Abstract
Purpose:
To determine the impact of age and gender on the clinicopathological characteristics of histologically confirmed bladder cancer in India.
Materials and Methods:
From January 2001 to June 2008, records of patients with bladder cancer were evaluated for age and gender at presentation, clinical symptoms, cystoscopic finding, history of smoking, and histopathological characteristics. A total of 561 patients were identified from the computer-based hospital information system and the case files of patients.
Results:
A total of 97% of the patients presented with painless hematuria. The mean age was 60.2 ± 4.4 years old (range: 18–90 years old) and the male to female ratio was 8.6:1. Transitional cell carcinoma (TCC) was the most common histological variety, which was present in 97.71% (470 of 481) of the patients. A total of 26% of the patients had muscle invasive disease at the time of presentation. However, 34.5% (166 of 481) of the patients did not show any evidence of detrusor muscle in their biopsy specimen. In patients with nonmuscle-invasive bladder carcinoma, 55% had p Ta while 45% had p T1. Overall, 44.7% (215 of 481) of the patients had low-grade disease. Among patients younger than 60 years old, low-grade (51.0% vs. 38.1%; P = 0.006) and low-stage (77.1% vs. 70.8%; P = 0.119) disease were more prevalent than in patients older than 60 years old. The incidence of smoking was much higher among males compared with females (74% vs. 22%).
Conclusion:
TCC is the predominant cancer, with significant male preponderance among Indian patients. Younger-aged patients have low-grade disease. Hematuria is the most common presentation and greater awareness is needed not to overlook bladder cancer.
Keywords: Age, bladder cancer, gender, smoking, transitional cell carcinoma
INTRODUCTION
Bladder cancer is one of the most common urological malignancies. As per the Indian cancer registry data in men, it is the ninth most common cancer accounting for 3.9% of all cancer cases.[1] The median age at diagnosis was 69 years old for males and 71 years old for females.[2] It is three times more common in men than in women, and 90% of the bladder tumors are transitional cell carcinoma (TCC).[3] Age, gender, and racial factors all affect the survival and prognosis of patients with bladder cancer.[4] A total of 40 to 45% of newly diagnosed bladder cancers are high-grade lesions, more than half of which are muscle invasive at the time of diagnosis.[5] To our knowledge, no large series has been presented to describe the demographic data of bladder cancer in India. Herein, we present the clinicopathological characteristics of bladder cancer with an analysis of the impact of age and gender on tumor biology.
MATERIALS AND METHODS
Patient population
From January 2001 to June 2008, data from all the patients with bladder cancer entered on the hospital information system and in the case record files were evaluated for age, gender, symptoms, history of smoking, and histopathological characteristics at the time of presentation. A total of 561 patients presented with bladder cancer and complete records were available for 481 patients, which formed the study group.
Treatment
When bladder cancer was identified on a cystoscopy, the location, number, and nature of the disease were recorded. As most of the patients were referred to us with the diagnosis, there was no consistent record on urine cytology. A transurethral resection of the bladder tumor (TURBT) was performed using Glycine (1.5%) as an irrigant. A deep biopsy was taken separately to include the detrusor muscle. A random biopsy was not performed as per the protocol and only suspicious lesions were biopsied. None of the patients had repeat a TURBT.
Pathology
The new World Health Organization (WHO) and International Society of Urological Pathology (ISUP) classifications were used for pathological grading. Data were recorded as Ta for papillary, urothelial-confined carcinoma, T1 for lamina-invasive carcinoma, and T2 for muscle-invasive carcinoma. In this study, for the purpose of statistical analysis, Grade 1 and Grade 2 were classified as low-grade and Grade 3 was classified as high-grade. The biopsy specimen in which there was no evidence of detrusor muscle was recorded separately. Data were analysed using SPSS software, Version 11.5 (SPSS, Chicago, IL, USA) and the χ2 test was used to compare the variables. A P-value of less than 0.05 was taken as significant.
RESULTS
Incidence
Of the 481 patients, 97% presented with painless hematuria. The next most common symptoms were dysuria and frequency. TCC was the most common variant seen in 97.71% of the patients [Table 1]. Twenty-six percent of the patients had muscle-invasive disease at the time of presentation while the remaining 74% had nonmuscle- invasive bladder carcinoma in which 40.3% had p Ta and 33.7% had p T1. Overall, 44.7% of the patients had low-grade disease. Detrusor muscle was not evident in 34.5% of the patients in their biopsy specimen.
Table 1.
Impact of age on tumor grade and stage
| <60 yrs (%) | >60 yrs (%) | P-value | |
|---|---|---|---|
| Number of patients | 245 | 236 | |
| Smokers | 170 (69.4) | 160 (67.8) | |
| Tumor histology | |||
| Transitional cell | 236 | 234 | |
| Squamous cell | 3 | 2 | |
| Adenocarcinoma | 6 | 0 | |
| Grade | |||
| Low | 125 (51.0) | 90 (38.1) | 0.006 |
| High | 120 (49.0) | 146 (61.9) | |
| Stage | |||
| Nonmuscle invasive (Ta + T1) | 189 (77.1) | 167 (70.8) | 0.119 |
| Ta | 102 (41.6) | 92 (39.0) | |
| T1 | 87 (35.5) | 75 (31.8) | |
| Invasive (T2) | 56 (22.9) | 69 (29.2) |
Age and gender
The median age at presentation was 60 years old (range: 18–90 years old) and the average age was 60.2 ± 4.4 yrs. The age-wise pathological distribution of bladder tumors are shown in Table 1. Only 5.19% (25 of 481) of the patients presented with bladder cancer at younger than 40 years of age while only 2 patients presented with less than 20 years of age [Figure 1]. Low-grade cancer was found more commonly in patients younger than 60 years old as compared with patients older than 60 years old (51.0% vs. 38.1%; P-value = 0.006). Although not statistically significant, nonmuscle- invasive bladder tumors, i.e., Ta and T1 were relatively more common in the younger age group (41.6% vs. 39.0% and 35.5% vs. 31.8%, respectively) and muscle-invasive tumors were more common in the group of patients who were older than 60 years old (29.2% vs. 22.9%; P = 0.119). The male to female ratio was 8.6:1. A total of 74% of the males and 22% of the females with bladder cancer smoked or had an intake of tobacco in some form. The incidence of various cancer stages and grades was not statistically different among both the gender groups. The pathological distribution of bladder cancer in males and females is shown in Table 2.
Figure 1.
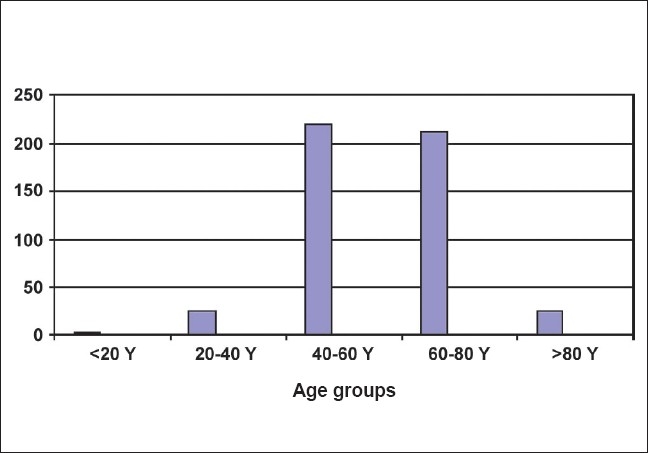
Age distribution of bladder tumors
Table 2.
Impact of gender on tumor grade and stage
| Male (%) | Female (%) | P-value | |
|---|---|---|---|
| Number of patients | 431 | 50 | |
| Smokers | 319 (74.0) | 11 (22.0) | |
| Grade | |||
| Low | 191 (44.3) | 29 (58.0) | 0.073 |
| High | 240 (55.7) | 21 (42.0) | |
| Stage | |||
| Nonmuscle invasive (Ta + T1) | 317 (73.5) | 39 (78.0) | |
| Ta | 172 (39.9) | 23 (46.0) | |
| T1 | 145 (33.6) | 16 (32.0) | |
| Invasive (T2) | 114 (26.5) | 11 (22.0) | 0.503 |
Treatment
Of the 481 patients, 199 patients were in the high-risk category, which included TaHG, T1LG, and T1HG. Of the 199 patients, 135 patients received Bacillus Calmette-Guérin (BCG) as induction therapy. A total of 19 patients received 40 mg, 48 patients received 80 mg, and 68 patients received 120 mg of BCG. Each patient received BCG weekly for 6 weeks. A significantly lower number of patients developed toxicity in the 40-mg arm. On Kaplan Meier analysis, time to recurrence and time to progression was not statistically significant in the three arms.
Of the 125 patients with muscle-invasive bladder cancer, 86 patients underwent a radical cystectomy and standard pelvic lymph node dissection with urinary diversion. Of the 86 patients, 50 patients had orthotopic neobladder while the remaining 36 underwent illeal conduit. A total of 18 patients died during the mean follow-up of 63 months (5–90 months).
DISCUSSION
Bladder cancer presents with painless hematuria in 80–85% of the patients. However, in reality, nearly all patients with cystoscopically detectable bladder cancer have at least microhematuria if enough urine samples are tested. In our study, 97.0% of the patients presented with gross hematuria. This high incidence may be due to a lack of screening for microscopic hematuria in the form of dipstick or flexible cystoscopy, even in high-volume centers.
TCC is the most common variant accounting for 90% of bladder cancer in the world literature.[3] Considerable variability is noted in the prevalence of squamous cell carcinoma (SCC) of the bladder in different parts of the world. SCC accounts for only 1% of bladder cancers in England, 3–7% in the United States, but as much as 75% in Egypt. Adenocarcinoma (AC) accounts for less than 2% of bladder cancer.[2] In our study, 97.71% of the patients had TCC, whereas SCC and AC accounted for 1.04% and 1.25% of the patients, respectively.
Cigarette smokers have at least a four times higher incidence of bladder cancer.[6,7] This risk has been observed in both the genders. Former cigarette smokers have a reduced incidence of bladder cancer compared with active smokers.[8] When comparing p53 mutations in the bladder cancer of smokers with those in the bladder cancer of patients who have never smoked, differences in the types or sites of mutations have not been seen, although a higher number of mutations occurred in smokers. This suggests that smoking might increase the amount of mutations in urothelial cells without necessarily directing the site or type of mutation.[9] In our study, 68.6% (330 of 481) of the patients were smokers. In one series reported on bladder cancer, the percentage of male smokers was 50% and 31% for female smokers.[10] Contrary to this, the incidence of smoking or intake of tobacco in any form in our patients with bladder cancer was much higher among males compared with females (74% vs. 22%). Bladder cancer is rare in people younger than 50 years of age, even though it can occur at any age. The incidence of cancer increases directly with age with the median age at diagnosis of around 70 years for each gender.[2] In our study, the median age was 60 years old.
Younger individuals present more frequently with low-grade and low-stage tumors than their elderly counterparts[11] and behave in an indolent fashion.[12] The same has been observed in our study. This is contrary to the common belief in malignancy that biological behavior of a cancer is more aggressive in younger age groups. This fact is underplayed in the literature and research should be carried out to find out the reasons for this. However, it has been observed that genetic alterations that are frequently seen in older adults are extremely rare in young patients. Urothelial neoplasms in children and young adults appear to be biologically distinct and lack genetic instability in most cases.[13]
In our study, the male to female ratio was 8.6:1, which is different from what is reported in the literature, i.e., 3:1–5:1.[14] A much higher incidence of tumors was detected in males compared with females. Possible explanations for the excessive risk in men include environmental and dietary exposures not yet identified and innate sexual characteristics such as anatomic differences, urination habits, or hormonal factors.[15,16] Compared with data available from the west, a disproportionate decrease of tumor incidences in females can be due to the decreased exposure to industrial carcinogens as fewer number of women work outside the home. It is also observed that there is a decreased exposure of females to tobacco intake, which has been the major predisposing factor for bladder cancer. Also, females tend to present to the hospital less because of social reasons.
In our series, 34.5% of the patients did not show evidence of detrusor muscle in their biopsy specimen. This is in concordance with the literature, in which the detrusor muscle was absent in 40–51% TUR specimens.[17] Not including the detrusor muscle in the biopsy specimen may lead to understaging in many patients.
CONCLUSION
In the Indian population, bladder cancer is predominantly TCC and more than 95% of the patients present with painless hematuria. Male preponderance is much more frequent in Indians than in other races. Younger patients present with low-grade disease for which further studies are required to understand this biological difference. Awareness is needed among the public and treating physicians as they tend to neglect the symptoms of hematuria, resulting in an advanced stage of bladder cancer at presentation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kurkure AP. Cancer incidence and patterns in urban Maharashtra. Consolidated report of the population based cancer registries. 2001 [Google Scholar]
- 2.Lynch CF, Cohen MB. Urinary system. Cancer. 1995;75:316–29. doi: 10.1002/1097-0142(19950101)75:1+<316::aid-cncr2820751314>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 3.Rabbani F, Cordon-Cardo C. Mutation of cell cycle regulators and their impact on superficial bladder cancer. Urol Clin North Am. 2000;27:83–102. doi: 10.1016/s0094-0143(05)70237-8. [DOI] [PubMed] [Google Scholar]
- 4.Madeb R, Messing EM. Gender, racial and age differences in bladder cancer incidence and mortality. Urol Oncol. 2004;22:86–92. doi: 10.1016/S1078-1439(03)00139-X. [DOI] [PubMed] [Google Scholar]
- 5.Messing EM, Young TB, Hunt VB, Gilchrist KW, Newton MA, Bram LL, et al. Comparison of bladder cancer outcome in men undergoing hematuria home screening versus those with standard clinical presentations. Urology. 1995;45:396–7. doi: 10.1016/s0090-4295(99)80006-5. [DOI] [PubMed] [Google Scholar]
- 6.Morrison AS. Advances in the etiology of urothelial cancer. Urol Clin North Am. 1984;11:557–66. [PubMed] [Google Scholar]
- 7.Burch JD, Rohan TE, Howe GR, Risch HA, Hill GB, Steele R, et al. Risk of bladder cancer by source and type of tobacco exposure: A case-control study. Int J Cancer. 1989;44:622–8. doi: 10.1002/ijc.2910440411. [DOI] [PubMed] [Google Scholar]
- 8.Augustine A, Hebert JR, Kabat GC, Wydner EL. Bladder cancer in relation to cigarette smoking. Cancer Res. 1988;48:4405–8. [PubMed] [Google Scholar]
- 9.Spruck 3rd CH, Rideout 3rd WM, Olumi AF, Ohneseit PF, Yang AS, Tsai YC, et al. Distinct pattern of p53 mutations in bladder cancer: Relationship to tobacco usage. Cancer Res. 1993;53:1162–6. [PubMed] [Google Scholar]
- 10.Wydner EL, Goldsmith K. The epidemiology of bladder cancer: A second look. Cancer. 1977;40:1246. doi: 10.1002/1097-0142(197709)40:3<1246::aid-cncr2820400340>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Wan J, Grosman HB. Bladder carcinoma in patients age 40 years or younger. Cancer. 1989;64:178–81. doi: 10.1002/1097-0142(19890701)64:1<178::aid-cncr2820640130>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 12.Linn JF, Sesterhann I, Mostofi FK, Schoenberg M. The molecular characteristics of bladder cancer in young patients. J Urol. 1998;159:1493–6. doi: 10.1097/00005392-199805000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Wild PJ, Giedl J, Stoehr R, Junker K, Boehm S, Van Oers JM, et al. none Genomic aberrations are rare in urothelial neoplasms of patients 19 years or younger. J Pathol. 2007;211:18–25. doi: 10.1002/path.2075. [DOI] [PubMed] [Google Scholar]
- 14.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 15.Hartge P, Harvey EB, Linehan WM, Silverman DT, Sullivan JW, Hoover RN, et al. Unexplained excess risk of bladder cancer in men. J Natl Cancer Inst. 1990;82:1636–40. doi: 10.1093/jnci/82.20.1636. [DOI] [PubMed] [Google Scholar]
- 16.Horn EP, Tucker MA, Lambert G, Silverman D, Zametkin D, Sinha R, et al. A study of gender-based cytochrome P450 1A2 variability: A possible mechanism for the male excess of bladder cancer. Cancer Epidemiol Biomarkers Prev. 1995;4:529–33. [PubMed] [Google Scholar]
- 17.Maruniak NA, Takezawa K, Murphy WM. Accurate pathological staging of urothelial neoplasms requires better cystoscopic sampling. J Urol. 2002;167:2404–7. [PubMed] [Google Scholar]


