Abstract
Nerve growth factor is known to stimulate neurite outgrowth and support neuronal survival during embryonic development. We have studied the expression of the nerve growth factor receptor, TrkA, at both mRNA and protein levels during the course of chicken retinal development. Furthermore, we have compared the expression of trkA mRNA with that of the 75-kD low-affinity neurotrophin receptor (p75NTR). RNase protection assay identified peak-levels of trkA mRNA in the late embryonic retina. Using in situ hybridization and immunohistochemistry, we found cells expressing TrkA in both the internal and the external part of the inner nuclear layer, corresponding to amacrine and horizontal cells, respectively. The TrkA-expressing amacrine cell has a unistratified dendritic arborization in the second sublamina of the inner plexiform layer, and may represent the stellate amacrine cell described by Cajal. The horizontal cells, possessing arciform dendrite processes in the outer plexiform layer, showed strong TrkA immunoreactivity in both dendrites and cell bodies. During the course of retinal development, the TrkA-expressing amacrine cells decreased in number, whereas the TrkA-expressing horizontal cells persisted. Because nerve growth factor was expressed where the horizontal cells, but not where the amacrine cells were located, these findings raise the question of whether nerve growth factor could locally support the survival of TrkA-expressing interneurons during retinal development.
Indexing terms: avian, interneuron, immunohistochemistry, P75 neurotrophin receptor
During development of the nervous system, neurons are initially formed in an excess, and the number of neurons is reduced during periods of naturally occurring neuronal death. The neurotrophins, including the nerve growth factor (NGF), are structurally related proteins that are known to support neuronal survival and to stimulate neurite outgrowth (Ebendal, 1992; Snider, 1994; Lewin and Barde, 1996). These factors are expressed in the target tissue of innervating neurons. This is particularly evident in the peripheral nervous system, where NGF has been shown to support survival of sympathetic neurons and a subset of sensory neurons. The innervating neurons in turn express TrkA, a receptor for NGF, which upon ligand-binding mediates the cellular effects. TrkA is a transmembrane glycoprotein of approximately 140 kD (Cordon-Cardo et al., 1991; Hempstead et al., 1991; Kaplan et al., 1991a; Nebreda et al., 1991), which contains an intracellular tyrosine kinase domain. NGF causes receptor dimerization upon binding TrkA, thereby initiating the signaling transduction cascade (Kaplan et al., 1991b). In addition to binding to TrkA, NGF and the other neurotrophins bind to the 75-kD low-affinity neurotrophin receptor (p75NTR; Rodriguez-Tébar et al., 1990). This receptor is a transmembrane glycoprotein distantly related to a family of cytokine receptors, and has been suggested to function as a co-receptor for TrkA, where p75NTR modulates and mediates high-affinity binding for NGF (Hempstead et al., 1991; Mahadeo et al., 1994; Verdi et al., 1994; Chao and Hempstead, 1995). Another suggested role for p75NTR is to induce cell death upon NGF binding in the absence of TrkA (Casaccia-Bonnefil et al., 1996; Frade et al., 1996). In addition to the target-derived support, neurotrophins may serve a local mode of action, autocrine or paracrine, amongst neurons in the peripheral ganglia (Ernfors and Persson, 1991; Schecterson and Bothwell, 1992). Less is known about the modes of action and the function of NGF and the other neurotrophins in the central nervous system. We have previously shown that NGF induces neurite outgrowth from retinal explants (Hallböök et al., 1996) and in the same study found that mRNA for NGF and TrkA is expressed in the external part of the inner nuclear layer (INL) of embryonic day 18 (E18) chicken retina. In the present study, we have analyzed in more detail the expression of trkA mRNA and TrkA-immunoreactivity (IR) in the developing retina from E4 to hatching. We have compared the TrkA with p75NTR expression, and based on the localization and morphology of the labeled cells, we discuss the identity of the TrkA-expressing cells in relation to previously described amacrine and horizontal cells, and the role of TrkA receptors in the developing chicken retina.
MATERIALS AND METHODS
Preparation and analysis of RNA
Only White Leghorn chicken embryos were used in this study and they were incubated at 37°C. The embryos were staged according to the series of Hamburger and Hamilton (1951), these stages are referred to in this study as days of incubation. Retinas from chicken embryos at ages E5.5–6/stage 28 (st28), E8.5–9/st35, E12/st38, E15/st41, E18/st44, and 5-day-old hatchlings (P5) were dissected, and total RNA prepared as described in detail previously (Williams et al., 1993). Efforts were made to minimize animal suffering and to reduce the number of animals used. The procedures were approved by the local ethics committee for the use of experimental animals in Uppsala. The experiments have been performed in accordance with the European Community guidelines. Briefly, 0.25 to 0.5 g of tissue was homogenized in 7.5 ml of extraction buffer, containing 5.5 M guanidinium isothiocyanate, 25 mM sodium citrate, pH 7.0, 0.5% sodium lauryl sarcosine, and 0.2 M β-mercaptoethanol, by using a Polytron homogenizer. The samples were layered on 4 ml of cesium trifluoroacetate (density, 1.51 g/ml) and centrifuged at 27,000 revolutions per minute at 15°C for 22 hours. The pelleted RNA was dissolved in 200 μl of water and phenol extracted prior to ethanol precipitation. The concentration of RNA was measured spectrophotometrically at 260 nm, and the integrity of the RNA was analyzed on agarose gels.
The RNase protection assay (RPA) was performed by using the RPAII Ribonuclease Protection Assay Kit (Ambion, Austin, TX). cRNA probes of trkA and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were in vitro transcribed from DNA templates cloned into the pBS SK M13+ and the pBS KS M13+ cloning vectors (Stratagene, La Jolla, CA), respectively. The 431-bp trkA template, synthesized by polymerase chain reaction, corresponds to nucleotides 1042–1473 of the full-length chicken trkA cDNA clone (Schröpel et al., 1995), which spans the transmembrane domain, as well as the juxtamembrane domain of the chicken TrkA receptor. The 202-bp GAPDH template corresponds to amino acid residues 224–292 in the chicken GAPDH (Panabières et al., 1984). The cRNA probe for trkA was in vitro transcribed with α-[32P]-UTP (Amersham, Buckinghamshire, UK) to a specific activity of approximately 6.8 × 108 counts per minute/μg by using T3 RNA polymerase (Promega, Madison, WI). The probe for GAPDH was in vitro transcribed to a specific activity of approximately 1.1 × 108 counts per minute/μg by using T7 RNA polymerase. trkA probe corresponding to 500,000 counts per minute and GAPDH probe corresponding to 200,000 counts per minute were hybridized to 10 μg of total RNA in 20 μl hybridization buffer containing 80% formamide, 0.3 M sodium acetate, 0.1 M sodium citrate and 1 mM EDTA. After hybridization, the samples were treated with 0.6 U ribonuclease A and 12 U ribonuclease T1 at 30°C for 60 minutes. Protected complementary RNA (cRNA) fragments were analyzed on a denaturing 5% polyacrylamide gel. The gel was dried and subsequently exposed to screens and scanned by using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). The images were quantified by using ImageQuant software and the trkA signal was normalized to the GAPDH mRNA content, which was used as an internal standard. We have previously confirmed the linearity in this assay by two-fold dilutions of RNA, and the relationship between the signal and the amount of RNA included, is constant (Hallböök et al., 1996).
Cryostat sectioning and in situ hybridization analysis
Embryos of embryonic ages ranging from E4/st23 to E17/st43 were removed from the eggs. The heads were dissected free from skin, feathers and beak, and were immediately frozen and stored at −70°C until sectioning. Ten-micrometer-thick sections of the total head, including both eyes, were cut using a cryostat (Leitz Digital 1702; Wetzlar, Federal Republic of Germany). The sections were thaw-mounted onto 50 mg/ml poly-L-lysine-coated slides, air dried for 30 minutes, and stored at −70°C before use.
For in situ hybridization, we used a synthetic oligonucleotide probe complementary to trkA mRNA corresponding to amino acids 658–673 of the chicken TrkA receptor (Bäckström et al., 1996). This includes the tyrosine-kinase domain of the receptor mRNA. We also used an oligonucleotide probe for the chicken p75NTR mRNA corresponding to amino acids 47–60 of the p75NTR receptor (Hallböök et al., 1990). Fifty nanograms of the respective oligonucleotide probes were labeled at their 3′ end with deoxyadenosine 5′-[α-35S]thiotriphosphate using terminal deoxynucleotidyl transferase (Promega) to a specific activity of approximately 1.5 × 109 counts per minute/μg. The probes were purified on a Nensorb 20 column (DuPont, Wilmington, Delaware) prior to use. In situ hybridization was performed as previously described (Hallböök et al., 1993) at 42°C. The hybridization solution contained 50% formamide, 4× standard saline citrate (SSC), 10% dextran sulfate, 0.275 mg/ml yeast tRNA, 0.06 M dithiotreitol, 0.1× Denhardt’s, and 0.5 mg/ml sonicated salmon sperm DNA. After hybridization, the sections were washed once for 15 minutes in 1× SSC with 0.05% Sarcosyl at 55°C, three times for 15 minutes in 0.5× SSC, and finally two 30-second washes in cold RNase-free water. The sections were dehydrated in ethanol and left to air dry. Subsequently they were dipped in Kodak NTB-2 photographic emulsion diluted 1:1 in distilled water and left to expose at 4°C for 4 to 6 weeks. The slides were developed and lightly counter stained with cresyl violet. As an in situ hybridization control, 100 times excess of nonlabeled oligonucleotide probe was added to the hybridization solution prior to hybridization to block the specific signals.
Immunohistochemistry
Eyes from embryos of ages E5/st26, E5.5–6/st28, E7–7.5/st31, E8/st34, E10/st36, E13/st39, E15/st41, E17/st43, and from hatchlings (P0) were dissected and fixed for 2 hours with 2% paraformaldehyde in 0.1 M phosphate buffer, pH 7.0. Then the eyes were cryoprotected using 30% sucrose in phosphate buffered saline (PBS), pH 7. Eyes were frozen in Tissuetek before being sectioned in a cryostat. Ten-micrometer-thick sections were collected and processed for immunohistochemistry. Sections were incubated overnight at 4°C with a rabbit polyclonal anti-chicken TrkA antibody (Lefcort et al., 1996; Oakley et al., 1997) diluted to 1 μg/ml in PBS, containing 0.3% Triton X-100 and 5% normal serum, and were subsequently washed twice with PBS for 5 minutes. The Vectastain kit (Vector Labs, Burlingame, CA) was used to visualize the TrkA antibody by using the peroxidase-3–3′-diaminobenzidine reaction according to the manufacturer’s instructions. Described briefly, the sections were incubated for 1 hour at room temperature with a 1:200 dilution of biotinylated anti-rabbit IgG (Vectastain) and were washed twice with PBS for 5 minutes. Sections were then incubated with pre-formed avidin-biotin-peroxidase complexes for another hour at room temperature, followed by two washes with PBS for 15 minutes at room temperature. The peroxidase complex was localized in the tissue by using diaminobenzidine tetrahydrochloride, which produced a brown precipitate. Sections were dehydrated in alcohols, cleared in xylene, and coverslipped with Permount. Images of the stained retinas were captured by using a digital scanner (Lumina Leaf System Inc., Southborough, MA) and the figures were processed for printing on a Tektronix Phaser II sdx color printer by using Adobe Photoshop (Adobe Systems Inc., Mountain View, CA). Minor (<5%) contrast and shade adjustments were done to equalize the different panels to each other.
RESULTS
We have analyzed the levels of trkA mRNA in the developing chicken retina using RPA. As shown in Figure 1, a fragment of 431 bp corresponding to nucleotides 1042–1473 of the full length chicken trkA cDNA clone (Schröpel et al., 1995), was protected after being hybridized to total RNA from retina, but not by control yeast tRNA. At E5.5–6, the earliest time point tested, we detected very low levels of trkA mRNA. At E8.5–9, E12, and E15, increasing levels were detected, and peak levels were reached at E18. By P5, the latest time point tested, the levels had decreased and were somewhat higher compared to the levels at E12.
Fig. 1.
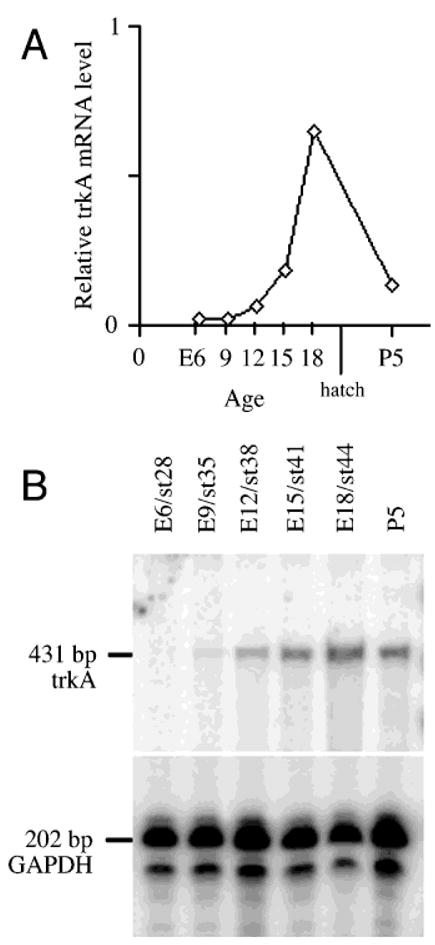
Levels of trkA mRNA in the developing retina. The graph (A) shows the relative levels of trkA mRNA in the chicken retina during development. The levels were measured using RNase protection assay on total RNA prepared from retinas of the indicated ages, and a representative gel is illustrated in B. Ten micrograms of total RNA of each age was analyzed in the assay, and as an internal standard, a probe for chicken glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used. Analysis of protected RNA fragments was made on a denaturing polyacrylamide gel, which was exposed to screens, and scanned by a PhosphorImager. E, embryonic day; P, postnatal day; st, Hamburger and Hamilton stage.
Cells located in the internal INL express TrkA
By using in situ hybridization analysis we found the first weak labeling for trkA mRNA diffusely spread over the INL in E7–7.5 retina. At E8.5–9 (Fig. 2A), cells located both in the external and the internal part of the INL were labeled for trkA mRNA. The location of these cells corresponded to that of future horizontal and amacrine cells, respectively. However, the labeled cells in the internal INL started to decrease in number after E8.5–9, and very few labeled cells were left in the internal INL in retinas of later developmental ages (Fig. 2C,E). Unlike the decrease of the labeling in the internal INL, the labeling in the external INL persisted. By E15, the distribution of labeled cells had spread from the central region of the retina out to the periphery. In order to compare different ages of the retina, we consistently show cells corresponding to a region 1 mm from the optic nerve exit on the temporal side. The oligonucleotide probe used to detect trkA mRNA was directed toward the part of the mRNA that encoded the intracellular tyrosine kinase domain of the receptor, corresponding to amino acids 658–673 of the chicken TrkA receptor (Bäckström et al., 1996). The specificity of the in situ hybridization analysis was shown by the capacity to out-compete all labeling with excess of cold oligonucleotide (Fig. 2H). Note that the pigment epithelium scattered light by itself in darkfield illumination microscopy (Fig. 2).
Fig. 2.
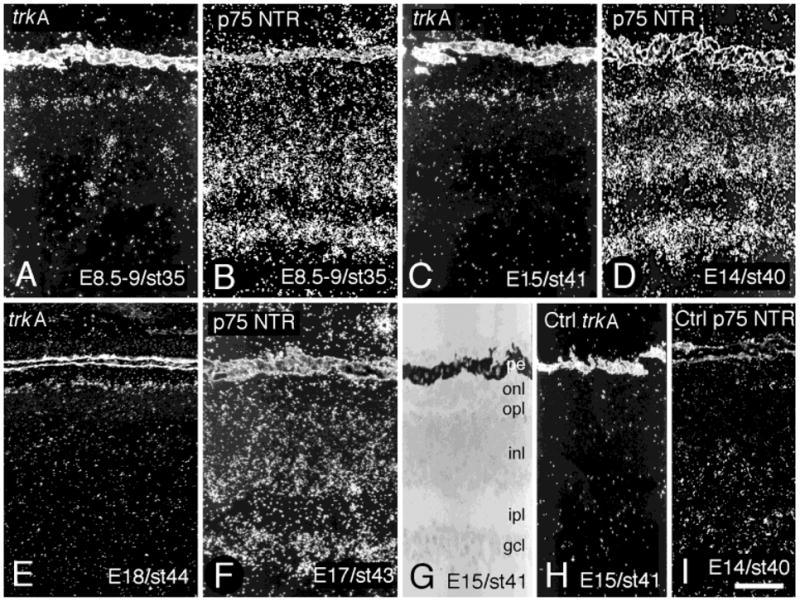
Expression of trkA and p75 neurotrophin receptor (p75NTR) mRNA in the developing chicken retina. In situ hybridization was used to study the cellular localization of trkA mRNA (A,C,E) and p75NTR mRNA (B,D,F) in chicken retinas of indicated ages. Note that trkA mRNA-labeled amacrine cells in the inner nuclear layer can no longer be found in E15 (C) or E18 (E) retina. Controls (H,I) show that cold oligonucleotide in 100 times excess, can out-compete the signal from the 35S-labeled oligonucleotide probes. A brightfield image, with a guide to the retinal layers is included in G. The depicted region is 1 mm from the optic nerve exit on the temporal side. pe, pigment epithelium; onl, outer nuclear layer; opl, outer plexiform layer; inl, inner nuclear layer; ipl, inner plexiform layer; gcl, ganglion cell layer. Scale bar = 50 μm (applies to all).
In order to study the cellular localization and morphology of TrkA-expressing cells in the cell layers of the developing chicken retina, we used a rabbit polyclonal antibody against the chicken TrkA protein (Figs. 3, 4). TrkA-IR cells were detected as early as E5.5–6 (Fig. 3B). These early cells had an irregular shape and appeared as separate cells diffusely spread over the internal part of the prospective INL. Some labeled cells seemed to follow the pattern of early migrating proliferating neuroblasts, because they appeared also in the more central part of the INL. The location and morphology of these TrkA-IR cells suggested that they were precursor amacrine cells. At E7–7.5, the IR cells appeared more organized and had increased in number (Fig. 3C). This is the age when the inner plexiform layer (IPL) is formed, and accordingly, TrkA-IR was found in the IPL. At E8, the intensity of the TrkA-IR increased on the cells as well as in the IPL, and by E10, most of the IR amacrine cells seemed to have taken their final positions in the internal INL (Fig. 3E). Neurite processes extending to the prospective second sublamina (terminology by Cajal, 1972) of the IPL showed strong TrkA-IR, however, weak IR could transiently be found in the prospective first sublamina. At E13, the number of TrkA-IR cells had decreased (Fig. 3F) and the IR in the IPL was weaker as well. By this age the cells had adopted a more regular and smooth shape, and by E15 very few TrkA-IR amacrine cells remained, sparsely distributed in the internal part of the INL (Fig. 3G). In addition, the intensity of the TrkA-IR in the IPL appeared much weaker. The few remaining TrkA-labeled amacrine cells in the late embryonic retina (E17 and hatchling), had a distinct TrkA-IR dendrite process, branching in the second sublamina of the IPL (Figs. 3H,I, 4C). The IR seen in the first sublamina at earlier ages had disappeared.
Fig. 3.
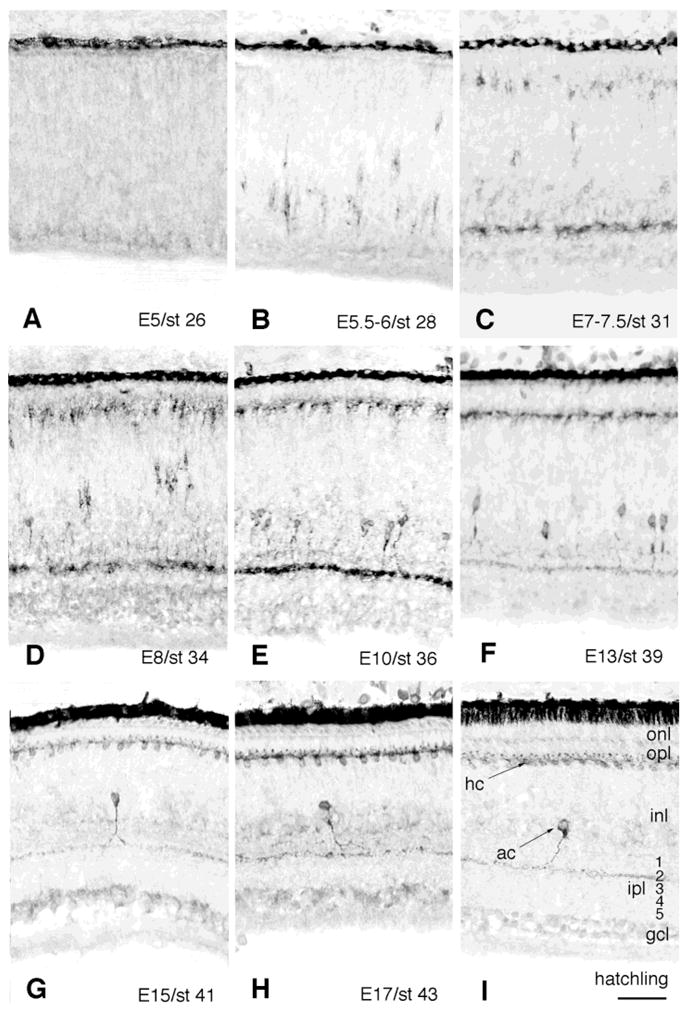
TrkA-immunoreactivity in the developing chicken retina. A polyclonal anti-chicken TrkA antibody was used to study TrkA-immunoreactive cells in chicken retinas of different embryonic ages ranging from E5 to hatching as indicated in the figure (A–I). Cells were visualized by using the peroxidase-3–3′-diaminobenzidine reaction. Note that the TrkA-immunoreactive amacrine cells start to disappear between E10 (E) and E13 (F), and that the TrkA-immunoreactive horizontal cells persist through the course of retinal development (C–I). Panel I includes a guide to the retinal layers. gcl, ganglion cell layer; ipl, inner plexiform layer; inl, inner nuclear layer; opl, outer plexiform layer; onl, outer nuclear layer. Numbers 1–5 indicate the sublamina of the IPL. ac, amacrine cell; hc, horizontal cell. Scale bar = 50 μm (applies to all).
Fig. 4.
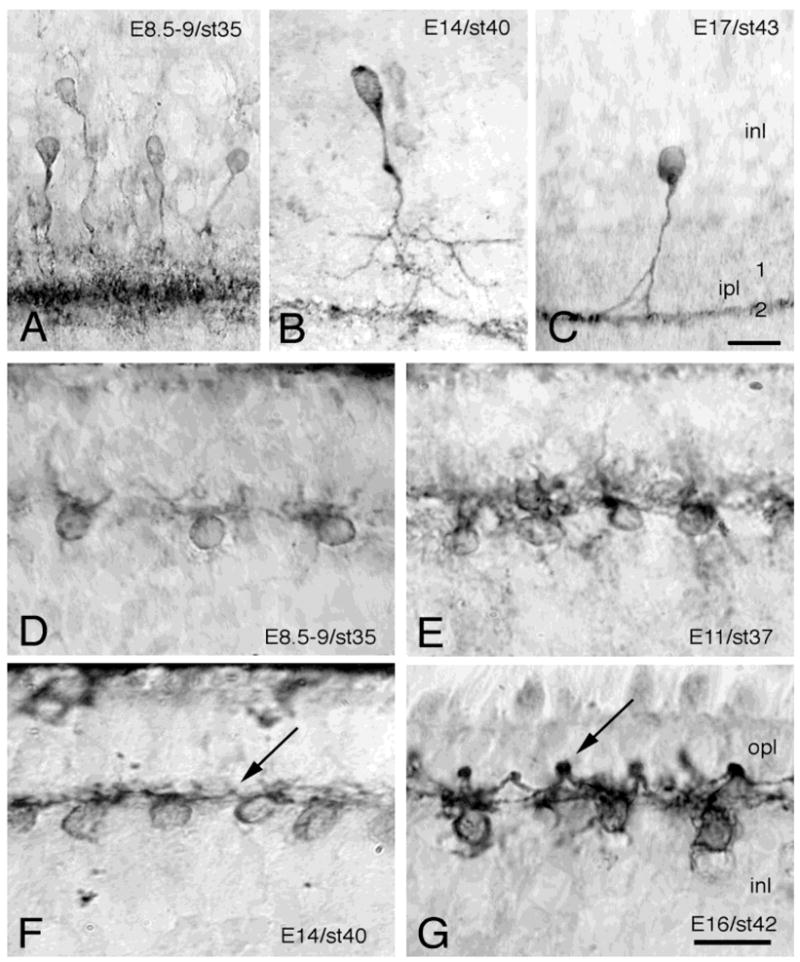
Developing TrkA-immunoreactive amacrine and horizontal cells. High-power magnification photomicrographs, showing TrkA-immunoreactive amacrine (A–C) and horizontal cells (D–G) by using immunohistochemistry with a polyclonal anti-chicken TrkA antibody on sections of indicated ages of the developing chicken retina. Cells were visualized using the peroxidase-3–3′-diaminobenzidine reaction. Note the broad band of strongly immunoreactive amacrine cell dendritic trees at E8.5–9 (A), which at later embryonic ages becomes restricted to a narrow zone in the second sublamina of the IPL (B,C). Note also the TrkA-immunoreactive arciform horizontal cell dendritic trees (F,G). The arrows are pointing at dendritic endings forming synaptic knobs, which show strong TrkA-immunoreactivity. inl, inner nuclear layer; ipl, inner plexiform layer; opl, outer plexiform layer. Scale bars = 20 μm in A–C, 10 μm in D–G.
Cells located in the external INL also express TrkA
In addition to the TrkA-labeled amacrine cells in the internal INL, cells were also found in the external part of the INL. Labeling for trkA mRNA was seen from E8–9 and onward (Fig. 2). Accordingly, the rabbit polyclonal anti-chicken TrkA antibody recognized cells in the very external part of the INL as early as E7–7.5 (Figs. 3C–I, 4D–G). This was 1 to 2 days later than the appearance of the TrkA-IR cells in the internal INL. These relatively weakly immunoreactive cells in the external INL appeared as separate, or loosely attached cells, regularly distributed on the border between the INL and the prospective outer plexiform layer (OPL). The location and morphology of the cells correspond to that of the horizontal cells. By this age, these TrkA-IR cells had grown short, irregular neurites toward the border between the INL and the developing OPL. This is in accordance with the time of formation of the OPL. In the E8 retina, additional TrkA-IR cells were found, and the IR was stronger compared to earlier ages (Fig. 3D). The cell bodies of these cells were clearly distinguishable, but the dendritic trees (Fig. 4D) looked rather diffuse and sparse in comparison with the dendritic trees seen at E10 and E11 (Figs. 3E, 4E). At E13, the TrkA-IR cells were localized to a narrow zone in the external part of the INL (Fig. 3F), and from E14 and onward (Fig. 3G–I) the cells appeared in a highly organized row of cells, like pearls on a string, on the border between the INL and the OPL (Figs. 3, 4). From E14 and onward, weakly IR “knob-like” structures could be seen between the cells (Fig. 4F,G). These knobs were found in the end of arciform dendritic processes. The intensity of the IR over these knobs and dendrites increased during later ages of development and was strongest by E16 and E17 (Figs. 3H, 4G). The cell bodies were clearly labeled as well. At this late age of development, the morphology of the TrkA-IR horizontal cells seemed established, with several arciform dendrites extending from each cell body. Most of these dendrites ended with the knob-like structure forming a contact with an adjacent horizontal cell. No axon or axon-like structure could be found.
p75NTR mRNA expression in the developing chicken retina
Labeling for p75NTR mRNA was found over the cells in the internal and external parts of the INL at E8.5–9, E14, and E17 (Fig. 2B,D,F). This pattern overlapped with the TrkA pattern. The strong expression of p75NTR mRNA at E14 in the external part of the INL has previously not been documented. In addition, labeling could be seen over the entire INL, and over the ganglion cell layer at E8.5–9, E14, and E17 (Fig. 2B,D,F). Controls prepared in the same way as for the trkA in situ hybridization analysis were negative (Fig. 2I).
DISCUSSION
In this study we report on the expression of the NGF-receptor TrkA and compare it with the expression of p75NTR in the developing chicken retina. Our results suggest that two different cell types express TrkA; amacrine and horizontal cells. We have used both in situ hybridization analysis and immunohistochemistry to identify TrkA-expressing cells. The results from these two analytical methods agree with each other, indicating that the in situ hybridization analysis and immunohistochemistry labeling were specific. Furthermore, the results from the RPA confirmed the expression of trkA mRNA during retinal development.
Number of TrkA-expressing amacrine cells decreases during development
A population of cells in the internal part of the INL is labeled for TrkA at E8.5–9. After this age, the number of TrkA-IR cells decreases, leaving only a few TrkA-IR cells in the internal INL during the later part of development. The location and morphology of these cells clearly suggest that they are amacrine cells (see Fig. 2A). The pattern of expression found in the present study differs from a previous study in rat (Ernfors et al., 1992) in which trkA mRNA labeling was suggested to be located in the ganglion cell layer of E18 rat retina. Two other studies have suggested the presence of TrkA-IR retinal ganglion cells in the developing rat retina (Zanellato et al., 1993; Rickman and Brecha, 1995). In both of these studies, an antibody directed to the carboxy terminal of the TrkA receptor, a pantrk antibody cross-reactive for all Trk receptors, was used. The discrepancies between our study and these other studies may be the result of species differences. Rickman and Brecha (1995) showed TrkA-IR in the INL of the rat retina, which agrees with our findings. Taken together, there are conflicting results on the expression of TrkA receptors in avian and mammalian retinas and more data are needed to sort out the similarities and differences between the species.
The TrkA-IR precursor cells found early in the retina, have an irregular morphological shape, and resemble multipodial amacrine neuroblasts, which have previously been described using the silver staining technique (Prada et al., 1987). The TrkA-IR putative amacrine cells appeared for the first time at E5.5–6, which is in accordance with the appearance of the multipodial neuroblast between E5 and E7. Precursors of amacrine cells (and also possibly of horizontal cells) are the only retinal cells that migrate freely during the period of proliferation in the developing retina (Prada and Ramírez, 1983). Our results indicated that in the early avian retina only a proportion of the total number of amacrine cells appeared to express TrkA. Thus, the TrkA-expressing amacrine cells could constitute a subpopulation of the total number of early amacrine cells. The age at which the TrkA-IR cells begin to take a position in the internal INL (E7–7.5), agrees with that for the multipodial amacrine neuroblast. From E8 and onward, the TrkA-IR cells start to grow neurite-like processes extending to the prospective second sublamina (Cajal, 1972) of the IPL (Figs. 3D, 4A). This is in good agreement with the findings that multipodial cells become “stalked” amacrine cells between E8 and E12, with unistratified dendrites in the IPL. One amacrine subtype denoted “stellate” amacrine cell (described by Cajal, 1972) resembles the TrkA-IR amacrine cell, and is unistratified, having an extensive stelliform dendritic tree with long dendrites extending in the second sublamina of the IPL. In transverse sections of the retina, the complete second sublamina of the IPL is TrkA-IR, and it is likely that this IR represents the long dendrites of the stellate amacrine cell.
The TrkA-IR on amacrine cells and their processes increases between E8 and E10, and during a period beginning from E10 to E13, the number of TrkA-IR amacrine cells clearly decreases at the same time as the IR over the IPL decreases (Fig. 3D–F). There are two conceivable explanations for the decrease in the number of TrkA-IR cells at this age. One is that the cells down-regulate their expression of the receptor. The other is that the TrkA-IR cells disappear due to cell death. In both mouse (Young, 1984) and rat (Voyvodic et al., 1995), extensive cell death has been found in the developing INL. This has not yet been studied in detail in chick, however, fragmented DNA as a sign of apoptosis can be isolated from chick retina (Ilschner and Waring, 1992) and pyknotic cells are found in the INL (Hughes and McLoon, 1979; Martín-Partido et al., 1988). This would imply that naturally occurring neuronal death occurs also in the chicken INL during development. In the newly hatched chicken, the remaining TrkA-IR amacrine cells are found mainly in the periphery of the retina, and in the adult retina these cells appear in a low number (Karten and Reichardt, 1995). NGF is synthesized in the embryonic retina (Ebendal and Persson, 1988; Frade et al., 1996; Hallböök et al., 1996) but the exact localization is not determined in the E8–E15 retina. By E18, NGF is produced exclusively by cells in the external part of the INL (Hallböök et al., 1996) and this raises the question whether the TrkA-expressing amacrine cells in the internal INL do not receive the NGF and consequently die. The function of the p75NTR is also not fully understood. It has been suggested that it contributes to high-affinity binding of NGF (Hempstead et al., 1991), and more recently, that it might induce cell death in the presence of NGF but in the absence of TrkA (Frade et al., 1996; Bredesen and Rabizadeh, 1997; Carter and Lewin, 1997). For both suggested functions, the question of whether the two receptors are co-expressed is relevant. The present study, as well as previous studies, show that the p75NTR is expressed in the developing avian retina (Large et al., 1989; Hallböök et al., 1990; von Bartheld et al., 1991). The expression of p75NTR mRNA is robust in the INL and overlaps with the TrkA-expression pattern (Fig. 2), suggesting that the TrkA-expressing cells also express p75NTR.
TrkA expression persists in horizontal cells
In the present study, we found TrkA-IR cells in the external INL from E7–7.5 and onward, which is in agreement with the in situ hybridization analysis (Fig. 2A). The location and morphology of these cells clearly suggested that they were horizontal cells. During chicken retinal development, horizontal cells are born between E3 and E6 (Kahn, 1974), migrating horizontal neuroblasts have been identified from E7–7.5 (Prada and Ramírez, 1983), and after E8.5 most horizontal cells have taken their final position in the external INL. This developmental schedule is in agreement with the pattern of the TrkA expression. From E8 and onward, the TrkA-IR horizontal cells develop complex and highly organized dendritic trees, but the cells do not seem to have any distinct axon. In the chicken retina, three different subtypes of horizontal cells have been previously described by means of their morphology and synaptic contacts (Cajal, 1972; Gallego, 1976; Génis-Gálvez et al., 1979). The TrkA-IR horizontal cells show similarities with the third of these subtypes, which was named “candelabrum-shaped” horizontal cell by Génis-Gálvez, et al. (1979). This cell type is located in the external INL and lacks an axon. The term candelabrum-shaped refers to the shape of the dendritic trees, with each consisting of 8 to 12 dendrites of variable length. The TrkA-IR horizontal cells develop what we term “synaptic knobs” at the end of their dendritic processes (Fig. 4F,G). In a similar manner, the candelabrum-shaped horizontal cells have terminal dendritic spines terminating in the most superficial strata of the OPL (Génis-Gálvez et al., 1979). The RPA shows that the trkA mRNA levels in total retina peak at late embryonic age. This is in agreement with stronger TrkA-IR on horizontal cells (Figs. 3H,I, 4G) at the later stages.
NGF is expressed in the E18 embryo in the narrow region of the retina where the TrkA-IR horizontal cells are located (Hallböök et al., 1996) and the receptors on these cells are likely to be exposed to their ligand. NGF is known to support neuronal survival and to stimulate neurite outgrowth in the peripheral nervous system and one possible function for TrkA on the horizontal cells could be to support their survival. This is tentatively suggested by the fact that the TrkA-IR horizontal cells, which have access to NGF remain, whereas the TrkA-IR amacrine cells decrease in number during the course of retinal development. NGF (Crowley et al., 1994) and TrkA(Smeyne et al., 1994) knock-out mice do not exhibit any increased cell death of cholinergic neurons in the basal forebrain during the limited time the mice are alive and the capacity of NGF to counteract death in the developing central nervous system is unclear. NGF can stimulate neurite outgrowth, so the TrkA receptor may well be involved in the formation of the dendrites and synaptic contacts in the OPL. A high degree of neuronal plasticity occurs in the E18 retina (Adler and Farber, 1986) and NGF has been suggested to participate in such events in other systems (Thoenen, 1995; Snider and Lichtman, 1996). We have previously found that NGF mRNA levels are modulated by light in the 5-day-old retina (Hallböök et al., 1996) and consequently it is suggested that NGF and TrkA may be involved in activity-dependent horizontal cell plasticity.
Acknowledgments
We thank Helena Vretman and Agnieszka Brzozowska-Prechtl for technical assistance and Alex Mercer for valuable comments on the manuscript.
Grant sponsor: Swedish Medical Research Council; Grant number: K94–12P-10660–02B; Grant sponsor: EU; Grant number: BMH4CT96–0976; Grant sponsor: NEI; Grant number: EY 06890; Grant sponsor: NINDS; Grant number: NS 24560; Grant sponsor: Göran Gustavsson’s Research Foundation; Grant number: 97:74; Grant sponsor: NIH; Grant number MH48300; Grant sponsor: Kronprinsessan Margaretas arbetsnämnd för synskadade; Grant number: 97:16.
LITERATURE CITED
- Adler R, Farber D. The retina: A model for cell biology studies. Cellular Neurobiology, Orlando: Academic Press; 1986. [Google Scholar]
- Bäckström A, Söderström S, Kylberg A, Ebendal T. Molecular cloning of the chicken trkA and its expression in early peripheral ganglia. J Neurosci Res. 1996;46:67–81. doi: 10.1002/(SICI)1097-4547(19961001)46:1<67::AID-JNR9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Bredesen DE, Rabizadeh S. p75NTR and apoptosis: Trk-dependent and Trk-independent effects. Trends Neurosci. 1997;20:287–290. doi: 10.1016/s0166-2236(96)01049-1. [DOI] [PubMed] [Google Scholar]
- Cajal SR. The Structure of the Retina. Springfield, Ill: C.C. Thomas; 1972. [Google Scholar]
- Carter BD, Lewin GR. Neurotrophins live or let die: Does p75NTR decide? Neuron. 1997;18:187–190. doi: 10.1016/s0896-6273(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P, Carter BD, Dobrowsky RT, Chao MV. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p 75. Nature. 1996;383:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- Chao MV, Hempstead BL. p75 and Trk: A two-receptor system. Trends Neurosci. 1995;18:321–326. [PubMed] [Google Scholar]
- Cordon-Cardo C, Tapley P, Jing SQ, Nanduri V, O’Rourke E, Lamballe F, Kovary K, Klein R, Jones KR, Reichardt LF, Barbacid M. The trk tyrosine kinase mediates the mitogenic properties of nerve growth factor and neurotrophin-3. Cell. 1991;66:173–183. doi: 10.1016/0092-8674(91)90149-s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, McMahon SB, Shelton DL, Levinson AD, Phillips HS. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76:1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- Ebendal T. Function and evolution in the NGF family and its receptors. J Neurosci Res. 1992;32:461–470. doi: 10.1002/jnr.490320402. [DOI] [PubMed] [Google Scholar]
- Ebendal T, Persson H. Detection of nerve growth factor mRNA in the developing chicken embryo. Development. 1988;102:101–106. doi: 10.1242/dev.102.1.101. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Merlio JP, Persson H. Cells expressing mRNA for neurotrophins and their receptors during embryonic rat development. Eur J Neurosci. 1992;4:1140–1158. doi: 10.1111/j.1460-9568.1992.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Persson H. Developmentally regulated expression of HDNF/NT-3 mRNA in rat spinal cord motoneurons and expression of BDNF mRNA in embryonic dorsal root ganglion. Eur J Neurosci. 1991;3:953–961. doi: 10.1111/j.1460-9568.1991.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Frade JM, Rodríguez-Tébar A, Barde YA. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature. 1996;383:166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- Gallego A. Comparative study of the horizontal cells in the vertebrate retina: Mammals and birds. In: Zettler F, Weiler R, editors. Proceedings in Life Sciences; “Neural Principles in Vision”. Berlin: Springer-Verlag; 1976. pp. 26–62. [Google Scholar]
- Génis-Gálvez JM, Prada F, Armengol JA. Evidence of three types of horizontal cells in the chicken retina. Jpn J Ophthalmol. 1979;23:378–387. [Google Scholar]
- Hallböök F, Ayer-Lelièvre C, Ebendal T, Persson H. Expression of nerve growth factor receptor mRNA during early development of the chicken embryo: emphasis on cranial ganglia. Development. 1990;108:693–704. doi: 10.1242/dev.108.4.693. [DOI] [PubMed] [Google Scholar]
- Hallböök F, Bäckström A, Kullander K, Ebendal T, Carri NG. Expression of neurotrophins and Trk receptors in the avian retina. J Comp Neurol. 1996;364:664–676. doi: 10.1002/(SICI)1096-9861(19960122)364:4<664::AID-CNE5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Hallböök F, Ibañez CF, Ebendal T, Persson H. Cellular localization of brain-derived neurotrophic factor and neurotrophin-3 mRNA in the early chicken embryo. Eur J Neurosci. 1993;5:1–14. doi: 10.1111/j.1460-9568.1993.tb00199.x. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hempstead BL, Martin-Zanca D, Kaplan DR, Parada LF, Chao MV. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991;350:678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Hughes WF, McLoon SC. Ganglion cell death during normal retinal development in the chick: Comparisons with cell death induced by early target field destruction. Exp Neurol. 1979;66:587–601. doi: 10.1016/0014-4886(79)90204-8. [DOI] [PubMed] [Google Scholar]
- Ilschner SU, Waring P. Fragmentation of DNA in the retina of chicken embryos coincides with retinal ganglion cell death. Biochem Biophys Res Commun. 1992;183:1056–1061. doi: 10.1016/s0006-291x(05)80297-9. [DOI] [PubMed] [Google Scholar]
- Kahn AJ. An autoradiographic analysis of the time of appearance of neurons in the developing chick neural retina. Dev Biol. 1974;38:30–40. doi: 10.1016/0012-1606(74)90256-5. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Hempstead BL, Martin-Zanca D, Chao MV, Parada LF. The trk proto-oncogene product: A signal transducing receptor for nerve growth factor. Science. 1991a;252:554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Martin-Zanca D, Parada LF. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991b;350:158–160. doi: 10.1038/350158a0. [DOI] [PubMed] [Google Scholar]
- Karten HJ, Reichardt LF. TrkA receptors reveal a new class of non-GABAergic horizontal cells in the retina. ARVO Abstr Invest Ophthalmol Vis Sci. 1995;36:S256. [Google Scholar]
- Large TH, Weskamp G, Helder JC, Radeke MJ, Misko TP, Shooter EM, Reichardt LF. Structure and developmental expression of the nerve growth factor receptor in the chicken central nervous system. Neuron. 1989;2:1123–1134. doi: 10.1016/0896-6273(89)90179-7. [DOI] [PubMed] [Google Scholar]
- Lefcort F, Clary DO, Rusoff AC, Reichardt LF. Inhibition of the NT-3 receptor TrkC, early in the chick embryogenesis, results in a severe reduction in multiple neuronal subpopulations in the dorsal root ganglia. J Neurosci. 1996;16:3704–3713. doi: 10.1523/JNEUROSCI.16-11-03704.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Mahadeo D, Kaplan L, Chao MV, Hempstead BL. High affinity nerve growth factor binding displays a faster rate of association than p140trk binding. Implications for multi-subunit polypeptide receptors. J Biol Chem. 1994;269:6884–6891. [PubMed] [Google Scholar]
- Martín-Partido G, Rodríguez-Gallardo L, Ignacio SA, Navascués J. Cell death in the ventral region of the neural retina during the early development of the chick embryo eye. Anat Rec. 1988;222:272–281. doi: 10.1002/ar.1092220308. [DOI] [PubMed] [Google Scholar]
- Nebreda AR, Martin-Zanca D, Kaplan DR, Parada LF, Santos E. Induction by NGF of meiotic maturation of Xenopus oocytes expressing the trk proto-oncogene product. Science. 1991;252:558–560. doi: 10.1126/science.1850550. [DOI] [PubMed] [Google Scholar]
- Oakley RA, Lefcort FB, Clary DO, Reichardt LF, Prevette D, Oppenheim RW, Frank E. Neurotrophin-3 promotes the differentiation of muscle spindle afferents in the absence of peripheral targets. J Neurosci. 1997;17:4262–4274. doi: 10.1523/JNEUROSCI.17-11-04262.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panabières F, Piechaczyk M, Rainer B, Dani C, Fort P, Riaad S, Marty L, Imbach JL, Jeanteur P, Blanchard JM. Complete nucleotide sequence of the messenger RNA coding for chicken muscle glyceraldehyde-3-phosphate dehydrogenase. Biochem Biophys Res Commun. 1984;118:767–773. doi: 10.1016/0006-291x(84)91461-x. [DOI] [PubMed] [Google Scholar]
- Prada C, Puelles L, Geniz-Gálvez JM, Ramírez G. Two modes of free migration of amacrine cell neuroblasts in the chick retina. Anat Embryol. 1987;175:281–287. doi: 10.1007/BF00309842. [DOI] [PubMed] [Google Scholar]
- Prada C, Ramírez G. A Golgi study of the cell cycle and early neuron and glia differentiation in the chick retina. In: Grisolíà S, editor. Ramón y Cajal’s Contribution to the Neurosciences. Amsterdam, NY: Elsevier Science Publishers; 1983. pp. 117–123. [Google Scholar]
- Rickman DW, Brecha NC. Expression of the proto-oncogene, trk, receptors in the developing rat retina. Vis Neurosci. 1995;12:215–222. doi: 10.1017/s0952523800007896. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Tébar A, Dechant G, Barde YA. Binding of brain-derived neurotrophic factor to the nerve growth factor receptor. Neuron. 1990;4:487–492. doi: 10.1016/0896-6273(90)90107-q. [DOI] [PubMed] [Google Scholar]
- Schecterson LC, Bothwell M. Novel roles for neurotrophins are suggested by BDNF and NT-3 mRNA expression in developing neurons. Neuron. 1992;9:449–463. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- Schröpel A, von Schank D, Dechant G, Barde YA. Early expression of the nerve growth factor receptor ctrkA in chick sympathetic and sensory ganglia. Mol Cell Neurosci. 1995;6:544–556. doi: 10.1006/mcne.1995.0006. [DOI] [PubMed] [Google Scholar]
- Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, Lira SA, Barbacid M. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994;368:246–249. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Snider WD, Lichtman JW. Are neurotrophins synaptotrophins? Mol Cell Neurosci. 1996;7:433–442. doi: 10.1006/mcne.1996.0031. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- Verdi JM, Birren SJ, Ibanez CF, Persson H, Kaplan DR, Benedetti M, Chao MV, Anderson DJ. p75LNGFR regulates Trk signal transduction and NGF-induced neuronal differentiation in MAH cells. Neuron. 1994;12:733–745. doi: 10.1016/0896-6273(94)90327-1. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS, Heuer JG, Bothwell M. Expression of nerve growth factor NGF receptors in the brain and retina of chick embryos: Comparison with cholinergic development. J Comp Neurol. 1991;310:103–129. doi: 10.1002/cne.903100110. [DOI] [PubMed] [Google Scholar]
- Voyvodic JT, Burne JF, Raff MC. Quantification of normal cell death in the rat retina: implications for clone composition in cell lineage analysis. Eur J Neurosci. 1995;7:2469–2478. doi: 10.1111/j.1460-9568.1995.tb01045.x. [DOI] [PubMed] [Google Scholar]
- Williams R, Bäckström A, Ebendal T, Hallböök F. Molecular cloning and cellular localization of trkC in the chicken embryo. Dev Brain Res. 1993;75:235–252. doi: 10.1016/0165-3806(93)90028-9. [DOI] [PubMed] [Google Scholar]
- Young RW. Cell death during differentiation of the retina in the mouse. J Comp Neurol. 1984;229:362–373. doi: 10.1002/cne.902290307. [DOI] [PubMed] [Google Scholar]
- Zanellato A, Comelli MC, Dal Toso R, Carmignoto G. Developing rat retinal ganglion cells express the functional NGF receptor p140trkA. Dev Biol. 1993;159:105–113. doi: 10.1006/dbio.1993.1224. [DOI] [PubMed] [Google Scholar]


