Abstract
Leukocyte migration from the blood into tissues is vital for immune surveillance and inflammation. During this diapedesis of leukocytes, the leukocytes bind to endothelial cell adhesion molecules and then migrate across the vascular endothelium. Endothelial cell adhesion molecules and their counter-receptors on leukocytes generate intracellular signals. This review focuses on the active function of endothelial cells during leukocyte-endothelial cell interactions. We include a discussion of the “outside-in” signals in endothelial cells, which are stimulated by antibody cross-linking or leukocyte binding to platelet-endothelial cell adhesion molecule-1, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1. Some of these signals in endothelial cells have been demonstrated to actively participate in leukocyte migration. We suggest that some of the adhesion molecule signals, which have not been assigned a function, are consistent with signals that stimulate retraction of lateral junctions, stimulate endothelial cell basal surface adhesion, or induce gene expression.
Keywords: leukocyte migration, signal transduction, PECAM-1, CD99, ICAM-1, VCAM-1, JAM
INTRODUCTION
Leukocytes migrate from the blood and into tissues during immune surveillance. In sites of inflammation, leukocytes of the innate immune system are the first to migrate across activated endothelium. Meanwhile, naive lymphocytes migrate into draining peripheral lymph nodes through specialized endothelium composed of high endothelial venule (HEV) cells. Within the peripheral lymph nodes, antigen-specific lymphocytes are activated. These lymphocytes traffic to the sites of inflammation, where they migrate across activated endothelium expressing adhesion molecules. The adhesion molecules regulate leukocyte adhesion and leukocyte migration into the tissue. During this process, the endothelial cell has an active role in regulating leukocyte migration. Upon leukocyte-endothelial cell interaction, the endothelial cell promotes the migration of the leukocytes through “outside-in” signals from adhesion molecules or removes leukocytes that are in early stages of apoptosis. In addition, cross-linking of some of the endothelial cell adhesion molecules stimulates signals that are consistent with a potential for increased endothelial cell basal surface attachment or induction of gene expression.
Tissue infiltration by circulating leukocytes is a three-step process involving rolling on the endothelium, attachment to the endothelium, followed by transmigration across the endothelial cells lining blood vessel walls. Leukocyte migration out of the blood is initiated by leukocyte rolling on the luminal side of the endothelium, as mediated by the low-affinity receptors selectins and addressins [1, 2]. In lieu of the selectin/addressin interaction, rolling can also be mediated by the interaction of leukocyte α4β1-integrin and vascular cell adhesion molecule-1 (VCAM-1)/CD106 [3]. Binding of selectins on leukocytes stimulates “outside-in” signals in leukocytes, increasing the affinity of the integrin family of receptors, which then bind to endothelial cell adhesion molecules such as intercellular adhesion molecule-1 [(ICAM-1)/CD54] and VCAM-1 [4–6]. Leukocyte integrin affinity is also rapidly increased by “inside-out” signals from leukocyte chemokine receptors triggered by chemokines displayed on the surface of endothelial cells [7]. With an increase in leukocyte integrin receptor affinity, leukocyte rolling is arrested. The arrested leukocyte is stimulated for chemotaxis by a chemokine gradient on the surface of the endothelial cells [8].
The set of adhesion molecules expressed by an endothelial cell depends on the stimulant(s) [9]. Furthermore, the combination of adhesion molecules expressed regulates the specificity of leukocyte homing to tissues [10]. HEV cells in peripheral lymph nodes constitutively express adhesion molecules required for lymphocyte migration [11]. These HEV cells are continuously activated to express adhesion molecules, as occlusion of the afferent lymphatics returns the HEV to a flattened cell morphology with loss of adhesion molecule expression [12]. In contrast, endothelial cells at sites of inflammation require activation to increase expression of the adhesion molecules. The adhesion events have been discussed in previous reviews [11, 13–17]. In comparison to our knowledge of the adhesion events on the luminal surface of the endothelium, mechanisms for the subsequent transendothelial migration have been reported more recently. This review will focus on the active participation of the endothelium during leukocyte-endothelial cell interactions.
The mode of leukocyte migration across endothelial cells has been a controversial topic for over 40 years, with most reports maintaining that they cross between cells, while a few others report them to transcytose through a pore in the cytoplasm of the endothelial cell [18–23]. Neutrophils have been shown to roll across the luminal surface of human umbilical vein endothelial cells (HUVECs) to bicellular and tricellular endothelial cell junctions [19]. Several laboratories have determined that neutrophils and monocytes migrate between endothelial cells [18–22]. It has also been reported that neutrophils, in response to the peptide N-formyl-methionyl-leucyl-phenylalanine, migrate through a pore in nonactivated, cutaneous endothelial cells, as determined by electron microscopy [23, 24]. In these electron micrographs, there is a thin layer of the endothelial cell over the neutrophils [23]. In addition, there is a “clear” zone without electron-dense material between the neutrophil membrane and the encompassing endothelial cell [23].
In addition, upon leukocyte-endothelial cell interaction, endothelial cells function in the removal of leukocytes at early stages of apoptosis. We have reported that leukocytes can be found within vacuolar structures of endothelial cells, but this occurs only when endothelial cells phagocytose apoptotic leukocytes [25, 26]. Apoptotic T cells, B cells, and mononuclear cells are phagocytosed by human tonsil microvascular endothelial cells in primary cultures, by mouse lymph node endothelial cells in vivo, and by mouse endothelial cell lines in vitro, as determined by studies using confocal microscopy, flow cytometry, and electron microscopy [25, 26]. This active function of endothelial cells may be a mechanism by which the endothelium is protected from localized vascular damage, which would occur if apoptotic leukocytes were to undergo necrosis. It is interesting that apoptotic leukocytes can be phagocytosed by endothelial cells prior to significant accumulation of apoptotic cell markers [27, 28]. In summary, endothelial cells modulate leukocyte migration by promoting leukocyte migration or removing leukocytes undergoing apoptosis.
During leukocyte migration, endothelial cells are not merely passive participants but are activated by leukocytes. Endothelial cell adhesion molecules provide a scaffold on which leukocytes can migrate as well as stimulate “outside-in” signal transduction in endothelial cells. Signals are activated by the endothelial cell adhesion molecules platelet-endothelial cell adhesion molecule-1 [(PECAM-1)/CD31], CD99, ICAM-1 (CD54), and VCAM-1 (CD106). Some of these signals have been demonstrated to cause localized alterations in endothelial cell junctions. Adhesion molecule signals result in alterations in the function of cell junction proteins and/or contractile forces in the endothelial cell, thereby opening an endothelial cell junction and permitting leukocyte migration into the tissue. During migration by neutrophils and monocytes, there is localized endothelial cell retraction of lateral junctions at the site of leukocyte migration [19–22]. Furthermore, the migration by monocytes is dependent on endothelial cell cytoskeletal changes. It has been reported that inhibition of the microfilaments in lung microvascular endothelial cells or leukotriene B4-treated pulmonary artery endothelial cells reduces monocyte and neutrophil transendothelial migration, respectively, indicating that contractile forces in endothelial cells have an active role in leukocyte migration [29, 30].
PECAM-1 AND CD99
Homophilic PECAM-1 adhesion at lateral borders of nonactivated endothelial cells participates in the formation of endothelial cell junctions [31]. PECAM-1 also mediates homophilic binding with PECAM-1 on leukocytes. Anti-PECAM-1 antibodies can block monocyte diapedesis across tumor necrosis factor α(TNF-α)-activated or interleukin (IL)-1β-activated endothelial cells [32], although there are examples of PECAM-1-independent models of lymphocyte, neutrophil, and monocyte migration [33, 34]. Anti-PECAM-1 antibodies arrest monocytes on the apical surface of cytokine-activated endothelium, whereas blocking another junction receptor CD99 arrests monocytes between these endothelial cells, suggesting a downstream function of CD99 in monocyte migration [32]. Although a signaling role for CD99 in endothelial cells during leukocyte migration has not been reported, CD99 may activate signals in endothelial cells, as in Jurkat T cells, it activates extracellular signal-regulated kinase (ERK), c-jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (MAPK) [35]. Moreover, ERK, JNK, and MAPK have been shown to stimulate other endothelial cell functions, including increases in vascular permeability and actin cytoskeletal remodeling [36–38].
PECAM-1 can activate signals that increase leukocyte adhesion. Antibody cross-linking of PECAM-1 on IL-4-treated HUVECs stimulates an increase in eosinophil adhesion [39]. This increased eosinophil binding is mediated by PECAM-1 “outside-in” stimulation of protein kinase C (PKC) and phosphoinositide 3-kinase (PI-3K), which activate αvβ3-integrin on the endothelial cell [39]. This binding stimulates an increase in the affinity of eosinophil α4β1-integrin, which then binds to VCAM-1 on the endothelium [39].
In addition to PECAM signals for increased adhesion molecule affinity, its “outside-in” signals may be involved in modulating PECAM-1 association with the cytoskeleton. PECAM-1 has recently been shown to recycle between the cell membrane and a cytoplasmic compartment juxtaposed to the membrane [20]. PECAM-1 is recruited to the endothelial cell surface at sites of monocyte transmigration [20]. This recruitment of PECAM-1 may be regulated by “outside-in” signals. Treating nonactivated HUVECs with an inducer of oxidative stress (tert-butylhydroperoxide) increases PECAM-1 phosphorylation, correlating with an increase in HL-60 leukocyte migration [40]. This increased PECAM-1 phosphorylation can be blocked with anti-PECAM-1 antibodies, antioxidants, or inhibitors of PKC, Ras, and glutathione synthesis [40]. Furthermore, PECAM-1 phosphorylation is regulated by phosphatases, as pharmacologic inhibition of ser/thr phosphatases augments oxidant-induced PECAM-1 phosphorylation in HUVECs [40]. Homophilic adhesion of PECAM-1 or antibody cross-linking of PECAM-1 on platelets or endothelial cells stimulates PECAM-1 binding to several intracellular proteins involved in signaling including Src homology (SH)2-containing phosphatase (SHP)-1, SHP-2, SH2 domain-containing inositol phosphatase, phospholipase C-γ, and PI-3K [41–44]. PECAM-1 also is linked to the cytoskeleton. PECAM-1, on nonactivated HUVECs, can be phosphorylated by PKC and linked to the cytoskeletal-associated proteins β- and γ-catenin through its immunoreceptor tyrosine-based activation motif [31]. PECAM-1 sequestering of the catenins may limit catenin translocation to the nucleus, thereby modulating gene expression [31].
PECAM-1 localization in endothelial cells is modulated by cytokines. Shaw et al. [45] have shown that PECAM-1 moves out of lateral junctions after treatment with TNF-α plus interferon-γ (IFN)-γ. However, cytokine-induced movement of PECAM-1 out of lateral junctions does not inhibit the ability of monocytes to migrate across these endothelial cells under laminar flow conditions, whereas anti-PECAM-1 antibodies still inhibit migration [45], suggesting that sufficient PECAM-1 is still available for migration. In summary, several signals have been reported for PECAM-1, some of which increase leukocyte affinity. Whether these PECAM-1 signals participate in leukocyte migration, endothelial cell basal surface adhesion, or gene expression has not been established. The activation of PI-3K and PKC has been shown in other signaling systems to stimulate focal adhesions on basal cell surfaces, gene expression, or leukocyte migration [46–54].
ICAM-1
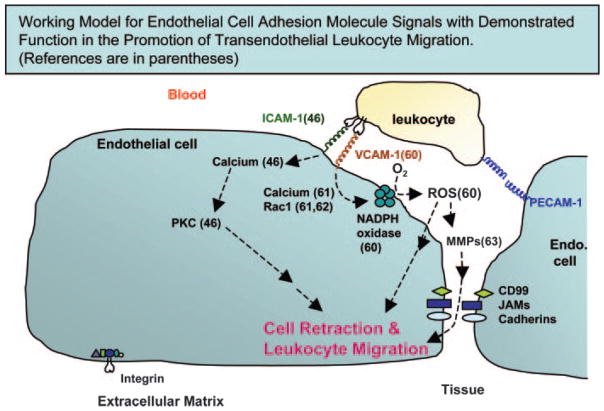
ICAM-1 expression is constitutive on many cell types but can be increased on endothelium by inflammatory mediators [55]. The first and third immunoglobulin-like domain of ICAM-1 bind to the counter-receptors lymphocyte function-associated antigen-1 (LFA-1; CD11a/CD18) and membrane-activated complex-1 (CD11b/CD18), respectively, on leukocytes [56]. In addition, ICAM-1 binds to fibrinogen on lymphocytes [57]. LFA-1 binding to green fluorescent protein (GFP)-labeled ICAM-1 in Chinese hamster ovary (CHO)-K1 cells causes redistribution of ICAM-1 to microvilli projections, and this is dependent on calcium [58]. A role for endothelial cell ICAM-1-mediated signals during lymphocyte migration has been reported. Etienne-Manneville et al. [46] demonstrated that chelating intracellular calcium or inhibition of PKC in IFN-γ-treated brain endothelial cell lines blocks ICAM-1-dependent lymphocyte migration without affecting lymphocyte adhesion. Antibody cross-linking of ICAM-1 also induces production of chemokines by HUVECs [59]. This ICAM-1-stimulated endothelial cell chemokine production is dependent on activation of ERK1 and ERK2 [59]. These data suggest that ICAM-1 induces endothelial cell signals that are required for lymphocyte migration (Fig. 1).
Fig. 1.
Stimulation of ICAM-1 and VCAM-1 activates “outside-in” signals, which are required for leukocyte migration. Intracellular calcium or PKC in brain endothelial cells is necessary for ICAM-1-dependent lymphocyte migration but not lymphocyte adhesion [46]. Antibody cross-linking of VCAM-1 or lymphocyte binding to VCAM-1 activates release of intracellular calcium, calcium channels, and Rac1, which are required for the VCAM-1 activation of endothelial cell nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [61]. This stimulation of NADPH oxidase is required for the VCAM-1-dependent activation of endothelial cell-associated matrix metalloproteinases (MMPs) [63]. VCAM-1-dependent lymphocyte migration requires VCAM-1-stimulated endothelial cell NADPH oxidase and endothelial cell-associated MMPs [60, 63]. ROS, Reactive oxygen species; JAMs, junctional adhesion molecules.
Other ICAM-1 signals have been reported to regulate the endothelial cell actin cytoskeleton. Antibody cross-linking of ICAM-1 on TNF-α-activated pulmonary microvascular endothelial cells activates xanthine oxidase and p38 MAPK, resulting in actin rearrangement [64]. The C-terminal peptide of ICAM-1 can bind directly to α-actinin, β-tubulin, and glycer-aldehyde-3-phosphate [65]. Antibody cross-linking of ICAM-1 on IFN-γ-stimulated brain endothelial cell lines or TNF-α-activated HUVECs also activates Src kinase and increases phosphorylation of the p60Src substrate cortactin, an actin-binding protein [46, 66–68]. This ICAM-1-stimulated phosphorylation of p60Src and cortactin requires PKC [46]. PKC and Rho are also required for anti-ICAM-1-stimulated phosphorylation of focal adhesion kinase (FAK), paxillin, and p130cas, as well as stress fiber formation in IFN-γ-stimulated brain endothelial cell lines [46, 67]. A downstream mediator of phosphorylated p130cas may be JNK [67]. Antibody cross-linking of ICAM-1 on TNF-α-activated pulmonary microvascular endothelial cells induces phosphorylation of another cytoskeletal protein ezrin [69]. ICAM-1 associates with the cytoskeletal protein ezrin through phosphytidylinositol 4,5-bisphosphate in ICAM-1-transfected Cos1 cells [70]. ICAM-1 also binds to the phosphatase SHP-2 in fibrinogen-stimulated HUVECs [71]. This phosphatase has been shown to regulate focal adhesions in embryonic fibroblast cell lines [72]. In summary, some of the many ICAM-1 receptor-mediated signals that regulate the cytoskeleton of the endothelial cell may be important for leukocyte migration or alternatively, regulation of focal adhesions, which are known to be modulated by paxillin and FAK.
VCAM-1
We have demonstrated that binding of lymphocytes or anti-VCAM-1-coated 10 μm beads to VCAM-1 (CD106) stimulates localized endothelial cell-shape changes and the “opening of a narrow passageway” through which leukocytes can migrate [33, 60]. Endothelial cell shape, endothelial cell viability, and vascular function can be regulated by endothelial cell NADPH oxidase [73–76]. Endothelial cells express NADPH oxidase subunits (gp91 phox, p22 phox, p47 phox, and p67 phox) [77, 78]. NADPH oxidase catalyzes production of superoxide, which dismutates to H2O2. We showed that lymphocyte binding to VCAM-1 or anti-VCAM-1-coated beads activates endothelial cell NADPH oxidase for the generation of ROS in murine endothelial cell lines [murine HEV (mHEV) cells], which constitutively express VCAM-1 and the chemokine monocyte chemoattractant protein (MCP)-1 [60, 79]. The anti-VCAM-1-stimulated generation of ROS is blocked by morpholino antisense against the catalytic subunit of NADPH oxidase in these endothelial cells [61]. Anti-VCAM-1-coated beads also stimulate ROS generation in primary cultures of IL-4-activated HUVECs [60]. van Wetering et al. [62] substantiated this VCAM-1 induction of ROS production. In contrast, antibody cross-linking of ICAM-1 and PECAM-1 does not activate endothelial cell NADPH oxidase [60, 80]. Importantly, the VCAM-1-stimulated endothelial cell NADPH oxidase activity is required for VCAM-1-dependent lymphocyte migration, as inhibition of endothelial cell NADPH oxidase by diphenyliodonium or apocynin blocks MCP-1-stimulated, VCAM-1-dependent lymphocyte migration without altering lymphocyte adhesion [60, 79] (Fig. 1). Furthermore, scavenging ROS with catalase and/or superoxide dismutase inhibits VCAM-1-dependent lymphocyte migration across the mHEV cell lines. In contrast, VCAM-1-dependent lymphocyte migration is not blocked by inhibition of the ROS-generating enzymes nitric oxide synthase, xanthine oxidase, or cytochrome p450 in endothelial cells [60].
VCAM-1-mediated activation of NADPH oxidase is dependent on a calcium flux and the small molecular weight G protein Rac1. We have shown that lymphocyte binding to VCAM-1 or anti-VCAM-1-coated 10 μm beads stimulates endothelial cell verapamil-sensitive calcium channels and the release of intracellular calcium, which are required for the activation of NADPH oxidase in the mHEV cell lines [61]. A calcium flux is induced in lipopolysaccharide (LPS)-stimulated HUVECs by VCAM-1-dependent monocyte adhesion to HUVECs or antibody cross-linking of VCAM-1 [81]. In addition, anti-VCAM-1-coated 10 μm beads stimulate endothelial cell Rac1 [61]. Transfection of endothelial cell lines with a dominant-negative Rac1 prevents anti-VCAM-1-stimulated generation of H2O2 [61] as well as the migration of U-937 cells across cytokine-activated HUVECs [62]. Thus, Rac1 likely is involved in the assembly of the active NADPH oxidase complex in endothelial cells as in neutrophils [82–84].
Low concentrations of H2O2 (1 μM) are generated in endothelial cell lines by lymphocyte binding to VCAM-1 or anti-VCAM-1-coated bead stimulation [85]. This is in contrast to the 50 –200 μM H2O2 released by neutrophils and macrophages for the destruction of pathogens [86, 87] or released in disease states such as atherosclerosis, pulmonary fibrosis, ischemia-reperfusion syndrome, and neurodegenerative diseases [88, 89]. Low levels of ROS generate rapid, transient, and reversible signals. This is important, as once a leukocyte reaches an endothelial cell junction, the process of transmigration occurs within a couple of minutes. Compartmentalization of ROS likely limits the proteins that are modified by ROS. In support of this, TNF-α-activated HUVECs generate ROS from the mitochondria and a nonmitochondrial Rac1-dependent process [74], yet the mitochondrial ROS induces apoptosis, whereas the Rac1-dependent ROS generation is protective against TNF-α-induced apoptosis [74]. Forman and Torres [90] propose that low levels of ROS function as signaling molecules, as they have a restricted location of action, their signals are transient, and their oxidation reactions are reversible. ROS modify thiolate anions (−S−) to form sulfenate (−SO−) as well as react with disulfide linkages [89]. These can be reduced back to their native state by intracellular thiols in the cell such as thioredoxin, peroxiredoxins, and glutathione [90]. Thus, ROS can reversibly and transiently activate “outside-in” signals in endothelial cells.
VCAM-1 activates changes in endothelial cell actin structure. Lorenzon et al. [81] reported the presence of actin stress fibers in LPS-activated endothelial cells upon VCAM-1 cross-linking or monocyte adhesion; however, the signals for the rearrangement of actin were not determined. We reported that the ROS production stimulated by lymphocyte binding to VCAM-1 or anti-VCAM-1-coated beads is required for endothelial cell actin coalescence at the site of adhesion to endothelial cell lines or TNF-α-activated HUVECs [60]. van Wetering et al. [62] demonstrated that the VCAM-1-stimulated ROS activates p38 MAPK with subsequent loss of β-catenin staining at the cell periphery and an increase in intercellular gaps in IL-1β-activated HUVECs.
We reported another function for the ROS generated during VCAM-1-dependent lymphocyte migration. Lymphocyte binding to VCAM-1, antibody cross-linking of VCAM-1, or exogenous 1 μM H2O2 activates within minutes MMPs associated with endothelial cell lines or IL-4-activated HUVECs [63]. Anti-VCAM-1 bead activation of these endothelial cell MMPs is not altered by laminar flow at the rate found in postcapillary venules (2 dynes/cm2) [63]. The endothelial cell-derived H2O2 also activates lymphocyte-associated MMPs but these MMPs are not activated until 2–5 h later [63]. Several other laboratories have also reported that lymphocyte MMPs are activated several hours after T cell binding to VCAM-1 or cross-linking of the ligand for VCAM-1, α4-integrin [91–93]. This suggests that H2O2 as well as α4-integrin signals stimulate a delayed activation of lymphocyte MMPs. It is most interesting that the endothelial cell-associated rather than the lymphocyte-associated MMPs are required for VCAM-1-dependent lymphocyte migration across VCAM-1-expressing endothelial cell lines as determined with pharmacological inhibitors of MMPs [63] (Fig. 1). Romanic and Madri [91] also demonstrated that tissue inhibitors of metalloproteinase-2 (TIMP-2) inhibition of MMP activity blocked T cell migration [91], although they did not determine whether inhibition with TIMP-2 was mediated by blocking lymphocyte or endothelial cell MMPs. In summary, the requirement for VCAM-1-stimulated endothelial cell ROS generation and endothelial cell-associated MMP activity during lymphocyte migration indicates that the endothelial cell has an active role in VCAM-1-dependent lymphocyte migration (Fig. 1).
MMPs associated with endothelial cells are likely more important than secreted MMPs during lymphocyte migration, as secreted MMPs would be removed from the site by the flow of blood. MMPs are held at the cell surface by membrane-type MMPs (MT-MMPs) and adhesion molecules. MMP-2 is held by MT1-MMP (MMP-14) [94]. MMP-9 and MMP-7 bind to cell-surface CD44 [95–97]. Pro-MMP-9 can bind to ICAM-1 [98]. MMP-1, MMP-2, and MMP-9 bind to α2β1-integrin on keratinocytes, αvβ3-integrin on endothelial cells, and α2-integrin on epithelial cells, respectively [99]. The endothelial cell-associated MMP-2 and MMP-9 likely degrade matrix and endothelial cell junction molecules at the site of transmigration. Endothelial cell junction proteins, such as vascular endothelial (VE)-cadherin, can be cleaved by MMPs, as demonstrated during endothelial cell apoptosis [100].
The anti-VCAM-1 or lymphocyte-stimulated VCAM-1 rapidly activates endothelial cell-associated MMPs without altering cell-associated levels of the MMPs or the TIMPs [63]. As this expression does not change and as low levels of exogenous H2O2 can directly activate purified MMPs [101], the H2O2 generated by VCAM-1 stimulation likely has a direct, rapid effect on the endothelial cell-associated MMPs. ROS activate purified MMPs by oxidizing the cysteine in the propeptide arm, opening the arm, and exposing the MMP active site [102]. This opening of the propeptide arm stimulates autocatalytic removal of the arm, forming an active MMP [103].
In contrast to a direct effect of H2O2 on endothelial cell-associated MMPs, H2O2 indirectly activates lymphocyte-associated MMPs. H2O2 activation of the lymphocyte-associated MMPs is mediated by the down-regulation of the expression of the relatively high levels of TIMPs on lymphocytes without altering expression of lymphocyte MMPs [63]. This results in a net increase in lymphocyte MMP activity. Given the several hours it takes to induce the increase in lymphocyte MMP activity and our studies indicating that lymphocyte MMPs are not required for VCAM-1-dependent lymphocyte migration, the stimulation of lymphocyte MMPs is likely involved in the subsequent leukocyte migration through extravascular tissue.
ENDOTHELIAL CELL JUNCTION PROTEINS
Endothelial cells have tight junctions, adherence (or adherens) junctions, complexus adherents, and gap junctions [104, 105]. Endothelial cell junctions dissociate during intercellular leukocyte migration. There have been recent advances in the participation of cell junction proteins during lymphocyte migration. The function of the endothelial cell junction molecules may be modulated by adhesion molecule signals or may be cleaved by MMPs. The junction protein claudin can recruit active MT-MMP/MMP-2 complexes to cell-cell borders in transfected COS cells and the embryonic kidney cell line 293T cells [106]. Therefore, claudin recruitment of MMPs may regulate localized adhesion molecule stimulation of MMPs in these junctions.
Endothelial cell tight junctions also contain junctional adhesion molecules (JAMs) [107], which have recently been assigned a new nomenclature that we use here to discuss the JAMs [107]. Anti-JAM-A decreases inflammatory infiltrates into the cerebrospinal fluid in a mouse model of meningitis [108]. Anti-JAM-A antibodies also inhibit stromal cell-derived factor (SDF)-1 and IL-8-induced chemotaxis of memory T cells and neutrophils across nonactivated HUVECs [109]. In contrast, anti-JAM-A antibodies do not inhibit neutrophil migration across JAM-A-expressing, TNF-α-activated HUVECs under conditions of laminar flow at 1 dyne/cm2 [45]. Furthermore, when TNF-α plus IFN-γ-treated HUVECs stimulate movement of JAM-A out of lateral junctions, there is no reduction in neutrophil migration under flow conditions [45]. JAM-A can bind to αLβ2-integrin (LFA-1) on leukocytes, but the contribution of the binding to αLβ2-integrin in the presence of other adhesion molecules has not been demonstrated [109]. In addition, purified recombinant JAM-A as well as JAM-A-transfected CHO cells mediate homophilic adhesion [110], but a functional role for homophilic binding has yet to be determined [18]. JAM-A interacts with other tight junction proteins, claudin and occludin, via the intracellular actin-binding protein zonula occludin-1 [110]. JAM-A also directly binds to the signaling molecule calcium/calmodulin-dependent serine protein kinase and indirectly binds to PKC via the cell polarity protein proteinase-activated receptor-3 [111, 112]. Thus, although JAM-A can participate in leukocyte migration, and JAM-A binds several signaling molecules, further studies are necessary to determine if these signaling molecules regulate JAM-A function during leukocyte migration.
JAM-B has also been shown to be involved in SDF-1-stimulated migration of peripheral blood lymphocytes across nonactivated HUVECs [113]. JAM-B is expressed primarily by endothelial cells in peripheral lymph nodes, but some arterioles express it in sites of inflammation and tumor foci [113]. In humans but not mice, JAM-B is also expressed on lymphocytes and monocytes [113]. Murine endothelial cell JAM-B can bind to JAM-C on leukocytes [18]. JAM-C is expressed by T cells, natural killer cells, dendritic cells, and peripheral lymph node endothelial cells [18]. As leukocytes in humans express JAM-B and JAM-C, it is not clear whether transendothelial migration is mediated by homophilic or heterophilic interactions or whether JAM involvement in transendothelial migration differs in humans and mice [18].
In adherens junctions, cadherins are linked to actin via catenins. Endothelial cells express N- and VE-cadherin, which mediate homophilic binding. In the absence of VE-cadherin, N-cadherin can replace the function of VE-cadherin in the adherens junctions [114]. Studies using VE-cadherin-GFP demonstrate that during neutrophil or monocyte migration across TNF-α-activated HUVECs, VE-cadherin moves laterally in the membrane so that it is out of the location of the leukocyte migration [22]. After migration, the VE-cadherin moves back into the junction [22]. In contrast, PECAM-1 in cell junctions is not displaced during leukocyte migration; instead, it can be recruited to the junction during migration of monocytes across TNF-α-activated HUVECs [20, 115]. Antibodies to VE-cadherin increase neutrophil transmigration across IL-1β-activated HUVECs or coronary venular endothelial cells in vitro and increase microvascular permeability in vitro and vivo [104, 116, 117]. The N-terminal domains of VE-cadherin can be cleaved by neutrophil cell-surface elastase [118]. This domain of VE-cadherin is thought to be critical for homophilic interactions of VE-cadherin, as demonstrated for another member of the cadherin family, C-cadherin [119]. VE-cadherin can also be cleaved by MMPs during TNF-α-induced apoptosis in HUVECs [100]. Therefore, when endothelial cell-associated MMPs or leukocyte proteases are activated during leukocyte migration, they may cleave cadherins, thus decreasing the homophilic binding in endothelial cell junctions at sites of leukocyte migration.
SUMMARY
In summary, endothelial cells play an active and essential role during transendothelial cell migration of leukocytes. Leukocytes activate endothelial cell signals that stimulate endothelial cell retraction during localized dissociation of the endothelial cell junctions. Signals for ICAM-1 and VCAM-1 have been defined as functioning in endothelial cell promotion of leukocyte migration (Fig. 1). ICAM-1-mediated signals activate an endothelial cell calcium flux and PKC, which are required for ICAM-1-dependent leukocyte migration. VCAM-1-mediated signals stimulate an endothelial cell intracellular calcium release, calcium channels, and Rac1, which then increase endothelial cell NADPH oxidase activity. This activation of NADPH oxidase yields the generation of low concentrations of H2O2, which activate endothelial cell-associated MMPs, and these MMPs are required for VCAM-1-dependent lymphocyte migration. How signals from several adhesion molecules are coordinated and whether there is functional redundancy in the downstream signals from these adhesion molecules are interesting avenues for future research. Signals such as PKC and PI-3K are activated by several adhesion molecules. After endothelial cell retraction, the adhesion molecules in the junctions and perhaps some of the other transmembrane junction proteins provide receptors on which the leukocytes can migrate.
Signals activated by endothelial cell adhesion molecules may also have important endothelial cell functions during inflammation, which are independent of endothelial cell junction retraction and are not required for leukocyte migration. We propose that some of the endothelial cell adhesion molecule signals are consistent with enhancement of endothelial cell basal surface adhesion, thus retaining vascular integrity during opening of endothelial cell junctions under flow conditions. Adhesion molecule signals through PI-3K, AKT, and Src may modulate integrin-mediated focal adhesions on the endothelial cell basal surface, as these signals have been reported to modulate focal adhesions in several cell types [47–51]. Furthermore, some of the adhesion molecule signals in endothelial cells may modulate gene expression. For instance, adhesion molecule signals such as p38 kinase, Akt, and PKC could also stimulate gene expression, as these signals have been shown to activate transcription factors including nuclear factor-κB in endothelial cells [52–54]. Furthermore, activation of ICAM-1 induces ERK1 and ERK2-dependent gene expression of chemokines by HUVECs [59]. In summary, endothelial cells demonstrate active function during leukocyte transendothelial migration involving adhesion molecule-stimulated “outside-in” signals as well as endothelial cell removal of apoptotic leukocytes via phagocytosis.
References
- 1.McEver RP. Selectins: lectins that initiate cell adhesion under flow. Curr Opin Cell Biol. 2002;14:581–586. doi: 10.1016/s0955-0674(02)00367-8. [DOI] [PubMed] [Google Scholar]
- 2.Vestweber D. Lymphocyte trafficking through blood and lymphatic vessels: more than just selectins, chemokines and integrins. Eur J Immunol. 2003;33:1361–1364. doi: 10.1002/eji.200324011. [DOI] [PubMed] [Google Scholar]
- 3.Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol. 1995;128:1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell JJ, Qin S, Bacon KB, Mackay CR, Butcher EC. Biology of chemokine and classical chemoattractant receptors: differential requirements for adhesion-triggering versus chemotactic responses in lymphoid cells. J Cell Biol. 1996;134:255–266. doi: 10.1083/jcb.134.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shamri R, Grabovsky V, Feigelson SW, Dwir O, Van Kooyk Y, Alon R. Chemokine stimulation of lymphocyte α 4 integrin avidity but not of leukocyte function-associated antigen-1 avidity to endothelial ligands under shear flow requires cholesterol membrane rafts. J Biol Chem. 2002;277:40027– 40035. doi: 10.1074/jbc.M206806200. [DOI] [PubMed] [Google Scholar]
- 6.Alon R, Feigelson S. From rolling to arrest on blood vessels: leukocyte tap dancing on endothelial integrin ligands and chemokines at sub-second contacts. Semin Immunol. 2002;14:93–104. doi: 10.1006/smim.2001.0346. [DOI] [PubMed] [Google Scholar]
- 7.Alon R, Grabovsky V, Feigelson S. Chemokine induction of integrin adhesiveness on rolling and arrested leukocytes local signaling events or global stepwise activation? Microcirculation. 2003;10:297–311. doi: 10.1038/sj.mn.7800195. [DOI] [PubMed] [Google Scholar]
- 8.Middleton J, Patterson AM, Gardner L, Schmutz C, Ashton BA. Leukocyte extravasation: chemokine transport and presentation by the endothelium. Blood. 2002;100:3853–3860. doi: 10.1182/blood.V100.12.3853. [DOI] [PubMed] [Google Scholar]
- 9.Pober JS, Kluger MS, Schechner JS. Human endothelial cell presentation of antigen and the homing of memory/effector T cells to skin. Ann N Y Acad Sci. 2001;941:12–25. doi: 10.1111/j.1749-6632.2001.tb03706.x. [DOI] [PubMed] [Google Scholar]
- 10.Lalor PF, Shields P, Grant A, Adams DH. Recruitment of lymphocytes to the human liver. Immunol Cell Biol. 2002;80:52– 64. doi: 10.1046/j.1440-1711.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 11.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 12.Hendriks HR, Korn C, Duijvestijn AM, Kraal G. Changes in lymphocyte binding and expression of HIS 22 of high endothelial venules in rat lymph nodes after occlusion of afferent lymph flow. Adv Exp Med Biol. 1988;237:485– 490. doi: 10.1007/978-1-4684-5535-9_74. [DOI] [PubMed] [Google Scholar]
- 13.Johnston B, Butcher EC. Chemokines in rapid leukocyte adhesion triggering and migration. Semin Immunol. 2002;14:83–92. doi: 10.1006/smim.2001.0345. [DOI] [PubMed] [Google Scholar]
- 14.Marshall D, Haskard DO. Clinical overview of leukocyte adhesion and migration: where are we now? Semin Immunol. 2002;14:133–140. doi: 10.1006/smim.2001.0350. [DOI] [PubMed] [Google Scholar]
- 15.Elangbam CS, Qualls CW, Jr, Dahlgren RR. Cell adhesion molecules— update. Vet Pathol. 1997;34:61–73. doi: 10.1177/030098589703400113. [DOI] [PubMed] [Google Scholar]
- 16.Ley K. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovasc Res. 1996;32:733–742. [PubMed] [Google Scholar]
- 17.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 18.Luscinskas FW, Ma S, Nusrat A, Parkos CA, Shaw SK. The role of endothelial cell lateral junctions during leukocyte trafficking. Immunol Rev. 2002;186:57– 67. doi: 10.1034/j.1600-065x.2002.18606.x. [DOI] [PubMed] [Google Scholar]
- 19.Gopalan PK, Burns AR, Simon SI, Sparks S, McIntire LV, Smith CW. Preferential sites for stationary adhesion of neutrophils to cytokine-stimulated HUVEC under flow conditions. J Leukoc Biol. 2000;68:47–57. [PubMed] [Google Scholar]
- 20.Mamdouh Z, Chen X, Pierini LM, Maxfield FR, Muller WA. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature. 2003;421:748–753. doi: 10.1038/nature01300. [DOI] [PubMed] [Google Scholar]
- 21.Muller WA. Migration of leukocytes across endothelial junctions: some concepts and controversies. Microcirculation. 2001;8:181–193. doi: 10.1038/sj/mn/7800078. [DOI] [PubMed] [Google Scholar]
- 22.Shaw SK, Bamba PS, Perkins BN, Luscinskas FW. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J Immunol. 2001;167:2323–2330. doi: 10.4049/jimmunol.167.4.2323. [DOI] [PubMed] [Google Scholar]
- 23.Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM. Neutrophils emigrate from venules by a transendothelial cell pathway in response to fMLP. J Exp Med. 1998;187:903–915. doi: 10.1084/jem.187.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoshi O, Ushiki T. Scanning electron microscopic studies on the route of neutrophil extravasation in the mouse after exposure to the chemotactic peptide N-formyl-methionyl-leucyl-phenylalanine (fMLP) Arch Histol Cytol. 1999;62:253–260. doi: 10.1679/aohc.62.253. [DOI] [PubMed] [Google Scholar]
- 25.Hess KL, Tudor KSRS, Johnson JD, Osati-Ashtiani F, Askew DS, Cook-Mills JM. Human and murine high endothelial venule cells phagocytose apoptotic leukocytes. Exp Cell Res. 1997;236:404– 411. doi: 10.1006/excr.1997.3745. [DOI] [PubMed] [Google Scholar]
- 26.Hess KL, Babcock GF, Askew DS, Cook-Mills JM. A novel flow cytometric method for quantifying phagocytosis of apoptotic cells. Cytometry. 1997;27:145–152. [PMC free article] [PubMed] [Google Scholar]
- 27.Hess KL, Johnson JD, Cook-Mills JM. Different orders for acquisition of apoptotic characteristics by leukocytes. J Leukoc Biol. 2001;70:405– 412. [PubMed] [Google Scholar]
- 28.Johnson JD, Hess KL, Cook-Mills JM. CD44, α-4 integrin, and fucoidin receptor-mediated phagocytosis of apoptotic leukocytes. J Leukoc Biol. 2003;74:810– 820. doi: 10.1189/jlb.0303092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kielbassa K, Schmitz C, Gerke V. Disruption of endothelial microfilaments selectively reduces the transendothelial migration of monocytes. Exp Cell Res. 1998;243:129–141. doi: 10.1006/excr.1998.4133. [DOI] [PubMed] [Google Scholar]
- 30.Garcia JG, Verin AD, Herenyiova M, English D. Adherent neutrophils activate endothelial myosin light chain kinase: role in transendothelial migration. J Appl Physiol. 1998;84:1817–1821. doi: 10.1152/jappl.1998.84.5.1817. [DOI] [PubMed] [Google Scholar]
- 31.Ilan N, Cheung L, Pinter E, Madri JA. Platelet-endothelial cell adhesion molecule-1 (CD31), a scaffolding molecule for selected catenin family members whose binding is mediated by different tyrosine and serine/threonine phosphorylation. J Biol Chem. 2000;275:21435–21443. doi: 10.1074/jbc.M001857200. [DOI] [PubMed] [Google Scholar]
- 32.Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002;3:143–150. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- 33.Cook-Mills JM, Gallagher JS, Feldbush TL. Isolation and characterization of high endothelial cell lines derived from mouse lymph nodes. In Vitro Cell Dev Biol Anim. 1996;32:167–177. doi: 10.1007/BF02723682. [DOI] [PubMed] [Google Scholar]
- 34.Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, Spellberg J, Luis de la Pompa J, Elia A, Wakeham A, Karan-Tamir B, Muller WA, Senaldi G, Zukowski MM, Mak TW. Genetic evidence for functional redundancy of platelet/endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J Immunol. 1999;162:3022–3030. [PubMed] [Google Scholar]
- 35.Hahn MJ, Yoon SS, Sohn HW, Song HG, Park SH, Kim TJ. Differential activation of MAP kinase family members triggered by CD99 engagement. FEBS Lett. 2000;470:350–354. doi: 10.1016/s0014-5793(00)01330-2. [DOI] [PubMed] [Google Scholar]
- 36.Yoshizumi M, Abe JI, Tsuchiya K, Berk BC, Tamaki T. Stress and vascular responses: atheroprotective effect of laminar fluid shear stress in endothelial cells: possible role of mitogen-activated protein kinases. J Pharmacol Sci. 2003;91:172–176. doi: 10.1254/jphs.91.172. [DOI] [PubMed] [Google Scholar]
- 37.Usatyuk PV, Natarajan V. Role of mitogen-activated protein kinases in 4-hydroxy-2-nonenal-induced actin remodeling and barrier function in endothelial cells. J Biol Chem. 2004;279:11789–11797. doi: 10.1074/jbc.M311184200. [DOI] [PubMed] [Google Scholar]
- 38.Ni CW, Wang DL, Lien SC, Cheng JJ, Chao YJ, Hsieh HJ. Activation of PKC-ε and ERK1/2 participates in shear-induced endothelial MCP-1 expression that is repressed by nitric oxide. J Cell Physiol. 2003;195:428– 434. doi: 10.1002/jcp.10259. [DOI] [PubMed] [Google Scholar]
- 39.Chiba R, Nakagawa N, Kurasawa K, Tanaka Y, Saito Y, Iwamoto I. Ligation of CD31 (PECAM-1) on endothelial cells increases adhesive function of αvβ3 integrin and enhances β1 integrin-mediated adhesion of eosinophils to endothelial cells. Blood. 1999;94:1319–1329. [PubMed] [Google Scholar]
- 40.Rattan V, Sultana C, Shen Y, Kalra VK. Oxidant stress-induced transendothelial migration of monocytes is linked to phosphorylation of PECAM-1. Am J Physiol. 1997;273:E453–E461. doi: 10.1152/ajpendo.1997.273.3.E453. [DOI] [PubMed] [Google Scholar]
- 41.Pumphrey NJ, Taylor V, Freeman S, Douglas MR, Bradfield PF, Young SP, Lord JM, Wakelam MJ, Bird IN, Salmon M, Buckley CD. Differential association of cytoplasmic signalling molecules SHP-1, SHP-2, SHIP and phospholipase C-γ1 with PECAM-1/CD31. FEBS Lett. 1999;450:77– 83. doi: 10.1016/s0014-5793(99)00446-9. [DOI] [PubMed] [Google Scholar]
- 42.Hua CT, Gamble JR, Vadas MA, Jackson DE. Recruitment and activation of SHP-1 protein-tyrosine phosphatase by human platelet endothelial cell adhesion molecule-1 (PECAM-1). Identification of immunoreceptor tyrosine-based inhibitory motif-like binding motifs and substrates. J Biol Chem. 1998;273:28332–28340. doi: 10.1074/jbc.273.43.28332. [DOI] [PubMed] [Google Scholar]
- 43.Pellegatta F, Chierchia SL, Zocchi MR. Functional association of platelet endothelial cell adhesion molecule-1 and phosphoinositide 3-kinase in human neutrophils. J Biol Chem. 1998;273:27768–27771. doi: 10.1074/jbc.273.43.27768. [DOI] [PubMed] [Google Scholar]
- 44.Jackson DE, Ward CM, Wang R, Newman PJ. The protein-tyrosine phosphatase SHP-2 binds platelet/endothelial cell adhesion molecule-1 (PECAM-1) and forms a distinct signaling complex during platelet aggregation. Evidence for a mechanistic link between PECAM-1- and integrin-mediated cellular signaling. J Biol Chem. 1997;272:6986– 6993. doi: 10.1074/jbc.272.11.6986. [DOI] [PubMed] [Google Scholar]
- 45.Shaw SK, Perkins BN, Lim YC, Liu Y, Nusrat A, Schnell FJ, Parkos CA, Luscinskas FW. Reduced expression of junctional adhesion molecule and platelet/endothelial cell adhesion molecule-1 (CD31) at human vascular endothelial junctions by cytokines tumor necrosis factor-α plus interferon-γ does not reduce leukocyte transmigration under flow. Am J Pathol. 2001;159:2281–2291. doi: 10.1016/s0002-9440(10)63078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Etienne-Manneville S, Manneville JB, Adamson P, Wilbourn B, Greenwood J, Couraud PO. ICAM-1-coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. J Immunol. 2000;165:3375–3383. doi: 10.4049/jimmunol.165.6.3375. [DOI] [PubMed] [Google Scholar]
- 47.Pribila JT, Shimizu Y. Signal transduction events regulating integrin function and T cell migration: new functions and complexity. Immunol Res. 2003;27:107–128. doi: 10.1385/ir:27:1:107. [DOI] [PubMed] [Google Scholar]
- 48.Haynes MP, Li L, Sinha D, Russell KS, Hisamoto K, Baron R, Collinge M, Sessa WC, Bender JR. Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitricoxide synthase activation by estrogen. J Biol Chem. 2003;278:2118–2123. doi: 10.1074/jbc.M210828200. [DOI] [PubMed] [Google Scholar]
- 49.Windham TC, Parikh NU, Siwak DR, Summy JM, McConkey DJ, Kraker AJ, Gallick GE. Src activation regulates anoikis in human colon tumor cell lines. Oncogene. 2002;21:7797–7807. doi: 10.1038/sj.onc.1205989. [DOI] [PubMed] [Google Scholar]
- 50.Bang OS, Kim EJ, Chung JG, Lee SR, Park TK, Kang SS. Association of focal adhesion kinase with fibronectin and paxillin is required for precartilage condensation of chick mesenchymal cells. Biochem Biophys Res Commun. 2000;278:522–529. doi: 10.1006/bbrc.2000.3831. [DOI] [PubMed] [Google Scholar]
- 51.Crossthwaite AJ, Valli H, Williams RJ. Inhibiting Src family tyrosine kinase activity blocks glutamate signalling to ERK1/2 and Akt/PKB but not JNK in cultured striatal neurones. J Neurochem. 2004;88:1127–1139. doi: 10.1046/j.1471-4159.2004.02257.x. [DOI] [PubMed] [Google Scholar]
- 52.Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1β-mediated up-regulation of HIF-1α via an NFκB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17:2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- 53.Pieper GM. Activation of nuclear factor-κB in cultured endothelial cells by increased glucose concentration: prevention by calphostin C. J Cardiovasc Pharmacol. 1997;30:528–532. doi: 10.1097/00005344-199710000-00019. [DOI] [PubMed] [Google Scholar]
- 54.Costanzo A, Moretti F, Burgio VL, Bravi C, Guido F, Levrero M, Puri PL. Endothelial activation by angiotensin II through NFκB and p38 pathways: involvement of NFκB-inducible kinase (NIK), free oxygen radicals, and selective inhibition by aspirin. J Cell Physiol. 2003;195:402– 410. doi: 10.1002/jcp.10191. [DOI] [PubMed] [Google Scholar]
- 55.Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999;66:876– 888. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- 56.Diamond MS, Staunton DE, Marlin SD, Springer TA. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 1991;65:961–971. doi: 10.1016/0092-8674(91)90548-d. [DOI] [PubMed] [Google Scholar]
- 57.Duperray A, Languino LR, Plescia J, McDowall A, Hogg N, Craig AG, Berendt AR, Altieri DC. Molecular identification of a novel fibrinogen binding site on the first domain of ICAM-1 regulating leukocyte-endothelium bridging. J Biol Chem. 1997;272:435–441. doi: 10.1074/jbc.272.1.435. [DOI] [PubMed] [Google Scholar]
- 58.Carman CV, Jun CD, Salas A, Springer TA. Endothelial cells proactively form microvilli-like membrane projections upon intercellular adhesion molecule 1 engagement of leukocyte LFA-1. J Immunol. 2003;171:6135– 6144. doi: 10.4049/jimmunol.171.11.6135. [DOI] [PubMed] [Google Scholar]
- 59.Sano H, Nakagawa N, Chiba R, Kurasawa K, Saito Y, Iwamoto I. Cross-linking of intercellular adhesion molecule-1 induces interleukin-8 and RANTES production through the activation of MAP kinases in human vascular endothelial cells. Biochem Biophys Res Commun. 1998;250:694– 698. doi: 10.1006/bbrc.1998.9385. [DOI] [PubMed] [Google Scholar]
- 60.Matheny HE, Deem TL, Cook-Mills JM. Lymphocyte migration through monolayers of endothelial cell lines involves VCAM-1 signaling via endothelial cell NADPH oxidase. J Immunol. 2000;164:6550–6559. doi: 10.4049/jimmunol.164.12.6550. [DOI] [PubMed] [Google Scholar]
- 61.Cook-Mills JM, Johnson JD, Deem TL, Ochi A, Wang L, Zheng Y. Calcium mobilization and Rac1 activation are required for VCAM-1 (vascular cell adhesion molecule-1) stimulation of NADPH oxidase activity. Biochem J. 2004;378:539–547. doi: 10.1042/BJ20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Wetering S, van den Berk N, van Buul JD, Mul FPJ, Lommerse I, Mous R, ten Klooster JP, Zwaginga JJ, Hordijk PL. VCAM-1-mediated Rac signaling controls endothelial cell-cell contacts and leukocyte transmigration. Am J Physiol Cell Physiol. 2003;285:C343–C352. doi: 10.1152/ajpcell.00048.2003. [DOI] [PubMed] [Google Scholar]
- 63.Deem TL, Cook-Mills JM. Vascular cell adhesion molecule-1 (VCAM-1) activation of endothelial cell matrix metalloproteinases: role of reactive oxygen species. Blood. 2004;104:2385–2393. doi: 10.1182/blood-2004-02-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Q, Doerschuk CM. The p38 mitogen-activated protein kinase mediates cytoskeletal remodeling in pulmonary microvascular endothelial cells upon intracellular adhesion molecule-1 ligation. J Immunol. 2001;166:6877– 6884. doi: 10.4049/jimmunol.166.11.6877. [DOI] [PubMed] [Google Scholar]
- 65.Federici C, Camoin L, Hattab M, Strosberg AD, Couraud PO. Association of the cytoplasmic domain of intercellular-adhesion molecule-1 with glyceraldehyde-3-phosphate dehydrogenase and β-tubulin. Eur J Biochem. 1996;238:173–180. doi: 10.1111/j.1432-1033.1996.0173q.x. [DOI] [PubMed] [Google Scholar]
- 66.Durieu-Trautmann O, Chaverot N, Cazaubon S, Strosberg AD, Couraud PO. Intercellular adhesion molecule 1 activation induces tyrosine phosphorylation of the cytoskeleton-associated protein cortactin in brain microvessel endothelial cells. J Biol Chem. 1994;269:12536–12540. [PubMed] [Google Scholar]
- 67.Etienne S, Adamson P, Greenwood J, Strosberg AD, Cazaubon S, Couraud PO. ICAM-1 signaling pathways associated with Rho activation in microvascular brain endothelial cells. J Immunol. 1998;161:5755–5761. [PubMed] [Google Scholar]
- 68.Tilghman RW, Hoover RL. The Src-cortactin pathway is required for clustering of E-selectin and ICAM-1 in endothelial cells. FASEB J. 2002;16:1257–1259. doi: 10.1096/fj.01-0969fje. [DOI] [PubMed] [Google Scholar]
- 69.Wang Q, Pfeiffer GR, II, Gaarde WA. Activation of SRC tyrosine kinases in response to ICAM-1 ligation in pulmonary microvascular endothelial cells. J Biol Chem. 2003;278:47731– 47743. doi: 10.1074/jbc.M308466200. [DOI] [PubMed] [Google Scholar]
- 70.Heiska L, Alfthan K, Gronholm M, Vilja P, Vaheri A, Carpen O. Association of ezrin with intercellular adhesion molecule-1 and -2 (ICAM-1 and ICAM-2). Regulation by phosphatidylinositol 4, 5-bisphosphate. J Biol Chem. 1998;273:21893–21900. doi: 10.1074/jbc.273.34.21893. [DOI] [PubMed] [Google Scholar]
- 71.Pluskota E, Chen Y, D’Souza SE. Src homology domain 2-containing tyrosine phosphatase 2 associates with intercellular adhesion molecule 1 to regulate cell survival. J Biol Chem. 2000;275:30029–30036. doi: 10.1074/jbc.M000240200. [DOI] [PubMed] [Google Scholar]
- 72.Yu DH, Qu CK, Henegariu O, Lu X, Feng GS. Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J Biol Chem. 1998;273:21125–21131. doi: 10.1074/jbc.273.33.21125. [DOI] [PubMed] [Google Scholar]
- 73.Hu Q, Zheng G, Zweier JL, Deshpande S, Irani K, Ziegelstein RC. NADPH oxidase activation increases the sensitivity of intracellular Ca2+ stores to inositol 1,4,5-trisphosphate in human endothelial cells. J Biol Chem. 2000;275:15749–15757. doi: 10.1074/jbc.M000381200. [DOI] [PubMed] [Google Scholar]
- 74.Deshpande SS, Angkeow P, Huang J, Ozaki M, Irani K. Rac1 inhibits TNF-α-induced endothelial cell apoptosis: dual regulation by reactive oxygen species. FASEB J. 2000;14:1705–1714. doi: 10.1096/fj.99-0910com. [DOI] [PubMed] [Google Scholar]
- 75.De KGW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- 76.Howard AB, Alexander RW, Nerem RM, Griendling KK, Taylor WR. Cyclic strain induces an oxidative stress in endothelial cells. Am J Physiol. 1997;272:C421–C427. doi: 10.1152/ajpcell.1997.272.2.C421. [DOI] [PubMed] [Google Scholar]
- 77.Meyer JW, Holland JA, Ziegler LM, Chang MM, Beebe G, Schmitt ME. Identification of a functional leukocyte-type NADPH oxidase in human endothelial cells: a potential atherogenic source of reactive oxygen species. Endothelium. 1999;7:11–22. doi: 10.3109/10623329909165308. [DOI] [PubMed] [Google Scholar]
- 78.Jones SA, O’Donnell VB, Wood JD, Broughton JP, Hughes EJ, Jones OT. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol. 1996;271:H1626–H1634. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- 79.Qureshi MH, Cook-Mills J, Doherty DE, Garvy BA. TNF-α-dependent ICAM-1- and VCAM-1-mediated inflammatory responses are delayed in neonatal mice infected with Pneumocystis carinii. J Immunol. 2003;171:4700– 4707. doi: 10.4049/jimmunol.171.9.4700. [DOI] [PubMed] [Google Scholar]
- 80.van Buul JD, Voermans C, van den Berg V, Anthony EC, Mul FPJ, van Wetering S, van der Schoot EC, Hordijk PL. Migration of human hematopoietic progenitor cells across bone marrow endothelium is regulated by vascular endothelial cadherin. J Immunol. 2002;168:588–596. doi: 10.4049/jimmunol.168.2.588. [DOI] [PubMed] [Google Scholar]
- 81.Lorenzon P, Vecile E, Nardon E, Ferrero E, Harlan JM, Tedesco F, Dobrina A. Endothelial cell E- and P-selectin and vascular cell adhesion molecule-1 function as signaling receptors. J Cell Biol. 1998;142:1381–1391. doi: 10.1083/jcb.142.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boivin D, Bilodeau D, Beliveau R. Regulation of cytoskeletal functions by Rho small GTP-binding proteins in normal and cancer cells. Can J Physiol Pharmacol. 1996;74:801– 810. [PubMed] [Google Scholar]
- 83.Leusen JH, de Klein A, Hilarius PM, Ahlin A, Palmblad J, Smith CI, Diekmann D, Hall A, Verhoeven AJ, Roos D. Disturbed interaction of p21-rac with mutated p67-phox causes chronic granulomatous disease. J Exp Med. 1996;184:1243–1249. doi: 10.1084/jem.184.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dorseuil O, Quinn MT, Bokoch GM. Dissociation of Rac translocation from p47phox/p67phox movements in human neutrophils by tyrosine kinase inhibitors. J Leukoc Biol. 1995;58:108–113. doi: 10.1002/jlb.58.1.108. [DOI] [PubMed] [Google Scholar]
- 85.Tudor KSRS, Hess KL, Cook-Mills JM. Cytokines modulate endothelial cell intracellular signal transduction required for VCAM-1-dependent lymphocyte transendothelial migration. Cytokine. 2001;15:196–211. doi: 10.1006/cyto.2001.0922. [DOI] [PubMed] [Google Scholar]
- 86.DeLeo FR, Quinn MT. Assembly of the phagocyte NADPH oxidase: molecular interaction of oxidase proteins. J Leukoc Biol. 1996;60:677– 691. doi: 10.1002/jlb.60.6.677. [DOI] [PubMed] [Google Scholar]
- 87.Cook-Mills JM, Wirth JJ, Fraker PJ. Possible roles for zinc in destruction of Trypanosoma cruzi by toxic oxygen metabolites produced by mononuclear phagocytes. In: Bendich A, Phillips M, Tengerdy R, editors. Antioxidant Nutrients and Immune Function. New York, NY: Plenum; 1990. pp. 111–121. [DOI] [PubMed] [Google Scholar]
- 88.Rattan V, Sultana C, Shen Y, Kalra VK. Oxidant stress-induced transendothelial migration of monocytes is linked to phosphorylation of PECAM-1. Am J Physiol. 1997;273:E453–E461. doi: 10.1152/ajpendo.1997.273.3.E453. [DOI] [PubMed] [Google Scholar]
- 89.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 90.Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med. 2002;166:S4–S8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 91.Romanic AM, Madri JA. The induction of 72-kD gelatinase in T cells upon adhesion to endothelial cells is VCAM-1 dependent. J Cell Biol. 1994;125:1165–1178. doi: 10.1083/jcb.125.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yakubenko VP, Lobb RR, Plow EF, Ugarova TP. Differential induction of gelatinase B (MMP-9) and gelatinase A (MMP-2) in T lymphocytes upon α (4) β (1)-mediated adhesion to VCAM-1 and the CS-1 peptide of fibronectin. Exp Cell Res. 2000;260:73– 84. doi: 10.1006/excr.2000.5002. [DOI] [PubMed] [Google Scholar]
- 93.Esparza J, Vilardell C, Calvo J, Juan M, Vives J, Urbano-Marquez A, Yague J, Cid MC. Fibronectin upregulates gelatinase B (MMP-9) and induces coordinated expression of gelatinase A (MMP-2) and its activator MT1-MMP (MMP-14) by human T lymphocyte cell lines. A process repressed through RAS/MAP kinase signaling pathways. Blood. 1999;94:2754–2766. [PubMed] [Google Scholar]
- 94.Zucker S, Drews M, Conner C, Foda HD, DeClerck YA, Langley KE, Bahou WF, Docherty AJ, Cao J. Tissue inhibitor of metalloproteinase-2 (TIMP-2) binds to the catalytic domain of the cell surface receptor, membrane type 1-matrix metalloproteinase 1 (MT1-MMP) J Biol Chem. 1998;273:1216–1222. doi: 10.1074/jbc.273.2.1216. [DOI] [PubMed] [Google Scholar]
- 95.Murray D, Morrin M, McDonnell S. Increased invasion and expression of MMP-9 in human colorectal cell lines by a CD44-dependent mechanism. Anticancer Res. 2004;24:489– 494. [PubMed] [Google Scholar]
- 96.Abecassis I, Olofsson B, Schmid M, Zalcman G, Karniguian A. RhoA induces MMP-9 expression at CD44 lamellipodial focal complexes and promotes HMEC-1 cell invasion. Exp Cell Res. 2003;291:363–376. doi: 10.1016/j.yexcr.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 97.Yu WH, Woessner JF, Jr, McNeish JD, Stamenkovic I. CD44 anchors the assembly of matrilysin/MMP-7 with heparin-binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodeling. Genes Dev. 2002;16:307–323. doi: 10.1101/gad.925702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fiore E, Fusco C, Romero P, Stamenkovic I. Matrix metalloproteinase 9 (MMP-9/gelatinase B) proteolytically cleaves ICAM-1 and participates in tumor cell resistance to natural killer cell-mediated cytotoxicity. Oncogene. 2002;21:5213–5223. doi: 10.1038/sj.onc.1205684. [DOI] [PubMed] [Google Scholar]
- 99.Seiki M. The cell surface: the stage for matrix metalloproteinase regulation of migration. Curr Opin Cell Biol. 2002;14:624– 632. doi: 10.1016/s0955-0674(02)00363-0. [DOI] [PubMed] [Google Scholar]
- 100.Herren B, Levkau B, Raines EW, Ross R. Cleavage of β-catenin and plakoglobin and shedding of VE-cadherin during endothelial apoptosis: evidence for a role for caspases and metalloproteinases. Mol Biol Cell. 1998;9:1589–1601. doi: 10.1091/mbc.9.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murphy G, Willenbrock F, Crabbe T, O’Shea M, Ward R, Atkinson S, O’Connell J, Docherty A. Regulation of matrix metalloproteinase activity. Ann N Y Acad Sci. 1994;732:31– 41. doi: 10.1111/j.1749-6632.1994.tb24722.x. [DOI] [PubMed] [Google Scholar]
- 103.Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci USA. 1990;87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tinsley JH, Wu MH, Ma W, Taulman AC, Yuan SY. Activated neutrophils induce hyperpermeability and phosphorylation of adherens junction proteins in coronary venular endothelial cells. J Biol Chem. 1999;274:24930–24934. doi: 10.1074/jbc.274.35.24930. [DOI] [PubMed] [Google Scholar]
- 105.Alexander JS, Elrod JW. Extracellular matrix, junctional integrity and matrix metalloproteinase interactions in endothelial permeability regulation. J Anat. 2002;200:561–574. doi: 10.1046/j.1469-7580.2002.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miyamori H, Takino T, Kobayashi Y, Tokai H, Itoh Y, Seiki M, Sato H. Claudin promotes activation of pro-matrix metalloproteinase-2 mediated by membrane-type matrix metalloproteinases. J Biol Chem. 2001;276:28204–28211. doi: 10.1074/jbc.M103083200. [DOI] [PubMed] [Google Scholar]
- 107.Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 108.Del Maschio A, De Luigi A, Martin-Padura I, Brockhaus M, Bartfai T, Fruscella P, Adorini L, Martino G, Furlan R, De Simoni MG, Dejana E. Leukocyte recruitment in the cerebrospinal fluid of mice with experimental meningitis is inhibited by an antibody to junctional adhesion molecule (JAM) J Exp Med. 1999;190:1351–1356. doi: 10.1084/jem.190.9.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ostermann G, Weber KSC, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the β (2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;3:151–158. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- 110.Bazzoni G, Martinez-Estrada OM, Mueller F, Nelboeck P, Schmid G, Bartfai T, Dejana E, Brockhaus M. Homophilic interaction of junctional adhesion molecule. J Biol Chem. 2000;275:30970–30976. doi: 10.1074/jbc.M003946200. [DOI] [PubMed] [Google Scholar]
- 111.Itoh M, Sasaki H, Furuse M, Ozaki H, Kita T, Tsukita S. Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J Cell Biol. 2001;154:491– 497. doi: 10.1083/jcb.200103047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martinez-Estrada OM, Villa A, Breviario F, Orsenigo F, Dejana E, Bazzoni G. Association of junctional adhesion molecule with calcium/calmodulin-dependent serine protein kinase (CASK/LIN-2) in human epithelial caco-2 cells. J Biol Chem. 2001;276:9291–9296. doi: 10.1074/jbc.M006991200. [DOI] [PubMed] [Google Scholar]
- 113.Johnson-Leger CA, Aurrand-Lions M, Beltraminelli N, Fasel N, Imhof BA. Junctional adhesion molecule-2 (JAM-2) promotes lymphocyte transendothelial migration. Blood. 2002;100:2479–2486. doi: 10.1182/blood-2001-11-0098. [DOI] [PubMed] [Google Scholar]
- 114.Navarro P, Ruco L, Dejana E. Differential localization of VE- and N-cadherins in human endothelial cells: VE-cadherin competes with N-cadherin for junctional localization. J Cell Biol. 1998;140:1475–1484. doi: 10.1083/jcb.140.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Allport JR, Ding H, Collins T, Gerritsen ME, Luscinskas FW. Endothelial-dependent mechanisms regulate leukocyte transmigration: a process involving the proteasome and disruption of the vascular endothelial-cadherin complex at endothelial cell-to-cell junctions. J Exp Med. 1997;186:517–527. doi: 10.1084/jem.186.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hordijk PL, Anthony E, Mul FP, Rientsma R, Oomen LC, Roos D. Vascular-endothelial-cadherin modulates endothelial monolayer permeability. J Cell Sci. 1999;112:1915–1923. doi: 10.1242/jcs.112.12.1915. [DOI] [PubMed] [Google Scholar]
- 117.Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, McDonald DM, Ward PA, Dejana E. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci USA. 1999;96:9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Boggon TJ, Murray J, Chappuis-Flament S, Wong E, Gumbiner BM, Shapiro L. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 2002;296:1308–1313. doi: 10.1126/science.1071559. [DOI] [PubMed] [Google Scholar]
- 119.Hermant B, Bibert S, Concord E, Dublet B, Weidenhaupt M, Vernet T, Gulino-Debrac D. Identification of proteases involved in the proteolysis of vascular endothelium cadherin during neutrophil transmigration. J Biol Chem. 2003;278:14002–14012. doi: 10.1074/jbc.M300351200. [DOI] [PubMed] [Google Scholar]