Abstract
Lymphocytes bound at endothelial cell junctions extravasate within minutes. Lymphocyte-endothelial cell binding is mediated by receptors such as vascular cell adhesion molecule 1 (VCAM-1). VCAM-1 activates endothelial cell nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in minutes, and this activity is required for VCAM-1–dependent lymphocyte migration. In this report, we examined mechanisms for activation of matrix metalloproteinases (MMPs) during VCAM-1–dependent lymphocyte migration. Lymphocyte binding to VCAM-1 rapidly activated endothelial cell-associated MMPs. Furthermore, inhibition of MMPs on the endothelial cells but not on the lymphocytes blocked VCAM-1–dependent lymphocyte migration across endothelial cells. The activation of endothelial cell MMPs required VCAM-1–stimulated endothelial cell NADPH oxidase activity as determined by scavenging of reactive oxygen species (ROS) and by pharmacologic or antisense inhibition of NADPH oxidase. Exogenous addition of 1 μM H2O2, the level of H2O2 generated by VCAM-1–stimulated endothelial cells, rapidly activated endothelial cell-associated MMPs. In contrast, activation of lymphocyte-associated MMPs was delayed by hours after binding to VCAM-1, and this activation was blocked by inhibition of endothelial cell ROS generation. There was also a delay in H2O2-induced decrease in lymphocyte-associated tissue inhibitors of metalloproteinases (TIMPs), resulting in an increase in MMP/TIMP ratio. In summary, this is the first report of a mechanism for ROS function in VCAM-1 activation of endothelial cell MMPs during VCAM-1–dependent lymphocyte migration.
Introduction
Lymphocytes migrate out of the blood between endothelial cells and into tissues where the lymphocytes can interact with antigen. Endothelial cells bind lymphocytes through cell surface adhesion molecules. One of these adhesion molecules is vascular cell adhesion molecule 1 (VCAM-1). It is important to understand VCAM-1 signaling because it is involved in several diseases. For example, VCAM-1 is required for eosinophil infiltration into the lung in experimental ovalbumin-induced asthma,1 as well as T-cell infiltration across the blood-brain barrier in experimental allergic encephalomyelitis (EAE).2 In addition, VCAM-1 functions in combination with other adhesion molecules during chronic inflammation and tumor metastasis. Understanding VCAM-1 signaling may have important implications for disease intervention.
We have reported that VCAM-1 signaling in endothelial cells is required for VCAM-1–dependent lymphocyte migration.3 Stimulation of VCAM-1 activates endothelial cell nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which catalyzes the release of low levels of reactive oxygen species (ROS) in cytokine-treated human umbilical vein endothelial cells (HUVECs) and in endothelial cell lines.4,5 These ROS are required for VCAM-1–stimulated endothelial cell actin restructuring and lymphocyte migration.3,6,7 Therefore, ROS are involved in modulating endothelial cell function to promote VCAM-1–dependent lymphocyte migration.
It has been reported that VCAM-1–dependent adhesion of a T-cell line activates lymphocyte matrix metalloproteinases (MMPs) after 5 hours.8 However, the mechanism(s) for VCAM-1 activation of lymphocyte MMPs is not known. It is also not known whether VCAM-1 signaling activates endothelial cell MMPs. Activated MMPs degrade extracellular matrix, cell surface receptors in cell-cell junctions, and tight junction proteins.9–11 MMP activation can be regulated by ROS. In smooth muscle cells, the latent form of MMP-2 (pro-MMP-2) is released after mechanical stretch-stimulated production of ROS by NADPH oxidase.12 In cell-free systems, low concentrations of ROS can activate pro-MMPs by oxidation of the sulfide bond in the prodomain of the MMP followed by release of this prodomain by autocatalytic cleavage.13 In this report, we demonstrate that VCAM-1 rapidly activates endothelial cell–associated MMPs and that this activation is mediated by endothelial cell–derived ROS. In addition, endothelial cell–derived ROS are involved in VCAM-1–stimulated activation of lymphocyte MMPs, and this lymphocyte MMP activation is delayed by several hours.
Materials and methods
Cells
The endothelial cell line mHEVc was previously derived from BALB/c mouse cervical lymph nodes and cultured as previously described.14 HUVECs obtained from Clonetics (Walkersville, MD) were grown in endothelial growth medium (Clonetics) plus 5% fetal calf serum (FCS) and were used at passage 4 to 6. Mouse BALB/c CL.7 fibroblasts, obtained from American Type Culture Collection (Manassas, VA), were grown in Dulbecco modified Eagle medium (DMEM) plus 10% FCS. Single cell suspensions of spleen cells were prepared from freshly isolated spleens of male 6- to 8-week-old BALB/c mice (Harlan Industries, Indianapolis, IN) as previously described.14 Where indicated, red blood cells were lysed by hypotonic shock as previously described.3 Cell viability was determined by trypan blue exclusion.
Antibody-coated beads
Streptavidin-coated 9.9-μm diameter beads (40 μL; Bangs Laboratories, Fishers, IN) were labeled with 6 μg biotin-conjugated goat antirat immunoglobulin (no. 3050-08; Southern Biotech, Birmingham, AL) in 75 μL phosphate-buffered saline (PBS) with gentle rocking for 1 hour at 4°C and then washed 3 times.3 These beads were incubated with 8 μg rat antimouse VCAM-1 (clone MVCAM.A) or a rat isotype control antibody (clone R35–95; PharMingen, San Diego, CA) in 40 μL PBS with gentle rocking for 1 hour at 4°C, washed, and suspended in 40 μL PBS. For experiments with HUVECs, the streptavidin-conjugated beads were coated with 12 μg biotin-conjugated goat antimouse immunoglobulin G1 (IgG1; no. 12062D; PharMingen) and 8 μg mouse antihuman VCAM-1(clone 51-10C9; PharMingen) or a control antibody (Ab; mouse antihuman platelet endothelial cell adhesion molecule 1 [PECAM-1]; clone C-20; Santa Cruz Biotechnology, Santa Cruz, CA).
Transwell migration assay
mHEVc cells were grown to confluence on Transwells with 12-μm pores (Costar, Cambridge, MA). Spleen cell migration was performed as previously described.3 The mHEVc cell monolayers block nonspecific accumulation of red blood cells in the lower Transwell chamber and block fluorescein isothiocyanate (FITC)–albumin diffusion.4 Spleen cell migration is stimulated by mHEV cell secretion of the chemokine monocyte chemoattractant protein 1 (MCP-1).15 The number of lymphocytes that migrate is linear from 0 to 24 hours followed by a plateau.4 Spleen cells that migrate are more than 95% lymphocytes,4 and the percentage of cells that migrate is consistent with other in vitro models with endothelial cell lines or cytokine-activated microvascular endothelial cells.16,17
Migration with laminar flow
Endothelial cells were grown to confluence on glass slides and attached to a parallel plate flow chamber.18 After red blood cell removal, spleen cells were added to the endothelial cells. The coculture was untreated or exposed to laminar flow at 2 dynes/cm2 at 37°C for 30 minutes, washed, and fixed with 3% paraformaldehyde for 1 hour. Lymphocyte migration was quantified by phase contrast microscopy.19 Lymphocyte migration was detected by 15 minutes and was linear at least to 60 minutes (data not shown).
Zymography for MMP activity
Endothelial cells or lymphocytes from static cultures or parallel plate flow chambers18 were washed and lysed with 120 mM Tris (tris(hydroxymethyl)aminomethane), pH 8.7, 0.25% Triton X-100, 0.01% NaN3.8 Lysates without reduction containing equal protein were electrophoresed overnight (70V, 25 mA, 4°C) through a native 7.5% polyacrylamide gel impregnated with gelatin (0.2 mg/mL). The gel was then incubated for 1 hour at 25°C in 2.5% Triton X-100 and washed twice for 20 minutes in water. For MMP degradation of gelatin, the gel was incubated for 16 to 24 hours at 37°C in 0.05 M Tris-HCl, pH 8, 0.005 M CaCl2. As a control, duplicate gels were incubated in 0.05 M Tris-HCl, pH 8, 0.01 M EDTA (ethylenediaminetetraacetic acid). The gels were fixed and stained in 50% methanol, 10% acetic acid, and 0.25% Coomassie blue R250. Bands were analyzed by Image J software (National Institutes of Health [NIH], Bethesda, MD).
Endothelial cell transfection
Endothelial cells in 24-well plates at 70% confluence were transfected with carboxyfluorescein-labeled morpholino-antisense oligomers to gp91 phox (5′-CTTCATTCACAGCCCAGTTCCCCAT-3′) or a carboxyfluorescein-labeled standard control morpholino-oligomer (5′-CCTCTTACCTCAgTTA-CAATTTATA-3′) according to the protocol from GENE TOOLS, LLC (Philomath, OR). For each well, morpholino oligomers (0.465 nmol) were incubated for 20 minutes with 31.3 μL water and 0.93 μL ethoxylated polyethylenimine (EPEI) for 20 minutes, and then mixed with 500 μL serum-free medium; 500 μL was added per well. After a 1-hour transfection, the medium was removed and replaced with fresh medium plus 20% FCS for 3 days.
Statistics
Data were analyzed by a one-way analysis of variance (ANOVA) followed by Tukey multiple comparisons test (SigmaStat; Jandel Scientific, San Ramon, CA).
Results
Endothelial cell matrix metalloproteinases are required for lymphocyte migration across an endothelial cell line
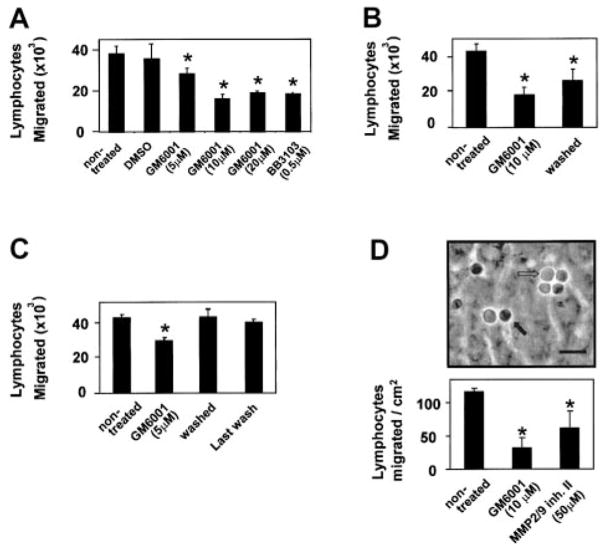
Spleen lymphocyte migration across the endothelial cell line mHEVc is dependent on VCAM-1/α4-integrin binding because blocking antibodies for these adhesion molecules inhibit lymphocyte adhesion and lymphocyte migration across the endothelial cells.20 These endothelial cells do not express other known ligands for lymphocyte binding such as intercellular adhesion molecule 1 (ICAM-1), PECAM-1, and E-selectin as determined by immunofluorescence labeling20 or cDNA microarray analysis (data not shown). The mHEV cells constitutively express VCAM-1, thus providing a model to investigate VCAM-1 signals without interference initiated by signals from other adhesion molecules or from signals for induction of VCAM-1 expression. MMPs are implicated in VCAM-1–dependent lymphocyte migration between microvascular endothelial cells derived from rat epididymal fat pads.8 However, whether endothelial cell MMPs play a role in promotion of lymphocyte migration is not known. We first determined whether blocking MMP activity inhibited VCAM-1–dependent lymphocyte migration across mHEVc cells. Confluent monolayers of mHEVc cells on Transwell membranes were pretreated for 30 minutes with the general MMP inhibitor GM6001 (10 μM; Chemicon, Temecula, CA) or the solvent dimethyl sulfoxide (DMSO; 0.1%), and then the number of accumulated spleen cells that had migrated by 24 hours was examined. Migration is dependent on VCAM-1 because anti–VCAM-1 antibodies, which block lymphocyte adhesion to the mHEVc cells, block lymphocyte migration.3,20 GM6001, but not the solvent, blocked this VCAM-1–dependent lymphocyte migration (Figure 1A). To determine whether MMPs on the endothelial cells or lymphocytes were required for lymphocyte migration, the mHEVc cells or spleen cells were pretreated for 30 minutes with 10 μM GM6001 and then washed 5 times. Pretreatment of the endothelial cells with GM6001 blocked lymphocyte migration (Figure 1B). GM6001 completely blocked VCAM-1 activation of endothelial cell MMP activity at 20 to 30 minutes (Figure 2E), whereas migration was reduced by 50% at 24 hours (Figure 1B), indicating that endothelial cell MMPs are important for lymphocyte migration. Pretreatment of lymphocytes with GM6001 did not block migration (Figure 1C). The pretreated lymphocytes were sufficiently washed because there was no inhibition when the medium from the last wash was added to nontreated lymphocytes (Figure 1C). Similarly, another MMP inhibitor BB3103 (0.5 μM; British Biotech Pharmaceuticals, Oxford, England) blocked lymphocyte migration (Figure 1A).
Figure 1. Inhibition of MMPs associated with endothelial cells but not lymphocytes blocks lymphocyte migration across endothelial cells.
(A) Confluent monolayers of mHEVc cells in 12-μm pore Transwells were treated for 30 minutes with GM6001 or BB3103, general MMP inhibitors, or the solvent control, 0.1% DMSO. (B) mHEVc cells were treated for 30 minutes with 10 μM GM6001, and then the inhibitor was left in or GM6001-treated mHEVc cells were washed 5 times to remove excess inhibitor. (C) Spleen cells were treated with 10 μM GM6001 for 30 minutes and then washed 5 times to remove the inhibitor. For pretreated lymphocytes that were not washed, the final concentration of GM6001 in the coculture was 5 μM. In addition, medium from the last wash was added to nontreated spleen cells to ensure that the inhibitor was sufficiently removed. (A–C) Spleen cells were added on top of the mHEVc monolayer. At 24 hours, cells were collected from the bottom chamber and counted. (D) Confluent monolayers of mHEVc cells on glass slides were treated for 30 minutes with 10 μM GM6001, 50 μM MMP-2/MMP-9 inhibitor II set ((2R)-[(4-Biphenylylsulfonyl)amino]-N-hydroxy-3-phenylpropionamide; Calbiochem), or the solvent control, 0.1% DMSO (data not shown), and then washed 5 times. In addition, medium from the last wash was added to nontreated spleen cells to ensure that the inhibitor was sufficiently removed (data not shown). Spleen lymphocytes were added to the monolayer, and the coculture was exposed to 2 dynes/cm2 laminar flow for 30 minutes. Medium was removed, and cells were fixed in 3% paraformalde-hyde for 1 hour. Lymphocyte migration was examined by phase contrast microscopy (micrograph). The image was acquired using an Olympus BH-2 microscope (SPlan 40 × objective lens with 0.7 aperture and a 3.3 × internal lens) equipped with an Olympus C-35AD-2 camera. The micrograph was digitized using a Nikon SLC D1X digital camera and Adobe Photoshop 7.0. Nonmigrated lymphocytes are phase light (open arrow). In contrast, migrated lymphocytes appear as phase dark (closed arrow). Bar, 15 μm. GM6001 and BB3103 had no effect on cell viability, as determined by trypan blue exclusion (data not shown). Data are presented as mean ± SD from a representative experiment of 2 experiments with duplicate samples. *P < .05 compared to nontreated and DMSO controls.
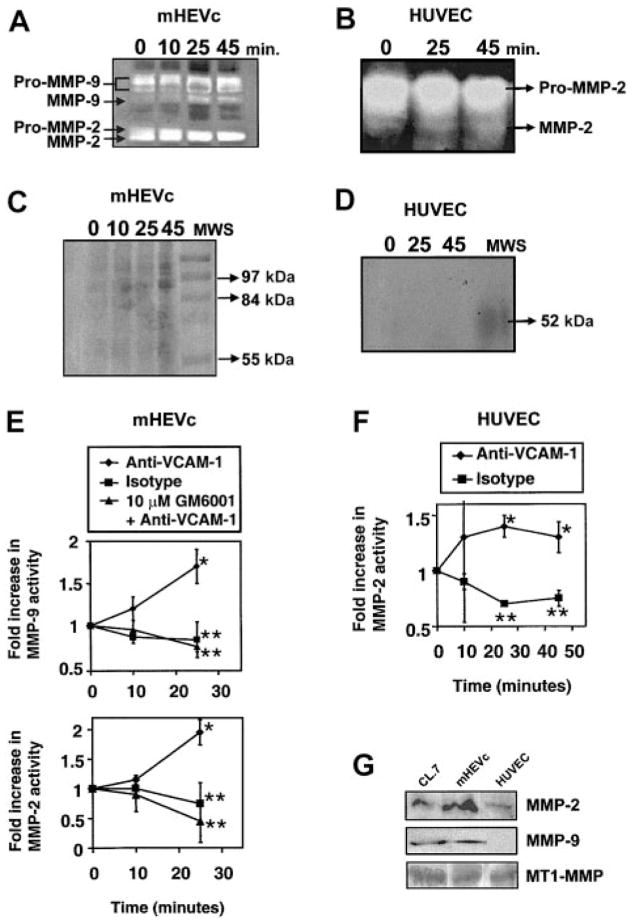
Figure 2. VCAM-1 activates endothelial cell–associated MMPs.
(A,C,E) mHEVc cells and (B,D,F) HUVECs were incubated with isotype antibody-coated beads or anti–VCAM-1 antibody-coated beads. mHEVc cells were also incubated in the presence or absence of 10 μM GM6001. At the indicated time points, the cells were washed. Equal cell numbers were lysed, nuclei were removed by centrifugation, and supernatants were examined by zymography. (A–B) Representative zymograms of VCAM-1 activation of endothelial cell–associated MMPs. Arrows label the latent forms (pro-MMP) and smaller active forms of the MMPs. The activity of MMP-9 and MMP-2 were compared with migration by purified standards (data not shown). (C–D) Same as for panels A and B except with 0.01 M EDTA, indicating the cation dependence of MMP degradation of gelatin. (E–F) Densitometry analysis of the active forms of MMPs in zymograms from 3 experiments. Fold increase is the change in the active form of MMP activity as compared with the nonstimulated cultures. Presented are the mean ± SD. *P < .05 compared with mHEVc cells at 0 minutes. **P < .05 compared with anti–VCAM-1–bead-stimulated cells. (G) Western blots for 1 (MT1)–MMP, MMP-2, and MMP-9 expression using rabbit antihuman MT1-MMP (a kind gift from Qing-Xiang Amy Sang, Florida State University)21 (1:200), affinity-purified rabbit antihuman MMP-9 (no. SA-106; Biomol, Plymouth Meeting, PA) (1:200), or purified rabbit antirat MMP-2 (1:200) (no. AB19015; Chemicon) followed by a horseradish peroxidase (HRP)–conjugated donkey antirabbit secondary antibody (Amersham Pharmacia Biotech, Piscataway, NJ) (1:4000) and enhanced chemiluminescence (ECL) detection.
Lymphocyte migration in vivo occurs primarily in postcapillary venules where shear stress is 2 dynes/cm2.22 Therefore, we determined whether laminar flow modulated lymphocyte migration. In addition, migration is detected within minutes in this assay because the accumulation of fewer migrated cells is needed to detect migration with this assay than the Transwell assay. The mHEVc cells were pretreated with 10 μM GM6001, 50 μM MMP-2/MMP-9 inhibitor II ((2R)-[(4-Biphenylylsulfonyl)amino]-N-hydroxy-3-phenylpropionamide; Calbiochem), or the vehicle control, 0.1% DMSO, for 30 minutes and then washed. Resting splenic lymphocytes were added, and 2 dynes/cm2 laminar shear stress was applied for 30 minutes. The cells were washed and fixed with 3% paraformaldehyde, and lymphocyte migration was examined by using phase contrast microscopy. The general MMP inhibitor, GM6001, as well as the MMP-2/MMP-9 inhibitor II set (Calbiochem) blocked lymphocyte migration across the endothelial cells (Figure 1D). DMSO and the last washes, which were added to nontreated cells, had no effect on lymphocyte migration (data not shown). Therefore, the endothelial cell MMPs rather than the lymphocyte MMPs were necessary for VCAM-1–dependent3,20 lymphocyte migration across the endothelial cells.
VCAM-1 activates MMPs associated with endothelial cells
Lymphocyte binding to VCAM-1 activates endothelial cell NADPH oxidase for the release of superoxide, resulting in the generation of low concentrations of H2O2.3,4 Low levels of H2O2 have been reported to activate purified MMPs.13 Therefore, we determined whether VCAM-1 stimulation of NADPH oxidase activates endothelial cell–associated MMPs. Cell-associated MMPs, rather than released MMPs, were investigated because, within a vessel lumen, released MMPs are likely rapidly removed from the localized site of lymphocyte-endothelial cell contact by the flow of blood. Lymphocyte stimulation of endothelial cell MMPs was not examined because, when lymphocytes were released from the endothelial cells with anti–VCAM-1 antibodies, there were significant numbers of lymphocytes with cell-associated MMPs remaining in the endothelial cell preparation. Therefore, to address activation of endothelial cell–associated MMPs, endothelial cell VCAM-1 was stimulated by cross-linking with anti–VCAM-1 antibody-coated beads (9.9 μm in diameter). Anti–VCAM-1–coated beads also provided stimulation of VCAM-1 without complications of signals from subsequent cell-cell interactions. We have previously reported that lymphocytes as well as anti–VCAM-1–antibody-coated beads bind to VCAM-1 and activate NADPH oxidase in the mHEVc cells and in cytokine-treated primary cultures of HUVECs.3 Furthermore, this indicates that VCAM-1, rather than VCAM-1 cross talk with other cell surface molecules, is sufficient for the signals examined. Beads coated with anti-CD44, which is expressed by the mHEVc cells, have no effect on NADPH oxidase activation.3 mHEVc cells were stimulated with anti–VCAM-1 beads, washed, lysed, and examined by zymography. At 15 to 25 minutes, the anti–VCAM-1 antibody-coated beads significantly increased 2-fold the active form of MMP-2 and MMP-9 that was associated with the mHEVc cells (Figure 2A,E and Figure 3). MMP-2 and MMP-9 expression were identified by Western blot (Figure 2G) and by comparison to purified MMP standards in zymograms (data not shown). The mHEVc cells also express transmembrane MT1-MMP (Figure 2G), which has little gelatinase activity as determined by zymography, but importantly, it holds both pro–MMP-2 and active MMP-2 at the cell surface.23 Incubation of the gel with EDTA blocked the enzymatic activity of MMP-2 and MMP-9, confirming cation dependence of the MMP activities (Figure 2C). VCAM-1 activation of MMPs was also examined in primary cultures of endothelial cells. Interleukin 4 (IL-4)–treated HUVECs expressed VCAM-1 (data not shown), and these cells expressed MMP-2 and MT1-MMP but not MMP-9 (Figure 2G). This is consistent with other reports that HUVECs primarily express MMP-2.24–27 Cross-linking VCAM-1 induced a significant increase in the active form of MMP-2 on HUVECs by 25 minutes (Figure 2B,F). In summary, within minutes, VCAM-1 stimulates MMP activation on endothelial cell lines and primary endothelial cells. This MMP activity is necessary for VCAM-1–dependent migration of lymphocytes across the endothelial cell lines.
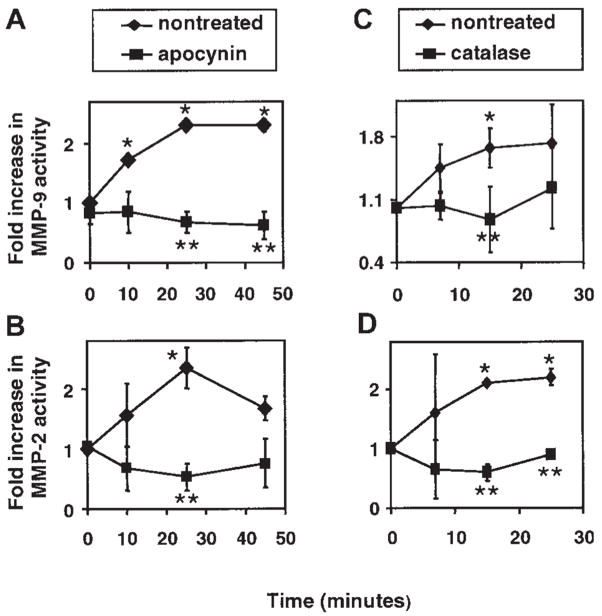
Figure 3. An inhibitor of NADPH oxidase and a ROS scavenger block VCAM-1 activation of endothelial cell MMP-2 and MMP-9.
(A,C) MMP-9 activity. (B,D) MMP-2 activity. mHEVc cells were incubated with anti–VCAM-1 antibody-coated beads in the presence and absence of apocynin (4 mM), an NADPH oxidase inhibitor, or catalase (5000 U/mL), a H2O2 scavenger. Apocynin and catalase had no effect on endothelial cell viability as determined by trypan blue exclusion (data not shown). At the times indicated the cells were washed, and equal cell numbers were lysed and analyzed by zymography as described for Figure 2. Data presented are the mean ± SD from 5 experiments. Symbols without error bars indicate that the SD was smaller than the symbol. *P < .05 compared with mHEVc cells at 0 minutes. **P < .05 compared with anti–VCAM-1–bead-stimulated cells.
VCAM-1 activation of endothelial cell MMPs is mediated by endothelial cell NADPH oxidase-generated ROS
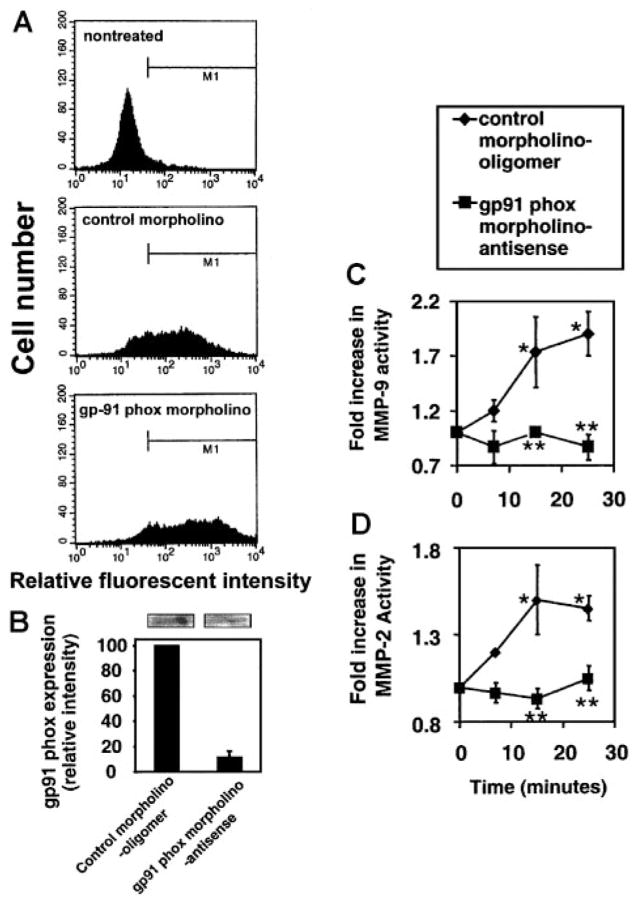
VCAM-1 activates endothelial cell generation of 1 μM H2O2.4 The VCAM-1–stimulated ROS are generated from endothelial cell NADPH oxidase but not nitric oxide synthase, xanthine oxidase, or cytochrome P450.3 NADPH oxidase catalyzes the synthesis of superoxide, which dismutates to H2O2. This ROS generation is necessary for VCAM-1–dependent lymphocyte migration,3 and endothelial cell MMPs are involved in VCAM-1–dependent lymphocyte migration (Figure 1). Furthermore, it has been reported that low levels of H2O2 activate purified MMP-2 and MMP-9.13 Therefore, it was determined whether ROS generated by VCAM-1–stimulated endothelial cell NADPH oxidase3 activates endothelial cell-associated MMPs. To address this, mHEVc cells were pre-treated for 30 minutes with apocynin (4 mM), an NADPH oxidase inhibitor, or with catalase (5000 U/mL), a H2O2 scavenger. We have previously reported that these concentrations of apocynin and catalase block mHEVc cell VCAM-1–stimulated generation of ROS and block VCAM-1–dependent lymphocyte migration.3 Apocynin and catalase did not alter VCAM-1 expression on the endothelial cells as determined by flow cytometry (data not shown). The inhibitors did not affect endothelial cell viability as determined by trypan blue exclusion (data not shown). Anti–VCAM-1 bead activation of endothelial cell MMP-2 and MMP-9 activity was inhibited by apocynin and catalase (Figure 3). To further examine endothelial cell NADPH oxidase as an intermediate in VCAM-1 activation of MMPs, the mHEVc cells were transfected with a carboxyfluorescein-tagged morpholino-anti-sense to gp91 phox or carboxyfluorescein-tagged control morpholino oligomer and then cultured for 3 days. gp91 phox is the catalytic subunit of the NADPH oxidase enzyme complex. The mHEVc cells were successfully transfected (80%–90% transfection) as determined by flow cytometry (Figure 4A), and gp91 phox protein expression at 72 hours was inhibited more than 90% as determined by densitometry analysis of Western blots (Figure 4B). We previously reported that this gp91 phox morpholino antisense inhibits VCAM-1 activation of endothelial cell ROS production.30 VCAM-1 activation of MMP-2 and MMP-9 was blocked in cells transfected with the gp91 phox morpholino antisense as compared with control morpholino-treated cells (Figure 4 C–D). Therefore, endothelial cell NADPH oxidase was required for VCAM-1 induction of endothelial cell MMPs. In summary, ROS from VCAM-1–activated endothelial cell NADPH oxidase stimulates endothelial cell MMP activity, and this MMP activity in turn is required for endothelial cell promotion of VCAM-1–dependent lymphocyte migration.
Figure 4. Morpholino-antisense oligomers for gp91 phox block VCAM-1 activation of endothelial cell MMP-2 and MMP-9.
mHEVc cells were transfected with carboxyfluorescein-labeled control morpholino oligomers and the carboxyfluorescein-labeled gp91 phox morpholino antisense oligomers. The cells were cultured for 3 days to allow for turnover of previously synthesized gp91 phox, stimulated with anti–VCAM-1–antibody-coated beads, washed at the times indicated, and scraped into lysis buffer; equal amounts of protein were examined by zymography. (A) Flow cytometry verifies the transfection of the mHEVc cells with the carboxyfluorescein-tagged morpholinos. (B) gp91 phox protein expression. Western blots were performed with equal protein loading (80 μg/lane) and using mouse antimouse gp-91phox (a kind gift from Drs D. Roos and E. van der Schoot, University of Amsterdam, The Netherlands)28 (1:200) followed by an HRP-conjugated rabbit antimouse secondary antibody (Amersham) and ECL detection. gp91 phox in the mouse has an apparent molecular mass of 58 kDa.29 Lanes from a representative Western blot are above the graph. The Western blots were analyzed with Image J software from NIH. Only 2 nonspecific bands (200–230 kDa) (data not shown) were present and exhibited similar intensity in all lanes as previously reported.30 (C) MMP-9 activity. (D) MMP-2 activity. (B–D) Data presented are the mean ± SD from 3 experiments. *P < .05 compared with mHEVc cells at 0 minutes. **P < .05 compared with control morpholino-oligomer–transfected mHEVc cells.
Exogenous H2O2 activates endothelial cell–associated MMPs
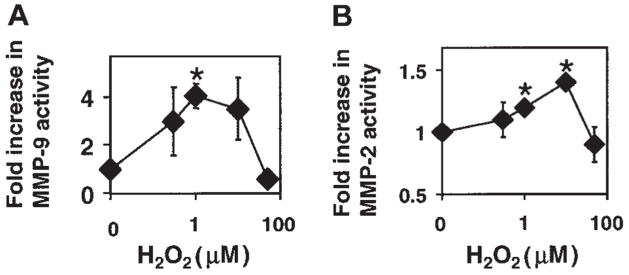
In cell-free experiments, low concentrations of ROS activate purified MMP-2 and MMP-9, and high concentrations of ROS (>50 μM) inhibit purified MMP activity.13 In cells, ROS can be generated extracellularly by the NADPH oxidase enzyme complex located in the endothelial cell plasma membrane. VCAM-1 stimulates the production of low levels of H2O2 by endothelial cells. Therefore, it was determined whether exogenous H2O2 at low levels could activate cell-associated MMPs. Low concentrations of H2O2 (1–10 μM) activated endothelial cell–associated MMP-2 and MMP-9 at 15 minutes (Figure 5). H2O2 (1 μM) did not alter expression of MMP-2, MMP-9, tissue inhibitor of metalloproteinase 1 (TIMP-1), or TIMP-2 at 15 minutes as determined by Western blot (data not shown), and there was very low TIMP expression by the endothelial cells as compared with lymphocytes, which is consistent with the literature.31,32 In contrast, 50 μM H2O2, a concentration produced by activated macrophages, inhibited endothelial cell MMP-2 and MMP-9 activity by 50% (Figure 5), which is consistent with previous reports that high levels of ROS inhibit cellular MMPs.13 Therefore, VCAM-1 activates endothelial cell NADPH oxidase for production of low concentrations of ROS4 that then can activate endothelial cell–associated MMPs.
Figure 5. Exogenous H2O2 activates endothelial cell–associated MMP-2 and MMP-9.
(A) MMP-9 activity. (B) MMP-2 activity. mHEVc cells were incubated with 0, 0.3, 1, 3, and 50 μM H2O2 for 10 minutes. The cells were washed, then equal cell numbers were lysed, and MMP activity was examined by zymography. H2O2 had no effect on cell viability (data not shown). Data presented are the mean ± SD from 5 experiments. *P < .05 compared with nonstimulated mHEVc cells.
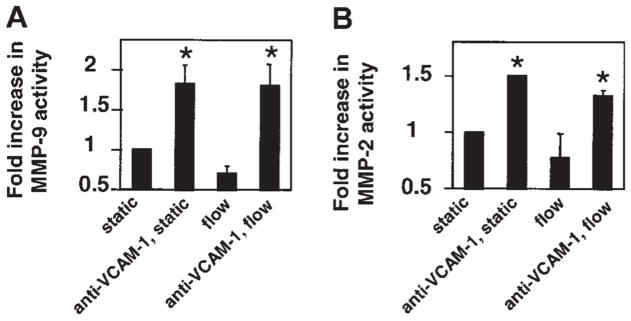
VCAM-1 activation of endothelial cell MMP-2 and MMP-9 was not altered by laminar shear stress at 2 dynes/cm2
Leukocyte transendothelial migration into inflammatory sites occurs primarily in postcapillary venules in which the laminar shear stress is 2 dynes/cm2.22 Therefore, it was determined whether this shear stress modulated VCAM-1 activation of endothelial cell-associated MMPs. The mHEVc cells were grown on glass slides, attached to a parallel plate flow chamber, and then stimulated with anti–VCAM-1–coated beads under a laminar shear stress of 2 dynes/cm2. This shear stress did not alter VCAM-1 activation of endothelial cell MMPs as compared with static conditions (Figure 6).
Figure 6. VCAM-1 activation of endothelial cell MMP-2 and MMP-9 is not altered by laminar flow.
Confluent endothelial cells on glass slides were incubated with anti–VCAM-1 beads and exposed to static conditions or 2 dynes/cm2 laminar flow for 15 minutes. Medium was removed and cells were examined by zymography. The same number of beads was bound for static and laminar flow conditions (data not shown). Data presented are the mean ± SD from 2 experiments. *P < .05 compared with control (nonstimulated, static mHEVc).
Lymphocyte MMP-9 activation is blocked by inhibition of endothelial cell NADPH oxidase
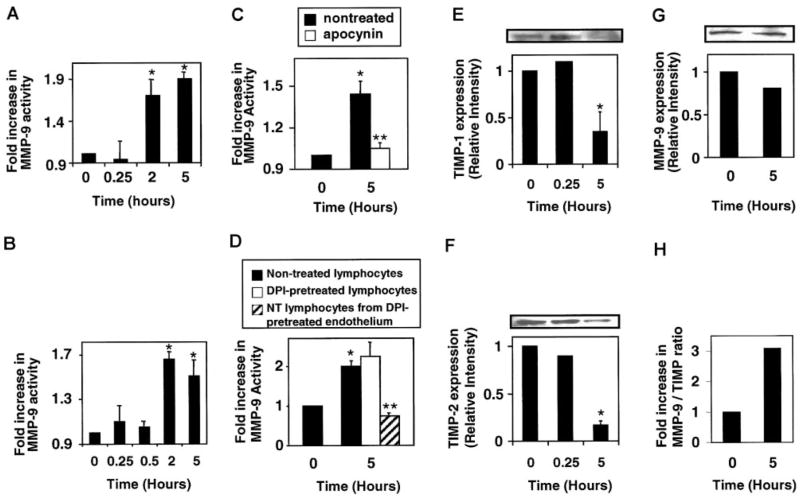
As ROS are released extracellularly and activate endothelial cell–associated MMPs, we determined whether these ROS also modulate MMP activity on the adherent lymphocytes. First, we examined activation of lymphocyte MMP activity after adhesion to mHEVc cells. Spleen lymphocytes were incubated with the mHEVc cells for 15 minutes and nonbound lymphocytes were removed, because at 15 minutes the maximal number of lymphocytes adhere to the endothelial cells.4 The bound lymphocytes and endothelial cells were incubated, and, at the time points indicated, lymphocyte binding was reversed with blocking anti–VCAM-1 antibodies.3 Lymphocyte-associated MMP activity was examined by zymography. Spleen lymphocyte MMP-9 activity was increased at 2 to 5 hours after addition to the mHEVc cells (Figure 7A), which is consistent with the more than 5 hours reported for VCAM-1 activation of lymphocyte MMPs.8 The less than 1% contaminating endothelial cells in this preparation did not contribute to the MMP activity observed for lymphocytes because, when this small number of remaining endothelial cells was obtained from a VCAM-1–stimulated monolayer and analyzed, there was no detectable endothelial cell MMP activity (data not shown). Thus, VCAM-1 stimulates a delayed activation of MMPs on lymphocytes as compared with the rapid activation of endothelial cell MMPs.
Figure 7. Lymphocyte-associated MMP-9 is activated by lymphocyte binding to VCAM-1.
Mouse spleen cells were isolated, and red blood cells were removed by hypotonic shock. (A) mHEVc cells were incubated with spleen cells at 37°C. Nonbound cells were removed after 15 minutes because this yielded maximal cell adhesion.4 At the times indicated, bound lymphocytes were collected by reversing their binding with blocking anti–VCAM-1 antibody3 and washed. Equal cell equivalents were examined by zymography as described in Figure 2. (B) Spleen cells were incubated with 1 μM H2O2 at 37°C. (C) mHEVc cells were pretreated with apocynin (4 mM) for 30 minutes. Nonbound cells were removed after 15 minutes. Bound lymphocytes were recovered and analyzed as described for panel A. (D) mHEVc cells or lymphocytes were pretreated with diphenyliodonium (DPI; 5 μM) for 30 minutes and washed prior to coculture with nontreated (NT) lymphocytes or nontreated mHEVc cells, respectively. Nonbound cells were removed after 15 minutes of coculture. Bound lymphocytes were recovered and analyzed as described for panel A. (E–H) Lymphocytes were incubated with 1 μM H2O2 as in panel B. Western blots were performed with equal cell number by using mouse antimouse TIMP-1 (clone 102B1) or TIMP-2 (clone 67-4H11) antibodies (1:1000; Oncogene, EMD Biosciences, La Jolla, CA) or a rabbit antimouse MMP-9 antibody (1:500; Biomol). (E) TIMP-1. (F) TIMP-2. (G) MMP-9. (H) MMP-9/TIMP ratio. Data presented are the mean ± SD from 2 experiments. *P < .05 compared with nonstimulated lymphocytes (0 hours). **P < .05 compared with nontreated lymphocytes at 5 hours.
It was determined whether ROS mediate VCAM-1–stimulated activation of lymphocyte MMPs. mHEVc cells were pretreated with the NADPH oxidase inhibitor apocynin or the irreversible flavoprotein inhibitor diphenyliodonium, DPI, for 30 minutes prior to the addition of lymphocytes. DPI-treated cells were washed. Apocynin was left in the coculture because it is a reversible inhibitor. Lymphocytes were added to the endothelial cells, and after 15 minutes nonadherent lymphocytes were removed. At 5 hours, anti–VCAM-1 antibody was added to release the bound lymphocytes. Lymphocyte MMP activity was examined by zymography. Apocynin blocked VCAM-1–stimulated lymphocyte MMP-9 activity (Figure 7C). DPI pretreatment of endothelial cells but not lymphocytes blocked VCAM-1 activation of lymphocyte MMPs (Figure 7D). Apocynin and DPI did not alter VCAM-1 expression or lymphocyte adhesion to the mHEVc cells.3 To determine whether extracellular ROS could activate lymphocyte-associated MMPs, exogenous H2O2 (1 μM) was added to spleen cells, and MMP was activity examined. Spleen cell MMP-9 was activated by 1 μM H2O2 but only after 2 to 5 hours (Figure 7B). Therefore, endothelial cell–derived ROS mediate a delayed activation of lymphocyte MMP-9 and a rapid activation of endothelial cell MMPs.
MMP activity is regulated by endogenous tissue inhibitors of metalloproteinases (TIMPs).33 It has been shown that leukocytes express high levels of TIMPs,32 and a decrease in TIMP expression can yield a net increase in MMP activity.34 Therefore, it was determined whether low levels of H2O2 such as those generated by VCAM-1 signaling could modulate MMP and TIMP expression in the resting splenic lymphocytes. Lymphocytes were incubated in the presence of 1 μM H2O2 for 0, 0.25, or 5 hours. The 0.25- and 5-hour time points were chosen because they coincide with the time for the increased MMP activity on the endothelial cells and lymphocytes, respectively (Figures 3 and 7). Equal cell numbers were lysed, and expression of TIMP-1, TIMP-2, and MMP-9 was analyzed by Western blot. H2O2 treatment did not increase lymphocyte MMP-9 expression (Figure 7G). In contrast, H2O2 induced a significant decrease in TIMP-1 and TIMP-2 protein expression at 5 hours, corresponding to the time for H2O2 stimulation of lymphocyte MMP activity (Figure 7E–F). Thus, the MMP/TIMP ratio was significantly increased at 5 hours (Figure 7H). These data suggest that the delayed increase in lymphocyte MMP-9 activity is, at least in part, due to a decrease in lymphocyte-associated TIMPs.
Discussion
We have shown for the first time that endothelial cell–associated MMP activity is increased within minutes after VCAM-1 cross-linking and that a mechanism for this activation is through endothelial cell NADPH oxidase-derived ROS. In addition, we demonstrated that endothelial cell–associated rather than lymphocyte-associated MMPs are necessary for VCAM-1–dependent lymphocyte migration across endothelial cells. VCAM-1 activation of NADPH oxidase and endothelial cell MMPs are required for VCAM-1–dependent lymphocyte migration because lymphocyte migration across mHEVc cells is blocked by (1) antibody inhibition of VCAM-1 binding3; (2) apocynin and DPI inhibition of endothelial cell NADPH oxidase activity but not inhibition of endothelial cell xanthine oxidase, nitric oxide synthase, or cytochrome P4503; (3) scavenging of ROS by catalase or superoxide dismutase3; and, as shown in this study, (4) inhibition of endothelial cell MMP activity. Endothelial cell MMP activity is downstream of VCAM-1 activation of NADPH oxidase because inhibition of NADPH oxidase or scavenging of ROS blocked VCAM-1 activation of endothelial cell MMP activity. These endothelial cell–generated ROS also mediate a delayed reduction in lymphocyte-associated TIMP expression and an increase in lymphocyte-associated MMP activity.
The level of H2O2 produced after VCAM-1 stimulation is approximately 1 μM, which is 50- to 200-fold lower than levels of H2O2 generated by macrophages and granulocytes.4 Most studies on ROS modulation of endothelial cell function focus on high levels of ROS for damage to endothelial cells.35 Furthermore, high levels of ROS induce changes in the entire endothelial cell with regard to actin fibers and tight junctions.35 In contrast, after VCAM-1 stimulation, low levels of ROS induce changes in endothelial cell actin structure only at the site of leukocyte adhesion.3,4,14 Localized structural changes in endothelial cells are consistent with endothelial cell retraction at the site of lymphocyte migration while maintaining general vascular integrity. In further support for concentration-dependent mechanisms for ROS, high levels of exogenous ROS (> 50 μM) inhibit purified MMP activity, whereas less than 10 μM ROS activate purified MMPs.13 We demonstrated that these ROS concentrations had similar opposing effects on activation of endothelial cell–associated MMPs.
ROS are generated at the cell surface by NADPH oxidase, and MMPs are bound to the cell surface. MMP-9 is held at the cell surface by CD44.36 MMP-2 binds to MT1-MMP at the cell surface through a tertiary complex involving TIMP-2. The N-terminal end of the TIMP molecule binds to the N-terminal end of MT1-MMP, inhibiting the MT1-MMP catalytic site.37 This allows the free carboxy-terminal end of the TIMP molecule to bind to the carboxy terminal end of the MMP-2 molecule, leaving the N-terminal MMP-2 catalytic site uninhibited.37 MMP-2 can then be activated by a nearby MT1-MMP37 or in this model by ROS. Thus, low levels of TIMPs participate in holding active MMP-2 at the cell surface, whereas high levels of TIMPs block the active site of MMP-2.37
MMP function has been implicated in lymphocyte transendothelial migration.8 However, whether lymphocyte or endothelial cell MMPs were involved in this migration was not defined. Our studies using MMP inhibitors indicate that endothelial cell but not lymphocyte MMPs are required for VCAM-1–dependent lymphocyte migration between endothelial cells. The time for VCAM-1–stimulated ROS production and activation of endothelial cell MMPs is consistent with the time for transendothelial lymphocyte migration. Subsequent to binding to endothelial cells, most of the cells that are attached to endothelium then roll to bicellular and tricellular endothelial cell junctions where they stop rolling and migrate between endothelial cells.38 Lymphocyte migration is an asynchronous process, but an individual lymphocyte, which had rolled to an endothelial cell junction, then migrates between the endothelial cells in less than 2 minutes.14 The maximal anti–VCAM-1–bead-stimulated endothelial cell MMP activity occurs in 15 minutes. These 15 minutes include the time for antibody-coated beads to settle asynchronously onto the endothelial cells and include the time for the antibodies to bind and activate VCAM-1. Therefore, the time for anti–VCAM-1–coated beads to stimulate endothelial cell MMP activity is similar in magnitude to the time for lymphocytes to migrate between the endothelial cells as compared with the several hour delay required for VCAM-1 activation of lymphocyte MMPs.
A role for ROS in activation of tumor cell MMPs has been reported. Antioxidants block an increase in pro-MMP secretion but not MMP mRNA by HL-60 leukemia cells at 3 days after butyric acid–induced cell differentiation.39 Overexpression of manganese superoxide dismutase, which scavenges superoxide, disrupts the ROS balance and increases MMP-2 secretion in MCF-7 breast cancer cells.40 The mechanism for the ROS modulation of MMPs in these tumor models was not defined. The VCAM-1–mediated activation of endothelial cell MMPs is likely a direct effect of ROS on MMPs because (1) activation occurred within minutes in our studies without altering MMP or TIMP expression and (2) low levels of exogenous H2O2 have been reported to directly activate purified MMPs.13,23 ROS oxidize the cysteine in the prodomain of MMPs, causing autocatalytic cleavage of the prodomain and activation of the MMP.33
In contrast to rapid endothelial cell MMP activation, MMP activity on lymphocytes is delayed by 2 hours after binding to VCAM-1 or after exogenous addition of 1 μM H2O2. Consistent with this finding, others have reported that T-cell MMP activation occurs 5 to 12 hours after adherence to endothelial cells or binding to ligands for α4-integrin.8,25,41 After adhesion, MMP-2 versus MMP-9 activation on T cells varies in the literature. Lymphocytes express MMP-9 with little to no MMP-225,42–49 unless under very restricted conditions.8,45,50 T cells that migrated across endothelial cells also exhibit increased MMP activity after 8 to 20 hours.8 Furthermore, the activation of T-cell MMPs by ligands such as fibronectin or VCAM-1 immobilized on plastic8,25,41 suggests that lymphocyte MMPs can be activated by “outside-in” signals from α4-integrins. Taken together with our studies, lymphocyte MMP activity can be activated by α4-integrin outside-in signals as well as through endothelial cell–derived ROS. The delayed activation of lymphocyte MMPs suggest that lymphocyte MMPs may participate in migration through extravascular tissue.
MMPs on endothelial cells are modulated by factors in the microenvironment such as the cytokine milieu.51 For example, large vein endothelial cells such as HUVECs express MMP-2 and little to no MMP-9, whereas microvascular endothelial cells, as shown in this study and others, express MMP-2 and MMP-9.52 However, even though MMP-9 is not expressed by IL-4–stimulated HUVECs, they can still promote lymphocyte migration.53 This may be due to a redundancy in function of MMPs. Redundancy of MMP activity for neovascularization and hematopoietic stem cell mobilization was recently demonstrated in vivo by using MMP-9 knockout (KO) mice, MMP-2 KO mice, and double KOs.54,55 When MMP-9 expression is induced in HUVECs, its activity can be increased with 1.3 to 8 μM H2O2 with minimal increase (1.2-fold) in MMP-9 mRNA.24 An area for future research is to determine whether other adhesion molecules such as CD44 or PECAM-1, which do not activate ROS signals,3,6 modulate MMPs, thus potentially providing a redundancy in signals that participate in activation of MMPs during migration.
Although the rapid VCAM-1–stimulated endothelial cell MMP activation is involved in VCAM-1–dependent lymphocyte migration, the delayed activation of lymphocyte MMPs is interesting and warrants some discussion of this delay. The ROS that activate lymphocyte MMPs are generated from the endothelial cells because pretreatment of the endothelial cells but not the lymphocytes with an irreversible inhibitor of flavoproteins, DPI, blocked VCAM-1 activation of lymphocyte MMPs. Because ROS are released by the endothelial cells at the endothelial cell surface with a bound lymphocyte, one might expect direct activation of lymphocyte-associated MMPs; however, our data and other reports indicate a delayed activation of lymphocyte MMPs after binding to VCAM-1 on endothelial cells.8,25,41 In addition, exogenous ROS activated lymphocyte MMPs after 5 hours. This delay suggests that indirect signals are necessary for activation of lymphocyte MMPs. The 1 μM H2O2 generated by VCAM-1 stimulation is too low form H2O2 (> 25 μM) activation of nuclear factor κB (NFκB)–induced MMP-9 mRNA in lymphocytes.56 Consistent with this finding, lymphocyte MMP-9 protein expression was not increased by VCAM-1 stimulation or H2O2. Lymphocytes have relatively high levels of endogenous inhibitors of MMPs, TIMPs.31,32 Indeed, 1 μM H2O2 reduced TIMP-1 and TIMP-2 expression in the resting splenic lymphocytes at 5 hours. Thus, a H2O2-induced increase in MMP/TIMP ratio mediates a net increase in MMP enzymatic activity. Thus, this report identifies a role for ROS in activation of lymphocyte MMPs.
In summary, this is the first report to demonstrate a function for endothelial cell MMPs after VCAM-1 binding. VCAM-1 signaling stimulates the release of low levels of H2O24 that result in endothelial cell MMP activation within minutes, and these endothelial cell MMPs are required for VCAM-1–dependent lymphocyte migration. Furthermore, the level of shear stress at postcapillary venules does not alter VCAM-1 activation of endothelial cell MMPs, and it has been reported that this level of shear stress does not alter endothelial cell generation of ROS.57 Our data are consistent with the working model that MMPs associated with the endothelial cell are directly activated by low levels of extracellular ROS generated by NADPH oxidase in the endothelial cell membrane. MMPs secreted by the endothelial cells in vivo are likely flushed from the site by the flow of blood. MMPs are anchored to cell surfaces by transmembrane MMPs, CD44, and integrins.23,33,36 The mHEVc cell line expresses CD4414 and the transmembrane MT1-MMP. MT1-MMP retains activated MMP-2 as well as pro–MMP-2 at the plasma membrane, whereas CD44 can bind activated MMP-9.23,36 By using a mechanism of MMP cell surface retention, localized ROS generation activates MMPs associated with the endothelial cell, likely at the site of lymphocyte binding, so that endothelial cells can retract only at the site of lymphocyte interaction.14 At this site, endothelial cell–associated MMPs could degrade several endothelial cell surface molecules that form endothelial cell junctions such as extracellular matrix and adhesion molecules.9,10 The rest of the endothelial cell maintains its cell shape and thus ensures that endothelium integrity remains relatively intact. This report contributes to our understanding of VCAM-1 signals and thus will aid in the design of modulators for VCAM-1–dependent functions in diseases such as asthma and EAE.
Acknowledgments
We thank Drs Amy Sang and Jeff Gidday for their suggestions. We also thank Jay Card for photographic assistance.
Supported by grant from the National Institutes of Health (RO1 HL68171; J.M.C-M.).
References
- 1.Chin JE, Hatfield CA, Winterrowd GE, et al. Airway recruitment of leukocytes in mice is dependent on alpha4-integrins and vascular cell adhesion molecule-1. Am J Physiol. 1997;272:L219–L229. doi: 10.1152/ajplung.1997.272.2.L219. [DOI] [PubMed] [Google Scholar]
- 2.Baron JL, Madri JA, Ruddle NH, Hashim G, Jane-way CA., Jr Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matheny HE, Deem TL, Cook-Mills JM. Lymphocyte migration through monolayers of endothelial cell lines involves VCAM-1 signaling via endothelial cell NADPH oxidase. J Immunol. 2000;164:6550–6559. doi: 10.4049/jimmunol.164.12.6550. [DOI] [PubMed] [Google Scholar]
- 4.Tudor KSRS, Hess KL, Cook-Mills JM. Cytokines modulate endothelial cell intracellular signal transduction required for VCAM-1-dependent lymphocyte transendothelial migration. Cytokine. 2001;15:196–211. doi: 10.1006/cyto.2001.0922. [DOI] [PubMed] [Google Scholar]
- 5.van Wetering S, van Buul JD, Quik S, et al. Reactive oxygen species mediate Rac-induced loss of cell-cell adhesion in primary human endothelial cells. J Cell Sci. 2002;115:1837–1846. doi: 10.1242/jcs.115.9.1837. [DOI] [PubMed] [Google Scholar]
- 6.van Buul JD, Voermans C, van den Berg V, et al. Migration of human hematopoietic progenitor cells across bone marrow endothelium is regulated by vascular endothelial cadherin. J Immunol. 2002;168:588–596. doi: 10.4049/jimmunol.168.2.588. [DOI] [PubMed] [Google Scholar]
- 7.van Wetering S, van den Berk N, van Buul JD, et al. VCAM-1-mediated Rac signaling controls endothelial cell-cell contacts and leukocyte transmigration. Am J Physiol Cell Physiol. 2003;285:C343–C352. doi: 10.1152/ajpcell.00048.2003. [DOI] [PubMed] [Google Scholar]
- 8.Romanic AM, Madri JA. The induction of 72-kD gelatinase in T cells upon adhesion to endothelial cells is VCAM-1 dependent. J Cell Biol. 1994;125:1165–1178. doi: 10.1083/jcb.125.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seiki M. The cell surface: the stage for matrix metalloproteinase regulation of migration. Curr Opin Cell Biol. 2002;14:624–632. doi: 10.1016/s0955-0674(02)00363-0. [DOI] [PubMed] [Google Scholar]
- 10.Alexander JS, Elrod JW. Extracellular matrix, junctional integrity and matrix metalloproteinase interactions in endothelial permeability regulation. J Anat. 2002;200:561–574. doi: 10.1046/j.1469-7580.2002.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lohmann C, Krischke M, Wegener J, Galla HJ. Tyrosine phosphatase inhibition induces loss of blood-brain barrier integrity by matrix metalloproteinase-dependent and -independent pathways. Brain Res. 2004;995:184–196. doi: 10.1016/j.brainres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Grote K, Flach I, Luchtefeld M, et al. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res. 2003;92:e80–e86. doi: 10.1161/01.RES.0000077044.60138.7C. [DOI] [PubMed] [Google Scholar]
- 13.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook-Mills JM, Gallagher JS, Feldbush TL. Isolation and characterization of high endothelial cell lines derived from mouse lymph nodes. In Vitro Cell Dev Biol. 1996;32:167–177. doi: 10.1007/BF02723682. [DOI] [PubMed] [Google Scholar]
- 15.Qureshi MH, Cook-Mills J, Doherty DE, Garvy BA. TNF-alpha-dependent ICAM-1- and VCAM-1-mediated inflammatory responses are delayed in neonatal mice infected with Pneumocystis carinii. J Immunol. 2003;171:4700–4707. doi: 10.4049/jimmunol.171.9.4700. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto H, Sedgwick JB, Busse WW. Differential regulation of eosinophil adhesion and transmigration by pulmonary microvascular endothelial cells. J Immunol. 1998;161:971–977. [PubMed] [Google Scholar]
- 17.May MJ, Ager A. ICAM-1-independent lymphocyte transmigration across high endothelium: differential up-regulation by interferon gamma, tumor necrosis factor-alpha and interleukin 1 beta. Eur J Immunol. 1992;22:219–226. doi: 10.1002/eji.1830220132. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence MB, McIntire LV, Eskin SG. Effect of flow on polymorphonuclear leukocyte/endothelial cell adhesion. Blood. 1987;70:1284–1290. [PubMed] [Google Scholar]
- 19.Ager A, Mistry S. Interaction between lymphocytes and cultured high endothelial cells: an in vitro model of lymphocyte migration across high endothelial venule endothelium. Eur J Immunol. 1988;18:1265–1274. doi: 10.1002/eji.1830180818. [DOI] [PubMed] [Google Scholar]
- 20.Tudor K-SRS, Deem TL, Cook-Mills JM. Novel α4-integrin ligands on an endothelial cell line. Biochem Cell Biol. 2000;78:99–113. [PubMed] [Google Scholar]
- 21.Li H, Bauzon DE, Xu X, Tschesche H, Cao J, Sang QA. Immunological characterization of cell-surface and soluble forms of membrane type 1 matrix metalloproteinase in human breast cancer cells and in fibroblasts. Mol Carcinogen. 1998;22:84–94. [PubMed] [Google Scholar]
- 22.Mariscalco MM, Tcharmtchi MH, Smith CW. P-selectin support of neonatal neutrophil adherence under flow: contribution of L-selectin, LFA-1, and ligand(s) for P-selectin. Blood. 1998;91:4776–4785. [PubMed] [Google Scholar]
- 23.Cao J, Sato H, Takino T, Seiki M. The C-terminal region of membrane type matrix metalloproteinase is a functional transmembrane domain required for pro-gelatinase A activation. J Biol Chem. 1995;270:801–805. doi: 10.1074/jbc.270.2.801. [DOI] [PubMed] [Google Scholar]
- 24.Belkhiri A, Richards C, Whaley M, McQueen SA, Orr FW. Increased expression of activated matrix metalloproteinase-2 by human endothelial cells after sublethal H2O2 exposure. Lab Invest. 1997;77:533–539. [PubMed] [Google Scholar]
- 25.Esparza J, Vilardell C, Calvo J, et al. Fibronectin upregulates gelatinase B (MMP-9) and induces coordinated expression of gelatinase A (MMP-2) and its activator MT1-MMP (MMP-14) by human T lymphocyte cell lines. A process repressed through RAS/MAP kinase signaling pathways. Blood. 1999;94:2754–2766. [PubMed] [Google Scholar]
- 26.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen M, Arkell J, Jackson CJ. Active and tissue inhibitor of matrix metalloproteinase-free gelatinase B accumulates within human microvascular endothelial vesicles. J Biol Chem. 1998;273:5400–5404. doi: 10.1074/jbc.273.9.5400. [DOI] [PubMed] [Google Scholar]
- 28.Verhoeven AJ, Bolscher BG, Meerhof LJ, et al. Characterization of two monoclonal antibodies against cytochrome b558 of human neutrophils. Blood. 1989;73:1686–1694. [PubMed] [Google Scholar]
- 29.Bjorgvinsdottir H, Zhen L, Dinauer MC. Cloning of murine gp91phox cDNA and functional expression in a human X-linked chronic granulomatous disease cell line. Blood. 1996;87:2005–2010. [PubMed] [Google Scholar]
- 30.Cook-Mills JM, Johnson JD, Deem TL, Ochi A, Wang L, Zheng Y. Calcium mobilization and Rac1 activation are required for VCAM-1 (vascular cell adhesion molecule-1) stimulation of NADPH oxidase activity. Biochem J. 2004;378:539–547. doi: 10.1042/BJ20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnatty RN, Taub DD, Reeder SP, et al. Cytokine and chemokine regulation of proMMP-9 and TIMP-1 production by human peripheral blood lymphocytes. J Immunol. 1997;158:2327–2333. [PubMed] [Google Scholar]
- 32.Trocme C, Gaudin P, Berthier S, Morel F. Regulation of TIMP-1 phenotypic expression in Ep-stein—Barr virus-immortalized B lymphocytes. Biochim Biophys Acta. 2002;1590:167–176. doi: 10.1016/s0167-4889(02)00210-0. [DOI] [PubMed] [Google Scholar]
- 33.Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–2154. [PubMed] [Google Scholar]
- 34.Pauschinger M, Chandrasekharan K, Schultheiss H-P. Myocardial remodeling in viral heart disease: possible interactions between inflammatory mediators and MMP-TIMP system. Heart Failure Rev. 2004;9:21–31. doi: 10.1023/B:HREV.0000011391.81676.3c. [DOI] [PubMed] [Google Scholar]
- 35.van der Goes A, Wouters D, van der Pol SM, et al. Reactive oxygen species enhance the migration of monocytes across the blood-brain barrier in vitro. FASEB J. 2001;15:1852–1854. doi: 10.1096/fj.00-0881fje. [DOI] [PubMed] [Google Scholar]
- 36.Yu Q, Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Develop. 1999;13:35–48. doi: 10.1101/gad.13.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zucker S, Drews M, Conner C, et al. Tissue inhibitor of metalloproteinase-2 (TIMP-2) binds to the catalytic domain of the cell surface receptor, membrane type 1-matrix metalloproteinase 1 (MT1-MMP) J Biol Chem. 1998;273:1216–1222. doi: 10.1074/jbc.273.2.1216. [DOI] [PubMed] [Google Scholar]
- 38.Gopalan PK, Burns AR, Simon SI, Sparks S, McIntire LV, Smith CW. Preferential sites for stationary adhesion of neutrophils to cytokine-stimulated HUVEC under flow conditions. J Leukoc Biol. 2000;68:47–57. [PubMed] [Google Scholar]
- 39.Richard D, Hollender P, Chenais B. Butyric acid increases invasiveness of HL-60 leukemia cells: role of reactive oxygen species. FEBS Lett. 2002;518:159–163. doi: 10.1016/s0014-5793(02)02690-x. [DOI] [PubMed] [Google Scholar]
- 40.Zhang HJ, Zhao W, Venkataraman S, et al. Activation of matrix metalloproteinase-2 by overexpression of manganese superoxide dismutase in human breast cancer MCF-7 cells involves reactive oxygen species. J Biol Chem. 2002;277:20919–20926. doi: 10.1074/jbc.M109801200. [DOI] [PubMed] [Google Scholar]
- 41.Yakubenko VP, Lobb RR, Plow EF, Ugarova TP. Differential induction of gelatinase B (MMP-9) and gelatinase A (MMP-2) in T lymphocytes upon alpha(4)beta(1)-mediated adhesion to VCAM-1 and the CS-1 peptide of fibronectin. Exp Cell Res. 2000;260:73–84. doi: 10.1006/excr.2000.5002. [DOI] [PubMed] [Google Scholar]
- 42.Hauzenberger D, Bergstrom SE, Klominek J, Sundqvist KG. Spectrum of extracellular matrix degrading enzymes in normal and malignant T lymphocytes. Anticancer Res. 1999;19:1945–1952. [PubMed] [Google Scholar]
- 43.Trocme C, Gaudin P, Berthier S, Barro C, Zaoui P, Morel F. Human B lymphocytes synthesize the 92-kDa gelatinase, matrix metalloproteinase-9. J Biol Chem. 1998;273:20677–20684. doi: 10.1074/jbc.273.32.20677. [DOI] [PubMed] [Google Scholar]
- 44.Montgomery AM, Sabzevari H, Reisfeld RA. Production and regulation of gelatinase B by human T-cells. Biochim Biophys Acta. 1993;1176:265–268. doi: 10.1016/0167-4889(93)90054-s. [DOI] [PubMed] [Google Scholar]
- 45.Knauper V, Will H, Lopez-Otin C, et al. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J Biol Chem. 1996;271:17124–17131. doi: 10.1074/jbc.271.29.17124. [DOI] [PubMed] [Google Scholar]
- 46.Weeks BS, Schnaper HW, Handy M, Holloway E, Kleinman HK. Human T lymphocytes synthesize the 92 kDa type IV collagenase (gelatinase B) J Cell Physiol. 1993;157:644–649. doi: 10.1002/jcp.1041570326. [DOI] [PubMed] [Google Scholar]
- 47.Leppert D, Waubant E, Galardy R, Bunnett NW, Hauser SL. T cell gelatinases mediate basement membrane transmigration in vitro. J Immunol. 1995;154:4379–4389. [PubMed] [Google Scholar]
- 48.Stetler-Stevenson M, Mansoor A, Lim M, et al. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in reactive and neoplastic lymphoid cells. Blood. 1997;89:1708–1715. [PubMed] [Google Scholar]
- 49.Xia M, Leppert D, Hauser SL, et al. Stimulus specificity of matrix metalloproteinase dependence of human T cell migration through a model basement membrane. J Immunol. 1996;156:160–167. [PubMed] [Google Scholar]
- 50.Kambara C, Nakamura T, Furuya T, et al. Vascular cell adhesion molecule-1-mediated matrix metalloproteinase-2 induction in peripheral blood T cells is up-regulated in patients with HTLV-I-associated myelopathy. J Neuroimmunol. 1999;99:242–247. doi: 10.1016/s0165-5728(99)00110-1. [DOI] [PubMed] [Google Scholar]
- 51.Mitola S, Strasly M, Prato M, Ghia P, Bussolino F. IL-12 regulates an endothelial cell-lymphocyte network: effect on metalloproteinase-9 production. J Immunol. 2003;171:3725–3733. doi: 10.4049/jimmunol.171.7.3725. [DOI] [PubMed] [Google Scholar]
- 52.Hanemaaijer R, Koolwijk P, le Clercq L, de Vree WJ, van Hinsbergh VW. Regulation of matrix metalloproteinase expression in human vein and microvascular endothelial cells. Effects of tumour necrosis factor alpha, interleukin 1 and phorbol ester. Biochem J. 1993;296:803–809. doi: 10.1042/bj2960803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dallaire MJ, Ferland C, Page N, Lavigne S, Davoine F, Laviolette M. Endothelial cells modulate eosinophil surface markers and mediator release. Eur Respir J. 2003;21:918–924. doi: 10.1183/09031936.03.00102002. [DOI] [PubMed] [Google Scholar]
- 54.Robinson SN, Pisarev VM, Chavez JM, Singh RK, Talmadge JE. Use of matrix metalloproteinase (MMP)-9 knockout mice demonstrates that MMP-9 activity is not absolutely required for G-CSF or Flt-3 ligand-induced hematopoietic progenitor cell mobilization or engraftment. Stem Cells. 2003;21:417–427. doi: 10.1634/stemcells.21-4-417. [DOI] [PubMed] [Google Scholar]
- 55.Lambert V, Wielockx B, Munaut C, et al. MMP-2 and MMP-9 synergize in promoting choroidal neovascularitzation. FASEB J. 2003;17:2290–2292. doi: 10.1096/fj.03-0113fje. [DOI] [PubMed] [Google Scholar]
- 56.Los M, Droge W, Stricker K, Baeuerle PA, Schulze-Osthoff K. Hydrogen peroxide as a potent activator of T lymphocyte functions. Eur J Immunol. 1995;25:159–165. doi: 10.1002/eji.1830250127. [DOI] [PubMed] [Google Scholar]
- 57.Wei Z, Costa K, Al-Mehdi AB, Dodia C, Muzykantov V, Fisher AB. Simulated ischemia in flow-adapted endothelial cells leads to generation of reactive oxygen species and cell signaling. Circ Res. 1999;85:682–689. doi: 10.1161/01.res.85.8.682. [DOI] [PubMed] [Google Scholar]