Abstract
Five cases of small-cell carcinoma (SCC) of prostate were identified, at the Rhode Island Hospital and the Miriam Hospital from 1984 to 2006, with an average age of 71 years at the time of diagnosis. Three of these patients had a prior diagnosis of prostatic adenocarcinoma, with all of the five patients receiving anti-androgen treatment. The average time between the diagnosis of adenocarcinoma and of SCC in these patients was 6.7 years. The PSA levels varied greatly, with two patients possessing markedly elevated levels and the remaining patients with normal levels. Approximately 3/5 patients developed liver metastases, 2/5 patients had bone metastases, and 1/5 patients developed carcinomatous meningitis. Of the four patients who expired, the median survival time after diagnosis of SCC was 3.6 months (0.5–12 months).
Keywords: Neuroendocrine carcinoma, small cell carcinoma, prostatic neoplasm
INTRODUCTION
Small-cell (neuroendocrine) carcinoma (SCNC) is a rare malignancy of neuroendocrine cell lineage arising in the human prostate, with an estimated incidence of 0.5–2% of all primary prostatic tumors. [1] As of 1997, there have been only 150 reported cases of small cell prostate carcinoma. This particular variant is typically aggressive, often exhibiting distant visceral or nodal metastases[2] with a median survival time ranging from 6 to 17 months following diagnosis,[3] a considerably more dismal prognosis than its adenocarcinoma counterpart. In contrast to most prostate adenocarcinomas, the small-cell variant does not usually secrete prostate-specific antigen (PSA), often causes lytic bone lesions, and more frequently is complicated by visceral metastases.[4]
Attention has been drawn to this particular type of prostate carcinoma since it occurs primarily in patients who have been diagnosed with prostatic adenocarcinoma previously. The development of neuroendocrine carcinomas is thought to be secondary to resistance of the anti-androgen therapy; a therapy widely used and generally successful in treating most adenocarcinomas of the prostate. The chemotherapeutic (using etoposide and cisplatin chemotherapy) response on these particular carcinomas and of SCCs of the lung was found to be similar.[5]
Aiming to further characterize this rare disease, we examined the cases of small-cell prostatic carcinoma diagnosed at our institution in the last twenty years. The following five cases provide examples of the varied ways this malignancy may present and progress.
CASE REPORTS
The pathology archives of the Rhode Island Hospital and The Miriam Hospital were searched for the cases of small-cell prostatic carcinoma diagnosed by tissue biopsy. Of the cases identified, we reviewed the records of 10 patients who were diagnosed between 1984 and 2006. Clinical data, including presenting symptoms, laboratory values, treatment, and survival were recorded. Due to insufficient data representation in the patients' charts, four of the 10 initial cases identified were excluded so as to fully characterize the clinical courses. One further case was excluded due to the patient having had a previous diagnosis of SCC of the lung and being unable to determine if the patient's prostatic biopsy represented a primary prostatic SCC or metastasis from his lung carcinoma. The remaining five patients had their biopsy specimens reviewed, confirming the diagnosis of SCC. The clinical features of those cases were then analysed in detail.
Case 1
The patient was a 50-year-old male when he was diagnosed with prostatic adenocarcinoma with bone metastases. Initial treatment included orchiectomy and stillbestrol administration. One year after diagnosis, due to advancement of this disease in spite of orchiectomy, the patient was started on monthly intramuscular injections of leuprolide 7.5 mg and was maintained on that medication for the next 6 years. At the age of 57, he presented to the hospital with pelvic pain and was found to have recurrence of this disease. The patient was then treated with pelvic radiation, and was started on mitoxantrone and hydrocortisone. A subsequent prostatic biopsy revealed SCC admixed with a small component of typical prostatic adenocarcinoma. The immunohistochemistry confirmed the presence of neuroendocrine differentiation, but the tumor was negative for prostatic acid phosphates, calcitonin, and ACTH. A PSA level was markedly elevated (2180 ng/ml).
The patient was treated for his SCC with vincristine and estramustine as an outpatient with once weekly treatments. One month later, he was readmitted to the hospital with mental status changes, a lumbar puncture was performed, and the spinal fluid studies revealed meningeal carcinomatosis, for which he was treated with intrathecal methotrexate. He was also found to have a hypodense lesion of his left parietal lobe consistent with metastatic disease. He subsequently developed disseminated intravascular coagulation (DIC) and expired shortly thereafter.
Case 2
The patient was a 69-year-old male with a past medical history of hypertension and diabetes mellitus who presented to the hospital institution with urinary retention, acute renal failure (BUN, 101 mg/dl; creatinine, 8.5 mg/dl), and bilateral hydronephrosis. The PSA and the alkaline phosphatase level were 3205 ng/ml and 123 U/l, respectively. A bone scan showed diffuse uptake in the ribs and lumbar spine consistent with the metastatic disease. No biopsy was performed during this admission, and the patient was treated with leuprolide and flutamide for presumed prostatic adenocarcinoma.
Three months later, the patient was admitted with symptoms of volume overload and urinary retention. A prostatic biopsy sample revealed SCC of the prostate with no identification of typical prostatic adenocarcinoma. The tumor was positive for cytokeratins and chromogranin A, and was negative for PSA and prostatic acid phosphatase. The serum PSA level during this admission was 209 ng/ml. The patient was subsequently discharged with a Foley catheter in place, and received treatment with cyclophosphamide, vincristine, and carboplatin as an outpatient.
He was readmitted eight months later with pelvic pain. A CT of the abdomen and pelvis revealed a large pelvic soft tissue mass arising from the prostate causing ureteral obstruction, and a liver mass consistent with metastatic disease. A transurethral resection revealed mixed SCC and a poorly differentiated adenocarcinoma. He received pelvic radiation therapy following that admission, but represented two weeks later with lower extremity weakness. Due to his poor condition, he was deemed not to be a candidate for further treatment, and was subsequently discharged with hospice care. Of note, during the patient's last admission, his PSA level was undetectable.
Case 3
A 78-year-old male with a past medical history of coronary artery disease, peripheral vascular disease, and prostatic adenocarcinoma status post resection and adjuvant chemotherapy with leuprolide one year earlier was admitted to the hospital for a right-below-the-knee amputation due to complications of his vasculopathy. During the postoperative period, he developed acute urinary retention and subsequently a TURP procedure was performed with the pathology revealing high-grade SCC. The tumor was positive for chromogranin A and synaptophysin, while stains for prostate-specific antigen and prostate-specific acid phosphatase were negative.
The patient represented to the hospital one month later with abdominal pain, fevers, and elevated liver function tests. A CT of the abdomen and pelvis revealed a single iliac lymph node and irregularity of the bladder wall, thought to represent invasion from the primary prostatic carcinoma. There was no evidence of a metastatic disease involving the liver. He was started on antibiotics for the provisional treatment of suspected cholangitis, but his condition deteriorated and he subsequently expired during that admission. The patient's infection was felt unlikely to be related to his underlying malignancy.
Case 4
The patient was an 80-year-old male and his past medical history was significant for prostatic adenocarcinoma, diagnosed 12 years earlier [Figure 1]. He had been treated with external beam radiation, followed by a transurethral resection nine years later, and subsequently a bicalutamide treatment. The current admission was precipitated by multiple episodes of urinary obstruction and hematuria that started 10 days prior to admission.
Figure 1.
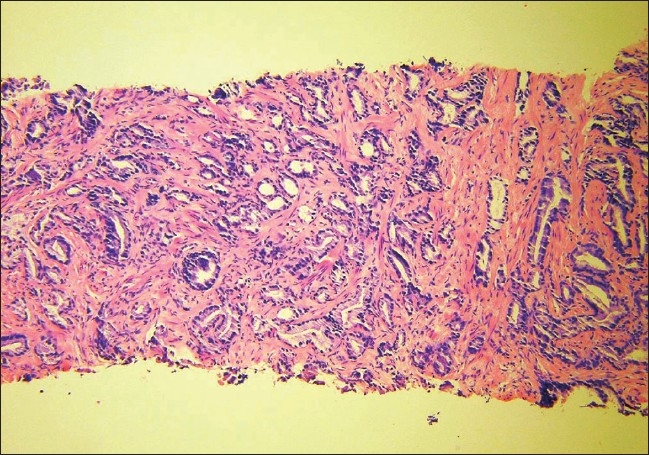
A 10× magnification H&E stain of the initial prostate biopsy of case 4 when he was diagnosed with prostatic adenocarcinoma ten years prior to this presentation.
On admission, he was afebrile with a physical examination significant for 3+ bilateral lower extremities pitting edema and bibasilar lung crackles. Laboratory values included a WBC of 22 900 /ul, with 25% bands and 10% eosinophils. Serum creatinine was 2.6 mg/dl, which increased from his baseline of 1.1 mg/dL. Urinalysis revealed 3+ leukocyte esterase, >100 RBC, and >100 WBC. Liver function tests revealed an elevated total bilirubin of 3.0 mg/dl, and a renal ultrasound showed bilateral hydronephrosis.
On the second day in hospital the patient underwent cystoscopy to evaluate the etiology of the obstructive renal failure and for prostate biopsy, which revealed poorly differentiated carcinoma. On day three, a liver ultrasound revealed marked heterogeneity concerning for metastatic disease. A left-sided percutaneous nephrostomy was performed on day four to relieve the obstructive hydronephrosis. On day nine, an abdominal MRI demonstrated diffuse metastatic disease of the liver and therefore a liver biopsy was performed. On hospital day 11, the carcinoembryonic antigen (CEA) was found to be 4155 U/ml, but PSA was undetectable. A noncontrast CT of the chest, abdomen, and pelvis revealed multiple bilateral pulmonary nodules consistent with diffuse metastases as well as enlarged lymph nodes in the chest and retroperitoneum. The liver biopsy demonstrated metastatic high-grade neuroendocrine carcinoma with mixed small and large cell features; the same histology was seen on subsequent review of the prostate biopsy [Figure 2]. He continued to become more encephalopathic and edematous, and was deemed not to be a candidate for chemotherapy given his extensive metastatic disease. He expired on hospital day 17 from presumed sepsis.
Figure 2.
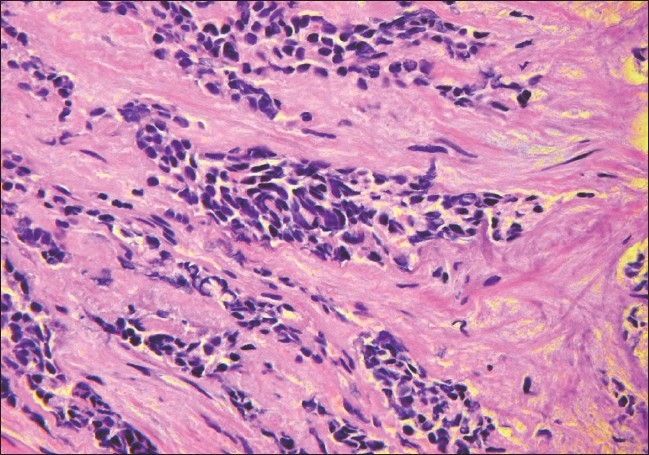
A 40× magnification H&E stain of case 4 at this presentation shows transition of the adenocarcinoma to a small cell neuroendocrine carcinoma.
Case 5
The patient was a 73-year-old male with a past history of hypertension, dyslipidemia, and diabetes mellitus. He presented to his urologist with multiple urinary tract infections over the preceding two years. The PSA was normal, but a nodule was detected in the left side of the prostate by ultrasound. The patient had a core biopsy performed revealing SCC without surrounding adenocarcinoma. The tumor was positive for cytokeratins, chromogranin A, and synaptophysin.
He was then referred to oncology clinic where he had a CT of his chest/abdomen/pelvis, MRI of the brain and spine, and a PET scan performed, all of which failed to reveal evidence of metastatic disease. The patient received four rounds of chemotherapy with etoposide and carboplatin, along with radiation treatment to the prostate. The patient enjoyed normal activities of daily living for 18 months following treatment, but during routine restaging was found to have extensive metastatic disease involving the brain, lungs, liver, and hilar lymph nodes. After these findings were discovered, the patient received high-dose corticosteroids to treat the vasogenic edema surrounding the brain metastasis. Upon discharge, the patient was scheduled to start treatment with topotecan.
DISCUSSION
In these case series we have reviewed five previously unreported cases of SCC of prostatic origin. The five cases differed greatly in their clinical presentations and in the subsequent course of their disease.
As noted in the previously reported series, prostate SCC is seen frequently in patients with a prior diagnosis of prostatic adenocarcinoma, similar to three of the five patients in our series. This pattern of presentation is thought to represent proliferation of the native neuroendocrine cells in response to androgen-ablation therapy, since neuroendocrine cells lack androgen receptor. Of the previously diagnosed adenocarcinoma patients studied, two of them had aggressive courses with metastases to the bone, brain, liver, spinal cord, and lungs, while the other patients had locally invasive disease. Reported sites of metastases include omentum, vocal cord, axillary lymph nodes, and periadrenal soft tissue.[6] The average time separating diagnosis of SCC from the previously diagnosed adenocarcinoma was 6.7 years (range 1–13 years.) The previously reported ranges are from 2 to 12 years.[7] These three cases illustrate that patients who have been previously diagnosed with adenocarcinoma of the prostate who develop aggressive disease after receiving androgen-ablation therapy should be suspected of developing SCC.
Small-cell carcinomas are known to release neuroendocrine secretory products. Abrahamsson reported that increased levels of neuroendocrine secretory products are associated with a poor prognosis in these patients.[8] In a case report, progastrin-releasing peptide was followed serially in a patient with SCC of the prostate and normal PSA levels to monitor disease progression.[9] In addition, serum chromogranin A levels were found to correlate with the extent of neuroendocrine differentiation in prostatic carcinomas, though neuron-specific enolase, chromogranin B and pancreastatin did not.[10] Unfortunately in our case series, levels of secretory products were not measured, and the patients clinical courses were only followed by serum PSA measurements, of which 2 were elevated. Other serum markers that may be elevated in patients with SCC include chromogranin A, chromogranin B, neuron-specific enolase, serotonin, and secretoneurin. [11,12] Carcinoembryonic antigen has also been found to be elevated in certain patients, including one of the above five patients, with neuroendocrine carcinoma. This may be the result of multiple phenotypes being expressed.[13]
Small-cell neuroendocrine differentiation likely represents one end of the spectrum of disease in prostatic carcinoma. It is postulated that androgen-ablation therapy enhances the progression of neuroendocrine differentiation, as these particular cells lack androgen receptors,[14] and leads to a progressive, hormone-refractory malignancy. Focal neuroendocrine differentiation is exceedingly common in prostatic adenocarcinomas, seen in 30–100% of cases,[8] and in 10% of all prostatic malignancies, multifocal neuroendocrine features may be seen.[15] In cases presented in the literature, these malignancies are divided between those that are pure SCCs and mixed carcinomas with small cell and adenocarcinoma differentiation. Abbas et al., reviewed 130 cases of SCC originating from the prostate, of which 52% were pure SCCs whereas 40% were associated with adenocarcinoma.[2] In our series, four of the five cases represented pure SCCs without features of adenocarcinoma; however, this may represent incomplete sampling of the tumors.
In confirming the diagnosis of SCC, immunohistochemical evaluation was performed on all the cases. In the patients sampled, 4/4 were positive for chromogranin A, 3/3 were positive for synaptophysin, and 3/3 were positive for cytokeratin cocktail (AE1, AE3, CAM5.2, KA4, and UC2/PR-10-11.) No samples stained positive for prostatic-specific antigen or prostatic-specific acid phosphatase. These results are consistent with those published by Petraki et al., in which 86% of biopsies of SCC stained positively for synaptophysin and 71% for chromogranin A.[16] Other neuropeptides that may be synthesized by neuroendocrine cells and stain positive immunohistochemically include somatostain, alpha-human chorionic gonadotropin, and parathyroid hormone-related protein.[17] In differentiating small-cell prostate carcinoma from adenocarcinoma, TTF-1 and CD56 are also helpful markers.[18]
With regard to treatment, in our group of five patients, three received treatment directed towards the neuroendocrine malignancy. Patient #1 was given a regimen of vincristine and estramustine for one month before representing to the hospital and receiving intrathecal methotrexate for carcinomatous meningitis. The regimen of Patient #2 consisted of vincristine, carboplatin, and cyclophosphamide. The medications of Patient #5 were etoposide and carboplatin, and this was the only patient of the three to survive even after 12 months from diagnosis of SCC. Once this patient was found to have metastatic disease, he was treated with topotecan, a drug normally reserved for the treatment of small-cell lung cancer in patients who have failed platinum-based regimens.
Treatment in patients with poorly differentiated neuroendocrine carcinomas arising from gastroenteropancreatic origin is usually with a combination of etoposide and cisplatin, as this regimen has shown a 41.5% objective response rate, although there are significant associated hematologic and neurologic toxicities.[19] Likewise, the typical chemotherapeutic regimen for neuroendocrine carcinomas of prostatic origin is based on treatments for SCC of the lung, and involves etoposide, cisplatin, and vincristine. Cisplatin is an inorganic metal complex, which kills cells in all stages of the cell cycle, inhibits DNA biosynthesis, and binds DNA by forming intrastrand crosslinks. Two of the patients in this study received carboplatin, a medication similar to cisplatin although associated with less gastrointestinal and renal side effects. Vincristine is an alkaloid derivative of the plant Vinca rosea, and functions as a mitotic spindle poison, causing arrest in the M phase of the cell cycle. Etoposide is a semisynthetic derivative of podophyllotoxin, which blocks cell division in the late S phase of the cell cycle by inhibiting topoisomerase II.
Studies have shown good response to combination of oral estramustine and etoposide,[20] while the addition of doxorubicin to a regimen of etoposide and cisplatin was found to cause higher toxicity and did not improve the outcome.[5] A recent study of two patients with SCC who received gemcitabine, docetaxel, and carboplatin showed an initial decrease in neuron-specific enolase levels in both patients, indicative of a favorable response.[21] A single case report also suggested that somatostatin, an antiproliferative hormone of hypothalamic origin, may be a useful adjunctive treatment for hormone-refractory prostate carcinoma.[22] It should be mentioned, however, that even though poorly differentiated neuroendocrine carcinomas have a high level of chemosensitivity, patients diagnosed with these malignancies often have advanced metastatic disease or a poor functional condition at the time of diagnosis, making treatment less effective, and in some cases, impossible.
CONCLUSION
Small-cell neuroendocrine carcinoma of the prostate is an aggressive malignancy, often presenting with advanced metastasis at the time of diagnosis, and may develop in patients with previously diagnosed adenocarcinoma who have received anti-androgen therapy. Treatment of this cancer typically involves a platinum-based regimen, although other experimental therapies have been used.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Yashi M, Terauhi F, Nukui A, Ochi M, Yuzawa M, Hara Y, et al. Small-cell neuroendocrine carcinoma as a variant form of prostate cancer recurrence: A case report and short literature review. Urol Oncol. 2006;24:313–7. doi: 10.1016/j.urolonc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Abbas F, Civantos F, Benedetto P, Soloway MS. Small cell carcinoma of the bladder and prostate. Urology. 1995;46:617–30. doi: 10.1016/S0090-4295(99)80290-8. [DOI] [PubMed] [Google Scholar]
- 3.Rubenstein JH, Katin MJ, Mangano MM, Dauphin J, Salenius SA, Dosoretz DE, et al. Small cell anaplastic carcinoma of the prostate: Seven new cases, review of the literature and discussion of a therapeutic strategy. Am J Clin Onc. 1997;20:376–80. doi: 10.1097/00000421-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka M, Suzuki Y, Takaoka K, Suzuki N, Murakami S, Matsuzaki O, et al. Progression of prostate cancer to neuroendocrine cell tumor. Int J Urol. 2001;8:431–6. doi: 10.1046/j.1442-2042.2001.00347.x. [DOI] [PubMed] [Google Scholar]
- 5.Papandeou CN, Daliani DD, Thall PF, Tu SM, Wang X, Reyes A, et al. Results of a phase II study with doxorubicin, etoposide and cisplatin in patients with fully characterized small-cell carcinoma of the prostate. J Clin On. 2002;20:3072–80. doi: 10.1200/JCO.2002.12.065. [DOI] [PubMed] [Google Scholar]
- 6.Tetu B, Ro JY, Ayala AG, Johnson DE, Logothetis CJ, Ordonez NE. Small cell carcinoma of the prostate, Part I: A clinicopathologic study of 20 cases. Cancer. 1987;59:1803–9. doi: 10.1002/1097-0142(19870515)59:10<1803::aid-cncr2820591019>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Evans AJ, Humphrey PA, Belanai J, van der Kwast TH, Srigley JR. Large cell neuroendocrine carcinoma of prostate: A clinicopathologic summary of 7 cases of a rare manifestation of advanced prostate cancer. Am J Surg Pathol. 2006;30:684–93. doi: 10.1097/00000478-200606000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Abrahamsson PA. Neuroendocrine differentiation in prostatic carcinoma. Prostate. 1999;39:135–48. doi: 10.1002/(sici)1097-0045(19990501)39:2<135::aid-pros9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 9.Yahsi M, Kurokawa S, Tokue A. Prostatic small-cell neuroendocrine carcinoma with disease progression monitored by measurement of serum progastrin-releasing peptide. BJU Int. 2000;86:1091–2. doi: 10.1046/j.1464-410x.2000.00951.x. [DOI] [PubMed] [Google Scholar]
- 10.Angelsen A, Syversen U, Haugen OA, Stridsberg M, Mjolnerod OK, Waldum HL. Neuroendocrine differentiation in carcinomas of the prostate: Do neuroendocrine serum markers reflect immunohistochemical findings? Prostate. 1997;30:1–6. doi: 10.1002/(sici)1097-0045(19970101)30:1<1::aid-pros1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 11.Sciarra A, Mariotti G, Gentile V, Voria G, Pastore A, Monti S, et al. Neuroendocrine differentiation in human prostatic tissue: Is it detectable and treatable? BJU Int. 2003;91:438–45. doi: 10.1046/j.1464-410x.2003.03066.x. [DOI] [PubMed] [Google Scholar]
- 12.Angelsen A, Syversen U, Stridsberg M, Haugen OA, Mjolnerod OK, Waldum HI. Use of neuroendocrine serum markers in the follow-up of patients with cancer of the prostate. Prostate. 1997;31:110–7. doi: 10.1002/(sici)1097-0045(19970501)31:2<110::aid-pros6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 13.Kinebuchi Y, Noguchi W, Irie K, Nakayama T, Kato H, Nishizawa O. Relapsed prostate cancer with neuroendocrine differentiation and high serum levels of carcinoembyonic antigen without elevation of prostrate-specific antigen: A case report. Int J Urol. 2007;14:147–9. doi: 10.1111/j.1442-2042.2007.01616.x. [DOI] [PubMed] [Google Scholar]
- 14.Jiborn T, Bjartell A, Abrahamsson PA. Neuroendocrine differentiation in prostatic carcinoma during hormonal treatment. Urology. 1998;51:585–9. doi: 10.1016/s0090-4295(97)00684-5. [DOI] [PubMed] [Google Scholar]
- 15.Abrahamsson PA. Neuroendocrine differentiation and hormone-refractory prostate cancer. Prostate Suppl. 1996;6:3–8. doi: 10.1002/(sici)1097-0045(1996)6+<3::aid-pros2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 16.Petraki C, Vaslamatzis M, Petraki K, Revelos K, Alevizopoulos N, Papanastasiou P, et al. Prostate cancer with small-cell morphology: An immunophenotypic subdivision. Scand J Urol Nephrol. 2005;39:455–63. doi: 10.1080/00365590500199855. [DOI] [PubMed] [Google Scholar]
- 17.Clegg N, Ferguson C, True LD, Arnold H, Moorman A, Quinn JE, et al. Molecular characterization of prostatic small-cell neuroendocrine carcinoma. Prostate. 2003;55:55–64. doi: 10.1002/pros.10217. [DOI] [PubMed] [Google Scholar]
- 18.Yao JL, Madeb R, Bourne P, Lei J, Yang X, Tickoo S, et al. Small cell carcinoma of the prostate: An immunohistochemical study. Am J Surg Pathol. 2006;30:705–12. doi: 10.1097/00000478-200606000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Mitry E, Baudin E, Ducreux M, Sabourin JC, Rufle P, Aparicio T, et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br J Cancer. 1999;81:1351–5. doi: 10.1038/sj.bjc.6690325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimopoulos MA, Panopoulos C, Bamia C, Deliveliotis C, Alivizatos G, Pantazopoulos D, et al. Oral estramustine and oral etoposide for hormone-refractory prostate cancer. Urology. 1997;50:754–8. doi: 10.1016/S0090-4295(97)00323-3. [DOI] [PubMed] [Google Scholar]
- 21.Aoki H, Ishidoya S, Ito A, Endoh M, Shimazui T, Arai Y. Experience of the treatment with gemcitabine, docetaxel, and carboplatin (GDC) chemotherapy for patients with small-cell carcinoma of the prostate. Int J Urol. 2006;13:1254–8. doi: 10.1111/j.1442-2042.2006.01514.x. [DOI] [PubMed] [Google Scholar]
- 22.Spieth ME, Lin YG, Nguyen TT. Diagnosing and treating small-cell carcinomas of prostatic origin. Clin Nucl Med. 2002;27:11–7. doi: 10.1097/00003072-200201000-00003. [DOI] [PubMed] [Google Scholar]


