb β 3 through cytoskeletal reorganization and tyrosine phosphorylation in human platelets. J Biol Chem. 1995;270:11927–11934. doi: 10.1074/jbc.270.20.11927. [DOI] [PubMed] [Google Scholar]
- 45.Groen A, Lemeer S, van der Wijk T, Overvoorde J, Heck AJR, Ostman A, Barford D, Slijper M, den Hertog J. Differential oxidation of protein-tyrosine phosphatases. J Biol Chem. 2005;280:10298–10304. doi: 10.1074/jbc.M412424200. [DOI] [PubMed] [Google Scholar]
- 46.Keshavan P, Deem TL, Schwemberger SJ, Babcock GF, Cook-Mills JM, Zucker SD. Unconjugated bilirubin inhibits VCAM-1-mediated transendothelial leukocyte migration. J Immunol. 2005;174:3709–3718. doi: 10.4049/jimmunol.174.6.3709. [DOI] [PubMed] [Google Scholar]
- 47.Cree B. Emerging monoclonal antibody therapies for multiple sclerosis. Neurologist. 2006;12:171–178. doi: 10.1097/01.nrl.0000204859.15501.6b. [DOI] [PubMed] [Google Scholar]
- 48.Bennett JL. Natalizumab and progressive multifocal leukoencephalopathy: migrating towards safe adhesion molecule therapy in multiple sclerosis. Neurol Res. 2006;28:291–298. doi: 10.1179/016164106X98189. [DOI] [PubMed] [Google Scholar]
- 49.Niino M, Bodner C, Simard ML, Alatab S, Gano D, Kim HJ, Trigueiro M, Racicot D, Guerette C, Antel JP, et al. Natalizumab effects on immune cell responses in multiple sclerosis. Ann Neurol. 2006;59:748–754. doi: 10.1002/ana.20859. [DOI] [PubMed] [Google Scholar]
- 50.Lanzarotto F, Carpani M, Chaudhary R, Ghosh S. Novel treatment options for inflammatory bowel disease targeting α4 integrin. Drugs. 2006;66:1179–1189. doi: 10.2165/00003495-200666090-00002. [DOI] [PubMed] [Google Scholar]