Abstract
Local delivery of brain-derived neurotrophic factor (BDNF) by genetically modified cells provides the unique opportunity to examine the effects of BDNF on adult dopaminergic and cholinergic neurons in vivo. Primary rat fibroblasts were genetically engineered to produce BDNF. Conditioned media from BDNF-transduced fibroblasts supported embryonic chick dorsal root ganglion neurons as well as rat fetal mesencephalic neurons. BDNF-transduced fibroblasts grafted to the rat brain survived and showed continued mRNA production for at least 2 weeks. The effects of BDNF-transduced fibroblast grafts on the dopaminergic and cholinergic systems were then assessed. BDNF-transduced fibroblasts grafted into the normal intact substantia nigra induced sprouting of tyrosine hydroxylase- and neurofilament-immunoreactive fibers into the graft. Fibroblast grafts implanted into the normal intact striatum and midbrain as well as the 6-hydroxydopamine-lesioned brain did not induce sprouting of dopaminergic fibers; neither did they affect drug-induced rotational behavior. BDNF-transduced fibroblasts did, however, significantly increase the homovanillic acid/dopamine ratio when grafted into the normal midbrain. Following transection of the fimbria-fornix, BDNF-transduced fibroblasts grafted into the septum were unable to rescue the septal cholinergic population, as did nerve growth factor-producing fibroblast grafts. Genetically modified fibroblast grafts may provide an effective, localized method of BDNF delivery in vivo to test biological effects of this factor on the central nervous system.
Indexing terms: cholinergic, dopaminergic, neurotrophic factor, NGF, transplantation
Neurotrophic factors are present in the central nervous system (CNS) and play important roles in neural development, differentiation, and survival (for reviews, see Barde, 1989; Thoenen, 1991). A family of related neurotrophic molecules, called neurotrophins, whose members affect overlapping as well as distinct populations of neurons, has been recently isolated. Members of the neurotrophin family include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophic factor-3 (NT-3), and neurotrophic factors-4/-5 (NT-4/5). Extensive conservation between species and sequence similarity between members (over 50% homology in amino acid identity) suggest important roles for neurotrophins in the CNS (Leibrock et al., 1989; Maisonpierre et al., 1990; Berkemeier et al., 1991).
Neurotrophins exert their effects through specific binding to cell surface receptors that exist in both low (Kd ~ 10−9)- and high (Kd ~ 10−11)-affinity forms (Meakin and Shooter, 1991). The low-affinity NGF receptor (p75NGFR) comprises a 75 kD protein (Chao et al., 1986) and binds all known members in the neurotrophin family. Specificity in binding is afforded by high-affinity binding sites on trk protooncogenes: trkA., trkB, and trkC. Neurotrophic factors appear to mediate their effects by inducing phosphorylation of tyrosine residues on trk molecules (Kaplan et al., 1991). However, there exists a great deal of controversy regarding what receptor components are necessary and sufficient to mediate signal transduction (for reviews, see Bothwell, 1991; Meakin and Shooter, 1992).
Although the role of neurotrophic factors in shaping the developing nervous system by their presence in limited amounts in target tissues has been best established (Barde, 1989), neurotrophic factors have also been implicated in maintaining adult neuronal populations. Neurotrophin mRNA and protein as well as receptors for neurotrophins are present in the adult nervous system (Korsching et al., 1985; Shelton and Reichardt, 1986), and adult neurons are able to transport retrogradely 125I-NGF (Schwab et al., 1979; Seiler and Schwab, 1984) and 125I-BDNF (Wiegand et al., 1991; DiStefano et al., 1992). Neurotrophic molecules promote neuronal survival when exogenously administered in amounts that exceed normal physiological levels. NGF, the best-characterized member of the neurotrophin family, has been extensively studied for its role in the cholinergic septohippocampal model, where administration of exogenous NGF to the axotomized septohippocampal projection rescues up to 90% of the transected septal cholinergic neurons (Hefti, 1986; Williams et al., 1986; Kromer, 1987; Gage et al., 1988). A decrease in neurotrophic factors during aging has also been hypothesized to predicate degenerative disorders such as Alzheimer’s disease (AD) and Parkinson’s disease (PD; Appel, 1981; Hefti, 1983), where the loss of dopaminergic neurons of the substantia nigra pars compacta (SNC) is correlated with the major motor disability of PD, and the loss of cholinergic neurons in the basal forebrain is associated with memory loss in AD (Bernheimer et al., 1973; Olton, 1990). Although the etiology and mechanisms underlying the selective degeneration of cholinergic and dopaminergic neuronal populations are unclear, the action of neurotrophic substances in protecting and regenerating damaged neurons has recently received attention for its potential therapeutic role (see Phelps, 1989).
BDNF promotes the survival and differentiation of fetal cholinergic neurons of the basal forebrain in vitro (Knusel et al., 1991; Nonomura and Hatanaka, 1992) as well as adult cholinergic neurons in vivo following septohippocampal transection (Knusel et al., 1992; Morse et al., 1993). BDNF also promotes the survival of fetal dopaminergic neurons and protects them from MPP+ and 6-hydroxydopamine (6-OHDA) toxicity in vitro (Hyman et al., 1991; Beck et al., 1992; Spina et al., 1992; Hyman et al., 1994). When applied to grafted fetal dopaminergic neurons, BDNF enhances the functional effect of the grafts in reducing amphetamine-induced rotation behavior but does not enhance graft survival (Sauer et al., 1993). The effect of BDNF on adult dopaminergic neurons is not well characterized but, when infused above the SNC following transection of the medial forebrain bundle (MFB), BDNF is unable to protect tyrosine hydroxylase (TH)-positive neurons from degenerative changes (Knusel et al., 1992). On the other hand, BDNF induces rotational behavior in the normal adult rat when infused into the SNC (Altar et al., 1992; Martin-Iverson et al., 1994; Shults et al., 1994), indicating a role for BDNF in the adult dopaminergic system.
Methods of examining the role of BDNF in vivo involve infusing the protein intraventricularly or intraparenchymally. It is thought that an abundance of truncated forms of the trkB receptor (Klein et al., 1990; Middlemas et al., 1991), however, limit the diffusion of BDNF in vivo. To circumvent this problem, we employed a technique involving the use of genetically engineered cells. Dermal fibroblasts were genetically altered to produce the neurotrophic factor of interest and subsequently grafted to the brain, where they act locally as sources of the trophic substance (Gage and Fisher, 1991; Gage et al., 1991). NGF, introduced to the brain by both genetically modified fibroblast cell lines (Rosenberg et al., 1988) and primary fibroblasts (Kawaja et al., 1992), is successful in rescuing cholinergic neurons following fimbria-fornix transection. We hypothesized that very discrete administration of BDNF using genetically modified fibroblasts would provide more localized and sustained delivery of BDNF than that of cannula infusion. Therefore, we tested the effects of localized BDNF delivery on adult dopaminergic and cholinergic neurons after chemical or mechanical lesions.
Intracerebral grafting of genetically modified fibroblasts also affords the unique opportunity of studying the ability of trophic molecules to promote neurite extension in vivo. With this approach, we can monitor axonal arborization and sprouting induced by localized production of trophic molecules in vivo. Grafts of NGF-producing fibroblasts (FF/NGF) implanted into the septum or striatum encourage the ingrowth of cholinergic fibers (Kawaja and Gage, 1991) and promote the regeneration of acetylcholinesterase (AChE)-positive axons when grafted into the lesioned fimbria-fornix cavity in a collagen matrix (Kawaja et al., 1992). Neurite extension into the grafts, although promoted by NGF, is thought to be mediated by astrocytic processes that provide a permissive substrate for axonal ingrowth. Therefore, delivery of neurotrophic factors by fibroblast grafts provides substrates for growth as well as trophic factors for neurite extension. We hypothesized that grafts of BDNF-transduced fibroblasts (FF/BDNF) implanted into the striatum or midbrain would analogously induce dopaminergic fiber ingrowth. Because BDNF binding is conferred by p75NGFR, which is expressed by cholinergic neurons of the basal forebrain sensitive to BDNF, we also investigated cholinergic ingrowth into FF/BDNF grafts in vivo.
MATERIALS AND METHODS
Construction of the plasmid including the BDNF gene
The gene encoding human BDNF was isolated by screening genomic clones with degenerate oligonucleotides derived from regions of amino acid sequence conserved among pig BDNF and NGF from several species. Sequence analysis identified a clone with near identity to pig BDNF, which was subsequently confirmed to be the gene for human BDNF (Jones and Reichardt, 1990). The entire coding region for human BDNF is present in one exon. An expression cassette was constructed by adding restriction enzyme sites and cloning into Bluescript vector.
Retroviral vector construction
The retroviral vector was derived from the Moloney murine leukemia virus in which the structural genes gag, pol, and env were replaced by the selectable marker aminoglycoside phosphotransferase (neomycin). Transcription is controlled by an internal Rous sarcoma virus (RSV) promoter (LRNL). The 830 bp EcoRI-BamHI fragment containing the entire coding region of human BDNF was ligated into the vector LRNL downstream from the viral 5′ long terminal repeat (LTR; Fig. 1: LBdnfRNL). The plasmid LBdnfRNL was then lipofected into the Ψ-2 ecotropic packaging cell line by Lipofectin (BRL/Gibco). Conditioned medium was then used to infect the amphotrophic cell line, PA-317. Clones of PA-317 cells were selected in the presence of 400 μg/ml of Geneticin (G-418 Sulfate; BRL/Gibco Laboratories), and the conditioned medium was used to infect Rat 1 cell lines to assay for virus titer. PA-317 clones showing the highest titer were selected and used to infect primary rat fibroblasts.
Fig. 1.

Expression cassette DNA coding for human brain-derived neurotrophic factor (BDNF) was inserted into a retroviral vector to produce an amphotrophic helper-free viral producer line. A 750 bp BDNF message is transcribed by retroviral long terminal repeat (LTR) promoter. The vector contains a selection marker aminoglycoside phosphotransferase (neo) driven by Rous sarcoma virus (RSV) promoter.
Primary culture of rat dermal fibroblasts
Skin biopsies were obtained from inbred Fischer 344 rats as previously described (Kawaja et al., 1991). Primary fibroblasts were grown in Dulbecco’s minimal essential medium (DMEM) supplemented with 10% fetal bovine serum, 0.3 mg/ml (2 mM) glutamate, 2.5 μg/ml amphotericin, and 40 mg/ml gentamycin (supplemented DMEM) in a 10% CO2 incubator. After reaching confluence, fibroblasts were passaged by trypsinization and propagated by replating at a density of 1:4. Passage number was carefully monitored. Primary fibroblasts (FF) were infected with the recombinant retrovirus containing the BDNF minigene by incubation in conditioned medium from the packaging cell lines in 4 μg/ml polybrene. After 24 hours, successfully transfected fibroblasts (FF/BDNF) were selected in the presence of 400 μg/ml G-418 and grown to confluence. FF/NGF- and β-galactosidase-transfected fibroblasts (FF/β-gal) engineered from identical biopsies served as control grafts. The retroviral vector and genetic modification of these cells have been previously described (Rosenberg et al., 1988).
Conditioned media were sometimes obtained from transduced and unmodified fibroblasts. Five milliliters of media (without G418; supplemented DMEM for chick DRG cultures, defined media for mesencephalic cultures) were conditioned by preconfluent (75%) fibroblasts in a T75 flask for 24 hours. The media incubated with the fibroblasts were filtered with a 0.45 μm filter to remove cell debris and obtain BDNF, β-gal, or uninfected fibroblast conditioned media (CM-BDNF, CM-β-gal, or CM-FF)
Northern blot analysis
Approximately 1 × 106 FF/BDNF and control FF were grown to 90% confluence and harvested in guanidinium thiocyanate solution. RNA was isolated by cesium chloride centrifugation (5.7 M) and precipitated with 3 M Na acetate in 100% EtOH. Fifteen micrograms of RNA were then electrophoresed in a 1.2% agarose gel (containing 5% formaldehyde) and transferred overnight to a nylon membrane. Membranes were hybridized overnight at 42°C with a 32P-labeled cDNA probe (950 bp BamHI-BstXI fragment) corresponding to most of the coding region. The size of the transcript was estimated by comparison with the 28 and 18S ribosomal bands.
Chick dorsal root ganglia assay
Dorsal root ganglia (DRG) were dissected from E9 chick embryos in cold buffered Hank’s solution (as described by Davies, 1989). Ganglia were suspended in 0.08% trypsin for 20 minutes at 37°C and gently triturated in supplemented DMEM. Cells were preplated for 2–4 hours on tissue culture-treated plastic to allow differential adhesion of nonneuronal cells. Plates were gently washed, and unattached cells were resuspended and plated at a density of 15,000 cells/cm2 in 24-well plates previously coated with poly-DL-ornithine (0.5 mg/ml) and laminin (10 mg/ml). Cultures were incubated in supplemented DMEM alone, CM-BDNF, CM-β-gal, or supplemented DMEM containing 100 ng/ml recombinant human (rh) BDNF or rhNGF in a 5% CO2 incubator. Cultures were allowed to survive for 48 hours, fixed with 4% paraformaldehyde, and photographed.
Fetal mesencephalic culture
Fetal mesencephalon was dissected from the mesencephalic flexure of E15 rat fetuses and dissociated in papain solution. Dissociated cells were plated at a concentration of 100,000 cells/cm2 on poly-D-lysine-coated plates in chemically defined medium after a 24 hour incubation in defined media supplemented with 10% fetal calf serum. Mesencephalic cultures were then incubated in defined media alone, CM-BDNF, CM-FF, or media containing rhBDNF at 50 ng/ml. Two parallel cultures were fixed at 3 and 5 days with 4% paraformaldehyde and immunostained with an antibody directed against TH (Boehringer-Mannheim; 1:200). Tyrosine hydroxylase-immunoreactive (TH-IR) cells and total cells were counted.
Surgical procedures: Grafting, 6-OHDA lesions, and fimbria-fornix transections
Female Fischer 344 rats (150–200 g) were anesthetized with a mixture of ketamine (75 mg/kg), acepromazine (0.75 mg/kg), and rompun (4 mg/kg) and placed in a Kopf stereotaxic apparatus for all surgical procedures. FF/BDNF, FF/NGF, and FF/β-gal were washed, trypsinized, and suspended in Dulbecco’s phosphate-buffered saline (PBS) at a concentration of 1 × 105 cells/μl. Two or three microliters of the fibroblast suspension were stereotaxically implanted at one or more sites in the normal intact striatum (A/P, +0.2; LAT, 2.0; D/V, 4.5; and A/P, 1.2; LAT, 2.0; D/V 4.5) or midbrain (A/P, −5.4; LAT, 1.5; D/V, 7.5) at a rate of 1 μl/minute (all coordinates given with reference to Bregma; Paxinos and Watson).
One week following grafting into the striatum, unilateral lesions of the substantia nigra ipsilateral to the graft site were made by stereotaxic injection (A/P, 4.2; LAT, 1.5; D/V, 7.8) of 12 mg 6-OHDA dissolved in 4 μl saline containing 0.5 mg/ml ascorbic acid. The efficacy of the lesion was tested by observing rotational behavior.
Complete unilateral aspirative lesions of the fimbria-fornix and supracollosal striae were made under visual inspection as previously described (Gage et al., 1983). Aspirative lesions, conducted with the aid of a surgical microscope, were made on the right side of the brain ipsilateral to the graft site. A summary of the grafting experiments discussed in the text is included in Table 1.
TABLE 1.
Summary of Grafting Experiments Completed
| Experiment | FF/BDNF vs. Control (n) | Lesion | Rotational test | Histology or biochemistry |
|---|---|---|---|---|
| Graft in normal striatum (Fig. 5) | 3 vs. 3 | None | None | 1,1 2, 3, and 6 weeks |
| Graft in normal striatum with subsequent 6-OHDA lesion in MFB | 11 vs. 11 | 8–13 days postgrafting | 2, 4, 6, 8, and 15 weeks after lesion (apomorphine); 3, 5, 7, and 16 weeks after lesion (amphetamine) | 10 (n = 6 each) and 16 weeks (n = 5 each) after lesion |
| Graft in normal midbrain (Fig. 6) | 10 vs. 8 | None | 2 and 4 days | 4 days |
| Graft in normal midbrain | 10 vs. 10 | None | 4 and 7 days | 1 week |
| Graft in normal midbrain (Figs. 7, 8) | 11 vs. 10 | None | 1, 4, and 6 weeks | 6 weeks |
| 6-OHDA lesion (Fig. 9) | 11 vs. 10 | 2 weeks postgrafting (after 2 week rotation test) | 1.5 and 2 weeks after grafting; 1.5, 2.5, 3.5, and 4.5 weeks after lesion | 8 weeks after grafting |
| Graft in septum following fimbria-fornix lesion (Figs. 10, 11) | 5 vs. 5 | At time of grafting | None | 4 weeks after grafting |
Boldface indicates data illustrated in this paper.
Behavioral analysis
Following subcutaneous injection of 0.1 mg/kg apomorphine or 5 mg/kg amphetamine, animals were placed in an automated rotameter, and the number of rotations/hour were recorded. Complete (360°) rotations were recorded for both clockwise and counterclockwise directions for 60 minutes. Rotational behavior was monitored at time points of 2, 4, and 7 days in intact adult rats with striatal grafts and 1, 4, and 6 weeks following grafting into the MFB. Behavioral analysis was also performed at weekly intervals in the 6-OHDA-lesioned rat beginning 2 weeks after grafting into the striatum. Apomorphine and amphetamine were used in alternate weeks.
Biochemistry: High-pressure liquid chromatography
Four days following grafting, rats were killed by rapid decapitation, and brains were immediately removed. The striata were dissected on ice, immediately frozen in liquid nitrogen, and stored at −80°C. For high-pressure liquid chromatography (HPLC), each unilateral frozen striatal tissue was extracted with 500 μl of 0.2 M perchloric acid (PCA) with sodium EDTA (0.1 mM) and sodium bisulfide (0.4 mM). Dopamine, 3,4-dihydroxyphenylacetic (DOPAC) and homovanillic acid (HVA) levels were assayed by injection of 50 μl PCA extracts onto a coulometric electrode array, gradient liquid chromatography system (CEAS model 55-0650; ESA, Bedford, MA).
Histological analysis
Rats were anesthetized (as described above) and transcardially perfused with 50 ml saline followed by 250 ml ice cold 4% paraformaldehyde at various time points following grafting (1, 2, 3, and 8 weeks for the intact striatum; 8 weeks for the 6-OHDA-lesioned brain; 4 weeks for the fimbria-fornix-lesioned brain). Brains were removed, post-fixed overnight, and transferred to 30% sucrose until equilibration. Forty micrometer sections were cut on a freezing sliding microtome and stored in cryoprotectant. Sections were stained with thionin to obtain a qualitative assessment of cells within the graft and host tissue, stained with picrofuchsin to visualize graft-derived collagen (Sheehan and Hrapchak, 1973), or processed for immunohistochemistry using the following procedure. Endogenous peroxidases were first blocked with 0.06% H2O2, and sections were permeabilized with 0.25% Triton X-100 and blocked with 10% horse serum (monoclonal antibodies) or 10% goat serum (polyclonal antibodies). Sections were incubated with either anti-rat TH monoclonal antibody (Boehringer-Mannheim), anti-rat neurofilament (NF; 200 kD) monoclonal antibody (Boehringer-Mannheim), anti-mouse p75NGFR monoclonal antibody, anti-rat glial fibrillary acidic protein (GFAP) monoclonal antibody (Boehringer-Mannheim), anti-rat OX-42 monoclonal antibody (Serotec), anti-choline acetyltransferase (ChAT) polyclonal antibody (a generous gift of David Armstrong), or anti-rat fibronectin (FN) polyclonal antibody (Telios) at dilutions of 1:200, 1:25, 1:100, 1:100, 1:500, 1:7,500, or 1:500, respectively. The antigen-antibody complex was reacted with horse anti-mouse or goat anti-rabbit secondary antibody coupled to biotin. Staining was developed by the avidin-biotin method (Vector Laboratories) with nickel intensification using 3,3-diaminobenzidine as the chromogen.
Quantification of p75NGFR- and ChAT-immunoreactive cells was done blindly in three to four sections throughout the rostral-caudal extent of the medial septum and ventral limb of the diagonal band. The amount of cell savings is represented as the ratio of ipsilateral/contralateral cell number. Only cells with a distinguishable process originating from the soma were included in the quantification.
In situ hybridization
Cells expressing mRNA for BDNF were identified by in situ hybridization. Complementary antisense RNA probes were transcribed from the linearized plasmid using T7 polymerase in the presence of 35S-CTP. Forty micrometer sections were mounted directly onto slides and hybridized overnight at 56°C. Sections were processed for emulsion autoradiography and counterstained with thionin.
RESULTS
Transcription of the BDNF transgene in vitro: Northern blot analysis
FF/BDNF have a doubling time of 48 hours in culture and exhibit contact inhibition, as do FF. To determine if BDNF mRNA is transcribed from the transgene in vitro, we performed Northern blot analysis on the cultured cells (Fig. 2). The hybridized blot shows message of 4.8 kb, which is the expected size of the retroviral transcript containing the BDNF minigene, RSV promoter, and neomycin resistance gene. No hybridization occurred in the lane representing FF. This result was repeatable using several different infections and biopsies.
Fig. 2.
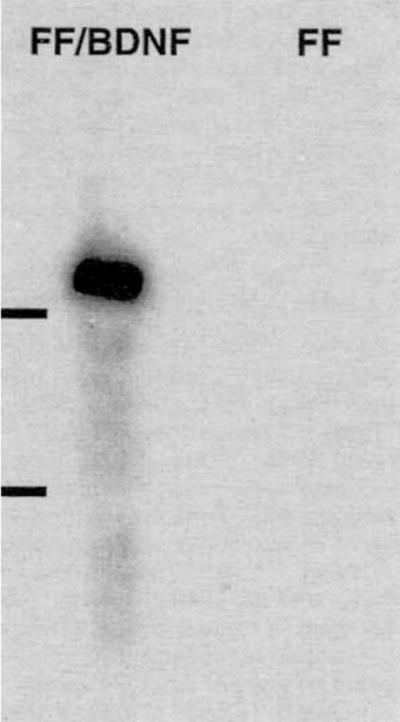
Northern blot analysis confirms expression of BDNF mRNAs in the lane representing FF/BDNF but not FF. The retroviral transcript containing the BDNF minigene is approximately 4.8 kb in size; 18 and 28S ribosomal bands are indicated.
Translation of BDNF mRNA in vitro
Embryonic chick dorsal root ganglion assay
After establishing that the BDNF transgene was transcribed in vitro, we sought to determine whether the mRNA was translated into biologically active protein and, moreover, secreted by FF/BDNF. We relied on two bioassays that are standard for assessing the presence of BDNF: cultures obtained from embryonic chick DRG and fetal rat mesencephalon. Lack of specific antibody available to us prevented us from performing Western blot analysis or immunohistochemistry.
To obtain a qualitative indication of the biological activity of BDNF secreted by our fibroblasts, we cultured E9 chick DRG in the presence of CM-BDNF. Control cultures were incubated in supplemented DMEM alone, CM-β-gal, 100 ng/ml rhBDNF, or 100 ng/ml rhNGF. Neurite outgrowth from phase-bright cells was evident several hours after plating, and extensive processes were apparent the next day. After 48 hours, cultures were fixed and photographed.
Two days after plating, DRG cultures incubated in rhNGF, rhBDNF, and CM-BDNF contained phase-bright cells with extensive processes and few (< 10%) nonneuronal cells. CM-β-gal also supported the survival of substantially fewer DRG neurons with shorter processes. Control cultures incubated in supplemented DMEM contained almost no phase-bright cells with long processes after 48 hours in vitro. CM-BDNF did not support the survival of E9 cultured sympathetic neurons (data not shown), indicating the specificity of BDNF protein produced by FF/BDNF.
Fetal rat mesencephalic culture
We assessed the effects of CM-BDNF on cultures derived from E15 rat mesencephalon. Cultures were incubated in defined media, CM-BDNF, CM-FF, or in the presence of defined media containing rhBDNF. After 5 days, cultures incubated in CM-BDNF contained more TH-positive neurons with more extensive processes (Fig. 3C) than cultures incubated in control media (Fig. 3A) or CM-FF (Fig. 3B).
Fig. 3.
CM-BDNF were examined for biological activity in E15 fetal mesencephalic cultures. Photographs show tyrosine hydroxylase (TH)-immunoreactive (IR) mesencephalic neurons cultured for 5 days in control (N2) media (A), CM-FF (B), and CM-BDNF (C). Scale bar = 100 μm.
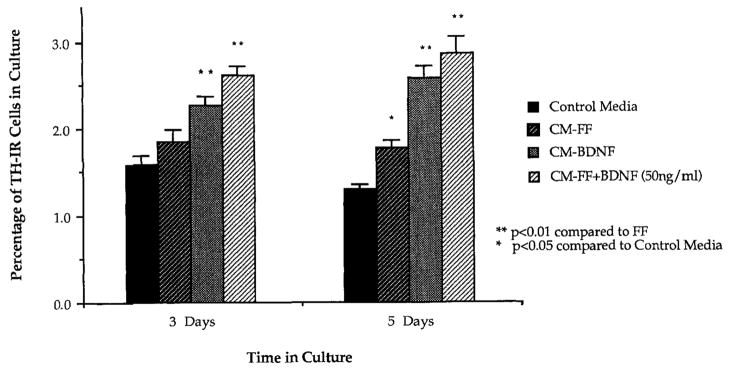
To obtain a more quantitative indication of the biological activity (and, therefore, of BDNF protein levels), we determined the number of TH-positive neurons in mesencephalic cultures. After 3 days in culture with control media, the percentage of TH-IR cells was 1.6% (±0.1%; SEM) and 1.3% (±0.06%; SEM) after 5 days. Cultures incubated with media conditioned by FF/BDNF showed a significant increase of TH-IR cells to 2.3% after 3 days in culture and to 2.6% after 5 days in culture. This effect was comparable to the effect of adding rhBDNF at 50 ng/ml to media conditioned by FF (Fig. 4). At 5 days in vitro, the percentage of TH-IR cells in cultures grown in CM-FF was increased compared to cultures exposed to defined media alone, similar to the effect of CM-β-gal on chick DRG cultures.
Fig. 4.
The number of TH-IR neurons was quantified in E15 fetal mesencephalic cultures. Cultures incubated with CM-BDNF contained more TH-IR cells than control cultures incubated with CM-FF at 3 and 5 days. The effect of media conditioned by FF/BDNF was similar to that of adding 50 ng/ml of rhBDNF to FF conditioned media. *P < .05; **P < .01 (ANOVA).
Effects of BDNF-transfected fibroblast grafts in vivo
Transcription of the BDNF transgene in vivo: In situ hybridization
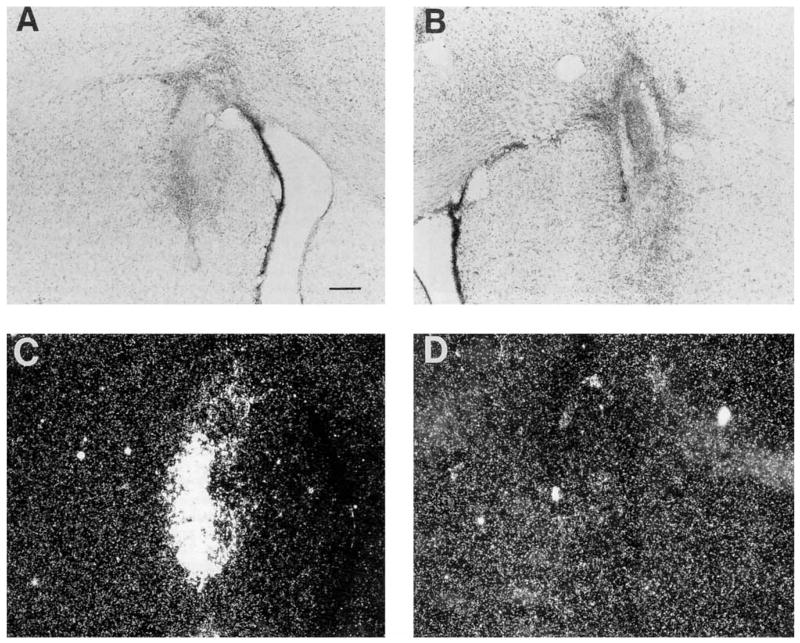
To investigate whether the BDNF transgene was transcribed in vivo, we performed in situ hybridization using riboprobes on tissue sections containing fibroblast grafts. Fisher 344 rats received bilateral grafts of FF/BDNF and FF/β-gal into the striatum. Thionin staining (Fig. 5A,B) depicts bilateral grafts in the same rat. One week following implantation, cells in the BDNF graft (Fig. 5C), but not in the contralateral β-gal graft (Fig. 5D), hybridized with the BDNF probe. The expected pattern of hybridization was observed in the hippocampus of the same rat. We observed hybridized cells within the grafts at 1 week following grafting, and hybridization was also seen (although to a lesser extent) at 2 weeks.
Fig. 5.
Nissl-stained sections of the striatum depict bilaterally placed FF/BDNF (A) and FF/b-gal (B) fibroblast grafts of one rat. In situ hybridization of the same tissue section with riboprobes shows BDNF mRNA expression in the FF/BDNF graft at 1 week in vivo (C). The control graft (FF/β-gal) does not hybridize with the BDNF probe (D). Scale bar = 200 μm.
Effects on the intact dopaminergic system
Having confirmed that FF/BDNF express BDNF mRNA in vitro and in vivo and that biologically active protein is secreted from the cells, we investigated the effects of FF/BDNF grafts on dopaminergic neurons in vivo. Grafts were stereotaxically placed in the target field striatum of normal adult rats. Because FF/NGF grafts encourage the ingrowth of cholinergic fibers (Kawaja and Gage, 1991), we analogously monitored the effect of the FF/BDNF grafts on dopaminergic neurite sprouting. Histological analysis of the grafts at time points covering 1–8 weeks showed good survival and placement of the fibroblast grafts but a lack of TH-IR processes within the grafts. Neurofilament staining was likewise negative (data not shown).
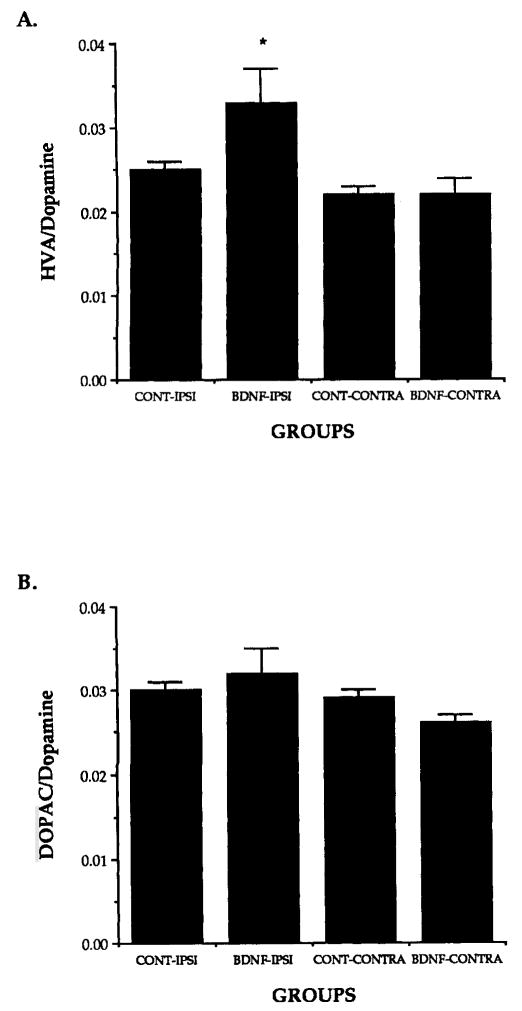
Following infusion of rhBDNF above the SNC, normal rats exhibit amphetamine-induced rotation behavior (Altar et al., 1992; Shults et al., 1994). We therefore assessed behavioral effects of FF/BDNF grafts in the midbrain (near the rostral end of the substantia nigra) by monitoring amphetamine-induced rotations. Because transgene expression driven by the retroviral promoter appears to be highest shortly after graft implantation (Palmer et al., 1991; Rang et al., unpublished data), rotational behavior was monitored at 2 days, 4 days, and 1 week following grafting. No behavioral changes were noted. Despite the lack of significant difference in rotation behavior, biochemical measurement of the dissected striatum 4 days after grafting showed evidence for increased turnover of dopamine by a significant increase in the HVA/dopamine ratio when grafts were placed next to the MFB (Fig. 6). Another experimental group with graft placement near the MFB showed no behavioral changes at longer time points of 1, 4, or 6 weeks following grafting.
Fig. 6.
A: FF/BDNF grafts produced a significant elevation of dopamine turnover as demonstrated by the ratio of the metabolite homovanillic acid (HVA)/dopamine in the striatum ipsilateral to FF/BDNF grafts (BDNF-IPSI) compared to contralateral sides of the same group (BDNF-CONTRA) and compared to the ipsilateral sides of the control grafts (CONT-IPSI). B: 3,4-dihydroxyphenylacetic acid (DOPAC)/dopamine ratios in the striatum, however, were not significantly elevated. *P < 0.05 (Tukey’s multiple comparison). BDNF-IPSI, FF/BDNF ipsilateral; BDNF-CONTRA, FF/BDNF contralateral; CONT-IPSI, FF/β-gal ipsilateral; CONT-CONTRA, FF/β-gal contralateral.
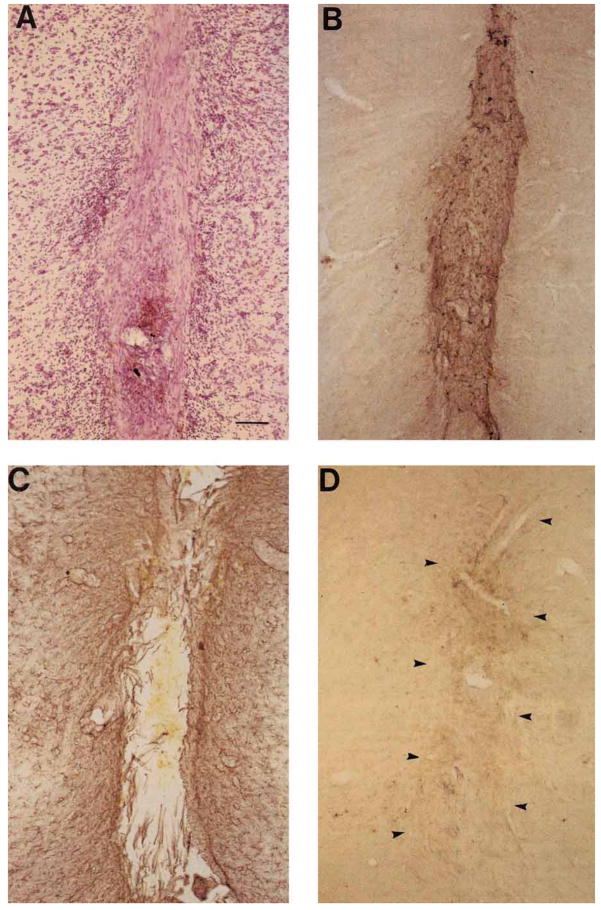
FF/BDNF grafts placed directly into the SNC in normal rats elicited sprouting of TH-IR and neurofilament immunoreactive (NF-IR) fibers when examined after 6 weeks (Fig. 7). Only those animals with FF/BDNF grafts placed directly in the SNC, partially disrupting the dopaminergic neurons, showed neuronal profiles observed within the graft (Fig. 7A,C). Similarly placed FF/β-gal grafts were devoid of TH-IR or NF-IR fibers (Fig. 7B,D). We assessed the viability of FF/BDNF grafts at 6 weeks following grafting, the longest time point shown in our study. Figure 8 depicts a typical FF/BDNF graft implanted into the ventral mesencephalon at 6 weeks. Coronal sections taken through the level of the substantia nigra were stained with picrofuchsin (pink) followed by a thionin counterstain (Fig. 8A) to visualize graft-associated collagen. At 6 weeks, grafts are healthy, as indicated by intense pink staining within the graft. Figure 8B also demonstrates graft viability with immunostaining directed against FN within the borders of the graft. Immunostaining directed against GFAP (Fig. 8C) showed astrocytic migration into the graft that has been previously shown to support axonal ingrowth (Kawaja et al., 1991). Mild macrophage infiltration was also observed as noted by OX-42 immunoreactivity (Fig. 8D).
Fig. 7.
Coronal sections at the level of the substantia nigra show FF/BDNF (A,C) and FF/β-gal (B,D) fibroblasts grafted into the ventral mesencephalon. Six weeks following implantation, coronal sections were immunostained for TH (A,B) and NF (C,D). TH-IR fibers (A) as well as NF-IR profiles (C) are evident in FF/BDNF grafts but not in FF/β-gal grafts (B,D). Scale bar = 50 μm.
Fig. 8.
Coronal sections at the level of the substantia nigra show a typical FF/BDNF graft 6 weeks following implantation into the ventral mesencephalon. Sections taken through one FF/BDNF graft are stained with picrofuchsin, followed by a thionin counterstain (A), and immuno-stained with antibodies directed against FN (B), GFAP (C), and OX-42 (D). Arrowheads in D indicate graft borders. Scale bar = 100 μm.
Effects on the 6-OHDA-lesioned dopaminergic system
BDNF has been shown to protect fetal dopaminergic neurons from the toxicity of MPP+ and 6-OHDA in vitro (Hyman et al., 1991; Spina et al., 1992). We therefore tested whether BDNF produced from FF/BDNF grafts placed in the striatum could protect against 6-OHDA toxicity. One week following grafting, a time at which transgene expression appears to be high and BDNF message is produced by the grafts in vivo, 6-OHDA lesions were made in the MFB. We monitored rotation behavior beginning 2 weeks after grafting. Behavioral analysis showed no differences between the FF/BDNF-grafted group and the control group in either amphetamine- or apomorphine-induced rotation (Fig. 9). Immunocytochemistry directed against TH revealed severe cell loss in the substantia nigra and a complete lack of TH-positive fibers in the ipsilateral striatum in both experimental and control groups. No differences in TH-IR profiles were observed in the FF/BDNF-grafted animals, even directly surrounding the graft. Grafting into the midbrain with subsequent ipsilateral 6-OHDA lesioning at similar time points also showed no significant behavioral or histological effects (data not shown).
Fig. 9.

Amphetamine (A)- and apomorphine (B)-induced rotation are plotted for animals that received 6-OHDA lesions in the medial forebrain bundle (MFB) 1 week following grafting of FF/BDNF or FF/β-gal into the striatum. Behavioral analysis showed no significant differences between the FF/BDNF-grafted group and the control group in either amphetamine- or apomorphine-induced rotation.
Effects on the intact basal forebrain cholinergic system
Grafts of FF/NGF induce the ingrowth of p75NGFR- and ChAT-immunoreactive fibers from the nucleus basalis when grafted into the intact striatum (Kawaja and Gage, 1991). Given that cholinergic neurons of the basal forebrain express p75NGFR (which binds BDNF) and respond to BDNF in vitro as well as in vivo, we monitored the sprouting of cholinergic fibers into striatal grafts. At time points covering 1–8 weeks, we observed no p75NGFR-, ChAT-, or NF-immunoreactive processes within the grafts (data not shown). Control (FF/β-gal) grafts were likewise devoid of neuronal fibers.
Effects on the fimbria-fornix lesioned basal forebrain cholinergic system
Cholinergic neurons of the medial septum primarily innervate the hippocampus via the fimbria-fornix, and transections of this afferent pathway result in the loss of cholinergic neurons. NGF, infused intraventricularly, is able to prevent the loss of approximately 90% of the cholinergic neurons. Intraventricular and intraparenchymal infusions of BDNF also rescue septal cholinergic neurons following lesions of the fimbria-fornix (Knusel et al., 1992; Morse et al., 1993). We investigated whether FF/BDNF grafts placed directly in the septum could avert cholinergic neuronal degeneration following fimbria-fornix lesions, as do grafts of FF/NGF (Rosenberg et al., 1988).
The effects of FF/BDNF grafts were examined 4 weeks following unilateral fimbria-fornix lesions. FF/NGF, which served as a positive control, grafted into the ipsilateral septum, encouraged the ingrowth of p75NGFR- and ChAT-immunoreactive fibers, but no immunoreactive profiles were seen in similarly placed FF/BDNF grafts (data not shown). FF/(β-gal grafts, which served as a negative control, were similarly devoid of immunoreactive fibers. Figure 10 depicts septal cholinergic neurons immunoreactive for p75NGFR and ChAT in each of the grafting paradigms (FF/BDNF, FF/NGF, FF/β-gal). Quantification of ChAT-positive cells in the medial septum revealed that about 50% of the septal neurons ipsilateral to the lesion were present in the animals grafted with FF/BDNF and FF/β-gal compared to the contralateral septum (Fig. 11). The cell savings elicited by the NGF grafts (approximately 80%) was significantly different from that of FF/β-gal and FF/BDNF grafted animals (P < 0.01). Quantification of p75NGFR-immunoreactive cells revealed a similar cell savings (over 90%) in the lesioned animals that received FF/NGF grafts as a positive control (again, significantly greater than FF/β-gal and FF/BDNF grafted animals; P < 0.01). FF/BDNF grafts tended to increase the proportion of ipsilateral/contralateral p75NGFR-positive cells above negative control values (68% vs. 60%), although not significantly.
Fig. 10.
Coronal sections through the medial septal region processed for p75NGFR (A,C,E) and ChAT immunohistochemistry (B,D,F) are shown 4 weeks following unilateral (right) aspirative lesions of the fimbria-fornix. A,B: Representative serial sections for a lesioned animal ipsilaterally grafted with FF/NGF at the time of lesion. C,D: FF/BDNF graft. E,F: FF/β-gal graft. Scale bar = 0.5 mm.
Fig. 11.
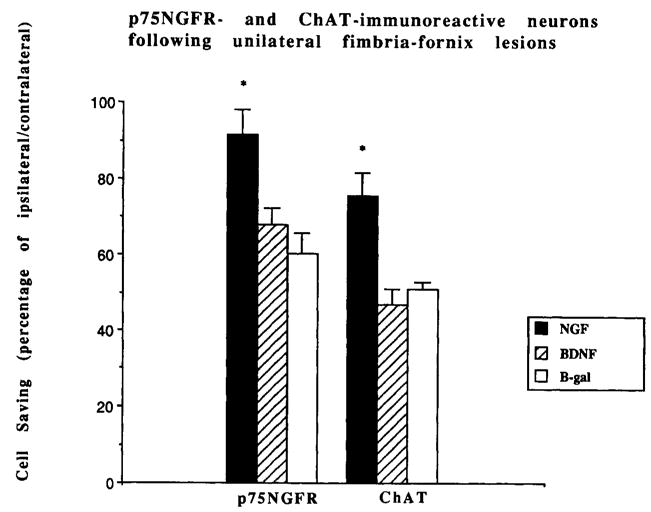
Percentage of ipsilateral/contralateral septal neurons quantified following fibroblast grafts and lesions of the fimbria-fornix. p75NGFR and ChAT-immunoreactive cells (±SEM) are depicted for each of the grafting conditions (FF/NGF, FF/BDNF, and FF/β-gal). *P < 0.01 (ANOVA).
DISCUSSION
Production of BDNF by genetically modified cells
In this study, we show that the genetic modification of fibroblasts to effect trophic factor delivery in vivo is a generalizable technique. Genetically engineered fibroblasts express mRNA corresponding to the BDNF transgene both in vitro and in vivo, as determined by Northern blot analysis and in situ hybridization. Like NGF, BDNF is transcribed in precursor form and subsequently proteolytically processed to yield the mature, biologically active protein (Jones and Reichardt, 1990). The BDNF minigene containing coding information for the precursor form is transcribed and apparently properly processed to secrete the mature, biologically active form. Our previous experience with NGF, which is encoded by a similar pre-pro sequence, suggests a similar capacity of primary fibroblast cells. Our evidence that NGF is secreted in the properly processed form is more direct, in that we can measure quantitatively amounts of secreted protein by ELISA. Lack of specific antibody available to us precluded similar quantitative demonstration for BDNF. Instead, we rely on data from bioassays to analyze conditioned media for the presence of biologically active BDNF.
BDNF supports the survival of embryonic chick DRG neurons and rat fetal mesencephalic neurons (Davies et al., 1986; Hyman et al., 1991). CM-BDNF likewise promote the survival of these neuronal populations. Moreover, the number of TH-IR cells in fetal mesencephalic cultures incubated in 50 ng/ml rhBDNF added to CM-FF was not different from that of cultures incubated in CM-BDNF (from 1 million preconfluent cells in a 24-hour time period in vitro; Fig. 4). Again, this is consistent with our previous experience with FF/NGF, where 106 FF/NGF produce between 10 and 50 ng/ml NGF over 24 hours (unpublished data).
Importantly, we also observed expression of the BDNF transgene in vivo. Riboprobes specific to the BDNF transcript hybridize with fibroblast cells in FF/BDNF, but not FF/β-gal grafts, for at least 1 to 2 weeks following grafting. Moreover, grafts of FF/BDNF remain viable and integrate well within the host tissue at least 6 weeks following implantation (Fig. 7). Picrofuchsin staining indicates graft production of collagen, whereas immunostaining with FN, which recognizes a group of structurally related glycoproteins, also indicates the production of fibroblast-specific proteins. Immunostaining with antibodies directed against GFAP demonstrates astrocytic migration into the grafts and, therefore, integration within the host brain. The minor macrophage infiltration observed with OX-42 immunostaining also indicates the integration as well as the viability of FF/BDNF grafts at the longest time point depicted in this study. These data, in combination with previous reports from our laboratory demonstrating long-term graft viability using ultrastructural analysis (Kawaja et al., 1991), suggest that the structural integrity of FF/BDNF grafts at 6 weeks was sufficient to pursue further grafting experiments.
BDNF effects on the adult dopaminergic system in vivo
BDNF promotes the survival of fetal dopaminergic neurons in vitro (Hyman et al., 1991; this study), but the role that BDNF plays in the adult dopaminergic system is unclear. We noted increased turn-over of dopamine as evidenced by a small but significant difference in HVA/dopamine ratios ipsilateral to FF/BDNF grafts placed near the substantia nigra. We did not, however, observe differences in amphetamine-induced rotation behavior following grafting into the midbrain. When rhBDNF protein is administered in vivo at 12.5 μg/day, several effects have been reported. BDNF infusion into the midbrain produces slight amphetamine-induced rotation behavior in the normal rat and enhances dopamine turn over in the ipsilateral striatum, as noted by increased HVA/dopamine ratios (Altar et al., 1992; Martin-Iverson et al., 1994). We believe that the differences between our data and the infusion data of Altar et al. (1992) are due to lesser amounts of BDNF introduced to the brain by somatic cell delivery. We expect that FF/BDNF grafts deliver approximately nanogram or lesser amounts of BDNF, as they consist originally of 2–3 × 105 cells/graft (with rough estimates of tens of nanograms produced by 106 cells in vitro). This fact, in and of itself, could explain the modest effect from our grafts, although our results parallel those obtained by high dose infusion. Because HVA levels reflect both intra- and extraneuronal dopamine metabolism, they may be one of the more sensitive parameters describing dopamine metabolism. The significance of augmented rotation behavior is ambiguous, however. Although BDNF apparently has a biochemical effect, behavioral effects observed following very high infusion concentrations are likely to be nonphysiological.
The most striking effect we observed was the sprouting of dopaminergic neurons of the substantia nigra into FF/BDNF grafts. TH-IR and NF-IR profiles are only evident when grafts are placed directly adjacent to, or partially in, the SNC. We did not observe sprouting of dopaminergic, neuronal profiles when grafts were stereotaxically placed in the striatum; neither does intraventricular infusion of BDNF induce sprouting of dopaminergic profiles (Knusel et al., 1992). Dopaminergic ingrowth is observed only when grafts directly interrupt the cell bodies of nigral neurons, suggesting that levels of BDNF are highest directly surrounding the graft. In situ hybridization studies show BDNF mRNA in the striatum, the target region of nigral neurons (Hofer et al., 1990), but BDNF mRNA is also expressed in the cell bodies of nigral neurons themselves (Friedman et al., 1991; Gall et al., 1992). This finding greatly complicates the classical view of neurotrophic factors but is interesting in light of our results. BDNF may function in an autocrine fashion as a locally derived, rather than target-derived, neurotrophic factor in vivo (Schecterson and Both well, 1992). Although retrograde transport of 125I-BDNF to the substantia nigra following infusion into the striatum has been reported (Wiegand et al., 1991), this may not be the normal mechanism by which BDNF exerts its effects on dopaminergic cell bodies.
BDNF also protects fetal dopaminergic neurons from MPP+ and 6-OHDA toxicity (Hyman et al., 1991; Spina et al., 1992). Protection may be mediated via the glutathione system, reducing the toxicity of free radicals generated from these toxins. Implications for the pathogenesis of and possible therapeutic approaches to PD are significant. We therefore addressed whether BDNF produced by fibroblast grafts could protect against 6-OHDA toxicity in vivo. We saw no sparing of TH-IR neurons or axons or evidence of dopaminergic sprouting into the grafts. The lack of sprouting probably reflects the fact that our lesions were complete and the toxicity too potent to overcome. FF/BDNF grafts do not protect dopaminergic neurons from 6-OHDA toxicity in vivo. This is consistent with reports that BDNF is not able to support dopaminergic neurons following transection of the MFB (Knusel et al., 1992). Protective effects of BDNF for dopaminergic neurons in vitro may not be an adequate model for the in vivo situation; however, higher doses of BDNF, other methods of administration, or BDNF’s introduction in combination with other trophic factors may be effective.
BDNF effects on cholinergic neurons in vivo
Some reports indicate that intraventricular and intraparenchymal infusions of microgram amounts of BDNF rescue cholinergic neurons following lesions of the fimbria-fornix (Knusel et al., 1992; Morse et al., 1993). However, more recent data suggest that the protective effect of BDNF is limited to p75NGFR induction (Koliatsos et al., 1994). Grafts of FF/BDNF did not protect ChAT-immunoreactive septal cells following fimbria-fornix lesions. Discrepancies between our results and those of others may be due to differences of several orders of magnitude (nanograms/day vs. micrograms/day) of delivery of BDNF to the septal cholinergic population. Despite the lack of protection of cholinergic cells by FF/BDNF grafts, as indicated by quantification of ChAT-immunoreactive neurons, we did observe a slight increase in the proportion of neurons (ipsilateral/contralateral) expressing p75NGFR. Widmer et al. (1993) recently showed more complete protection of septal neurons expressing p75NGFR than ChAT with BDNF infusion, and data from Koliatsos et al. (1994) suggest that the effect of BDNF on lesioned cholinergic neurons is primarily that of p75NGFR induction. The slight trend towards increased p75NGFR expression we observed is consistent with these findings. Up-regulation of p75NGFR, which occurs with neurotrophin administration in vivo (Cavicchioli et al., 1989; Higgins et al., 1989), may indicate small differences brought about by expression of the BDNF transgene.
In vivo delivery of BDNF by genetically modified fibroblasts
On the surface, there appear to be some discrepancies between our data and findings from infusion studies. Three explanations may account for these disparities: 1) FF/BDNF grafts do not secrete biologically active protein in vivo or do so only very transiently; 2) FF/BDNF are not able to produce enough of the correctly processed protein to produce the expected result in vivo; and 3) given that the amount of protein is sufficient to produce the expected effect, it is not able to reach the cell population of interest.
It is likely for several reasons that FF/BDNF grafts secrete BDNF protein in vivo. BDNF mRNA and protein are produced in vitro, and expression of BDNF mRNA is seen in vivo for up to 2 weeks. NGF, which is similar in signal sequence, size, and homology, is secreted from FF/NGF grafts in vivo, as indicated by cholinergic sprouting into the grafts and a sparing of septal cholinergic neurons following fimbria-fornix transection. Additionally, grafts of FF/BDNF induce dopaminergic sprouting and increase HVA/dopamine ratios when grafted into the midbrain. FF/BDNF grafts may also increase expression of the p75NGFR in the fimbria-fornix lesioned brain. We acknowledge, however, that limits to the amount of transgene produced exist, and stable, long-term gene expression in fibroblasts, as well as graft survival and integration into host tissue, are additional factors that must be considered.
The more likely explanation for differences between our in vivo findings and those described by others is that the level of BDNF produced by genetically modified fibroblasts is not comparable to amounts of BDNF infused. The small but significant differences we see in the HVA/dopamine ratios in FF/BDNF-grafted rats are consistent with this interpretation. Likewise, the trend towards increased expression of p75NGFR may indicate small differences brought about by expression of the BDNF transgene.
Another major factor concerning the bioavailability of BDNF in both our studies and infusion studies is that diffusion of BDNF is limited in vivo. Truncated forms of trkB lacking the catalytic kinase domain (Klein et al., 1990; Middlemas et al., 1991) may be responsible for sequestering the protein. Experiments involving the infusion of BDNF following fimbria-fornix lesions illustrate the importance of this phenomenon. Cholinergic neurons are rescued after axotomy following daily infusion of microgram amounts of BDNF (Knusel et al., 1992; Morse et al., 1993). However, the preventive effect of BDNF on degeneration of cholinergic cells after axotomy is significantly more pronounced when the trophic molecule is infused intraparenchymally rather than intraventricularly (Morse et al., 1993), presumably because intraparenchymal infusion circumvents the amount of BDNF that can bind to the truncated trkB receptor present on the ventricular wall. Because of the presence of the truncated form of the trkB receptor in the parenchyma as well, the proximity of the injection site is a crucial determinant. In support of this, sprouting of TH-, NF-IR processes is only observed in grafts directly adjacent to the cell bodies of nigral neurons.
Our results suggest that genetically modified fibroblasts may provide an effective method of delivering trophic molecules in vivo. FF/BDNF grafts afford the possibility of discrete, localized delivery, which is especially important in light of truncated forms of trkB receptors that limit diffusion of trophic molecules in vivo. Moreover, fibroblast grafts allow us to study uniquely the neurite-promoting properties of trophic molecules in vivo. Despite limits in the amount and length of time of trophic factor expression by fibroblasts in vivo, we observe dopaminergic sprouting into FF/BDNF grafts implanted into the SNC as well as increased HVA/dopamine levels. Additionally, there is a trend towards increased expression of the p75NGFR in septal cholinergic neurons following fimbria-fornix transection. It remains unclear whether or not BDNF has a protective effect on adult dopaminergic neurons in vivo and is potentially therapeutic for PD. When extrapolating these data to clinical situations, however, it should be emphasized that PD is a chronic process involving cell death over a long time period, whereas 6-OHDA toxicity causes acute degeneration of dopaminergic neurons. We are yet in need of better models of neurodegenerative processes to test the effects of substances like BDNF.
Acknowledgments
We thank Mary Lynn Gage for her helpful suggestions regarding the manuscript. This work was supported by PHS grant AG00408 and American Academy of Neurology Research Fellowship to U.J.K., NIH grants 5P01 AG10435 and 5R37 AG08514 to F.H.G., and NIH grant MH48200 to K.R.J. and L.F.R. and by a Merit Review Grant from the Department of Veteran’s Affairs to C.W.S. C.A.L.-P. was supported by NIH grant 5T32 AG00216.
Abbreviations
- ChAT
choline acetyltransferase
- CM-β-gal
β-gal conditioned media
- CM-BDNF
brain-derived neurotrophic factor-conditioned media
- CM-FF
uninfected fibroblast conditioned media
- FF
fibroblasts
- FF/β-gal
β-gal-transduced fibroblasts
- FF/BDNF
brain-derived neurotrophic factor-transduced fibroblasts
- FF/NGF
nerve growth factor-transduced fibroblasts
- FN
fibronectin
- GFAP
glial fibrillary acidic protein
- NF
neurofilament
- p75NGFR
low-affinity NGF receptor
- TH
tyrosine hydroxylase
LITERATURE CITED
- Altar CA, Boylan CB, Jackson C, Hershenson S, Miller J, Wiegand SJ, Lindsay RM, Hyman C. Brain-derived neurotrophic factor augments rotational behavior and nigrostriatal dopamine turnover in vivo. Proc Natl Acad Sci USA. 1992;89:11347–11351. doi: 10.1073/pnas.89.23.11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel SH. A unifying hypothesis for the cause of amyotrophic lateral sclerosis, Parkinsonism, and Alzheimer disease. Ann Neurol. 1981;68:2417–2420. doi: 10.1002/ana.410100602. [DOI] [PubMed] [Google Scholar]
- Barde YA. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Beck KD, Knusel B, Winslow JW, Rosenthal A, Burton LE, Nikolics K, Hefti F. Pretreatment of dopaminergic neurons in culture with brain-derived neurotrophic factor attenuates toxicity of 1-methyl-4-phenylpyridinium. Neurodegeneration. 1992;1:27–36. [Google Scholar]
- Berkemeier LR, Winslow JW, Kaplan DR, Nikolics K, Goeddel DV, Rosenthal A. Neurotrophin-5: A novel neurotrophic factor that activates trk and trkB. Neuron. 1991;7:857–866. doi: 10.1016/0896-6273(91)90287-a. [DOI] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O. Brain dopamine and the syndromes of Parkinson and Huntington—Clinical, morphological, and neurochemical correlations. J Neurol Sci. 1973;20:415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Bothwell M. Keeping track of neurotrophin receptors. Cell. 1991;65:915–918. doi: 10.1016/0092-8674(91)90540-f. [DOI] [PubMed] [Google Scholar]
- Cavicchioli L, Flanigan TP, Vantini G, Fusco M, Polato P, Toffano G, Walsh FS, Leon A. NGF amplifies expression of NGF receptor messenger RNA in forebrain cholinergic neurons of rats. Eur J Neurosci. 1989;1:258–262. doi: 10.1111/j.1460-9568.1989.tb00793.x. [DOI] [PubMed] [Google Scholar]
- Chao MV, Bothwill MA, Ross AH, Koprowski H, Lanahan AA, Buck R, Sehgal A. Gene transfer and molecular cloning of the human NGF receptor. Science. 1986;232:518–521. doi: 10.1126/science.3008331. [DOI] [PubMed] [Google Scholar]
- Davies AM. Neurotrophic factor bioassay using dissociated neurons. In: Rush RA, editor. Nerve Growth Factors. IBRO Handbook Series: Methods in the Neurosciences. New York: John Wiley and Sons; 1989. pp. 95–109. [Google Scholar]
- Davies AM, Thoenen H, Barde YA. The response of chick sensory neurons to brain-derived neurotrophic factor. J Neurosci. 1986;6:1897–1904. doi: 10.1523/JNEUROSCI.06-07-01897.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano PS, Friedman B, Radziejewski C, Alexander C, Boland P, Schick CM, Lindsay RM, Wiegand SJ. The neurotrophins BDNF, NT-3, and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron. 1992;8:983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- Friedman WJ, Olson L, Persson H. Cells that express brain-derived neurotrophic factor mRNA in the developing postnatal rat brain. Eur J Neurosci. 1991;3:688–697. doi: 10.1111/j.1460-9568.1991.tb00854.x. [DOI] [PubMed] [Google Scholar]
- Gage FH, Fisher LJ. Intracerebral grafting: A tool for the neurobiologist. Neuron. 1991;6:1–12. doi: 10.1016/0896-6273(91)90116-h. [DOI] [PubMed] [Google Scholar]
- Gage FH, Bjorklund A, Stenevi U. Reinnervation of the partially deafferented hippocampus by compensatory collateral sprouting from spared cholinergic and noradrenergic afferents. Brain Res. 1983;268:27–37. doi: 10.1016/0006-8993(83)90387-6. [DOI] [PubMed] [Google Scholar]
- Gage FH, Armstrong DM, Williams LR, Varon S. Morphological response of axotomized septal neurons to nerve growth factor. J Comp Neurol. 1988;269:147–155. doi: 10.1002/cne.902690112. [DOI] [PubMed] [Google Scholar]
- Gage FH, Kawaja MD, Fisher LJ. Genetically modified cells: Applications for intracerebral grafting. Trends Neurosci. 1991;14:328–333. doi: 10.1016/0166-2236(91)90156-o. [DOI] [PubMed] [Google Scholar]
- Gall CM, Gold SJ, Isackson PJ, Seroogy KB. Brain-derived neurotrophic factor and neurotrophin-3 mRNAs are expressed in ventral midbrain regions containing dopaminergic neurons. Mol Cell Neurosci. 1992;2:53–63. doi: 10.1016/1044-7431(92)90009-q. [DOI] [PubMed] [Google Scholar]
- Hefti F. Is Alzheimer Disease caused by lack of nerve growth factor? Ann Neurol. 1983;13:109–110. doi: 10.1002/ana.410130127. [DOI] [PubMed] [Google Scholar]
- Hefti F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J Neurosci. 1986;8:2155–2162. doi: 10.1523/JNEUROSCI.06-08-02155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Koh S, Chen KS, Gage FH. NGF induction of NGF receptor gene expression and cholinergic neuronal hypertrophy within the basal forebrain of the adult rat. Neuron. 1989;3:247–256. doi: 10.1016/0896-6273(89)90038-x. [DOI] [PubMed] [Google Scholar]
- Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990;9:2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto S, Lindsay RM. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Hyman C, Juhasz M, Jackson C, Wright P, Ip NY, Lindsay RM. Overlapping and distinct actions of the neurotrophins BDNF, NT-3, and NT-4/5 on cultured dopamineric and GABAergic neurons of the ventral mesencephalon. J Neurosci. 1994;14:335–347. doi: 10.1523/JNEUROSCI.14-01-00335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KR, Reichardt LF. Molecular cloning of a human gene that is a member of the nerve growth factor family. Proc Natl Acad Sci USA. 1990;87:8060–8064. doi: 10.1073/pnas.87.20.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DR, Martin-Zanca DM, Parada LF. Tyrosine phosphorylation of and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991;350:158–160. doi: 10.1038/350158a0. [DOI] [PubMed] [Google Scholar]
- Kawaja MD, Gage FH. Reactive astrocytes are substrates for the growth of adult CNS axons in the presence of elevated levels of nerve growth factor. Neuron. 1991;7:1019–1030. doi: 10.1016/0896-6273(91)90346-2. [DOI] [PubMed] [Google Scholar]
- Kawaja MD, Fagan AM, Firestein BL, Gage FH. Intracerebral grafting of cultured autologous fibroblasts into the rat striatum: An assessment of graft size and ultrastructure. J Comp Neurol. 1991;307:695–706. doi: 10.1002/cne.903070414. [DOI] [PubMed] [Google Scholar]
- Kawaja MD, Rosenberg MB, Yoshida K, Gage FH. Somatic gene transfer of nerve growth factor promotes the survival of axotomized septal neurons and the regeneration of their axons in adult rats. J Neurosci. 1992;12:2849–2864. doi: 10.1523/JNEUROSCI.12-07-02849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Conway D, Parada LF, Barbacid M. The trkB tyrosine kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell. 1990;61:647–656. doi: 10.1016/0092-8674(90)90476-u. [DOI] [PubMed] [Google Scholar]
- Knusel B, Winslow JW, Rosenthal A, Burton LE, Seid DP, Nikolics K, Hefti F. Promotion of central cholinergic and dopaminergic neuron differentiation by brain-derived neurotrophic factor but not neurotrophin 3. Proc Natl Acad Sci USA. 1991;88:961–965. doi: 10.1073/pnas.88.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knusel B, Beck KD, Winslow JW, Rosenthal A, Burton A, Widmer HR, Nikolics K, Hefti F. Brain-derived neurotrophic factor administration protects basal forebrain cholinergic but not nigral dopaminergic neurons from degenerative changes after axotomy in the adult rat brain. J Neurosci. 1992;12:4391–4402. doi: 10.1523/JNEUROSCI.12-11-04391.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliatsos VE, Price DL, Gouras GK, Cayouette MH, Burton LE, Winslow JW. Highly selective effects of nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 on intact and injured basal forebrain magnocellular neurons. J Comp Neurol. 1994;343:247–262. doi: 10.1002/cne.903430206. [DOI] [PubMed] [Google Scholar]
- Korsching S, Auburger G, Heumann R, Scott J, Theonen H. Levels of nerve growth factor and its mRNA in the central nervous system of the rat correlate with cholinergic innervation. EMBO J. 1985;4:1389–1393. doi: 10.1002/j.1460-2075.1985.tb03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromer L. Nerve growth factor treatment after brain injury prevents neuronal death. Science. 1987;235:214–216. doi: 10.1126/science.3798108. [DOI] [PubMed] [Google Scholar]
- Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P, Thoenen H, Barde YA. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989;341:149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Belluscio L, Squinto S, Ip NY, Furth ME, Lindsay RM, Yancopoulos GD. Neurotrophin-3: A neurotrophic factor related to NGF and BDNF. Science. 1990;247:1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- Martin-Iverson MT, Todd KG, Altar CA. Brain-derived neurotrophic factor and neurotrophin-3 activate striatal dopamine and serotonin metabolism and related behaviors: Interactions with amphetamine. J Neurosci. 1994;14:1262–1270. doi: 10.1523/JNEUROSCI.14-03-01262.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meakin SO, Shooter EM. Molecular investigation of the high-affinity nerve growth factor receptor. Neuron. 1991;6:153–163. doi: 10.1016/0896-6273(91)90130-r. [DOI] [PubMed] [Google Scholar]
- Meakin SO, Shooter EM. The nerve growth factor family of receptors. Trends Neurosci. 1992;15:323–331. doi: 10.1016/0166-2236(92)90047-c. [DOI] [PubMed] [Google Scholar]
- Middlemas DS, Lindberg RA, Hunter T. TrkB, a neural receptor protein-tyrosine kinase: Evidence for a full-length and two truncated receptors. Mol Cell Biol. 1991;11:143–153. doi: 10.1128/mcb.11.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse JK, Wiegand SJ, Anderson K, You Y, Cai N, Carnahan J, Miller J, DiStefano PS, Altar CA, Lindsay RM, Anderson RF. Brain derived neurotrophic factor (BDNF) prevents the degeneration of medial septal cholinergic neurons following fimbria transection. J Neurosci. 1993;13:4146–4156. doi: 10.1523/JNEUROSCI.13-10-04146.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonomura T, Hatanaka H. Neurotrophic effect of brain-derived neurotrophic factor on basal forebrain cholinergic neurons in culture from postnatal rats. Neurosci Res. 1992;14:226–233. doi: 10.1016/0168-0102(92)90083-o. [DOI] [PubMed] [Google Scholar]
- Olton DS. Dementia: Animal models of the cognitive impairments following damage to the basal forebrain cholinergic system. Brain Res Bull. 1990;25:499–502. doi: 10.1016/0361-9230(90)90242-r. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Rosman GJ, Osborne WRA, Miller AD. Genetically modified fibroblasts persist long after transplantation but gradually inactivate introduced genes. Proc Natl Acad Sci USA. 1991;88:1330–1334. doi: 10.1073/pnas.88.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps CH. Trophic factors and Alzheimer’s disease. Neurobiol Aging. 1989;10:584–586. doi: 10.1016/0197-4580(89)90139-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg MB, Friedmann T, Robertson RC, Tuszynski M, Wolff JA, Breakefield XO, Gage FH. Grafting genetically modified cells to the damaged brain: Restorative effects of NGF expression. Science. 1988;242:1575–1578. doi: 10.1126/science.3201248. [DOI] [PubMed] [Google Scholar]
- Sauer H, Fischer W, Nikkhah G, Weigand SJ, Brundin P, Lindsay RM, Bjorklund A. Brain-derived neurotrophic factor enhances function rather than survival of intrastriatal dopamine cell-rich grafts. Brain Res. 1993;626:37–4. doi: 10.1016/0006-8993(93)90560-a. [DOI] [PubMed] [Google Scholar]
- Schecterson LC, Bothwell M. Novel roles for neurotrophins are suggested by BDNF and NT-3 mRNA expression in developing neurons. Neuron. 1992;9:449–463. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Otten U, Agid Y, Thoenen H. Nerve growth factor (NGF) in the rat CNS: Absence of specific retrograde axonal transport and tyrosine hydroxylase induction in locus coeruleus and substantia nigra. Brain Res. 1979;168:473–483. doi: 10.1016/0006-8993(79)90303-2. [DOI] [PubMed] [Google Scholar]
- Seiler M, Schwab ME. Specific retrograde transport of nerve growth factor (NGF) from neocortex to nucleus basalis in the rat. Brain Res. 1984;300:33–39. doi: 10.1016/0006-8993(84)91338-6. [DOI] [PubMed] [Google Scholar]
- Sheehan DC, Hrapchak BB. Theory and Practice of Histology. St. Louis: C.V. Mosby Co; 1973. p. 107. [Google Scholar]
- Shelton DL, Reichardt LF. Studies on the expression of the beta-nerve growth factor (NGF) gene in the central nervous system: Level and regional distribution of NGF mRNA suggest that NGF functions as a trophic factor for several distinct populations of neurons. Proc Natl Acad Sci USA. 1986;83:2714–2718. doi: 10.1073/pnas.83.8.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shults CW, Matthews RT, Altar CA, Hill LR, Langlais PJ. A single intramesencephalic injection of brain-derived neurotrophic factor induces persistent rotational asymmetry in rats. Exp Neurol. 1994;125:183–194. doi: 10.1006/exnr.1994.1023. [DOI] [PubMed] [Google Scholar]
- Spina MB, Squinto SP, Miller J, Lindsay RM, Hyman C. Brain-derived neurotrophic factor protects dopamine neurons against 6-hydroxydopamine and N-methyl-4-phenylpyridinium ion toxicity: Involvement of the glutathione system. J Neurochem. 1992;59:99–106. doi: 10.1111/j.1471-4159.1992.tb08880.x. [DOI] [PubMed] [Google Scholar]
- Theonen H. The changing scene of neurotrophic factors. Trends Neurosci. 1991;14:165–170. doi: 10.1016/0166-2236(91)90097-e. [DOI] [PubMed] [Google Scholar]
- Widmer HR, Knusel B, Hefti F. BDNF protection of basal forebrain cholinergic neurons after axotomy: Complete protection of p75NGFR-positive cells. Neuroreport. 1993;4:363–366. doi: 10.1097/00001756-199304000-00005. [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Alexander C, Lindsay RM, DiStefano PS. Axonal transport of [125I]-labeled neurotrophins in the central nervous system. Soc Neurosci Abstr. 1991:446. [Google Scholar]
- Williams LW, Varon S, Peterson GM, Wictorin K, Fischer W, Bjorklund A, Gage FH. Continuous infusion of nerve growth factor prevents basal forebrain neuronal death after fimbria fornix transection. Proc Natl Acad Sci USA. 1986;83:9231–9235. doi: 10.1073/pnas.83.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]