Abstract
Aim:
To determine the prevalence of testosterone deficiency syndrome (TDS) in healthy Indian men employed in a hospital aged above 40 years.
Materials and Methods:
A general medical health check-up camp was organized for all male employees above 40 years age working in surgical departments. After clinical history and systemic inquiry, subjects were requested to fill the St. Louis University's ADAM Questionnaire based on which the total and free-serum testosterone estimation was then done.
Results:
One hundred fifty seven healthy volunteers enrolled for the study (mean age 53.1 years; range 40–60). The androgen decline in the aging male (ADAM) Questionnaire detected 106 men (67.5%) to be symptomatic for TDS. Serum testosterone estimation in these subjects revealed 41/106 to have low free-serum testosterone levels and 32/106 to have low total-serum testosterone. In 11 and 6 cases, respectively, the serum free- and total-testosterone levels were found to be low although the subjects were asymptomatic for TDS.
Conclusions:
The prevalence of symptomatic biochemical hypogonadism was 26.1%. The higher prevalence of symptoms alone of TDS was unusual. It could be because of the nature of the questionnaire. Free-serum testosterone may be a better single test to diagnose symptomatic hypogonadism than total-serum testosterone.
Keywords: Aging men, andropause, hypogonadism, India, prevalence
INTRODUCTION
Andropause, Androgen Decline in the Aging Male (ADAM), or testosterone deficiency syndrome (TDS) is a true clinical entity characterized by symptoms of erectile dysfunction, decreased libido, osteoporosis, and general weakness. However, its occurrence is still poorly documented and most literature is from European or American continents. Three questionnaires have been developed to detect TDS in adult men: (1) St. Louis University's ADAM; (2) the Aging Male Survey (AMS); and (3) Massachusetts Male Aging Study (MMAS) questionnaire. We report a study in a small number of men to determine the prevalence of TDS in healthy individuals above 40-years of age using the St. Louis University's ADAM questionnaire.
MATERIALS AND METHODS
In order to assess by a standardized questionnaire and obtain blood sample for serum testosterone estimation in working men population, a general health check-up camp was advertised and mandated for all male workers (surgical technicians, peons, and ward assistants with regular working duration not exceeding 8 h) between 40 and 60 years of age for the Departments of Surgical Sciences comprising of the Departments of General Surgery, Department of Urology, Department of Plastic Surgery, Department of Neurosurgery, Department of Plastic Surgery, Department of Pediatric Surgery, and Department of Cardiothoracic Surgery. Ethical clearance for the study was obtained from the institutional ethics committee and in accordance with the Declaration of Helsinki. This camp was organized for a one-week period in April 2006. All participants were asked to fill a proforma in which the volunteers were asked to fill personal information like age, occupation, co-morbid conditions, drug intake, and past illnesses. After physical examination, some investigations like urine examination, hemogram, serum creatinine estimation, liver function tests, electrocardiogram, and ultrasonography of the abdomen were performed as part of the free camp on all volunteers. All the participants were also invited to participate in a study on andropause after signing an informed consent. We had an interviewer-administered vernacular version of the St. Louis University's ADAMs Questionnaire [Table 1]. Serum testosterone (total and free) level estimation was performed simultaneously using ELISA (DRG International, Inc., USA. Fax: (908)233 0758, e-mail: corp@drg-international.com) in the Department of Pathology, the sample for which was withdrawn between 8 a.m. and 11 a.m. All tests were performed in duplicate and quality assessment was done with the help of a third party quality control sera. Within-assay variability was determined and was found to be 6%. To calculate the sample size, the prevalence of TDS was taken as 10%. To detect this prevalence in Indian men with a precision of 5% and alfa level of 0.05, the study included approximately 138 cases. Univariate analysis was done to assess the distribution of baseline variables. To assess association of TDS or low-serum testosterone levels, Chi-square test and t-test was used for categorical and continuous variables, respectively.
Table 1.
The androgen deficiency in aging male Questionnaire: A positive answer represents yes to question 1 or question 7 or any 3 other questions
| Do you have a decrease in libido? | Yes/No |
| Do you have a lack of energy? | Yes/ No |
| Do you have a decrease in strength and/or endurance? | Yes/ No |
| Have you lost height? | Yes/ No |
| Have you noticed a decreased enjoyment of life? | Yes/ No |
| Are you sad and/or grumpy? | Yes/ No |
| Are your erections less strong? | Yes/ No |
| Have you noticed a recent deterioration in your ability to play sports? | Yes/ No |
| Are you falling asleep after dinner? | Yes/ No |
| Has there been a recent deterioration in your work performance? | Yes/ No |
RESULTS
Of the 180 listed male workers between ages 40 and 60 years, 170 participated in the study. Of these, 157 agreed to participate in the TDS study. The mean age was 53.1 years (n = 157; range 40–60). Twenty-three volunteers had other co-morbid conditions like diabetes (12 cases), hypertension (12 cases), and tuberculosis (1 case). None of the volunteers were on drugs that could affect the serum testosterone levels. The mean body weight was 64.3 kg (range 54–78) and the mean height was 167.4 cm (range 158–180 cm). On the basis of St. Louis Questionnaire, of the 157 subjects, 106 (67.5%) tested positive for symptoms of TDS (mean age 53.5 years; range 40–60). Of these 106 symptomatic cases, 41 and 32 subjects were found to have less than normal serum-free (mean serum-free testosterone 5.55 pg/ml; range 3.09–7.08) and total testosterone (mean serum-total testosterone 1.5 ng/ml; range 1.1–2.5) levels. The other 65 symptomatic subjects had normal total-and free-testosterone levels (mean age 51.8 years, range 40–60). Eleven asymptomatic subjects (mean age 55.1 years; range 46–60) were found to have low free-serum testosterone levels (6.4 pg/ml; range 4.1–7.4) and in 6 of these cases low total-serum testosterone was identified (mean 1.4 ng/ml; range 1.2–2.5). The risk of TDS was 70% significantly lower in the age group of 40–50 years as compared to 51–60 years (RR = 0.3, 95% CI = 0.1–0.8). The risk of TDS was 2.9 times higher in those having at least one symptom as compared to those not having any symptom (RR = 2.9, 95% CI = 1.8–4.6) and this was statistically significant. The response to various questions in men with biochemically confirmed TDS in shown in Table 2. The association of different variables between andropause and non-andropause patients is shown in Table 3 and Table 4. A scatter plot for free-serum testosterone levels is shown in Figure 1 and bar diagram of serum free testosterone [Figure 2].
Table 2.
Responses according to andropause/Non-andropause
| Quisstion No. | Low testosterone | Normal testosterone | Chi-square | P value | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| 1 | Yes | 32 | 78.0 | 19 | 16.4 | 52.53 | 0.000* |
| No | 9 | 22.0 | 97 | 83.6 | |||
| 2 | Yes | 22 | 53.7 | 27 | 23.3 | 13.03 | 0.000* |
| No | 19 | 46.3 | 89 | 76.7 | |||
| 3 | Yes | 33 | 80.5 | 21 | 18.1 | 52.25 | 0.000* |
| No | 8 | 19.5 | 95 | 81.9 | |||
| 4 | Yes | 7 | 17.1 | 9 | 7.8 | 2.94 | 0.23 |
| No | 24 | 58.5 | 78 | 67.2 | |||
| Could not understand | 10 | 24.4 | 29 | 25.0 | |||
| 5 | Yes | 0 | 0.0 | 18 | 15.5 | 8.67 | 0.01* |
| No | 39 | 95.1 | 87 | 75.0 | |||
| Could not understand | 2 | 4.9 | 11 | 9.5 | |||
| 6 | Yes | 8 | 19.5 | 11 | 9.5 | 2.87 | 0.09 |
| No | 33 | 80.5 | 105 | 90.5 | |||
| 7 | Yes | 34 | 82.9 | 36 | 31.0 | 33.02 | 0.000* |
| No | 7 | 17.1 | 80 | 69.0 | |||
| 8 | Yes | 0 | 0.0 | 17 | 14.7 | 6.74 | 0.02* |
| No | 37 | 90.2 | 89 | 76.7 | |||
| Could not understand | 4 | 9.8 | 10 | 8.6 | |||
| 9 | Yes | 0 | 0.0 | 6 | 5.2 | 8.19 | 0.02* |
| No | 35 | 85.4 | 106 | 91.4 | |||
| Could not understand | 6 | 14.6 | 4 | 3.4 | |||
| 10 | Yes | 1 | 2.4 | 2 | 1.7 | 1.15 | 0.56 |
| No | 40 | 97.6 | 111 | 95.7 | |||
| Could not understand | 0 | 0.0 | 3 | 2.6 | |||
Significant
Table 3.
Association between different variables with andropause and non-andropause patients
| Variable | Andropause | Non-andropause | RR (95%CI) |
| Age | |||
| 40-50 | 5/47 (10.6) | 42/47 (89.4) | 0.3(0.1-0.8) |
| 51-60 | 36/110(32.7) | 74/110(67.3) | |
| Type of disease | |||
| Diabetic | 8/12 (66.7) | 4/12 (33.3) | 1.3(0.7-2.7) |
| Hypertensive | 6/12 (50.0) | 6/12 (50.0) |
*Significant, values in the parentheses are the percentage, RR = relative risk, CI = confidence interval
Table 4.
Mean ± SD of height, weight, and BMI of studied patients
| Variable | Andropause (n = 41) | Non-andropause (n = 116) | t-value | P value |
| Height | 166.5 ± 15.19 | 167.72 ± 5.49 | 1.23 | 0.22 |
| Weight | 66.44 ± 6.57 | 63.58 ±6.89 | 2.31 | 0.02* |
| BMI | 24.03 ±2.77 | 22.68 ±2.95 | 2.55 | 0.01* |
Figure 1.
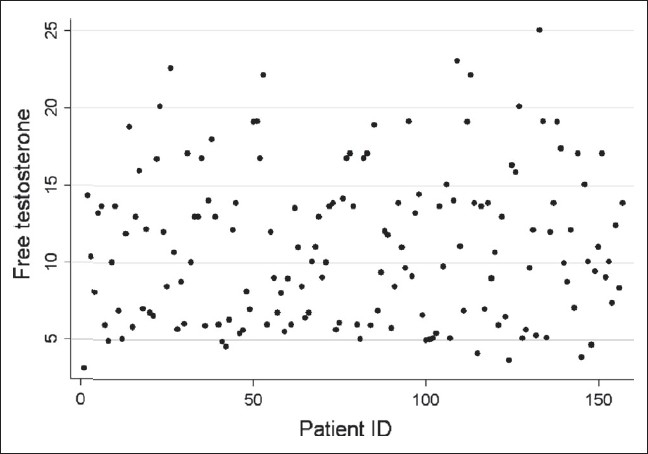
Scatter plot of free testosterone for each patient
Figure 2.
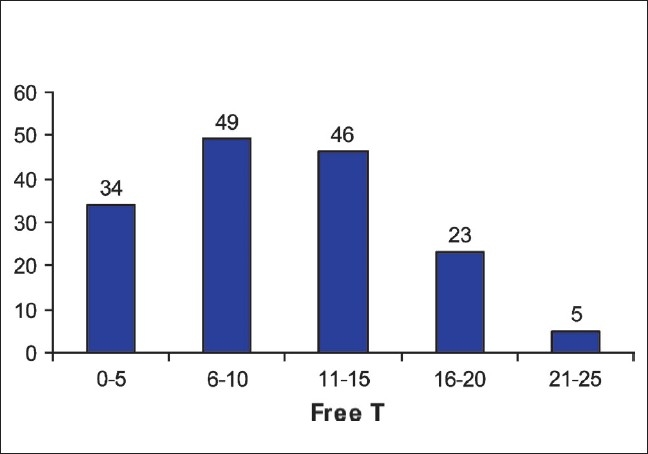
Bar diagram of serum free testosterone
DISCUSSION
The exact prevalence of TDS is not known. As the life span of humans is increasing, the prevalence of this condition is also on the rise. The Massachusetts Male Aging Study reported a crude incidence rate of 12.3 per 1000 person-years, leading to a prevalence of 481 000 new cases of ADAM per year in American men aged between 40 and 69 years.[1] The prevalence of TDS has not been reported from most Asian countries.[2–4] To estimate the prevalence of this condition in India, we performed a pilot study in a small number of healthy volunteers working in the surgical departments of our hospital. The volunteers were asked to attend a free health checkup camp and before attending the camp they did not even know that they will be evaluated for TDS too. However, when they reported for the checkup, they were invited to participate in TDS study. In this way, we were able to avoid the bias that would have appeared had we advertised that it will be an ‘andrology’ checkup camp. So the chances of selection bias that volunteers with some sexual problems would attend the camp were minimized.
We used the simpler St. Louis University's questionnaire, the validity of which has been tested and reported previously.[5–7] The ADAM questionnaire in the original validation had a sensitivity and specificity of 88 and 60%, respectively, against the serum bioavailable testosterone levels.[5] The sensitivity and specificity of AMS has been reported to be 83 and 39%, respectively;[8] however, in another study from Europe none of the three AMS domains correlated significantly with serum levels of total, bioavailable, or free testosterone in men older than 70 years.[9] The MMAS has shown a sensitivity and specificity of 60 and 59%, respectively. These questionnaires are useful as screening tools but fall short of expectation for diagnostic purposes.[10] Morley et al., compared the ADAM, AMS, and MMAS questionnaires using bioavailable testosterone as the ‘biochemical gold standard’ for diagnosis of hypogonadism. The sensitivity for ADAM, AMS, and MMAS was found to be 97, 83, and 60%, respectively and the specificity for ADAM, AMS, and MMAS was 30, 39, and 59%, respectively.[11]
In our study, an unusually high number (67.5%) of volunteers reported symptoms of ADAM on the basis of St. Louis University's Questionnaire. This could be because of the nature of the questionnaire. The questionnaire was structured on a ‘Yes/No’ format and the volunteers did not have the scope of saying that they had only mild symptoms. Many volunteers who had mild symptoms could have been picked up as symptomatic for androgen deficiency on the basis of this questionnaire. Of interest, 58.5, 95, 80.5, 90, 85.4, and 97.6% of men gave a ‘No’ answer to questions number 4, 5, 6, 8, 9, and 10, respectively. For possible explanation of this finding, a larger study in a more heterogenous group of people is required. Some questions like ‘Have you noticed a recent deterioration in your ability to play sports?’ may not be relevant in some countries like India where most people above 40-years of age do not play sports. Similarly, many of our volunteers reported that answers to questions 2, 3, 8, and 10 were quite close to each other [Table 1]. Some investigators have, therefore, developed shorter versions of ADAM questionnaire.[12,13]
Due to the low specificity of the St. Louis questionnaire, a substantial number of men tested positive for andropause symptoms based on this questionnaire but did have low-serum androgens. It is therefore likely that the population of men with symptoms of TDS but who did not meet the criteria for the diagnosis had some other medical or psychological co-morbidity that was contributing to the symptoms.
On the basis of biochemical evaluation, 52 subjects (33.1%) were found to have low free-serum testosterone levels. However, the total-serum testosterone levels were found to be low in 32 subjects only. This is because the normal total-serum testosterone levels have a wide normal range and do not decline as rapidly as do free and bioavailable (free and albumin-bound) testosterone.[14–17] The more pronounced decrease in free than in total testosterone is explained by the age-dependent increase in the binding capacity of SHBG (1.2% per year). Many investigators recommend measurement of bioavailable or free testosterone as a better predictor for diagnosing andropause.[18,19] The ideal test in men suspected of hypogonadism is the measurement of free testosterone by the equilibrium dialysis method.[19] This method, however, is difficult to perform, not automated, and largely inaccessible to most clinicians. Measurement of ‘free testosterone’ by radioimmunoassay is widely available but is unreliable.[19] We have evaluated serum testosterone levels using the ELISA, which is a reliable method.[20]
The reported prevalence of biochemical hypogonadism is about 7% of the age group less than 60 years old but increases to 20% in those over 60 years of age.[21] Our study in a small group of healthy men revealed symptoms of TDS in 67.5% and biochemical hypogonadism in 33.1%. However, symptomatic hypogonadism was seen in only 26.1% (in 41 out of 157 cases). The prevalence of biochemical hypogonadism found in our study (26.1%) is similar to that found in a study of 316 Canadian physicians aged 40–62 years. Low bioavailable testosterone levels (< 70 ng/dl) were present in 25% of these men; the questionnaire identified this group with a sensitivity of 88% and a specificity of 60%.[5] However, our incidence is much higher than prevalence of 6–9.5% reported for community-dwelling men aged 40–70 years from France.[22]
Serum testosterone levels show diurnal variation and there is also substantial variation (~20%) from week-to-week.[23] Therefore, two testosterone measurements at least a week or two apart are recommended for diagnosing TDS and starting treatment. We measured serum testosterone at a single point which may have overestimated or underestimated this condition. The relationship between symptoms of TDS and hormonal levels in complex are still not fully understood and there is a lack of correlation between the clinical picture and the most commonly used biochemical confirmatory tests.[24] Additional questionnaires (CES-D, Beck Depression Inventory, etc) for depression and additional biochemical data (like thyroid hormone levels) that may influence the response to ADAM questionnaire were not done. However, no patient had clinical manifestation of hypo- or hyperthyroidism. The population selected for this study was men working in hospital and is unlikely to be a representative sample of the general male population in India. Another study using other validated questionnaires such as the AMS scale or MMAS and more detailed measurement of testosterone levels in a larger number of men is required to determine the prevalence of this condition in Indian men.
CONCLUSION
Symptomatic hypogonadism was found to be at least as common as that reported in the Western literature. The higher prevalence of patients with symptoms of hypogonadism but with normal serum testosterone found in our patients could be because of the nature of the ADAM questionnaire. Free-serum testosterone levels may be a better single test to diagnose TDS.
Acknowledgments
This study was supported by a research grant given by Research Cell, King George's Medical University, Lucknow, India.
Footnotes
Source of Support: Research grant given by Research Cell, King George's Medical University, Lucknow, India.
Conflict of Interest: None declared.
REFERENCES
- 1.Araujo AB, O'Donnell AB, Brambilla DJ, Simpson WB, Longcope C, Matsumoto AM, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: Estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metabol. 2004;89:5920–6. doi: 10.1210/jc.2003-031719. [DOI] [PubMed] [Google Scholar]
- 2.Li JY, Li XY, Li IM, Zhang GK, Ma FL, Liu ZM, et al. Decline of serum levels of free testosterone in aging healthy Chinese men. Aging Male. 2005;8:203–6. doi: 10.1080/13685530500356010. [DOI] [PubMed] [Google Scholar]
- 3.Reyes JA, 3rd, Tan DA, Quimpo JA, Tan-Garcia J, Garcia LA, Gonzaga FP, et al. The Philippine male aging survey. Aging Male. 2004;7:227–35. doi: 10.1080/13685530400004116. [DOI] [PubMed] [Google Scholar]
- 4.Ichioka K, Nishiyama H, Yoshimura K, Itoh N, Okubo K, Terai A. Aging Males’ Symptoms scale in Japanese men attending a multiphasic health screening clinic. Urology. 2006;67:589–93. doi: 10.1016/j.urology.2005.09.057. [DOI] [PubMed] [Google Scholar]
- 5.Morley JE, Charlton E, Patrick P, Kaiser FE, Cadeau P, McCready D, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism. 2000;49:1239–42. doi: 10.1053/meta.2000.8625. [DOI] [PubMed] [Google Scholar]
- 6.Tancredi A, Reginster JY, Schleich F, Pire G, Maassen P, Luyckx F, et al. Interest of the androgen deficiency in aging males (ADAM) questionnaire for the identification of hypogonadism in elderly community-dwelling male volunteers. Eur J Endocrinol. 2004;151:355–60. doi: 10.1530/eje.0.1510355. [DOI] [PubMed] [Google Scholar]
- 7.Morales A, Spevack M, Emerson L, Kuzmarov I, Casey R, Black A, et al. Adding to the controversy: Pitfalls in the diagnosis of testosterone deficiency syndromes with questionnaires and biochemistry. Aging Male. 2007;10:57–65. doi: 10.1080/13685530701342686. [DOI] [PubMed] [Google Scholar]
- 8.Moore C, Huebler D, Zimmermann T, Heinemann LA, Saad F, Thai DM. The Aging Male Symptom Scale (AMS) as outcome measure for treatments of androgen deficiency. Eur Urol. 2004;46:80–7. doi: 10.1016/j.eururo.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 9.T'sjoen G, Goemaere S, De Meyere M, Kaufman JM. Perception of male aging symptoms, health and well being in elderly community-dwelling men is not related to circulating androgen levels. Psychoneuroendocrinology. 2004;29:201–14. doi: 10.1016/s0306-4530(03)00023-4. [DOI] [PubMed] [Google Scholar]
- 10.Morales A, Morley J, Heaton JP. In: Androgen deficiency in the aging male. Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Saunders Elsevier: Philadelphia; 2007. pp. p. 850–62. Campbell-Walsh Urology. Vol 1. [Google Scholar]
- 11.Morley JE, Perry HM, 3rd, Kevorkian RT, Patrick P. Comparison of screening questionnaires for the diagnosis of hypogonadism. Maturitas. 2006;53:424–9. doi: 10.1016/j.maturitas.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Chu LW, Tam S, Kung AW, Lam TP, Lee A, Wong RL, et al. A shorter version of the ADAM questionnaire for androgen deficiency in Chinese men. J Gerontol A Biol Sci Med Sci. 2008;63:426–31. doi: 10.1093/gerona/63.4.426. [DOI] [PubMed] [Google Scholar]
- 13.Tancredi A, Legros JJ, Pire G, Maassen P, Luyckx F, Reginster JY. Analysis of the discrimnant ability of shorter versions of the French ADAM questionnaire. Aging Male. 2007;10:159–64. doi: 10.1080/13685530701433121. [DOI] [PubMed] [Google Scholar]
- 14.Vermeulen A. Androgens in the aging male. J Clin Endocrinol Metab. 1991;73:221–4. doi: 10.1210/jcem-73-2-221. [DOI] [PubMed] [Google Scholar]
- 15.Ferrini RL, Barrett-Connor E. Sex hormones and age: A cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol. 1998;147:750–4. doi: 10.1093/oxfordjournals.aje.a009519. [DOI] [PubMed] [Google Scholar]
- 16.Morley JE, Kaiser F, Raum WJ, Perry HM, 3rd, Flood JF, Jensen J. Potentially predictive and manipulable blood stream correlates of ageing in the healthy human male: Progressive decreases in bioavailable testosterone, dehydroepiandrosterone sulfate, and the ratio of insulin- like growth factor 1 to growth hormone. Proc Natl Acad Sci U S A. 1997;94:7537–42. doi: 10.1073/pnas.94.14.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nahoul K, Roger M. Age related decline of plasma bioavailable testosterone in adult men. J Steroid Biochem. 1990;35:293–9. doi: 10.1016/0022-4731(90)90287-3. [DOI] [PubMed] [Google Scholar]
- 18.Tenover JS. Declining testicular function in aging men. Int J Impot Res. 2003;15:S3–8. doi: 10.1038/sj.ijir.3901029. [DOI] [PubMed] [Google Scholar]
- 19.Morales A, Lunenfeld B. International Society for the Study of the Aging Male. Investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male. 2002;5:74–86. [PubMed] [Google Scholar]
- 20.Tietz NW. Textbook of chemical chemistry. Philadelphia: Saunders; 1986. [Google Scholar]
- 21.Vermeulen A, Kaufman JM. Aging of the hypothalamo-pituitary-testicular axis in men. Horm Res. 1995;43:25–8. doi: 10.1159/000184233. [DOI] [PubMed] [Google Scholar]
- 22.Tostain JL, Blanc F. Testosterone deficiency: A common, unrecognized syndrome. Nat Clin Pract Urol. 2008;5:388–96. doi: 10.1038/ncpuro1167. [DOI] [PubMed] [Google Scholar]
- 23.Morley JE, Patrick P, Perry HM., 3rd Evaluation of assays available to measure free testosterone. Metabolism. 2002;51:554–9. doi: 10.1053/meta.2002.31975. [DOI] [PubMed] [Google Scholar]
- 24.Morales A, Spevack M, Emerson L, Kuzmarov I, Casey R, Black A, Tremblay R. Adding to the controversy: Pitfalls in the diagnosis of testosterone deficiency syndromes with questionnaires and biochemistry. Aging Male. 2007;10:57–65. doi: 10.1080/13685530701342686. [DOI] [PubMed] [Google Scholar]


