SUMMARY
In order to assess the in vivo function of integrins containing the β8 subunit, we have generated integrin β8- deficient mice. Ablation of β8 results in embryonic or perinatal lethality with profound defects in vascular development. Sixty-five percent of integrin β8-deficient embryos die at midgestation, with evidence of insufficient vascularization of the placenta and yolk sac. The remaining 35% die shortly after birth with extensive intracerebral hemorrhage. Examination of brain tissue from integrin β8-deficient embryos reveals abnormal vascular morphogenesis resulting in distended and leaky capillary vessels, as well as aberrant brain capillary patterning. In addition, endothelial cell hyperplasia is found in these mutant brains. Expression studies show that integrin β8 transcripts are localized in endodermal cells surrounding endothelium in the yolk sac and in periventricular cells of the neuroepithelium in the brain. We propose that integrin β8 is required for vascular morphogenesis by providing proper cues for capillary growth in both yolk sac and embryonic brain. This study thus identifies a molecule crucial for vascular patterning in embryonic yolk sac and brain.
Keywords: Integrin, Vasculature, Embryo, Brain, Angiogenesis, Mouse
INTRODUCTION
The vertebrate vascular network is essential for embryonic survival. The basic structural unit, a blood vessel, is assembled through two processes, vasculogenesis and angiogenesis, followed by the recruitment of vascular support cells (Risau, 1997). Proper patterning of the vascular network, however, requires further morphogenic events, including vessel branching and coupling, vessel guidance within target tissues, and arterial or venous specification.
Recent studies have identified several factors that function in regulating vasculogenesis and angiogenesis. Basic fibroblast growth factor (bFGF) and its receptor (FGFR) are required for the differentiation of mesodermal progenitors into endothelial cells (Flamme and Risau, 1992). Vascular endothelial growth factor (VEGF) (Carmeliet et al., 1996; Ferrara et al., 1996) and its receptors VEGFR2/Kdr (Shalaby et al., 1995), VEGFR1/Flt1 (Fong et al., 1995), and VEGF-R3/Flt4 (Dumont et al., 1998) are essential for early endothelial cell differentiation and for both vasculogenesis and angiogenesis. Angiopoietins (Ang1 and Ang2) and their receptors Tie2/Tek and Tie1 promote the recruitment of support cells and vessel stability during angiogenesis (Dumont et al., 1994; Puri et al., 1995; Sato et al., 1995; Suri et al., 1996). In addition, platelet derived growth factor β (PDGFβ) and its receptors, as well as transforming growth factor β (TGFβ) and its signaling mediators, are necessary for the differentiation and recruitment of vascular support cells to developing blood vessels (Dickson et al., 1995; Lindahl et al., 1997; Goumans et al., 1999; Hellström et al., 1999). Despite significant advances in our understanding of the mechanisms underlying blood vessel assembly, the mechanisms that regulate patterning of the vascular network are still poorly characterized.
More recently, factors regulating intersomitic vessel branching and patterning have been identified through genetic studies. Ephrins and Eph receptors have been found to mediate artery/vein boundary specification (Wang et al., 1998; Adams et al., 1999; Adams et al., 2001), vessel assembly, and vascular morphogenesis and branching (Adams et al., 2001; Adams et al., 1999). Delta/Notch signaling is required for intersomitic blood vessel branching and morphogenesis (Xue et al., 1999; Krebs et al., 2000). Ca2+/calcineurin/NFAT are necessary for the recruitment of smooth muscle cells to the dorsal aorta and for intersomitic vascular branching and patterning (Graef et al., 2001).
While molecules required for intersomitic vessel patterning have been identified, molecules that regulate patterning of a complex vascular network within an organ such as the brain are completely uncharacterized. The developing neuroepithelium lacks intrinsic vasculature. Capillary vessels penetrate the neuroepithelium in response to angiogenic factor(s), such as VEGF, expressed by neuroepithelial cells, and extend in a parallel and organized fashion during subsequent development (Breier et al., 1992). They branch regularly and anastomose to form an organized network (Marin-Padilla, 1988). Thus, the development of the brain vascular network provides an ideal system for studies on patterning of the vascular network.
Vascular patterning processes are likely to be regulated by cell-matrix and cell-cell interactions, and to be influenced by environmental cues. Integrins are important extracellular matrix (ECM) transmembrane receptors that participate in a variety of cellular events including proliferation, differentiation, adhesion, migration and cell survival (Schwartz et al., 1995). Integrin receptors are heterodimeric molecules that consist of α and β subunits. A subset of integrins including α vβ3, α vβ5,α 5β1,α 2β1,α vβ1 and α 1β1 have been implicated in vascular development (Hynes and Bader, 1997). Of particular interest is integrin α vβ3, which is expressed on endothelial cells. α vβ3 antagonists and blocking monoclonal antibodies inhibit angiogenesis in vitro, suggesting that this integrin may play a crucial role in vascular development (Eliceiri and Cheresh, 1999). However, neither integrin αv nor integrin β3 mutant mice exhibit obvious defects in angiogenesis during development (Bader et al., 1998; Hodivala-Dilke et al., 1999).
Analysis of the integrin β8 sequence indicates that it is highly conserved among species, but is quite divergent from other integrin β subunits, suggesting that it may have unique functions (Moyle et al., 1991). At present, the integrin αv is the only partner identified for integrin β8. The integrin αvβ8 has been shown to bind vitronectin, and may also bind laminin and collagen IV in cell culture (Moyle et al., 1991; Nishimura et al., 1994; Venstrom and Reichardt, 1995). β8 mRNA is expressed in adult brain, kidney and placenta (Moyle et al., 1991), and its protein is reportedly localized to brain synapses, glial cells (Milner et al., 1997a; Milner et al., 1997b; Nishimura et al., 1998), as well as to the suprabasal cells of the epidermis during eyelid morphogenesis (Stepp, 1999).
To dissect integrin β8 functions in vivo, we have generated β8-deficient mice. This mutation results in embryonic lethality with defective vascular development in the placenta and yolk sac or perinatal lethality with intracerebral hemorrhage. Analyses of mutant brain tissue demonstrate that capillaries are formed, but are aberrantly patterned with hyperproliferative endothelial cells. In addition, neuroepithelial cells appear disorganized in the mutant. Finally, integrin β8 mRNA is localized to neuroepithelial cells, but has not been detected in the vasculature. Consequently, we propose that integrins containing β8 provide proper environmental cues for patterning of the embryonic brain vascular network.
MATERIALS AND METHODS
Generation of integrin β8-deficient mice
A 6.5 kb XbaI genomic fragment with a PGK-neo-cassette (1.4 kb) inserted to replace an EcoRI fragment was used to generate the targeted allele following the standard procedures (Fig. 1A). For Southern blots, a 672 bp DNA fragment located at 5′ and a 700 bp DNA fragment at 3′ end of the targeting construct were used. Three independently targeted ES cell lines were identified (Fig. 1B), of which one gave the germline transmission. Transmission of the targeted integrin β8 locus was confirmed by Southern blot. Chimeric males were bred to C57BL/6J and 129/Sv females. For Northern blots, total RNAs were extracted from P0 brains using RNAzol B (LPS Industries, Moonachie, NJ). A 300 bp fragment from the 3′UTR of integrin β8 cDNA (K. M. and L. F. R., unpublished) was used as a probe.
Fig. 1.
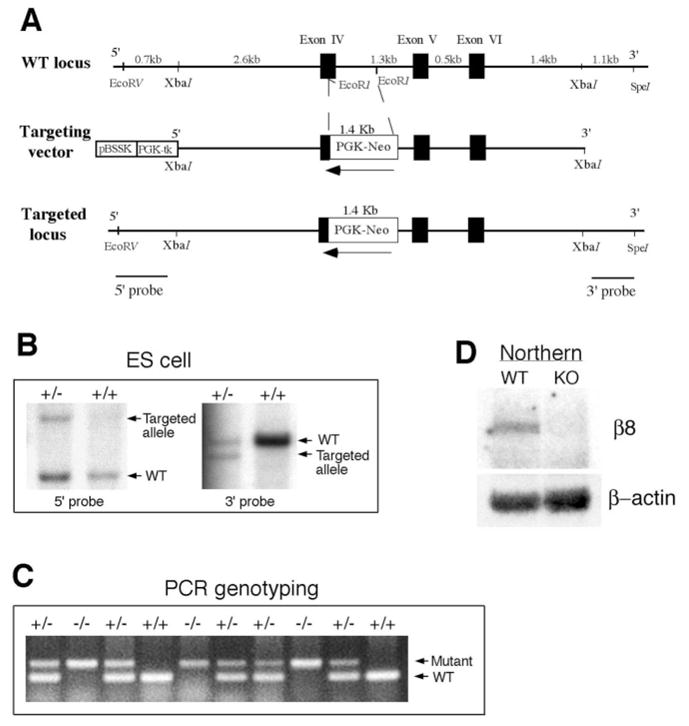
Generation of integrin β8-deficient mice. (A) Schematic drawing of integrin β8 genomic region encompassing Exons IV, V and VI in the wild type and mutant. Black boxes represent exons and white box represents the PGK-neor-cassette. Arrows indicate the transcriptional orientation of the cassette. 5′ and 3′ probes for Southern blot analyses are indicated. (B) Identification of ES clones containing a mutated β8 allele with 5′ and 3′ probes by Southern blot analyses. The wild-type and mutant alleles are labeled. (C) PCR analysis of genotypes from a heterozygous intercross. Mutant and wild-type amplification products are indicated. (D) Northern blot analysis showing the absence of integrin β8 transcripts in homozygous mutant mice. Comparable amounts of wild-type and mutant total RNA were loaded, as indicated by the presence of equal amounts of β-actin RNA. RT-PCR analysis further verified that no functional transcript is expressed in the mutant (data not shown).
PCR genotyping
Yolk sac DNA was used for PCR analyses with primers specific for the wild-type and targeted alleles [wild-type primers DW74 (5′ATTATCTGGTTGATGTGTCAGC3′) and JW153 (5′AGAGAGGAACAAATATCCTTCCC3′); mutant primers DW56 (5′AGAGGCCACTTGTGTAGCGCCAAG3′) and DW94 (5′GGAGGCATACAGTCTAAATTGT3′)]. PCR conditions as described by the manufacturer of AmpliTaq Polymerase (Perkin-Elmer Cetus, Norwalk, CT) were used to generate fragments from wild-type (330 bp) and targeted (450 bp) alleles on 1% agarose gels. Tie2/lacZ heterozygotes (Schlaeger et al., 1997) were identified by PCR using primers: lacZFor (5′-TCGTTTGCCGTCTGAATTTGACCTG-3′) and lacZRev (5′-TGACCATGCAGAGGATGATGCTCG- 3′).
Morphological and histological analysis
Whole-mount embryos were photographed on a dissecting microscope. For histology, embryos were fixed in Carnoy’s solution or in 4% paraformaldehyde (PFA) in PBS. Fixed embryos were either used directly for vibratome sectioning or were dehydrated and embedded in paraffin. Sections (150 μm) were used in free-floating immunohistochemistry. Paraffin wax embedded sections (7 μm) were either stained with Hematoxylin and Eosin or were used for immunohistochemistry.
Immunohistochemistry
Immunohistological methods were used as described previously (Jones et al., 1994). Primary antibodies were used as follows: anti- CD31 (PECAM1) (Pharmagen, MEC 13.3) at 1:150 dilution; anti-fibronectin (Sigma) at 1:2000 dilution; anti-laminin (Sigma, #L9393) at 1:4000 dilution; RC2 (Hybridoma bank, Iowa city, IA) at 1:20 dilution; and anti-desmin (DAKO, #A0611) at 1:50 dilution.
Electron microscopy
Embryos were fixed overnight in 2.5% glutaraldehyde, 1% PFA in 0.1 M sodium cacodylate buffer, pH 7.4. Brains were sectioned at 1 mm using a vibratome and processed using standard techniques.
In situ hybridization
In situ hybridization was performed on fresh frozen sections as described (Schaeren-Wiemers and Gerfin-Moser, 1993). The digoxigenin RNA labeling and detection kit from Roche Biochemicals (Indianapolis, IN) was used. A 5.5 kb integrin β8 cDNA fragment was used as template.
Endothelial cell quantification
Capillary endothelial cell nuclei were counted using 5 μm paraffin sections of brains. Sections were stained with DAPI, biotinylated isolectin BS (L-2140; Sigma) and anti-BrdU antibody (BD pharmagen) [methods were modified from Hellstorm et al. (Hellstorm et al., 2001)]. Images were taken from the ganglionic eminence using a 40× objective on a Nikon Eclipse E600 microscope and overlaid in Adobe Photoshop 6.0.
VEGF ELISA assay
VEGF was assayed following the procedures of Hellstrom et al. (Hellstrom et al., 2001), using the mouse VEGF ELISA detection kit QuantikineM (R & D Systems, Minneapolis, MN).
RESULTS
Generation of integrin β8-deficient mice
The gene for integrin β8 was inactivated by replacing sequences encoding 45 amino acids at the C terminus of exon IV and part of the intron sequences with a neomycin-resistance (neor)- expression cassette. Exon IV encodes a von Willebrand factor-A-like domain that has been shown to form part of the ligand binding site in other integrin heterodimers (Tuckwell and Humphries, 1997; Xiong et al., 2001). This alteration results in a frameshift in addition to a deletion, abolishing the production of functional β8 protein (Fig. 1).
Integrin β8-deficient mice die either by E11.5 or perinatally
Mice heterozygous for integrin β8-deficiency appeared normal and were fertile. Genotypic analyses of over 150 weaning-age pups from heterozygous intercrosses identified no homozygous mutants, indicating that integrin β8-deficiency results in embryonic or perinatal lethality. Examination of embryos collected at different developmental stages revealed that approximately two-thirds of homozygous embryos die between E9.5–E11.5, while the remaining die within days of birth (Table 1). Thus, the integrin β8 mutant allele is a recessive lethal mutation.
Table 1.
Genotypes of progeny from heterozygous integrin β8 intercrosses
| Integrin β8 genotype* |
||||||
|---|---|---|---|---|---|---|
| Age | +/+ | +/− | −/− | Number of viable −/−† | Expected number of viable −/− | Expected −/− progeny (%) |
| E9.5 | 27 (31) | 39 (45) | 20 (24) | 20 | 22 | 91 |
| E10.5 | 48 (24) | 101 (50) | 52 (26) | 41‡ | 50 | 82 |
| E11.5 | 37 (32) | 54 (46) | 26 (22)§ | 12 | 30 | 40 |
| E12.5 | 23 (25) | 52 (57) | 16 (18) | 9 | 25 | 36 |
| E13.5 | 25 (25) | 65 (65) | 10 (10) | 10 | 30 | 33 |
| E14.5 | 14 (45) | 14 (45) | 3(10) | 3 | 9 | 33 |
| E15.5 | 12 (36) | 18 (55) | 3 (9) | 3 | 10 | 30 |
| E16.5 | 11 (35) | 17 (55) | 3 (10) | 3 | 9 | 33 |
| P0–P4 | 58 (32)¶ | 103 (57)¶ | 20 (10) | 20** | 54 | 36 |
Numbers are given with percentages in brackets.
E, embryonic day; P, postnatal day.
Expected viable −/− calculated as if the sum of (+/+)+(+/−) is 75%.
Heart is beating.
28 embryos had pale yolk sacs; 15 had dilated pericardiac cavities; 6 had no obvious vasculature; 5 had loose allantois-chorion connections.
Many embryos were deteriorating or being absorbed.
Two +/+ and two +/− embryos had cleft palate and died. The rest were viable and fertile.
Two had a cleft palate. All had intracerebral hemorrhages.
To characterize phenotypic defects in the homozygotes, embryos were harvested from embryonic day 9.5 (E9.5) to birth (Table 1, Fig. 2). The normal Mendelian ratio of 1:2:1 of wild type, heterozygous and homozygous mutant embryos was observed between E9.5 and E10.5. Integrin β8-deficient embryos at E9.5 were largely indistinguishable from wild-type and heterozygous littermates (data not shown). At E10.5, however, approximately half of the integrin β8-deficient embryos had pale yolk sacs with poorly developed vasculature (Fig. 2A,B). In some extreme cases, no obvious vasculature was observed in the yolk sacs (data not shown). Approximately 20% of E10.5 mutant embryos had no heart beat and were presumed to be dead. Of the living embryos, approximately half were smaller than their wild-type littermates (Fig. 2C,D) and often had a dilated pericardiac cavity with adjacent hemorrhage. Except for these defects, these E10.5 mutant embryos were morphologically similar to their wild-type littermates with the presence of developed telencephalic and mesencephalic vesicles, optic vesicles, otic pits, brachial arches, and forelimb and hindlimb buds (Fig. 2D). The remaining half of E10.5 mutant embryos appeared normal. At E11.5, homozygotes fell into two main categories: ~30–40% appeared morphologically normal (designated class B) while the rest were deteriorating and partially absorbed (designated class A) (data not shown).
Fig. 2.
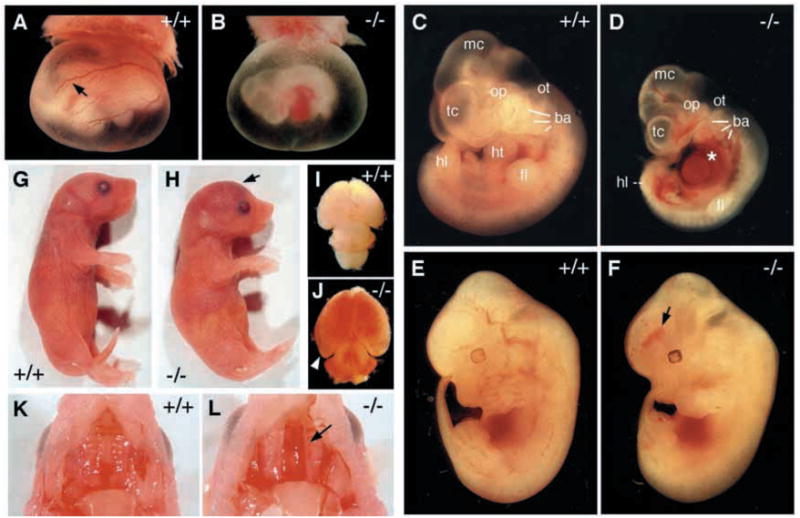
Phenotypic defects in integrin β8-deficient mice. (A,B) E10.5 yolk sacs. The vasculature is less prominent and often pale in mutant embryos (B) when compared with wild type (A, arrow). (C,D) Side views of E10.5 embryos. The class A mutant embryo exhibits a smaller body size (D) compared with a wild-type littermate (C). An example of enlarged pericardiac cavities is shown in D (*). (E,F) Side views of E12.5 embryos. Intracerebral hemorrhage in a class B mutant embryo is shown (F, arrow) when compared with its wild-type littermate (E). (G,H) Side views of P0 wild-type (G) and mutant (H) pups. Severe hemorrhage in the mutant head is obvious (H, arrow). (I,J) The P0 mutant brain (J) shows characteristics of hydrocephalus (arrowhead) and exhibits visible hemorrhage compared with a wild type (I). (K,L) A cleft palate is obvious in a mutant neonate (L, arrow), but is absent in a wild-type littermate (K). mc, metencephalon; tc, telencephalon; op, optic vesicle; ot, otic pit; ba, bronchial arches; he, heart; fl, forelimb; hl, hindlimb.
Almost all class B mutant embryos developed intracerebral hemorrhage by E12.5 (Fig. 2F), but were otherwise morphologically identical to their wild-type littermates. The intracerebral hemorrhage appeared first in the ganglionic eminence, and later in the telencephalon, mesencephalon and myelencephalon (data not shown). Most of these mutants survived gestation (Table 1), giving rise to viable neonates (Fig. 2H). These mutant pups, however, had severe intracerebral hemorrhage (Fig. 2J) and died shortly after birth. Additionally, ~10% of these had a cleft palate (Fig. 2L).
This analysis indicates that deficiency of integrin β8 leads to lethality at two different developmental stages. Class A integrin β8-deficient embryos (~60%) died by E11.5 with small body size and pale yolk sacs. Class B integrin β8-deficient embryos (~40%) survived to birth, but died perinatally with evidence of massive intracerebral hemorrhage and occasional cleft palate. The morphological characteristics mentioned above are used to define two mutant classes for further analyses hereafter.
Impaired vascular development in class A integrin β8-deficient mutants
The small body size and abnormal yolk sac vasculature of E10.5 class A mutants suggests a defect in extra-embryonic tissues. In wild-type embryos, the chorionic plate is well developed and traversed by the allantoic vessels by E10.5. The placenta has developed an apparent labyrinthine layer where fetal blood vessels invade and actively interdigitate with maternal blood vessels to form a sponge-like network for nutrient and O2 exchange (Fig. 3A). Examination of placental tissue from class A mutants indicates that while the chorionic plate (cp) and labyrinthine trophoblast layer appear normal, the labyrinthine layer is dramatically reduced in thickness and is less vascularized compared with wild-type littermates. Furthermore, fewer fetal blood vessels containing embryonic nucleated erythrocytes (cells stained a darker red color) penetrate into the labyrinthine area and the sponge-like vascular network is missing (Fig. 3B).
Fig. 3.
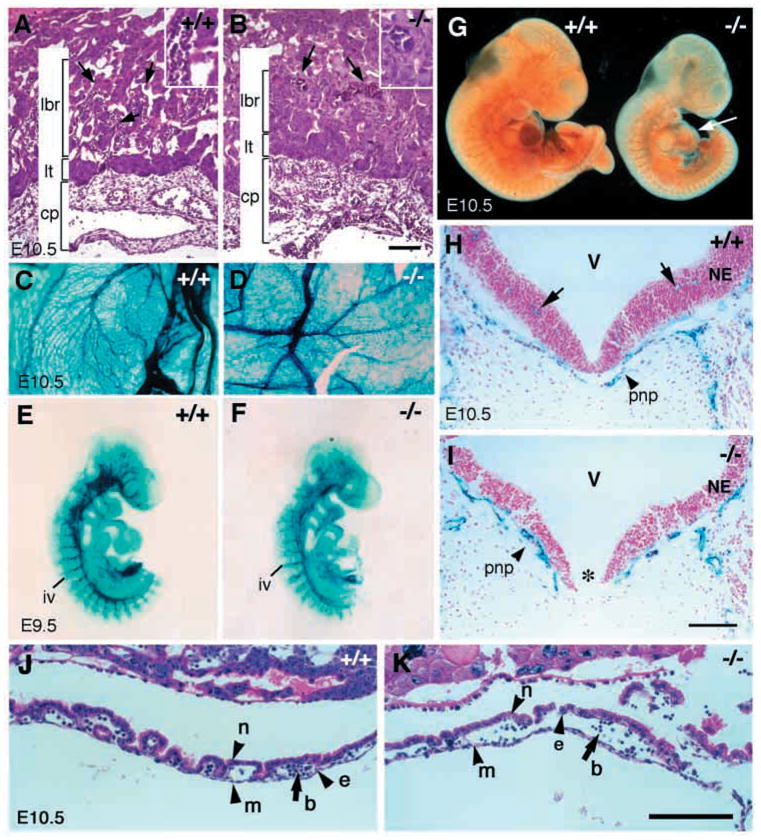
Angiogenesis defects in class A integrin β8-deficient mutants. (A,B) Hematoxylin and Eosin staining of transverse sections of placentas from an E10.5 wild type (A) and a mutant (B). While the chorionic plate (cp) and labyrinthine trophoblast layer (lt) are comparable in mutant and wild-type littermates, the labyrinthine layer (lbr) is reduced in the mutant. While the interdigitation of fetal blood vessels (A, arrows) and maternal blood vessels in wild-type embryos is elaborate, only a few fetal blood vessels have penetrated into the labyrinthine layer in the mutant (B, arrows). Inserts in A,B provide higher magnification photos of the vasculature. (C,D) Vasculature of E10.5 yolk sac in wild-type (C) and mutant (D) as depicted by X-gal staining of a Tie2:lacZ reporter gene in these mice. (E–G) Vascular patterns revealed by whole-mount staining of β-galactosidase activity in E9.5 embryos expressing the Tie2:lacZ reporter gene (E,F) and whole-mount immunohistochemistry with anti- PECAM antibody in E10.5 embryos (G). An E9.5 mutant embryo (E) shows no obvious abnormalities in vascular pattern compared with a wild-type littermate (F) (the tails of the embryos in both E and F were used for genotyping). E10.5 mutant embryos (G, right) have similar vascular patterns as wild-type littermates (G, left) except for reduced vasculature development in the heart (G, arrow). (H,I) X-gal staining of transverse sections of E10.5 neural tubes in wild-type (H) and mutant embryos (I) with the Tie2:lacZ reporter gene reveals the vasculature (blue) and cell nuclei labeled with Nuclear Fast Red (pink). While perineural plexuses are present in both the wild type and the mutant (H,I, arrowheads), there is no apparent penetration of vessels into the neural tube in the mutant when compared with the wild-type embryo (H, arrows). Note that the floor plate in the mutant (I, *) is absent. cp, chorionic plate; lt, labyrinthine trophoblast; lbr, labyrinthine layer; iv, intersomitic vessel; pnp, perineural plexus; V, ventricle; NE, neuroepithelium. (J,K) Hematoxylin and Eosin staining of E10.5 yolk sac showing presence of endothelial cells (e), mesothelial cells (m), blood cells (b) and endoderm cells (n) in both wild type (J) and mutant (K). Scale bars: 100 μm in A,B,H,I (500 μm in insets); 150 μm in J,K.
The yolk sac vasculature of E10.5 mutants was analyzed using a mouse strain carrying a Tie2:lacZ reporter gene that is expressed in all embryonic endothelial cells (Schlaeger et al., 1997). The Tie2:lacZ reporter allele was crossed into the integrin β8-deficient strain and mutant embryos were examined using X-gal staining. The vascular pattern of the yolk sac in E9.5 integrin β8-deficient embryos appears grossly normal (data not shown). At E10.5, the vasculature in the wild-type yolk sac develops a complex pattern with primary and secondary branches from the major vessels (Fig. 3C). In the yolk sacs of class A mutants, while the primary vascular plexuses are present, the vessels are less complex, with reduced branching compared with wild-type littermates (Fig. 3C,D), indicating that vasculogenesis occurred, but further vascular morphogenesis was abnormal. Immunohistochemical staining with an antibody recognizing platelet endothelial cell adhesion molecule 1 (PECAM1), a specific marker for vascular endothelial cells (Baldwin et al., 1994), confirmed the abnormal yolk sac vascular pattern in the class A mutant embryos (data not shown). When we compared E10.5 yolk sac in wild-type and mutant embryos, we found endodermal (n), endothelial (e) and mesothelial (m) cells in both wild-type (Fig. 3J) and mutant (Fig. 3K) yolk sacs. No apparent abnormality other than dilated endothelium in the mutant yolk sac was observed. At E10.5, the yolk sacs of class B mutants are indistinguishable from those of wild-type littermates (data not shown). Together, these observations suggest that vascular morphogenesis in the placenta and yolk sac is defective in class A mutants, but relatively normal in class B mutants (data not shown).
To examine vascular development within the embryo proper, we employed whole-mount immunohistochemical staining with anti-PECAM as well as X-gal staining in embryos carrying the Tie2:lacZ reporter gene. As shown in Fig. 3E–G, formation of the vasculature in class A mutants is similar to that in wild-type littermates at E9.5 and E10.5, although the heart ventricles appeared less vascularized at E10.5 in mutants (Fig. 3G). Major blood vessels such as the dorsal aorta, cardinal veins, intersomitic arteries and intracerebral arteries are present in mutant embryos (data not shown), suggesting that the initial stages of vascular development occur normally in mutant embryos. During normal development, the perineural vascular plexus is formed in mesenchyme adjacent to the neuroepithelium through vasculogenesis. Shortly after E9.0, capillary vessels begin to invade the neuroepithelium towards the ventricle via angiogenesis. Capillary vessels anastomose as they extend into the neuroepithelium and the vascular network within the embryonic brain becomes evident by E10.5 (Herken et al., 1989). To characterize intracerebral vascular development, transverse brain sections from class A mutant embryos carrying the Tie2:lacZ reporter gene were stained with X-gal. While the perineural plexus is formed in both wild-type and mutant embryos, capillary vessels have not penetrated the neuroepithelium in mutants by E10.5 (Fig. 3H,I). This indicates that vasculogenesis occurs normally, but angiogenesis in the brain is blocked or delayed. Interestingly, the floor plate is absent in class A mutants (three out of three) (Fig. 3I), suggesting that defects in neural tube development occur in these mutants. Thus, class A integrin β8-deficient embryos exhibit defective vascular morphogenesis in the placenta and yolk sac. In addition, angiogenesis within the nervous system and neural tube development are not normal.
Integrin β8 is expressed in cells surrounding endothelium in the placenta and yolk sac
The vascular defects in the placenta and yolk sac could result from the absence of integrin β8 from endothelial cells, from cells surrounding endothelium, or both. To identify cells expressing integrin β8, in situ hybridization was performed on E9.5 placenta and yolk sac (Fig. 4A–D). Integrin β8 transcripts are present in most of the cells in the placenta with relatively higher expression in trophoblast giant cells (Fig. 4A). Closer examination of yolk sacs revealed that integrin β8 is most strongly expressed in the endodermal cells (n) surrounding the endothelial cells (Fig. 4D). This suggests that β8-integrins in endodermal cells regulate the patterning of endothelial cells during vascular morphogenesis in the yolk sac.
Fig. 4.
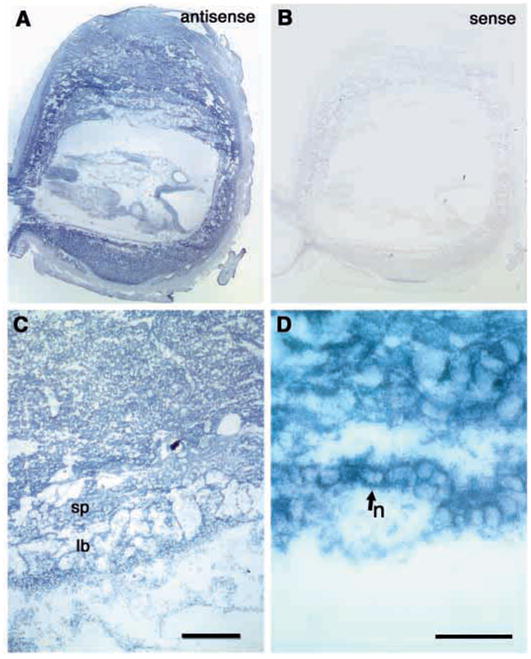
Integrin β8 expression in E9.5 placenta tissue. (A–D) Sections of E9.5 placenta hybridized with integrin β8 antisense (A,C,D) or sense (B) probes showing localization of integrin β8 to most of placenta tissues, notably in the trophoblast giant cells (A), labyrinthine layer and spongiotrophoblast layer (C). In the yolk sac, integrin β8 appears to be expressed specifically in the endoderm cells (D, arrow). sp, spongiotrophoblast layer; lb, labyrinthine layer. Scale bar: 200 μm in C; 50 μm in D.
Normal capillary blood vessel assembly but defective vascular patterning in class B mutants
We chose to focus our analysis of intra-embryonic phenotypes on class B mutants for the following reasons. Class A mutants die by E11.5 with prominent defects in placental and yolk sac vascularization. The placental defect is likely responsible for the early lethality and at least some of the intra-embryonic phenotypes of class A mutant embryos. In addition, the placenta includes multiple cell types of both maternal and embryonic origin. By contrast, the early embryonic brain consists primarily of only two cell types, endothelial cells and neuroepithelial cells, both of embryonic origin, facilitating analysis of vascular defects in class B mutants.
In class B integrin β8-deficient embryos, intracerebral hemorrhage appears initially bilaterally in the ganglionic eminence by E12.5 (Fig. 2F) and later becomes apparent in the diencephalon, cortex and mantle layer in the hindbrain (data not shown). To characterize intracerebral phenotypes, we analyzed wild-type and mutant brains at E11.5 (Fig. 5A–D) and E14.5 (Fig. 5E–H). Strikingly, bilateral cavitation was observed in the subventricular area surrounding all four ventricles. The cavitation was most apparent in the ganglionic eminence (Fig. 5B) and diencephalon (Fig. 5D) at E11.5 and became more severe at E14.5 (Fig. 5F,H). Capillary blood vessels appeared grossly normal at E11.5 and became distended and filled with blood cells at E14.5 (Fig. 5F,H). Hemorrhage was visible at E11.5 in the ganglionic eminence and diencephalon, and became evident in the area surrounding all four ventricles at E14.5 (Fig. 5F,H, data not shown). Thus, the neuroepithelium and its capillary vessels are abnormal in class B mutants.
Fig. 5.
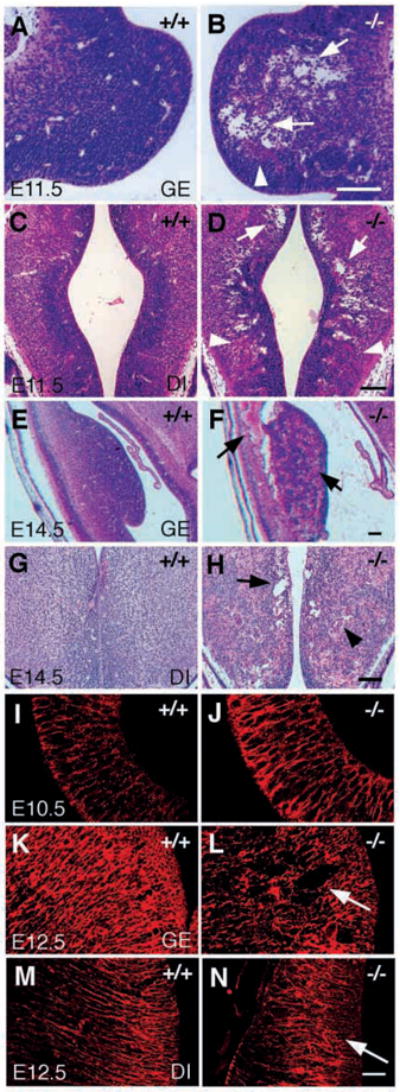
Abnormal cavitation and radial glial organization in the brains of class B integrin β8-deficient mutants. (A–H) Hematoxylin and Eosin staining of transverse sections of E11.5 brain (A–D) and E14.5 brain (E–H), showing abnormal cavitation in integrin β8-deficient mutant brains (B,D,F,H, arrows) compared with the wild-type littermates (A,C,E,G). Hemorrhage is visible in the ganglionic eminence (B, arrowhead) and diencephalon (D, arrowhead) of an E11.5 mutant brain and becomes much more severe in the E14.5 mutant brain (F, arrow; H, arrowhead). (I–N) Radial glial organization characterized by immunohistochemical staining using the RC2 antibody. Radial glial cells look grossly normal in an E10.5 mutant brain (J) compared with a wild-type littermate (I). However, they are apparently disorganized in the ganglionic eminence (L, arrow) and diencephalon (N, arrow) in the E12.5 mutant brain when compared with the same regions of E12.5 wild-type brains (K,M). GE, ganglionic eminence; DI, diencephalon. Scale bars: 100 μm in A–H; 50 μm in I–N.
Distended capillary blood vessels could result from abnormal basement membranes or defects in recruitment of pericytes. The vascular basement membranes were examined using antibodies recognizing laminin, fibronectin, collagen IV and perlecan, all of which are core components of the basement membrane. Integrin β8-deficient embryos at E12.5 express all four proteins, which are localized similarly in both wild-type and mutant embryos (data not shown). Anti-laminin staining outlines thin, elongated capillary vessels present in E12.5 wild-type brain (Fig. 6A). In E12.5 mutants, the capillary blood vessels are distended and form excessive branches (Fig. 6B). In addition, laminin expression is discontinuous in mutant capillaries (arrows in Fig. 6B). By E14.5, while capillaries in wild-type embryos are thin and isolated (Fig. 6C), the capillaries in mutants are present in aggregates, forming complex, tortuous structures (Fig. 6D). Blood cells are observed both inside and outside of these capillaries (blue staining cells) (Fig. 6B,D). This observation is further confirmed by analysis of double-labeled capillary blood vessels in the E12.5 brain with anti-PECAM and anti-fibronectin (Fig. 6E1–3, F1–3). Endothelial cells in the wild-type brain form regular thin capillary tubes, while in the mutant they form distended, disorganized capillary aggregates. Fibronectin surrounds the capillaries, but is discontinuous in some regions. Occasionally, blood cells are observed at a site of discontinuity indicating a potential point of hemorrhage (Fig. 6F2). Thus, in mutants, there are discontinuities, but no general failure in basement membrane assembly.
Fig. 6.
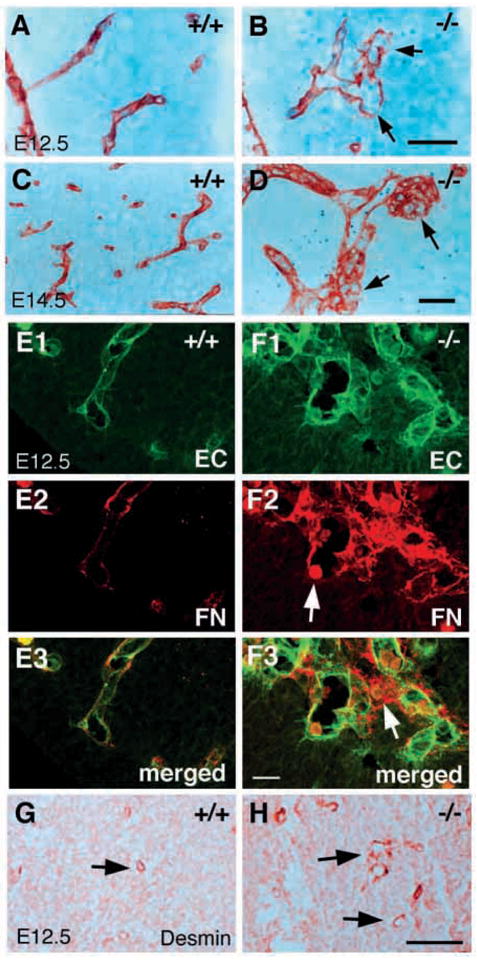
Abnormal brain capillary morphologies in class B integrin β8-deficient mutants. (A–D) Paraffin wax embedded sections of E12.5 (A,B) and E14.5 (C,D) brains stained with anti-laminin antibodies that show the abnormal morphologies of capillary vessels in the integrin β8-deficient mutant (B,D) when compared with a wild-type littermate (A,C). Discontinuous basement membranes are indicated by arrows (B). Aggregates of capillary vessels are visible (D, arrow). (E–F) Projected confocal images of E12.5 brain capillary vessels double labeled with anti-PECAM antibody (green) and anti- FN (red) antibodies. Capillary vessels in an integrin β8-deficient mutant (F1–3) exhibit irregular distended morphologies and are often conjoined when compared with those in wild-type littermates (E1–3). The basement membrane is discontinuous and a blood cell is captured at a potential hemorrhage site (F2, arrow) in the mutant. Note that a blood cell is present clearly outside of the capillaries, indicating hemorrhage in a nearby location (F3, arrow). (G,H) Pericytes recognized with anti-desmin are present and recruited to the capillaries in the brains of E12.5 wild-type (G, arrow) and mutant embryos (H, arrow). Scale bars: 50 μm in A–D; 20 μm in E,F; 100 μm in G,H.
Pericytes were detected using an antibody recognizing desmin, a pericyte-specific intermediate filament protein in the brain. Pericytes are present and are recruited to the vascular capillaries in both wild-type and mutant embryos at E12.5 (Fig. 6G,H), indicating no failure in recruitment and differentiation of pericytes in mutants.
Examination of capillary endothelial cell ultrastructure using electron microscopy identified numerous morphological abnormalities in E12.5 mutant brains (Fig. 7B). Unlike endothelial cells in wild-type brain, which are well connected to each other and their surroundings (Fig. 7A), endothelial cells from integrin β8-deficient mutants possess abundant membrane protrusions (Fig. 7B,C). In addition, large empty spaces surrounding the endothelial cells are evident, suggesting that endothelial cells are poorly attached to the ECM and to cells in their environment. Furthermore, endothelial cell membranes in the mutants are often thin and fenestrated (Fig. 7D). However, cell-cell junctions between endothelial cells appear normal and pericytes are present (data not shown).
Fig. 7.
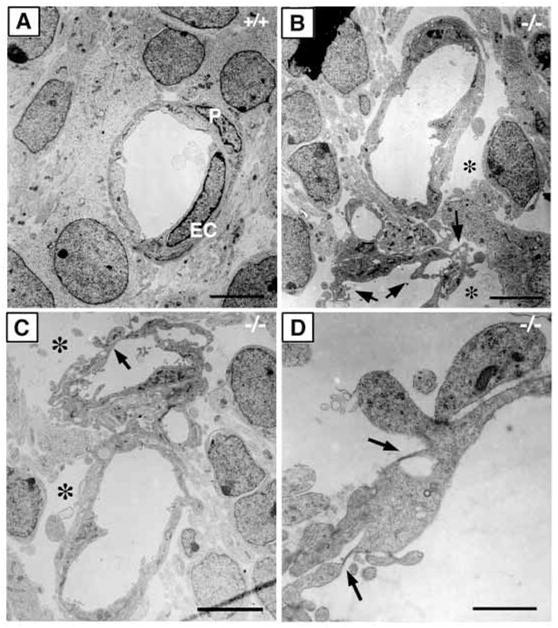
Abnormal endothelial cell morphology in class B integrin β8- deficient brains. (A–D) Electron micrographs of capillary structure in E12.5 wild-type (A) and integrin β8-deficient mutant (B–D) brains. In contrast to the wild type (A), the endothelial cells in the mutant display abundant active membrane protrusions (B, arrows) and large empty spaces surrounding the capillaries (B,C, *). In addition, fenestrations (D, arrows) are often seen in the mutant endothelium. P, pericyte; EC, endothelial cell. Scale bars: 5 μm in A–C; 1 μm in D.
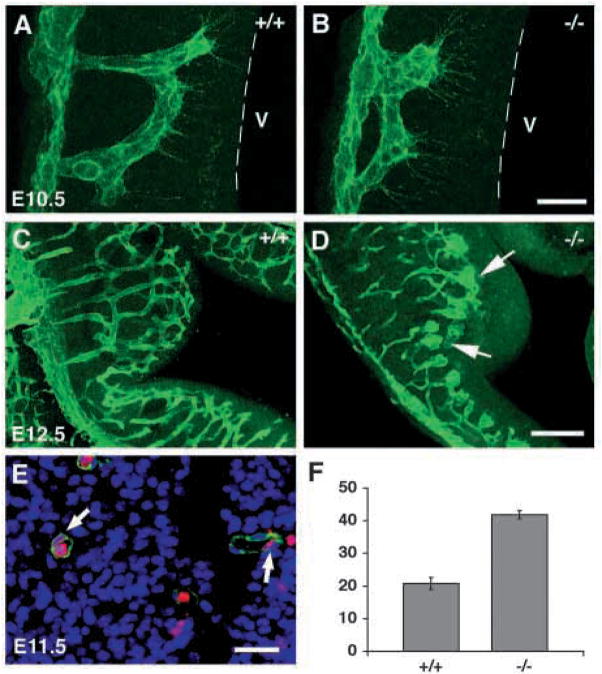
To investigate vascular development and patterning in more detail, we analyzed the brain capillary network. In E10.5 wild-type brains, capillary vessels have penetrated into the neuroepithelium and coupling of vessels has occurred near the ventricle (Fig. 8A). By contrast, capillary vessels in mutant brains extend for a shorter distance into the neuroepithelium and couple further from the ventricle (Fig. 8B). By E12.5, capillary vessels in wild-type brains have penetrated deeply into the ganglionic eminence and cortical areas, and have anastomosed at sites immediately adjacent to the ventricles (Fig. 8C). Capillary vessel growth in mutant brains, however, appears tortuous and irregular. These vessels appears to have stalled before reaching the ventricle, resulting in failure to form an organized anastomotic network. In addition, the mutant capillaries appeared bulbous and tortuous at the termini of these projections (Fig. 8D). These observations suggest that capillary vessels in integrin β8-deficient mutants develop abnormally after entering the neuroepithelium, resulting in a disorganized vascular network. Either vascular guidance cue(s) in the brain are defective or capillary endothelial cells cannot interpret these signals.
Fig. 8.
Abnormal capillary vascular patterning and endothelial cell hyperplasia in class B integrin β8-deficient brains. (A–D) Projected confocal images of brain capillary vessels stained with anti-PECAM in E10.5 (A,B) and E12.5 (C,D) embryos. Wild-type capillary vessels grow close to and couple near the ventricle (A, broken line marks boundary of ventricle, V). However, capillary vessels extend a shorter distance into the neuroepithelium and couple further away from the ventricle in the mutant (B). At E12.5, wild-type capillary vessels have branched and anastomosed to form an organized network (C); while in the mutant, capillary vessels show bulbous distentions and have not invaded regions of neuroepithelium immediately adjacent to the ventricle (D, arrows). (E) A brain capillary stained for isolectin BS (staining endothelial cell, green), DAPI (staining nuclei, blue) and BrdU (labeling proliferating cell, red, arrows). (F) The quantification of endothelial cell nuclei per vessel cross section in E11.5 wild-type and mutant brains. The percentage of BrdU-labeled endothelial cell nuclei in total endothelial cell nuclei scored is shown in the histogram (n=3). The error bars represent the s.e.m. Wild-type and mutant values are significantly different (P<0.005). Scale bars: 20 μm in A,B; 100 μm in C,D; 25 μm in E.
Endothelial cell hyperplasia in class B β8-deficient mutants
The ultrastructural analysis of endothelial cells in Class B mutants suggests that endothelial cells are hyperactive (Fig. 7). To examine their proliferation rate, we measured the percentage of endothelial cell nuclei labeled with BrdU in cross-sections of the E11.5 ganglionic eminence (Fig. 8E). We observed a twofold increase in the percentage of BrdU-labeled endothelial cells in the mutant compared with the wild type (n=3, Fig. 8F). In one of the three mutant embryos, hyperproliferating endothelial cells are observed in the absence of vascular hemorrhage, suggesting that endothelial cell hyperproliferation precedes brain hemorrhage in class B mutants.
Interestingly, overexpression of VEGF can result in endothelial cell proliferation with highly fenestrated and ‘glomeruloid-like’ vessels (Cheng et al., 1997; Sundberg et al., 2001), similar to those observed here. To determine whether elevated expression of VEGF might account for the defective vasculature observed in the brains of the integrin β8 mutants, we measured VEGF levels in the brains of E12.5 and E14.5 control and mutant mice. A significant increase of VEGF in the mutant brains was not seen at either age [E12.5 wild type, 24.6±8.3 ng/g; E12.5 mutant, 22.4±1.8 ng/g (n=3); E14.5 wild type, 28.5±6.1 ng/g; E14.5 mutant, 30.6±0.8 ng/g (n=2)]. We were unable to localize VEGF within the brain by immunohistochemistry.
Abnormal neuroepithelial organization in class B β8- deficient mutants
Radial glial cells are the predominant cell type populating the early neuroepithelium. Their cell bodies reside near the internal ventricular surface and their processes extend radially towards the external surface at the pia (Rakic, 1972). Their processes also wrap around developing brain capillary vessels (Noctor et al., 2001), suggesting they may regulate capillary development. Neuroepithelial cell organization was examined with the radial glial cell-specific antibody RC2 (Edwards et al., 1990). The alignment of radial glial cell processes appears normal in E10.5 mutant embryos (Fig. 4J), but becomes aberrant in the ganglionic eminence and diencephalon before E12.5 (Fig. 4L,N). In contrast to wild-type brains, where radial glial processes extend in a parallel fashion from the ventricle towards the pial surface (Fig. 4K,M), large cavitations with disruptions of radial glia processes are observed in the ganglionic eminences of mutants (Fig. 4L). In the diencephalons, radial glial processes appear tortuous and disconnected from the pial surface (Fig. 4N). When examined by RC2 and anti-PECAM double labeling, cavitations observed within disorganized radial glial cells are not necessarily associated with sites of hemorrhage, suggesting that they are not simply a secondary consequence of hemorrhage (data not shown). TUNEL analyses of E12.5 brain sections detected no excessive apoptosis of radial glial cells in mutant embryos (data not shown).
Integrin β8 is expressed in periventricular neuroepithelial cells
The defects observed in integrin β8-deficient embryos might result from the absence of integrin β8 function within endothelial cells, neuroepithelial cells or both. The phenotype could reflect a defect in cell adhesion, basement membrane organization or cell signaling. In situ hybridization analysis indicated that the integrin β8 transcript is localized to the embryonic brain in the periventricular zone at E10.5 (Fig. 7A,B) and in periventricular cells surrounding all four brain ventricles at E12.5 and E13.5 (Fig. 7C–F). As the predominant cell type adjacent to the ventricles, radial glial cells must express integrin β8. If integrin β8 were expressed in endothelial cells, obvious labeling of the capillary vasculature outside of periventricular area would be expected at E12.5 and E13.5, but none was observed (Fig. 7C–F). The failure to label the vascular network inside or outside of the brain suggests that expression of integrin β8 in neuroepithelial cells regulates capillary vessel growth in the early embryonic brain.
DISCUSSION
With a genetic approach, we have found that absence of integrin β8 results in embryonic or perinatal lethality with profound vascular defects. The majority of mutants (class A) die during midgestation with defective vascularization in the placenta and yolk sac as well as abnormal angiogenesis in the neuroepithelium. Approximately 35% of mutants (class B) die perinatally with intracerebral hemorrhage and abnormal vasculature in the brain. Unusually high proliferation of endothelial cells is observed and apparently precedes hemorrhage in class B mutant brains. In addition, vascular patterning and organization of the neuroepithelium are disrupted in class B mutant brains. Finally, expression studies localize integrin β8 transcripts to endodermal cells in the yolk sac and periventricular neuroepithelial cells in the early embryonic brain. We propose that integrins containing the β8 subunit are required in some tissues for vascular morphogenesis by providing non-autonomous cellular cues for capillary patterning. This study thus identifies a molecule crucial for proper patterning of the embryonic yolk sac and brain vasculature.
Integrin β8 and brain vascular patterning
Class B integrin β8-deficient embryos develop extensive intracerebral hemorrhage, suggesting that there is defective development of the vasculature. Consistent with this, we observed in the mutant brains aberrant capillary vessel growth and patterning (Fig. 8A–D), distended capillary blood vessels with discontinuous basement membranes (Fig. 6), and abnormal endothelial morphology (Fig. 7) and proliferation (Fig. 8E,F). The abnormal vascular morphogenesis is unlikely to be an incidental consequence of defective neuroepithelial cell organization because aberrant vasculature and hemorrhage are not observed in Pax6-deficient mice, which also exhibit defective radial glial cell differentiation and organization (Gotz et al., 1998). Therefore, β8 integrins are likely to perform a more direct role in regulating brain capillary growth and patterning. To regulate vascular morphogenesis, β8 integrins can either act from neuroepithelial cells to direct capillary growth or act from endothelial cells to respond to guidance signals. The localization of β8 on neuroepithelial cells, but not endothelial cells (Fig. 9) suggests that β8 integrins function in a cell non-autonomous fashion on neuroepithelial cells.
Fig. 9.
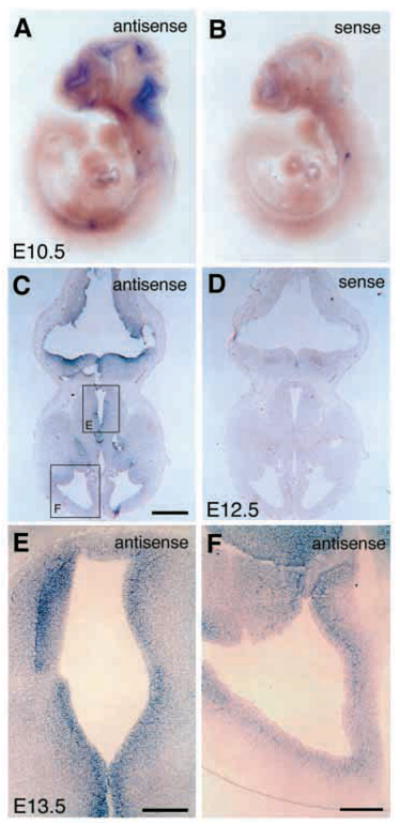
Integrin β8 is expressed in periventricular cells of the embryonic brain. (A,B) Whole-mount in situ hybridization analyses shows integrin β8 mRNA expression in tissues surrounding the ventricles of E10.5 embryonic brain (A, antisense; B, sense). (C,D) Transverse sections of E12.5 brains hybridized with integrin β8 antisense (C) or sense (D) probes showing localization of integrin β8 to periventricular cells in the neuroepithelium. (E,F) Similar areas to those shown in C at E12.5 (C, box E and box F) are shown at a higher magnification in an E13.5 brain hybridized with an integrin β8 antisense probe (E,F). Note the absence of integrin β8 mRNA from the vascular cells of the brain (A) and inside of the brain area (C,E). Scale bar: 500 μm in C and D; 200 μm in E,F.
In the absence of integrin β8, organization of the neuroepithelium is abnormal as shown by cavitations in periventricular tissues as well as disorganization of the neuroepithelial cells (Fig. 4). Some of cavitations and disorganized neuroepithelial cells are observed outside of the hemorrhage area. Nevertheless, as the disorganization of the neuroepithelium is intimately associated with the vascular defects in class B mutant brains, it may be a secondary consequence of defective and leaky vasculature. Consistent with this possibility, electron microscopy analysis of neuroepithelial cell morphology in the E12.5 brain showed no apparent abnormalities (data not shown).
How might β8 integrins function to generate or regulate vascular guidance cues? One possibility is that β8 integrins could bind to a receptor expressed on capillary endothelial cells and directly function as cell-tethered guidance molecules. Numerous examples of transmembrane receptors functioning as guidance molecules have been documented in studies of axonal growth and guidance (Yu and Bargmann, 2001). Alternatively, β8 integrins could indirectly regulate vascular morphogenesis by affecting the production and distribution of angiogenic factors that are required for capillary growth. Notably, VEGF is expressed in periventricular neuroepithelial cells and bioactive forms of surface bound VEGF are released by proteolysis (Breier et al., 1992; Ferrara, 1999). Even though overexpression of VEGF can result in endothelial cell and vascular phenotypes similar to those of the integrin β8 mutant (Cheng et al., 1997; Sundberg et al., 2001), we did not detect a significant increase in total VEGF levels in the brains of mutant compared with wild-type embryos. Thus, β8 integrins must regulate endothelial cell proliferation and vasculogenesis through a different pathway. Additionally, β8 integrins could regulate capillary growth by organizing extracellular matrix substrates necessary for capillary migration and growth. There is evidence in class B mutants that the capillary basement membrane is discontinuous, indicating that extracellular matrix organization may be defective. In other experimental systems, β1 integrins have been shown to be required for laminin assembly (Colognato et al., 1999; Wu, 1997).
Integrin αvβ8
Like integrin β8 mutants, there are two classes of integrin αv mutant mice with a majority dying embryonically at E10.5– E11.5 with placental defects and a minority dying perinatally with intracerebral hemorrhage (Bader et al., 1998). Integrin αv is reportedly expressed in radial glial cells beginning at E10.5 (Hirsch et al., 1994). These similarities indicate that integrin α vβ8 is required for normal vascular development in the placenta and embryonic brain, and for the proper organization of the neuroepithelium. In addition to the β8 subunit, the αv subunit can associate with multiple other integrin β subunits (β1, β3, β5, β6). However, mice that lack β3 (Hodivala-Dilke et al., 1999), β5 (Huang et al., 2000), or β6 (Huang et al., 1996) are viable and exhibit no obvious defects in vascular development. This suggests that other α v-containing integrins can not compensate for the absence of α vβ8 during early development.
In addition to the similarities described above, there are several differences between the integrin β8 and the integrin αv mutant phenotypes. The yolk sac vascular defect in integrin β8- deficient mutants has not been reported in integrin α v-null mice. Although not the only interpretation, it thus seems possible that an additional unidentified α subunit pairs with integrin β8 in the yolk sac. Notably, 24 integrin α and 9 integrin β genes have been identified in the human genome sequencing project, of these six α and one β subunits were not identified previously and thus the proteins encoded by these sequences have not been characterized (Venter and Adams, 2001). The aberrant angiogenesis and absent floor plate detected in the E10.5 neuroepithelium of integrin β8 class A mutants, and the abnormal capillary growth and vascular patterning in integrin β8 class B mutants have not been reported in integrin αv knockout mice (Bader et al., 1998). If these differences are confirmed by further analyses of integrin α v-null embryos, they suggest that other integrin α subunits are likely to participate with integrin β8 in these developmental processes. Alternatively, overexpression of one of the other αv partners in the absence of β8 could account for some of the phenotypic features.
Integrin α v-null neonates develop a cleft palate and intestinal hemorrhage with 100% penetrance; however, intestinal hemorrhage was never observed and palatal defects were only observed rarely in integrin β8 mutants. This suggests that the absence of other α v-containing heterodimers is responsible for these phenotypes.
Vascular morphogenesis in embryonic brain
What are the molecular bases of brain vascular patterning cues regulated by integrin β8? Studies of integrins α vβ1, α vβ5 and α vβ6 have shown that each can bind to TGFβ1 (Munger et al., 1998; Munger et al., 1999). Recent biochemical studies indicate that integrin α vβ8 can bind and activate TGFβ1 signaling in cultured cells (Mu et al., 2002). A mutation in endoglin, a TGFβ1 receptor involved in its signaling, results in brain hemorrhage in humans (McAllister et al., 1994). We are currently investigating the possibility that TGFβ1 signaling is involved in regulating vascular development in the embryonic brain. Interestingly, mice bearing mutations in PDGFB and PDGF receptor-β (PDGFRβ) have intracerebral hemorrhage beginning at E16.5 (later than that in integrin β8-deficient mice) and show endothelial cell hyperproliferation at E11.5 (Hellstrom et al., 2001). These phenotypes are believed to result from defective differentiation and recruitment of pericytes, which are dependent upon PDGF-/PDGFRβ signaling (Hellström et al., 1999; Lindahl et al., 1997). It is possible that integrin β8 functions upstream to affect PDGFB/PDGFRβ signaling. In addition, intracerebral hemorrhages have been observed in mice carrying mutations in transcription factors, such as Id1,3 (Lyden et al., 1999), Fli1 (Spyropoulos et al., 2000) and CBP (Tanaka et al., 2000), and components of the Notch signaling pathway, such as presenilin 1 (Shen et al., 1997) and Numb (Zhong et al., 2000). However, the cellular defects associated with intracerebral hemorrhages in these mutants have not been well characterized. Comparisons of the brain capillary defects of these mutants to those of integrin β8-deficient mutants may help to elucidate the mechanisms regulating brain vascular development.
Brain vascular patterning is a complex process whose regulating mechanisms are poorly characterized. We show here that ablation of integrin β8 results in abnormal vascular capillary growth and patterning in the embryonic brain. Thus, integrin β8 is essential for normal development of the vascular network in the embryonic brain. In addition, the periventricular hemorrhage observed in the integrin β8-deficient mutant brain is reminiscent of that associated with germinal matrix hemorrhage (GMH), which affects premature human babies (Sherwood et al., 1978). Further analyses of the expression and functions of integrin β8 should help to unravel the regulatory mechanisms necessary to establish an organized capillary network in the embryonic brain and may also shed light on the cause of GMH.
Acknowledgments
We thank Tom Sato for Tie2:lacZ reporter mice; Steve Nishimura for sharing unpublished observations and critical reading of the manuscript; Ivy Hsieh for assistance on EM analysis; Donald McDonald for extensive insightful discussions; and Zhen Huang, Ursula Fuenfschilling and Ravi Majeti for helpful comments on the manuscript. This work was supported by NIH grant NS19090 and by the Howard Hughes Medical Institute (L. F. R.). J. Zhu is the recipient of an N.I.H. N.R.S.A. Fellowship. L. F. R. is an investigator of the Howard Hughes Medical Institute.
References
- Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RH, Diella F, Hennig S, Helmbacher F, Deutsch U, Klein R. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell. 2001;104:57–69. doi: 10.1016/s0092-8674(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- Baldwin HS, Shen HM, Yan HC, DeLisser HM, Chung A, Mickanin C, Trask T, Kirschbaum NE, Newman PJ, Albelda SM, et al. Platelet endothelial cell adhesion molecule-1 (PECAM- 1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development. 1994;120:2539–2553. doi: 10.1242/dev.120.9.2539. [DOI] [PubMed] [Google Scholar]
- Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Cheng SY, Nagane M, Huang HS, Cavenee WK. Intracerebral tumor-associated hemorrhage caused by overexpression of the vascular endothelial growth factor isoforms VEGF121 and VEGF165 but not VEGF189. Proc Natl Acad Sci USA. 1997;94:12081–12087. doi: 10.1073/pnas.94.22.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato H, Winkelmann DA, Yurchenco PD. Laminin polymerization induces a receptor-cytoskeleton network. J Cell Biol. 1999;145:619–631. doi: 10.1083/jcb.145.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development. 1995;121:1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897– 1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- Edwards MA, Yamamoto M, Caviness VS., Jr Organization of radial glia and related cells in the developing murine CNS. An analysis based upon a new monoclonal antibody marker. Neuroscience. 1990;36:121–144. doi: 10.1016/0306-4522(90)90356-9. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Cheresh DA. The role of alphav integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Invest. 1999;103:1227–1230. doi: 10.1172/JCI6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999;77:527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Flamme I, Risau W. Induction of vasculogenesis and hematopoiesis in vitro. Development. 1992;116:435–439. doi: 10.1242/dev.116.2.435. [DOI] [PubMed] [Google Scholar]
- Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Gotz M, Stoykova A, Gruss P. Pax6 controls radial glia differentiation in the cerebral cortex. Neuron. 1998;21:1031–1044. doi: 10.1016/s0896-6273(00)80621-2. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Zwijsen A, van Rooijen MA, Huylebroeck D, Roelen BA, Mummery CL. Transforming growth factor-beta signalling in extraembryonic mesoderm is required for yolk sac vasculogenesis in mice. Development. 1999;126:3473–3483. doi: 10.1242/dev.126.16.3473. [DOI] [PubMed] [Google Scholar]
- Graef IA, Chen F, Chen L, Kuo A, Crabtree GR. Signals Transduced by Ca2+/Calcineurine and NFATc3/c4 pattern the developing vasculature. Cell. 2001;105:863–875. doi: 10.1016/s0092-8674(01)00396-8. [DOI] [PubMed] [Google Scholar]
- Hellström M, Kaln M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herken R, Götz W, Wattjes KH. Initial development of capillaries in the neuroepithelium of the mouse. J Anat. 1989;164:85–92. [PMC free article] [PubMed] [Google Scholar]
- Hirsch E, Gullberg D, Balzac F, Altruda F, Silengo L, Tarone G. Alpha v integrin subunit is predominantly located in nervous tissue and skeletal muscle during mouse development. Dev Dyn. 1994;201:108– 120. doi: 10.1002/aja.1002010203. [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Griffiths M, Wu J, Farese RV, Jr, Sheppard D. Normal development, wound healing, and adenovirus susceptibility in beta5-deficient mice. Mol Cell Biol. 2000;20:755–759. doi: 10.1128/mcb.20.3.755-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, Farese RV, Jr, Sheppard D. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133:921–928. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO, Bader BL. Targeted mutations in integrins and their ligands: their implications for vascular biology. Thromb Haemost. 1997;78:83–87. [PubMed] [Google Scholar]
- Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, et al. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O’Reilly R, Bader BL, Hynes RO, Zhuang Y, Manova K, et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature. 1999;401:670–677. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Cerebral Cortex. New York: Plenum; 1988. [Google Scholar]
- McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J, et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8:345–351. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- Milner R, Frost E, Nishimura S, Delcommenne M, Streuli C, Pytela R, Ffrench-Constant C. Expression of alpha vbeta3 and alpha vbeta8 integrins during oligodendrocyte precursor differentiation in the presence and absence of axons. Glia. 1997a;21:350–360. [PubMed] [Google Scholar]
- Milner R, Wilby M, Nishimura S, Boylen K, Edwards G, Fawcett J, Streuli C, Pytela R, ffrench-Constant C. Division of labor of Schwann cell integrins during migration on peripheral nerve extracellular matrix ligands. Dev Biol. 1997b;185:215–228. doi: 10.1006/dbio.1997.8547. [DOI] [PubMed] [Google Scholar]
- Moyle M, Napier MA, McLean JW. Cloning and expression of a divergent integrin subunit beta 8. J Biol Chem. 1991;266:19650– 19658. [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin αvβ8 mediates epithelial homeostasis through MT1- MMP-dependent activation of TGF-β1. J Cell Biol. 2002 doi: 10.1083/jcb.200109100. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Harpel JG, Giancotti FG, Rifkin DB. Interactions between growth factors and integrins: latent forms of transforming growth factor-beta are ligands for the integrin alphavbeta1. Mol Biol Cell. 1998;9:2627–2638. doi: 10.1091/mbc.9.9.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Nishimura SL, Sheppard D, Pytela R. Integrin alpha v beta 8. Interaction with vitronectin and functional divergence of the beta 8 cytoplasmic domain. J Biol Chem. 1994;269:28708–28715. [PubMed] [Google Scholar]
- Nishimura SL, Boylen KP, Einheber S, Milner TA, Ramos DM, Pytela R. Synaptic and glial localization of the integrin alphavbeta8 in mouse and rat brain. Brain Res. 1998;791:271–282. doi: 10.1016/s0006-8993(98)00118-8. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Puri MC, Rossant J, Alitalo K, Bernstein A, Partanen J. The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J. 1995;14:5884–5891. doi: 10.1002/j.1460-2075.1995.tb00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- Schaeren-Wiemers N, Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- Schlaeger TM, Bartunkova S, Lawitts JA, Teichmann G, Risau W, Deutsch U, Sato TN. Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. Proc Natl Acad Sci USA. 1997;94:3058–3063. doi: 10.1073/pnas.94.7.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Ann Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- Sherwood A, Hopp A, Smith JF. Cellular reactions to subependymal plate haemorrhage in the human neonate. Neuropathol Appl Neurobiol. 1978;4:245–261. doi: 10.1111/j.1365-2990.1978.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Spyropoulos DD, Pharr PN, Lavenburg KR, Jackers P, Papas TS, Ogawa M, Watson DK. Hemorrhage, impaired hematopoiesis, and lethality in mouse embryos carrying a targeted disruption of the Fli1 transcription factor. Mol Cell Biol. 2000;20:5643–5652. doi: 10.1128/mcb.20.15.5643-5652.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp MA. Alpha9 and beta8 integrin expression correlates with the merger of the developing mouse eyelids. Dev Dyn. 1999;214:216–228. doi: 10.1002/(SICI)1097-0177(199903)214:3<216::AID-AJA5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Sundberg C, Nagy JA, Brown LF, Feng D, Eckelhoefer IA, Manseau EJ, Dvorak AM, Dvorak HF. Glomeruloid microvascular proliferation follows adenoviral vascular permeability factor/vascular endothelial growth factor-164 gene delivery. Am J Pathol. 2001;158:1145–1160. doi: 10.1016/S0002-9440(10)64062-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Naruse I, Hongo T, Xu M, Nakahata T, Maekawa T, Ishii S. Extensive brain hemorrhage and embryonic lethality in a mouse null mutant of CREB-binding protein. Mech Dev. 2000;95:133–145. doi: 10.1016/s0925-4773(00)00360-9. [DOI] [PubMed] [Google Scholar]
- Tuckwell DS, Humphries MJ. A structure prediction for the ligand-binding region of the integrin beta subunit: evidence for the presence of a von Willebrand factor A domain. FEBS Lett. 1997;400:297–303. doi: 10.1016/s0014-5793(96)01368-3. [DOI] [PubMed] [Google Scholar]
- Venstrom K, Reichardt L. Beta 8 integrins mediate interactions of chick sensory neurons with laminin-1, collagen IV, and fibronectin. Mol Biol Cell. 1995;6:419–431. doi: 10.1091/mbc.6.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter JC, Adams MD. The sequence of the human genome. Science. 2001;291:1304–1350. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Wu C. Roles of integrins in fibronectin matrix assembly. Histol Histopathol. 1997;12:233–240. [PubMed] [Google Scholar]
- Xiong J, Stehle T, Diefenbach B, Zhang R, Reinhardt D, Scott DL, Joachimiak A, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin αvβ3. Science. 2001;294:339– 345. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Gao X, Lindsell CE, Norton CR, Chang B, Hicks C, Gendron-Maguire M, Rand EB, Weinmaster G, Gridley T. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum Mol Genet. 1999;8:723–730. doi: 10.1093/hmg/8.5.723. [DOI] [PubMed] [Google Scholar]
- Yu TW, Bargmann CI. Dynamic regulation of axon guidance. Nat Neurosci. 2001;4 (Suppl):1169–1176. doi: 10.1038/nn748. [DOI] [PubMed] [Google Scholar]
- Zhong W, Jiang MM, Schonemann MD, Meneses JJ, Pedersen RA, Jan LY, Jan YN. Mouse numb is an essential gene involved in cortical neurogenesis. Proc Natl Acad Sci USA. 2000;97:6844–6849. doi: 10.1073/pnas.97.12.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]